The Effectiveness of Liquid-Phase Microextraction of Beta-Blockers from Aqueous Matrices for Their Analysis by Chromatographic Techniques
Abstract
1. Introduction
2. Results
2.1. Optimization of DLLME and SFOME Conditions
2.2. Validation of SFOME-LC-PDA Method
2.3. Validation of DLLME-GC-MS Method
2.4. Analysis of Wastewater
2.5. Comparison with Other Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Extraction by SFOME and DLLME Protocol
3.4. Enrichment Factor and Extraction Recovery
3.5. Statistical Approach Used for DLLME and SFOME Optimization
3.6. Sample Collection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DLLME | Dispersive Liquid–Liquid Microextraction |
| SFOME | Solidification Of Floating Organic Droplet Microextraction |
| GC | Gas Chromatography |
| LC | Liquid Chromatography |
| GC–MS | Gas Chromatography coupled with Mass Spectrometry |
| HPLC–PDA | High-Performance Liquid Chromatography with Photodiode Array Detector |
| LOQ | Limit of Detection |
| LOD | Limit of Quantification |
| SPE | Solid-Phase Extraction |
| SPME | Solid-Phase Microextraction |
| SBSE | Stir-Bar Sorptive Extraction |
| FPSE | Fabric-Phase Sorbent Extraction |
| µQuEChERS | Micro Quick, Easy, Cheap, Effective, Rugged, and Safe |
| SDME | Single-Drop Microextraction |
| HF-LPME | Hollow-Fiber Liquid-Phase Microextraction |
| ATE | Atenolol |
| NAD | Nadolol |
| PIN | Pindolol |
| ACE | Acebutolol |
| MET | Metoprolol |
| BIS | Bisoprolol |
| PRP | Propranolol |
| BTX | Betaxolol |
| RSD | Relative Standard Deviation |
| SD | Standard Deviation |
| S | the Slope of each the Calibration Curve |
| ICH | International Committee for Harmonization |
| EF | Enrichment Factor |
| ER | Enrichment Recovery |
| BSTFA | N,O-bis(trimethylsilyl)trifluoroacetamide |
| TMCS | Trimethylchlorosilane |
| TMS | trimethylsilyl |
| DLLME-SFO | Dispersive Liquid–Liquid Microextraction based on Solidification pf Floating Organic Droplet |
| VA DLPME-SFOD | Vortex Assisted Dispersive Liquid-Phase Microextraction with Solidification of Floating Organic Droplet |
| TDLLME | Tandem Dispersive Liquid–Liquid Microextraction |
| AALLME-SFOD | Air-Assisted Liquid–Liquid Microextraction with Solidification of Floating Organic Droplet |
| ACN | Acetonitrile |
| MS | Mass Spectrometry |
| PDA | Photodiode Array Detector |
| USA | United States of America |
References
- Xu, J.; Sun, H.; Zhang, Y.; Alder, A.C. Occurrence and Enantiomer Profiles of Β-Blockers in Wastewater and a Receiving Water Body and Adjacent Soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130. [Google Scholar] [CrossRef]
- Yıldırım, S.; Sellitepe, H.E. Vortex Assisted Liquid-Liquid Microextraction based on in Situ Formation of a Natural Deep Eutectic Solvent by Microwave Irradiation for the Determination of Beta-Blockers in Water Samples. J. Chromatogr. A 2021, 1642, 462007. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Sorouraddin, M.H.; Farajzadeh, M.A.; Somi, M.H.; Fazeli-Bakhtiyari, R. Determination of Five Antiarrhythmic Drugs in Human Plasma by Dispersive Liquid–Liquid Microextraction and High-Performance Liquid Chromatography. Talanta 2015, 134, 681–689. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; Fent, K. Cardiovascular Drugs and Lipid Regulating Agents in Surface Waters at Global Scale: Occurrence, Ecotoxicity and Risk Assessment. Sci. Total Environ. 2020, 729, 138770. [Google Scholar] [CrossRef]
- Nisyriou, S.; Zacharis, C.K. Microextraction-Based Techniques for the Determination of Beta-Blockers in Biological Fluids: A Review. Separations 2025, 12, 14. [Google Scholar] [CrossRef]
- Lee, H.-B.; Sarafin, K.; Peart, T.E. Determination of β-blockers and β2-agonists in Sewage by Solid-Phase Extraction and Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2007, 1148, 158–167. [Google Scholar] [CrossRef]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-Blockers in the Environment: Part II. Ecotoxicity study. Sci. Total Environ. 2014, 493, 1122–1126. [Google Scholar] [CrossRef]
- Gonçalves, R.; Ribeiro, C.; Cravo, S.; Cunha, S.C.; Pereira, J.A.; Fernandes, J.O.; Afonso, C.; Tiritan, M.E. Multi-Residue Method for Enantioseparation of Psychoactive Substances and Beta Blockers by Gas Chromatography–Mass Spectrometry. J. Chromatogr. B 2019, 1125, 121731. [Google Scholar] [CrossRef]
- Pană, M.; Crăciun, P.; Nicolae, C.; Tăerel, A.-E.; Lupuliasa, D. The Cardiovascular Market in Romania During 1998–2017 Period—Analysis and Evolution. Farmacia 2019, 67, 922–930. [Google Scholar] [CrossRef]
- Yi, M.; Sheng, Q.; Sui, Q.; Lu, H. β-Blockers in the Environment: Distribution, Transformation, and Ecotoxicity. Environ. Poll. 2020, 266, 115269. [Google Scholar] [CrossRef] [PubMed]
- Iancu, V.-I.; Radu, G.-L.; Scutariu, R. A New Analytical Method for the Determination of Beta-Blockers and one Metabolite in the Influents and Effluents of Three Urban Wastewater Treatment Plants. Anal. Methods 2019, 11, 4668–4680. [Google Scholar] [CrossRef]
- Alder, A.C.; Schaffner, C.; Majewsky, M.; Klasmeier, J.; Fenner, K. Fate of β-Blocker Human Pharmaceuticals in Surface Water: Comparison of Measured and Simulated Concentrations in The Glatt Valley Watershed, Switzerland. Water Res. 2010, 44, 936–948. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, Ecotoxicological Effects and Risk Assessment of Antihypertensive Pharmaceutical Residues in the Aquatic Environment—A Review. Chemosphere 2015, 138, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Gomez, M.J.; Aguera, A.; Fernandez-Alba, A.R. LC-MS Analysis of Basic Pharmaceuticals (Beta-Blockers and Anti-Ulcer Agents) in Wastewater and Surface Water. TrAC Trends Anal. Chem. 2007, 26, 581–594. [Google Scholar] [CrossRef]
- Gil García, M.D.; Canada Canada, F.; Culzonia, M.J.; Vera-Candioti, L.; Siano, G.G.; Goicoechea, H.C.; Martínez Galera, M. Chemometric Tools Improving the Determination of Anti-Inflammatory and Antiepileptic Drugs in River and Wastewater by Solid-Phase Microextraction and Liquid Chromatography Diode Array Detection. J. Chromatogr. A 2009, 1216, 5489–5496. [Google Scholar] [CrossRef]
- Unceta, N.; Sampedro, M.C.; Bakar, N.K.A.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Multi-Residue Analysis of Pharmaceutical Compounds in Wastewaters by Dual Solid-Phase Microextraction Coupled to Liquid Chromatography Electrospray Ionization Ion Trap Mass Spectrometry. J. Chromatogr. A 2010, 1217, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; He, M.; Chen, B.; Hu, B. Membrane Protected C18 Coated Stir Bar Sorptive Extraction Combined with High Performance Liquid Chromatography-Ultraviolet Detection for the Determination of Non-Steroidal Anti-Inflammatory Drugs in Water Samples. J. Chromatogr. A 2016, 1472, 27–34. [Google Scholar] [CrossRef]
- Arismendi, D.; Becerra-Herrera, M.; Cerrato, I.; Richter, P. Simultaneous Determination of Multiresidue and Multiclass Emerging Contaminants in Waters by Rotating-Disk Sorptive Extraction–Derivatization Gas Chromatography/Mass Spectrometry. Talanta 2019, 201, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Kalaboka, M.; Chrimatopoulos, C.; Jiménez-Holgado, C.; Boti, V.; Sakkas, V.; Albanis, T. Exploring the Efficiency of UHPLC-Orbitrap MS for the Determination of 20 Pharmaceuticals and Acesulfame K in Hospital and Urban Wastewaters with the Aid of FPSE. Separations 2020, 7, 46. [Google Scholar] [CrossRef]
- Misolas, A.; Sleiman, M.; Sakkas, V. Application of Fabric Phase Sorptive Extraction as a Green Method for the Analysis of 10 Anti-Diabetic Drugs in Environmental Water Samples. Molecules 2024, 29, 4834. [Google Scholar] [CrossRef] [PubMed]
- Berlioz-Barbier, A.; Baudot, R.; Wiest, L.; Gust, M.; Garric, J.; Cren-Olivé, C.; Buleté, A. MicroQuEChERS–Nanoliquid Chromatography–Nanospray–Tandem Mass Spectrometry for the Detection and Quantification of Trace Pharmaceuticals in Benthic Invertebrates. Talanta 2015, 132, 796–802. [Google Scholar] [CrossRef]
- Wicht, A.J.; Heye, K.; Schmidt, A.; Oehlmann, J.; Huhn, C. The Wastewater Micropollutant Carbamazepine in Insectivorous Birds—An Exposure Estimate. Anal. Bioanal. Chem. 2022, 414, 4909–4917. [Google Scholar] [CrossRef]
- Farhadi, K.; Hatami, M.; Forough, M.; Molaei, R. Dispersive Liquid–Liquid Microextraction of Propranolol Enantiomers from Human Plasma Based on the Solidification of a Floating Organic Droplet. Bioanalysis 2013, 5, 701–710. [Google Scholar] [CrossRef]
- Kiszkiel-Taudul, I.; Starczewska, B. Single Drop Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry (SDME-LC-MS/MS) for Determination of Ranitidine in Water Samples. Microchem. J. 2019, 145, 936–941. [Google Scholar] [CrossRef]
- Ramos Payán, M.; Bello López, M.Á.; Fernández-Torres, R.; Callejón Mochón, M.; Gómez Ariza, J.L. Application of Hollow Fiber-Based Liquid-Phase Microextraction (HF-LPME) for the Determination of Acidic Pharmaceuticals in Wastewaters. Talanta 2010, 82, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Determination of Selected Antiretroviral Drugs in Wastewater, Surface Water and Aquatic Plants Using Hollow Fibre Liquid Phase Microextraction and Liquid Chromatography—Tandem Mass Spectrometry. J. Hazard. Mater. 2020, 382, 121067. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Asghari, A.; Bazregar, M.; Rajabi, M. Rapid Determination of Some Beta-Blockers in Complicated Matrices by Tandem Dispersive Liquid-Liquid Microextraction Followed by High Performance Liquid Chromatography. ABC 2016, 408, 8163–8176. [Google Scholar] [CrossRef]
- Hryniewicka, M.; Starczewska, B.; Gołębiewska, A. Determination of Budesonide and Sulfasalazine in Water and Wastewater Samples Using DLLME-SFO-HPLC-UV Method. Water 2019, 11, 1581. [Google Scholar] [CrossRef]
- AlSalem, H.S.; Algethami, K.F.; Ali, M.A.; Mansour, F.R. Development of an Ecofriendly Dispersive Liquid Phase Microextraction Method for The Preconcentration of Β-Blockers in Waste and Environmental Samples. Microchem. J. 2024, 205, 111279. [Google Scholar] [CrossRef]
- Notardonato, I.; Avino, P. Dispersive Liquid–Liquid Micro Extraction: An Analytical Technique Undergoing Continuous Evolution and Development—A Review of the Last 5 Years. Separations 2024, 11, 203. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.-R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of Organic Compounds in Water Using Dispersive Liquid–Liquid Microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.-I.; Huang, S.-D. Dispersive Liquid–Liquid Microextraction Method based on Solidification of Floating Organic Drop Combined with Gas Chromatography with Electron-Capture or Mass Spectrometry Detection. J. Chromatogr. A 2008, 1211, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Beldean-Galea, M.S.; Coman, V.; Thiébaut, D.; Vial, J. Determination of Four Acidic Nonsteroidal Anti-Inflammatory Drugs in Wastewater Samples by Dispersive Liquid-Liquid Microextraction Based on Solidification of Floating Organic Droplet and High-Performance Liquid Chromatography. J. Sep. Sci. 2015, 38, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Kalajahi, M.; Fazeli-Bakhtiyari, R.; Amiri, M.; Golmohammadi, A.; Afrasiabi, A.; Khoubnasabjafari, M.; Jouyban, A. Dispersive Liquid–Liquid Microextraction based on Solidification of Floating Organic Droplet Followed by Spectrofluorimetry for Determination of Carvedilol in Human Plasma. Bioanalysis 2013, 5, 437–448. [Google Scholar] [CrossRef]
- Farahmand, F.; Ghasemzadeh, B.; Naseri, A. Air-Assisted Liquid–Liquid Microextraction Using Floating Organic Droplet Solidification for Simultaneous Extraction and Spectrophotometric Determination of some Drugs in Biological Samples Through Chemometrics Methods. Spectrochim. Acta—A. Mol. Biomol. Spectrosc. 2018, 188, 72–79. [Google Scholar] [CrossRef]
- Beldean-Galea, M.S.; Klein, R.; Coman, M.-V. Simultaneous Determination of Four Nonsteroidal Anti-Inflammatory Drugs and Three Estrogen Steroid Hormones in Wastewater Samples by Dispersive Liquid–Liquid Microextraction based on Solidification of Floating Organic Droplet and HPLC. J. AOAC Int. 2020, 103, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Beldean-Galea, M.S.; Vial, J.; Thiébaut, D.; Coman, M.-V. Analysis of Multiclass Organic Pollutant in Municipal Landfill Leachate by Dispersive Liquid-Liquid Microextraction and Comprehensive Two-Dimensional Gas Chromatography Coupled with Mass Spectrometry. Environ. Sci. Pollut. Res. 2020, 27, 9535–9546. [Google Scholar] [CrossRef] [PubMed]
- Herghelegiu, M.C.; Pănescu, V.A.; Bocoș-Bințințan, V.; Coman, R.-T.; Berg, V.; Lyche, J.L.; Bruzzoniti, M.C.; Beldean-Galea, M.S. Simultaneous Determination of Steroids and Nsaids, Using DLLME-SFO Extraction and HPLC Analysis, in Milk and Eggs Collected from Rural Roma Communities in Transylvania, Romania. Molecules 2024, 29, 96. [Google Scholar] [CrossRef] [PubMed]
- Candioti, L.V.; De Zan, M.M.; Cámara, M.S.; Goicoeche, H.C. Experimental Design and Multiple Response Optimization. Using the Desirability Function in Analytical Methods Development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef]
- Validation of Analytical Procedures: Text and Methodology, Q2(R1). 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guideline-validation-analytical-procedures-step-5-revision-1_en.pdf (accessed on 18 November 2024).
- Herghelegiu, M.-C.; Ernault, A.; Beldean-Galea, M.S.; Coman, M.-V. HPLC-PDA versus GC-MS in the Analysis of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs in Wastewater. Stud. Univ. Babes-Bolyai Chem. 2023, 68, 19–35. [Google Scholar] [CrossRef]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021 v2. 2021. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 15 November 2024).
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and Opportunities of Experimental Design for the Dispersive Liquid–Liquid Microextraction Method—A Review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef]
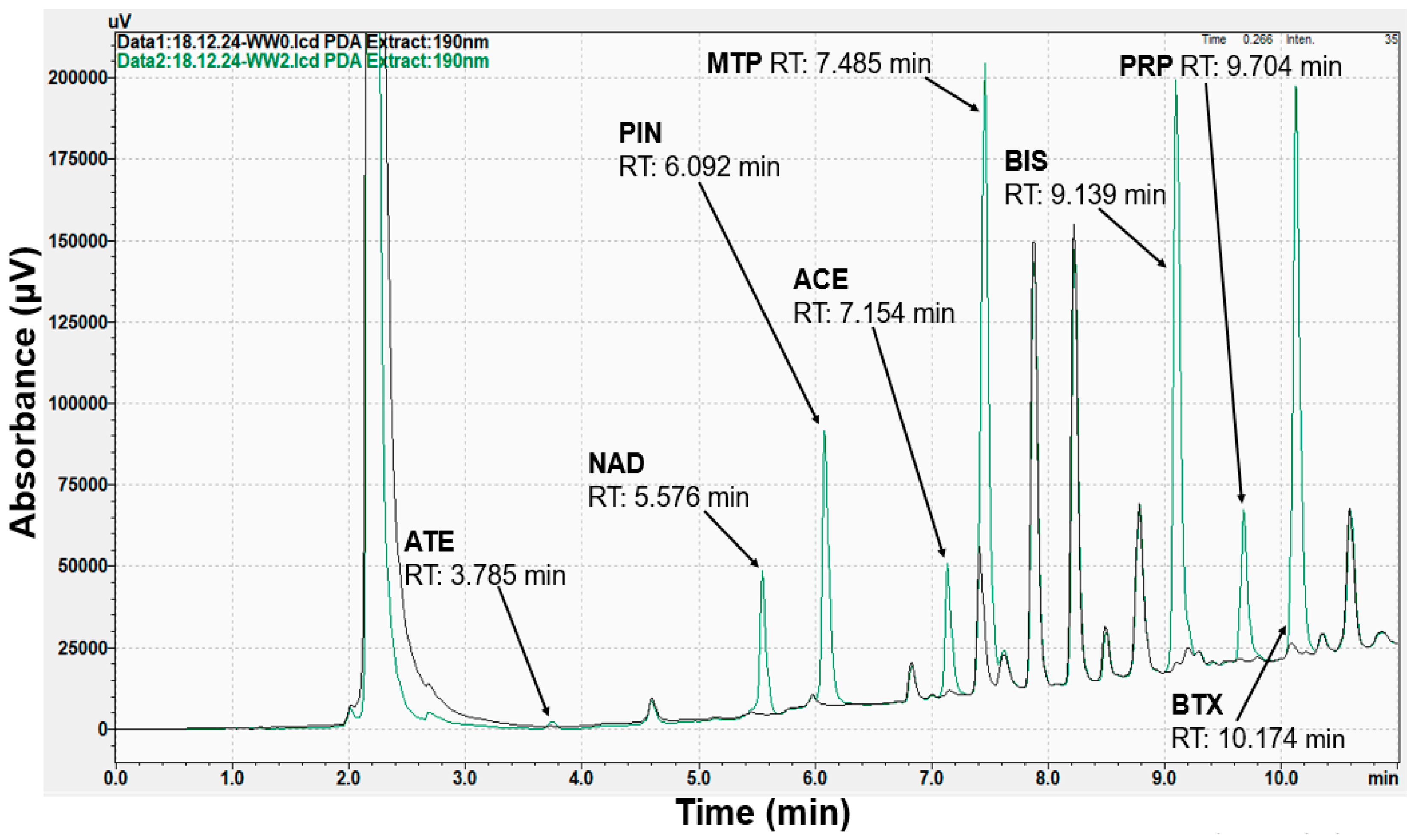
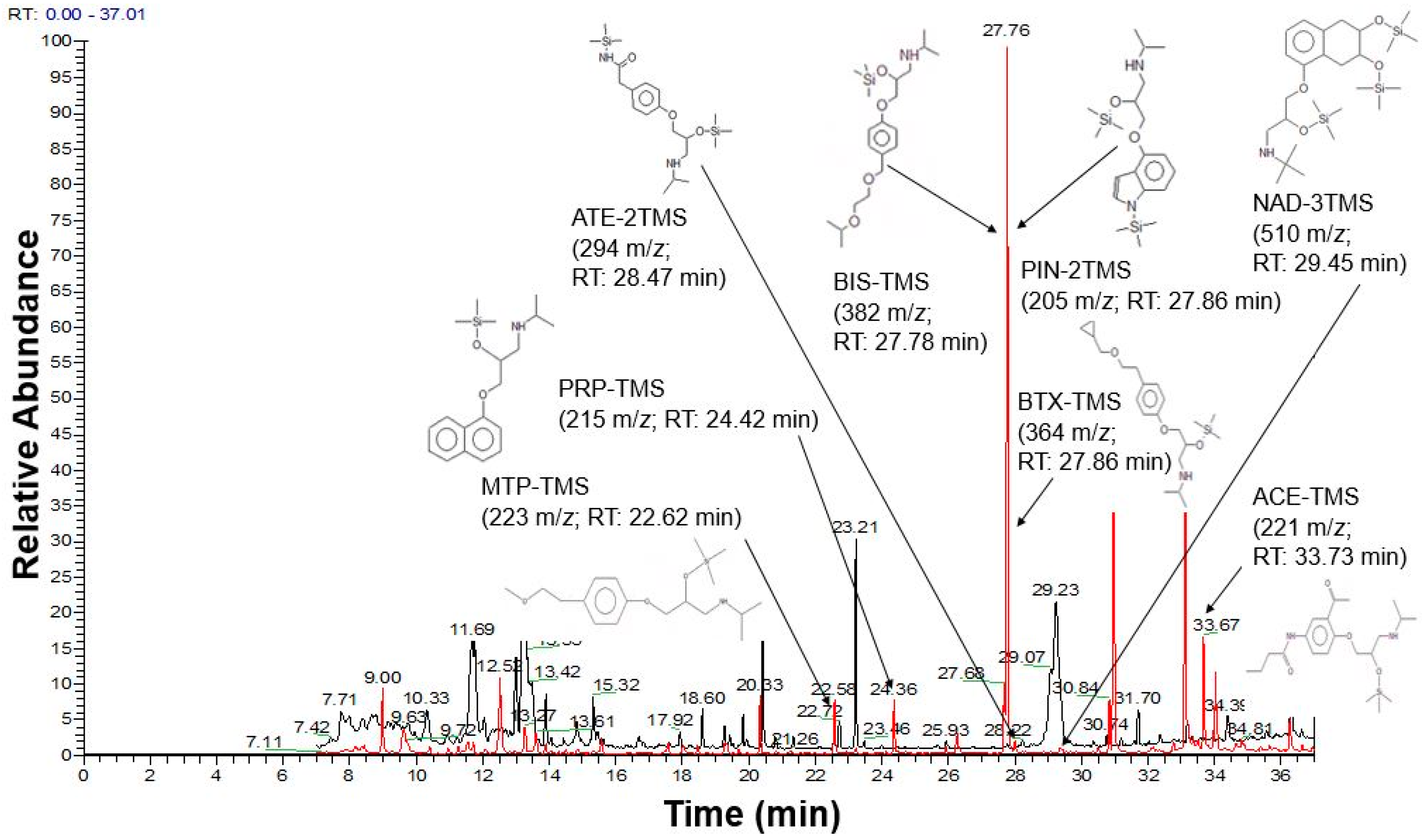
| Beta-Blockers | Abbreviation | Extraction Recovery (%) | |||||
|---|---|---|---|---|---|---|---|
| SFOME | DLLME | ||||||
| Minimum | Maximum | Optimum | Minimum | Maximum | Optimum | ||
| Atenolol | ATE | 3.24 | 13.94 | 8.59 | - | - | - |
| Nadolol | NAD | 8.34 | 24.91 | 16.62 | 5.31 | 8.51 | 6.91 |
| Pindolol | PIN | 37.10 | 84.88 | 60.99 | 64.58 | 110.07 | 87.32 |
| Acebutolol | ACE | 26.25 | 73.03 | 49.64 | −14.78 | 134.97 | 60.10 |
| Metoprolol | MTP | 40.14 | 94.59 | 67.36 | 98.82 | 161.62 | 130.22 |
| Bisoprolol | BIS | 45.56 | 119.46 | 82.51 | 99.69 | 181.64 | 69.94 |
| Propranolol | PRP | 67.42 | 136.69 | 102.05 | 106.36 | 151.00 | 128.68 |
| Betaxolol | BTX | 65.94 | 134.52 | 100.23 | 82.79 | 152.40 | 117.59 |
| Beta-Blockers | Calibration Curve Equation | SD | R2 | LOD (µg/mL) | LOQ (µg/mL) | Precision (RSD%) | ER (%) | EF | |
|---|---|---|---|---|---|---|---|---|---|
| Intra- | Inter- | ||||||||
| ATE | y = 168,393x + 180,171 | 5764.51 | 0.9983 | 0.11 | 0.34 | 2.64 | 6.23 | 14.71 | 15.01 |
| NAD | y = 119,820x + 194,465 | 2386.55 | 0.9991 | 0.07 | 0.20 | 2.91 | 2.37 | 29.85 | 30.46 |
| PIN | y = 73,452x + 116,174 | 2877.82 | 0.9963 | 0.13 | 0.39 | 3.93 | 4.45 | 68.79 | 70.20 |
| ACE | y = 72,148x + 22,066 | 2612.56 | 0.9998 | 0.12 | 0.36 | 1.23 | 2.37 | 59.99 | 61.22 |
| MTP | y = 130,487x + 203,807 | 5902.37 | 0.9950 | 0.15 | 0.45 | 4.35 | 5.02 | 74.50 | 76.02 |
| BIS | y = 130,628x + 142,398 | 5005.23 | 0.9974 | 0.13 | 0.38 | 0.73 | 2.73 | 81.45 | 83.11 |
| PRP | y = 131,203x + 32,456 | 5322.12 | 0.9998 | 0.13 | 0.41 | 4.84 | 6.38 | 86.81 | 88.58 |
| BTX | y = 127,756x + 107,522 | 4001.82 | 0.9981 | 0.10 | 0.31 | 4.87 | 6.10 | 87.55 | 89.34 |
| Derivatized Beta-Blockers | Calibration Curve Equation | SD | R2 | LOD (µg/mL) | LOQ (µg/mL) | Precision (RSD%) | ER (%) | EF | |
|---|---|---|---|---|---|---|---|---|---|
| Intra- | Inter- | ||||||||
| ATE-2TMS | y = 341,497x + 79,567 | 27,525.77 | 0.9995 | 0.27 | 0.81 | - | - | - | - |
| NAD–3TMS | y = 2E + 06x – 995,402 | 92,698.55 | 0.9999 | 0.15 | 0.46 | 7.38 | 10.74 | 3.14 | 11.79 |
| PIN-2TMS | y = 567,017x + 191,180 | 118,922.34 | 0.9983 | 0.69 | 2.10 | 12.36 | 13.59 | 53.04 | 198.89 |
| ACE-TMS | y = 219,605x – 332,187 | 8649.25 | 0.9999 | 0.13 | 0.39 | 8.33 | 11.08 | 56.89 | 212.75 |
| MTP-TMS | y = 2E + 06x + 2E + 06 | 182,859.84 | 0.9984 | 0.30 | 0.91 | 10.34 | 11.17 | 78.91 | 220.91 |
| BIS-TMS | y = 637,676x – 181,116 | 47,182.95 | 0.9997 | 0.24 | 0.74 | 12.60 | 14.26 | 92.1 | 243.97 |
| PRP-TMS | y = 2E + 06x + 2E + 06 | 188,228.14 | 0.9984 | 0.31 | 0.94 | 8.82 | 11.48 | 71.6 | 212.75 |
| BTX-TMS | y = 531,846x + 4113.8 | 35,621.76 | 0.9981 | 0.22 | 0.67 | 12.44 | 14.91 | 74.6 | 217.17 |
| Beta-Blockers | SFOME | DLLME | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spiked Amount (ng) | Wastewater | ER (%) | EF | Spiked Amount (ng) | Wastewater | ER (%) | EF | |||
| Initial | Found | Initial | Found | |||||||
| ATE | 625 | 0.69 | 10.75 | 1.58 | 1.61 | 625 | nd | nd | - | - |
| NAD | 6.06 | 128.54 | 19.20 | 19.60 | 0.04 | 29.64 | 3.95 | 11.85 | ||
| PIN | 5.36 | 421.28 | 65.22 | 66.55 | 0.04 | 475.07 | 63.34 | 190.03 | ||
| ACE | 1.67 | 377.54 | 58.94 | 60.14 | nd | 494.67 | 65.96 | 197.87 | ||
| MTP | 117.87 | 554.90 | 68.53 | 69.92 | 1.28 | 599.56 | 79.94 | 239.82 | ||
| BIS | 17.14 | 550.40 | 83.61 | 85.32 | nd | 615.78 | 82.10 | 246.31 | ||
| PRP | 7.04 | 558.04 | 86.40 | 88.16 | 0.20 | 540.56 | 72.07 | 216.22 | ||
| BTX | 17.03 | 553.64 | 84.14 | 85.86 | nd | 615.94 | 82.13 | 246.38 | ||
| Beta-Blockers | Method | Matrix | Sample Volume (mL) | Extraction Solvent (mL) | LOQ (µg/mL) | ER (%) | EF | Ref. |
|---|---|---|---|---|---|---|---|---|
| metoprolol, propranolol, carvedilol, diltiazem, verapamil | DLLME | plasma | 0.66 | 100 µL dichloromethane | 0.007–0.019 | 4.4–10.8 | 33–82 | [3] |
| racemic propranolol | DLLME-SFO | plasma | 0.5 | 40 µL of 1-undecanol | 0.05 | 13.3–13.0 | 16.7–16.2 | [23] |
| metoprolol, propranolol | TDLLME | plasma, wastewater | 10 | 103 µL of 1,2-dichloroethane and 45 µL of aqueous extractant phase with pH value of 2.0 | 0.028–0.003 | 34–45 | 75–100 | [27] |
| atenolol, acebutolol, bisoprolol, betaxolol | VA DLPME-SFOD | river water, wastewater | 5 | 50 µL of dodecanol | 0.01–0.07 | - | 23–55 | [29] |
| carvedilol | DLLME-SFO | plasma | 1 | 75 µL of 1-undecanol | 0.04 1 | - | - | [34] |
| atenolol, propranolol, carvedilol | AALLME-SFOD | urine | 1 | 125 µL of 1-dodecanol and 160 µL of water with 1000 mg/L Triton X-100 | 0.26–0.30 | 64.22–94.57 | 11.24–16.55 | [35] |
| atenolol, nadolol, pindolol, acebutolol, metoprolol, bisoprolol, propranolol, betaxolol | SFOME | wastewater | 10 | 100 µL of 1-undecanol | 0.20–0.45 | 58.94–86.40 | 60.92–88.16 | This study. |
| DLLME | 75 µL chloroform | 0.39–1.23 | 63.34–82.13 | 190.03–246.38 |
| Factors | Values for DLLME and SFOME | ||
|---|---|---|---|
| Lowest (–1) | Central Point (0) | Highest (+1) | |
| NaCl (g) (X1) | 0.5 | 1.25 | 2 |
| Disperser (µL) (X2) | 100 | 175 | 250 |
| Extractant (µL) (X3) | 50 | 75 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beldean-Galea, M.S.; Herghelegiu, M.-C.; Pănescu, V.-A.; Vial, J.; Bruzzoniti, M.C.; Coman, M.-V. The Effectiveness of Liquid-Phase Microextraction of Beta-Blockers from Aqueous Matrices for Their Analysis by Chromatographic Techniques. Molecules 2025, 30, 1016. https://doi.org/10.3390/molecules30051016
Beldean-Galea MS, Herghelegiu M-C, Pănescu V-A, Vial J, Bruzzoniti MC, Coman M-V. The Effectiveness of Liquid-Phase Microextraction of Beta-Blockers from Aqueous Matrices for Their Analysis by Chromatographic Techniques. Molecules. 2025; 30(5):1016. https://doi.org/10.3390/molecules30051016
Chicago/Turabian StyleBeldean-Galea, Mihail Simion, Mihaela-Cătălina Herghelegiu, Vlad-Alexandru Pănescu, Jérôme Vial, Maria Concetta Bruzzoniti, and Maria-Virginia Coman. 2025. "The Effectiveness of Liquid-Phase Microextraction of Beta-Blockers from Aqueous Matrices for Their Analysis by Chromatographic Techniques" Molecules 30, no. 5: 1016. https://doi.org/10.3390/molecules30051016
APA StyleBeldean-Galea, M. S., Herghelegiu, M.-C., Pănescu, V.-A., Vial, J., Bruzzoniti, M. C., & Coman, M.-V. (2025). The Effectiveness of Liquid-Phase Microextraction of Beta-Blockers from Aqueous Matrices for Their Analysis by Chromatographic Techniques. Molecules, 30(5), 1016. https://doi.org/10.3390/molecules30051016







