Urea Derivatives as H2S Scavengers
Abstract
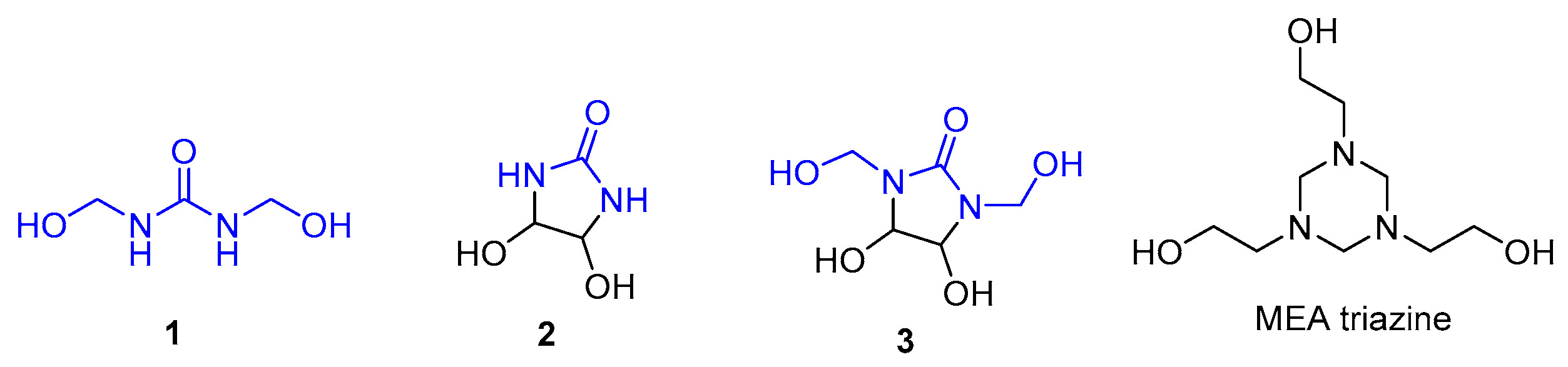
:1. Introduction
2. Results
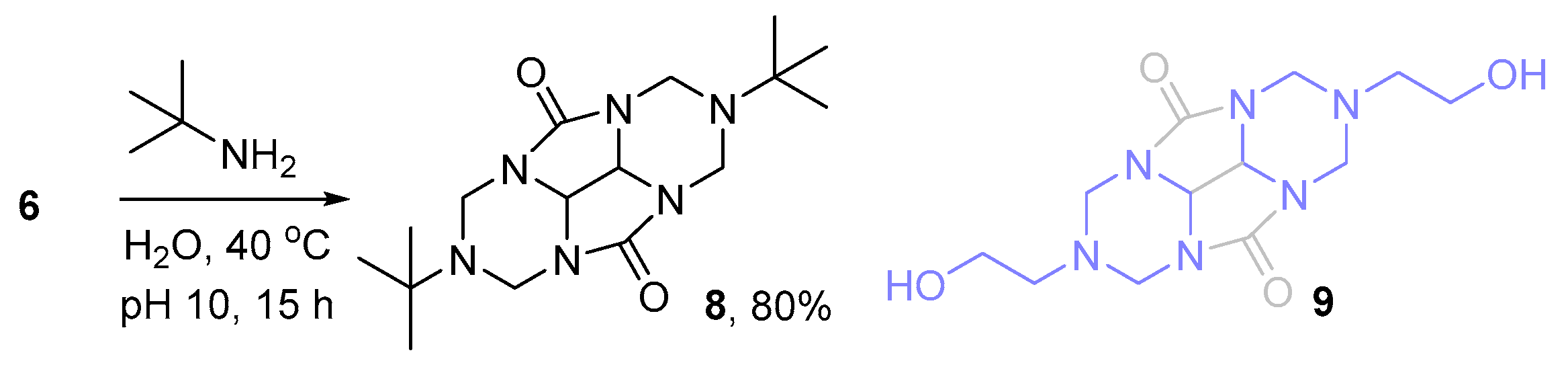
2.1. Synthesis of DMDHEU and Derivatives Thereof
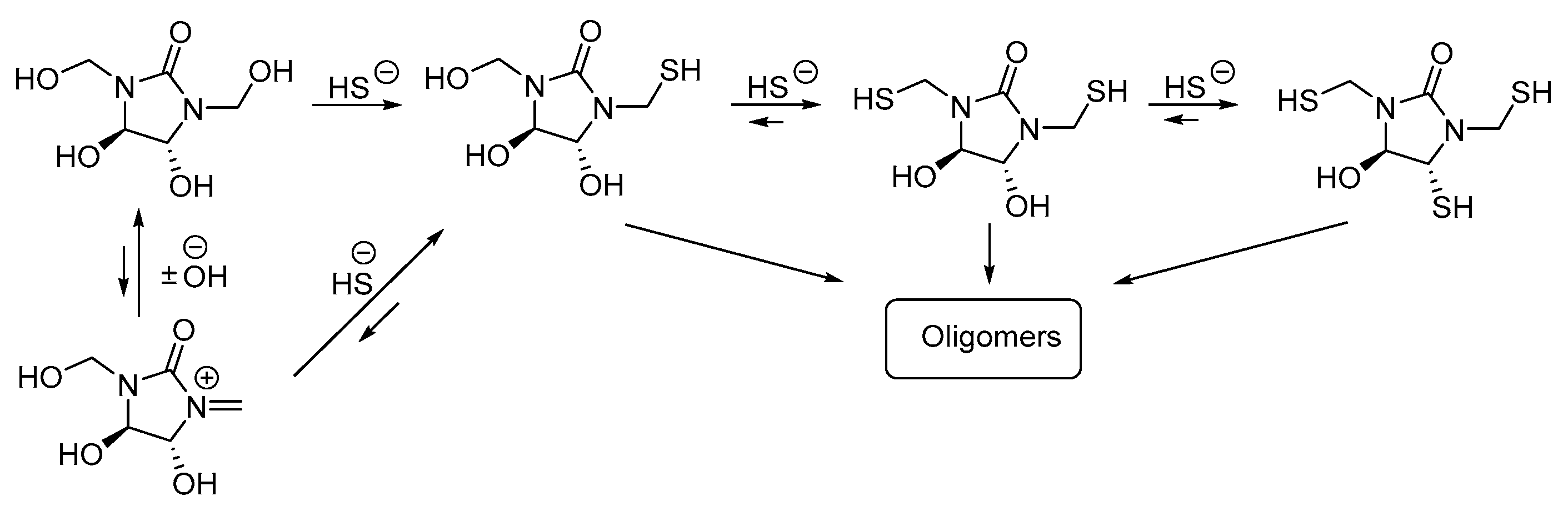
2.2. Raman Spectroscopic Study
3. Materials and Methods
3.1. General Synthetic Methods
3.2. Chemical Synthesis
3.3. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pudi, A.; Rezaei, M.; Signorini, V.; Andersson, M.P.; Baschetti, M.G.; Mansouri, S.S. Hydrogen Sulfide Capture and Removal Technologies: A Comprehensive Review of Recent Developments and Emerging Trends. Sep. Purif. Technol. 2022, 298, 121448. [Google Scholar] [CrossRef]
- Marriott, R.A.; Pirzadeh, P.; Marrugo-Hernandez, J.J.; Raval, S. Hydrogen Sulfide Formation in Oil and Gas. Can. J. Chem. 2015, 94, 406–413. [Google Scholar] [CrossRef]
- Kelland, M. Production Chemicals for the Oil and Gas Industry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume 1, ISBN 9780429195921. [Google Scholar]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429195945. [Google Scholar]
- Agbroko, O.W.; Piler, K.; Benson, T.J. A Comprehensive Review of H2S Scavenger Technologies from Oil and Gas Streams. ChemBioEng Rev. 2017, 4, 339–359. [Google Scholar] [CrossRef]
- Wylde, J.J.; Taylor, G.N.; Sorbie, K.S.; Samaniego, W.N. Synthesis, Reaction Byproduct Characterization, and Mechanistic Understanding of Hemiformal Hydrogen Sulfide Scavengers: Part 2─Identification of Byproducts, Reaction Mechanisms, and Suppression Thereof. Energy Fuels 2022, 36, 851–860. [Google Scholar] [CrossRef]
- Taylor, G.; Smith-Gonzalez, M.; Wylde, J.; Oliveira, A.P. H2S Scavenger Development During the Oil and Gas Industry Search for an MEA Triazine Replacement in Hydrogen Sulfide Mitigation and Enhanced Monitoring Techniques Employed During Their Evaluation. In Proceedings of the SPE International Conference on Oilfield Chemistry, Galveston, TX, USA, 8–9 April 2019; p. D011S006R001. [Google Scholar]
- Bakke, J.M.; Buhaug, J.; Riha, J. Hydrolysis of 1,3,5-Tris(2-Hydroxyethyl)Hexahydro-s-Triazine and Its Reaction with H2S. Ind. Eng. Chem. Res. 2001, 40, 6051–6054. [Google Scholar] [CrossRef]
- Madsen, H.T.; Søgaard, E.G. Fouling Formation During Hydrogen Sulfide Scavenging With 1,3,5-Tri-(Hydroxyethyl)-Hexahydro-s-Triazine. Pet. Sci. Technol. 2014, 32, 2230–2238. [Google Scholar] [CrossRef]
- Koue, A.M.; Montero, F.; Skjolding, L.M.; Kucheryavskiy, S.; Maschietti, M.; Pedersen, C.M. Multifunctional Biomass-Based Chemicals: H2S Scavenging. Green Chem. 2024, 26, 1984–1989. [Google Scholar] [CrossRef]
- Romero, I.; Montero, F.; Kucheryavskiy, S.; Wimmer, R.; Andreasen, A.; Maschietti, M. Temperature- and PH-Dependent Kinetics of the Aqueous Phase Hydrogen Sulfide Scavenging Reactions with MEA-Triazine. Ind. Eng. Chem. Res. 2023, 62, 8269–8280. [Google Scholar] [CrossRef]
- Hazarika, A.; Maji, T.K. Effect of Different Crosslinkers on Properties of Melamine Formaldehyde-Furfuryl Alcohol Copolymer/Montmorillonite Impregnated Softwood (Ficus hispida). Polym. Eng. Sci. 2013, 53, 1394–1404. [Google Scholar] [CrossRef]
- Ho, L.-Y.; Kan, C.-W. Effect of Resin Finishing on Some Properties of 100% Cotton Light Weight Woven Fabric. Coatings 2022, 12, 1791. [Google Scholar] [CrossRef]
- Li, B.; Dong, Y.; Wang, P.; Cui, G. Release Behavior and Kinetic Evaluation of Formaldehyde from Cotton Clothing Fabrics Finished with DMDHEU-Based Durable Press Agents in Water and Synthetic Sweat Solution. Text. Res. J. 2015, 86, 1738–1749. [Google Scholar] [CrossRef]
- Vail, S.L.; Barker, R.H.; Mennitt, P.G. Formation and Identification of Cis- and Trans-Dihydroxyimidazolidinones from Ureas and Glyoxal. J. Org. Chem. 1965, 30, 2179–2182. [Google Scholar] [CrossRef]
- Lavado, N.; de la Concepción, J.; Gallego, M.; Babiano, R.; Cintas, P. From Prebiotic Chemistry to Supramolecular Oligomers: Urea–Glyoxal Reactions. Org. Biomol. Chem. 2019, 17, 5826–5838. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Ketcham, R.; Nematollahi, J. Synthesis of Unsymmetrically Substituted Hexahydroimidazo [4,5-d] Imidazole-2,5-Diones and Elucidation of Reaction Pathways. Synth. Commun. 1979, 9, 863–870. [Google Scholar] [CrossRef]
- Grillon, E.; Gallo, R.; Pierrot, M.; Boileau, J.; Wimmer, E. Isolation and X-Ray Structure of the Intermediate Dihydroxyimidazolidine(DHI) in the Synthesis of Glycoluril from Glyoxal and Urea. Tetrahedron Lett. 1988, 29, 1015–1016. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Chikunov, I.E.; Lyssenko, K.A.; Baranov, V. V Glycolurils in α-Ureido- and α-Aminoalkylation Reactions. 3 **. N-(Hydroxymethyl)Glycolurils in Reactions with Aliphatic Amines and Amino Acids *. Chem. Heterocycl. Compd. 2014, 50, 1322–1331. [Google Scholar] [CrossRef]
- Wingard, L.A.; Johnson, E.C.; Sabatini, J.J. Efficient Method for the Cycloaminomethylation of Glycoluril. Tetrahedron Lett. 2016, 57, 1681–1682. [Google Scholar] [CrossRef]
- Clark, J.C.; Trevino, M.; Karas, L.J.; Gallardo, J.M.; Anantaneni, P.; Passos, R.C.; Burrell, C.; Rana, G. Available online: https://patents.google.com/patent/WO2019014415A1/en (accessed on 14 January 2025).
- Jin, P.; Liu, Z.; Weers, J.J.; Ramachandran, S.; Jani, J.; Lehrer, S.; Brooks, J. Synergistic Effects Among Mercaptan Scavengers. 2021. Available online: https://patents.google.com/patent/US11802246B2/en (accessed on 14 January 2025).
- Svec, J.; Dusek, M.; Fejfarova, K.; Stacko, P.; Klán, P.; Kaifer, A.E.; Li, W.; Hudeckova, E.; Sindelar, V. Anion-Free Bambus[6]Uril and Its Supramolecular Properties. Chem. A Eur. J. 2011, 17, 5605–5612. [Google Scholar] [CrossRef] [PubMed]
- Stancl, M.; Hodan, M.; Sindelar, V. Glycoluril Trimers: Selective Synthesis and Supramolecular Properties. Org. Lett. 2009, 11, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Kucheryavskiy, S.; Maschietti, M. Experimental Study of the Aqueous Phase Reaction of Hydrogen Sulfide with MEA-Triazine Using In Situ Raman Spectroscopy. Ind. Eng. Chem. Res. 2021, 60, 15549–15557. [Google Scholar] [CrossRef]


| Entry | Compound | Temperature/°C | Percentage Decrease in HS− Concentration in 1 h |
|---|---|---|---|
| 1 | DMU 1 | 50 | 20% * |
| 2 | DMU 1 | 75 | 50% |
| 3 | 2 | 25 | 0% |
| 4 | 2 | 75 | 30% |
| 5 | DMDHEU 3 | 25 | 25% |
| 6 | DMDHEU 3 | 75 | 80% |
| 7 | 5 | 50 | 0% |
| 8 | 6 | 50 | 13% |
| 9 | 6 | 75 | 20% |
| 10 | 7 | 50 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koue, A.M.; Szlek, K.A.; Kucheryavskiy, S.; Maschietti, M.; Pedersen, C.M. Urea Derivatives as H2S Scavengers. Molecules 2025, 30, 906. https://doi.org/10.3390/molecules30040906
Koue AM, Szlek KA, Kucheryavskiy S, Maschietti M, Pedersen CM. Urea Derivatives as H2S Scavengers. Molecules. 2025; 30(4):906. https://doi.org/10.3390/molecules30040906
Chicago/Turabian StyleKoue, Asger Munk, Karolina Agata Szlek, Sergey Kucheryavskiy, Marco Maschietti, and Christian Marcus Pedersen. 2025. "Urea Derivatives as H2S Scavengers" Molecules 30, no. 4: 906. https://doi.org/10.3390/molecules30040906
APA StyleKoue, A. M., Szlek, K. A., Kucheryavskiy, S., Maschietti, M., & Pedersen, C. M. (2025). Urea Derivatives as H2S Scavengers. Molecules, 30(4), 906. https://doi.org/10.3390/molecules30040906







