Nanomaterials for Energy Storage Systems—A Review
Abstract
:1. Introduction
2. Nanotechnology and Nanomaterials
3. Nanomaterials in Battery Technologies
3.1. Nanotechnology Application in Lithium-Ion Batteries (LiBs)
3.1.1. Negative Electrodes
Insertion
- i.
- Carbon nanotubes (CNTs)
- ii.
- Graphene
- iii.
- Spinel Li4Ti5O12 (LTO)
- iv.
- Titanium oxide (TiO2)
Conversion
- i.
- Oxides of iron
- ii.
- Cobalt oxides
Alloys
- i.
- Silicon
- ii.
- Germanium
3.1.2. Positive Electrode
Monoanion
- i.
- Lithium cobalt oxide (LCO)
- ii.
- Lithium manganese oxide (LiMn2O4)
Polyanion
3.1.3. Electrolyte

3.1.4. Separator
3.2. Nanotechnology Application in Sodium–Sulfur Batteries
3.2.1. Electrode Materials
3.2.2. Electrolyte and Separator
3.3. Nanotechnology Application in Redox Flow Batteries
3.3.1. Electrode Materials
3.3.2. Electrolyte Materials
3.3.3. Separator Materials
3.4. Nanotechnology Application in Supercapacitors
4. Manufacturing Approaches for Nanomaterial Applications
4.1. Top–Down
4.1.1. Ball-Milling Method
4.1.2. Sputtering
4.2. Bottom–Up Approaches
4.2.1. Chemical Vapor Deposition (CVD)
4.2.2. Sol–Gel Approach

4.2.3. Hybrid Approaches
5. Challenges and Perspectives
5.1. Electrode Rupture
5.2. Electron Transportation in Particles
5.3. Low Coulombic Efficiency
6. Economic Analysis
7. Sustainability and Environmental Concerns
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Lee, D.; Ağbulut, Ü.; Kumar, S.; Thapa, S.; Thakur, A.; Jilte, R.D.; Saleel, C.A.; Shaik, S. Different Energy Storage Techniques: Recent Advancements, Applications, Limitations, and Efficient Utilization of Sustainable Energy. J. Therm. Anal. Calorim. 2024, 149, 1895–1933. [Google Scholar] [CrossRef]
- Vaghela, P.; Pandey, V.; Sircar, A.; Yadav, K.; Bist, N.; Kumari, R. Energy Storage Techniques, Applications, and Recent Trends: A Sustainable Solution for Power Storage. MRS Energy Sustain. 2023, 10, 261–276. [Google Scholar] [CrossRef]
- Tondan, H.; Singh, A.K. Advances in Energy Harvesting and Storage Materials: Unlocking the Potential of Solid-State Nanomaterials for Renewable Energy Technologies. In Futuristic Trends in Physical Sciences Volume 3 Book 4; Iterative International Publishers, Selfypage Developers Pvt Ltd.: Chikkamagaluru, Karnataka, 2024; pp. 21–32. ISBN 978-93-5747-671-3. [Google Scholar]
- Alonzo, S.M.M.; Bentley, J.; Desai, S.; Bastakoti, B.P. Hydrothermal Synthesis of Hierarchical Microstructure Tungsten Oxide/Carbon Nanocomposite for Supercapacitor Application. Sci. Rep. 2023, 13, 21732. [Google Scholar] [CrossRef]
- Elzein, B. Nano Revolution: Tiny Tech, Big Impact: How Nanotechnology Is Driving SDGs Progress. Heliyon 2024, 10, e31393. [Google Scholar] [CrossRef] [PubMed]
- Charchi, N.; Li, Y.; Huber, M.; Kwizera, E.A.; Huang, X.; Argyropoulos, C.; Hoang, T. Small Mode Volume Plasmonic Film-Coupled Nanostar Resonators. Nanoscale Adv. 2020, 2, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Lovell, M. Computational Fluid Dynamics Analysis of a Direct Write Manufacturing Process. Int. J. Nanomanuf. 2009, 3, 171. [Google Scholar] [CrossRef]
- Cordeiro, J.; Desai, S. The Leidenfrost Effect at the Nanoscale. J. Micro Nano-Manuf. 2016, 4, 041001. [Google Scholar] [CrossRef]
- Cordeiro, J.; Desai, S. The Effect of Water Droplet Size, Temperature, and Impingement Velocity on Gold Wettability at the Nanoscale. J. Micro Nano-Manuf. 2017, 5, 031008. [Google Scholar] [CrossRef]
- Rodrigues, J.; Desai, S. The Nanoscale Leidenfrost Effect. Nanoscale 2019, 11, 12139–12151. [Google Scholar] [CrossRef]
- Gaikwad, A.; Desai, S. Molecular Dynamics Investigation of the Deformation Mechanism of Gold with Variations in Mold Profiles during Nanoimprinting. Materials 2021, 14, 2548. [Google Scholar] [CrossRef]
- Adarkwa, E.; Desai, S. Scalable Droplet Based Manufacturing Using In-Flight Laser Evaporation. J. Nanoeng. Nanomanuf. 2016, 6, 87–92. [Google Scholar] [CrossRef]
- Fialkova, S.; Yarmolenko, S.; Krishnaswamy, A.; Sankar, J.; Shanov, V.; Schulz, M.J.; Desai, S. Nanoimprint Lithography for Next-Generation Carbon Nanotube-Based Devices. Nanomaterials 2024, 14, 1011. [Google Scholar] [CrossRef] [PubMed]
- Gohar, O.; Zubair Khan, M.; Bibi, I.; Bashir, N.; Tariq, U.; Bakhtiar, M.; Ramzan Abdul Karim, M.; Ali, F.; Bilal Hanif, M.; Motola, M. Nanomaterials for Advanced Energy Applications: Recent Advancements and Future Trends. Mater. Des. 2024, 241, 112930. [Google Scholar] [CrossRef]
- Hai, T.; Abidi, A.; Wang, L.; Abed, A.M.; Mahmoud, M.Z.; Tag El Din, E.M.; Smaisim, G.F. Simulation of Solar Thermal Panel Systems with Nanofluid Flow and PCM for Energy Consumption Management of Buildings. J. Build. Eng. 2022, 58, 104981. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Liu, B.; Khalid, I.; Patra, I.; Kuzichkin, O.R.; Sivaraman, R.; Turki Jalil, A.; Sagban, R.; Fadhil Smaisim, G.; Majdi, H.S.; Hekmatifar, M. The Effect of Hydrophilic and Hydrophobic Surfaces on the Thermal and Atomic Behavior of Ammonia/Copper Nanofluid Using Molecular Dynamics Simulation. J. Mol. Liq. 2022, 364, 119925. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured Anode Materials for Lithium Ion Batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on Recent Progress of Nanostructured Anode Materials for Li-Ion Batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Lee, J.K.; Oh, C.; Kim, N.; Hwang, J.-Y.; Sun, Y.-K. Rational Design of Silicon-Based Composites for High-Energy Storage Devices. J. Mater. Chem. A 2016, 4, 5366–5384. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A Review of Carbon Materials and Their Composites with Alloy Metals for Sodium Ion Battery Anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Zhang, N.Q.; Sun, K.N. 3D Self-Supported Nanoarchitectured Arrays Electrodes for Lithium-Ion Batteries. J. Nanomater. 2012, 2012, 905157. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, S.; Liu, Y.; Tang, Y.; Yang, T.; Wang, X.; Wang, Z.; Zhao, Z.; Qiu, J. Rational Design of Metal Oxide Hollow Nanostructures Decorated Carbon Nanosheets for Superior Lithium Storage. J. Mater. Chem. A 2016, 4, 17718–17725. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A Review of Advanced and Practical Lithium Battery Materials. J. Mater. Chem. 2011, 21, 9938. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Liu, S.-H.; Li, F.; Bai, S.; Liu, C.; Tan, J.; Cheng, H.-M. Electrochemical Performance of Pyrolytic Carbon-Coated Natural Graphite Spheres. Carbon 2006, 44, 2212–2218. [Google Scholar] [CrossRef]
- Hong, Z.; Wei, M. Layered Titanate Nanostructures and Their Derivatives as Negative Electrode Materials for Lithium-Ion Batteries. J. Mater. Chem. A 2013, 1, 4403. [Google Scholar] [CrossRef]
- Wagemaker, M.; Mulder, F.M. Properties and Promises of Nanosized Insertion Materials for Li-Ion Batteries. Acc. Chem. Res. 2013, 46, 1206–1215. [Google Scholar] [CrossRef]
- Chen, Z.; Belharouak, I.; Sun, Y.-K.; Amine, K. Titanium-Based Anode Materials for Safe Lithium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 959–969. [Google Scholar] [CrossRef]
- Moretti, A.; Kim, G.-T.; Bresser, D.; Renger, K.; Paillard, E.; Marassi, R.; Winter, M.; Passerini, S. Investigation of Different Binding Agents for Nanocrystalline Anatase TiO2 Anodes and Its Application in a Novel, Green Lithium-Ion Battery. J. Power Sources 2013, 221, 419–426. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Z.; Pourpoint, F.; Armstrong, A.R.; Grey, C.P.; Bruce, P.G. Nanoparticulate TiO2 (B): An Anode for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, H.; Cho, J. MoS2 Nanoplates Consisting of Disordered Graphene-like Layers for High Rate Lithium Battery Anode Materials. Nano Lett. 2011, 11, 4826–4830. [Google Scholar] [CrossRef]
- Desai, S.; Mohan, R.; Sankar, J.; Tiano, T. Understanding Conductivity in a Composite Resin with Single Wall Carbon Nanotubes (SWCNTs) Using Design of Experiments. Int. J. Nanomanuf. 2008, 2, 292. [Google Scholar] [CrossRef]
- Desai, S.; Craps, M.; Esho, T. Direct Writing of Nanomaterials for Flexible Thin-Film Transistors (fTFTs). Int. J. Adv. Manuf. Technol. 2013, 64, 537–543. [Google Scholar] [CrossRef]
- Senami, M.; Ikeda, Y.; Fukushima, A.; Tachibana, A. Theoretical Study of Adsorption of Lithium Atom on Carbon Nanotube. AIP Adv. 2011, 1, 042106. [Google Scholar] [CrossRef]
- Zhao, J.; Buldum, A.; Han, J.; Ping Lu, J. First-Principles Study of Li-Intercalated Carbon Nanotube Ropes. Phys. Rev. Lett. 2000, 85, 1706–1709. [Google Scholar] [CrossRef]
- Pierard, N.; Fonseca, A.; Konya, Z.; Willems, I.; Van Tendeloo, G.; Nagy, J.B. Production of Short Carbon Nanotubes with Open Tips by Ball Milling. Chem. Phys. Lett. 2001, 335, 1–8. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Kim, J.-C.; Seo, S.-D.; Lee, S.; Lee, J.-H.; Kim, D.-W. A Binder-Free Ge-Nanoparticle Anode Assembled on Multiwalled Carbon Nanotube Networks for Li-Ion Batteries. Chem. Commun. 2012, 48, 7061. [Google Scholar] [CrossRef]
- Du, C.; Pan, N. Supercapacitors Using Carbon Nanotubes Films by Electrophoretic Deposition. J. Power Sources 2006, 160, 1487–1494. [Google Scholar] [CrossRef]
- Li, J.; Zhitomirsky, I. Electrophoretic Deposition of Manganese Dioxide–Carbon Nanotube Composites. J. Mater. Process. Technol. 2009, 209, 3452–3459. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Y.; Gong, K.; Mao, L.; Guo, Z.; Chen, Y. Electrostatic Layer-by-Layer Assembled Carbon Nanotube Multilayer Film and Its Electrocatalytic Activity for O2 Reduction. Langmuir 2004, 20, 8781–8785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Yabuuchi, N.; Gallant, B.M.; Chen, S.; Kim, B.-S.; Hammond, P.T.; Shao-Horn, Y. High-Power Lithium Batteries from Functionalized Carbon-Nanotube Electrodes. Nat. Nanotechnol. 2010, 5, 531–537. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, B.-S.; Chen, S.; Shao-Horn, Y.; Hammond, P.T. Layer-by-Layer Assembly of All Carbon Nanotube Ultrathin Films for Electrochemical Applications. J. Am. Chem. Soc. 2009, 131, 671–679. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. Large Reversible Capacity of High Quality Graphene Sheets as an Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Su, F.-Y.; He, Y.-B.; Li, B.; Chen, X.-C.; You, C.-H.; Wei, W.; Lv, W.; Yang, Q.-H.; Kang, F. Could Graphene Construct an Effective Conducting Network in a High-Power Lithium Ion Battery? Nano Energy 2012, 1, 429–439. [Google Scholar] [CrossRef]
- Liu, Y.; Artyukhov, V.I.; Liu, M.; Harutyunyan, A.R.; Yakobson, B.I. Feasibility of Lithium Storage on Graphene and Its Derivatives. J. Phys. Chem. Lett. 2013, 4, 1737–1742. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-Based Electrochemical Energy Conversion and Storage: Fuel Cells, Supercapacitors and Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384. [Google Scholar] [CrossRef]
- Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Li Storage Properties of Disordered Graphene Nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, D.; Fu, Y.; Wang, X. CoFe2O4-Graphene Nanocomposite as a High-Capacity Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2012, 83, 166–174. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, Y.; Wang, J.; Jiang, K.; Fan, S. Conformal Fe3O4 Sheath on Aligned Carbon Nanotube Scaffolds as High-Performance Anodes for Lithium Ion Batteries. Nano Lett. 2013, 13, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Kim, K.-H.; Jung, D.-W.; Singh, K.; Oh, E.-S.; Chung, J.S. Liquid Phase Co-Exfoliated MoS2–Graphene Composites as Anode Materials for Lithium Ion Batteries. J. Power Sources 2013, 244, 280–286. [Google Scholar] [CrossRef]
- Li, X.; Song, H.; Wang, H.; Zhang, Y.; Du, K.; Li, H.; Huang, J. A Nanocomposite of Graphene/MnO2 Nanoplatelets for High-Capacity Lithium Storage. J. Appl. Electrochem. 2012, 42, 1065–1070. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Ramaprabhu, S. Facile Synthesis of SnO2 Nanoparticles Dispersed Nitrogen Doped Graphene Anode Material for Ultrahigh Capacity Lithium Ion Battery Applications. J. Mater. Chem. A 2013, 1, 3865. [Google Scholar] [CrossRef]
- Shen, L.; Uchaker, E.; Zhang, X.; Cao, G. Hydrogenated Li4Ti5O12 Nanowire Arrays for High Rate Lithium Ion Batteries. Adv. Mater. 2012, 24, 6502–6506. [Google Scholar] [CrossRef]
- Meng, X.; Banis, M.N.; Geng, D.; Li, X.; Zhang, Y.; Li, R.; Abou-Rachid, H.; Sun, X. Controllable Atomic Layer Deposition of One-Dimensional Nanotubular TiO2. Appl. Surf. Sci. 2013, 266, 132–140. [Google Scholar] [CrossRef]
- Madian, M.; Klose, M.; Jaumann, T.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J.; Giebeler, L. Anodically Fabricated TiO2–SnO2 Nanotubes and Their Application in Lithium Ion Batteries. J. Mater. Chem. A 2016, 4, 5542–5552. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, S.; Jia, Y. First-Principles Investigation of Phonon Dynamics and Electrochemical Performance of TiO2-x Oxides Lithium-Ion Batteries. Int. J. Hydrogen Energy 2020, 45, 6207–6216. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Binetti, E.; Krueger, S.; Striccoli, M.; Winter, M.; Passerini, S. Percolating Networks of TiO2 Nanorods and Carbon for High Power Lithium Insertion Electrodes. J. Power Sources 2012, 206, 301–309. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, X.; Lu, L.; Wu, X.; Wang, F. Recent Developments of Nanomaterials and Nanostructures for High-Rate Lithium Ion Batteries. ChemSusChem 2020, 13, 5361–5407. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chen, J.S.; Luan, D.; Boey, F.Y.C.; Madhavi, S.; Lou, X.W. (David) Graphene-Supported Anatase TiO2 Nanosheets for Fast Lithium Storage. Chem. Commun. 2011, 47, 5780. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sa, Q.; Chen, J.; Wang, Y.; Jung, H.; Yin, Y. Porous TiO2/C Nanocomposite Shells As a High-Performance Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 6478–6483. [Google Scholar] [CrossRef]
- Xin, X.; Zhou, X.; Wu, J.; Yao, X.; Liu, Z. Scalable Synthesis of TiO2/Graphene Nanostructured Composite with High-Rate Performance for Lithium Ion Batteries. ACS Nano 2012, 6, 11035–11043. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Xu, K.; Xia, Y. Facile Synthesis of Hierarchically Porous Li4Ti5O12 Microspheres for High Rate Lithium Ion Batteries. J. Mater. Chem. 2010, 20, 6998. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent Developments in Nanostructured Anode Materials for Rechargeable Lithium-Ion Batteries. Energy Environ. Sci. 2011, 4, 2682. [Google Scholar] [CrossRef]
- Li, X.; Wang, C. Engineering Nanostructured Anodes via Electrostatic Spray Deposition for High Performance Lithium Ion Battery Application. J. Mater. Chem. A 2013, 1, 165–182. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Lou, X.W. (David) Iron-Oxide-Based Advanced Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1300958. [Google Scholar] [CrossRef]
- Tanaka, S.; Kaneti, Y.V.; Septiani, N.L.W.; Dou, S.X.; Bando, Y.; Hossain, M.S.A.; Kim, J.; Yamauchi, Y. A Review on Iron Oxide-Based Nanoarchitectures for Biomedical, Energy Storage, and Environmental Applications. Small Methods 2019, 3, 1800512. [Google Scholar] [CrossRef]
- Khan, I.; Khalil, A.; Khanday, F.; Shemsi, A.M.; Qurashi, A.; Siddiqui, K.S. Synthesis, Characterization and Applications of Magnetic Iron Oxide Nanostructures. Arab. J. Sci. Eng. 2018, 43, 43–61. [Google Scholar] [CrossRef]
- Ito, S.; Yui, Y.; Mizuguchi, J. Electrical Properties of Semiconductive α-Fe2O3 and Its Use as the Catalyst for Decomposition of Volatile Organic Compounds. Mater. Trans. 2010, 51, 1163–1167. [Google Scholar] [CrossRef]
- Xie, S.T.; Lu, F.; Liu, S.J.; Zheng, L.Q.; Jin, M.L.; Zhou, G.F.; Shui, L.L. Imidazolium ionic liquid induced one-step synthesis of α-Fe2O3 nanorods and nanorod assemblies for lithium-ion battery. APL Mater. 2016, 4, 126107. [Google Scholar] [CrossRef]
- Xue, W.D.; Shi, X.Q.; Xia, H. Ultrafine Fe2O3 Nanoflakes Grafted on TiO2 Nanosheet Arrays as Advanced Anodes for Lithium-Ion Batteries. Sci. Adv. Mater. 2016, 8, 1293. [Google Scholar] [CrossRef]
- Zhu, Y.R.; Li, J.Y.; Yun, X.R.; Zhou, W.; Xi, L.J.; Li, N.; Hu, Z.L. Hydrothermal Synthesis of Nanoflake-Assembled (Ni0.5Co0.5)0.85Se Microspheres as the Cathode and Reduced Graphene Oxide/Porous Fe2O3 Nanospheres Composite as the Anode for Novel Alkaline Aqueous Batteries. ACS Sustain. Chem. Eng. 2020, 8, 561–572. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Fan, H.; Zhu, Z.; Jiang, J.; Ding, R.; Hu, Y.; Huang, X. Iron Oxide-Based Nanotube Arrays Derived from Sacrificial Template-Accelerated Hydrolysis: Large-Area Design and Reversible Lithium Storage. Chem. Mater. 2010, 22, 212–217. [Google Scholar] [CrossRef]
- Shen, L.; Song, H.; Cui, H.; Wen, X.; Wei, X.; Wang, C. Fe3O4–Carbon Nanocomposites via a Simple Synthesis as Anode Materials for Rechargeable Lithium Ion Batteries. CrystEngComm 2013, 15, 9849. [Google Scholar] [CrossRef]
- Ma, X.-H.; Feng, X.-Y.; Song, C.; Zou, B.-K.; Ding, C.-X.; Yu, Y.; Chen, C.-H. Facile Synthesis of Flower-like and Yarn-like α-Fe2O3 Spherical Clusters as Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2013, 93, 131–136. [Google Scholar] [CrossRef]
- Koo, B.; Xiong, H.; Slater, M.D.; Prakapenka, V.B.; Balasubramanian, M.; Podsiadlo, P.; Johnson, C.S.; Rajh, T.; Shevchenko, E.V. Hollow Iron Oxide Nanoparticles for Application in Lithium Ion Batteries. Nano Lett. 2012, 12, 2429–2435. [Google Scholar] [CrossRef]
- Mitra, S.; Poizot, P.; Finke, A.; Tarascon, J.-M. Growth and Electrochemical Characterization versus Lithium of Fe3O4 Electrodes Made by Electrodeposition. Adv. Funct. Mater. 2006, 16, 2281–2287. [Google Scholar] [CrossRef]
- Kang, N.; Park, J.H.; Choi, J.; Jin, J.; Chun, J.; Jung, I.G.; Jeong, J.; Park, J.; Lee, S.M.; Kim, H.J.; et al. Nanoparticulate Iron Oxide Tubes from Microporous Organic Nanotubes as Stable Anode Materials for Lithium Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 6626–6630. [Google Scholar] [CrossRef]
- Yin, L.; Gao, Y.J.; Jeon, I.; Yang, H.; Kim, J.-P.; Jeong, S.Y.; Cho, C.R. Rice-Panicle-like γ-Fe2O3@C Nanofibers as High-Rate Anodes for Superior Lithium-Ion Batteries. Chem. Eng. J. 2019, 356, 60–68. [Google Scholar] [CrossRef]
- Wu, C.; Yin, P.; Zhu, X.; OuYang, C.; Xie, Y. Synthesis of Hematite (α-Fe2O3) Nanorods: Diameter-Size and Shape Effects on Their Applications in Magnetism, Lithium Ion Battery, and Gas Sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Co3O4 Nanoflake as High Capacity Anode Materials for Superior Lithium Storage Performance. Int. J. Electrochem. Sci. 2022, 17, 220454. [Google Scholar] [CrossRef]
- Prieto, P.; Marco, J.F.; Serrano, A.; Manso, M.; De La Figuera, J. Highly Oriented (111) CoO and Co3O4 Thin Films Grown by Ion Beam Sputtering. J. Alloys Compd. 2019, 810, 151912. [Google Scholar] [CrossRef]
- Michalska, M.; Xu, H.; Shan, Q.; Zhang, S.; Dall’Agnese, Y.; Gao, Y.; Jain, A.; Krajewski, M. Solution Combustion Synthesis of a Nanometer-Scale Co3O4 Anode Material for Li-Ion Batteries. Beilstein J. Nanotechnol. 2021, 12, 424–431. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the Capacity Limit of Lithium Cobalt Oxide in Lithium Ion Batteries via Lanthanum and Aluminium Doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Lou, X.W.; Deng, D.; Lee, J.Y.; Feng, J.; Archer, L.A. Self-Supported Formation of Needlelike Co3O4 Nanotubes and Their Application as Lithium-Ion Battery Electrodes. Adv. Mater. 2008, 20, 258–262. [Google Scholar] [CrossRef]
- Guan, H.; Wang, X.; Li, H.; Zhi, C.; Zhai, T.; Bando, Y.; Golberg, D. CoO Octahedral Nanocages for High-Performance Lithium Ion Batteries. Chem. Commun. 2012, 48, 4878. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and Bulk-Silicon-Based Insertion Anodes for Lithium-Ion Secondary Cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Gowda, S.R.; Shaijumon, M.M.; Ajayan, P.M. Hybrid Nanostructures for Energy Storage Applications. Adv. Mater. 2012, 24, 5045–5064. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-Alloy Based Anode Materials for Li Secondary Batteries. Chem. Soc. Rev. 2010, 39, 3115. [Google Scholar] [CrossRef] [PubMed]
- Zamfir, M.R.; Nguyen, H.T.; Moyen, E.; Lee, Y.H.; Pribat, D. Silicon Nanowires for Li-Based Battery Anodes: A Review. J. Mater. Chem. A 2013, 1, 9566. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Y.; Li, P.; Peng, C.; Yin, H.; Li, W.; Wang, D. Electrochemical Conversion of Silica Nanoparticles to Silicon Nanotubes in Molten Salts: Implications for High-Performance Lithium-Ion Battery Anode. ACS Appl. Nano Mater. 2021, 4, 7028–7036. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent Progress of Advanced Anode Materials of Lithium-Ion Batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Franco Gonzalez, A.; Yang, N.-H.; Liu, R.-S. Silicon Anode Design for Lithium-Ion Batteries: Progress and Perspectives. J. Phys. Chem. C 2017, 121, 27775–27787. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing Nanostructured Si Anodes for High Energy Lithium Ion Batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, C.; Song, L.; Zheng, Y.; Long, T. Research Progress of Nano-Silicon-Based Materials and Silicon-Carbon Composite Anode Materials for Lithium-Ion Batteries. J. Solid State Electrochem. 2022, 26, 1125–1136. [Google Scholar] [CrossRef]
- Bogart, T.D.; Chockla, A.M.; Korgel, B.A. High Capacity Lithium Ion Battery Anodes of Silicon and Germanium. Curr. Opin. Chem. Eng. 2013, 2, 286–293. [Google Scholar] [CrossRef]
- Rudawski, N.G.; Yates, B.R.; Holzworth, M.R.; Jones, K.S.; Elliman, R.G.; Volinsky, A.A. Ion Beam-Mixed Ge Electrodes for High Capacity Li Rechargeable Batteries. J. Power Sources 2013, 223, 336–340. [Google Scholar] [CrossRef]
- Chockla, A.M.; Klavetter, K.C.; Mullins, C.B.; Korgel, B.A. Solution-Grown Germanium Nanowire Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 4658–4664. [Google Scholar] [CrossRef]
- Zhang, H.; Braun, P.V. Three-Dimensional Metal Scaffold Supported Bicontinuous Silicon Battery Anodes. Nano Lett. 2012, 12, 2778–2783. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Im, H.S.; Cho, Y.J.; Jung, C.S.; Jang, D.M.; Myung, Y.; Kim, H.S.; Back, S.H.; Lim, Y.R.; Lee, C.-W.; et al. High-Yield Gas-Phase Laser Photolysis Synthesis of Germanium Nanocrystals for High-Performance Photodetectors and Lithium Ion Batteries. J. Phys. Chem. C 2012, 116, 26190–26196. [Google Scholar] [CrossRef]
- Park, M.; Cho, Y.; Kim, K.; Kim, J.; Liu, M.; Cho, J. Germanium Nanotubes Prepared by Using the Kirkendall Effect as Anodes for High-Rate Lithium Batteries. Angew. Chem. Int. Ed. 2011, 50, 9647–9650. [Google Scholar] [CrossRef]
- Yuan, F.-W.; Yang, H.-J.; Tuan, H.-Y. Alkanethiol-Passivated Ge Nanowires as High-Performance Anode Materials for Lithium-Ion Batteries: The Role of Chemical Surface Functionalization. ACS Nano 2012, 6, 9932–9942. [Google Scholar] [CrossRef]
- Hassoun, J.; Lee, K.-S.; Sun, Y.-K.; Scrosati, B. An Advanced Lithium Ion Battery Based on High Performance Electrode Materials. J. Am. Chem. Soc. 2011, 133, 3139–3143. [Google Scholar] [CrossRef]
- Chen, P.; Shen, J.; Wang, T.; Dai, M.; Si, C.; Xie, J.; Li, M.; Cong, X.; Sun, X. Zeolitic Imidazolate Framework-67 Based Separator for Enhanced High Thermal Stability of Lithium Ion Battery. J. Power Sources 2018, 400, 325–332. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Olkhovskii, D.; Rumyantsev, A.; Maximov, M. Applications and Advantages of Atomic Layer Deposition for Lithium-Ion Batteries Cathodes: Review. Batteries 2022, 8, 184. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Hussain, O.M. Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries. Materials 2019, 12, 2687. [Google Scholar] [CrossRef]
- Cao, B.; Li, T.; Zhao, W.; Yin, L.; Cao, H.; Chen, D.; Li, L.; Pan, F.; Zhang, M. Correlating Rate-Dependent Transition Metal Dissolution between Structure Degradation in Li-Rich Layered Oxides. Small 2023, 19, 2301834. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, T.; Sow, C.H.; Shen, Z.X.; Lim, C.T.; Subba Rao, G.V.; Chowdari, B.V.R. α-Fe2O3 Nanoflakes as an Anode Material for Li-Ion Batteries. Adv. Funct. Mater. 2007, 17, 2792–2799. [Google Scholar] [CrossRef]
- Liu, K.; Tan, S.; Moon, J.; Jafta, C.J.; Li, C.; Kobayashi, T.; Lyu, H.; Bridges, C.A.; Men, S.; Guo, W.; et al. Insights into the Enhanced Cycle and Rate Performances of the F-Substituted P2-Type Oxide Cathodes for Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000135. [Google Scholar] [CrossRef]
- Tabuchi, M.; Ado, K.; Kobayashi, H.; Sakaebe, H.; Kageyama, H.; Masquelier, C.; Yonemura, M.; Hirano, A.; Kanno, R. Preparation of LiCoO2 and LiCo1−xFexO2 Using Hydrothermal Reactions. J. Mater. Chem. 1999, 9, 199–204. [Google Scholar] [CrossRef]
- Jiao, F.; Shaju, K.M.; Bruce, P.G. Synthesis of Nanowire and Mesoporous Low-Temperature LiCoO2 by a Post-Templating Reaction. Angew. Chem. Int. Ed. 2005, 44, 6550–6553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wan, C.; Wu, Y.; Jiang, C.; Zhu, Y. Synthesis and Characterization of Ultrafine LiCoO2 Powders by a Spray-Drying Method. J. Power Sources 2000, 85, 294–298. [Google Scholar] [CrossRef]
- Maugeri, L.; Simonelli, L.; Iadecola, A.; Joseph, B.; Okubo, M.; Honma, I.; Wadati, H.; Mizokawa, T.; Saini, N.L. Temperature Dependent Local Structure of LiCoO2 Nanoparticles Determined by Co K-Edge X-Ray Absorption Fine Structure. J. Power Sources 2013, 229, 272–276. [Google Scholar] [CrossRef]
- Okubo, M.; Hosono, E.; Kim, J.; Enomoto, M.; Kojima, N.; Kudo, T.; Zhou, H.; Honma, I. Nanosize Effect on High-Rate Li-Ion Intercalation in LiCoO2 Electrode. J. Am. Chem. Soc. 2007, 129, 7444–7452. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, B.; Yang, Y.; Cao, A. Toward High-Areal-Capacity Electrodes for Lithium and Sodium Ion Batteries. Adv. Energy Mater. 2022, 12, 2201834. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Lithium Insertion into Manganese Spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- Gummow, R.J.; De Kock, A.; Thackeray, M.M. Improved Capacity Retention in Rechargeable 4 V Lithium/Lithium-Manganese Oxide (Spinel) Cells. Solid State Ion. 1994, 69, 59–67. [Google Scholar] [CrossRef]
- Liu, H.K.; Wang, G.X.; Guo, Z.; Wang, J.; Konstantinov, K. Nanomaterials for Lithium-Ion Rechargeable Batteries. J. Nanosci. Nanotechnol. 2006, 6, 1–15. [Google Scholar] [CrossRef]
- Mao, Y.F.; Xiao, S.H.; Liu, J.P. Nanoparticle-assembled LiMn2O4 hollow microspheres as high-performance lithium-ion battery cathode. Mater. Res. Bull. 2017, 96, 437. [Google Scholar] [CrossRef]
- Xia, H.; Xia, Q.; Lin, B.; Zhu, J.; Seo, J.K.; Meng, Y.S. Self-Standing Porous LiMn2O4 Nanowall Arrays as Promising Cathodes for Advanced 3D Microbatteries and Flexible Lithium-Ion Batteries. Nano Energy 2016, 22, 475–482. [Google Scholar] [CrossRef]
- Lee, S.; Oshima, Y.; Suzuki, K.; Kanno, R.; Hosono, E.; Zhou, H.; Takayanagi, K. Lithium Transport of Fast Battery Cycles in a LiMn2O4 Cathode Imaged By Operando Eels. ECS Meet. Abstr. 2016, MA2016-03, 216. [Google Scholar] [CrossRef]
- Labyedh, N.; Mattelaer, F.; Detavernier, C.; Vereecken, P.M. 3D LiMn2O4 Thin-Film Electrodes for High Rate All Solid-State Lithium and Li-Ion Microbatteries. J. Mater. Chem. A 2019, 7, 18996–19007. [Google Scholar] [CrossRef]
- Wang, C.; Hong, J. Ionic/Electronic Conducting Characteristics of LiFePO4 Cathode Materials. Electrochem. Solid-State Lett. 2007, 10, A65. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Chou, C.-J.; Lin, C.-H.; Yang, Y.-C.; Kao, H.-M. Encapsulation of LiFePO4 Nanoparticles into 3D Interpenetrating Ordered Mesoporous Carbon as a High-Performance Cathode for Lithium-Ion Batteries Exceeding Theoretical Capacity. ACS Appl. Energy Mater. 2019, 2, 1121–1133. [Google Scholar] [CrossRef]
- Xin, Y.-M.; Xu, H.-Y.; Ruan, J.-H.; Li, D.-C.; Wang, A.-G.; Sun, D.-S. A Review on Application of LiFePO4 Based Composites as Electrode Materials for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2021, 16, 210655. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Ding, Y.; Yu, G. Self-Assembled LiFePO4 Nanowires with High Rate Capability for Li-Ion Batteries. Chem. Commun. 2014, 50, 9569. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Opra, D.P.; Zemnukhova, L.A.; Sinebryukhov, S.L.; Kedrinskii, I.A.; Patrusheva, O.V.; Sergienko, V.I. Electrochemical Performance of Klason Lignin as a Low-Cost Cathode-Active Material for Primary Lithium Battery. J. Energy Chem. 2015, 24, 346–352. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Peng, H.-J.; Zhang, Q.; Huang, J.-Q.; Liu, X.-F.; Wang, L.; He, X.; Zhu, W.; Wei, F. Hierarchical Carbon Nanotube/Carbon Black Scaffolds as Short- and Long-Range Electron Pathways with Superior Li-Ion Storage Performance. ACS Sustain. Chem. Eng. 2014, 2, 200–206. [Google Scholar] [CrossRef]
- Sasikumar, M.; Krishna, R.H.; Raja, M.; Therese, H.A.; Balakrishnan, N.T.M.; Raghavan, P.; Sivakumar, P. Titanium Dioxide Nano-Ceramic Filler in Solid Polymer Electrolytes: Strategy towards Suppressed Dendrite Formation and Enhanced Electrochemical Performance for Safe Lithium Ion Batteries. J. Alloys Compd. 2021, 882, 160709. [Google Scholar] [CrossRef]
- Bhattacharyya, A.J.; Maier, J. Second Phase Effects on the Conductivity of Non-Aqueous Salt Solutions: “Soggy Sand Electrolytes”. Adv. Mater. 2004, 16, 811–814. [Google Scholar] [CrossRef]
- Besenhard, J.O. (Ed.) Handbook of Battery Materials; Wiley VCH: Weinheim, Germany, 1999. [Google Scholar]
- Croce, F.; Sacchetti, S.; Scrosati, B. Advanced, Lithium Batteries Based on High-Performance Composite Polymer Electrolytes. J. Power Sources 2006, 162, 685–689. [Google Scholar] [CrossRef]
- Shin, W.-K.; Cho, J.; Kannan, A.G.; Lee, Y.-S.; Kim, D.-W. Cross-Linked Composite Gel Polymer Electrolyte Using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/Garnet Composite Electrolytes for Solid-State Lithium Batteries: From “Ceramic-in-Polymer” to “Polymer-in-Ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Sun, Y.; Zhan, X.; Hu, J.; Wang, Y.; Gao, S.; Shen, Y.; Cheng, Y.-T. Improving Ionic Conductivity with Bimodal-Sized Li7La3Zr2O12 Fillers for Composite Polymer Electrolytes. ACS Appl. Mater. Interfaces 2019, 11, 12467–12475. [Google Scholar] [CrossRef]
- Croce, F.; Scrosati, B. Nanocomposite Lithium Ion Conducting Membranes. Ann. N. Y. Acad. Sci. 2003, 984, 194–207. [Google Scholar] [CrossRef]
- Pampal, E.S.; Stojanovska, E.; Simon, B.; Kilic, A. A Review of Nanofibrous Structures in Lithium Ion Batteries. J. Power Sources 2015, 300, 199–215. [Google Scholar] [CrossRef]
- Fu, D.; Luan, B.; Argue, S.; Bureau, M.N.; Davidson, I.J. Nano SiO2 Particle Formation and Deposition on Polypropylene Separators for Lithium-Ion Batteries. J. Power Sources 2012, 206, 325–333. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Zhang, J. A Waterborne superLEphilic and Thermostable Separator Based on Natural Clay Nanorods for High-Voltage Lithium-Ion Batteries. Mater. Today Energy 2020, 16, 100420. [Google Scholar] [CrossRef]
- Poorshakoor, E.; Darab, M. Advancements in the Development of Nanomaterials for Lithium-Ion Batteries: A Scientometric Review. J. Energy Storage 2024, 75, 109638. [Google Scholar] [CrossRef]
- Manuel Stephan, A.; Nahm, K.S. Review on Composite Polymer Electrolytes for Lithium Batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, S.H.; Kim, D.-W. Enhancement of Thermal Stability and Cycling Performance in Lithium-Ion Cells through the Use of Ceramic-Coated Separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Kim, M.; Nho, Y.-C.; Park, J.H. Electrochemical Performances of Inorganic Membrane Coated Electrodes for Li-Ion Batteries. J. Solid State Electrochem. 2010, 14, 769–773. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, P.; Shi, C.; Chen, L.; Dai, J.; Zhao, J. The functional separator coated with core–shell structured silica–poly (methyl methacrylate) sub-microspheres for lithium-ion batteries. J. Membr. Sci. 2015, 474, 148. [Google Scholar] [CrossRef]
- Kim, M.; Han, G.Y.; Yoon, K.J.; Park, J.H. Preparation of a Trilayer Separator and Its Application to Lithium-Ion Batteries. J. Power Sources 2010, 195, 8302–8305. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mateti, S.; Cai, Q.; Sultana, I.; Fan, Y.; Wang, X.; Hou, C.; Chen, Y. High Temperature and High Rate Lithium-Ion Batteries with Boron Nitride Nanotubes Coated Polypropylene Separators. Energy Storage Mater. 2019, 19, 352–359. [Google Scholar] [CrossRef]
- Oshima, T.; Kajita, M.; Okuno, A. Development of Sodium-Sulfur Batteries. Int. J. Appl. Ceram. Technol. 2004, 1, 269–276. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, Y.; Zhang, Y.; Lei, Y.; Lai, W.; Chou, S.; Liu, H.; Dou, S.; Wang, Y. A Critical Review on Room-Temperature Sodium-Sulfur Batteries: From Research Advances to Practical Perspectives. Adv. Mater. 2024, 36, 2402337. [Google Scholar] [CrossRef]
- Chu, C.; Li, R.; Cai, F.; Bai, Z.; Wang, Y.; Xu, X.; Wang, N.; Yang, J.; Dou, S. Recent Advanced Skeletons in Sodium Metal Anodes. Energy Environ. Sci. 2021, 14, 4318–4340. [Google Scholar] [CrossRef]
- Sun, Z.; Ye, Y.; Zhu, J.; Zhou, E.; Xu, J.; Liu, M.; Kong, X.; Jin, S.; Ji, H. Regulating Sodium Deposition through Gradiently-Graphitized Framework for Dendrite-Free Na Metal Anode. Small 2022, 18, 2107199. [Google Scholar] [CrossRef]
- Mubarak, N.; Rehman, F.; Ihsan-Ul-Haq, M.; Xu, M.; Li, Y.; Zhao, Y.; Luo, Z.; Huang, B.; Kim, J. Highly Sodiophilic, Defect-Rich, Lignin-Derived Skeletal Carbon Nanofiber Host for Sodium Metal Batteries. Adv. Energy Mater. 2022, 12, 2103904. [Google Scholar] [CrossRef]
- Ye, W.; Li, X.; Zhang, B.; Liu, W.; Cheng, Y.; Fan, X.; Zhang, H.; Liu, Y.; Dong, Q.; Wang, M. Superfast Mass Transport of Na/K Via Mesochannels for Dendrite-Free Metal Batteries. Adv. Mater. 2023, 35, 2210447. [Google Scholar] [CrossRef]
- Wang, G.; Yu, F.; Zhang, Y.; Zhang, Y.; Zhu, M.; Xu, G.; Wu, M.; Liu, H.-K.; Dou, S.-X.; Wu, C. 2D Sn/C Freestanding Frameworks as a Robust Nucleation Layer for Highly Stable Sodium Metal Anodes with a High Utilization. Nano Energy 2021, 79, 105457. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, K.; Xia, F.; Dahunsi, O.; Gao, S.; Li, B.; Wang, R.; Lu, S.; Qin, W.; Cheng, Y.; et al. Sodiated NaxSnSb Nanoparticles Embedded in N-Doped Graphene Sponges Direct Uniform Na Nucleation and Smooth Plating for High Efficiency Na Metal Batteries. J. Mater. Chem. A 2021, 9, 6123–6130. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Y.; Li, H.; Xu, J.; Zhang, Z. In Situ Construction of Sodiophilic Alloy Interface Enabled Homogenous Na Nucleation and Deposition for Sodium Metal Anode. J. Electrochem. Soc. 2022, 169, 080521. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, S.; Yang, Y. Strategies for Polysulfide Immobilization in Sulfur Cathodes for Room-Temperature Sodium–Sulfur Batteries. Small 2021, 17, 2100057. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, Y.; Yu, Y. Frontiers for Room-Temperature Sodium–Sulfur Batteries. ACS Energy Lett. 2021, 6, 529–536. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Zou, R.; Huang, Y.; Lai, H.; Chen, Z.; Peng, X. Cover Image, Volume 5, Number 1, January 2023. Carbon Energy 2023, 5, e325. [Google Scholar] [CrossRef]
- Li, D.; Gong, B.; Cheng, X.; Ling, F.; Zhao, L.; Yao, Y.; Ma, M.; Jiang, Y.; Shao, Y.; Rui, X.; et al. An Efficient Strategy toward Multichambered Carbon Nanoboxes with Multiple Spatial Confinement for Advanced Sodium–Sulfur Batteries. ACS Nano 2021, 15, 20607–20618. [Google Scholar] [CrossRef]
- Bai, R.; Lin, Q.; Li, X.; Ling, F.; Wang, H.; Tan, S.; Hu, L.; Ma, M.; Wu, X.; Shao, Y.; et al. Toward Complete Transformation of Sodium Polysulfides by Regulating the Second-Shell Coordinating Environment of Atomically Dispersed Fe. Angew. Chem. Int. Ed. 2023, 62, e202218165. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wu, C.; Lu, X.; Hua, W.; Li, S.; Liang, Y.; Liu, H.; Lai, W.; Gu, Q.; Cai, X.; et al. Streamline Sulfur Redox Reactions to Achieve Efficient Room-Temperature Sodium–Sulfur Batteries. Angew. Chem. Int. Ed. 2022, 61, e202200384. [Google Scholar] [CrossRef]
- Tang, W.; Zhong, W.; Wu, Y.; Qi, Y.; Guo, B.; Liu, D.; Bao, S.-J.; Xu, M. Vanadium Carbide Nanoparticles Incorporation in Carbon Nanofibers for Room-Temperature Sodium Sulfur Batteries: Confining, Trapping, and Catalyzing. Chem. Eng. J. 2020, 395, 124978. [Google Scholar] [CrossRef]
- Cai, W.; Li, G.; Zhang, K.; Xiao, G.; Wang, C.; Ye, K.; Chen, Z.; Zhu, Y.; Qian, Y. Conductive Nanocrystalline Niobium Carbide as High-Efficiency Polysulfides Tamer for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1704865. [Google Scholar] [CrossRef]
- Ma, L.; Hendrickson, K.E.; Wei, S.; Archer, L.A. Nanomaterials: Science and Applications in the Lithium–Sulfur Battery. Nano Today 2015, 10, 315–338. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Fan, X.; Gao, T.; Luo, C.; Ma, Z.; Suo, L.; Wang, C. High-Performance All-Solid-State Lithium–Sulfur Battery Enabled by a Mixed-Conductive Li2S Nanocomposite. Nano Lett. 2016, 16, 4521–4527. [Google Scholar] [CrossRef]
- Nagata, H.; Chikusa, Y. An All-Solid-State Sodium–Sulfur Battery Operating at Room Temperature Using a High-Sulfur-Content Positive Composite Electrode. Chem. Lett. 2014, 43, 1333–1334. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Nuli, Y.; Holze, R. Room Temperature Na/S Batteries with Sulfur Composite Cathode Materials. Electrochem. Commun. 2007, 9, 31–34. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ahn, H.-J.; Kim, I.-P.; Kim, K.-W.; Ahn, J.-H.; Park, C.-W.; Ryu, H.-S. The Short-Term Cycling Properties of Na/PVdF/S Battery at Ambient Temperature. J. Solid State Electrochem. 2008, 12, 861–865. [Google Scholar] [CrossRef]
- Wei, S.; Xu, S.; Agrawral, A.; Choudhury, S.; Lu, Y.; Tu, Z.; Ma, L.; Archer, L.A. A Stable Room-Temperature Sodium–Sulfur Battery. Nat. Commun. 2016, 7, 11722. [Google Scholar] [CrossRef]
- Sahgong, S.H.; Senthilkumar, S.T.; Kim, K.; Hwang, S.M.; Kim, Y. Rechargeable Aqueous Na–Air Batteries: Highly Improved Voltage Efficiency by Use of Catalysts. Electrochem. Commun. 2015, 61, 53–56. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; Ponce De León, C.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in Redox Flow Batteries, Remaining Challenges and Their Applications in Energy Storage. RSC Adv. 2012, 2, 10125. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in Flow Battery Research and Development. J. Electrochem. Soc. 2011, 158, R55. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Zhang, J. Nanofiltration (NF) Membranes: The next Generation Separators for All Vanadium Redox Flow Batteries (VRBs)? Energy Environ. Sci. 2011, 4, 1676. [Google Scholar] [CrossRef]
- Eckroad, S. Handbook of Energy Storage for Transmission or Distribution Applications; EPRI 1007189; EPRI: Palo Alto, CA, USA, 2022. [Google Scholar]
- Bartolozzi, M. Development of Redox Flow Batteries. A Historical Bibliography. J. Power Sources 1989, 27, 219–234. [Google Scholar] [CrossRef]
- Ponce De León, C.; Frías-Ferrer, A.; González-García, J.; Szánto, D.A.; Walsh, F.C. Redox Flow Cells for Energy Conversion. J. Power Sources 2006, 160, 716–732. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Williams, R.; Li, J. Report of UK-China Workshops on the Future of Energy Storage: Technologies and Policy; Royal Academy of Engineering: London, UK, 2012; ISBN 978-1-903496-91-6. [Google Scholar]
- Arenas, L.F.; Walsh, F.C.; De León, C.P. General Aspects and Fundamentals of Flow Batteries. In Flow Batteries; Roth, C., Noack, J., Skyllas-Kazacos, M., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 69–97. ISBN 978-3-527-34922-7. [Google Scholar]
- Long, Y.; Ding, M.; Jia, C. Application of Nanomaterials in Aqueous Redox Flow Batteries. ChemNanoMat 2021, 7, 699–712. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazakos, M. Chemical Modification and Electrochemical Behaviour of Graphite Fibre in Acidic Vanadium Solution. Electrochim. Acta 1991, 36, 513–517. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Kwon, Y. Performance Enhancement in Vanadium Redox Flow Battery Using Platinum-Based Electrocatalyst Synthesized by Polyol Process. Electrochim. Acta 2013, 114, 439–447. [Google Scholar] [CrossRef]
- Li, B.; Gu, M.; Nie, Z.; Shao, Y.; Luo, Q.; Wei, X.; Li, X.; Xiao, J.; Wang, C.; Sprenkle, V.; et al. Bismuth Nanoparticle Decorating Graphite Felt as a High-Performance Electrode for an All-Vanadium Redox Flow Battery. Nano Lett. 2013, 13, 1330–1335. [Google Scholar] [CrossRef]
- Jiang, H.R.; Zeng, Y.K.; Wu, M.C.; Shyy, W.; Zhao, T.S. A Uniformly Distributed Bismuth Nanoparticle-Modified Carbon Cloth Electrode for Vanadium Redox Flow Batteries. Appl. Energy 2019, 240, 226–235. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.S.; Zeng, L.; Zhou, X.L.; Zeng, Y.K. Copper Nanoparticle-Deposited Graphite Felt Electrodes for All Vanadium Redox Flow Batteries. Appl. Energy 2016, 180, 386–391. [Google Scholar] [CrossRef]
- Mehboob, S.; Mehmood, A.; Lee, J.-Y.; Shin, H.-J.; Hwang, J.; Abbas, S.; Ha, H.Y. Excellent Electrocatalytic Effects of Tin through in Situ Electrodeposition on the Performance of All-Vanadium Redox Flow Batteries. J. Mater. Chem. A 2017, 5, 17388–17400. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, X.; Fan, X.; Zhao, L.; Liu, J.; Yan, C. CeO2 Embedded Electrospun Carbon Nanofibers as the Advanced Electrode with High Effective Surface Area for Vanadium Flow Battery. Electrochim. Acta 2016, 215, 57–65. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, X.; Cheng, G.; Li, Y.; Li, C.; He, Z.; Zhu, J.; Meng, W.; Zhou, H.; Dai, L.; et al. Electrocatalytic Activity of MnO2 Nanosheet Array-Decorated Carbon Paper as Superior Negative Electrode for Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 322, 134754. [Google Scholar] [CrossRef]
- He, Z.; Li, M.; Li, Y.; Li, C.; Yi, Z.; Zhu, J.; Dai, L.; Meng, W.; Zhou, H.; Wang, L. ZrO2 Nanoparticle Embedded Carbon Nanofibers by Electrospinning Technique as Advanced Negative Electrode Materials for Vanadium Redox Flow Battery. Electrochim. Acta 2019, 309, 166–176. [Google Scholar] [CrossRef]
- He, Z.; Dai, L.; Liu, S.; Wang, L.; Li, C. Mn3O4 Anchored on Carbon Nanotubes as an Electrode Reaction Catalyst of V(IV)/V(V) Couple for Vanadium Redox Flow Batteries. Electrochim. Acta 2015, 176, 1434–1440. [Google Scholar] [CrossRef]
- Ejigu, A.; Edwards, M.; Walsh, D.A. Synergistic Catalyst–Support Interactions in a Graphene–Mn3O4 Electrocatalyst for Vanadium Redox Flow Batteries. ACS Catal. 2015, 5, 7122–7130. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mousavihashemi, S.; Murcia-López, S.; Flox, C.; Andreu, T.; Morante, J.R. High-Power Positive Electrode Based on Synergistic Effect of N- and WO3-Decorated Carbon Felt for Vanadium Redox Flow Batteries. Carbon 2018, 136, 444–453. [Google Scholar] [CrossRef]
- Bayeh, A.W.; Chang, Y.-C.; Liu, T.-R.; Chen, H.-Y.; Huang, H.-C.; Kabtamu, D.M.; Wang, C.-H. Novel Metal Oxide Electrode Materials for Vanadium Redox Flow Battery Application. ECS Meet. Abstr. 2019, MA2019-01, 8. [Google Scholar] [CrossRef]
- Xiang, Y.; Daoud, W.A. Binary NiCoO2-Modified Graphite Felt as an Advanced Positive Electrode for Vanadium Redox Flow Batteries. J. Mater. Chem. A 2019, 7, 5589–5600. [Google Scholar] [CrossRef]
- Mehboob, S.; Ali, G.; Shin, H.-J.; Hwang, J.; Abbas, S.; Chung, K.Y.; Ha, H.Y. Enhancing the Performance of All-Vanadium Redox Flow Batteries by Decorating Carbon Felt Electrodes with SnO2 Nanoparticles. Appl. Energy 2018, 229, 910–921. [Google Scholar] [CrossRef]
- Zhang, R.; Li, K.; Ren, S.; Chen, J.; Feng, X.; Jiang, Y.; He, Z.; Dai, L.; Wang, L. Sb-Doped SnO2 Nanoparticle-Modified Carbon Paper as a Superior Electrode for a Vanadium Redox Flow Battery. Appl. Surf. Sci. 2020, 526, 146685. [Google Scholar] [CrossRef]
- Fetyan, A.; El-Nagar, G.A.; Derr, I.; Kubella, P.; Dau, H.; Roth, C. A Neodymium Oxide Nanoparticle-Doped Carbon Felt as Promising Electrode for Vanadium Redox Flow Batteries. Electrochim. Acta 2018, 268, 59–65. [Google Scholar] [CrossRef]
- Busacca, C.; Blasi, O.D.; Giacoppo, G.; Briguglio, N.; Antonucci, V.; Blasi, A.D. High Performance Electrospun Nickel Manganite on Carbon Nanofibers Electrode for Vanadium Redox Flow Battery. Electrochim. Acta 2020, 355, 136755. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Li, D.; Xiao, Q.; Jing, W. 3D Flower-like Molybdenum Disulfide Modified Graphite Felt as a Positive Material for Vanadium Redox Flow Batteries. RSC Adv. 2020, 10, 17235–17246. [Google Scholar] [CrossRef]
- Ma, D.; Hu, B.; Wu, W.; Liu, X.; Zai, J.; Shu, C.; Tadesse Tsega, T.; Chen, L.; Qian, X.; Liu, T.L. Highly Active Nanostructured CoS2/CoS Heterojunction Electrocatalysts for Aqueous Polysulfide/Iodide Redox Flow Batteries. Nat. Commun. 2019, 10, 3367. [Google Scholar] [CrossRef]
- Gao, M.; Huang, S.; Zhang, F.; Lee, Y.M.; Huang, S.; Wang, Q. Successive Ionic Layer Adsorption and Reaction–Deposited Copper Sulfide Electrocatalyst for High-Power Polysulfide-Based Aqueous Flow Batteries. Mater. Today Energy 2020, 18, 100540. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.R.; Wu, M.C.; Fan, X.Z.; Chao, C.Y.H.; Zhao, T.S. Aligned Hierarchical Electrodes for High-Performance Aqueous Redox Flow Battery. Appl. Energy 2020, 271, 115235. [Google Scholar] [CrossRef]
- Jiang, Y.; Cheng, G.; Li, Y.; He, Z.; Zhu, J.; Meng, W.; Zhou, H.; Dai, L.; Wang, L. Superior Electrocatalytic Performance of Porous, Graphitic, and Oxygen-Functionalized Carbon Nanofiber as Bifunctional Electrode for Vanadium Redox Flow Battery. Appl. Surf. Sci. 2020, 525, 146453. [Google Scholar] [CrossRef]
- Chung, Y.; Noh, C.; Kwon, Y. Role of Borate Functionalized Carbon Nanotube Catalyst for the Performance Improvement of Vanadium Redox Flow Battery. J. Power Sources 2019, 438, 227063. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, Q.; Wu, C.; Liu, Y.; Ding, M.; Ye, J.; Cheng, Y.; Jia, C. Graphene Coated Carbon Felt as a High-Performance Electrode for All Vanadium Redox Flow Batteries. Surf. Coat. Technol. 2019, 358, 153–158. [Google Scholar] [CrossRef]
- Daugherty, M.C.; Gu, S.; Aaron, D.S.; Chandra Mallick, B.; Gandomi, Y.A.; Hsieh, C.-T. Decorating Sulfur and Nitrogen Co-Doped Graphene Quantum Dots on Graphite Felt as High-Performance Electrodes for Vanadium Redox Flow Batteries. J. Power Sources 2020, 477, 228709. [Google Scholar] [CrossRef]
- Opar, D.O.; Nankya, R.; Lee, J.; Jung, H. Assessment of Three-Dimensional Nitrogen-Doped Mesoporous Graphene Functionalized Carbon Felt Electrodes for High-Performance All Vanadium Redox Flow Batteries. Appl. Surf. Sci. 2020, 531, 147391. [Google Scholar] [CrossRef]
- Hu, G.; Jing, M.; Wang, D.-W.; Sun, Z.; Xu, C.; Ren, W.; Cheng, H.-M.; Yan, C.; Fan, X.; Li, F. A Gradient Bi-Functional Graphene-Based Modified Electrode for Vanadium Redox Flow Batteries. Energy Storage Mater. 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Tang, Y.; Bian, H.; Ng, T.; Zhang, W.; Lee, C. Graphene-Nanowall-Decorated Carbon Felt with Excellent Electrochemical Activity Toward VO2+/VO2+ Couple for All Vanadium Redox Flow Battery. Adv. Sci. 2016, 3, 1500276. [Google Scholar] [CrossRef]
- Park, M.; Jeon, I.; Ryu, J.; Baek, J.; Cho, J. Exploration of the Effective Location of Surface Oxygen Defects in Graphene-Based Electrocatalysts for All-Vanadium Redox-Flow Batteries. Adv. Energy Mater. 2015, 5, 1401550. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y. Semi-Solid Lithium Rechargeable Flow Battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Fan, L.; Beidaghi, M.; Boota, M.; Pomerantseva, E.; Kumbur, E.C.; Gogotsi, Y. Composite Manganese Oxide Percolating Networks As a Suspension Electrode for an Asymmetric Flow Capacitor. ACS Appl. Mater. Interfaces 2014, 6, 8886–8893. [Google Scholar] [CrossRef]
- Aberoumand, S.; Woodfield, P.; Shi, G.; Kien Nguyen, T.; Nguyen, H.-Q.; Li, Q.; Shabani, B.; Viet Dao, D. Thermo-Electro-Rheological Behaviour of Vanadium Electrolyte-Based Electrochemical Graphene Oxide Nanofluid Designed for Redox Flow Battery. J. Mol. Liq. 2021, 338, 116860. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. Electroactive Graphene Nanofluids for Fast Energy Storage. 2D Mater. 2016, 3, 031004. [Google Scholar] [CrossRef]
- Kim, J.; Park, H. Enhanced Mass Transfer in Nanofluid Electrolytes for Aqueous Flow Batteries: The Mechanism of Nanoparticles as Catalysts for Redox Reactions. J. Energy Storage 2021, 38, 102529. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, H.; Sun, P.; Ma, Q.; Lu, M.; Su, H.; Yang, W.; Xu, Q. Research Progress on Nanoparticles Applied in Redox Flow Batteries. Battery Energy 2022, 1, 20220023. [Google Scholar] [CrossRef]
- Akter, T.; Desai, S. Developing a Predictive Model for Nanoimprint Lithography Using Artificial Neural Networks. Mater. Des. 2018, 160, 836–848. [Google Scholar] [CrossRef]
- Gaikwad, A.; Desai, S. Understanding Material Deformation in Nanoimprint of Gold Using Molecular Dynamics Simulations. Am. J. Eng. Appl. Sci. 2018, 11, 837–844. [Google Scholar] [CrossRef]
- Desai, S.; Lovell, M. Modeling Fluid–Structure Interaction in a Direct Write Manufacturing Process. J. Mater. Process. Technol. 2012, 212, 2031–2040. [Google Scholar] [CrossRef]
- Desai, S.; Lovell, M. Multiphysics Modeling of a Piezoelectric Bimorph Disc in a Direct Write Fabrication Process; ASMEDC: Orlando, FL, USA, 2005; pp. 437–442. [Google Scholar]
- Odujole, J.; Desai, S. Atomistic Investigation of Material Deformation Behavior of Polystyrene in Nanoimprint Lithography. Surfaces 2020, 3, 649–663. [Google Scholar] [CrossRef]
- Gaikwad, A.; Odujole, J.; Desai, S. Atomistic Investigation of Process Parameter Variations on Material Deformation Behavior in Nanoimprint Lithography of Gold. Precis. Eng. 2020, 64, 7–19. [Google Scholar] [CrossRef]
- Marquetti, I.; Desai, S. Molecular Modeling the Adsorption Behavior of Bone Morphogenetic Protein-2 on Hydrophobic and Hydrophilic Substrates. Chem. Phys. Lett. 2018, 706, 285–294. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion Exchange Membranes for Vanadium Redox Flow Battery (VRB) Applications. Energy Environ. Sci. 2011, 4, 1147. [Google Scholar] [CrossRef]
- Sukkar, T.; Skyllas-Kazacos, M. Membrane Stability Studies for Vanadium Redox Cell Applications. J. Appl. Electrochem. 2004, 34, 137–145. [Google Scholar] [CrossRef]
- Qiu, J.; Li, M.; Ni, J.; Zhai, M.; Peng, J.; Xu, L.; Zhou, H.; Li, J.; Wei, G. Preparation of ETFE-Based Anion Exchange Membrane to Reduce Permeability of Vanadium Ions in Vanadium Redox Battery. J. Membr. Sci. 2007, 297, 174–180. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Chen, J.; Qian, P.; Zhai, Y. Modification of Nafion Membrane Using Interfacial Polymerization for Vanadium Redox Flow Battery Applications. J. Membr. Sci. 2008, 311, 98–103. [Google Scholar] [CrossRef]
- Zeng, J.; Jiang, C.; Wang, Y.; Chen, J.; Zhu, S.; Zhao, B.; Wang, R. Studies on Polypyrrole Modified Nafion Membrane for Vanadium Redox Flow Battery. Electrochem. Commun. 2008, 10, 372–375. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Schwenzer, B.; Kim, S.; Yang, Z.; Thevuthasan, S.; Liu, J.; Graff, G.L.; Hu, J. Investigation of Local Environments in Nafion–SiO2 Composite Membranes Used in Vanadium Redox Flow Batteries. Solid State Nucl. Magn. Reson. 2012, 42, 71–80. [Google Scholar] [CrossRef]
- Ling, X.; Jia, C.; Liu, J.; Yan, C. Preparation and Characterization of Sulfonated Poly(Ether Sulfone)/Sulfonated Poly(Ether Ether Ketone) Blend Membrane for Vanadium Redox Flow Battery. J. Membr. Sci. 2012, 415–416, 306–312. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, Y.; Li, W.; Manthiram, A. Hydrocarbon Blend Membranes with Suppressed Chemical Crossover for Redox Flow Batteries. RSC Adv. 2012, 2, 5554. [Google Scholar] [CrossRef]
- Xing, D.; Zhang, S.; Yin, C.; Zhang, B.; Jian, X. Effect of Amination Agent on the Properties of Quaternized Poly(Phthalazinone Ether Sulfone) Anion Exchange Membrane for Vanadium Redox Flow Battery Application. J. Membr. Sci. 2010, 354, 68–73. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, C.; Xing, D.; Yang, D.; Jian, X. Preparation of Chloromethylated/Quaternized Poly(Phthalazinone Ether Ketone) Anion Exchange Membrane Materials for Vanadium Redox Flow Battery Applications. J. Membr. Sci. 2010, 363, 243–249. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.; Peng, J.; Xu, L.; Li, J.; Zhai, M. Study on the Chemical Stability of the Anion Exchange Membrane of Grafting Dimethylaminoethyl Methacrylate. J. Membr. Sci. 2011, 376, 70–77. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; Ma, J.; Qiu, J.; Peng, J.; Li, J.; Zhai, M. A Novel Amphoteric Ion Exchange Membrane Synthesized by Radiation-Induced Grafting α-Methylstyrene and N,N-Dimethylaminoethyl Methacrylate for Vanadium Redox Flow Battery Application. J. Membr. Sci. 2012, 407–408, 184–192. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Wei, W. Silica Modified Nanofiltration Membranes with Improved Selectivity for Redox Flow Battery Application. Energy Env. Sci 2012, 5, 6299–6303. [Google Scholar] [CrossRef]
- Chmiola, J.; Gogotsi, P.; Weerasooriya, R.; Gogotsi, Y. Low-Cost Supercapacitors for Household Electrical Energy Storage and Harvesting. ECS Meet. Abstr. 2008, MA2008-02, 46. [Google Scholar] [CrossRef]
- Boyea, J.M.; Camacho, R.E.; Turano, S.; Ready, W. Carbon Nanotube-Based Supercapacitors: Technology and Markets. Nanotechnol. Law Bus. 2007, 4, 19–28. [Google Scholar]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef]
- Liang, Y.; Tao, Z.; Chen, J. Organic Electrodes: Organic Electrode Materials for Rechargeable Lithium Batteries (Adv. Energy Mater. 7/2012). Adv. Energy Mater. 2012, 2, 702. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of Electrochemical Capacitor Electrode Materials: A Review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a Carbon Nitride Structure for Visible-Light Catalysis by Copolymerization. Angew. Chem. Int. Ed. 2010, 49, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed Synthesis of 3D Covalent Organic Frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Yoshikawa, H.; Awaga, K. Monitoring the Solid-State Electrochemistry of Cu(2,7-AQDC) (AQDC = Anthraquinone Dicarboxylate) in a Lithium Battery: Coexistence of Metal and Ligand Redox Activities in a Metal–Organic Framework. J. Am. Chem. Soc. 2014, 136, 16112–16115. [Google Scholar] [CrossRef]
- Li, S.-L.; Xu, Q. Metal–Organic Frameworks as Platforms for Clean Energy. Energy Environ. Sci. 2013, 6, 1656. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Zhang, S.; Chen, J. Shape-Controlled Synthesis and Lithium-Storage Study of Metal-Organic Frameworks Zn4O(1,3,5-Benzenetribenzoate)2. J. Power Sources 2006, 160, 542–547. [Google Scholar] [CrossRef]
- Férey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.; Grenèche, J.; Tarascon, J. Mixed-Valence Li/Fe-Based Metal–Organic Frameworks with Both Reversible Redox and Sorption Properties. Angew. Chem. Int. Ed. 2007, 46, 3259–3263. [Google Scholar] [CrossRef]
- Xu, X.; Tang, J.; Qian, H.; Hou, S.; Bando, Y.; Hossain, M.S.A.; Pan, L.; Yamauchi, Y. Three-Dimensional Networked Metal–Organic Frameworks with Conductive Polypyrrole Tubes for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 38737–38744. [Google Scholar] [CrossRef]
- Béguin, F.; Frąckowiak, E. Supercapacitors: Materials, Systems, and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-3-527-32883-3. [Google Scholar]
- Reenu; Sonia; Phor, L.; Kumar, A.; Chahal, S. Electrode Materials for Supercapacitors: A Comprehensive Review of Advancements and Performance. J. Energy Storage 2024, 84, 110698. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kumar, N.; Jena, A.; Mishra, S.; Lee, C.-P.; Lee, S.-Y.; Park, S.-J. Recent Progress in Graphene and Its Derived Hybrid Materials for High-Performance Supercapacitor Electrode Applications. RSC Adv. 2024, 14, 1284–1303. [Google Scholar] [CrossRef] [PubMed]
- Arif, K.; Shakoor, A.; Awais, M.; Dashi, M.; Ajat Khel, B.K.; Sami Ur Rehman, B.S.P.; Masood, K.; Hussain, F.; Alam, W.; Atif, M. Graphene-Based Materials for Electrochemical Energy Storage Devices-EESDs, Opportunities and Future Perspective. In The 2-Dimensional World of Graphene; Khanna, V., Chaudhary, V., Munawar, A., Umapathi, R., Singh, K., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 160–176. ISBN 978-981-5238-93-8. [Google Scholar]
- Mohite, D.D.; Chavan, S.S.; Lokhande, P.E.; Sutar, K.B.; Dubal, S.; Rednam, U.; Ali Al-Asbahi, B.; Anil Kumar, Y. Metal Oxide-Based Nanocomposites as Advanced Electrode Materials for Enhancing Electrochemical Performance of Supercapacitors: A Comprehensive Review. Mater. Today Proc. 2024, in press. [Google Scholar] [CrossRef]
- Pilo, M.I.; Sanna, G.; Spano, N. Conducting Polymers in Amperometric Sensors: A State of the Art over the Last 15 Years with a Focus on Polypyrrole-, Polythiophene-, and Poly(3,4-Ethylenedioxythiophene)-Based Materials. Chemosensors 2024, 12, 81. [Google Scholar] [CrossRef]
- Abdalla, S.; Telfah, A.; Ferjani, H.; Tavares, C.J.; Etzkorn, J. Electrical Conductivity Enhancement in Polyaniline Films via Mixed Ion-electron Conductive Fillers. J. Appl. Polym. Sci. 2024, 141, e56072. [Google Scholar] [CrossRef]
- Bahaa, A.; Alhammadi, A.; Lethesh, K.C.; Susantyoko, R.A.; Bamgbopa, M.O. Ionic Liquid Electrolyte Selection for High Voltage Supercapacitors in High-Temperature Applications. Front. Chem. 2024, 12, 1349864. [Google Scholar] [CrossRef]
- Ding, L.; Tan, Y.; Li, G.; Zhang, K.; Wang, X. A Healable Quasi-Solid Polymer Electrolyte with Balanced Toughness and Ionic Conductivity. Chem.–Eur. J. 2024, 30, e202400584. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; He, L.; Sun, Y.; Huang, Q.; Xu, S.; Qiu, X.; Wei, T. A Gel Polymer Electrolyte Based on IL@NH2-MIL-53 (Al) for High-Performance All-Solid-State Lithium Metal Batteries. Chin. J. Chem. Eng. 2024, 69, 47–55. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Yang, G.; Yang, H.; Nan, H.; Ma, G.; Xu, S. A Polyvinylidene Fluoride-Hexafluoropropylene (PVDF-HFP)/Carboxylated g-C3N4 Composite Separator for High-Performance Lithium-Ion Batteries. ChemistrySelect 2024, 9, e202304054. [Google Scholar] [CrossRef]
- Zhang, Y.; He, R.; Li, Y.; Liu, H.; Liu, H.; Zhang, X.-X. Fabrication of Coaxially Electrospun PEI@PVDF-HFP Fibrous Membrane as Flame-Retardant and Anti-Shrink Separator for Lithium-Ion Battery. Mater. Lett. 2024, 361, 136063. [Google Scholar] [CrossRef]
- Atkins, P.; Ratcliffe, G.; Wormald, M.; Paula, J.D. Ion Transport across Membranes. In Physical Chemistry for the Life Sciences; Oxford University Press: Oxford, UK, 2023; ISBN 978-0-19-883010-8. [Google Scholar]
- Lu, W.; Yuan, Z.; Zhao, Y.; Zhang, H.; Zhang, H.; Li, X. Porous Membranes in Secondary Battery Technologies. Chem. Soc. Rev. 2017, 46, 2199–2236. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-Based Metallic Nanoparticles: Synthesis, Characterization and Applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Prasad Yadav, T.; Manohar Yadav, R.; Pratap Singh, D. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Bentley, J.; Desai, S.; Bastakoti, B.P. Porous Tungsten Oxide: Recent Advances in Design, Synthesis, and Applications. Chem.–Eur. J. 2021, 27, 9241–9252. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry; Gale, P.A., Steed, J.W., Eds.; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0-470-74640-0. [Google Scholar]
- Ayyub, P.; Chandra, R.; Taneja, P.; Sharma, A.K.; Pinto, R. Synthesis of Nanocrystalline Material by Sputtering and Laser Ablation at Low Temperatures. Appl. Phys. Mater. Sci. Process. 2001, 73, 67–73. [Google Scholar] [CrossRef]
- Son, H.H.; Seo, G.H.; Jeong, U.; Shin, D.Y.; Kim, S.J. Capillary Wicking Effect of a Cr-Sputtered Superhydrophilic Surface on Enhancement of Pool Boiling Critical Heat Flux. Int. J. Heat Mass Transf. 2017, 113, 115–128. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering Deposition of Nanoparticles onto Liquid Substrates: Recent Advances and Future Trends. Coord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Muñoz-García, J.; Vázquez, L.; Cuerno, R.; Sánchez-García, J.A.; Castro, M.; Gago, R. Toward Functional Nanomaterials; Springer: New York, NY, USA, 2009; pp. 323–398. [Google Scholar]
- Stokes, K.; Clark, K.; Odetade, D.; Hardy, M.; Goldberg Oppenheimer, P. Advances in Lithographic Techniques for Precision Nanostructure Fabrication in Biomedical Applications. Discov. Nano 2023, 18, 153. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of Carbon Nanotubes by Catalytic Chemical Vapour Deposition: A Review on Carbon Sources, Catalysts and Substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Barreca, D.; Fois, E.; Gasparotto, A.; Maccato, C.; Oriani, M.; Tabacchi, G. The Early Steps of Molecule-to-Material Conversion in Chemical Vapor Deposition (CVD): A Case Study. Molecules 2021, 26, 1988. [Google Scholar] [CrossRef]
- Wu, Q.; Wongwiriyapan, W.; Park, J.-H.; Park, S.; Jung, S.J.; Jeong, T.; Lee, S.; Lee, Y.H.; Song, Y.J. In Situ Chemical Vapor Deposition of Graphene and Hexagonal Boron Nitride Heterostructures. Curr. Appl. Phys. 2016, 16, 1175–1191. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The Evolution of ‘Sol–Gel’ Chemistry as a Technique for Materials Synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A Review of Photocatalysts Prepared by Sol-Gel Method for VOCs Removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, I.-S.; Lee, J.H.; Kim, D.H.; Kim, D.W.; Kim, J.Y.; Shin, H.; Lee, J.-K.; Jung, H.S.; Park, N.-G.; et al. Two-Step Sol−Gel Method-Based TiO2 Nanoparticles with Uniform Morphology and Size for Efficient Photo-Energy Conversion Devices. Chem. Mater. 2010, 22, 1958–1965. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–Gel-Deposited ZnO Thin Films: A Review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Hossain, N.; Mobarak, M.H.; Mimona, M.A.; Islam, M.A.; Hossain, A.; Zohura, F.T.; Chowdhury, M.A. Advances and Significances of Nanoparticles in Semiconductor Applications—A Review. Results Eng. 2023, 19, 101347. [Google Scholar] [CrossRef]
- Desai, S.; Shankar, M.R. Polymers, Composites and Nano Biomaterials: Current and Future Developments. In Bio-Materials and Prototyping Applications in Medicine; Bártolo, P., Bidanda, B., Eds.; Springer: Boston, MA, USA, 2008; pp. 15–26. ISBN 978-0-387-47682-7. [Google Scholar]
- Desai, S.; Shankar, M.R. Emerging Trends in Polymers, Composites, and Nano Biomaterial Applications. In Bio-Materials and Prototyping Applications in Medicine; Bártolo, P.J., Bidanda, B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 19–34. ISBN 978-3-030-35875-4. [Google Scholar]
- Desai, S.; Bidanda, B.; Bártolo, P.J. Emerging Trends in the Applications of Metallic and Ceramic Biomaterials. In Bio-Materials and Prototyping Applications in Medicine; Bártolo, P.J., Bidanda, B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–17. ISBN 978-3-030-35875-4. [Google Scholar]
- Aldawood, F.K.; Parupelli, S.K.; Andar, A.; Desai, S. 3D Printing of Biodegradable Polymeric Microneedles for Transdermal Drug Delivery Applications. Pharmaceutics 2024, 16, 237. [Google Scholar] [CrossRef]
- Adarkwa, E.; Roy, A.; Ohodnicki, J.; Lee, B.; Kumta, P.N.; Desai, S. 3D Printing of Drug-Eluting Bioactive Multifunctional Coatings for Orthopedic Applications. Int. J. Bioprinting 2023, 9, 661. [Google Scholar] [CrossRef]
- Perkins, J.; Hong, Y.; Ye, S.-H.; Wagner, W.R.; Desai, S. Direct Writing of Bio-Functional Coatings for Cardiovascular Applications: Direct Writing of Bio-Functional Coatings for Cardiovascular App. J. Biomed. Mater. Res. A 2014, 102, 4290–4300. [Google Scholar] [CrossRef]
- Desai, S.; Perkins, J.; Harrison, B.S.; Sankar, J. Understanding Release Kinetics of Biopolymer Drug Delivery Microcapsules for Biomedical Applications. Mater. Sci. Eng. B 2010, 168, 127–131. [Google Scholar] [CrossRef]
- Kumar Parupelli, S.; Saudi, S.; Bhattarai, N.; Desai, S. 3D Printing of PCL-Ceramic Composite Scaffolds for Bone Tissue Engineering Applications. Int. J. Bioprinting 2023, 9, 0196. [Google Scholar] [CrossRef]
- Godja, N.-C.; Munteanu, F.-D. Hybrid Nanomaterials: A Brief Overview of Versatile Solutions for Sensor Technology in Healthcare and Environmental Applications. Biosensors 2024, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Parupelli, S.K.; Desai, S. Hybrid Additive Manufacturing (3D Printing) and Characterization of Functionally Gradient Materials via in Situ Laser Curing. Int. J. Adv. Manuf. Technol. 2020, 110, 543–556. [Google Scholar] [CrossRef]
- Nandipati, M.; Ogunsanya, M.; Desai, S. Predictive Models for 3D Inkjet Material Printer Using Automated Image Analysis and Machine Learning Algorithms. Manuf. Lett. 2024, 41, 810–821. [Google Scholar] [CrossRef]
- Elhoone, H.; Zhang, T.; Anwar, M.; Desai, S. Cyber-Based Design for Additive Manufacturing Using Artificial Neural Networks for Industry 4.0. Int. J. Prod. Res. 2020, 58, 2841–2861. [Google Scholar] [CrossRef]
- Almakaeel, H.; Albalawi, A.; Desai, S. Artificial Neural Network Based Framework for Cyber Nano Manufacturing. Manuf. Lett. 2018, 15, 151–154. [Google Scholar] [CrossRef]
- Almakayeel, N.; Desai, S.; Alghamdi, S.; Qureshi, M.R.N.M. Smart Agent System for Cyber Nano-Manufacturing in Industry 4.0. Appl. Sci. 2022, 12, 6143. [Google Scholar] [CrossRef]
- Desai, S.; Dean, C.; Desai, Y. Cyber-Enabled Concurrent Material and Process Selection in a Flexible Design for Manufacture Paradigm. Int. J. Adv. Manuf. Technol. 2018, 97, 1719–1731. [Google Scholar] [CrossRef]
- Aljabali, B.A.; Shelton, J.; Desai, S. Genetic Algorithm-Based Data-Driven Process Selection System for Additive Manufacturing in Industry 4.0. Materials 2024, 17, 4544. [Google Scholar] [CrossRef]
- Olowe, M.; Ogunsanya, M.; Best, B.; Hanif, Y.; Bajaj, S.; Vakkalagadda, V.; Fatoki, O.; Desai, S. Spectral Features Analysis for Print Quality Prediction in Additive Manufacturing: An Acoustics-Based Approach. Sensors 2024, 24, 4864. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and Challenges of Nanomaterials for Lithium-Based Rechargeable Batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Yoda, M.; Garden, J.-L.; Bourgeois, O.; Haque, A.; Kumar, A.; Deyhle, H.; Hieber, S.; Müller, B.; Cano-Sarabia, M.; Maspoch, D.; et al. Nanomaterials for Electrical Energy Storage Devices. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 1597–1608. ISBN 978-90-481-9750-7. [Google Scholar]
- Zhang, X.; Ju, Z.; Zhu, Y.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; Yu, G. Multiscale Understanding and Architecture Design of High Energy/Power Lithium-Ion Battery Electrodes. Adv. Energy Mater. 2021, 11, 2000808. [Google Scholar] [CrossRef]
- Yang, M.; Parupelli, S.K.; Xu, Z.; Desai, S. Understanding the Effect of Dispersant Rheology and Binder Decomposition on 3D Printing of a Solid Oxide Fuel Cell. Micromachines 2024, 15, 636. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Parupelli, S.K.; Xu, Z.; Desai, S. Three-Dimensional-Printed Composite Structures: The Effect of LSCF Slurry Solid Loading, Binder, and Direct-Write Process Parameters. Materials 2024, 17, 2822. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.; Desai, S.; Kumar, D.; Sankar, J. Fabrication of Micro Single Chamber Solid Oxide Fuel Cell Using Photolithography and Pulsed Laser Deposition. J. Fuel Cell Sci. Technol. 2015, 12, 021004. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.; Desai, S.; Kumar, D.; Sankar, J. Fabrication of Novel Single-Chamber Solid Oxide Fuel Cells Towards Green Technology. In Volume 14: Processing and Engineering Applications of Novel Materials; ASMEDC: Lake Buena Vista, FL, USA, 2009; pp. 61–66. [Google Scholar]
- Rajaram, G.; Desai, S.; Xu, Z.; Pai, D.M.; Sankar, J. Systematic Studies on NI-YSZ Anode Material for Solid Oxide Fuel Cell (SOFCs) Applications. Int. J. Manuf. Res. 2008, 3, 350. [Google Scholar] [CrossRef]
- Vargas-Bernal, R. Introductory Chapter: Hybrid Nanomaterials. In Hybrid Nanomaterials—Flexible Electronics Materials; Vargas-Bernal, R., He, P., Zhang, S., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-337-7. [Google Scholar]
- Kim, H.-J.; Krishna, T.; Zeb, K.; Rajangam, V.; Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A Comprehensive Review of Li-Ion Battery Materials and Their Recycling Techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Addu, S.K.; Zhu, J.; Ng, K.Y.S.; Deng, D. A Family of Mesocubes. Chem. Mater. 2014, 26, 4472–4485. [Google Scholar] [CrossRef]
- Deng, D.; Lee, J.Y. Meso-Oblate Spheroids of Thermal-Stabile Linker-Free Aggregates with Size-Tunable Subunits for Reversible Lithium Storage. ACS Appl. Mater. Interfaces 2014, 6, 1173–1179. [Google Scholar] [CrossRef]
- Zhu, J.; Ng, K.Y.S.; Deng, D. Hollow Cocoon-Like Hematite Mesoparticles of Nanoparticle Aggregates: Structural Evolution and Superior Performances in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ng, K.Y.S.; Deng, D. Micro Single Crystals of Hematite with Nearly 100% Exposed {104} Facets: Preferred Etching and Lithium Storage. Cryst. Growth Des. 2014, 14, 2811–2817. [Google Scholar] [CrossRef]
- Zhu, J.; Ng, K.Y.S.; Deng, D. Porous Olive-like Carbon Decorated Fe3O4 Based Additive-Free Electrodes for Highly Reversible Lithium Storage. J Mater. Chem. A 2014, 2, 16008–16014. [Google Scholar] [CrossRef]
- Deng, D.; Lee, J.Y. Linker-Free 3D Assembly of Nanocrystals with Tunable Unit Size for Reversible Lithium Ion Storage. Nanotechnology 2011, 22, 355401. [Google Scholar] [CrossRef]
- Deng, D. Li-ion Batteries: Basics, Progress, and Challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching High-Performance Potassium-Ion Batteries via Advanced Design Strategies and Engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery Materials for Ultrafast Charging and Discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhang, B.; Miao, L.; Cai, J.; Peng, L.; Huang, Y.; Jiang, J.; Huang, Y.; Zhang, L.; et al. Nitrogen-Rich Hard Carbon as a Highly Durable Anode for High-Power Potassium-Ion Batteries. Energy Storage Mater. 2017, 8, 161–168. [Google Scholar] [CrossRef]
- Yan, C.; Yao, Y.; Chen, X.; Cheng, X.; Zhang, X.; Huang, J.; Zhang, Q. Lithium Nitrate Solvation Chemistry in Carbonate Electrolyte Sustains High-Voltage Lithium Metal Batteries. Angew. Chem. 2018, 130, 14251–14255. [Google Scholar] [CrossRef]
- Tai, Z.; Liu, Y.; Zhang, Q.; Zhou, T.; Guo, Z.; Liu, H.K.; Dou, S.X. Ultra-Light and Flexible Pencil-Trace Anode for High Performance Potassium-Ion and Lithium-Ion Batteries. Green Energy Environ. 2017, 2, 278–284. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Desai, S. The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects. Bioengineering 2023, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.; Olowe, M.; Desai, S. Deformation Mechanism of Aluminum, Copper, and Gold in Nanoimprint Lithography Using Molecular Dynamics Simulation. Nanomaterials 2023, 13, 3104. [Google Scholar] [CrossRef] [PubMed]
- Nandipati, M.; Fatoki, O.; Desai, S. Bridging Nanomanufacturing and Artificial Intelligence—A Comprehensive Review. Materials 2024, 17, 1621. [Google Scholar] [CrossRef]
- Hoenig, A.; Roy, K.; Acquaah, Y.T.; Yi, S.; Desai, S.S. Explainable AI for Cyber-Physical Systems: Issues and Challenges. IEEE Access 2024, 12, 73113–73140. [Google Scholar] [CrossRef]
- Desai, S. Methods and Apparatus of Manufacturing Micro and Nano-Scale Features. U.S. Patent US8573757B2, 5 November 2013. [Google Scholar]
- Mai, L.; Tian, X.; Xu, X.; Chang, L.; Xu, L. Nanowire Electrodes for Electrochemical Energy Storage Devices. Chem. Rev. 2014, 114, 11828–11862. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Kalaiselvam, S.; Harikrishnan, S.; Elayaperumal, A. Sustainable Thermal Energy Storage Technologies for Buildings: A Review. Renew. Sustain. Energy Rev. 2012, 16, 2394–2433. [Google Scholar] [CrossRef]
- Inshakova, E.; Inshakova, A.; Goncharov, A. Engineered Nanomaterials for Energy Sector: Market Trends, Modern Applications and Future Prospects. IOP Conf. Ser. Mater. Sci. Eng. 2020, 971, 032031. [Google Scholar] [CrossRef]
- Shapira, P.; Youtie, J. The Economic Contributions of Nanotechnology to Green and Sustainable Growth. In Green Processes for Nanotechnology; Basiuk, V.A., Basiuk, E.V., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 409–434. ISBN 978-3-319-15460-2. [Google Scholar]
- Lee, Y.J.; Yi, H.; Kim, W.-J.; Kang, K.; Yun, D.S.; Strano, M.S.; Ceder, G.; Belcher, A.M. Fabricating Genetically Engineered High-Power Lithium-Ion Batteries Using Multiple Virus Genes. Science 2009, 324, 1051–1055. [Google Scholar] [CrossRef]
- Chen, H.; Armand, M.; Demailly, G.; Dolhem, F.; Poizot, P.; Tarascon, J. From Biomass to a Renewable LiXC6O6 Organic Electrode for Sustainable Li-Ion Batteries. ChemSusChem 2008, 1, 348–355. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life Cycle Assessment of Lithium-Ion Batteries for Plug-in Hybrid Electric Vehicles—Critical Issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Sonoc, A.; Jeswiet, J.; Soo, V.K. Opportunities to Improve Recycling of Automotive Lithium Ion Batteries. Procedia CIRP 2015, 29, 752–757. [Google Scholar] [CrossRef]
- Olawore, O.; Ogunmola, M.; Desai, S. Engineered Nanomaterial Coatings for Food Packaging: Design, Manufacturing, Regulatory, and Sustainability Implications. Micromachines 2024, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Huo, H.; Ma, Y.; Wang, L.; Yin, G.; Zuo, P.; Gao, Y. Intelligent Dual-Anode Strategy for High-Performance Lithium-Ion Batteries. Device 2024, 2, 100501. [Google Scholar] [CrossRef]
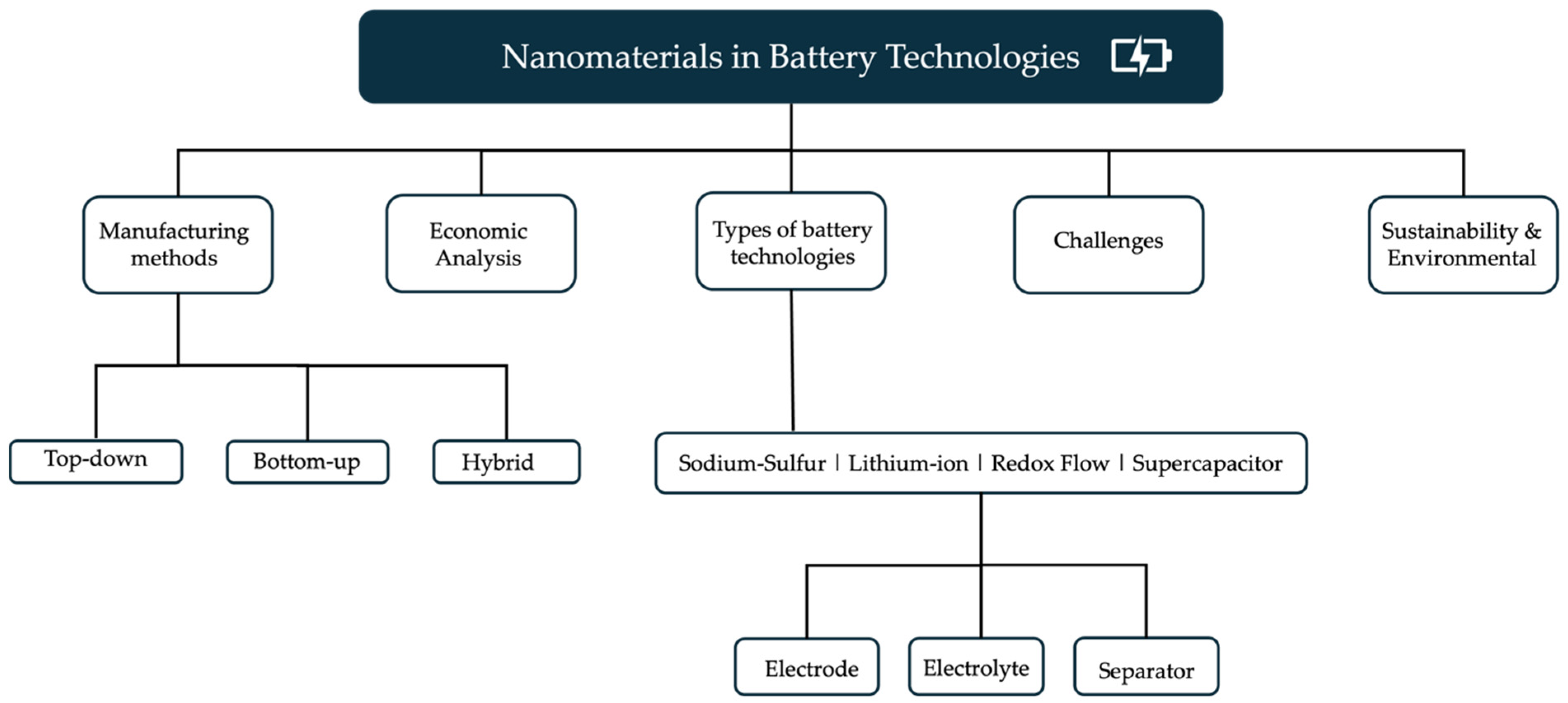

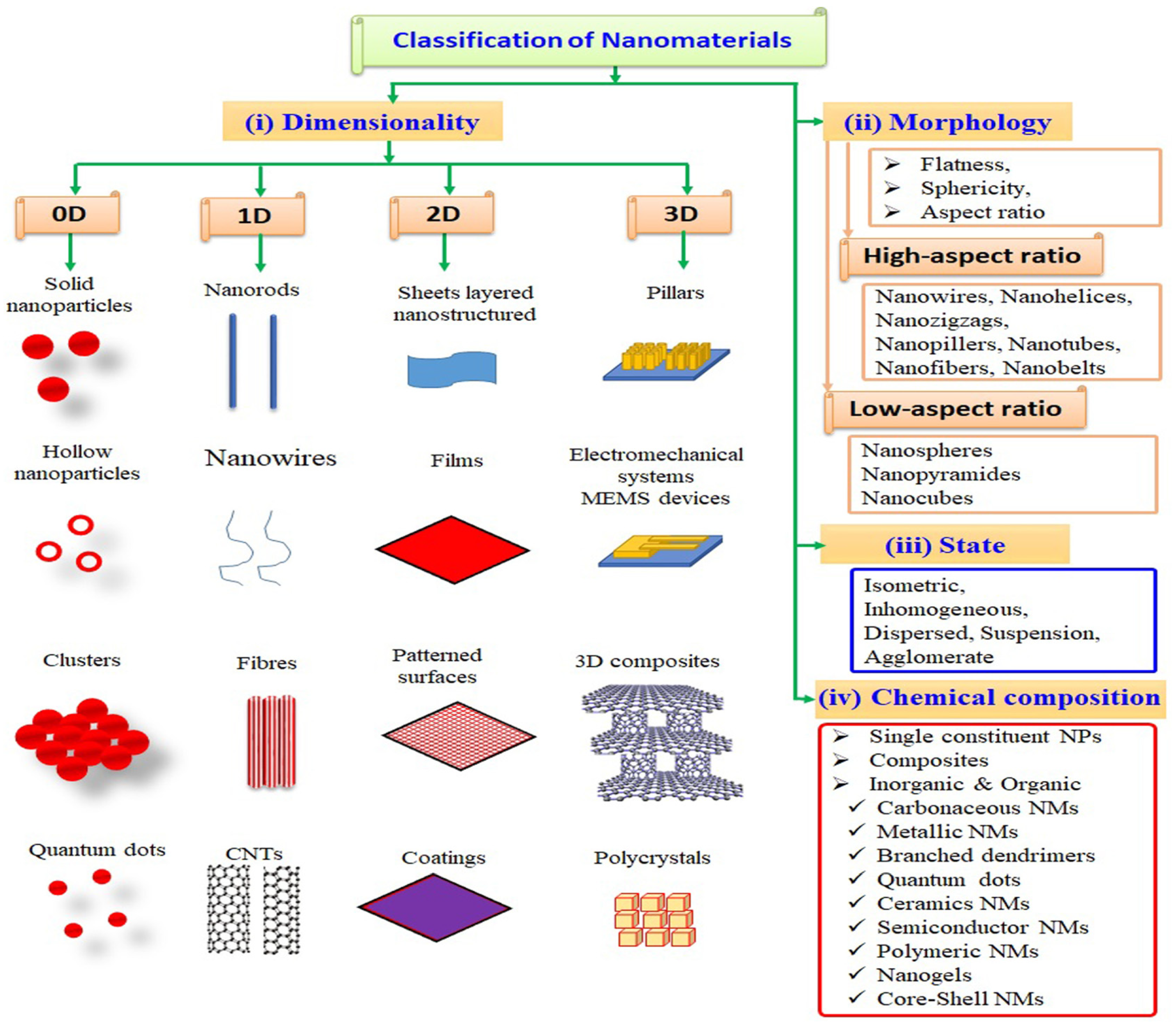
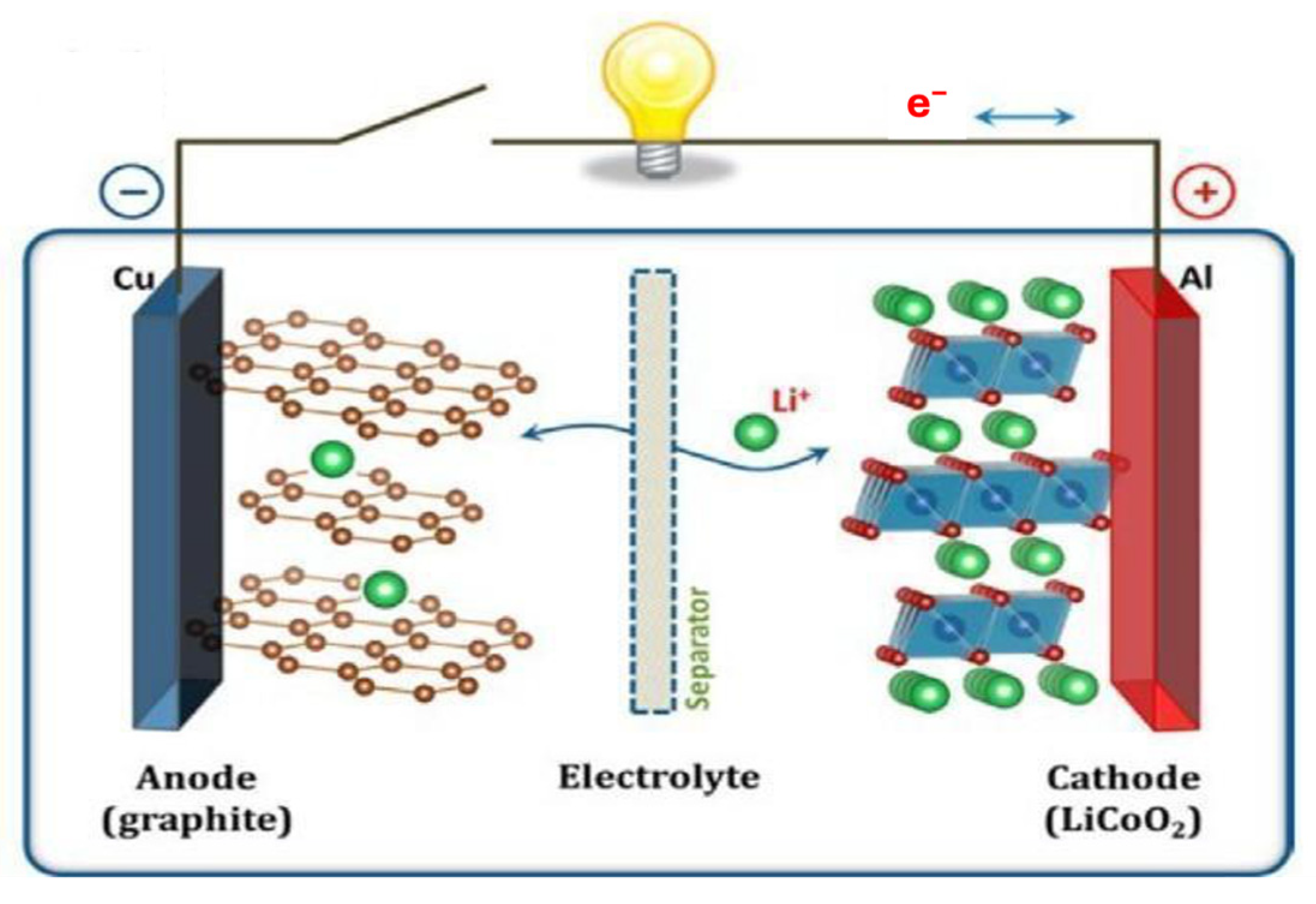
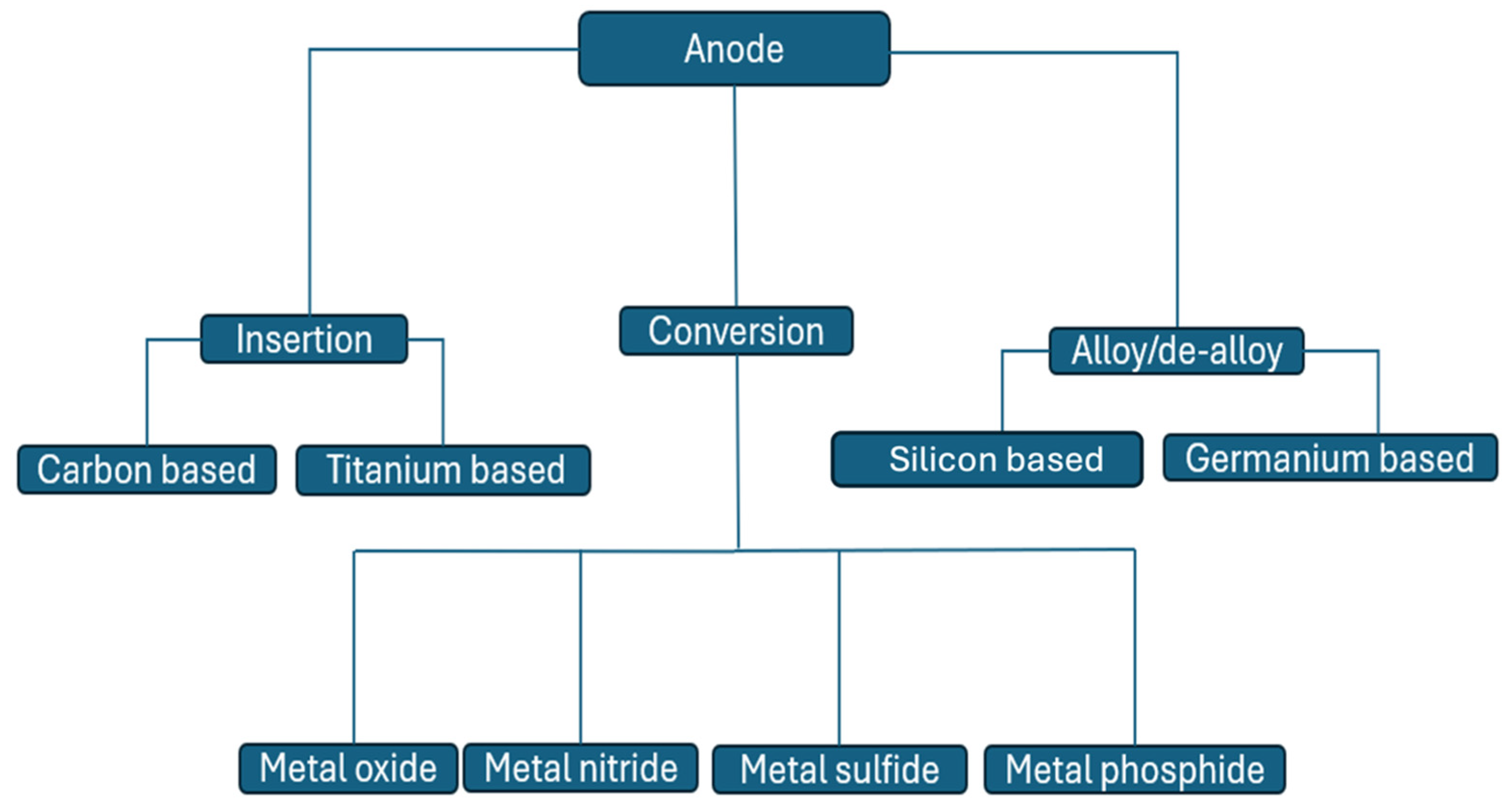
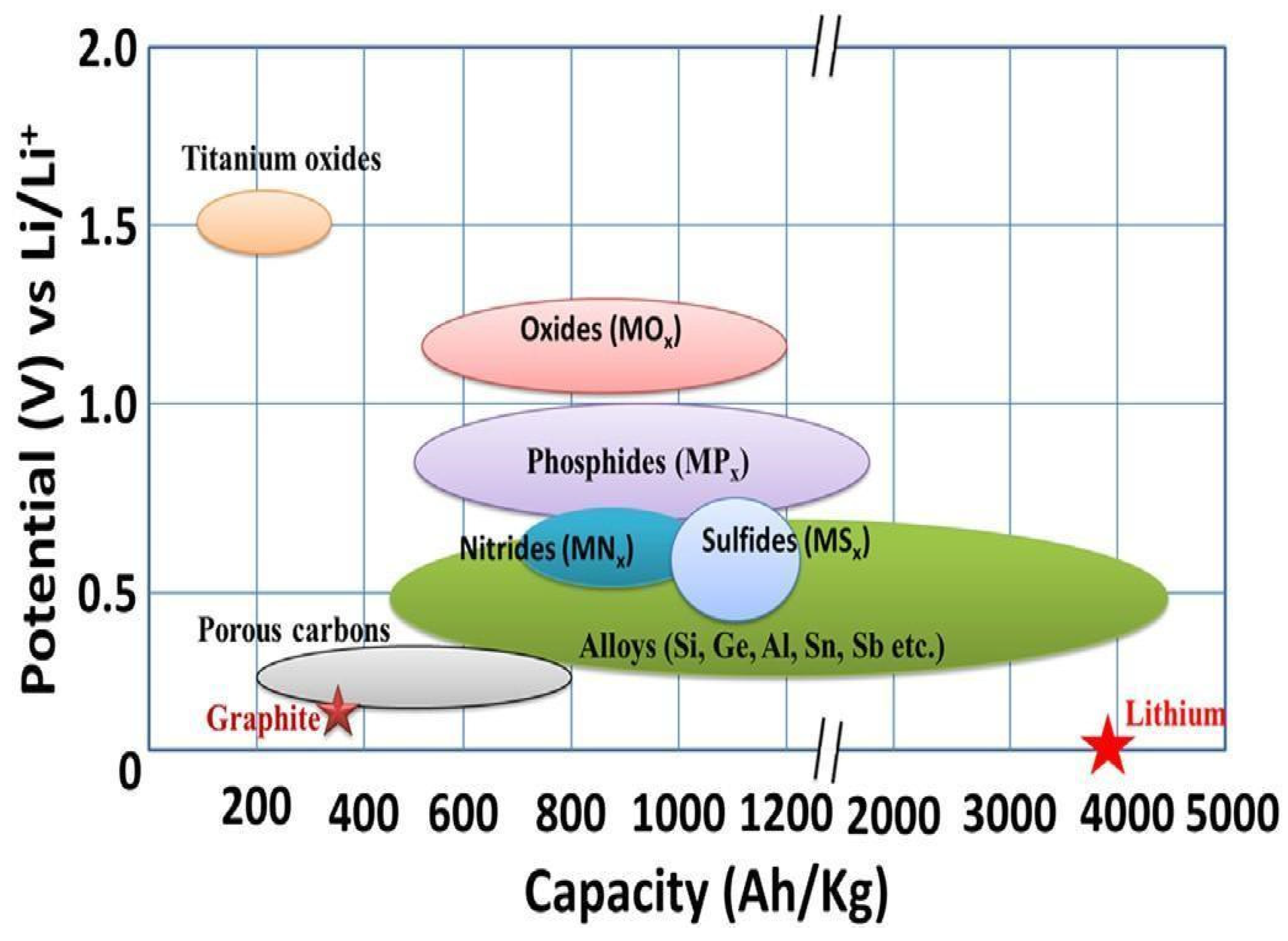
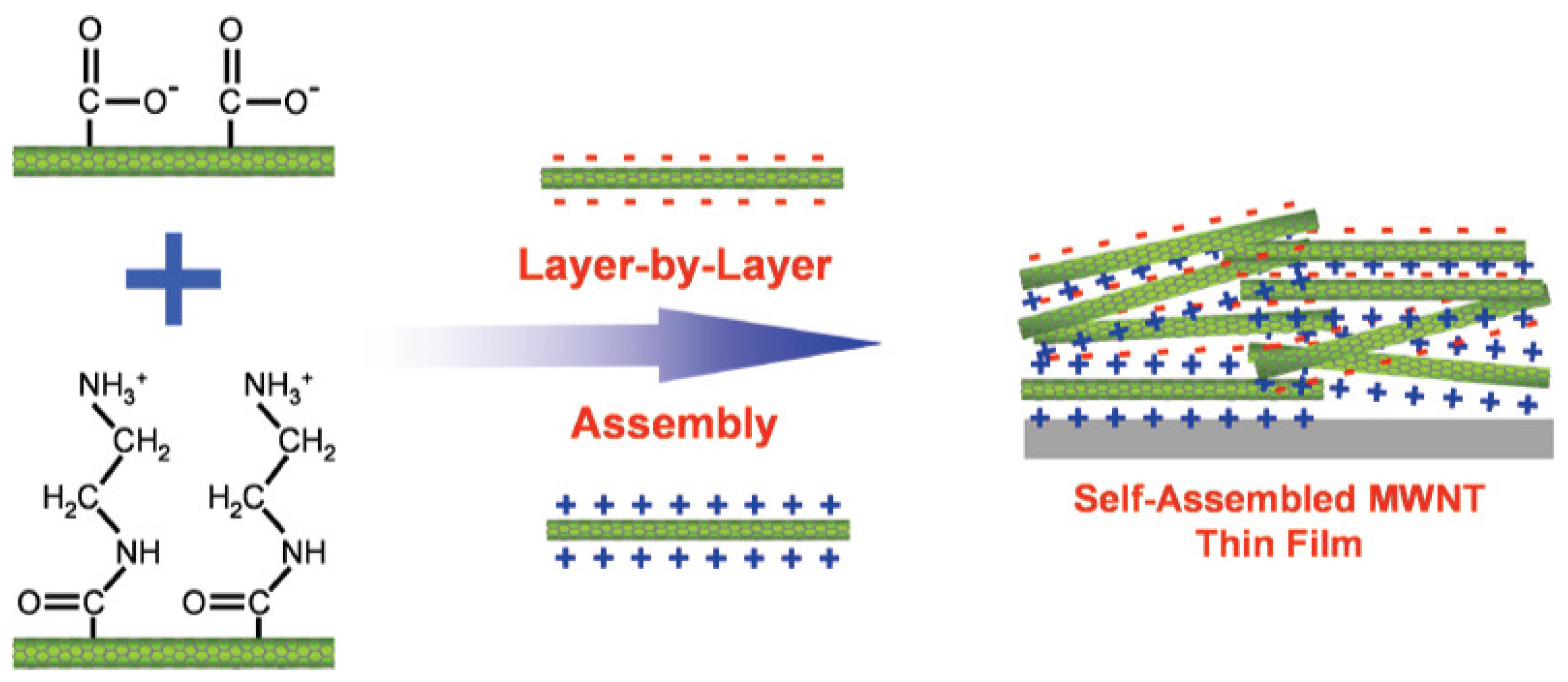
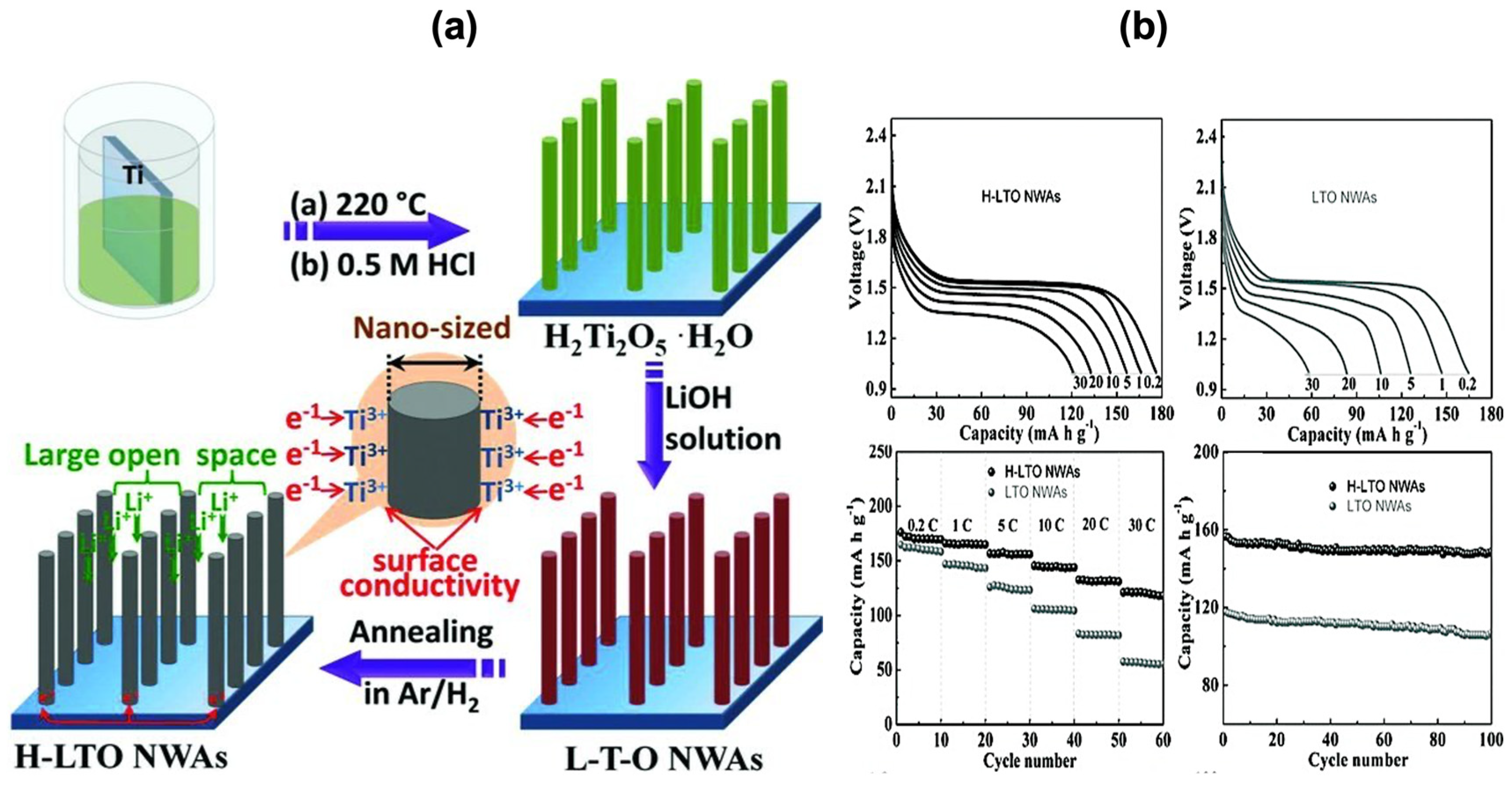
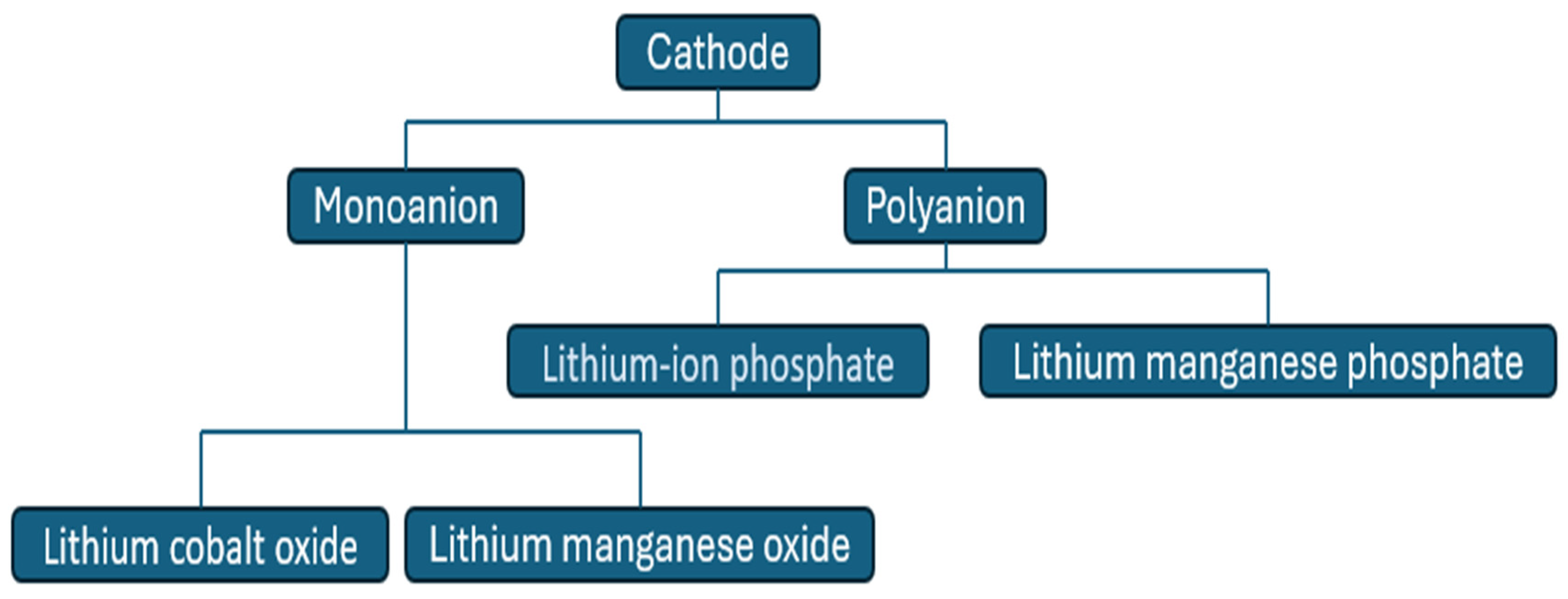
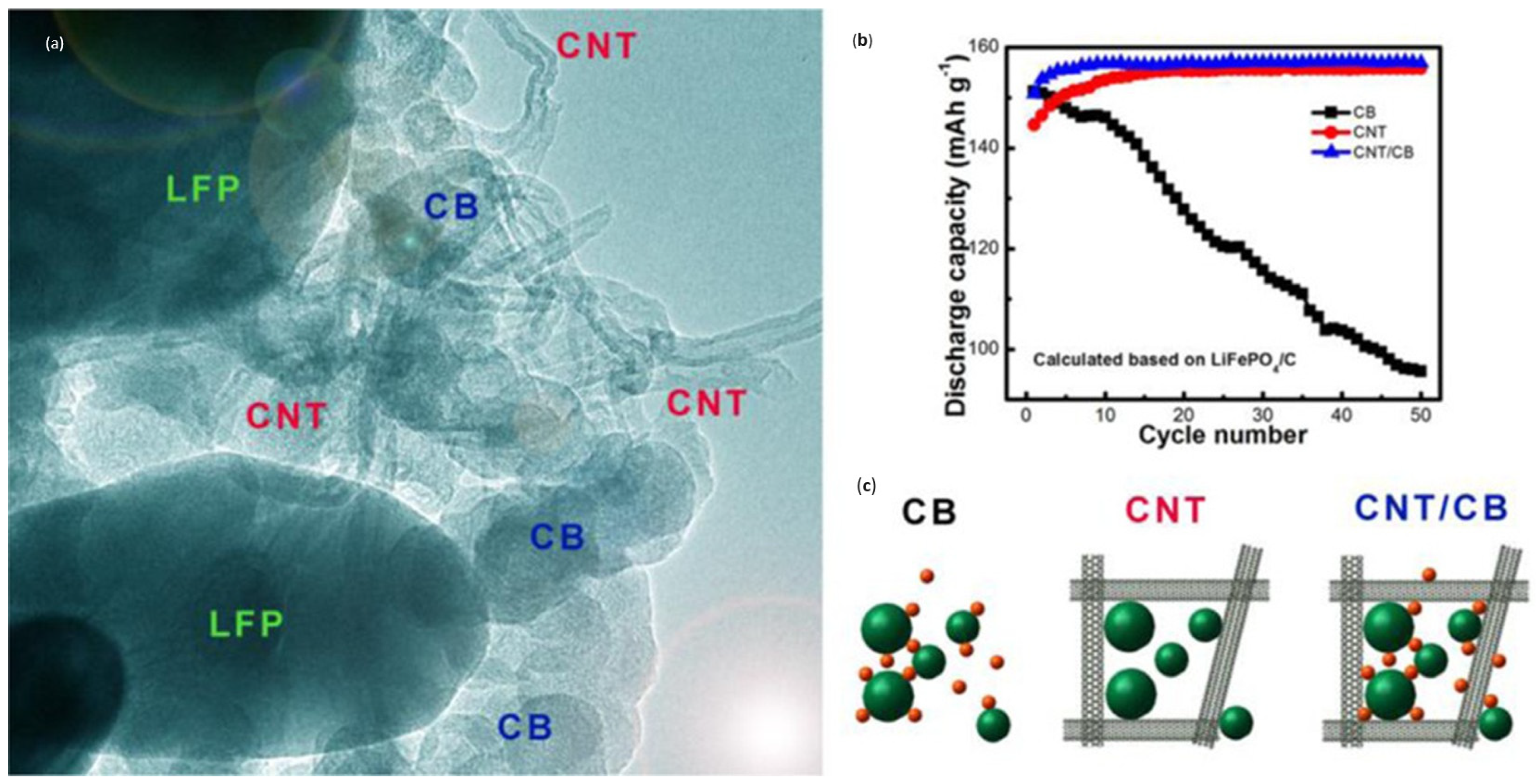
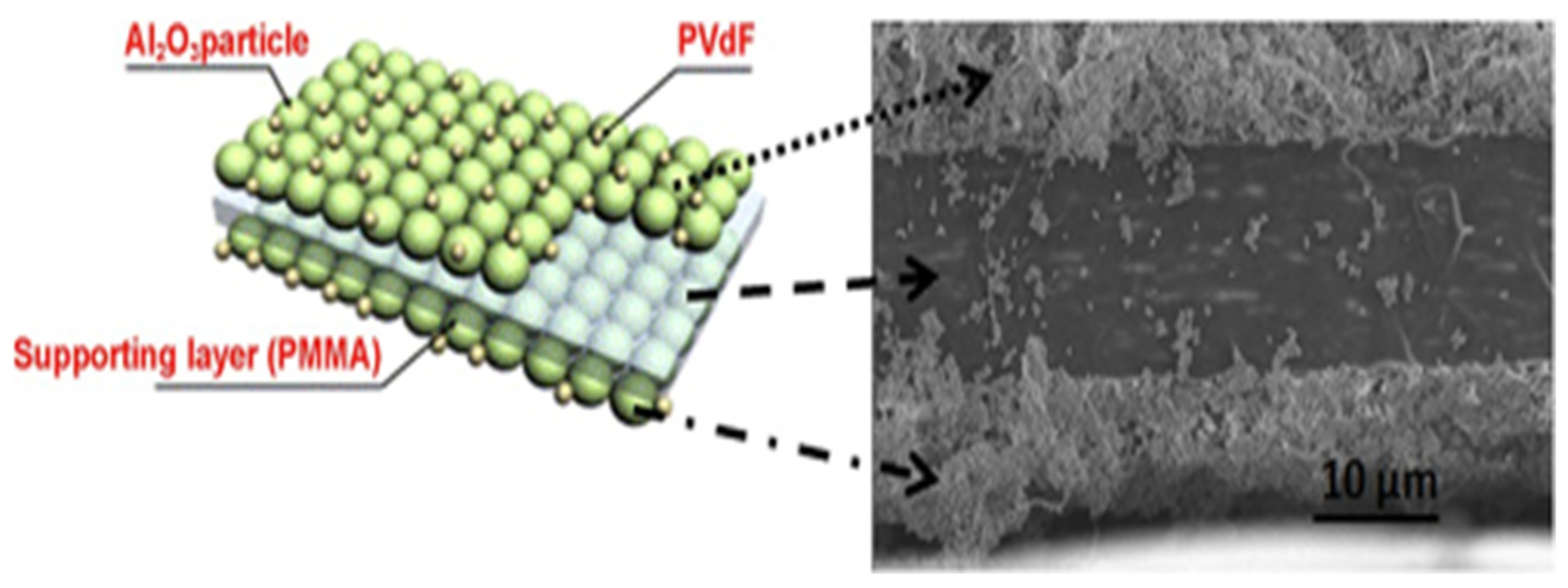

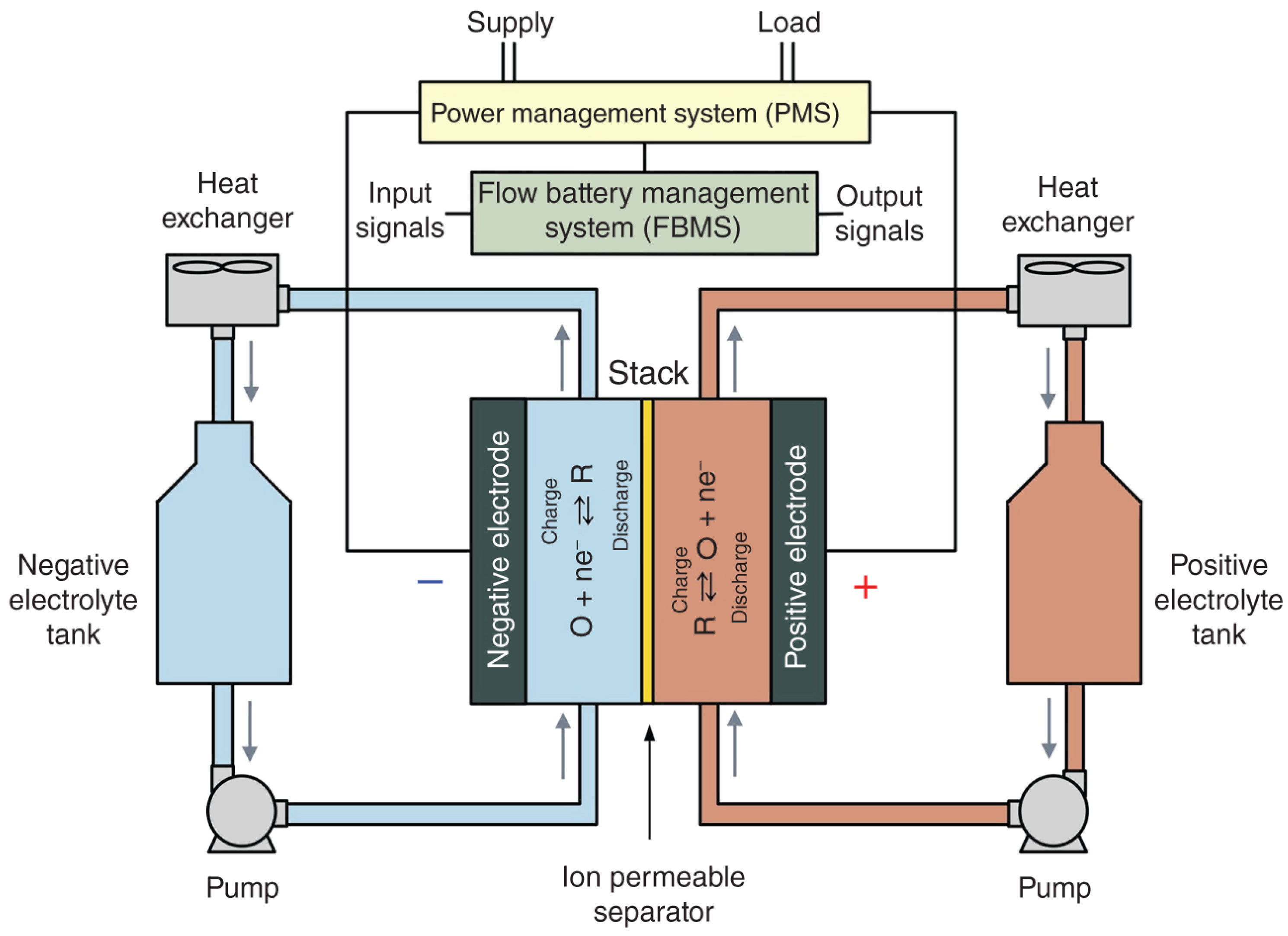
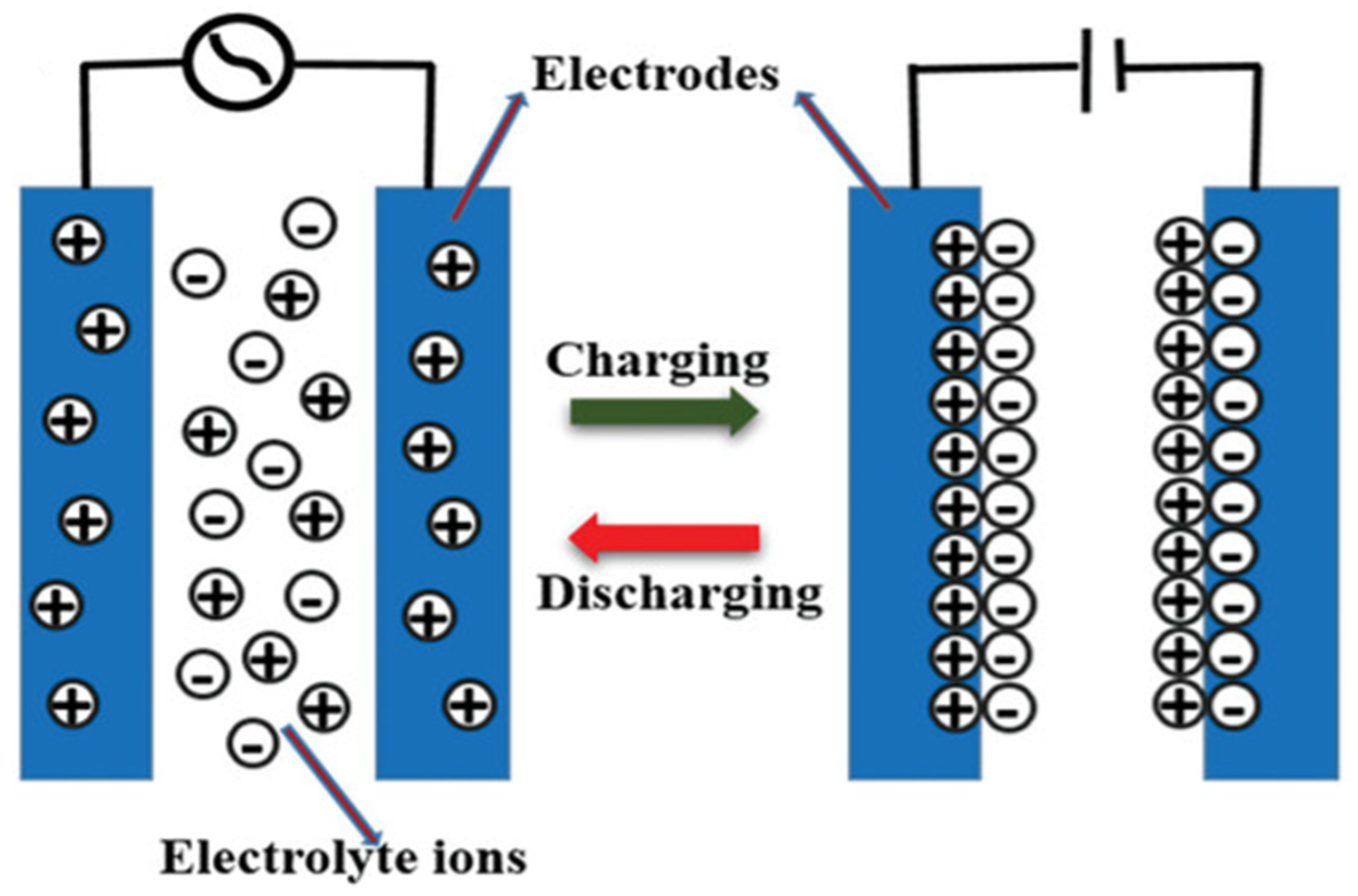
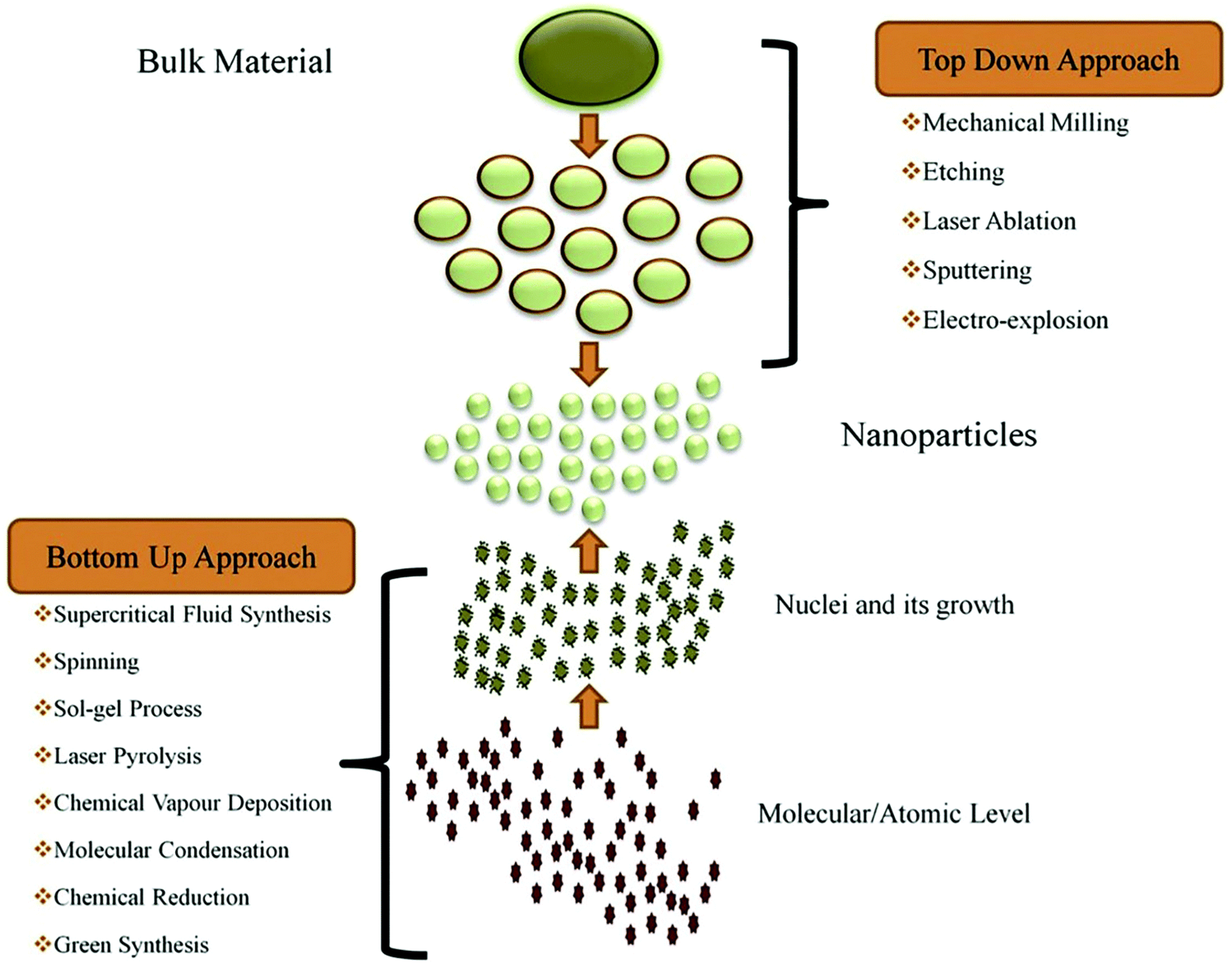
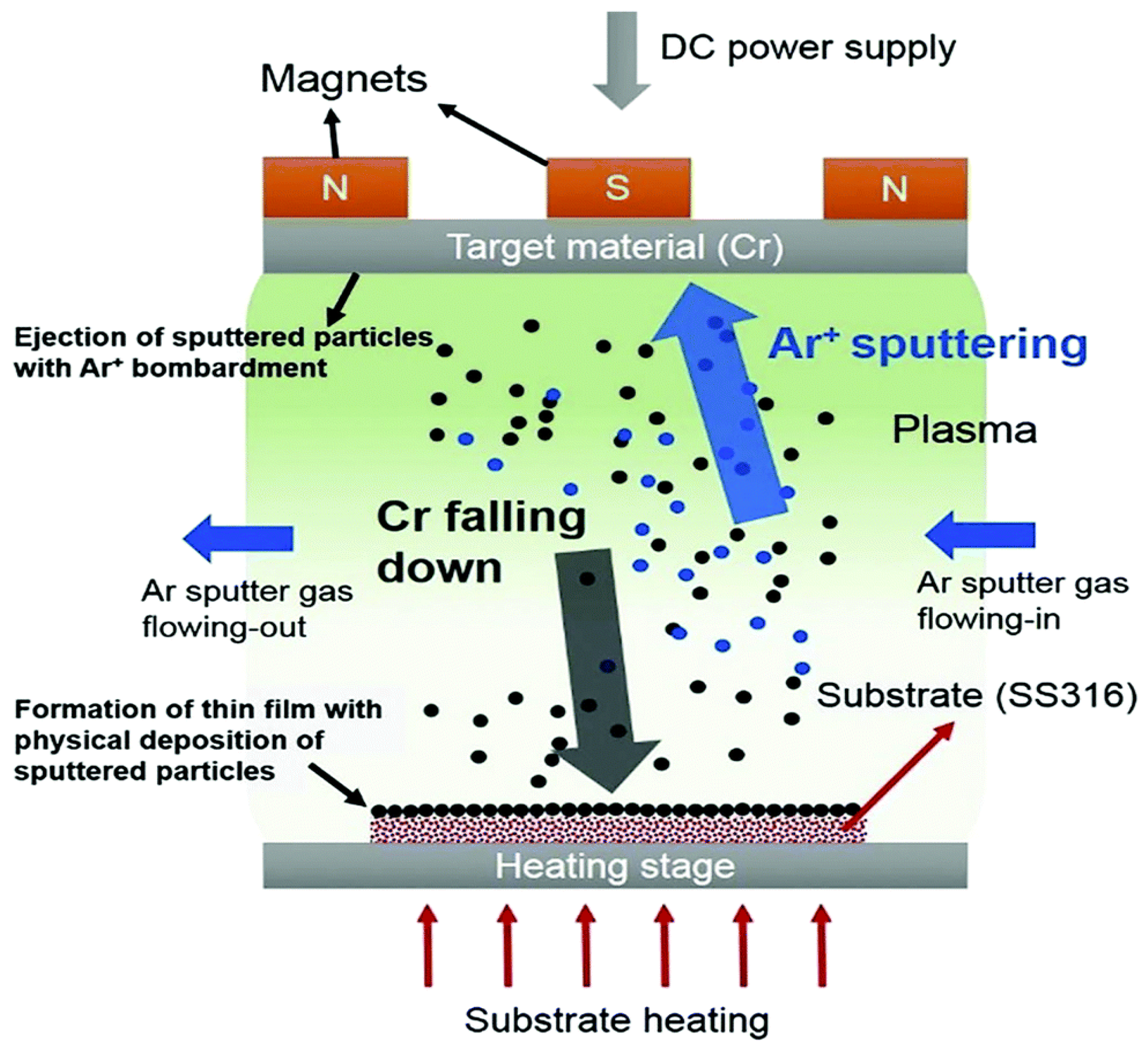
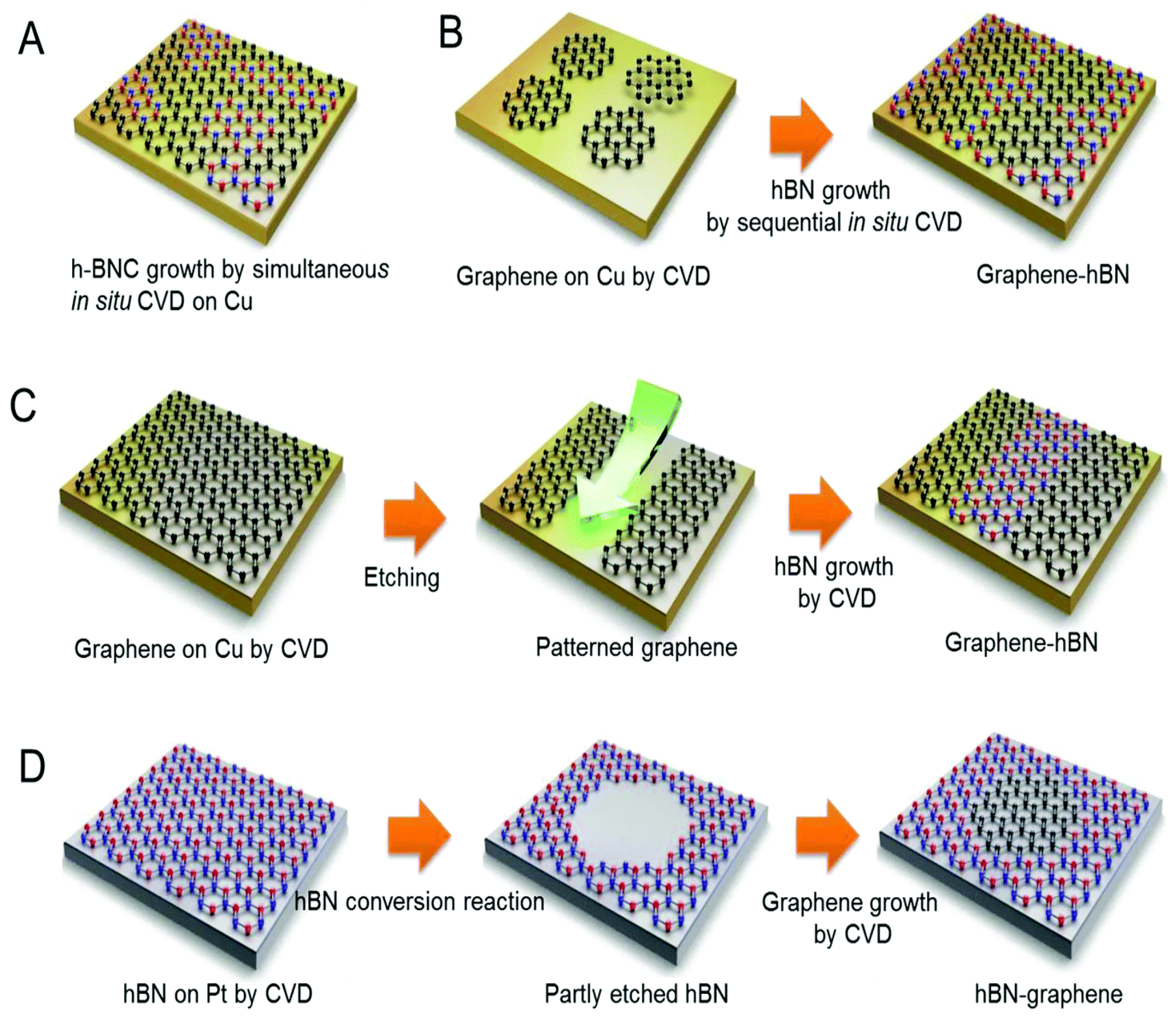
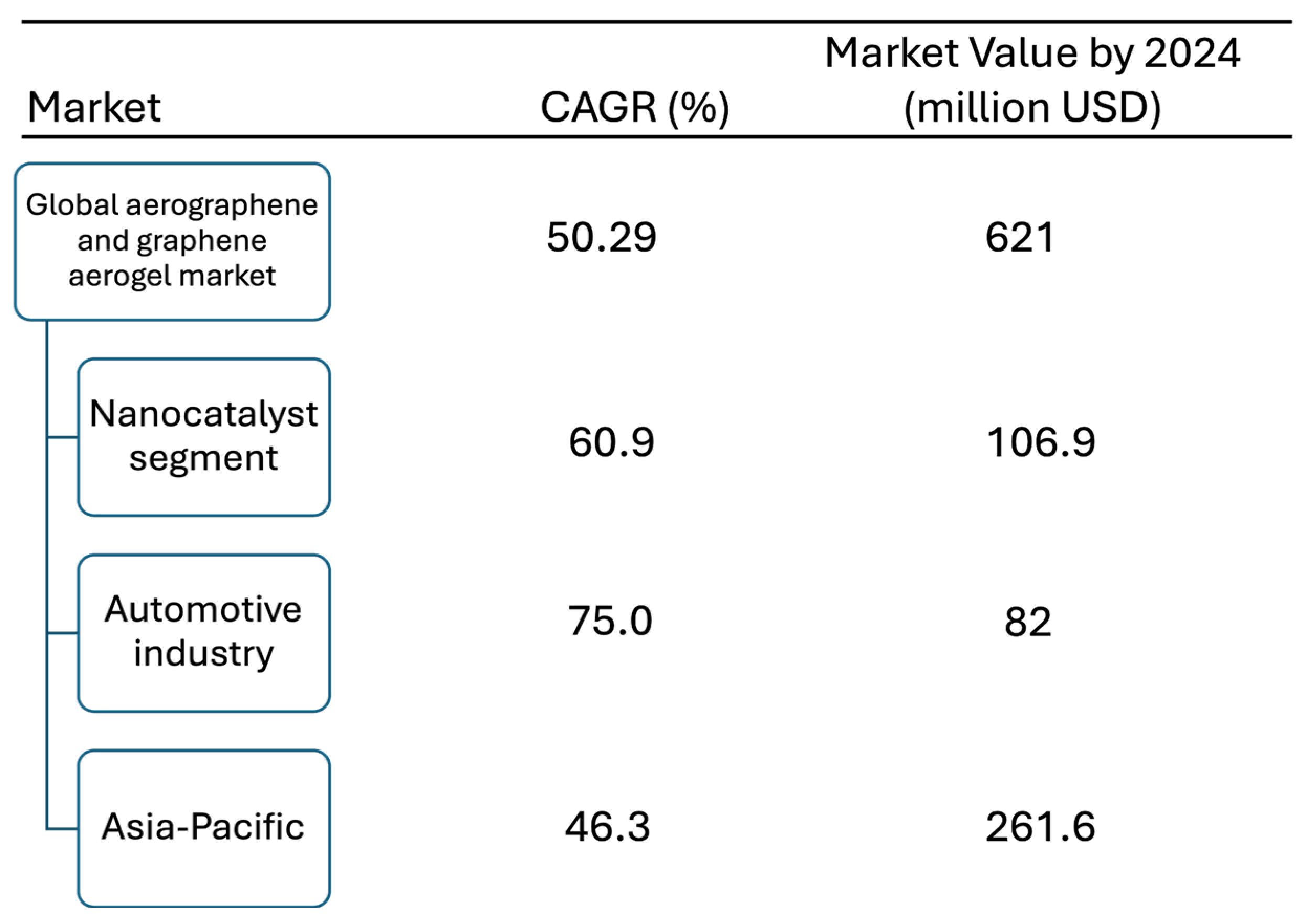
| Material | Advantages | Disadvantages |
|---|---|---|
| Carbon | High electronic conductivity | Low specific capacity |
| Nice hierarchical structure | Low-rate capacity | |
| Abundant and low-cost resources | Safety issues | |
| Alloys | High specific capacity (400–2300 mA h g−1) | Low electronic conductivity |
| Good security | Large volume change (100%) | |
| Transition metal oxides | High specific capacity (600–1000 mA h g−1) | Low coulombic efficiency |
| Nice stability | Large potential hysteresis | |
| Silicon | Highest specific capacity (3579 mA h g−1) | Large volume change (>300%) |
| Rich, low-cost, a from a clean resource |
| Material | Electrochemical Performance | Ref. | |
|---|---|---|---|
| Insertion | Carbon-based | Significant reversible cycles, chemical stability, electrochemical stability, thermal stability. | [28] |
| Carbon-based (with coating) | Improved performance due to thinner and denser SEI film | [29] | |
| Titanium-based | Minor safety issues, less toxicity, little volume change (2–3%), extended cycle life | [30,31,32,33] | |
| Carbon Nanotubes (CNTs) | - High conductivity and stability - Can absorb Li-ions on both internal and external surfaces | [21,36,38] | |
| Graphene | High conductivity, structural flexibility, higher charge mobility, lightweight, good surface area | [46,47] | |
| Conversion | Iron Oxides (Fe2O3, Fe3O4) | High theoretical capacity (~1000 mAh/g), non-toxicity, excellent reversible capacities | [62,66,68,69,70,71] |
| Cobalt Oxides (CoO, Co3O4) | High theoretical capacity (Co3O4: 890 mAh/g, CoO: 715 mAh/g), good capacity retention | [84,85,86,87,88,89] | |
| Alloys | Silicon (Si) | - High theoretical specific capacity (4211 mAh/g) - High volumetric capacity (9786 mAh/cm3) - Abundant and environmentally friendly | [62,90,91,92,93,94,95,96,97] |
| Silicon Nanowires | - Can withstand greater volume changes due to their nanoscale size - Direct growth on the current collector allows for quick charge transfer | [22] | |
| Germanium (Ge) | - High electrical conductivity (10,000 times greater than Si) - High theoretical capacity (1623 mAh/g) - High Li-ion diffusion rate | [62,92,100,101,102] | |
| Germanium Nanowires | - Can effectively suppress volume changes due to their nanoscale size - Enhanced electrochemical performance with high reversible capacity and cycling stability | [101,103,104,105] |
| Component | Nanomaterials | Electrochemical Performance |
|---|---|---|
| Electrolyte | Solid-state electrolytes (e.g., FSA-Na) | These membranes serve as both electrolyte and separator, enhancing stability and controlling the shuttle effect [167]. |
| Separator | Solid-state electrolyte membranes | These membranes help prevent polysulfide shuttling and improve safety in sodium–sulfur batteries [167]. |
| Cathode | Vanadium carbide nanoparticles in carbon nanofibers (VC-CNFs) | These materials enhance electrochemical performance, acting as chemical trappers and electrocatalysts to mitigate the shuttle effect and improve reaction kinetics [165]. |
| Nanocomposite catalytic cathodes | Incorporation of various nanomaterials (metal oxides, sulfides, single atoms) into porous carbon hosts accelerates the conversion of sulfur species and enhances reaction kinetics [161,162]. | |
| Anode | Nanostructured host materials | These materials help mitigate dendrite growth and volume expansion during cycling of sodium metal anodes [152]. |
| Transition metal nanoparticles or single atoms | These enhance sodiophilicity and improve the stability of the interphase of the solid electrolyte [156,157,158]. |
| Component | Nanomaterials | Key Features | Electrochemical Performance |
|---|---|---|---|
| Electrodes | Carbon-based nanomaterials [208,209,210,211] | High surface area | Enhanced mass transport |
| Superior conductivity | Improved charge transfer | ||
| Chemical stability | Increased electrocatalytic activity, leading to better cell performance | ||
| Metal nanoparticles (e.g., platinum, palladium, and gold) [184,185] | High conductivity | Improved efficiency | |
| Electrocatalytic activity | Enhanced durability | ||
| High cost and side reactions are concerns | |||
| Metal oxide nanoparticles (e.g., CeO2 and MnO2) [193,194,195,196,197] | Economical | Enhanced reaction kinetics | |
| High catalytic activity | Bifunctional catalytic behavior for both positive and negative reactions | ||
| Electrolytes | Carbon-based nanofluids (e.g., incorporating graphene and carbon nanotubes) [216,217,218] | Large surface area | Improved conductivity |
| Porous configuration | Enhanced electrochemical reaction kinetics | ||
| Good conductivity | |||
| Metal-based nanoparticles [215] | Rapid electron migration | Enhanced electron transport within the electrolyte | |
| Improved battery performance | |||
| Suspended nanofluids [214] | Intermediate properties between liquids and solids | Improved electrochemical reaction kinetics | |
| Better ion transport | |||
| Separators | Organic nanomaterials (e.g., poly(4-vinyl pyridine), polypyrroles, and polyaniline) [230,231,232] | Enhanced selectivity | Reduced crossover of active species |
| Improved ionic conductivity | Better overall battery performance | ||
| Inorganic nanoparticles (e.g., SiO2 and ZrP) [230,231,232] | Modification of the nafion matrix | Hinders passage of larger active species | |
| Creation of tortuous paths for ion transport | Increases efficiency of ion transport |
| Component | Nanomaterials | Electrochemical Performance |
|---|---|---|
| Electrodes | Carbon-based nanomaterials | Improve conductivity and surface area, resulting in increased capacity and quicker rates of charging and discharging [260]. |
| Metal oxides (e.g., RuO2 and MnO2) | Metal oxides, with their high capacitance and stability, are ideal for high-performance supercapacitors [261]. | |
| Conductive polymers (e.g., polyaniline and polypyrrole) | Provide adequate electrical conductivity and easy processing, contributing to the overall performance of the electrode [262,263]. | |
| MOFs and COFs | Tunable chemical composition, excellent stability, enhanced conductivity, high surface area, and porosity [250,251]. | |
| Electrolytes | Ionic liquids | Ionic liquids have a wide electrochemical window and high ionic conductivity, making them ideal for supercapacitor applications [264]. |
| Gel electrolytes | Gel electrolytes incorporate the benefits of solid and liquid electrolytes, providing excellent ionic conductivity and enhanced safety [265,266]. | |
| Separators | Nanofibers (e.g., polyvinylidene fluoride (PVDF) nanofibers) | Possess exceptional mechanical strength and can enhance the ionic conductivity of the separator [267,268]. |
| Porous membranes | Allow for efficient ion transport while preventing electrical short circuits between the electrodes [269,270]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.; Mia, M.F.; Wiggins, J.; Desai, S. Nanomaterials for Energy Storage Systems—A Review. Molecules 2025, 30, 883. https://doi.org/10.3390/molecules30040883
Mohammed H, Mia MF, Wiggins J, Desai S. Nanomaterials for Energy Storage Systems—A Review. Molecules. 2025; 30(4):883. https://doi.org/10.3390/molecules30040883
Chicago/Turabian StyleMohammed, Habeeb, Md Farouq Mia, Jasmine Wiggins, and Salil Desai. 2025. "Nanomaterials for Energy Storage Systems—A Review" Molecules 30, no. 4: 883. https://doi.org/10.3390/molecules30040883
APA StyleMohammed, H., Mia, M. F., Wiggins, J., & Desai, S. (2025). Nanomaterials for Energy Storage Systems—A Review. Molecules, 30(4), 883. https://doi.org/10.3390/molecules30040883







