Salicylaldehyde Benzoylhydrazones with Anticancer Activity and Selectivity: Design, Synthesis, and In Vitro Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of Methoxysalicylaldehyde Methoxybenzoylhydrazones and In Silico Drug Likeness Evaluation
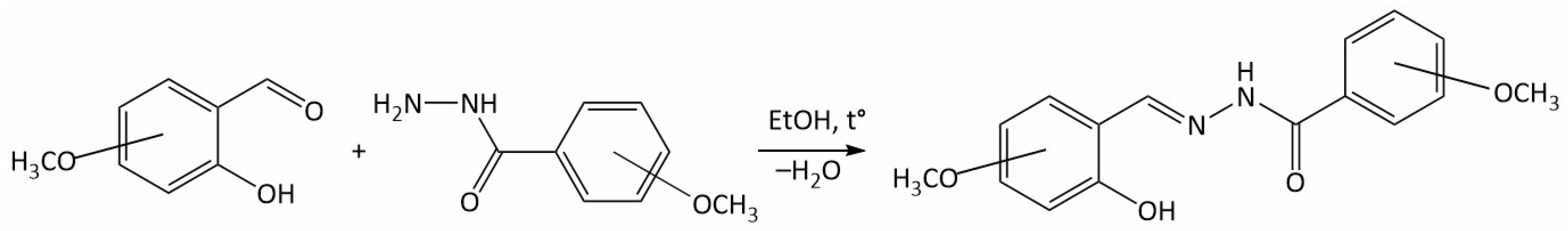
2.2. Synthesis of Dimethoxy Hydrazones
2.3. In Vitro Anticancer Activity and Selectivity
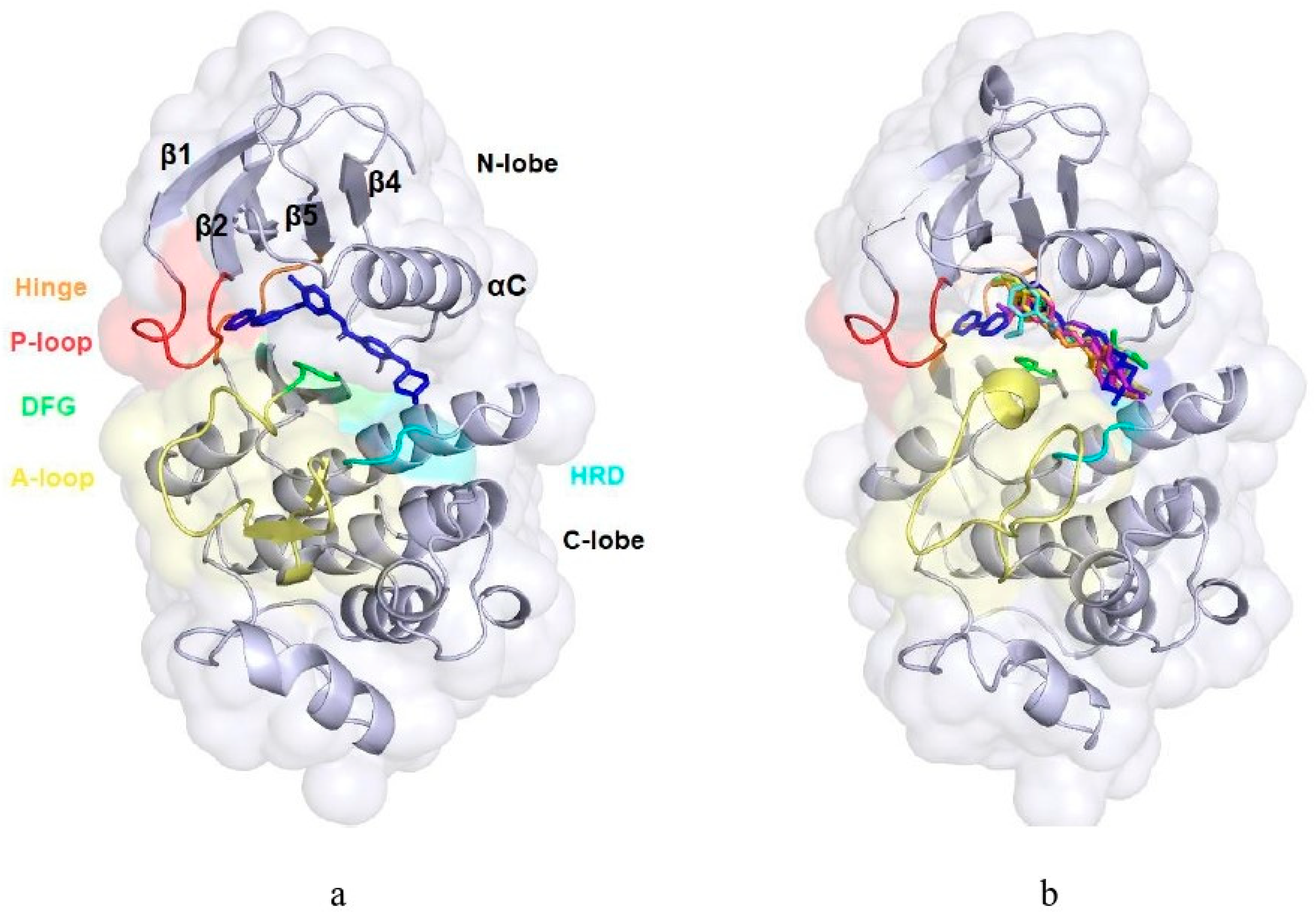
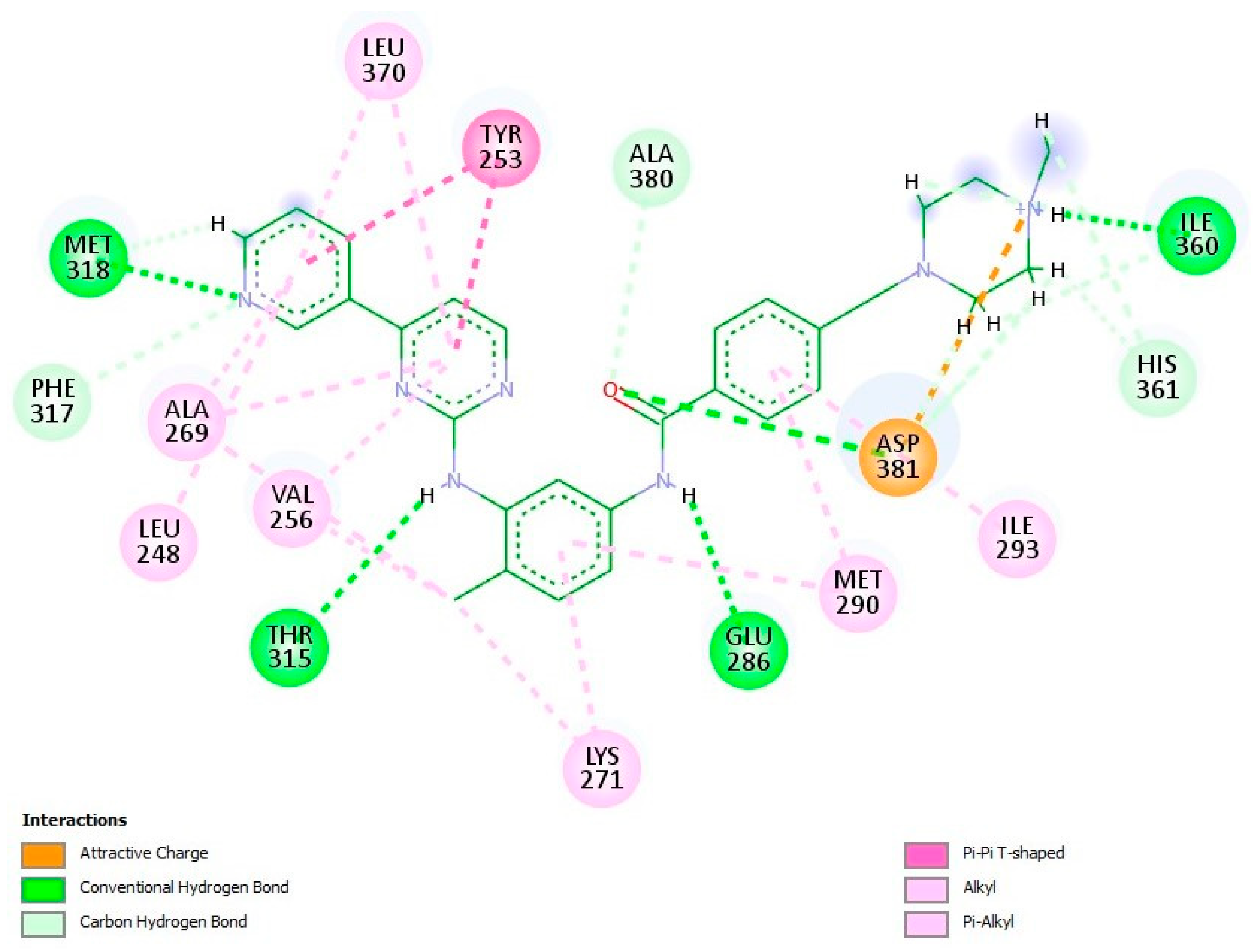
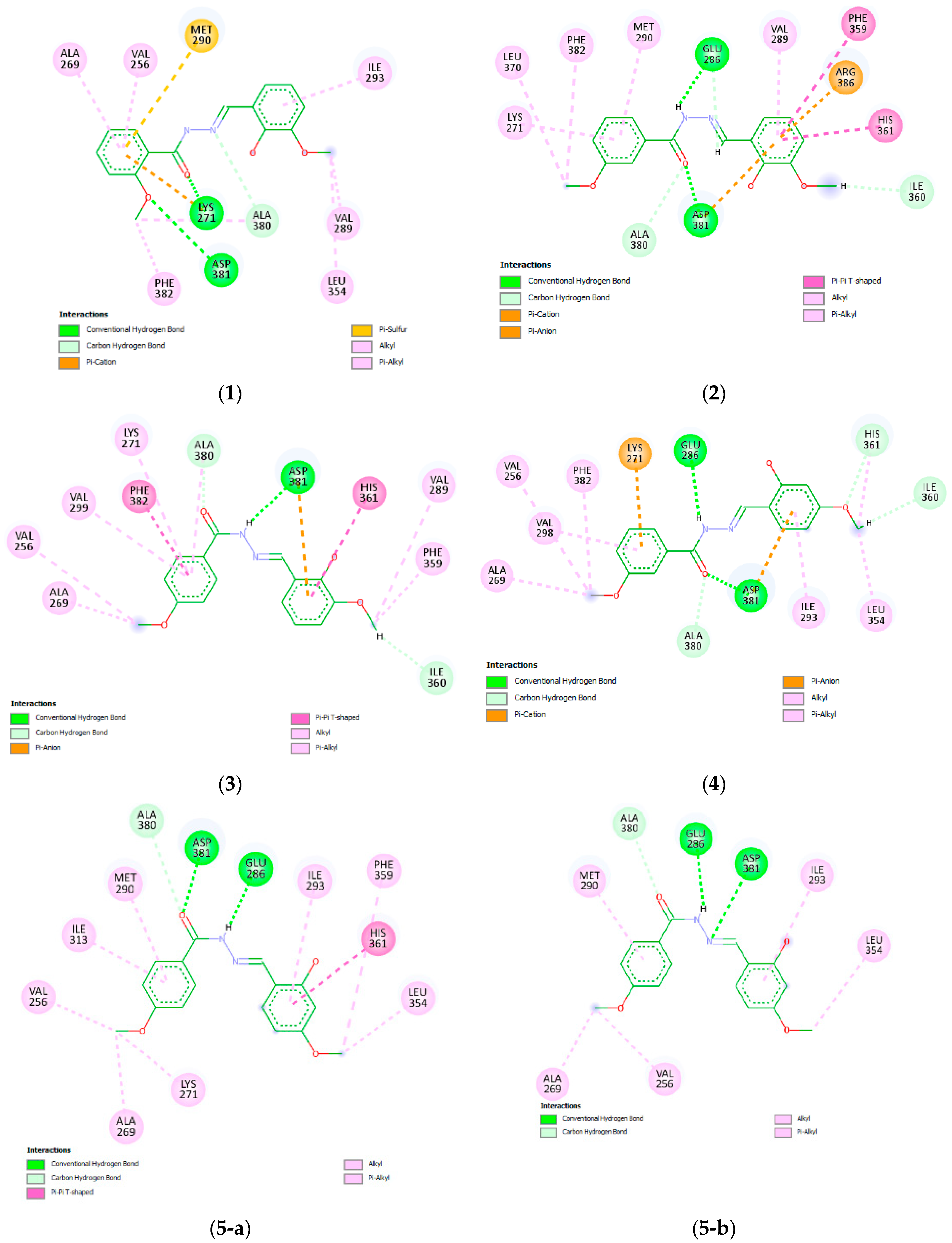
2.4. In Silico Modeling of the Interactions Between the Methoxy Salicylaldehyde Methoxybenzoylhydrazones and Human cAbl Tyrosine Kinase
3. Materials and Methods
3.1. Materials
3.2. Characterization of Dimethoxy Hydrazones
- N′-(2-hydroxy-3-methoxybenzylidene)-2-methoxybenzohydrazide (1)
- N′-(2-hydroxy-3-methoxybenzylidene)-3-methoxybenzohydrazide (2)
- N′-(2-hydroxy-3-methoxybenzylidene)-4-methoxybenzohydrazide (3)
- N′-(2-hydroxy-4-methoxybenzylidene)-3-methoxybenzohydrazide (4)
- N′-(2-hydroxy-4-methoxybenzylidene)-4-methoxybenzohydrazide (5)
3.3. Cell Lines and Culture Conditions
3.4. Mosmann’s MTT Test for Assessing Cell Viability
3.5. In Silico Evaluation, Molecular Docking, and Molecular Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes Dis. 2021, 8, 655–661. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Parte, S.; Are, A.C.; Batra, S.K.; Ponnusamy, M.P. Tumor microenvironment enriches the stemness features: The architectural event of therapy resistance and metastasis. Mol. Cancer 2022, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Brahmer, J.; Antonia, S.; Mok, T.; Peters, S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J. Clin. Oncol. 2022, 40, 598–610. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Skórzewska, M.; Gęca, K.; Polkowski, W.P. A Clinical Viewpoint on the Use of Targeted Therapy in Advanced Gastric Cancer. Cancers 2023, 15, 5490. [Google Scholar] [CrossRef] [PubMed]
- El-Sayes, N.; Vito, A.; Mossman, K. Tumor Heterogeneity: A Great Barrier in the Age of Cancer Immunotherapy. Cancers 2021, 13, 806. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Moyer, C.L.; Brown, P.H. Targeting nuclear hormone receptors for the prevention of breast cancer. Front. Med. 2023, 10, 1200947. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, Y.; Zheng, L.; Xiao, H.; Ouyang, L.; Wang, G.; Sun, Q. Small molecules targeting protein–protein interactions for cancer therapy. Acta Pharm. Sin. B 2023, 13, 4060–4088. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Nikolova-Mladenova, B.; Halachev, N.; Iankova, R.; Momekov, G.; Ivanov, D. Synthesis, characterization and cytotoxic activity of new salicylaldehyde benzoylhydrazone derivatives as potential anti-proliferative agents. Arzneimittelforschung 2011, 61, 714–718. [Google Scholar] [CrossRef]
- Nikolova-Mladenova, B.; Momekov, G.; Ivanov, D.; Bakalova, A. Design and drug-like properties of new 5-methoxysalicylaldehyde based hydrazones with anti-breast cancer activity. J. Appl. Biomed. 2017, 15, 233–240. [Google Scholar] [CrossRef]
- Nikolova-Mladenova, B.; Bakalova, A.; Momekov, G.; Ivanov, D. Design, drug-likeness and cytotoxicity of some bromosalic-ylaldehyde aroylhydrazones. J. Med. Biolog. Sci. 2015, 2, 16–20. [Google Scholar]
- Nikolova-Mladenova, B.; Momekov, G.; Gerasimova, T.; Topashka-Ancheva, M. Comparative evaluation of in silico and in vitro pharmacological activity of some 5-nitrosalicylaldehyde-derived hydrazones. J. Med. Biol. Sci. 2014, 1, 44–48. [Google Scholar]
- Nikolova-Mladenova, B.; Momekov, G.; Zhivkova, Z.; Doytchinova, I. Design, Synthesis and Cytotoxic Activity of Novel Salicylaldehyde Hydrazones against Leukemia and Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7352. [Google Scholar] [CrossRef]
- Nikolova-Mladenova, B.I.; Angelova, S.E. Ga (III) complexes of methoxy substituted aroylhydrazones: Synthesis, character-ization and DFT calculations. Bulg. Chem. Comm. 2017, 49, 237–242. [Google Scholar]
- Huo, L.-H.; Gao, S.; Zhao, H.; Zhao, J.-G.; Zain, S.M.; Ng, S.W. 3-Methoxysalicylaldehyde 4-methoxybenzoylhydrazone monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, o1538–o1540. [Google Scholar] [CrossRef]
- Ferraresi-Curotto, V.; Echeverría, G.A.; Piro, O.E.; Pis-Diez, R.; González-Baró, A.C. Structural, spectroscopic and DFT study of 4-methoxybenzohydrazide Schiff bases. A new series of polyfunctional ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 137, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; Choudhary, M.I. Acylhydrazide Schiff bases: Synthesis and antiglycation activity. J. Chem. Soc. Pak. 2013, 34, 929–937. [Google Scholar]
- Melnyk, P.; Leroux, V.; Sergheraert, C.; Grellier, P. Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg. Med. Chem. Lett. 2005, 16, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-F.; Liu, Q.-F.; Liu, J.-L.; Sun, M.-H.; Ma, J.-J. Synthesis, crystal structure, and insulin-like activity of [N′-(2-hydroxy-3-methoxybenzylidene)-2-methoxybenzohydrazonato](1,10-phenanthroline)oxovanadium(IV) methanol solvate. Inorg. Nano-Met. Chem. 2017, 47, 1585–1589. [Google Scholar] [CrossRef]
- Liu, H.-M.; Zhang, L.-H.; Cui, P.-L.; Zhang, D.-N.; Ma, J.-J. [N′-(2-Hydroxy-3-methoxybenzylidene)-2-methoxybenzohydrazonato](quinolin-8-olato)oxovanadium(V): Synthesis, Crystal Structure and Thermal Property. Synth. React. Inorg. Met. Nano-Met. Chem. 2013, 43, 873–876. [Google Scholar] [CrossRef]
- Qian, S.-S.; Zhen, M.-M.; Zhao, Y.; Zhang, N.; You, Z.-L.; Zhu, H.-L. Two water-coordinated mononuclear molybdenum(vi) oxo complexes with similar tridentate hydrazone ligands: Synthesis and crystal structures. J. Chil. Chem. Soc. 2013, 58, 1647–1650. [Google Scholar] [CrossRef]
- Asha, T.; Kurup, M.P. Synthesis, spectroscopy, electrochemistry, crystal structures and in vitro cytotoxicity of mononuclear molybdenum(VI) complexes incorporating tridentate ONO donor aroylhydrazone with auxiliary coordination site. Inorg. Chim. Acta 2018, 483, 44–52. [Google Scholar] [CrossRef]
- Asha, T.; Kurup, M. DMSO coordinated dioxidomolybdenum(VI) complexes chelated with 3-methoxybenzhydrazone related ligands: Synthesis, structural studies and in vitro cytotoxicity. Polyhedron 2019, 169, 151–161. [Google Scholar] [CrossRef]
- Jin, N.-Y. Syntheses, crystal structures, and catalytic properties of dioxomolybdenum(VI) complexes with hydrazone ligands. J. Coord. Chem. 2012, 65, 4013–4022. [Google Scholar] [CrossRef]
- Balsa, L.M.; Ferraresi-Curotto, V.; Lavecchia, M.J.; Echeverría, G.A.; Piro, O.E.; García-Tojal, J.; Pis-Diez, R.; González-Baró, A.C.; León, I.E. Anticancer activity of a new copper(ii) complex with a hydrazone ligand. Structural and spectroscopic characterization, computational simulations and cell mechanistic studies on 2D and 3D breast cancer cell models. Dalton Trans. 2021, 50, 9812–9826. [Google Scholar] [CrossRef]
- Thilagavathi, N.; Manimaran, A.; Priya, N.P.; Sathya, N.; Jayabalakrishnan, C. Synthesis, characterization, electrochemical, catalytic and antimicrobial activity studies of hydrazone Schiff base ruthenium(II) complexes. Appl. Organomet. Chem. 2009, 24, 301–307. [Google Scholar] [CrossRef]
- Zhivkova, Z.; Doytchinova, I. Prediction of Steady-State Volume of Distribution of Acidic Drugs by Quantitative Structure–Pharmacokinetics Relationships. J. Pharm. Sci. 2012, 101, 1253–1266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhivkova, Z.; Doytchinova, I. Quantitative structure—Plasma protein binding relationships of acidic drugs. J. Pharm. Sci. 2012, 101, 4627–4641. [Google Scholar] [CrossRef] [PubMed]
- Zhivkova, Z.; Doytchinova, I. Quantitative Structure—Clearance Relationships of Acidic Drugs. Mol. Pharm. 2013, 10, 3758–3768. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural Basis of the Drug-binding Specificity of Human Serum Albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef]
- Berellini, G.; Waters, N.J.; Lombardo, F. In silico Prediction of Total Human Plasma Clearance. J. Chem. Inf. Model. 2012, 52, 2069–2078. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Cortes, J.E. Chronic Myeloid Leukemia: Diagnosis and Treatment. Mayo Clin. Proc. 2006, 81, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Zubay, G.; Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia: Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lympoblastic leukemia with th. N. Engl. J. Med. 2001, 344, 1038–1042. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Cortes, J. Molecular biology of bcr-abl1–positive chronic myeloid leukemia. Blood 2009, 113, 1619–1630. [Google Scholar] [CrossRef]
- Arter, C.; Trask, L.; Ward, S.; Yeoh, S.; Bayliss, R. Structural features of the protein kinase domain and targeted binding by small-molecule inhibitors. J. Biol. Chem. 2022, 298, 102247. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-H.; Shiao, H.-Y.; Tu, C.-H.; Liu, P.-M.; Hsu, J.T.-A.; Amancha, P.K.; Wu, J.-S.; Coumar, M.S.; Chen, C.-H.; Wang, S.-Y.; et al. Protein Kinase Inhibitor Design by Targeting the Asp-Phe-Gly (DFG) Motif: The Role of the DFG Motif in the Design of Epidermal Growth Factor Receptor Inhibitors. J. Med. Chem. 2013, 56, 3889–3903. [Google Scholar] [CrossRef] [PubMed]
- Cowan-Jacob, S.W.; Fendrich, G.; Floersheimer, A.; Furet, P.; Liebetanz, J.; Rummel, G.; Rheinberger, P.; Centeleghe, M.; Fabbro, D.; Manley, P.W. Structural biology contributions to the discovery of drugs to treat chronic myelogenous leukaemia. Acta Crystallogr. Sect. D Struct. Biol. 2006, 63, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Raymer, B.; Bhattacharya, S.K. Lead-like Drugs: A Perspective. J. Med. Chem. 2018, 61, 10375–10384. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.R.d.S.; Moreira-Filho, J.T.; Neves, B.J.; Maidana, R.L.B.R.; Guimarães, A.C.R.; Furnham, N.; Andrade, C.H.; Silva, F.P. In silico Strategies to Support Fragment-to-Lead Optimization in Drug Discovery. Front. Chem. 2020, 8, 93. [Google Scholar] [CrossRef]
- BIOVIA. Dassault Systèmes, Discovery Studio Visualizer, v21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Atanasova, M.; Dimitrov, I.; Ivanov, S.; Georgiev, B.; Berkov, S.; Zheleva-Dimitrova, D.; Doytchinova, I. Virtual Screening and Hit Selection of Natural Compounds as Acetylcholinesterase Inhibitors. Molecules 2022, 27, 3139. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.-Y.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling Using Modeller. Curr. Protoc. Bioinform. 2006, 15, 5–6. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC Model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Ciccotti, G.; Ryckaert, J. Molecular dynamics simulation of rigid molecules. Comput. Phys. Rep. 1986, 4, 346–392. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, A.; Simonson, T. A molecular mechanics model for imatinib and imatinib:kinase binding. J. Comput. Chem. 2009, 31, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Seeliger, M.A.; Eastwood, M.P.; Frank, F.; Xu, H.; Jensen, M.Ø.; Dror, R.O.; Kuriyan, J.; Shaw, D.E. A conserved protonation-dependent switch controls drug binding in the Abl kinase. Proc. Natl. Acad. Sci. USA 2009, 106, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, P.; Lyczek, A.; Paung, Y.; Mingione, V.R.; Iacob, R.E.; de Waal, P.W.; Engen, J.R.; Seeliger, M.A.; Shan, Y.; Shaw, D.E. Structural mechanism of a drug-binding process involving a large conformational change of the protein target. Nat. Commun. 2023, 14, 1885. [Google Scholar] [CrossRef]
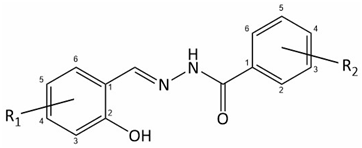 | ID | R1 | R2 |
| 1 | 3-methoxy | 2-methoxy | |
| 2 | 3-methoxy | 3-methoxy | |
| 3 | 3-methoxy | 4-methoxy | |
| 4 | 4-methoxy | 3-methoxy | |
| 5 | 4-methoxy | 4-methoxy |
| Property | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Mw g/mol | 300.31 | 300.31 | 300.31 | 300.31 | 300.31 |
| pKa | 8.40 | 8.39 | 8.47 | 8.01 | 8.08 |
| fA | 0.09 | 0.09 | 0.08 | 0.20 | 0.17 |
| logP | 3.31 | 3.75 | 3.45 | 3.86 | 3.56 |
| logD7.4 | 3.24 | 3.71 | 3.38 | 3.77 | 3.42 |
| PSA Å | 80.15 | 80.15 | 80.15 | 80.15 | 80.15 |
| FRB | 6 | 6 | 6 | 6 | 6 |
| HBDs | 2 | 2 | 2 | 2 | 2 |
| HBAs | 6 | 6 | 6 | 6 | 6 |
| R5 | 0 | 0 | 0 | 0 | 0 |
| Water solubility | Moderately soluble | Moderately soluble | Moderately soluble | Moderately soluble | Moderately soluble |
| GI absorption | High | High | High | High | High |
| Oral BA | INSATU | INSATU | INSATU | INSATU | INSATU |
| BA score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| BBB permeability | No | No | No | No | No |
| CYP inhibition | No | No | 1A2 | 1A2, 2C9 | No |
| P-gp substrate | No | No | No | No | No |
| Drug likeness | Yes | Yes | Yes | Yes | Yes |
| Lead likeness | Yes | Yes | Yes | Yes | Yes |
| Synthetic Accessibility | 2.72 | 2.72 | 2.62 | 2.69 | 2.63 |
| fu | 0.016 | 0.013 | 0.015 | 0.012 | 0.014 |
| CL L/h/kg | 0.45 | 0.43 | 0.44 | 0.39 | 0.43 |
| VDss L/kg | 0.208 | 0.206 | 0.208 | 0.209 | 0.211 |
| t1/2 h | 0.32 | 0.34 | 0.32 | 0.37 | 0.34 |
| Cell Line | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| HL-60 | 11.2 ± 2.1 | 3.6 ± 0.3 | 2.4 ± 0.1 | 2.1 ± 0.2 | 4.7 ± 0.5 |
| BV-173 | 33.5 ± 3.4 | 2.7 ± 0.2 | 2.7 ± 0.2 | 2.2 ± 0.3 | 1.4 ± 0.2 |
| K-562 | 20.4 ± 2.9 | 2.1 ± 0.1 | 2.8 ± 0.4 | 1.6 ± 0.2 | 1.5 ± 0.1 |
| AR-230 | 50.5 ± 6.6 | 5.9 ± 0.7 | >100 | 4.5 ± 0.4 | 1.1 ± 0.2 |
| SKW-3 | 4.3 ± 0.5 | 1.4 ± 0.1 | 2.7 ± 0.3 | 0.8 ± 0.1 | 0.5 ± 0.1 |
| MCF-7 | 30.1 ± 3.2 | 25.6 ± 2.8 | 36.1 ± 4.4 | 23.3 ± 2.7 | 21.3 ± 1.9 |
| MDA-MB-231 | 22.0 ± 2.8 | 26.9 ± 3.3 | 23.5 ± 3.4 | 19.8 ± 3.1 | 60.1 ± 5.0 |
| HEK-293 | >100 | 16.8 ± 2.3 | >100 | 8.6 ± 1.5 | 10.4 ± 1.1 |
| Cell Line | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LE | SI | LE | SI | LE | SI | LE | SI | LE | SI | |
| HL-60 | 0.225 | >9 | 0.247 | 5 | 0.255 | >42 | 0.258 | 4 | 0.242 | 2 |
| BV-173 | 0.203 | >3 | 0.253 | 6 | 0.240 | >19 | 0.257 | 4 | 0.266 | 7 |
| K-562 | 0.213 | >5 | 0.258 | 8 | 0.252 | >36 | 0.263 | 5 | 0.265 | 7 |
| AR-230 | 0.195 | >2 | 0.238 | 3 | >0.182 | >1 | 0.243 | 2 | 0.271 | 9 |
| SKW-3 | 0.244 | >23 | 0.266 | 12 | 0.253 | >37 | 0.277 | 11 | 0.286 | 21 |
| Avg. leukemia | 0.216 | >8 | 0.252 | 7 | 0.237 | >27 | 0.260 | 5 | 0.266 | 9 |
| MCF-7 | 0.206 | >3 | 0.209 | 1 | 0.202 | >3 | 0.211 | 0 | 0.212 | 0 |
| MDA-MB-231 | 0.212 | >5 | 0.208 | 1 | 0.210 | >4 | 0.214 | 0 | 0.192 | 0 |
| Avg. breast cancer | 0.209 | >4 | 0.209 | 1 | 0.206 | >4 | 0.213 | 0 | 0.202 | 0 |
| Avg. all cancers | 0.214 | >7 | 0.240 | 5 | 0.228 | >20 | 0.246 | 4 | 0.248 | 7 |
| HEK-293 | >0.182 | - * | 0.217 | - * | >0.182 | - * | 0.230 | - * | 0.226 | - * |
| Compound | ChemPLP Score | ChemPLP Score/Molecular Weight |
|---|---|---|
| Imatinib | 122.312 | 0.248 |
| 1 | 70.373 | 0.234 |
| 2 | 71.499 | 0.238 |
| 3 | 73.266 | 0.244 |
| 4 | 71.747 | 0.239 |
| 5 | 67.669 | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova-Mladenova, B.; Mihaylova, R.; Atanasova, M.; Zhivkova, Z.; Doytchinova, I. Salicylaldehyde Benzoylhydrazones with Anticancer Activity and Selectivity: Design, Synthesis, and In Vitro Evaluation. Molecules 2025, 30, 1015. https://doi.org/10.3390/molecules30051015
Nikolova-Mladenova B, Mihaylova R, Atanasova M, Zhivkova Z, Doytchinova I. Salicylaldehyde Benzoylhydrazones with Anticancer Activity and Selectivity: Design, Synthesis, and In Vitro Evaluation. Molecules. 2025; 30(5):1015. https://doi.org/10.3390/molecules30051015
Chicago/Turabian StyleNikolova-Mladenova, Boryana, Rositsa Mihaylova, Mariyana Atanasova, Zvetanka Zhivkova, and Irini Doytchinova. 2025. "Salicylaldehyde Benzoylhydrazones with Anticancer Activity and Selectivity: Design, Synthesis, and In Vitro Evaluation" Molecules 30, no. 5: 1015. https://doi.org/10.3390/molecules30051015
APA StyleNikolova-Mladenova, B., Mihaylova, R., Atanasova, M., Zhivkova, Z., & Doytchinova, I. (2025). Salicylaldehyde Benzoylhydrazones with Anticancer Activity and Selectivity: Design, Synthesis, and In Vitro Evaluation. Molecules, 30(5), 1015. https://doi.org/10.3390/molecules30051015








