Performance and Mechanism of Hydrolyzed Keratin for Hair Photoaging Prevention
Abstract
1. Introduction
2. Result and Discussion
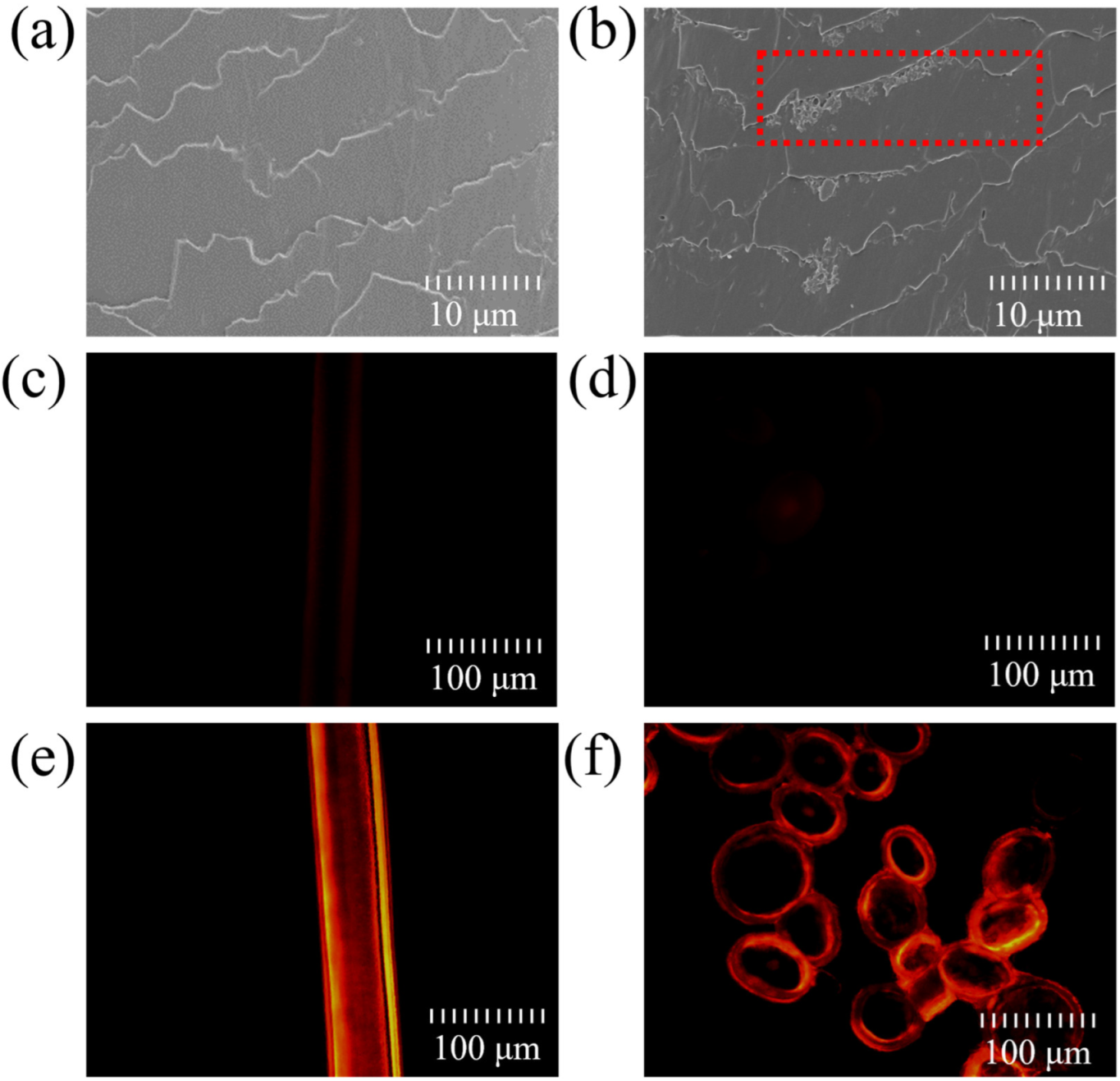
2.1. Deposition and Penetration Behavior of Hydrolyzed Keratin
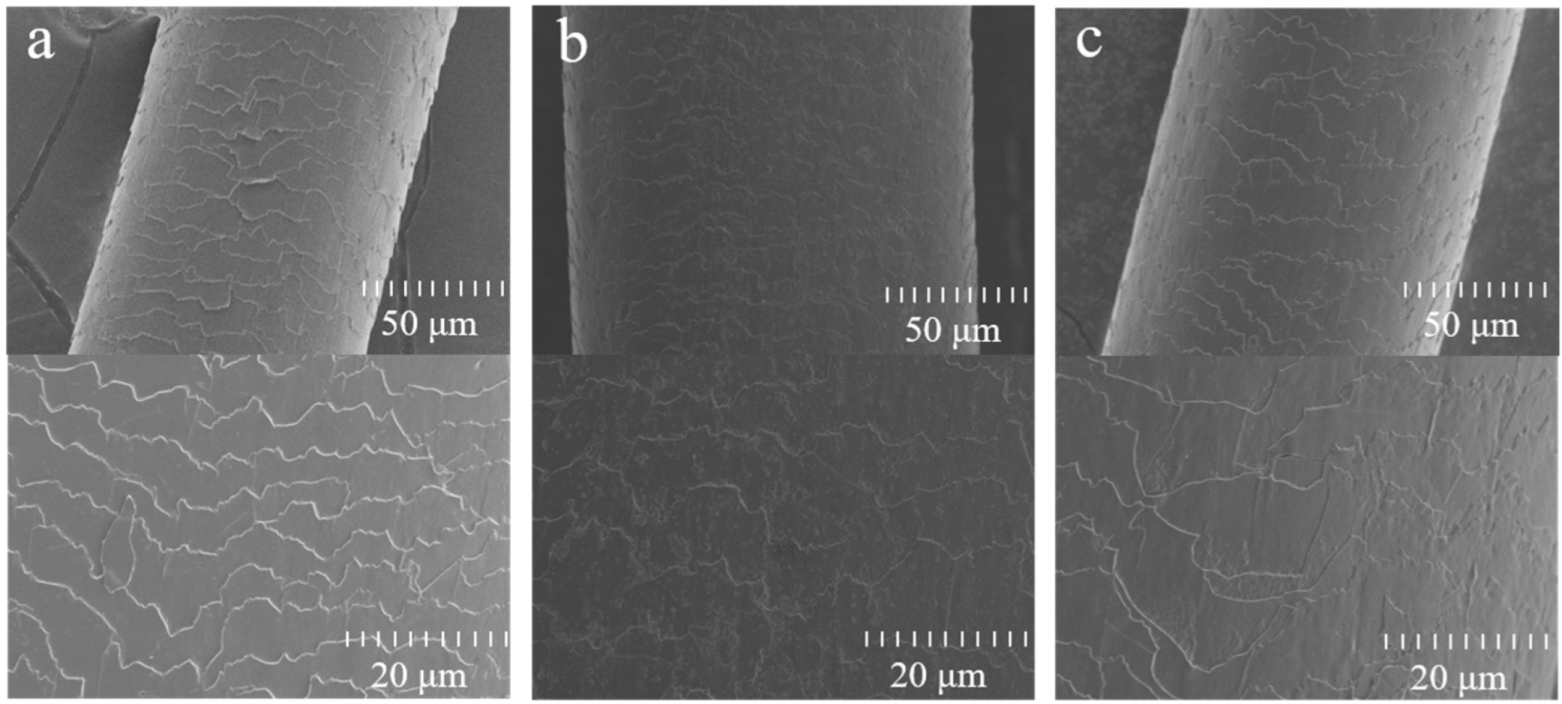
2.2. UV Protection Performance of Hydrolyzed Keratin
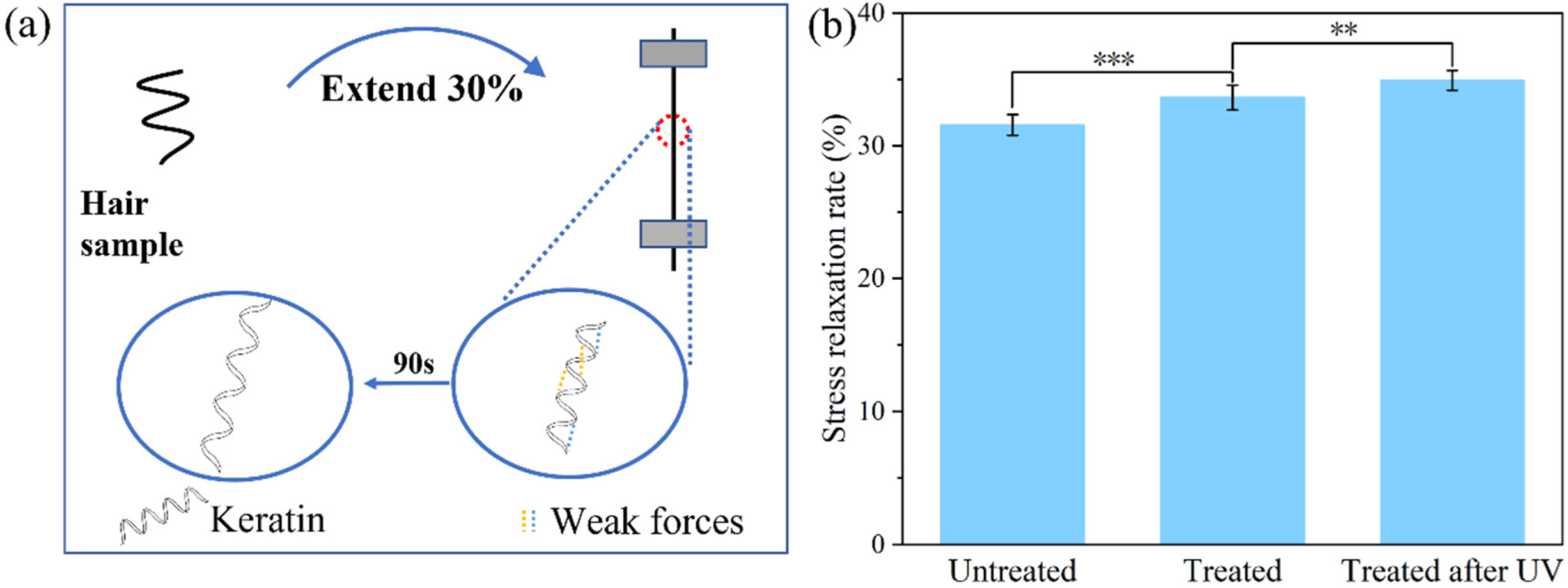
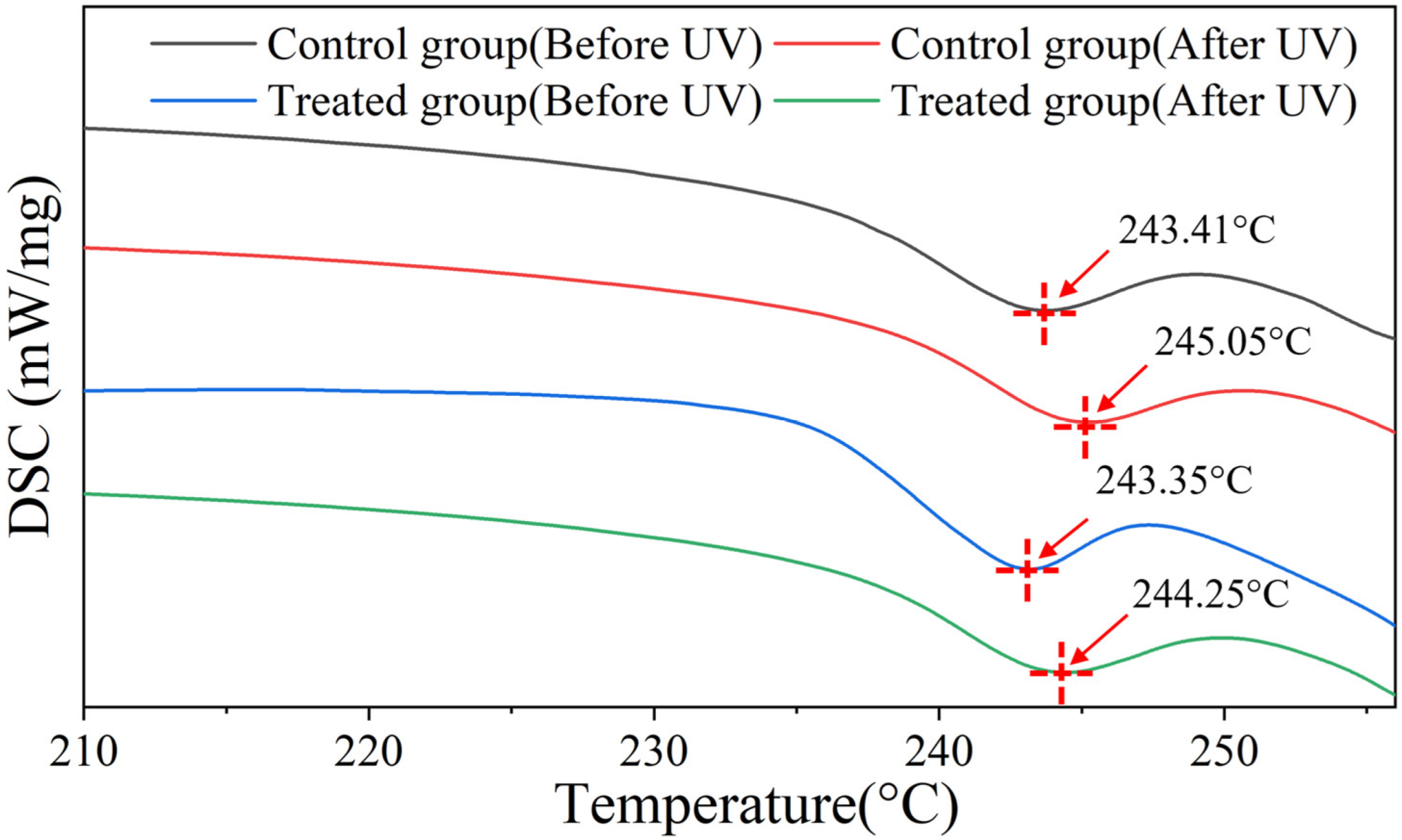
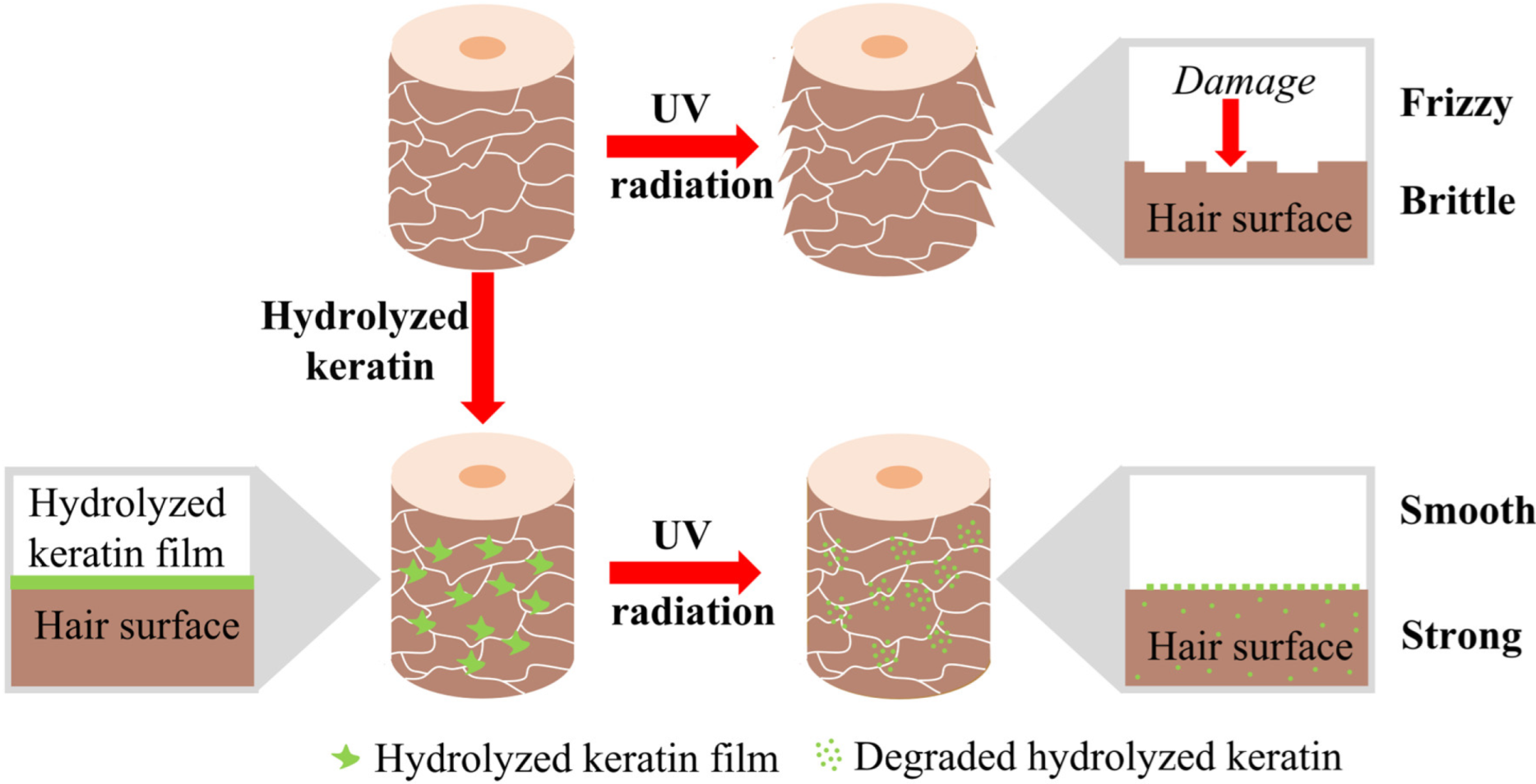
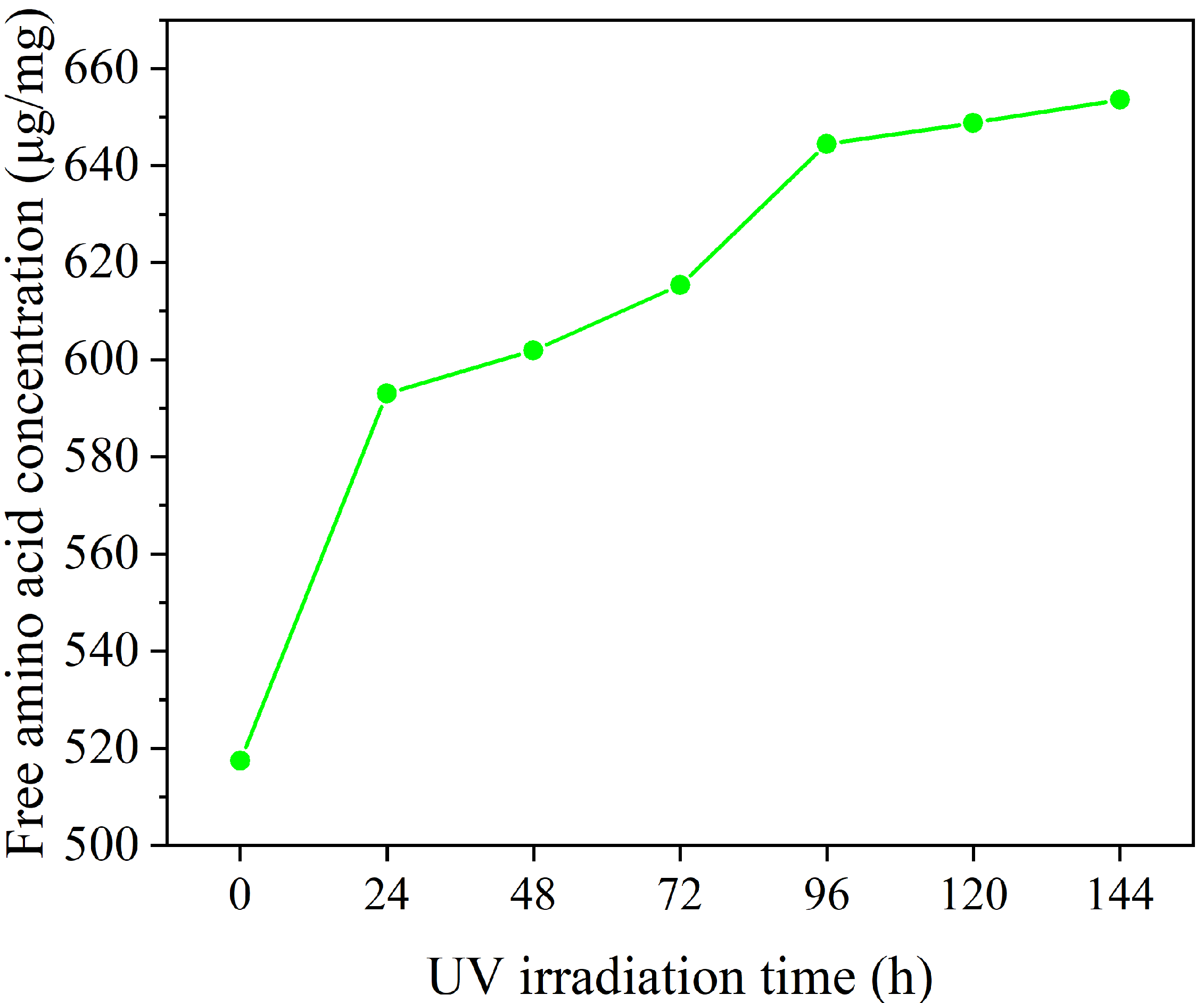
2.3. Mechanism Investigation
3. Experimental Method
3.1. Materials
3.2. SEM Characterization
3.3. Hydrolyzed Keratin Permeability Test
3.4. Tensile Tests
3.5. Stress Relaxation
3.6. DSC
3.7. Hydrolyzed Keratin Degradation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nogueira, A.C.S.; Nakano, A.K.; Joekes, I. Impairment of hair mechanical properties by sun exposure and bleaching treatments. J. Cosmet. Sci. 2004, 55, 533–537. [Google Scholar]
- Imai, T. The influence of hair bleach on the ultrastructure of human hair with special reference to hair damage. Okajimas Folia Anat. Jpn. 2011, 88, 1–9. [Google Scholar] [CrossRef]
- Dyer, J.M.; Bell, F.; Koehn, H.; Vernon, J.A.; Cornellison, C.D.; Clerens, S.; Harland, D.P. Redox proteomic evaluation of bleaching and alkali damage in human hair. Int. J. Cosmet. Sci. 2013, 35, 555–561. [Google Scholar] [CrossRef]
- Contreras, F.; Ermolenkov, A.; Kurouski, D. Infrared analysis of hair dyeing and bleaching history. Anal. Methods 2020, 12, 3741–3747. [Google Scholar] [CrossRef]
- Hyun, J.W. Analysis of Morphological changes on the hair surface by heat perm treatment method. J. Korean Soc. Cosmetol. 2023, 29, 449–455. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Estibalitz, F.; Barba, C.; Alonso, C.; Martí, M.; Parra, J.L.; Coderch, L. Photodamage determination of human hair. J. Photochem. Photobiol. B Biol. 2012, 106, 101–106. [Google Scholar]
- Robbins, C.R.; Bahl, M.K. Analysis of hair by electron spectroscopy for chemical analysis. J. Cosmet. Sci. 1984, 35, 379–390. [Google Scholar]
- Ji, J.H.; Park, T.S.; Lee, H.J.; Kim, Y.-D.; Pi, L.-Q.; Jin, X.-H.; Lee, W.-S. The ethnic differences of the damage of hair and integral hair lipid after ultra violet radiation. Ann. Dermatol. 2013, 25, 54–60. [Google Scholar] [CrossRef]
- Hoting, E.; Zimmermann, M.; Höcker, H. Photochemical alterations in human hair. II: Analysis of melanin. J. Cosmet. Sci. 1995, 46, 181–190. [Google Scholar]
- Richena, M.; Rezende, C.A. Effect of photodamage on the outermost cuticle layer of human hair. J. Photochem. Photobiol. B Biol. 2015, 153, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.E.; Kilburn, M.; Schroeder, M.; Martin, B.G.H.; Hawes, C.; Marsh, J.M.; Grovenor, C.R.M. Copper and calcium uptake in colored hair. Int. J. Cosmet. Sci. 2009, 60, 337–345. [Google Scholar] [CrossRef]
- Tang, Y.; Dyer, J.M.; Deb-Choudhury, S.; Li, Q. Trace metal ions in hair from frequent hair dyers in China and the associated effects on photo-oxidative damage. J. Photochem. Photobiol. B Biol. 2016, 156, 35–40. [Google Scholar] [CrossRef]
- Naqvi, K.R.; Marsh, J.M.; Godfrey, S.; Davis, M.G.; Flagler, M.J.; Hao, J.; Chechik, V. The role of chelants in controlling Cu (II)-induced radical chemistry in oxidative hair colouring products. Int. J. Cosmet. Sci. 2013, 35, 41–49. [Google Scholar] [CrossRef]
- Michelli, F.D.; André, R.B.; Maria, V.R.V. Effects of solar radiation on hair and photoprotection. J. Photochem. Photobiol. B Biol. 2015, 153, 240–246. [Google Scholar]
- Schlosser, A. Silicones used in permanent and semi-permanent hair dyes to reduce the fading and color change process of dyed hair occurred by wash-out or UV radiation. J. Cosmet. Sci. 2004, 55, S123–S131. [Google Scholar] [CrossRef]
- Pande, C.M.; Albrecht, L.; Yang, B. Hair photoprotection by dyes. J. Cosmet. Sci. 2001, 52, 377–389. [Google Scholar]
- Fernández, E.; Martínez-Teipel, B.; Armengol, R.; Barba, C.; Coderch, L. Efficacy of antioxidants in human hair. J. Photochem. Photobiol. B Biol. 2012, 117, 146–156. [Google Scholar] [CrossRef]
- Dario, M.F.; Pahl, R.; de Castro, J.R.; de Lima, F.S.; Kaneko, T.M.; Pinto, C.A.; Baby, A.R.; Velasco, M.V.R. Efficacy of Punica granatum L. hydroalcoholic extract on properties of dyed hair exposed to UVA radiation. J. Photochem. Photobiol. B Biol. 2013, 120, 142–147. [Google Scholar] [CrossRef]
- Davis, S.L.; Marsh, J.M.; Kelly, C.P.; Li, L.; Tansky, C.S.; Fang, R.; Simmonds, M.S.J. Protection of hair from damage induced by ultraviolet irradiation using tea (Camellia sinensis) extracts. J. Cosmet. Dermatol. 2022, 21, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Sahib, S.; Jungman, E.; Aquis Hairsciences Inc. Composition for Improving Hair Health. US2020/0069551A1, 5 March 2020. [Google Scholar]
- Cruz, C.F.; Azoia, N.G.; Matamá, T.; Cavaco-Paulo, A. Peptide-protein interactions within human hair keratins. Int. J. Biol. Macromol. 2017, 101, 805–814. [Google Scholar] [CrossRef]
- Tinoco, A.; Gonçalves, J.; Silva, C.; Loureiro, A.; Gomes, A.C.; Cavaco-Paulo, A.; Ribeiro, A. Keratin-based particles for protection and restoration of hair properties. Int. J. Cosmet. Sci. 2018, 40, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Malinauskyte, E.; Shrestha, R.; Cornwell, P.A.; Gourion-Arsiquaud, S.; Hindley, M. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int. J. Cosmet. Sci. 2021, 43, 26–37. [Google Scholar] [CrossRef]
- Wiesche, E.S.; Körner, A.; Schäfer, K.; Wortmann, F.J. Prevention of hair surface aging. J. Cosmet. Sci. 2011, 62, 237–249. [Google Scholar]
- Camargo, F.B., Jr.; Minami, M.M.; Rossan, M.R.; Magalhães, W.V.; Porto Ferreira, V.T.; Maia Campos, P.M.B.G. Prevention of chemically induced hair damage by means of treatment based on proteins and polysaccharides. J. Cosmet. Dermatol. 2022, 21, 827–835. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, R.; Lvov, Y. Halloysite/keratin nanocomposite for human hair photoprotection coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yamazaki, J.; Okita, N.; Shimotori, M.; Igarashi, K.; Sano, T. Mechanism of cuticle hole development in human hair due to UV-radiation exposure. Cosmetics 2018, 5, 24. [Google Scholar] [CrossRef]
- Barba, C.; Scott, S.; Roddick-Lanzilotta, A.; Kelly, R.; Manich, A.M.; Parra, J.L.; Coderch, L. Restoring important hair properties with wool keratin proteins and peptides. Fibers Polym. 2010, 11, 1055–1061. [Google Scholar] [CrossRef]
- Chandrashekara, M.N.; Ranganathaiah, C. Chemical and photochemical degradation of human hair: A free-volume microprobe study. J. Photoch. Photobio. B 2010, 101, 286–294. [Google Scholar] [CrossRef]
- Fan, J.; Yu, W.; Bian, M.; Yue, Z.; Wang, J.; Chang, K. Study on the efficacy and mechanism of an amino acid combination in hair care. China Surfactant Deterg. Cosmet. 2024, 54, 1059–1068. [Google Scholar]
- Masayuki, O.; Kazutaka, I.; Noriyuki, T.; Aoyagi, S. Investigation of the damage on the outermost hair surface using ToF-SIMS and XPS. Surf. Interface Anal. 2012, 44, 736–739. [Google Scholar]
- Flinders, B.; Cuypers, E.; Zeijlemaker, H.; Tytgat, J.; Heeren, R.M.A. Preparation of longitudinal sections of hair samples for the analysis of cocaine by MALDI-MS/MS and TOF-SIMS imaging. Drug Test. Anal. 2015, 7, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Kempson, I.M.; Skinner, W.M. ToF-SIMS analysis of elemental distributions in human hair. Sci. Total Environ. 2005, 338, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.L.; Harkey, M.R.; Chihong, Z.; Jones, R.T.; Jacob, P. Incorporation of isotopically labeled cocaine and metabolites into human hair: 1. dose-response relationships. J. Anal. Toxicol. 1996, 20, 1–12. [Google Scholar] [CrossRef]
- Brunner, A.; Minamitake, Y.; Göpferich, A. Labelling peptides with fluorescent probes for incorporation into degradable polymers. Eur. J. Pharm. Biopharm. 1998, 45, 265–273. [Google Scholar] [CrossRef]
- Antunes, E.; Cruz, C.F.; Azoia, N.G.; Cavaco-Paulo, A. Insights on the mechanical behavior of keratin fibrils. Int. J. Biol. Macromol. 2016, 89, 477–483. [Google Scholar] [CrossRef]
- Wortmann, F.J.; Quadflieg, J.M.; Wortmann, G. Comparing hair tensile testing in the wet and the dry state: Possibilities and limitations for detecting changes of hair properties due to chemical and physical treatments. Int. J. Cosmet. Sci. 2022, 44, 421–430. [Google Scholar] [CrossRef]
- Lima, C.R.R.d.C.; Machado, L.D.B.; Velasco, M.V.R.; Matos, J.D.R. DSC measurements applied to hair studies. J. Therm. Anal. Calorim. 2018, 132, 1429–1437. [Google Scholar] [CrossRef]
- Gu, H.j.; Peng, L.; Jiang, W.C.; Tan, Y.-M.; Zhou, G.-J.; Kan, H.-D.; Chen, R.-J.; Zou, Y. Impact of solar ultraviolet radiation on daily outpatient visits of atopic dermatitis in Shanghai, China. Environ. Sci. Pollut. Res. 2021, 28, 18081–18088. [Google Scholar] [CrossRef]
- Abernathy, D.G.; Spedding, G.; Starcher, B. Analysis of protein and total usable nitrogen in beer and wine using a microwell ninhydrin assay. J. Inst. Brew. 2009, 115, 122–127. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Wu, L.; Wang, J.; Bian, X.; Chen, C.; Chang, K. Performance and Mechanism of Hydrolyzed Keratin for Hair Photoaging Prevention. Molecules 2025, 30, 1182. https://doi.org/10.3390/molecules30051182
Fan J, Wu L, Wang J, Bian X, Chen C, Chang K. Performance and Mechanism of Hydrolyzed Keratin for Hair Photoaging Prevention. Molecules. 2025; 30(5):1182. https://doi.org/10.3390/molecules30051182
Chicago/Turabian StyleFan, Jiayi, Lei Wu, Jing Wang, Xiaoying Bian, Chongchong Chen, and Kuan Chang. 2025. "Performance and Mechanism of Hydrolyzed Keratin for Hair Photoaging Prevention" Molecules 30, no. 5: 1182. https://doi.org/10.3390/molecules30051182
APA StyleFan, J., Wu, L., Wang, J., Bian, X., Chen, C., & Chang, K. (2025). Performance and Mechanism of Hydrolyzed Keratin for Hair Photoaging Prevention. Molecules, 30(5), 1182. https://doi.org/10.3390/molecules30051182







