Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection
Abstract
:1. Introduction
2. Results and Discussion
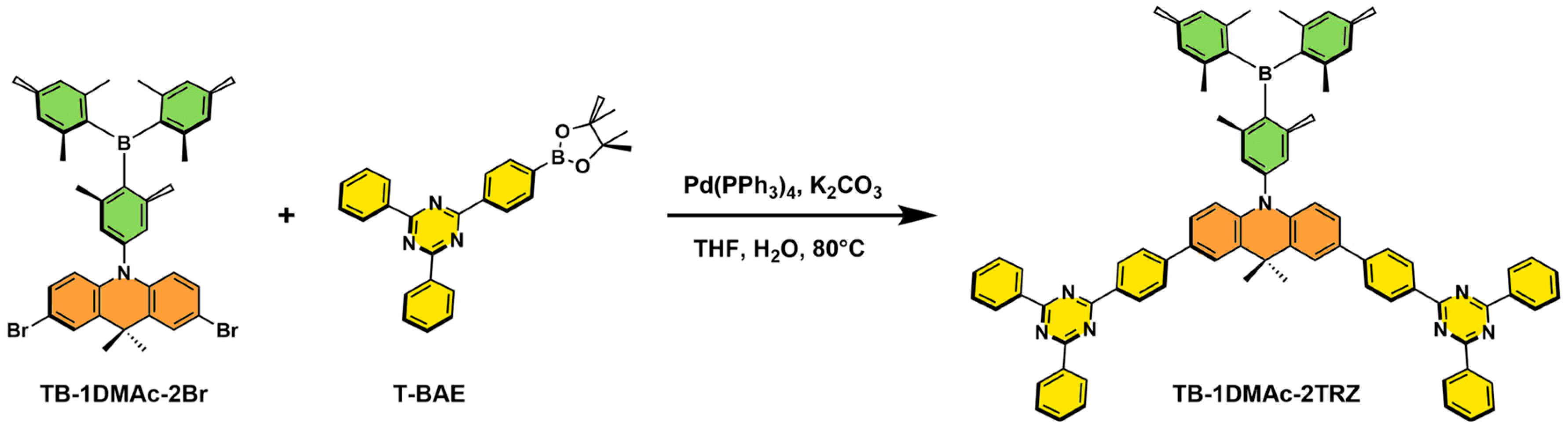
2.1. Synthesis and Characterization
2.2. Photophysical Properties
2.3. Detection of Fluoride Ions
2.4. Theoretical Calculations
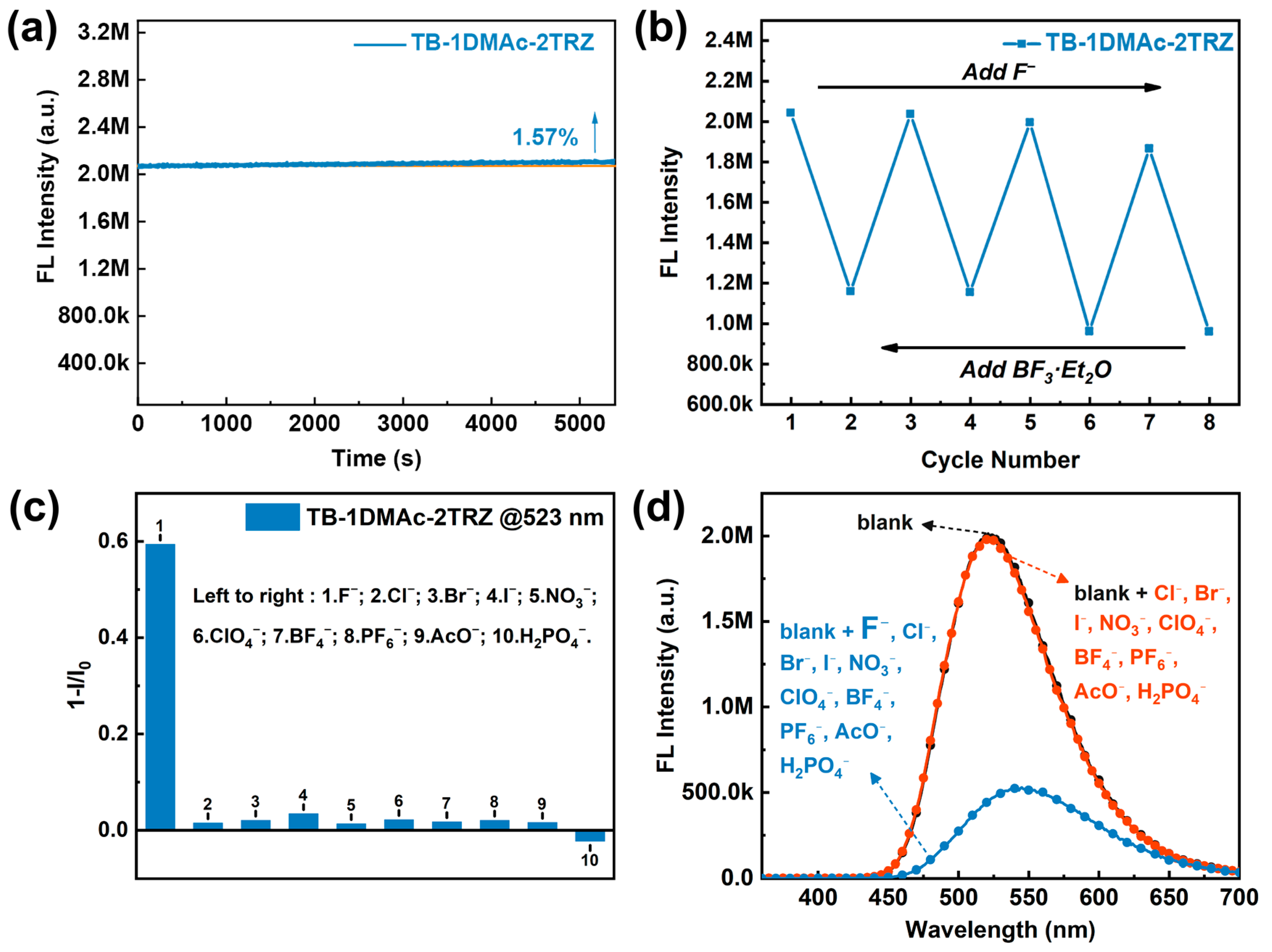
2.5. Photostability, Repeatability, Selectivity, and Anti-Interference Analysis
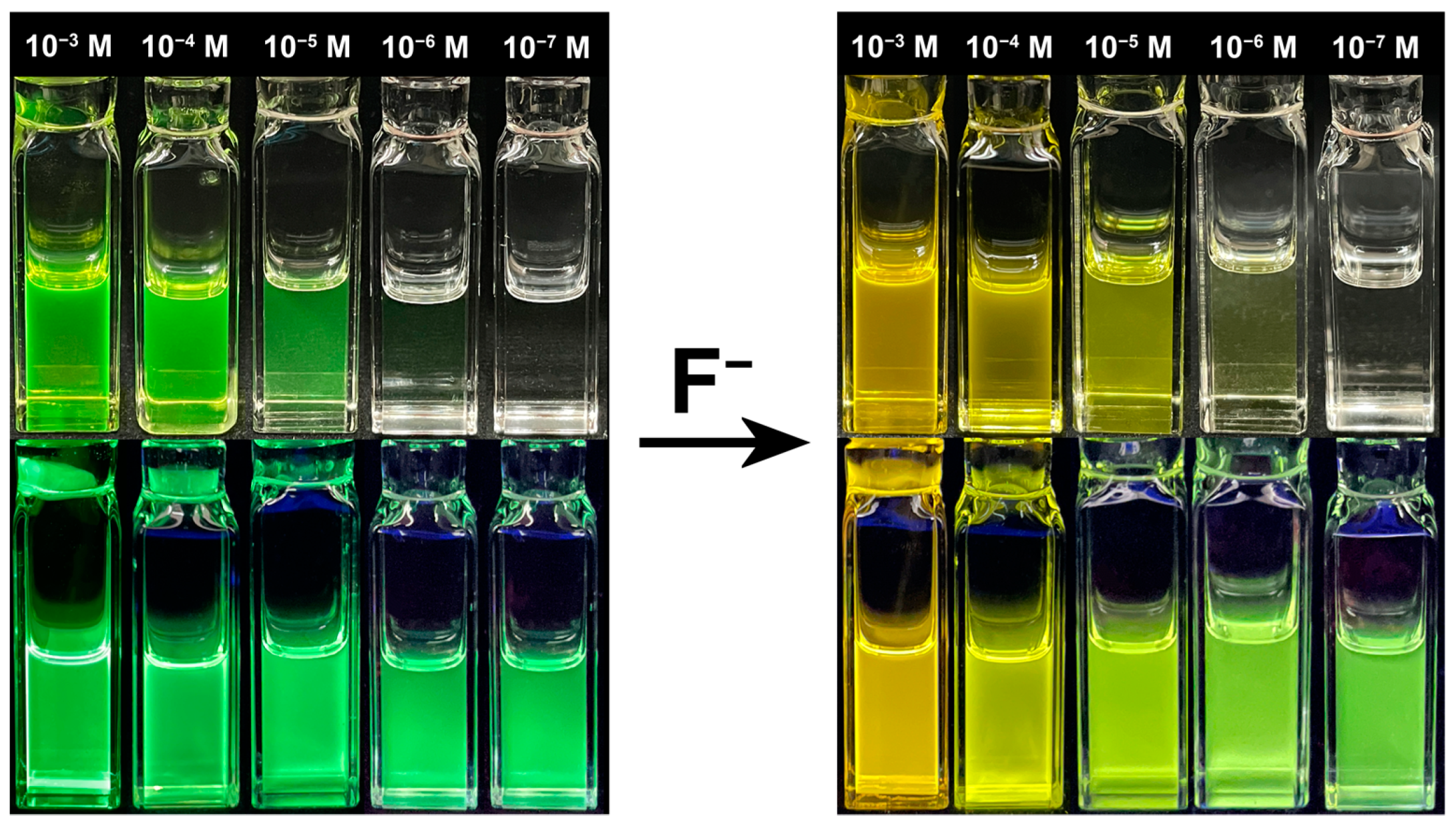
2.6. Visual Applications for Fluoride Ion Detection
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, R.; Dwivedi, S.K.; Mishra, H.; Misra, A. Imidazole-coumarin containing D–A type fluorescent probe: Synthesis photophysical properties and sensing behavior for F− and CN− anion. Dye. Pigm. 2020, 175, 108163. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Z.; Dang, J.; Sun, Y.; Zhou, G.; Wong, W.-Y. High performance solution-processed organic yellow light-emitting devices and fluoride ion sensors based on a versatile phosphorescent Ir(III) complex. Mater. Chem. Front. 2019, 3, 376–384. [Google Scholar] [CrossRef]
- Muthusamy, S.; Rajalakshmi, K.; Ahn, D.-H.; Kannan, P.; Zhu, D.; Nam, Y.-S.; Choi, K.Y.; Luo, Z.; Song, J.-W.; Xu, Y. Spontaneous detection of F− and viscosity using a multifunctional tetraphenylethene-lepidine probe: Exploring environmental applications. Food Chem. 2025, 466, 142147. [Google Scholar] [CrossRef]
- Sha, H.; Yan, B. Terbium-based metal-organic frameworks through energy transfer modulation for visual logical sensing zinc and fluorine ions. Talanta 2023, 257, 124326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Feng, X.; Zhao, Y. An experimental and theoretical study on mechanistic insights into urolithin-based ratiometric fluorescent probe for instant quantitative detection of fluoride ions. Talanta 2024, 276, 126220. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, Z.; Wei, X.; Han, Q.; Wang, H.; Yang, X.; Xu, S.; Wang, X. Engineering a triplet exciton enhanced photoluminescence self-assembled nanoprobe containing block copolymer and Tb3+/Ga3+ complex for fluoride ion detection in serum. Sens. Actuators B 2022, 366, 131975. [Google Scholar] [CrossRef]
- Ali, R.; Gupta, R.C.; Dwivedi, S.K.; Misra, A. Excited state proton transfer (ESIPT) based molecular probe to sense F− and CN− anions through a fluorescence “turn-on” response. New J. Chem. 2018, 42, 11746–11754. [Google Scholar] [CrossRef]
- Ayoob, S.; Gupta, A.K. Fluoride in drinking water: A review on the status and stress effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-A.; Mani, V.; Huang, S.-T. Design and synthesis of ultrasensitive off-on fluoride detecting fluorescence probe via autoinductive signal amplification. Analyst 2015, 140, 346–352. [Google Scholar] [CrossRef]
- Brugnara, A.; Topić, F.; Rissanen, K.; de la Lande, A.; Colasson, B.; Reinaud, O. Selective recognition of fluoride anion in water by a copper(II) center embedded in a hydrophobic cavity. Chem. Sci 2014, 5, 3897–3904. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Recognition and sensing of fluoride anion. Chem. Commun. 2009, 2809–2829. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Highlights on contemporary recognition and sensing of fluoride anion in solution and in the solid state. Chem. Soc. Rev. 2013, 42, 2016–2038. [Google Scholar] [CrossRef] [PubMed]
- Day, J.K.; Bresner, C.; Coombs, N.D.; Fallis, I.A.; Ooi, L.-L.; Aldridge, S. Colorimetric fluoride ion sensing by polyborylated ferrocenes: Structural influences on thermodynamics and kinetics. Inorg. Chem. 2008, 47, 793–804. [Google Scholar] [CrossRef]
- Langton, M.J.; Serpell, C.J.; Beer, P.D. Anion recognition in water: Recent advances from a supramolecular and macromolecular perspective. Angew. Chem. Int. Ed. 2016, 55, 1974–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, Y.; Zheng, J.; Li, J.; Zhao, W.; Yang, S.; Yang, R. Self-assembly of graphene oxide with a silyl-appended spiropyran dye for rapid and sensitive colorimetric detection of fluoride ions. Anal. Chem. 2013, 85, 11456–11463. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Li, W.-Y.; Gu, J.-A.; Lin, C.-M.; Huang, S.-T. Electrochemical off-on ratiometric chemodosimeters for the selective and rapid detection of fluoride. Talanta 2015, 131, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Morés, S.; Monteiro, G.C.; da Silva Santos, F.; Carasek, E.; Welz, B. Determination of fluorine in tea using high-resolution molecular absorption spectrometry with electrothermal vaporization of the calcium mono-fluoride CaF. Talanta 2011, 85, 2681–2685. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef]
- Zhu, B.; Yuan, F.; Li, R.; Li, Y.; Wei, Q.; Ma, Z.; Du, B.; Zhang, X. A highly selective colorimetric and ratiometric fluorescent chemodosimeter for imaging fluoride ions in living cells. Chem. Commun. 2011, 47, 7098–7100. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.T.; Xie, D.; Martinez, D.; Yu, M.; Que, E.L. A dual-responsive probe for detecting cellular hypoxia using 19F magnetic resonance and fluorescence. Chem. Commun. 2019, 55, 8860–8863. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Wang, Y.; Wang, X.; Ma, H.; Li, P.; Song, W.; Xia Han, X.; Zhao, B. Direct detection of fluoride ions in aquatic samples by surface-enhanced raman scattering. Talanta 2018, 178, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Guo, D.; Wang, N.; Wu, S.; Zhang, P.; Zhu, Y. Detection of trace fluoride in serum and urine by online membrane-based distillation coupled with ion chromatography. J. Chromatogr. A 2017, 1500, 145–152. [Google Scholar] [CrossRef]
- Musijowski, J.; Szostek, B.; Koc, M.; Trojanowicz, M. Determination of fluoride as fluorosilane derivative using reversed-phase HPLC with UV detection for determination of total organic fluorine. J. Sep. Sci. 2010, 33, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Yu, Z.; Phillips, B.L.; Wang, H.; Ji, J.; Pan, B.; Li, W. Molecular-scale investigation of fluoride sorption mechanism by nanosized hydroxyapatite using 19F solid-state NMR spectroscopy. J. Colloid Interface Sci. 2019, 557, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhao, Q.; Xu, W.J.; Huang, W. Fluoride probe based on organoboron compounds. Prog. Chem. 2008, 20, 1708–1715. [Google Scholar]
- Qi, Y.; Cao, X.; Zou, Y.; Yang, C. Multi-resonance organoboron-based fluorescent probe for ultra-sensitive, selective and reversible detection of fluoride ions. J. Mater. Chem. C 2021, 9, 1567–1571. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Lin, Q.; Huang, Q.; Liu, Y. Triarylboron-based fluorescent probe exhibiting simultaneous turn-on/turn off color-tunable emission for the highly sensitive detection of fluoride ion. J. Mol. Struct. 2022, 1257, 132537. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, Y.; Du, X.; Li, L.; Li, Q.; Mao, H. Annealing temperature-dependent photoelectrochemical property of zinc oxide/graphene nanocomposite and the application for fabricating a “signal-off” photoelectrochemical aptasensing for ATP. IEEE Sens. J. 2023, 23, 6489–6498. [Google Scholar] [CrossRef]
- Ai, Y.; Li, Y.; Fu, H.L.K.; Chan, A.K.W.; Yam, V.W.W. Aggregation and tunable color emission behaviors of L-glutamine-derived platinum (II) bipyridine complexes by hydrogen-bonding, π–π stacking and metal–metal interactions. Chem. Eur. J. 2019, 25, 5251–5258. [Google Scholar] [CrossRef]
- Cheng, F.; Bonder, E.M.; Jäkle, F. Electron-deficient triarylborane block copolymers: Synthesis by controlled free radical polymerization and application in the detection of fluoride ions. J. Am. Chem. Soc. 2013, 135, 17286–17289. [Google Scholar] [CrossRef]
- Gao, H.; Li, Z.; Pang, Z.; Qin, Y.; Liu, G.; Gao, T.; Dong, X.; Shen, S.; Xie, X.; Wang, P. Rational molecular design strategy for high-efficiency ultrapure blue TADF emitters: Symmetrical and rigid sulfur-bridged boron-based acceptors. ACS Appl. Mater. Interfaces 2023, 15, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kye, M.; Jung, J.Y.; Lim, Y.-b.; Yoon, J. Design for a small molecule based chemosensor containing boron and pyridine moieties to detect HF. Sens. Actuators B 2018, 255, 2621–2627. [Google Scholar] [CrossRef]
- Lee, H.; Jana, S.; Kim, J.; Lee, S.U.; Lee, M.H. Donor-acceptor-appended triarylboron lewis acids: Ratiometric or time-resolved turn-on fluorescence response toward fluoride binding. Inorg. Chem. 2020, 59, 1414–1423. [Google Scholar] [CrossRef]
- Madayanad Suresh, S.; Hall, D.; Beljonne, D.; Olivier, Y.; Zysman-Colman, E. Multiresonant thermally activated delayed fluorescence emitters based on heteroatom-doped nanographenes: Recent advances and prospects for organic light-emitting diodes. Adv. Funct. Mater. 2020, 30, 1908677. [Google Scholar] [CrossRef]
- Tao, L.-M.; Guo, Y.-H.; Huang, X.-M.; Wang, C.-K. Theoretical studies on two-photon absorption properties of newly synthesized triaryl boron-based A-π-A and triaryl nitrogen-based D-π-D quadrupolar compounds. Chem. Phys. Lett. 2006, 425, 10–15. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Triarylborane-based materials for OLED applications. Molecules 2017, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Liu, S.; Yan, Q.; Wei, Y.; Wang, J.; Lan, Y.; Tan, J. Empowering boronic acids as hydroxyl synthons for aryne induced three-component coupling reactions. Chem. Sci. 2023, 14, 4278–4287. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Agou, T.; Kobayashi, J.; Kawashima, T.; Gabbaï, F.P. Fluoride ion complexation by a cationic borane in aqueous solution. Chem. Commun. 2007, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shimoyama, D.; Zhang, N.; Jia, Y.; Hu, G.; Li, C.; Yin, X.; Wang, N.; Jäkle, F.; Chen, P. A new platform of B/N-doped cyclophanes: Access to a π-conjugated block-type B3N3 macrocycle with strong dipole moment and unique optoelectronic properties. Angew. Chem. Int. Ed. 2022, 61, e202200612. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Xia, H.; Ding, X.; Luo, X.; Liu, X.; Mu, Y. Triarylboron-linked conjugated microporous polymers: Sensing and removal of fluoride ions. Chem. Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-Q.; Zhan, B.; Zhao, J.; Guo, Y.; Zu, B.; Li, Y.; He, C. Modular enantioselective assembly of multi-substituted boron-stereogenic bodipys. Nat. Chem. 2024, 17, 83–91. [Google Scholar] [CrossRef]
- Sun, F.; Tan, S.; Cao, H.-J.; Lu, C.-s.; Tu, D.; Poater, J.; Solà, M.; Yan, H. Facile construction of new hybrid conjugation via boron cage extension. J. Am. Chem. Soc. 2023, 145, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Ahmad, M.; Singla, N.; Kumar, G.; Singh, P.; Luxami, V.; Kaur, N.; Kumar, S. Chemodosimeters for optical detection of fluoride anion. Coord. Chem. Rev. 2020, 405, 213138. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, B.; Chen, J.; Duan, X. Fluorescent sensing of fluoride in cellular system. Theranostics 2015, 5, 173. [Google Scholar] [CrossRef]
- Manjare, S.T.; Kim, Y.; Churchill, D.G. Selenium-and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cao, X.; Zou, Y.; Yang, C. Color-tunable tetracoordinated organoboron complexes exhibiting aggregation-induced emission for the efficient turn-on detection of fluoride ions. Mater. Chem. Front. 2021, 5, 2353–2360. [Google Scholar] [CrossRef]
- Wang, H.; Da, L.; Yang, L.; Chu, S.; Yang, F.; Yu, S.; Jiang, C. Colorimetric fluorescent paper strip with smartphone platform for quantitative detection of cadmium ions in real samples. J. Hazard. Mater. 2020, 392, 122506. [Google Scholar] [CrossRef]
- Hu, K.; Lian, G.; Wang, Y.; Shao, T.; Zhou, M.; Liu, Y.; Jin, G. Rich fluorine trident type rigid donor-acceptor (D-A) tricarbazoles-triazine structures: An effective strong acid affinity fluorescent dye. Dye. Pigm. 2022, 201, 110254. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, S.; Wang, R.; Zhang, L.; Ma, Q.; Ren, X.; Gao, Y.; Wang, Z.; Guo, Z.; Zhang, C. Covalent triazine frameworks for advanced energy storage: Challenges and new opportunities. Energy Environ. Sci. 2023, 16, 3181–3213. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Li, W.; Zhang, J.; Luo, M.; Du, S.; Zhang, X.; Xu, S.; Ge, Z. Multiple charge transfer processes enable blue emitter for highly efficient OLEDs. Adv. Opt. Mater. 2023, 11, 2300017. [Google Scholar] [CrossRef]
- Xie, F.M.; Li, H.Z.; Zhang, K.; Shen, Y.; Zhao, X.; Li, Y.Q.; Tang, J.X. A dislocated twin-locking acceptor-donor-acceptor configuration for efficient delayed fluorescence with multiple through-space charge transfer. Angew. Chem. Int. Ed. 2022, 61, e202213823. [Google Scholar] [CrossRef]
- Suzuki, N.; Suda, K.; Yokogawa, D.; Kitoh-Nishioka, H.; Irle, S.; Ando, A.; Abegão, L.M.G.; Kamada, K.; Fukazawa, A.; Yamaguchi, S. Near infrared two-photon-excited and -emissive dyes based on a strapped excited-state intramolecular proton-transfer (ESIPT) scaffold. Chem. Sci. 2018, 9, 2666–2673. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, D.; Lee, T.; Lee, J.; Jung, J.; Yoo, S.; Lee, M.H. Impact of boryl acceptors in para-acridine-appended triarylboron emitters on blue thermally activated delayed fluorescence OLEDs. Dye. Pigm. 2021, 188, 109224. [Google Scholar] [CrossRef]
- Buss, B.L.; Lim, C.H.; Miyake, G.M. Dimethyl dihydroacridines as photocatalysts in organocatalyzed atom transfer radical polymerization of acrylate monomers. Angew. Chem. Int. Ed. 2020, 59, 3209–3217. [Google Scholar] [CrossRef]
- Wu, J.; Lai, G.; Li, Z.; Lu, Y.; Leng, T.; Shen, Y.; Wang, C. Novel 2,1,3-benzothiadiazole derivatives used as selective fluorescent and colorimetric sensors for fluoride ion. Dye. Pigm. 2016, 124, 268–276. [Google Scholar] [CrossRef]
- Cugnasca, B.S.; Duarte, F.; Petrarca de Albuquerque, J.L.; Santos, H.M.; Luis Capelo-Martínez, J.; Lodeiro, C.; Dos Santos, A.A. Precision detection of cyanide, fluoride, and hydroxide ions using a new tetraseleno-BODIPY fluorescent sensor. J. Photochem. Photobiol. A 2024, 457, 115881. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Pei, X.; Qu, M.; Zhang, J.; Hu, W.; Zhang, Y.; Wu, W.; Pei, S. A multifunctional near-infrared fluorescent probe based on benzothiazole structure for fluoride-ion detection. Spectrochim. Acta Part A 2025, 324, 125009. [Google Scholar] [CrossRef]
- Zhou, M.-G.; Chen, L.; Zhou, J.; Zhong, X.; Yuan, M.-S. A fluorescence probe based on naphthalimide-functionalized triarylboron for fluoride ion detection. Inorg. Chem. Commun. 2024, 170, 113392. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.-C.; Bai, J.; Fang, H.; Wu, F.-Y.; Xiao, Q. A “turn-on” fluorescent probe based on BODIPY dyes for highly selective detection of fluoride ions. Dye. Pigm. 2021, 190, 109347. [Google Scholar] [CrossRef]
- Xiao, L.; Ren, L.; Jing, X.; Li, Z.; Wu, S.; Guo, D. A selective naphthalimide-based colorimetric and fluorescent chemosensor for “naked-eye” detection of fluoride ion. Inorg. Chim. Acta 2020, 500, 119207. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Ding, L.; Gao, J. Reusable colorimetric and fluorescent chemosensors based on 1,8-naphthalimide derivatives for fluoride ion detection. Spectrochim. Acta Part A 2020, 237, 118395. [Google Scholar] [CrossRef]
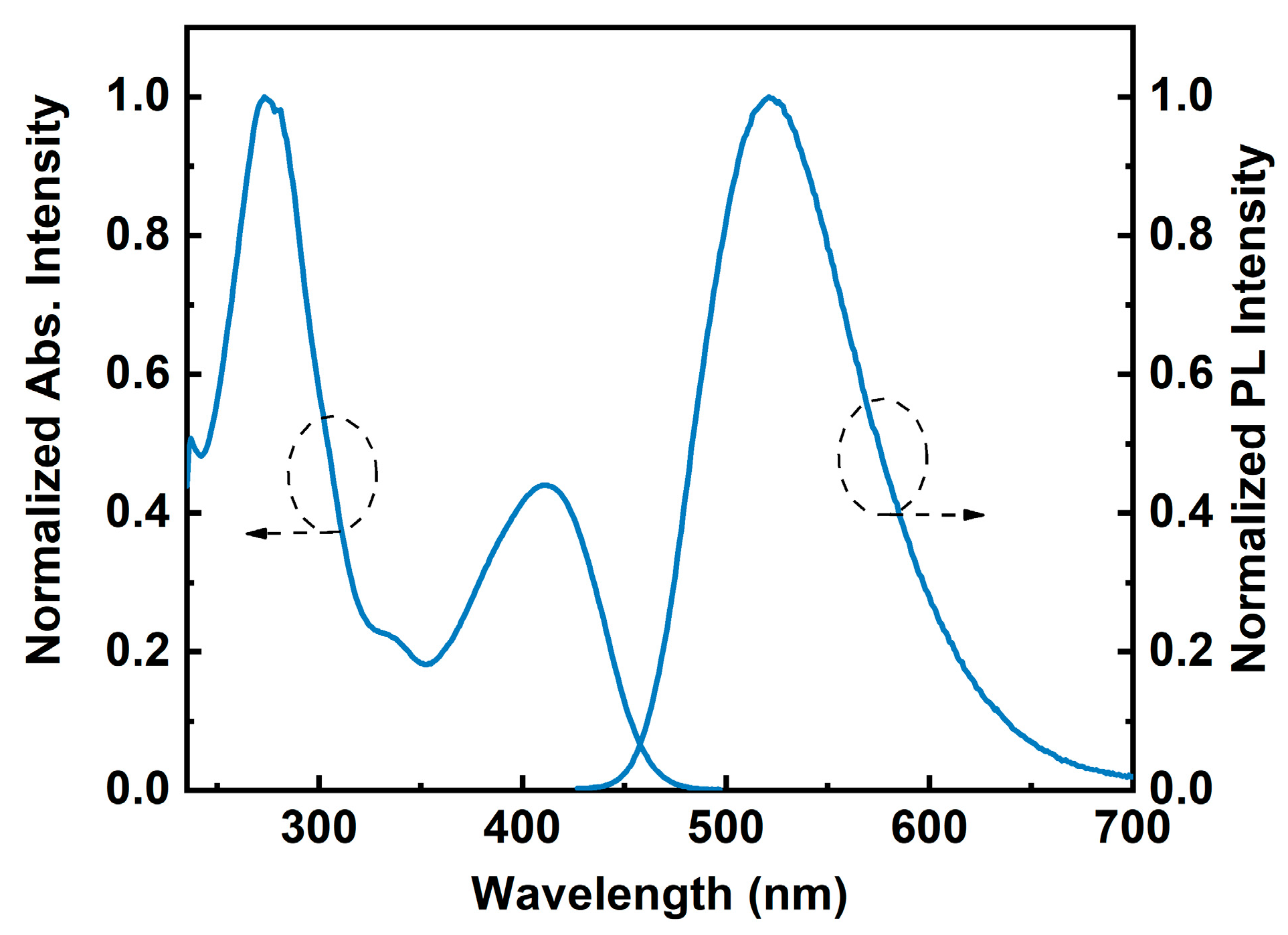

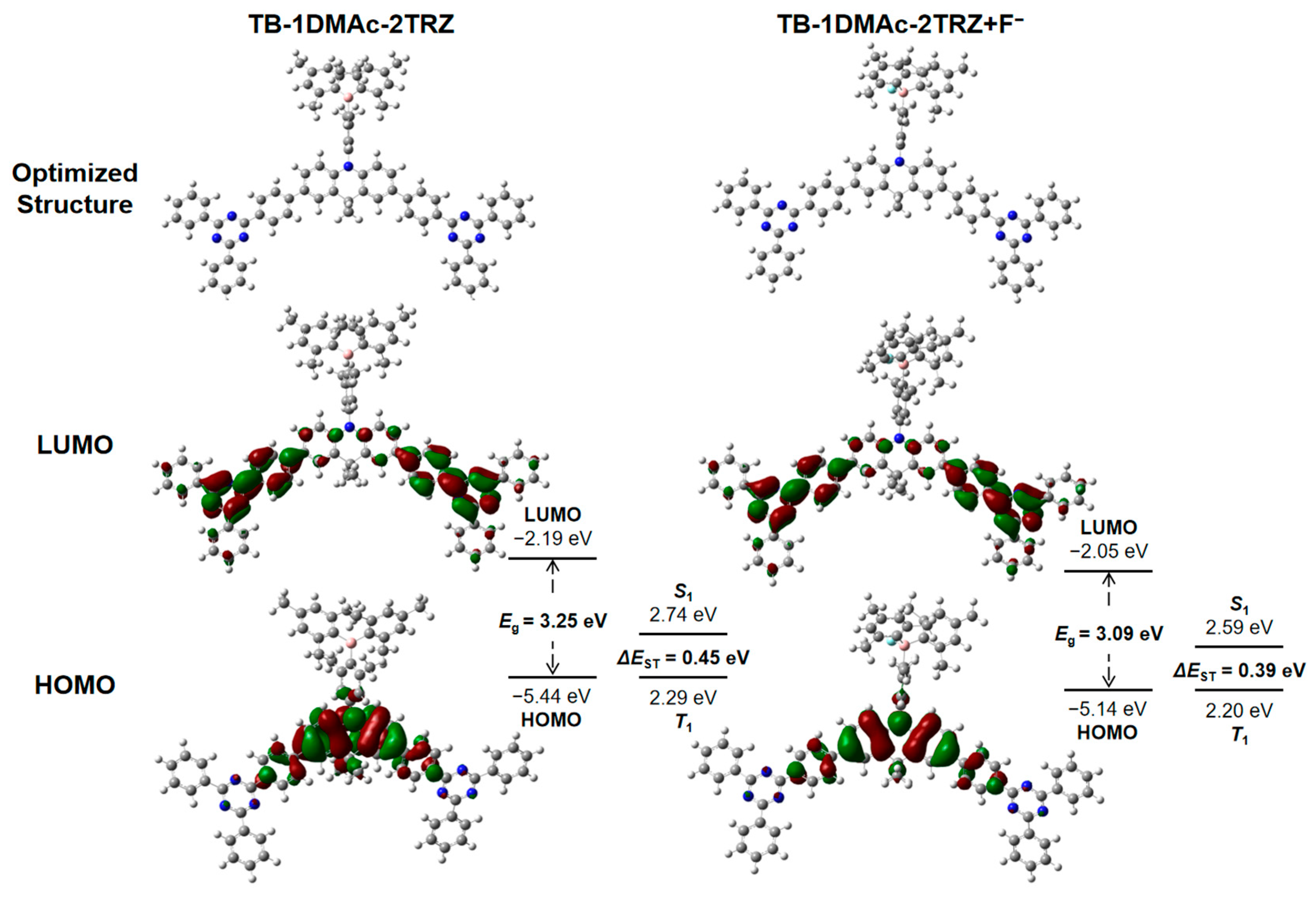
| Compound | λabs 1 [nm] | λem 1 [nm] | Td 2 [°C] | Tg 3 [°C] | ES/ET 4 [eV] | ΔEST 5 [eV] | HOMO 6/LUMO 7 [eV] | Eg 8 [eV] |
|---|---|---|---|---|---|---|---|---|
| TB-1DMAc-2TRZ | 272/411 | 522 | 432 | 206 | 2.74/2.29 | 0.45 | −5.44/−2.19 | 3.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Wang, J.; Liu, Y. Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection. Molecules 2025, 30, 879. https://doi.org/10.3390/molecules30040879
Tang L, Wang J, Liu Y. Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection. Molecules. 2025; 30(4):879. https://doi.org/10.3390/molecules30040879
Chicago/Turabian StyleTang, Lei, Jiaoyun Wang, and Yuan Liu. 2025. "Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection" Molecules 30, no. 4: 879. https://doi.org/10.3390/molecules30040879
APA StyleTang, L., Wang, J., & Liu, Y. (2025). Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection. Molecules, 30(4), 879. https://doi.org/10.3390/molecules30040879







