Electroanalytical Approaches to Combatting Food Adulteration: Advances in Non-Enzymatic Techniques for Ensuring Quality and Authenticity
Abstract
1. Introduction
| Foodstuff/Beverage | Adulterants | Reference |
|---|---|---|
| Olive oil | Seed oils (e.g., sunflower, soybean, sesame, corn, and hazelnut oil), olive oil of lower grade (e.g., olive pomace oil, lampante olive oil). | [1,11] |
| Honey | Sugar, rice syrups, barley syrups, corn syrups, rice molasses, and less expensive honey (e.g., polyfloral). | [2,12,13] |
| Milk and dairy products | Water, starch, glucose and other sugars, soybean and pea protein isolates, boric acid, salicylic acid, benzoic acid, melamine, urea, maltodextrose, cheese whey (byproduct of cheese production), hydrogen peroxide, and reconstituted skim milk powder. Different milk species (cow’s, sheep’s, and buffalo’s milk). | [1,2,8,14] |
| Wine | Synthetic sweeteners (e.g., saccharin), sugar, ethanol, flavor, water, synthetic dyes, and apple juice. | [1,3,5,6,7] |
| Whiskey | Alcohol (non-drinking or cereal alcohol), water caramel, dyes, flavors, beverages of lower commercial value, whiskey of different brands, aging, and blending (lower cost). | [1,4] |
| Beer | Flavor, different brands, and fermentation type (lower cost). | [15] |
| Fruit juices | Dilution with water, addition of other, less expensive fruit juices (e.g., lemon and grape fruit juice in orange juice), glucose-fructose syrup, formaldehyde, artificial flavor agents and dyes (e.g., rhodamine B), and salicylic acid. | [16,17,18,19,20,21] |
| Coffee | Grains (e.g., soybean, corn, barley, rice, triticale, rye, and chicory), brown sugar, defective coffee beans, coffee processing byproducts (e.g., coffee husks, sticks, and used coffee grounds), and cheaper varieties (e.g., Robusta (Coffea canephora) in Arabica (Coffea arabica)). | [22,23] |
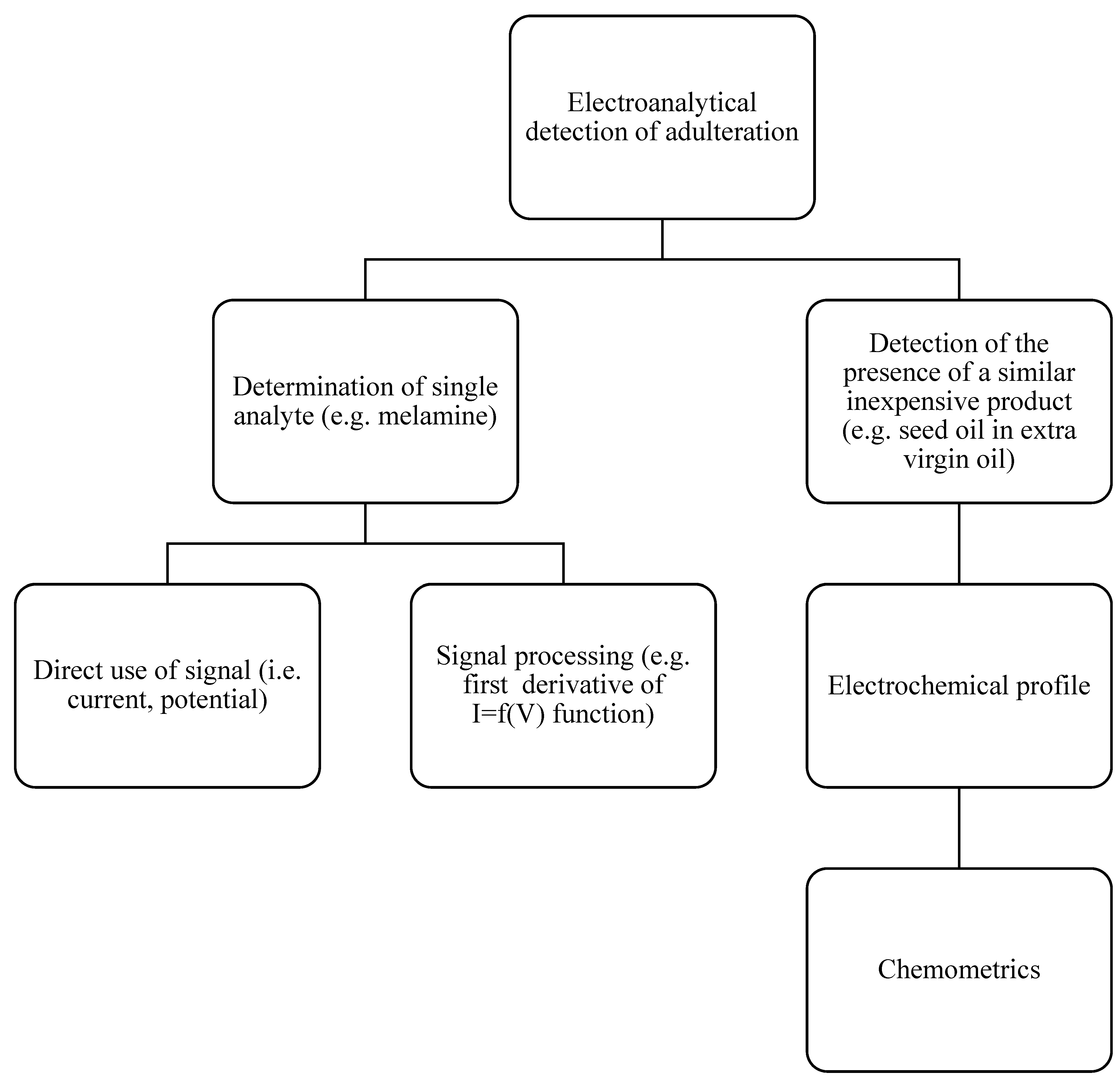
2. Analytical Strategy for Detection/Quantification of Food Adulteration
- ▪
- ▪
- Non-Specific Fingerprinting: Instead of targeting specific analytes, this approach uses the entire analytical signal (e.g., spectrum or voltammogram) as a multivariate representation of the sample’s chemical composition. Non-specific fingerprints can be generated using techniques like Ultra-Violet (UV-Vis) Spectrometry [26,27], Fourier Transform Infrared Spectrometry (FT-IR) [28], Fluorescence Spectrometry [26], Mid-Infrared Spectroscopy (MIR) [29], Raman Spectrometry [26], Nuclear Magnetic Resonance (NMR) [26,30], chromatography [31], Mass Spectrometry [32], or even Differential Scanning Calorimetry [33].
3. Olive Oil
4. Honey
5. Milk and Dairy Products
6. Wines
7. Other Alcoholic Beverages
8. Fruit Juices
9. Coffee
10. Future Trends and Perspectives
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ATLD | Alternating Trilinear Decomposition |
| DPV | Differential Pulse Voltammetry |
| EDTA | Ethylenediaminetetraacetic Acid |
| EIS | Electrochemical Impedance Spectroscopy |
| EMA | Economically Motivated Adulteration |
| FPCA | Functional Principal Component Analysis |
| GCE | Glassy Carbon Electrode |
| HCA | Hierarchical Cluster Analysis |
| K-NN | K-nearest Neighbors |
| LDA | Linear Discriminant Analysis |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MWCNT | Multi-walled Carbon Nanotube |
| NMR | Nuclear Magnetic Resonance (NMR) Spectroscopy |
| PCA | Principal Component Analysis |
| PLS | Partial Least Squares |
| PLS-DA | Partial Least Squares-Discriminant Analysis |
| PLS-LDA | Partial Least Squares-Linear Discriminant Analysis |
| POPD | Poly-Orthophenylene Diamine |
| rGO | Reduced Graphene Oxide |
| RTIL | Room Temperature Ionic Liquid |
| SIMCA | Soft Independent Modeling of Class Analogy |
| SVM | Support Vector Machine |
| SVMDA | Support Vector Machine Discriminant Analysis |
| SWV | Square Wave Voltammetry |
References
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef] [PubMed]
- Anagaw, Y.K.; Ayenew, W.; Limenh, L.W.; Geremew, D.T.; Worku, M.C.; Tessema, T.A.; Simegn, W.; Mitku, M.L. Food adulteration: Causes, risks, and detection techniques-review. SAGE Open Med. 2024, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burda, K.; Collins, M. Adulteration of wine with sorbitol and apple juice. J. Food Prot. 1991, 54, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Novakowski, W.; Bertotti, M.; Paixao, T.R.L.C. Use of copper and gold electrodes as sensitive elements for fabrication of an electronic tongue: Discrimination of wines and whiskies. Microchem. J. 2011, 99, 145–151. [Google Scholar] [CrossRef]
- Lin, S.; Salcido-Keamo, S. Fraud in wine and other alcoholic beverages (Chapter 12). In Food Fraud; Hellberg, R.S., Everstine, K., Sklare, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 233–247. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, F.; Gutierrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Fang, Y.; Ma, T. Real wine or not? Protecting wine with traceability and authenticity for consumers: Chemical and technical basis, technique applications, challenge, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 6783–6808. [Google Scholar] [CrossRef]
- Liveri, M.; Tsantili-Kakoulidou, A.; Tsopelas, F. Identification of white wine adulteration with apple juice and apple cider using cyclic voltammetry on a screen-printed electrode aided by chemometric analysis. J. Food Compos. Anal. 2024, 136, 106751. [Google Scholar] [CrossRef]
- Azad, T.; Ahmed, S. Common milk adulteration and their detection techniques. Int. J. Food Contam. 2016, 3, 22. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectrometry (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Tsopelas, F.; Konstantopoulos, D.; Tsantili-Kakoulidou, A. Voltammetric fingerprinting of oils and its combination with chemometrics for the detection of extra virgin olive oil adulteration. Anal. Chim. Acta 2018, 1015, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Veloso, A.C.A.; Sousa, M.E.B.C.; Estevinho, L.; Dias, L.G.; Peres, A.M. Honey evaluation using electronic tongues: An overview. Chemosensors 2018, 6, 28. [Google Scholar] [CrossRef]
- Sobrino-Gregorio, L.; Bataller, R.; Soto, J.; Escriche, I. Monitoring honey adulteration with sugar syrups using an automatic pulse voltammetric tongue. Food Control 2018, 91, 254–260. [Google Scholar] [CrossRef]
- Nikolaou, P.; Deskoulidis, E.; Topoglidis, E.; Tsantili-Kakoulidou, A.; Tsopelas, F. Application of chemometrics for detection and modeling of adulteration of fresh cow milk with reconstituted skim milk powder using voltammetric fingerprinting on a graphite/ SiO2 hybrid electrode. Talanta 2020, 206, 120223. [Google Scholar] [CrossRef]
- Harris, N.; Viejo, C.G.; Zhang, J.; Pang, A.; Hernandez-Brenes, C.; Fuentes, S. Enhancing beer authentication, quality, and control assessment using non-invasive spectroscopy through bottle and machine learning modeling. Food Sci. 2025, 90, e17670. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Mao, Y.; Qu, L. Simple voltammetric determination oh rhodamine B by using the glassy carbon electrode in fruit juice and preserved fruit. Food Anal. Methods 2013, 6, 1665–1670. [Google Scholar] [CrossRef]
- Kundu, M.; Prasad, S.; Krishnan, P.; Gajjala, S. A novel electrochemical biosensor based on hematite (α-Fe2O3) flowerlike nanostructures for sensitive determination of formaldehyde adulteration in fruit juices. Food Bioprocess. Technol. 2019, 12, 1659–1671. [Google Scholar] [CrossRef]
- Detpisuttitham, W.; Phanthong, C.; Ngamchana, S.; Rijiravanich, P.; Surareyngchai, W. Electrochemical Detection of Salicylic Acid in Pickled Fruit/Vegetable and Juice. J. Anal. Test. 2020, 4, 291–297. [Google Scholar] [CrossRef]
- Wojcik, S.; Jakubowska, M. Deep neural networks in profiling of apple juice adulteration based on voltammetric signal of the iridium quadruple-disk electrode. Chemom. Intell. Lab. Syst. 2021, 209, 104246. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Z.; Liao, X. A review of fruit juice authenticity assessments: Targeted and untargeted analyses. Crit. Rev. Food Sci. Nutr. 2022, 62, 6081–6102. [Google Scholar] [CrossRef]
- Monago-Marana, O.; Zapardiel Palenzuela, A.; Crevillen, A.G. Untargeted authentication of fruit juices based on electrochemical fingerprints combined with chemometrics. Adulteration of orange juice as case of study. LWT 2024, 209, 116797. [Google Scholar] [CrossRef]
- Daniel, D.; Silva Lopes, F.; dos Santos, V.B.; do Lago, C.L. Detection of coffee adulteration with soybean and corn by capillary electrophoresis-tandem mass spectrometry. Food Chem. 2018, 243, 305–310. [Google Scholar] [CrossRef] [PubMed]
- De Morais, T.C.B.; Rodrigues, D.R.; de Carvalho Polari Souto, U.T.; Lemos, S.G. A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chem. 2019, 273, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Green, H.S.; Li, X.; De Pra, M.; Lovejou, K.S.; Steiner, F.; Acworth, I.N.; Wang, S.C. A rapid method for the detection of extra virgin olive oil adulteration using UHPLC-CAD profiling of triacylglycerols. Food Control 2020, 107, 106773. [Google Scholar] [CrossRef]
- Lymperopoulou, T.; Balta-Brouma, K.; Tsakanika, L.A.; Tzia, C.; Tsantili-Kakoulidou, A.; Tsopelas, F. Identification of lentils (Lens culinaris Medik) from Eglouvi (Lefkada, Greece) based on rare earth elements profile combined with chemometrics. Food Chem. 2014, 447, 138965. [Google Scholar] [CrossRef] [PubMed]
- Ranaweera, K.R.R.; Capone, D.L.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. A review of wine authentication using spectroscopic approaches in combination with chemometrics. Molecules 2021, 26, 4334. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Sheashea, M.; Zhao, C.; Maamoun, A.A. UV fingerprinting approaches for quality control analyses of food and functional food coupled to chemometrics: A comprehensive analysis of novel trends and applications. Foods 2022, 11, 2867. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R.; Amit; Kumari, S.; Sharma, S.; Kelly, S.; Cannavan, A.; Singh, D.K. Recent trends in the use of FTIR spectroscopy integrated with chemometrics for the detection of edible oil adulteration. Vib. Spectrosc. 2021, 113, 103222. [Google Scholar] [CrossRef]
- Ceniti, C.; Spina, A.A.; Piras, C.; Oppedisano, F.; Tilocca, B.; Roncada, P.; Britti, D.; Moritu, V.M. Recent advances in the determination of milk adulterants and contaminants by Mid-Infrared Spectroscopy. Foods 2023, 12, 2917. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, P.A.; Fauhl-Hassek, C.; Riedl, J.; Esslinger, S.; Bontempo, L.; Camin, F. NMR spectroscopy in wine authentication: An official control perspective. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2040–2062. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Rodriguez, L.; Ortega-Gavilan, F.; Martin-Torres, S.; Arroyo-Cerezo, A.; Jimenez-Carvelo, A.M. Chromatographic fingerprinting and food identity/quality: Potentials and challenges. J. Agric. Food Chem. 2021, 69, 14428–14434. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lu, W.; Cai, Z.; Bao, L.; Hartmann, C.; Gao, B.; Yu, L. Rapid detection of milk adulteration using intact protein flow injection mass spectrometric fingerprints combined with chemometrics. Food Chem. 2018, 240, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Farah, J.S.; Cavalcanti, R.N.; Guimaraes, J.T.; Balthazar, C.F.; Coimbra, P.T.; Pimentel, T.C.; Esmerino, E.A.; Duarte, M.C.K.H.; Freitas, M.Q.; Granato, D.; et al. Differential scanning calorimetry coupled with machine learning technique: An effective approach to determine the milk authenticity. Food Control 2021, 121, 107585. [Google Scholar] [CrossRef]
- Oliveri, P. Class-modeling in food analytical chemistry: Development, sampling, optimization and validation issues—A tutorial. Anal. Chim. Acta 2017, 982, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dominguez, R.; Sayago, A.; Fernandez-Recamales, A. An overview on the application of chemometrics tools in food authenticity and traceability. Foods 2022, 11, 3940. [Google Scholar] [CrossRef]
- Rodionova, O.Y.; Oliveri, P.; Malegori, C.; Pomerantsev, A.L. Chemometrics as an efficient tool for food authentication: Golden pillars for building reliable models. Trends Food Sci. Technol. 2024, 147, 104429. [Google Scholar] [CrossRef]
- Forina, M.; Oliveri, P.; Lanteri, S.; Casale, M. Class-modeling techniques, classic and new, for old and new problems. Chemom. Intell. Lab. Syst. 2008, 93, 132–148. [Google Scholar] [CrossRef]
- Mutz, Y.S.; do Rosario, D.; Silva, L.R.G.; Galvan, D.; Stefano, J.S.; Janegitz, B.C.; Weitz, D.A.; Bernandes, P.C.; Conte-Junior, C.A. Lab-made 3D printed electrochemical sensors coupled with chemometrics for Brazilian coffee authentication. Food Chem. 2023, 403, 134411. [Google Scholar] [CrossRef] [PubMed]
- Kalogiouri, N.P.; Aalizadeh, R.; Dasenaki, M.E.; Thomaidis, N.S. Application of high resolution mass spectrometric methods coupled with chemometric techniques in olive oil authenticity studies—A review. Anal. Chim. Acta 2020, 1134, 150–173. [Google Scholar] [CrossRef]
- Wojcik, S.; Kopec, J.; Bas, B.; Jakubowska, M. A new approach in voltammetric profiling of wines and whiskies based on a useful faradaic signal component. Anal. Methods 2019, 11, 5984–5996. [Google Scholar] [CrossRef]
- Hilding-Ohlsson, A.; Fauerbach, J.A.; Sacco, N.J.; Bonetto, M.C.; Corton, E. Voltamperometric discrimination of urea and melamine adulterated skimmed milk powder. Sensors 2012, 12, 12220–12234. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M. Rapid fingerprinting of white wine oxidizable fraction and classification of white wines using disposable screen printed sensors and derivative voltammetry. Food Chem. 2016, 212, 837–843. [Google Scholar] [CrossRef]
- Szydlowska-Czerniak, A. Rapeseed and its products—Sources of bioactive compounds: A review of their characteristics and analysis. Crit. Rev. Food Sci. Nutr. 2013, 53, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellon, J.; Lopez-Yerena, A.; Dominguez-López, I.; Siscart-Serra, A.; Fraga, N.; Samano, S.; Lopez-Sabater, C.; Lamuela-Raventos, R.M.; Vallverdu-Queralt, A.; Perez, M. Extra Virgin Olive Oil: A Comprehensive Review of Efforts to Ensure Its Authenticity, Traceability, and Safety. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2639–2664. [Google Scholar] [CrossRef] [PubMed]
- Marx, Í.M.G.; Casal, S.; Rodrigues, N.; Cruz, R.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Does Water Addition during the Industrial Milling Phase Affect the Chemical-Sensory Quality of Olive Oils? The Case of Cv. Arbequina Oils. Food Chem. 2022, 395, 133570. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D. Olive Oil Minor Constitutents and Health; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Karagozlu, M.; Adun, P.; Ozsoz, M.; Asir, S. Detection of adulteration in cold-pressed olive oil by voltammetric analysis of alpha-tocopherol on single-use pencil graphite electrode. Int. J. Food Sci. Technol. 2024, 59, 1781–1788. [Google Scholar] [CrossRef]
- Tsopelas, F.; Ochsenkühn-Petropoulou, M.; Zikos, N.; Spyropoulos, E.; Andreadou, I. Electrochemical study of some non-steroidal anti-inflammatory drugs: Solvent effect and antioxidant activity. J. Solid. State Electrochem. 2011, 15, 1099–1108. [Google Scholar] [CrossRef]
- Oliveri, P.; Baldo, M.A.; Daniele, S.; Forina, M. Development of a voltammetric electronic tongue for discrimination of edible oils. Anal. Bioanal. Chem. 2009, 395, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Baldo, M.A.; Oliveri, P.; Simonetti, R.; Daniele, S. Voltammetric behavior of ferrocene in olive oils mixed with a phosphonium-based ionic liquid. J. Electroanal. Chem. 2014, 731, 43–48. [Google Scholar] [CrossRef]
- Baldo, M.A.; Oliveri, P.; Simonetti, R.; Daniele, S. A novel electroanalytical approach based on the use of a room temperature ionic liquid for the determination of olive oil acidity. Talanta 2016, 161, 881–887. [Google Scholar] [CrossRef]
- Baldo, M.A.; Oliveri, P.; Fabris, S.; Malegori, C.; Daniele, S. Fast determination of extra-virgin olive oil acidity by voltammetry and partial least squares regression. Anal. Chim. Acta 2019, 1056, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.; Rodriguez-Mendez, M.L.; de Saja, J.A. Modified carbon electrodes for discrimination of vegetable oils. Sens. Actuators B 2005, 111–112, 403–409. [Google Scholar] [CrossRef]
- Apetrei, C.; Gutierez, F.; Rodriguez-Mendez, M.L.; de Saja, J.A. Novel method based on carbon paste electrodes for the evaluation of bitterness in extra virgin olive oils. Sens. Actuators B Chem. 2007, 121, 567–575. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Detection of olive oil adulteration using a voltammetric e-tongue. Comput. Electron. Agric. 2014, 108, 148–154. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Classification and antioxidant activity evaluation of edible oils by using nanomaterial-based electrochemical sensors. Int. J. Mol. Sci. 2023, 24, 3010. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, X.; Yuan, L.; Han, E.; Zhou, L.; Zhou, A. Determination of Chinese Angelica honey adulterated with rice syrup by an electrochemical sensor and chemometrics. Anal. Methods 2013, 5, 2324–2328. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J. Classification of monofloral honeys by voltammetric electronic tongue with chemometrics method. Electrochim. Acta 2011, 56, 4907–4915. [Google Scholar] [CrossRef]
- Tiwari, K.; Tudu, B.; Bandyopadhyay, R.; Chatterjee, A. Identification of monofloral honey using voltammetric electronic tongue. J. Food Eng. 2013, 117, 205–210. [Google Scholar] [CrossRef]
- Tiwari, K.; Biswas, S.; Tudu, B.; Bandhopadhyay, R.; Pramanik, P. Development of metal oxide-modified carbon paste based sensor for honey analysis using electronic tongue. Mater. Today-Proc. 2017, 4, 9500–9504. [Google Scholar] [CrossRef]
- Tiwari, K.; Tudu, B.; Bandyopadhyay, R.; Chatterjee, A.; Pramanik, P. Voltammetric sensor for electrochemical determination of the floral origin of honey based on a zinc oxide nanoparticle modified carbon paste electrode. J. Sens. Sens. Syst. 2018, 7, 319–329. [Google Scholar] [CrossRef]
- Giordano, G.F.; Vicentini, M.B.R.; Murer, R.C.; Augusto, F.; Ferrão, M.F.; Helferd, G.A.; da Costa, A.B.; Gobbi, A.L.; Hantao, L.W.; Lima, R.S. Point-of-use electroanalytical platform based on homemade potentiostat and smartphone for multivariate data processing. Electrochim. Acta 2016, 219, 170–177. [Google Scholar] [CrossRef]
- Guellis, C.; Valerio, D.C.; Bessegato, G.G.; Boroski, M.; Dragunski, J.C.; Lindino, C.A. Non-targeted method to detect honey adulteration: Combination of electrochemical and spectrophotometric responses with principal component analysis. J. Food Compos. Anal. 2020, 89, 103466. [Google Scholar] [CrossRef]
- Wojcik, S.; Ciepiela, F.; Jakubowska, M. Computer vision analysis of sample colors versus quadruple-disk iridium-platinum voltammetric e-tongue for recognition of natural honey adulteration. Measurement 2023, 209, 112514. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Liao, W. Technique potential for classification of honey by electronic tongue. J. Food Eng. 2009, 94, 260–266. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J. Tracing floral and geographical origins of honeys by potentiometric and voltammetric electronic tongue. Comput. Electron. Agric. 2014, 108, 112–122. [Google Scholar] [CrossRef]
- Dias, L.A.; Peres, A.M.; Vilas-Boas, M.; Rocha, M.A.; Estevinho, L.; Machado, A.A. An electronic tongue for honey classification. Microchim. Acta 2008, 163, 97–102. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Scattareggia, C.; Chiofalo, V. Botanical origin identification of Sicilian honeys based on artificial senses and multi-sensor data fusion. Eur. Food Res. Technol. 2018, 244, 117–125. [Google Scholar] [CrossRef]
- Juan-Borras, M.; Soto, J.; Gil-Sanchez, L.; Pascual-Mate, A.; Escriche, I. Antioxidant activity and physico-chemical parameters for the differentiation of honey using a potentiometric electronic tongue. J. Sci. Food Agric. 2017, 97, 2215–2222. [Google Scholar] [CrossRef]
- Dias, L.G.; Bruni, A.R.S.; Anizelli, C.P.; Zangirolami, M.; Lima, P.C.; Estevinho, L.M.; Bona, E. Semi-quantitative discrimination of honey adulterated with cane sugar solution by an ETongue. Chem. Biodiversity 2022, 19, e202200698. [Google Scholar] [CrossRef]
- Bougrini, M.; Tahri, K.; Saidi, T.; El Hassani, N.E.; Bouchikhi, B.; El Bari, N. Classification of Honey According to Geographical and Botanical Origins and Detection of Its Adulteration Using Voltammetric Electronic Tongue. Food Anal. Method 2016, 9, 2161–2173. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Martinez-Bisbal, M.C.; Soto, J.; Borras, M.J.; Martinez-Manez, R.; Escriche, I. Monofloral honey authentication by voltammetric electronic tongue: A comparison with 1H NMR spectroscopy. Food Chem. 2022, 383, 132460. [Google Scholar] [CrossRef]
- Ciursa, P.; Oroian, M. Voltammetric e-tongue for honey adulteration detection. Sensors 2021, 21, 5059. [Google Scholar] [CrossRef] [PubMed]
- Leon-Medina, J.X.; Acosta-Opayome, D.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.M.; Anaya, M.; Tibaduiza, D.A. Intelligent electronic tongue system for the classification of genuine and false honeys. Int. J. Food Prop. 2023, 26, 327–343. [Google Scholar] [CrossRef]
- Mohebbi, M.; Ghanbari, K.; Nejabati, F. Electrochemical sensor based on EDTA-functionalized polyorthophylene diamine g-C3N4 nanocomposite for determination of melamine in milk. J. Electroanal. Chem. 2023, 946, 117757. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S.; Wong, N.K. Development of melamine sensor based on ionic liquid/nanoparticles/chitosan with modified gold electrode for determination of melamine in milk product. Sens. Bio-Sens. Res. 2015, 4, 16–22. [Google Scholar] [CrossRef][Green Version]
- Rovina, K.; Siddiquee, S. Electrochemical sensor based rapid determination of melamine using ionic liquid/zinc oxide nanoparticles/chitosan/gold electrode. Food Control 2016, 59, 801–808. [Google Scholar] [CrossRef]
- Heydarian-Dehkordi, N.; Saei-Dehkordi, S.S.; Izadi, Z.; Ghasemi-Varnamkhasti, M. Development of an ultrasensitive molecularly imprinted poly-(ortho-phenylenediamine) based sensor for the determination of melamine adulteration in milk and infant formula. Food Sci. Nutr. 2022, 10, 3154–3164. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, W.R.; Paixao, T.R.L.C. Use of copper electrode for melamine quantification in milk. Electrochim. Acta 2014, 117, 379–384. [Google Scholar] [CrossRef]
- Esmaeily, Z.; Madrakian, T.; Afkhami, A.; Ghoorchian, A.; Ghasemzadeh-Mohammadi, V. Electropolymerization as an electrochemical preconcentration approach for the determination of melamine in milk samples. Electrochim. Acta 2021, 390, 138897. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Khraibah, N.H. Development of a highly sensitive voltammetric sensor for trace determination of melamine residues in milk and water samples. Microchem. J. 2020, 157, 105087. [Google Scholar] [CrossRef]
- Daizy, M.; Tarafder, C.; Al-Mamun, M.R.; Liu, X.; Aly Saad Aly, M.; Khan, M.Z.H. Electrochemical detection of melamine by using reduced grapheme oxide-copper nanoflowers modified glassy carbon electrode. ACS Omega 2019, 4, 20324–20329. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, Y.T.; Li, Y.H.; Bao, T.; Sun, T.S.; Li, D.D.; Luo, X.K.; Fan, H.T. A electrochemical sensor for melamine detection based on copper-melamine complex using OMC modified glassy carbon electrode. Food Anal. Methods 2018, 11, 546–555. [Google Scholar] [CrossRef]
- Feng, N.; Wang, Y.; Li, W. Solid-phase microwave synthesis of nickel sulfide/nickel oxide/carbon nanotubes for the electrochemical determination of melamine by differential pulse voltammetry (DPV). Anal. Lett. 2024; in press. [Google Scholar] [CrossRef]
- An, Q.Q.; Feng, X.Z.; Zhou, Z.F.; Zhan, T.; Lian, S.F.; Zhy, J.; Han, G.C.; Chen, Z.; Kraatz, H.B. One step construction of an electrochemical sensor for melamine detection in milk towards an integrated portable system. Food Chem. 2022, 383, 132403. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Chen, M.; Ge, H.; Lu, Z.; Liu, X.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens. Bioelectron. 2017, 87, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, H.; Dong, G.; Yang, R.; Li, L.; Liu, Y.; Wu, H.; Zhang, W. Discrimination of milk adulterated with urea using voltammetric electronic tongue coupled with PCA-LSSVM. Int. J. Electrochem. Sci. 2015, 10, 10119–10131. [Google Scholar] [CrossRef]
- Sha, R.; Komori, K.; Badhulika, S. Graphene-Polyaniline composite based ultra-sensitive electrochemical sensor for non-enzymatic detection of urea. Electrochim. Acta 2017, 233, 44–51. [Google Scholar] [CrossRef]
- Shyamala, V.; Radha, S.; Kiruthika, R.; Acchutharaman, K.R. Experimental investigation of a mixed metal oxide (MMO)-based sensor for the detection of adulterants (urea and melamine) in milk at room temperature. J. Mater. Sci. Mater. Electron. 2024, 35, 1968. [Google Scholar] [CrossRef]
- Silva, R.A.B.; Montes, R.H.O.; Richter, E.M.; Munoz, R.A.A. Rapid and selective determination of hydrogen peroxide residues in milk by batch injection analysis with amperometric detection. Food Chem. 2012, 133, 200–204. [Google Scholar] [CrossRef]
- Palsaniya, S.; Lal Jat, B.; Mukherji, S. Amperometry sensor for real time detection of hydrogen peroxide adulteration in food samples. Electrochim. Acta 2023, 462, 142724. [Google Scholar] [CrossRef]
- Shalini Devi, K.S.; Mahalakshmi, V.T.; Ghosh, A.R.; Kumar, A.S. Unexpected co-immobilization of lactoferrin and methylene blue from milk solution on a Nafion/MWCNT modified electrode and application to hydrogen peroxide and lactoferrin biosensing. Electrochim. Acta 2017, 244, 26–37. [Google Scholar] [CrossRef]
- Fangmeier, M.; Kemerich, G.T.; Machado, B.L.; Maciel, M.J.; de Souza, C.F.V. Effects of cow, goat, and buffalo milk on the characteristics of cream cheese with whey retention. Food Sci. Technol. 2019, 39, 122–128. [Google Scholar] [CrossRef]
- Demiati; Wahyuni, W.T.; Rafi, M.; Putra, B.R. The detection of goat milk adulteration with cow milk using a combination of voltammetric fingerprints and chemometrics analysis. Chem. Pap. 2023, 77, 4307–4317. [Google Scholar] [CrossRef]
- Xue, D.; Zhao, H. Machine learning and electrochemistry techniques for detecting adulteration of goat milk with cow milk. J. Food Meas. Charact. 2024, 18, 6012–6019. [Google Scholar] [CrossRef]
- Geana, E.I.; Artem, V.; Apetrei, C. Discrimination and classification of wines based on polypyrrole modified screen-printed carbon electrodes coupled with multivariate data analysis. J. Food Compos. Anal. 2021, 96, 103704. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Ksenzhek, O.; Petrova, S.; Kolodyazhny, M. Redox spectra of wines. Electroanalysis 2007, 19, 389–392. [Google Scholar] [CrossRef]
- Mannino, S.; Brenna, O.; Buratti, S.; Cosio, M.S. A new method for the evaluation of the “antioxidant power” of wines. Electroanalysis 1998, 10, 908–912. [Google Scholar] [CrossRef]
- Blasco, A.J.; Rogerio, M.C.; Gonzalez, M.C.; Escarpa, A. “Electrochemical index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- Sanchez Arribas, A.; Martinez-Fernandez, M.; Moreno, M.; Bermejo, E.; Zapardiel, A.; Chicharro, M. Analysis of total polyphenols in wines by FIA with highly stable amperometric detection using carbon nanotube-modified electrodes. Food Chem. 2013, 136, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Sanchez Arribas, A.; Gonzalez, L.; Bermejo, E.; Zapardiel, A.; Chicharro, M. Flow injection analysis with amperometric detection of polyphenols at carbon nanotube/ polyvinylpyrrolidone-modified electrodes as classification tool for white wine varieties. Microchem. J. 2021, 162, 105836. [Google Scholar] [CrossRef]
- Parra, V.; Arrieta, A.A.; Fernandez-Escudero, J.A.; Rodriguez-Mendez, M.L.; De Saja, J.A. Electronic tongue based on chemically modified electrodes and voltammetry for the detection of adulterations in wines. Sens. Actuators B 2006, 118, 448–453. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Fu, M.; Li, G.; Lou, Z. Classification of chinese yellow wines by chemometric analysis of cyclic voltammogram of copper electrodes. Sensors 2005, 5, 529–536. [Google Scholar] [CrossRef]
- Schreyer, S.K.; Mikkelsen, S.R. Chemometric analysis of square wave voltammograms for classification and quantitation of untreated beverage samples. Sens. Actuators B Chem. 2000, 71, 147–153. [Google Scholar] [CrossRef]
- Kutyla-Olesiuk, K.; Wawrzyniak, U.E.; Janczyk, M.; Wroblewski, W. Electrochemical sensor arrays for the analysis of wine production. Procedia Eng. 2014, 87, 580–583. [Google Scholar] [CrossRef]
- Merkyte, V.; Morozova, K.; Boselli, E.; Scampicchio, M. Fast and simultaneous determination of antioxidant activity, total phenols and bitterness of red wines by a multichannel amperometric electronic tongue. Electroanalysis 2018, 30, 314–319. [Google Scholar] [CrossRef]
- Gonzalez-Calabuig, A.; del Valle, M. Voltammetric electronic tongue to identify Brett character in wines. On-site quantification of its ethylphenol metabolites. Talanta 2018, 179, 70–74. [Google Scholar] [CrossRef]
- Dominguez-Renedo, O.; Navarro-Cunado, A.M.; Melendez-Alvarez, M.E.; Alonso-Lomillo, M.A. Current state of electrochemical sensors in wine analysis for early diagnosis. TrAC Trends Anal. Chem. 2023, 168, 117349. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, L.; Zheng, Q.; Gao, F.; Chen, X.; Yang, Z.; Qin, Y.; Yu, Y. Diffentiating between ageing times of typical Chinese liquors by steady-state microelectrode voltammetry. Microchem. J. 2019, 151, 104244. [Google Scholar] [CrossRef]
- Rosello, A.; Serrano, N.; Diaz-Cruz, J.M.; Arino, C. Discrimination of beers by cyclic voltammetry using a single carbon screen-printed electrode. Electroanalysis 2021, 33, 864–872. [Google Scholar] [CrossRef]
- Lvova, L.; Paolesse, R.; Di Natale, C.; D’Amico, A. Detection of alcohols in beverages: An application of porphyrin-based Electronic tongue. Sens. Actuators B 2006, 118, 439–447. [Google Scholar] [CrossRef]
- Ortenero, J.R.; Dugos, N.P.; Soriano, A.N. A review on the application of voltammetry in the determination of various substances in fruit juices. Appl. Sci. Eng. Prog. 2023, 16, 5700. [Google Scholar] [CrossRef]
- Statista Database, Juices-Worldwide. Available online: https://www.statista.com (accessed on 12 January 2025).
- Ahmadi, S.; Hasanzadeh, M.; Ghasempour, Z. Sub-micro electrochemical recognition of carmoisine, sunset yellow, and tartrazine in fruit juices using P(β-CD/Arg)/CysA-AuNPs/AuE. Food Chem. 2023, 402, 134501. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, A.A.; Arrieta, P.L.; Mendoza, J.M. Analysis of coffee adulterated with roasted corn and roasted soybean using voltammetric electronic tongue. Acta Sci. Pol. Technol. Aliment. 2019, 18, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.R.; Fragoso, W.D.; Lemos, S.G. Electronic tongue based on a single impedimetric sensor and complex numbers-supervised pattern recognition. Electrochim. Acta 2021, 397, 139312. [Google Scholar] [CrossRef]
- Mertiri, M.; Hrbac, J.; Prodromidis, M.; Economou, A.; Kokkinos, C. Digital fabrication of 3D printed bismuth sparked sensors for electrochemical sensing. Appl. Mater. Today 2024, 39, 102289. [Google Scholar] [CrossRef]
- Ragazou, K.; Lougkovois, R.; Katseli, V.; Kokkinos, C. Fully integrated 3D-printed electronic device for the on-field determination of antipsychotic drug quetiapine. Sensors 2021, 21, 4753. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsopelas, F. Electroanalytical Approaches to Combatting Food Adulteration: Advances in Non-Enzymatic Techniques for Ensuring Quality and Authenticity. Molecules 2025, 30, 876. https://doi.org/10.3390/molecules30040876
Tsopelas F. Electroanalytical Approaches to Combatting Food Adulteration: Advances in Non-Enzymatic Techniques for Ensuring Quality and Authenticity. Molecules. 2025; 30(4):876. https://doi.org/10.3390/molecules30040876
Chicago/Turabian StyleTsopelas, Fotios. 2025. "Electroanalytical Approaches to Combatting Food Adulteration: Advances in Non-Enzymatic Techniques for Ensuring Quality and Authenticity" Molecules 30, no. 4: 876. https://doi.org/10.3390/molecules30040876
APA StyleTsopelas, F. (2025). Electroanalytical Approaches to Combatting Food Adulteration: Advances in Non-Enzymatic Techniques for Ensuring Quality and Authenticity. Molecules, 30(4), 876. https://doi.org/10.3390/molecules30040876






