Continuous In-Situ Polymerization of Complex-Based Films for High-Performance Electrochromic Devices
Abstract
1. Introduction
2. Results and Discussion
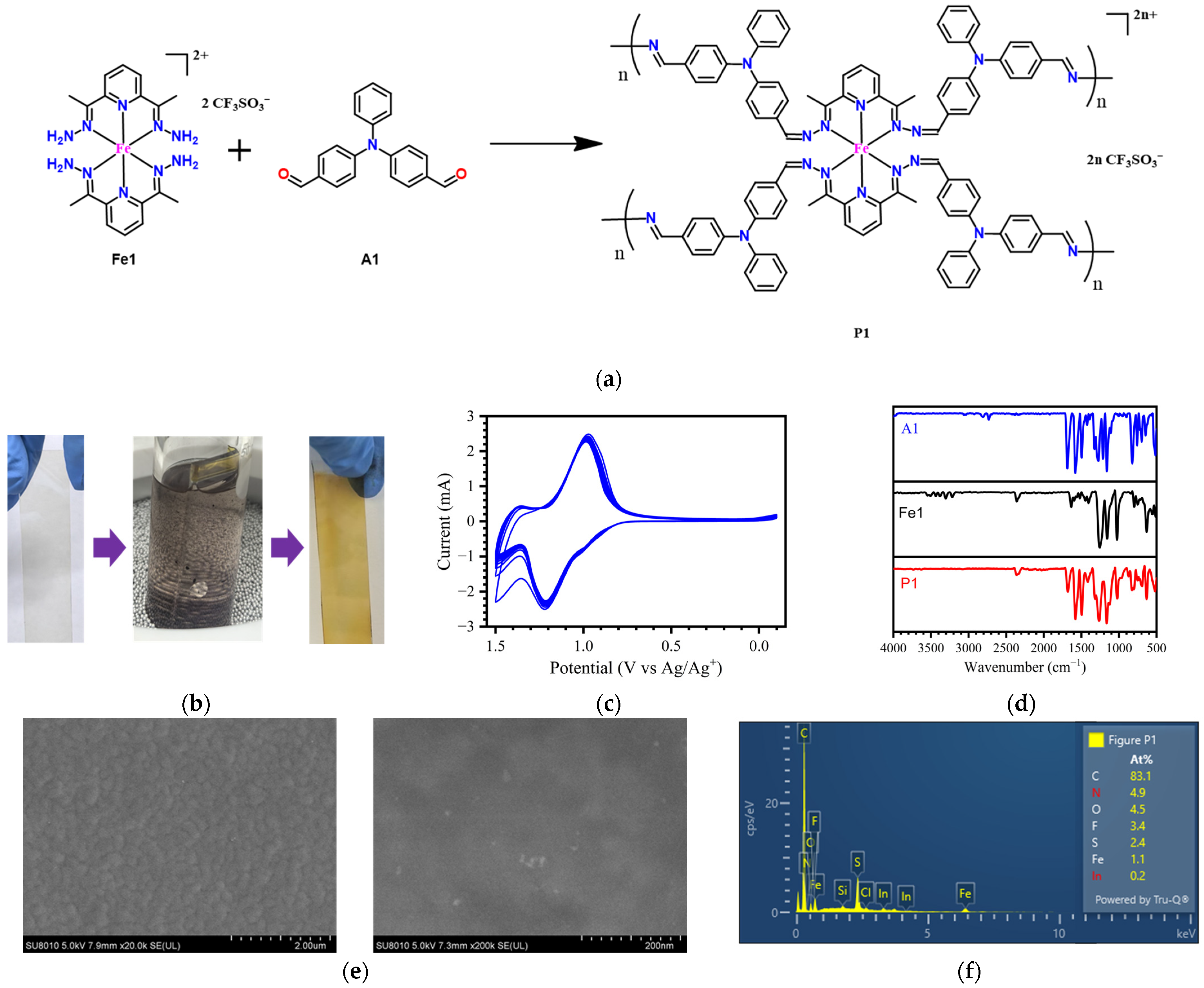
2.1. Continuous In Situ Polymerization of Electrochromic Film on an ITO Substrate
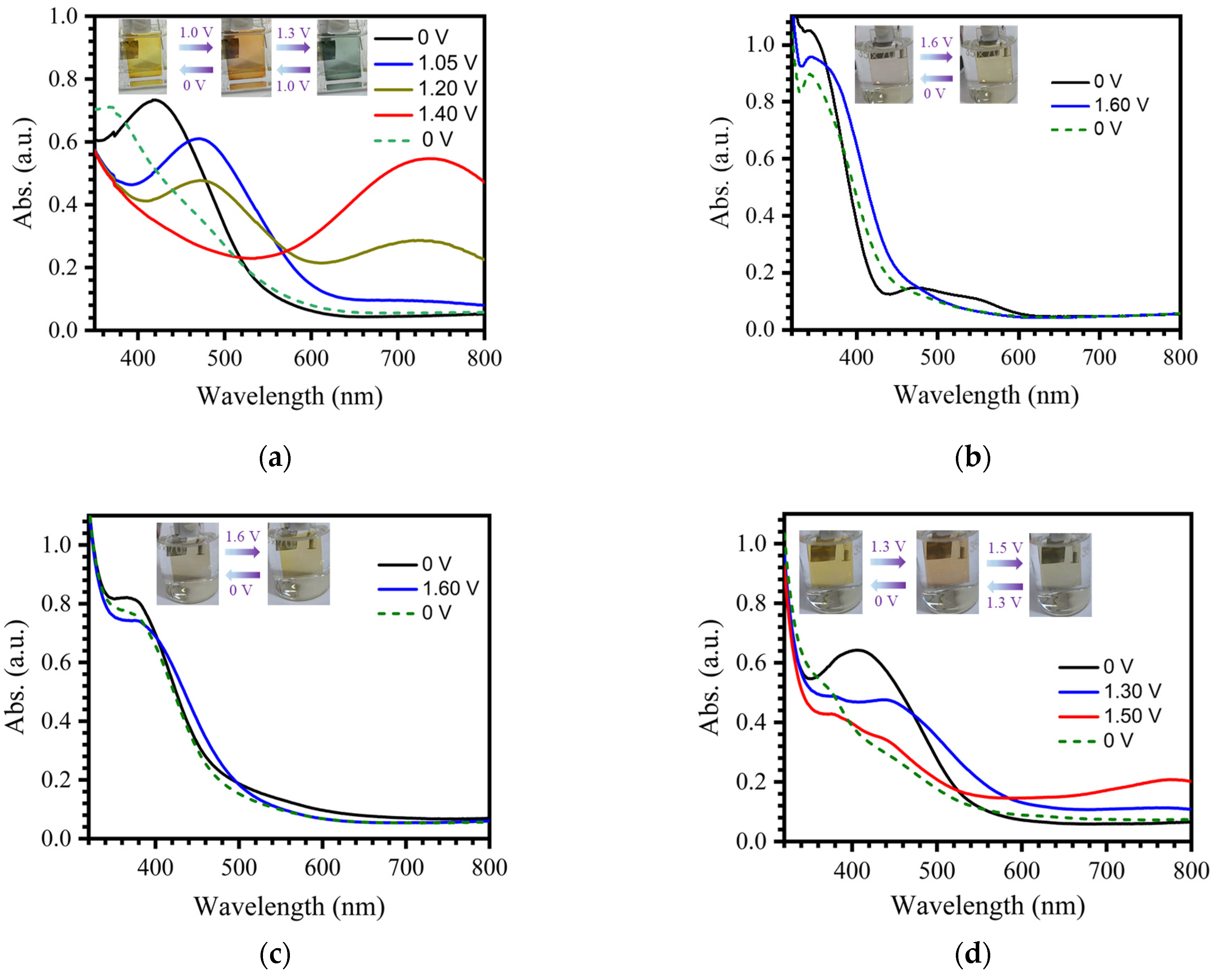
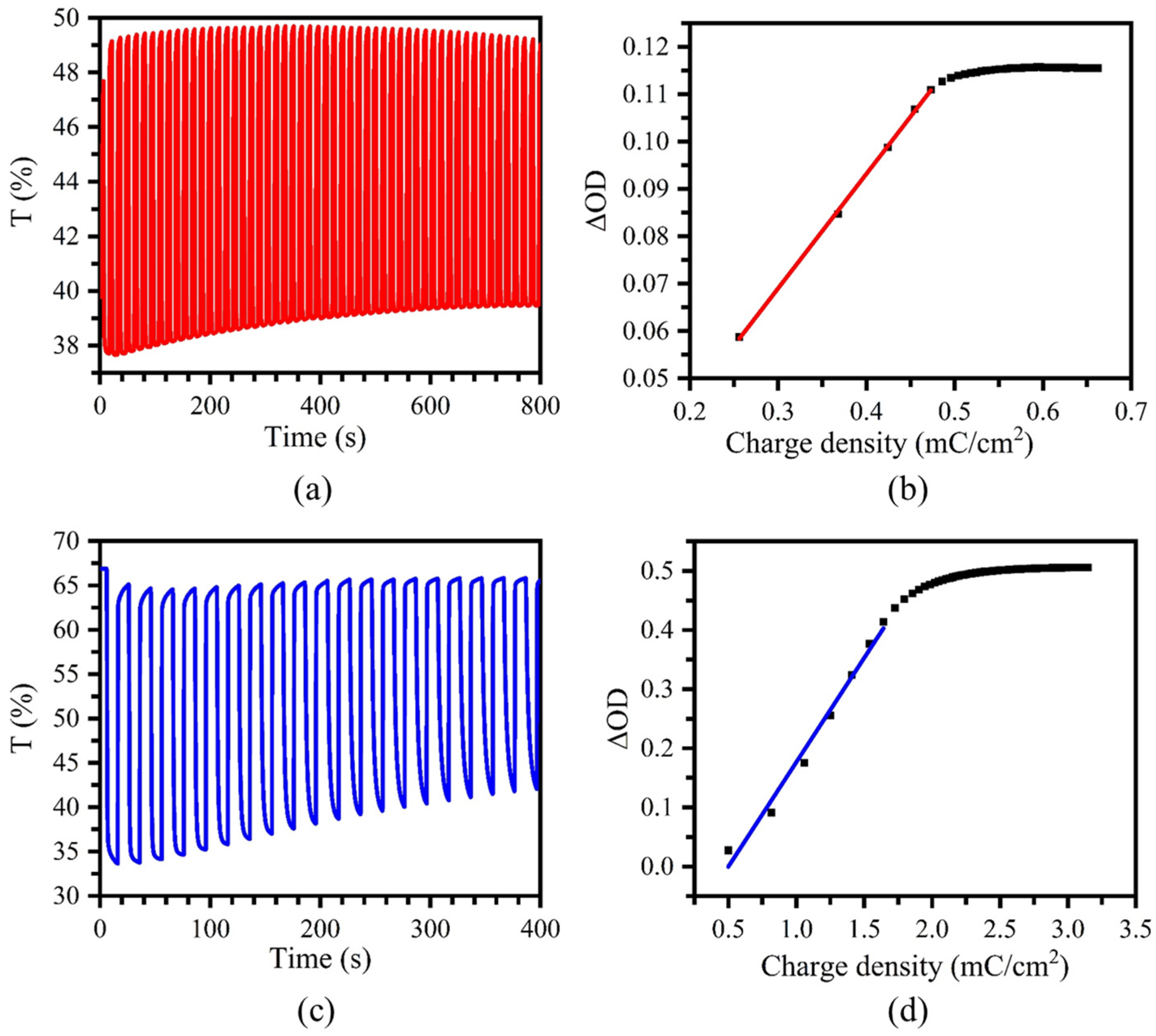
2.2. Spectroelectrochemical Properties of Electrochromic Films
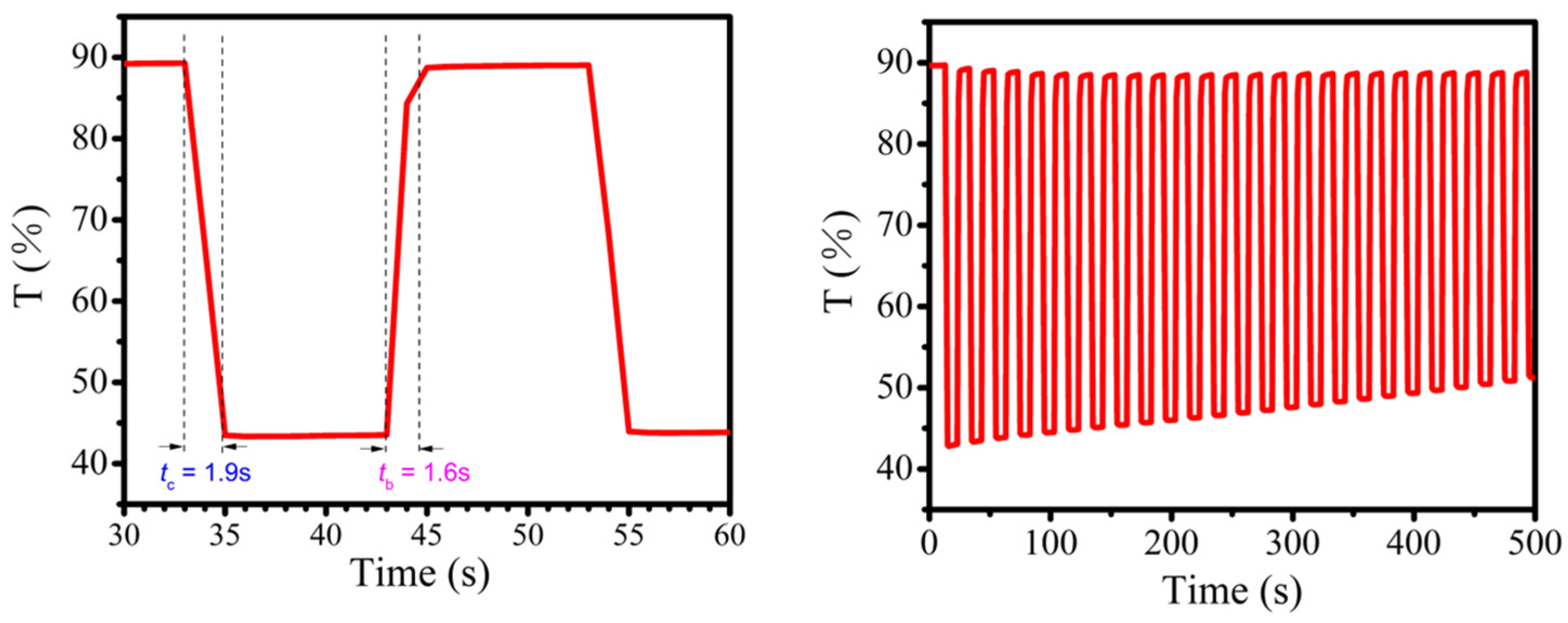
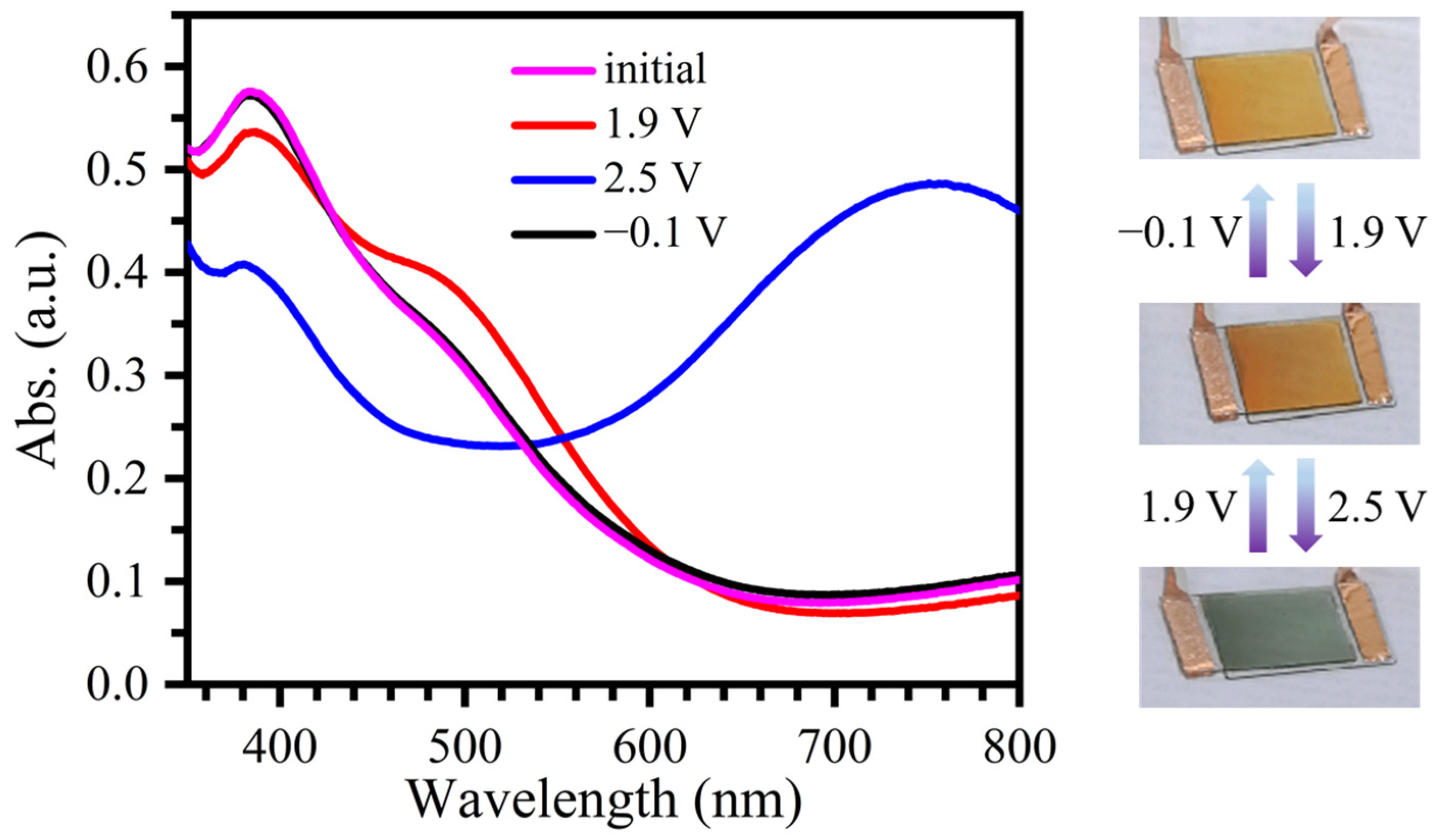
2.3. Fabrication and Characterization of Electrochromic Devices
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECDs | electrochromic devices |
| ITO | indium tin oxide |
| SEM | scanning electron microscopy |
| EDS | energy-dispersive X-ray spectroscopy |
| LMCT | ligand-to-metal charge transfer |
| tc | coloration time |
| tb | bleaching time |
| CE | coloration efficiency |
| ∆OD | change of the optical density |
| ∆Q | quantity of injected electric charges |
References
- Banasz, R.; Wałęsa-Chorab, M. Polymeric complexes of transition metal ions as electrochromic materials: Synthesis and properties. Coord. Chem. Rev. 2019, 389, 1–18. [Google Scholar] [CrossRef]
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A Review on Recent Advances in Electrochromic Devices: A Material Approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Cannavale, A.; Ayr, U.; Fiorito, F.; Martellotta, F. Smart Electrochromic Windows to Enhance Building Energy Efficiency and Visual Comfort. Energies 2020, 13, 1449. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C. Special topic on recent progress in electrochromism. Sci. China Chem. 2016, 60, 1–2. [Google Scholar] [CrossRef]
- Tong, Z.; Tian, Y.; Zhang, H.; Li, X.; Ji, J.; Qu, H.; Li, N.; Zhao, J.; Li, Y. Recent advances in multifunctional electrochromic energy storage devices and photoelectrochromic devices. Sci. China Chem. 2016, 60, 13–37. [Google Scholar] [CrossRef]
- Park, S.-I.; Quan, Y.-J.; Kim, S.-H.; Kim, H.; Kim, S.; Chun, D.-M.; Lee, C.S.; Taya, M.; Chu, W.-S.; Ahn, S.-H. A review on fabrication processes for electrochromic devices. Int. J. Precis. Eng. Manuf.-Green Technol. 2016, 3, 397–421. [Google Scholar] [CrossRef]
- Xu, J.W.; Chua, M.H.; Shah, K.W. (Eds.) Electrochromic Smart Materials: Fabrication and Applications; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Rosseinsky, D.R.; Monk, P.M.S. (Eds.) Electrochromic Materials and Devices; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Kumar, A.; Otley, M.T.; Alamar, F.A.; Zhu, Y.; Arden, B.G.; Sotzing, G.A. Solid-state electrochromic devices: Relationship of contrast as a function of device preparation parameters. J. Mater. Chem. C 2014, 2, 2510–2516. [Google Scholar] [CrossRef]
- Kline, W.M.; Lorenzini, R.G.; Sotzing, G.A. A review of organic electrochromic fabric devices. Color. Technol. 2014, 130, 73–80. [Google Scholar] [CrossRef]
- Li, X.; Perera, K.; He, J.; Gumyusenge, A.; Mei, J. Solution-processable electrochromic materials and devices: Roadblocks and strategies towards large-scale applications. J. Mater. Chem. C 2019, 7, 12761–12789. [Google Scholar] [CrossRef]
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Shao, X.; Yang, Y.; Huang, Q.; Dai, D.; Fu, H.; Gong, G.; Zhang, C.; Ouyang, M.; Li, W.; Dong, Y. Soluble polymer facilely self-grown in situ on conducting substrates at room temperature towards electrochromic applications. Dalton Trans. 2023, 52, 15440–15446. [Google Scholar] [CrossRef] [PubMed]
- Wałęsa-Chorab, M.; Skene, W.G. On-substrate polymerization—A versatile approach for preparing conjugated polymers suitable as electrochromes and for metal ion sensing. RSC Adv. 2014, 4, 19053. [Google Scholar] [CrossRef]
- Napierala, S.; Kubicki, M.; Walesa-Chorab, M. Toward Electrochromic Metallopolymers: Synthesis and Properties of Polyazomethines Based on Complexes of Transition-Metal Ions. Inorg. Chem. 2021, 60, 14011–14021. [Google Scholar] [CrossRef] [PubMed]
- Sicard, L.; Navarathne, D.; Skalski, T.; Skene, W.G. On-Substrate Preparation of an Electroactive Conjugated Polyazomethine from Solution-Processable Monomers and its Application in Electrochromic Devices. Adv. Funct. Mater. 2013, 23, 3549–3559. [Google Scholar] [CrossRef]
- Banasz, R.; Kubicki, M.; Walesa-Chorab, M. Yellow-to-brown and yellow-to-green electrochromic devices based on complexes of transition metal ions with a triphenylamine-based ligand. Dalton Trans. 2020, 49, 15041–15053. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Dong, J.; Bai, Y.; Gao, W.; Shang, S.; Wang, X.; Kuang, J.; Du, C.; Zou, Y.; et al. Two-dimensional covalent organic framework films prepared on various substrates through vapor induced conversion. Nat. Commun. 2022, 13, 1411. [Google Scholar] [CrossRef]
- Stockhausen, V.; Ghilane, J.; Martin, P.; Trippe-Allard, G.; Randriamahazaka, H.; Lacroix, J.C. Grafting Oligothiophenes on Surfaces by Diazonium Electroreduction: A Step toward Ultrathin Junction with Well-Defined Metal/Oligomer Interface. J. Am. Chem. Soc. 2009, 131, 14920–14927. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Schaming, D.; Martin, P.; Lacroix, J.C. Highly Resolved Nanostructured PEDOT on Large Areas by Nanosphere Lithography and Electrodeposition. ACS Appl. Mater. Interfaces 2015, 7, 21673–21681. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Sun, X.; Lafolet, F.; Audibert, J.F.; Miomandre, F.; Lemercier, G.; Loiseau, F.; Lacroix, J.C. Unprecedented Self-Organized Monolayer of a Ru(II) Complex by Diazonium Electroreduction. J. Am. Chem. Soc. 2016, 138, 9381–9384. [Google Scholar] [CrossRef]
- Maldonado, S.; Smith, T.J.; Williams, R.D.; Morin, S.; Barton, E.; Stevenson, K.J. Surface Modification of Indium Tin Oxide via Electrochemical Reduction of Aryldiazonium Cations. Langmuir 2006, 22, 2884–2891. [Google Scholar] [CrossRef]
- Kobak, R.Z.U.; Akyüz, D.; Koca, A. Substituent effects to the electrochromic behaviors of electropolymerized metallophthalocyanine thin films. J. Solid State Electrochem. 2016, 20, 1311–1321. [Google Scholar] [CrossRef]
- Patra, A.; Bendikov, M.; Chand, S. Poly(3,4-ethylenedioxyselenophene) and its derivatives: Novel organic electronic materials. Acc. Chem. Res. 2014, 47, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Nad, S.; Paul, B.; Malik, S. Effect of substituents of naphthalene diimide derivatives on electrochromic behaviours observed in proto-type devices. J. Electroanal. Chem. 2023, 946, 117729. [Google Scholar] [CrossRef]
- Stockhausen, V.; Trippé-Allard, G.; Van Quynh, N.; Ghilane, J.; Lacroix, J.-C. Grafting π-Conjugated Oligomers Incorporating 3,4-Ethylenedioxythiophene (EDOT) and Thiophene Units on Surfaces by Diazonium Electroreduction. J. Phys. Chem. C 2015, 119, 19218–19227. [Google Scholar] [CrossRef]
- Brooksby, P.A.; Downard, A.J. Electrochemical and Atomic Force Microscopy Study of Carbon Surface Modification via Diazonium Reduction in Aqueous and Acetonitrile Solutions. Langmuir 2004, 20, 5038–5045. [Google Scholar] [CrossRef]
- Dai, J.; Zu, Y.; Yang, Y.; Yang, C.; Yu, Y.; Zhang, S.; Hou, J. Thermal Cross-Linking preparation of Aqueous-Based cathodic films for enhanced stability in organic electrochromic devices. Chem. Eng. J. 2024, 487, 150473. [Google Scholar] [CrossRef]
- Ling, H.; Zhang, J.; Wang, Y.; Zeng, X. One-step achieving high performance all-solid-state and all-in-one flexible electrochromic supercapacitor by polymer dispersed electrochromic device strategy. J. Colloid Interface Sci. 2024, 665, 969–976. [Google Scholar] [CrossRef]
- Rim, M.; Kang, D.G.; Kim, W.; Jang, J.; Oh, M.; Wi, Y.; Park, S.; Tran, D.T.; Ha, M.; Jeong, K.U. Encryptable Electrochromic Smart Windows: Uniaxially Oriented and Polymerized Hierarchical Nanostructures Constructed by Self-Assembly of Tetrathiafulvalene-Based Reactive Mesogens. ACS Nano 2023, 17, 14750–14760. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-B.; Deng, H.-T.; Zhang, L.-Y.; Wei, J.-H.; Dai, F.-R.; Chen, Z.-N. Continuous In-Situ Polymerization of Complex-Based Films for High-Performance Electrochromic Devices. Molecules 2025, 30, 1099. https://doi.org/10.3390/molecules30051099
Liu Y-B, Deng H-T, Zhang L-Y, Wei J-H, Dai F-R, Chen Z-N. Continuous In-Situ Polymerization of Complex-Based Films for High-Performance Electrochromic Devices. Molecules. 2025; 30(5):1099. https://doi.org/10.3390/molecules30051099
Chicago/Turabian StyleLiu, Yang-Bo, Hao-Tian Deng, Li-Yi Zhang, Jing-Hao Wei, Feng-Rong Dai, and Zhong-Ning Chen. 2025. "Continuous In-Situ Polymerization of Complex-Based Films for High-Performance Electrochromic Devices" Molecules 30, no. 5: 1099. https://doi.org/10.3390/molecules30051099
APA StyleLiu, Y.-B., Deng, H.-T., Zhang, L.-Y., Wei, J.-H., Dai, F.-R., & Chen, Z.-N. (2025). Continuous In-Situ Polymerization of Complex-Based Films for High-Performance Electrochromic Devices. Molecules, 30(5), 1099. https://doi.org/10.3390/molecules30051099






