The Pharmaceutical and Pharmacological Potential Applications of Bilosomes as Nanocarriers for Drug Delivery
Abstract
1. Introduction
2. Literature Search Methodology
3. Nanovesicular Drug Delivery Systems: A Comparative Short Review
4. Bilosomes and Bilosome Technologies
4.1. Bilosome Formulations—Composition and Types
4.2. Methods of Preparation and Industrial Potential of Bilosomes
5. Administration Routes and Therapeutic Applications
5.1. Transdermal Application
5.2. Vaccines
5.3. Oral Application
5.4. Intranasal Administration
5.5. Ocular Application
5.6. Buccal Application
5.7. Vaginal Application
6. Limitations of the Use of Bilosomes
7. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Bansal, M.; Shaw, J. Nanosizing techniques for improving bioavailability of drugs. J. Control. Release 2017, 260, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Sindhuja, D.; Ganesh, G.N.K. Nanoparticulate targeted drug delivery systems—A review. Int. J. Res. Pharm. Sci. 2020, 11, 2505–2518. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Ren, Y.; Nie, L.; Zhu, S.; Zhang, X. Nanovesicles-Mediated Drug Delivery for Oral Bioavailability Enhancement. Int. J. Nanomed. 2022, 17, 4861–4877. [Google Scholar] [CrossRef]
- Gangavarapu, A.; Tapia-Lopez, L.V.; Sarkar, B.; Pena-Zacarias, J.; Badruddoza, A.Z.M.; Nurunnabi, M. Lipid nanoparticles for enhancing oral bioavailability. Nanoscale 2024, 16, 18319–18338. [Google Scholar] [CrossRef]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The Influence of Nanoparticle Properties on Oral Bioavailability of Drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef]
- Saadh, M.J.; Mustafa, M.A.; Kumar, S.; Gupta, P.; Pramanik, A.; Rizaev, J.A.; Shareef, H.K.; Alubiady, M.H.; Al-Abdeen, S.H.; Shakier, H.G.; et al. Advancing therapeutic efficacy: Nanovesicular delivery systems for medicinal plant-based therapeutics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 7229–7254. [Google Scholar] [CrossRef]
- Mühlberg, E.; Burtscher, M.; Umstätter, F.; Fricker, G.; Mier, W.; Uhl, P. Trends in liposomal nanocarrier strategies for the oral delivery of biologics. Nanomedicine 2021, 16, 1813–1832. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Mirtaleb, M.S.; Shahraky, M.K.; Ekrami, E.; Mirtaleb, A. Advances in biological nano-phospholipid vesicles for transdermal delivery: A review on applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102331. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Salehi Moghaddam, Z.; Hejabi, F.; Heidari, F.; Quazi, S.; Farasati Far, B. Niosomes: A novel targeted drug delivery system for cancer. Med. Oncol. 2022, 39, 240. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Arshad, R.; Ur Rehman, A.; Ahmed, N.; Akhtar, H. Recent Developments in Oral Delivery of Vaccines Using Nanocarriers. Vaccines 2023, 11, 490. [Google Scholar] [CrossRef]
- Sharma, S.; Garg, A.; Agrawal, R.; Chopra, H.; Pathak, D. A Comprehensive Review on Niosomes as a Tool for Advanced Drug Delivery. Pharm. Nanotechnol. 2024, 12, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sui, Z.; Jiang, Q.; Jiang, Y. Functional Evaluation of Niosomes Utilizing Surfactants in Nanomedicine Applications. Int. J. Nanomed. 2024, 19, 10283–10305. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Marzi, M.; Abdulabbas, H.T.; Jasim, S.A.; Kouhpayeh, S.A.; Barbaresi, S.; Ahmadi, S.; Ghasemian, A. Bilosomes as Nanocarriers for the Drug and Vaccine Delivery against Gastrointestinal Infections: Opportunities and Challenges. J. Funct. Biomater. 2023, 14, 453. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Ahmad, J.; Singhal, M.; Amin, S.; Rizwanullah, M.; Akhter, S.; Kamal, M.A.; Haider, N.; Midoux, P.; Pichon, C. Bile Salt Stabilized Vesicles (Bilosomes): A Novel Nano-Pharmaceutical Design for Oral Delivery of Proteins and Peptides. Curr. Pharm. Des. 2017, 23, 1575–1588. [Google Scholar] [CrossRef]
- Mondal, D.; Mandal, R.P.; De, S. Addressing the Superior Drug Delivery Performance of Bilosomes—A Microscopy and Fluorescence Study. ACS Appl. Bio Mater. 2022, 5, 3896–3911. [Google Scholar] [CrossRef]
- Pradnya, P.-S.; Supriya, L.; Riya, C.; Drushti, R. Bilosomes: Superior Vesicular Carriers. Curr. Drug Ther. 2020, 15, 312–320. [Google Scholar] [CrossRef]
- Abdel-moneum, R.; Abdel-Rashid, R.S. Bile salt stabilized nanovesicles as a promising drug delivery technology: A general overview and future perspectives. J. Drug Deliv. Sci. Technol. 2023, 79, 104057. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Rathnanand, M.; Tippavajhala, V.K. Unlocking the Potential of Bilosomes and Modified Bilosomes: A Comprehensive Journey into Advanced Drug Delivery Trends. AAPS PharmSciTech 2023, 24, 238. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Rheima, A.M.; Kadhim, M.M.; Ahmed, N.N.; Mohammed, S.H.; Abbas, F.H.; Abed, Z.T.; Mahdi, Z.M.; Abbas, Z.S.; Hachim, S.K.; et al. An overview of nanoparticles in drug delivery: Properties and applications. S. Afr. J. Chem. Eng. 2023, 46, 233–270. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Omran, S.; Hazzah, H.A.; Abdallah, O.Y. Anionic versus cationic bilosomes as oral nanocarriers for enhanced delivery of the hydrophilic drug risedronate. Int. J. Pharm. 2019, 564, 410–425. [Google Scholar] [CrossRef]
- Nemati, M.; Fathi-Azarbayjani, A.; Al-Salami, H.; Roshani Asl, E.; Rasmi, Y. Bile acid-based advanced drug delivery systems, bilosomes and micelles as novel carriers for therapeutics. Cell Biochem. Funct. 2022, 40, 623–635. [Google Scholar] [CrossRef]
- di Gregorio, M.C.; Travaglini, L.; Del Giudice, A.; Cautela, J.; Pavel, N.V.; Galantini, L. Bile Salts: Natural Surfactants and Precursors of a Broad Family of Complex Amphiphiles. Langmuir 2019, 35, 6803–6821. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Functional, Diagnostic and Therapeutic Aspects of Bile. Clin. Exp. Gastroenterol. 2022, 15, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Kecman, S.; Škrbić, R.; Badnjevic Cengic, A.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Potentials of human bile acids and their salts in pharmaceutical nano delivery and formulations adjuvants. Technol. Health Care 2020, 28, 325–335. [Google Scholar] [CrossRef]
- Kovacevic, B.; Jones, M.; Ionescu, C.; Walker, D.; Wagle, S.; Chester, J.; Foster, T.; Brown, D.; Mikov, M.; Mooranian, A.; et al. The emerging role of bile acids as critical components in nanotechnology and bioengineering: Pharmacology, formulation optimizers and hydrogel-biomaterial applications. Biomaterials 2022, 283, 121459. [Google Scholar] [CrossRef] [PubMed]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef]
- Gvoic, M.; Vukmirovic, S.; Al-Salami, H.; Mooranian, A.; Mikov, M.; Stankov, K. Bile acids as novel enhancers of CNS targeting antitumor drugs: A comprehensive review. Pharm. Dev. Technol. 2021, 26, 617–633. [Google Scholar] [CrossRef]
- Elnaggar, Y.S. Multifaceted applications of bile salts in pharmacy: An emphasis on nanomedicine. Int. J. Nanomed. 2015, 10, 3955–3971. [Google Scholar] [CrossRef]
- Imam, S.S.; Gilani, S.J.; Zafar, A.; Jumah, M.N.B.; Alshehri, S. Formulation of Miconazole-Loaded Chitosan-Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics 2023, 15, 581. [Google Scholar] [CrossRef]
- Karunakaran, B.; Gupta, R.; Patel, P.; Salave, S.; Sharma, A.; Desai, D.; Benival, D.; Kommineni, N. Emerging Trends in Lipid-Based Vaccine Delivery: A Special Focus on Developmental Strategies, Fabrication Methods, and Applications. Vaccines 2023, 11, 661. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Kesharwani, P.; Mody, N.; Jain, A.; Sharma, S. Formulation by design (FbD): An emerging approach to design vesicular nanocarriers. In Micro-and Nanotechnologies-Based Product Development; CRC Press: Boca Raton, FL, USA, 2021; pp. 15–31. [Google Scholar]
- Jain, A.; Hurkat, P.; Jain, S.K. Development of liposomes using formulation by design: Basics to recent advances. Chem. Phys. Lipids 2019, 224, 104764. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, B.; Porfire, A.; Muntean, D.M.; Vlase, L.; Lupuţ, L.; Licarete, E.; Sesarman, A.; Alupei, M.C.; Banciu, M.; Achim, M.; et al. Optimization of prednisolone-loaded long-circulating liposomes via application of Quality by Design (QbD) approach. J. Liposome Res. 2018, 28, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Cheng, M.; Hu, K.; Feng, J.; Tu, L. Vesicular drug delivery systems for oral absorption enhancement. Chin. Chem. Lett. 2024, 35, 109129. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Raina, N.; Rani, R.; Thakur, V.K.; Gupta, M. New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective. ACS Omega 2023, 8, 19145–19167. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Özcan Bülbül, E.; Okur, M.E.; Karantas, I.D.; Üstündağ Okur, N. The Application of Nanogels as Efficient Drug Delivery Platforms for Dermal/Transdermal Delivery. Gels 2023, 9, 753. [Google Scholar] [CrossRef]
- Richard, C.; Cassel, S.; Blanzat, M. Vesicular systems for dermal and transdermal drug delivery. RSC Adv. 2020, 11, 442–451. [Google Scholar] [CrossRef]
- Research and Markets. Skin Care Products—Global Strategic Business Report. Available online: https://www.researchandmarkets.com/reports/338704/skin_care_products_global_strategic_business (accessed on 3 April 2024).
- Cheng, T.; Tai, Z.; Shen, M.; Li, Y.; Yu, J.; Wang, J.; Zhu, Q.; Chen, Z. Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System. Pharmaceutics 2023, 15, 2165. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Bilosomes as a novel carrier for the cutaneous delivery for dapsone as a potential treatment of acne: Preparation, characterization and in vivo skin deposition assay. J. Liposome Res. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Abbas, H.; El Sayed, N.S.; Ali, M.E.; Elsheikh, M.A. Integrated lecithin-bile salt nanovesicles as a promising approach for effective skin delivery of luteolin to improve UV-induced skin damage in Wistar Albino rats. Colloids Surf. B Biointerfaces 2022, 211, 112299. [Google Scholar] [CrossRef] [PubMed]
- Zewail, M.; Gaafar, P.M.E.; Youssef, N.; Ali, M.E.; Ragab, M.F.; Kamal, M.F.; Noureldin, M.H.; Abbas, H. Novel Siprulina platensis Bilosomes for Combating UVB Induced Skin Damage. Pharmaceuticals 2022, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Alsofany, J.M.; El-Nabarawi, M.A. Clove Oil Endorsed Transdermal Flux of Dronedarone Hydrochloride Loaded Bilosomal Nanogel: Factorial Design, In vitro Evaluation and Ex vivo Permeation. AAPS PharmSciTech 2022, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.M. Exploitation of transdermal nanobilosomal gel platforms for ameliorating anti-diabetic activity of empagliflozin following I-optimal design. J. Drug Deliv. Sci. Technol. 2023, 84, 104455. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ali, A.A.; Khalil, N.M.; Elsisi, A.A. Evaluation of Metformin Hydrochloride Tailoring Bilosomes as an Effective Transdermal Nanocarrier. Int. J. Nanomed. 2022, 17, 1185–1201. [Google Scholar] [CrossRef]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. Transdermal Delivery of Ondansetron Hydrochloride via Bilosomal Systems: In Vitro, Ex Vivo, and In Vivo Characterization Studies. AAPS PharmSciTech 2018, 19, 2276–2287. [Google Scholar] [CrossRef]
- Zafar, A.; Imam, S.S.; Alruwaili, N.K.; Yasir, M.; Alsaidan, O.A.; Alshehri, S.; Ghoneim, M.M.; Khalid, M.; Alquraini, A.; Alharthi, S.S. Formulation and Evaluation of Topical Nano-Lipid-Based Delivery of Butenafine: In Vitro Characterization and Antifungal Activity. Gels 2022, 8, 133. [Google Scholar] [CrossRef]
- Mosallam, S.; Sheta, N.M.; Elshafeey, A.H.; Abdelbary, A.A. Fabrication of Highly Deformable Bilosomes for Enhancing the Topical Delivery of Terconazole: In Vitro Characterization, Microbiological Evaluation, and In Vivo Skin Deposition Study. AAPS PharmSciTech 2021, 22, 74. [Google Scholar] [CrossRef]
- Albash, R.; El-Nabarawi, M.A.; Refai, H.; Abdelbary, A.A. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: In-vitro characterization, ex-vivo permeation and in-vivo assessment. Int. J. Nanomed. 2019, 14, 6555–6574. [Google Scholar] [CrossRef]
- Almutairy, B.K.; Khafagy, E.S.; Aldawsari, M.F.; Alshetaili, A.; Alotaibi, H.F.; Abu Lila, A.S. Tailoring of Bilosomal Nanogel for Augmenting the Off-Label Use of Sildenafil Citrate in Pediatric Pulmonary Hypertension. ACS Omega 2024, 9, 19536–19547. [Google Scholar] [CrossRef]
- Peddapalli, H.; Radha, G.V.; Chinnaiyan, S.K. Formulation optimization and PK/PD evaluation of novel valsartan bilosomes enhancing transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105400. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Sideek, S.A.; El-Nassan, H.B.; Fares, A.R.; Elkasabgy, N.A.; ElMeshad, A.N. Cross-Linked Alginate Dialdehyde/Chitosan Hydrogel Encompassing Curcumin-Loaded Bilosomes for Enhanced Wound Healing Activity. Pharmaceutics 2024, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef]
- AbuBakr, A.H.; Hassan, H.; Abdalla, A.; Khowessah, O.M.; Abdelbary, G.A. Therapeutic potential of cationic bilosomes in the treatment of carrageenan-induced rat arthritis via fluticasone propionate gel. Int. J. Pharm. 2023, 635, 122776. [Google Scholar] [CrossRef]
- Abou Assi, R.; Abdulbaqi, I.M.; Tan, S.M.; Wahab, H.A.; Darwis, Y.; Chan, S.-Y. Breaking barriers: Bilosomes gel potentials to pave the way for transdermal breast cancer treatment with Tamoxifen. Drug Dev. Ind. Pharm. 2023, 1–12. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Almutairy, B.K.; Abu Lila, A.S. Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation. Polymers 2023, 15, 677. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, A.; Kocova El-Arini, S.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef]
- Mahmoud, T.M.; Nafady, M.M.; Farouk, H.O.; Mahmoud, D.M.; Ahmed, Y.M.; Zaki, R.M.; Hamad, D.S. Novel Bile Salt Stabilized Vesicles-Mediated Effective Topical Delivery of Diclofenac Sodium: A New Therapeutic Approach for Pain and Inflammation. Pharmaceuticals 2022, 15, 1106. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Gazar, A.A.; Abdelbary, A.A.; Elshafeey, A.H.; Mosallam, S. Brij® integrated bilosomes for improving the transdermal delivery of niflumic acid for effective treatment of osteoarthritis: In vitro characterization, ex vivo permeability assessment, and in vivo study. Int. J. Pharm. 2023, 640, 123024. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef]
- Abdelalim, L.R.; Abdallah, O.Y.; Elnaggar, Y.S.R. High efficacy, rapid onset nanobiolosomes of sildenafil as a topical therapy for erectile dysfunction in aged rats. Int. J. Pharm. 2020, 591, 119978. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions-An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef]
- Abbas, H.; Gad, H.A.; Khattab, M.A.; Mansour, M. The Tragedy of Alzheimer’s Disease: Towards Better Management via Resveratrol-Loaded Oral Bilosomes. Pharmaceutics 2021, 13, 1635. [Google Scholar] [CrossRef]
- Kwong, K.W.; Xin, Y.; Lai, N.C.; Sung, J.C.; Wu, K.C.; Hamied, Y.K.; Sze, E.T.; Lam, D.M. Oral Vaccines: A Better Future of Immunization. Vaccines 2023, 11, 1232. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, V.; Kesharwani, P. Bilosomes in the context of oral immunization: Development, challenges and opportunities. Drug Discov. Today 2016, 21, 888–899. [Google Scholar] [CrossRef]
- Zhao, K.; Xie, Y.; Lin, X.; Xu, W. The Mucoadhesive Nanoparticle-Based Delivery System in the Development of Mucosal Vaccines. Int. J. Nanomed. 2022, 17, 4579–4598. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wang, J. Mucosal vaccine delivery: A focus on the breakthrough of specific barriers. Acta Pharm. Sin. B 2022, 12, 3456–3474. [Google Scholar] [CrossRef]
- Trincado, V.; Gala, R.P.; Morales, J.O. Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems. Vaccines 2021, 9, 1177. [Google Scholar] [CrossRef]
- Baker, J.R., Jr.; Farazuddin, M.; Wong, P.T.; O’Konek, J.J. The unfulfilled potential of mucosal immunization. J. Allergy Clin. Immunol. 2022, 150, 1–11. [Google Scholar] [CrossRef]
- Singh, P.; Prabakaran, D.; Jain, S.; Mishra, V.; Jaganathan, K.S.; Vyas, S.P. Cholera toxin B subunit conjugated bile salt stabilized vesicles (bilosomes) for oral immunization. Int. J. Pharm. 2004, 278, 379–390. [Google Scholar] [CrossRef]
- Jain, S.; Indulkar, A.; Harde, H.; Agrawal, A.K. Oral mucosal immunization using glucomannosylated bilosomes. J. Biomed. Nanotechnol. 2014, 10, 932–947. [Google Scholar] [CrossRef]
- Shukla, A.; Singh, B.; Katare, O.P. Significant systemic and mucosal immune response induced on oral delivery of diphtheria toxoid using nano-bilosomes. Br. J. Pharmacol. 2011, 164, 820–827. [Google Scholar] [CrossRef]
- Conacher, M.; Alexander, J.; Brewer, J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine 2001, 19, 2965–2974. [Google Scholar] [CrossRef]
- Mann, J.F.S.; Ferro, V.A.; Mullen, A.B.; Tetley, L.; Mullen, M.; Carter, K.C.; Alexander, J.; Stimson, W.H. Optimisation of a lipid based oral delivery system containing A/Panama influenza haemagglutinin. Vaccine 2004, 22, 2425–2429. [Google Scholar] [CrossRef]
- Mann, J.F.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef]
- Bennett, E.; Mullen, A.B.; Ferro, V.A. Translational modifications to improve vaccine efficacy in an oral influenza vaccine. Methods 2009, 49, 322–327. [Google Scholar] [CrossRef]
- Wilkhu, J.S.; McNeil, S.E.; Anderson, D.E.; Perrie, Y. Characterization and optimization of bilosomes for oral vaccine delivery. J. Drug Target. 2013, 21, 291–299. [Google Scholar] [CrossRef]
- Shukla, A.; Khatri, K.; Gupta, P.N.; Goyal, A.K.; Mehta, A.; Vyas, S.P. Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes). JPPS 2008, 11, 59–66. [Google Scholar] [CrossRef]
- Shukla, A.; Katare, O.P.; Singh, B.; Vyas, S.P. M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int. J. Pharm. 2010, 385, 47–52. [Google Scholar] [CrossRef]
- Mann, J.F.S.; Scales, H.E.; Shakir, E.; Alexander, J.; Carter, K.C.; Mullen, A.B.; Ferro, V.A. Oral delivery of tetanus toxoid using vesicles containing bile salts (bilosomes) induces significant systemic and mucosal immunity. Methods 2006, 38, 90–95. [Google Scholar] [CrossRef]
- Jain, S.; Harde, H.; Indulkar, A.; Agrawal, A.K. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine 2014, 10, 431–440. [Google Scholar] [CrossRef]
- Gebril, A.; Obeid, M.A.; Bennett, E.M.; Pujol, A.; Chovel, M.L.; Mahy, T.; Acevedo, R.; Ferro, V.A. Mucosal and systemic immune responses following mucosal immunisation of tetanus toxoid entrapped in lipid nanoparticles prepared by microwave reactor. Eur. J. Pharm. Biopharm. 2022, 171, 11–18. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef]
- Nicze, M.; Borówka, M.; Dec, A.; Niemiec, A.; Bułdak, Ł.; Okopień, B. The Current and Promising Oral Delivery Methods for Protein- and Peptide-Based Drugs. Int. J. Mol. Sci. 2024, 25, 815. [Google Scholar] [CrossRef]
- Patel, V.; Lalani, R.; Bardoliwala, D.; Ghosh, S.; Misra, A. Lipid-Based Oral Formulation Strategies for Lipophilic Drugs. AAPS PharmSciTech 2018, 19, 3609–3630. [Google Scholar] [CrossRef]
- Spleis, H.; Sandmeier, M.; Claus, V.; Bernkop-Schnürch, A. Surface design of nanocarriers: Key to more efficient oral drug delivery systems. Adv. Colloid Interface Sci. 2023, 313, 102848. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631. [Google Scholar] [CrossRef]
- Binsuwaidan, R.; Sultan, A.A.; Negm, W.A.; Attallah, N.G.M.; Alqahtani, M.J.; Hussein, I.A.; Shaldam, M.A.; El-Sherbeni, S.A.; Elekhnawy, E. Bilosomes as Nanoplatform for Oral Delivery and Modulated In Vivo Antimicrobial Activity of Lycopene. Pharmaceuticals 2022, 15, 1043. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, R.V.; Woods, S.; Butcher, W.; McGahon, J.; Khadke, S.; Perrie, Y.; Williamson, E.D.; Roberts, C.W. Exploitation of the bilosome platform technology to formulate antibiotics and enhance efficacy of melioidosis treatments. J. Control. Release 2019, 298, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Teiama, M.; Magdy, B.; Sakran, W. Development of a Novel Bilosomal System for Improved Oral Bioavailability of Sertraline Hydrochloride: Formulation Design, In Vitro Characterization, and Ex Vivo and In Vivo Studies. AAPS PharmSciTech 2022, 23, 188. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Hadal Alotaibi, N.; Alharbi, K.S.; Afzal, M.; Ali, R.; Alshehri, S.; Alzarea, S.I.; Elmowafy, M.; et al. Bioactive Apigenin loaded oral nano bilosomes: Formulation optimization to preclinical assessment. Saudi Pharm. J. 2021, 29, 269–279. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Eid, H.M.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Abdelgawad, M.A.; Rateb, M.E.; Ali, M.R.A.; Alsalahat, I.; Abou-Taleb, H.A. Bilosomes as a promising nanoplatform for oral delivery of an alkaloid nutraceutical: Improved pharmacokinetic profile and snowballed hypoglycemic effect in diabetic rats. Drug Deliv. 2022, 29, 2694–2704. [Google Scholar] [CrossRef]
- Ayogu, I.J.; Ogbonna, O.; Ayolugbe, C.I.; Attama, A.A. Evaluation of the pharmacodynamic activity of insulin from bilosomal formulation. Curr. Drug Deliv. 2009, 6, 415–418. [Google Scholar] [CrossRef]
- Nallamothu, B.; Kuche, K.; Ghadi, R.; Chaudhari, D.; Jain, S. Enhancing oral bioavailability of insulin through bilosomes: Implication of charge and chain length on apical sodium-dependent bile acid transporter (ASBT) uptake. Int. J. Biol. Macromol. 2023, 252, 126565. [Google Scholar] [CrossRef]
- Soliman, M.O.; El-Kamel, A.H.; Shehat, M.G.; Bakr, B.A.; El-Moslemany, R.M. Lactoferrin decorated bilosomes for the oral delivery of quercetin in type 2 diabetes: In vitro and in vivo appraisal. Int. J. Pharm. 2023, 647, 123551. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Ahmad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2018, 111, 409–417. [Google Scholar] [CrossRef]
- Arzani, G.; Haeri, A.; Daeihamed, M.; Bakhtiari-Kaboutaraki, H.; Dadashzadeh, S. Niosomal carriers enhance oral bioavailability of carvedilol: Effects of bile salt-enriched vesicles and carrier surface charge. Int. J. Nanomed. 2015, 10, 4797–4813. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Yasir, M.; Alsaidan, O.A.; Alquraini, A.; Rawaf, A.; Alsuwayt, B.; Anwer, M.K.; Alshehri, S.; et al. Development and Optimization of Nanolipid-Based Formulation of Diclofenac Sodium: In Vitro Characterization and Preclinical Evaluation. Pharmaceutics 2022, 14, 507. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Almalki, R.K.H.; Hussain, A.; Bukhari, S.I.; Mahdi, W.A.; Qamar, W. Formulation of Chitosan-Coated Apigenin Bilosomes: In Vitro Characterization, Antimicrobial and Cytotoxicity Assessment. Polymers 2022, 14, 921. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Cheng, Y.; Qian, H. Bilosomes: A controlled delivery system for the sustained release of torularhodin during digestion in the small intestine both in vitro and in vivo. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130055. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Asfour, M.H.; Salama, A.A.A. Improved hepatoprotective activity of silymarin via encapsulation in the novel vesicular nanosystem bilosomes. Drug Dev. Ind. Pharm. 2017, 43, 2043–2054. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Yasir, M.; Ghoneim, M.M.; Alshehri, S.; Anwer, M.K.; Almurshedi, A.S.; Alanazi, A.S. Development and evaluation of luteolin loaded pegylated bilosome: Optimization, in vitro characterization, and cytotoxicity study. Drug Deliv. 2021, 28, 2562–2573. [Google Scholar] [CrossRef]
- Alruwaili, N.K.; Zafar, A.; Alsaidan, O.A.; Yasir, M.; Mostafa, E.M.; Alnomasy, S.F.; Rawaf, A.; Alquraini, A.; Alomar, F.A. Development of surface modified bilosomes for the oral delivery of quercetin: Optimization, characterization in-vitro antioxidant, antimicrobial, and cytotoxicity study. Drug Deliv. 2022, 29, 3035–3050. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Abd El-Halim, S.M.; Beshay, B.Y.; Zaki, I.; Abourehab, M.A.S. ‘Poly phenolic phytoceutical loaded nano-bilosomes for enhanced caco-2 cell permeability and SARS-CoV 2 antiviral activity’: In-vitro and insilico studies. Drug Deliv. 2023, 30, 2162157. [Google Scholar] [CrossRef]
- Joseph Naguib, M.; Moustafa Kamel, A.; Thabet Negmeldin, A.; Elshafeey, A.H.; Elsayed, I. Molecular docking and statistical optimization of taurocholate-stabilized galactose anchored bilosomes for the enhancement of sofosbuvir absorption and hepatic relative targeting efficiency. Drug Deliv. 2020, 27, 996–1009. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Fayad, E.; Althobaiti, F.; Zaki, I.; Abu Almaaty, A.H. Statistical optimization of bile salt deployed nanovesicles as a potential platform for oral delivery of piperine: Accentuated antiviral and anti-inflammatory activity in MERS-CoV challenged mice. Drug Deliv. 2021, 28, 1150–1165. [Google Scholar] [CrossRef]
- Hegazy, H.; Amin, M.M.; Fayad, W.; Zakaria, M.Y. TPGS surface modified bilosomes as boosting cytotoxic oral delivery systems of curcumin against doxorubicin resistant MCF-7 breast cancer cells. Int. J. Pharm. 2022, 619, 121717. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Alharbi, W.S.; Alfaleh, M.A.; Al-Hejaili, O.D.; Aldawsari, H.M.; Eid, B.G.; Bakhaidar, R.; Drago, F.; Caraci, F.; et al. Development of an Icariin-Loaded Bilosome-Melittin Formulation with Improved Anticancer Activity against Cancerous Pancreatic Cells. Pharmaceuticals 2021, 14, 1309. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Caruso, G.; Al-Rabia, M.W.; Badr-Eldin, S.M.; Aldawsari, H.M.; Asfour, H.Z.; Alshehri, S.; Alzaharani, S.H.; Alhamdan, M.M.; Rizg, W.Y.; et al. Piceatannol-Loaded Bilosome-Stabilized Zein Protein Exhibits Enhanced Cytostatic and Apoptotic Activities in Lung Cancer Cells. Pharmaceutics 2021, 13, 638. [Google Scholar] [CrossRef]
- Kharouba, M.; El-Kamel, A.; Mehanna, R.; Thabet, E.; Heikal, L. Pitavastatin-loaded bilosomes for oral treatment of hepatocellular carcinoma: A repurposing approach. Drug Deliv. 2022, 29, 2925–2944. [Google Scholar] [CrossRef]
- Youness, R.A.; Al-Mahallawi, A.M.; Mahmoud, F.H.; Atta, H.; Braoudaki, M.; Fahmy, S.A. Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells. Polymers 2023, 15, 1464. [Google Scholar] [CrossRef]
- Mondal, D.; Bagchi, A.; Biswas, S.; Dagar, T.; Biswas, A.; Bagchi, A.; De, S. Vesicle-Encapsulated Rolipram (PDE4 Inhibitor) and Its Anticancer Activity. ACS Appl. Bio Mater. 2024, 7, 369–378. [Google Scholar] [CrossRef]
- Parashar, P.; Rana, P.; Dwivedi, M.; Saraf, S.A. Dextrose modified bilosomes for peroral delivery: Improved therapeutic potential and stability of silymarin in diethylnitrosamine-induced hepatic carcinoma in rats. J. Liposome Res. 2019, 29, 251–263. [Google Scholar] [CrossRef]
- Matloub, A.A.; Salama, A.H.; Aglan, H.A.; AbouSamra, M.M.; ElSouda, S.S.M.; Ahmed, H.H. Exploiting bilosomes for delivering bioactive polysaccharide isolated from Enteromorpha intestinalis for hacking hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2018, 44, 523–534. [Google Scholar] [CrossRef]
- Naseri, A.; Taymouri, S.; Hosseini Sharifabadi, A.; Varshosaz, J. Chrysin loaded bilosomes improve the hepatoprotective effects of chrysin against CCl4 induced hepatotoxicity in mice. J. Biomater. Appl. 2023, 38, 509–526. [Google Scholar] [CrossRef]
- Maheshwari, R.; Bhatt, L.K.; Wairkar, S. Enhanced Oral Bioavailability of Progesterone in Bilosome Formulation: Fabrication, Statistical Optimization, and Pharmacokinetic Study. AAPS PharmSciTech 2024, 25, 29. [Google Scholar] [CrossRef]
- Guan, P.; Lu, Y.; Qi, J.; Wu, W. Readily restoring freeze-dried probilosomes as potential nanocarriers for enhancing oral delivery of cyclosporine A. Colloids Surf. B Biointerfaces 2016, 144, 143–151. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Z.; Xu, J.; Zhang, J.; Sun, R.; Zhou, J.; Lu, Y.; Gong, Z.; Huang, J.; Shen, X.; et al. Improving the ameliorative effects of berberine and curcumin combination via dextran-coated bilosomes on non-alcohol fatty liver disease in mice. J. Nanobiotechnol. 2021, 19, 230. [Google Scholar] [CrossRef]
- Narayanan, V.A.; Sharma, A.; Rajesh, K.S.; Arunraj, T.R.; Gururaj, M.P.; Parasuraman, S.; John, A. Bilosomes as a Potential Carrier to Enhance Cognitive Effects of Bacopa monnieri Extract on Oral Administration. J. Health Allied Sci. NU 2022, 13, 421–430. [Google Scholar] [CrossRef]
- Dighe, S.; Jog, S.; Momin, M.; Sawarkar, S.; Omri, A. Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics 2023, 16, 58. [Google Scholar] [CrossRef]
- Ingielewicz, A.; Szymczak, R.K. Intranasal Therapy in Palliative Care. Pharmaceutics 2024, 16, 519. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Wong, C.Y.J.; Baldelli, A.; Hoyos, C.M.; Tietz, O.; Ong, H.X.; Traini, D. Insulin Delivery to the Brain via the Nasal Route: Unraveling the Potential for Alzheimer’s Disease Therapy. Drug Deliv. Transl. Res. 2024, 14, 1776–1793. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; El-Feky, Y.A.; Al-Sawahli, M.M.; Ali, M.E.; Fayez, A.M.; Abbas, H. A Brain-Targeted Approach to Ameliorate Memory Disorders in a Sporadic Alzheimer’s Disease Mouse Model via Intranasal Luteolin-Loaded Nanobilosomes. Pharmaceutics 2022, 14, 576. [Google Scholar] [CrossRef]
- Abbas, H.; Refai, H.; El Sayed, N.; Rashed, L.A.; Mousa, M.R.; Zewail, M. Superparamagnetic iron oxide loaded chitosan coated bilosomes for magnetic nose to brain targeting of resveratrol. Int. J. Pharm. 2021, 610, 121244. [Google Scholar] [CrossRef]
- Salem, H.F.; Ali, A.A.; Hegazy, A.M.; Sadek, A.A.; Aboud, H.M. Harnessing of Doxylamine Succinate/Pyridoxine Hydrochloride-Dual Laden Bilosomes as a Novel Combinatorial Nanoparadigm for Intranasal Delivery: In Vitro Optimization and In Vivo Pharmacokinetic Appraisal. J. Pharm. Sci. 2022, 111, 794–809. [Google Scholar] [CrossRef]
- Salem, H.F.; Moubarak, G.A.; Ali, A.A.; Salama, A.A.A.; Salama, A.H. Budesonide-Loaded Bilosomes as a Targeted Delivery Therapeutic Approach Against Acute Lung Injury in Rats. J. Pharm. Sci. 2023, 112, 760–770. [Google Scholar] [CrossRef]
- El Taweel, M.M.; Aboul-Einien, M.H.; Kassem, M.A.; Elkasabgy, N.A. Intranasal Zolmitriptan-Loaded Bilosomes with Extended Nasal Mucociliary Transit Time for Direct Nose to Brain Delivery. Pharmaceutics 2021, 13, 1828. [Google Scholar] [CrossRef]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Peng, C.; Kuang, L.; Zhao, J.; Ross, A.E.; Wang, Z.; Ciolino, J.B. Bibliometric and visualized analysis of ocular drug delivery from 2001 to 2020. J. Control. Release 2022, 345, 625–645. [Google Scholar] [CrossRef]
- Tian, B.; Bilsbury, E.; Doherty, S.; Teebagy, S.; Wood, E.; Su, W.; Gao, G.; Lin, H. Ocular Drug Delivery: Advancements and Innovations. Pharmaceutics 2022, 14, 1931. [Google Scholar] [CrossRef]
- Yang, Y.; Lockwood, A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp. Eye Res. 2022, 218, 109006. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Zafar, A.; Yasir, M.; Alzarea, S.I.; Alqinyah, M.; Khalid, M. Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels 2022, 8, 687. [Google Scholar] [CrossRef]
- Zafar, A.; Alsaidan, O.A.; Imam, S.S.; Yasir, M.; Alharbi, K.S.; Khalid, M. Formulation and Evaluation of Moxifloxacin Loaded Bilosomes In-Situ Gel: Optimization to Antibacterial Evaluation. Gels 2022, 8, 418. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Yousry, C.; Zikry, P.M.; Salem, H.M.; Basalious, E.B.; El-Gazayerly, O.N. Integrated nanovesicular/self-nanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: Statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 801–814. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]
- Nemr, A.A.; El-Mahrouk, G.M.; Badie, H.A. Hyaluronic acid-enriched bilosomes: An approach to enhance ocular delivery of agomelatine via D-optimal design: Formulation, in vitro characterization, and in vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2022, 29, 2343–2356. [Google Scholar] [CrossRef]
- Sakr, M.G.; El-Zahaby, S.A.; Al-Mahallawi, A.M.; Ghorab, D.M. Fabrication of betaxolol hydrochloride-loaded highly permeable ocular bilosomes (HPOBs) to combat glaucoma: In vitro, ex vivo & in vivo characterizations. J. Drug Deliv. Sci. Technol. 2023, 82, 104363. [Google Scholar] [CrossRef]
- Nair, V.S.; Srivastava, V.; Bhavana, V.; Yadav, R.; Rajana, N.; Singh, S.B.; Mehra, N.K. Exploring Penetration Ability of Carbonic Anhydrase Inhibitor-Loaded Ultradeformable Bilosome for Effective Ocular Application. AAPS PharmSciTech 2023, 24, 157. [Google Scholar] [CrossRef]
- Anand, R.; Kumar, A. Significant biopolymers and their applications in buccal mediated drug delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 1203–1218. [Google Scholar] [CrossRef]
- Bahrami, K.; Lee, E.; Morse, B.; Lanier, O.L.; Peppas, N.A. Design of nanoparticle-based systems for the systemic delivery of chemotherapeutics: Alternative potential routes via sublingual and buccal administration for systemic drug delivery. Drug Deliv. Transl. Res. 2024, 14, 1173–1188. [Google Scholar] [CrossRef]
- Nair, V.V.; Cabrera, P.; Ramírez-Lecaros, C.; Jara, M.O.; Brayden, D.J.; Morales, J.O. Buccal delivery of small molecules and biologics: Of mucoadhesive polymers, films, and nanoparticles—An update. Int. J. Pharm. 2023, 636, 122789. [Google Scholar] [CrossRef]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.E.; Keum, T.; Noh, G.; Choi, Y.W.; Lee, S. Facilitated permeation of insulin across TR146 cells by cholic acid derivatives-modified elastic bilosomes. Int. J. Nanomed. 2018, 13, 5173–5186. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Abu Lila, A.S.; Sallam, N.M.; Sanad, R.A.; Ahmed, M.M.; Ghorab, M.M.; Alotaibi, H.F.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; et al. Preparation and Characterization of a Novel Mucoadhesive Carvedilol Nanosponge: A Promising Platform for Buccal Anti-Hypertensive Delivery. Gels 2022, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, M.F.; Khafagy, E.S.; Alotaibi, H.F.; Abu Lila, A.S. Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation. Polymers 2022, 14, 4184. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Petrova, V.A.; Skorik, Y.A. Biopolymer Drug Delivery Systems for Oromucosal Application: Recent Trends in Pharmaceutical R&D. Int. J. Mol. Sci. 2024, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Dilnawaz, F. Nanocarriers For Vaginal Drug Delivery. Recent Pat. Drug Deliv. Formul. 2019, 13, 3–15. [Google Scholar] [CrossRef]
- Xie, L.; Li, Y.; Liu, Y.; Chai, Z.; Ding, Y.; Shi, L.; Wang, J. Vaginal Drug Delivery Systems to Control Microbe-Associated Infections. ACS Appl. Bio Mater. 2023, 6, 3504–3515. [Google Scholar] [CrossRef]
- Albash, R.; Elmahboub, Y.; Baraka, K.; Abdellatif, M.M.; Alaa-Eldin, A.A. Ultra-deformable liposomes containing terpenes (terpesomes) loaded fenticonazole nitrate for treatment of vaginal candidiasis: Box-Behnken design optimization, comparative ex vivo and in vivo studies. Drug Deliv. 2020, 27, 1514–1523. [Google Scholar] [CrossRef]
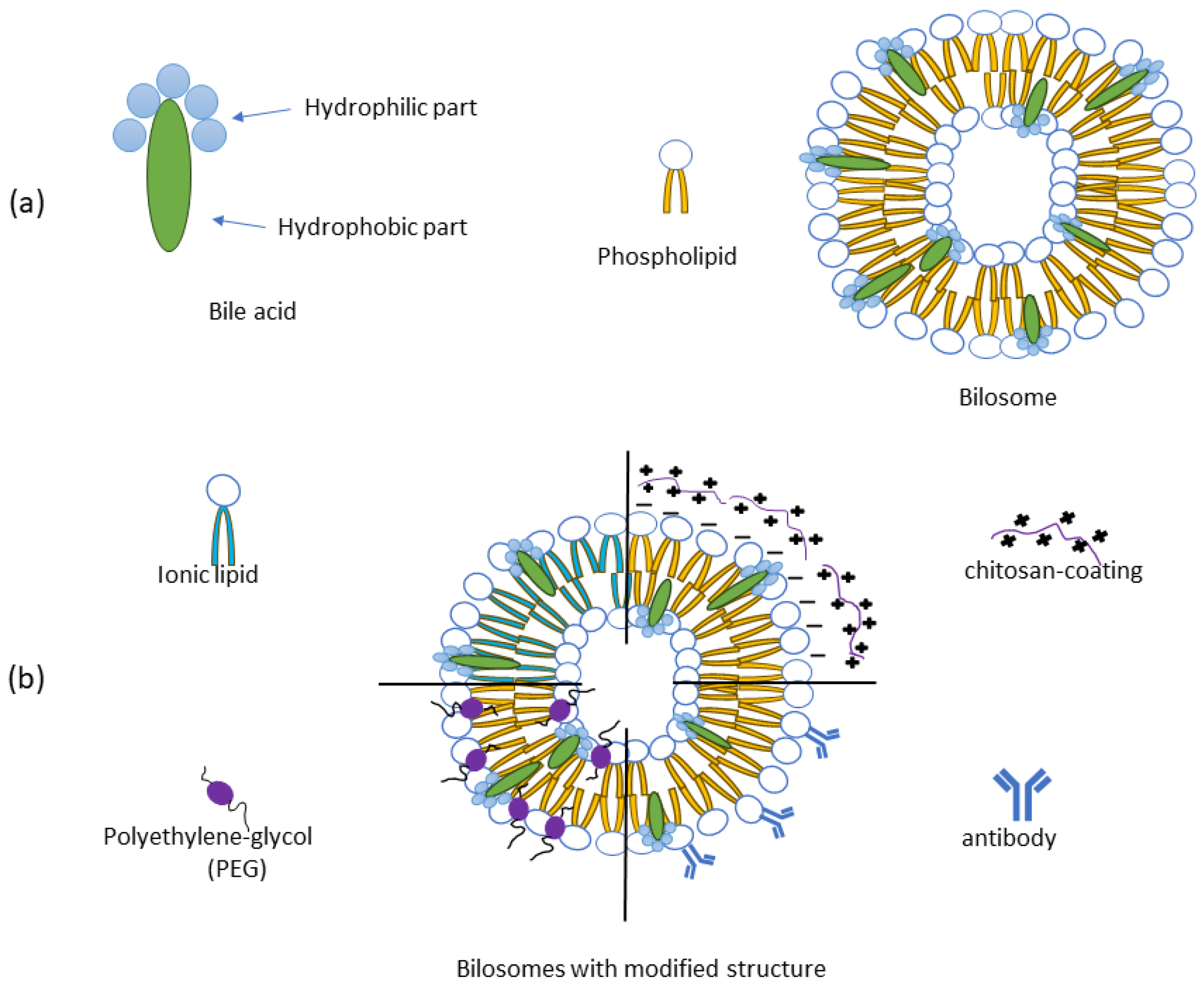
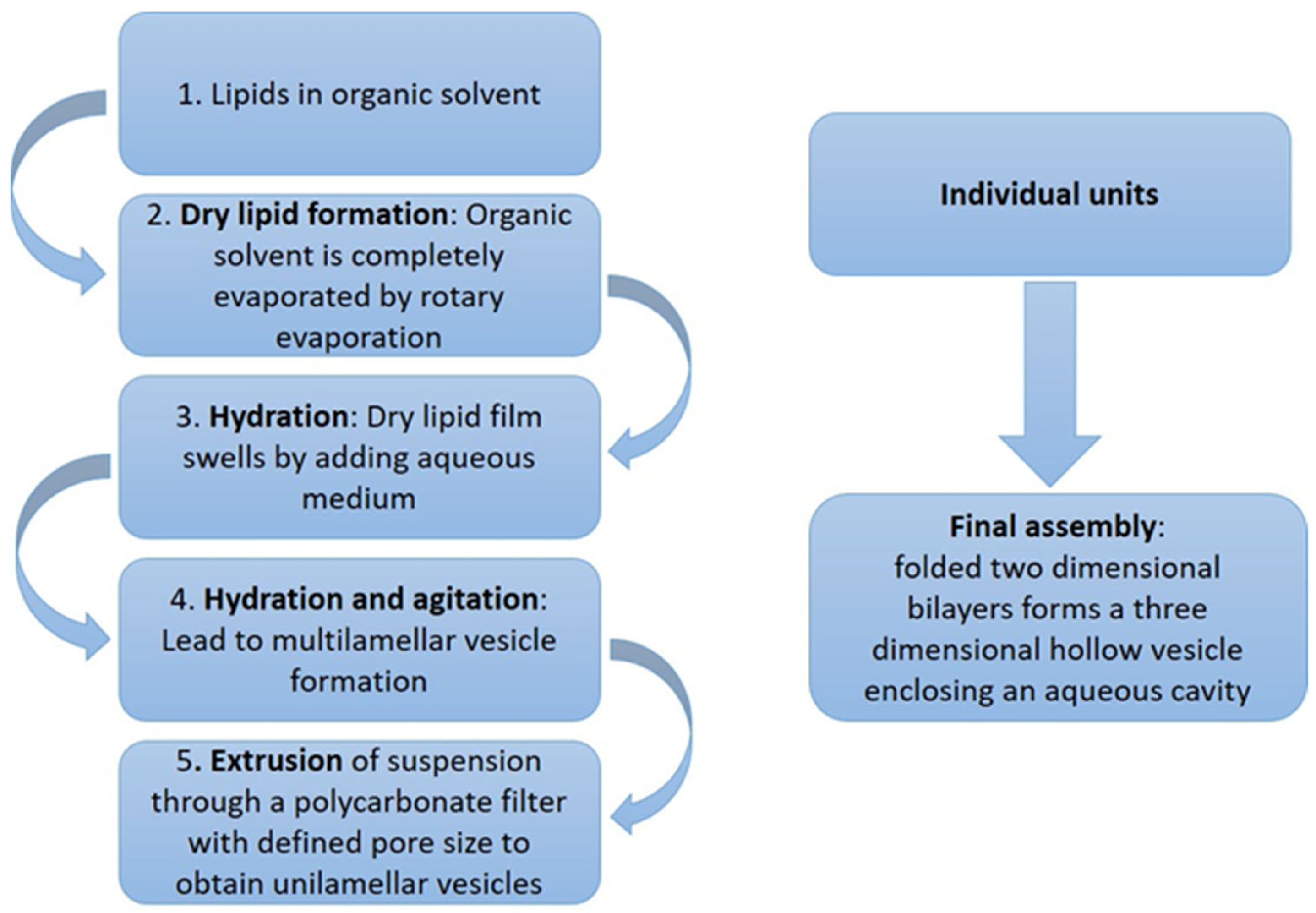
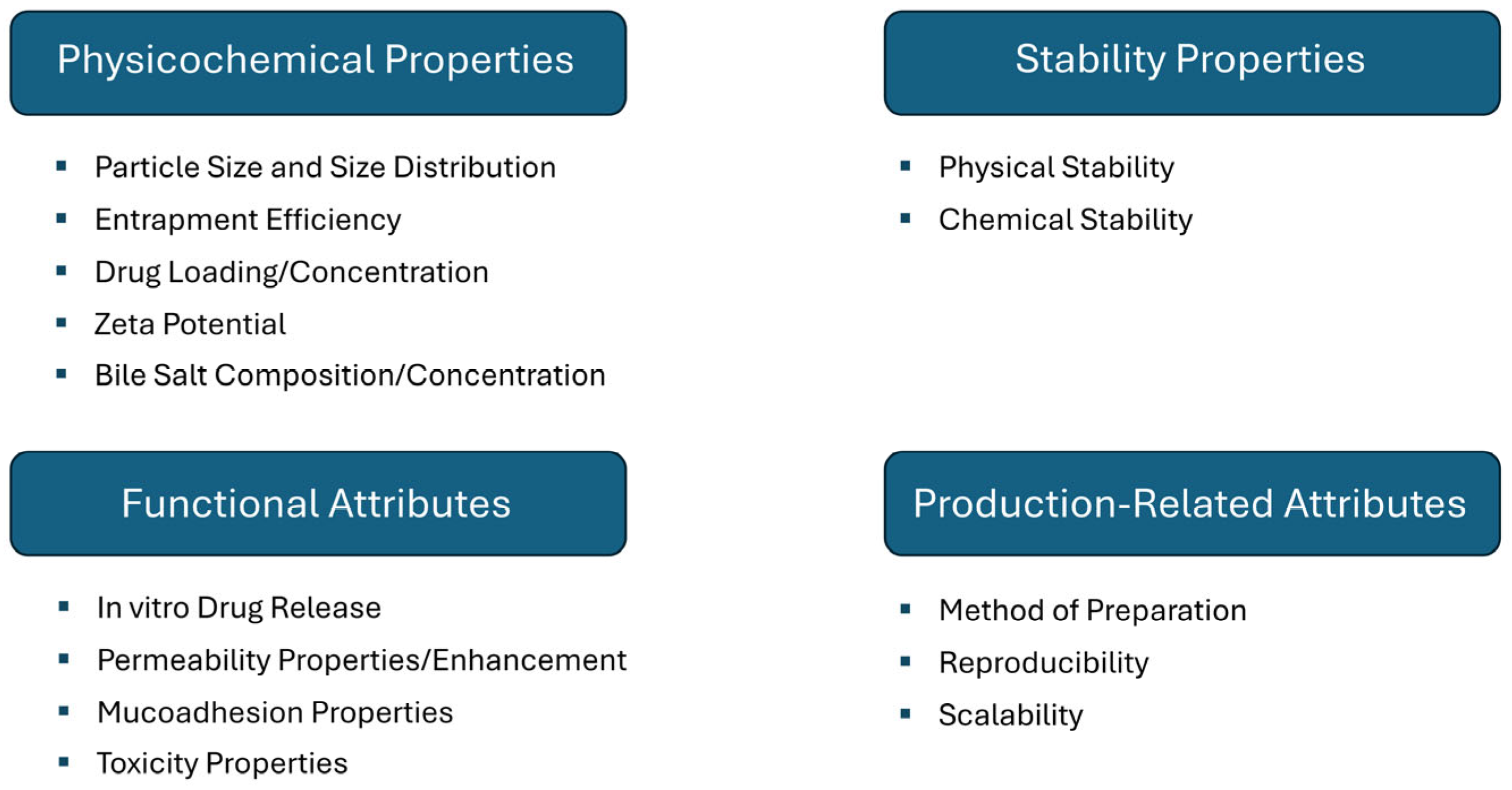
| Active Substance | Investigated Activity a | Bilosome Composition | Formation Technique/Optimization | Optimal Formulation | Irritability Test | Ref. |
|---|---|---|---|---|---|---|
| Dapsone | antiacne | Span 60:CHOL (5:1, 10:1 molar ratio), SDC, SC, STC (0.25–0.5 M) | Thin-film hydration/ 31∙22 full factorial design | Span 60:CHOL 5:1 molar ratio, SDC 0.25 M | Histopathological study (safe) | [52] |
| Luteolin | antiaging | L-α-PC:CHOL 4:1 weight ratio, SDC (0%, 10%, 25% w/w of PC) | Thin-film hydration | / | Draize test (score < 2), histopathological study (safe) | [53] |
| Spirulina platensis | antiaging | L-α-PC, CHOL, SDC (10 mg, 25 mg) | Thin-film hydration | / | Draize test (score < 2), histopathological study (safe) | [54] |
| Dronedarone | antiarrhythmic | Span 40:CHOL (1:1 molar ratio), SDC (0.2% w/v), clove oil (1.5–3% w/v), Tween 60 (25–50 mg), Tween 80 (25–50 mg), NaCMC (1% w/v) | Ethanol injection/ 23 full factorial design | Tween 60 (50 mg), clove oil (3% w/v) | pH within the non-irritating range | [55] |
| Empagliflozin | antidiabetic | Span 40, Span 60, CHOL (Span:CHOL mixture 100–300 mg, Span:CHOL 3–9 ratio) SDC, STGC (5–25 mg), Carbopol 940 (0.5–1%), Carbopol 934 (1–1.5%), HPMC (5%), NaCMC (5%) | Modified injection method/I-optimal design | Span 60:CHOL 9:1 mixture 139.59 mg, STGC 25 mg, Carbopol 940 1% (gel:bilosome 1:1) | Histopathological study (safe) | [56] |
| Metformin hydrochloride | antidiabetic | CHOL, Span 40, Span 60, SC, SDC, STC (8 mg, 14 mg) | Solvent evaporation/ 31∙22 full factorial design | Span 60, SDC 8 mg | / | [57] |
| Ondansetron hydrochloride | antiemetic | Span 60, Span 80, CHOL (Span:CHOL 7:0, 7:1, 7:3 molar ratio), SDC (0–5% w/v) | Thin-film hydration/ 32∙21 full factorial design | Span 60:CHOL 7:1, SDC 2.5% w/v | In vivo histopathological study (safe) | [58] |
| Butenafine | antifungal | PC (1.5–4.5%), Span 60 (30–60 mg) CHOL, SDC (15–25 mg), Carbopol 940 (1% w/v) | Thin-film hydration/ Box-Behnken design | PC 3.5%, Span 60 45 mg, SDC 22 mg | HET-CAM test (score 0) | [59] |
| Miconazole nitrate | antifungal | CHOL (20 mg, 40 mg), Tween 80 (2%, 4%), SDC (20 mg, 30 mg), Carbopol 934P (0.5–1% w/v), Chitosan (0.5% w/v) | Thin-film hydration/ Box-Behnken 33 statistical design (BBD) | CHOL 30 mg, Tween 80 4%, SDC 30 mg, Carbopol 934P 1% | HET-CAM (score 0) | [39] |
| Terconazole | antifungal | CHOL (25 mg), Span 60 (100 mg), SDC, SGC (5–15 mg), Brij-93, Cremophor EL (5 mg) | Thin-film hydration/ 24 complete factorial design | CHOL 25 mg, Span 60 100 mg, SDC 5 mg, Brij 93 5 mg | Histopathological study (safe) | [60] |
| Olmesartan medoxomil | antihypertensive | L-α-PC, Cholesterol (25 mg), Span 60 (50 mg), SDC (5 mg), STC (15 mg), Brijo20 (15–25 mg), Brij 52 (15–25 mg) | Thin-film hydration/ 24 full factorial design | STC (15 mg), Brij 52 (15 mg) | In vivo histopathological study (normal) | [61] |
| Sildenafil citrate | antihypertensive | SPC (200–400 mg), CHOL (20 mg), Span 60 (40–60 mg), SDC (10–30 mg), HPMC (2% w/w) | Thin-film hydration/ 33 Box-Behnken design | SPC 251.5 mg, SDC 30 mg, Span 60 60 mg | / | [62] |
| Valsartan | antihypertensive | Span 20, Span 40, Span 60, CHOL (Span:CHOL 1:1, 8:2, 2:8), SDC (5–20 mg), Carbopol 940 (1% w/v) | Thin-film hydration/ 33 full factorial design | Span 60:CHOL 8:2, SDC 20 mg | HET-CAM test (score 0), histopathological study (safe) | [63] |
| Berberine chloride | anti-inflammatory | Soybean lecithin (2.5–5% w/v), CHOL, SDC (5–15% w/v), Chitosan (0–0.25% w/v), Carbopol 974 NF (2%) | Thin-film hydration/ 33 Box-Behnken design | Soybean lecithin 5%, SDC 5%, Chitosan 0.16% | Histopathological study (safe) | [64] |
| Curcumin | anti-inflammatory | CHOL, Span 60 (CHOL:Span 1:5, 1:10 molar ratio), SC, SDC (5 mg, 10 mg), Alginate dialdehyde/Chitosan cross-linked hydrogel | Thin-film hydration/ 23 factorial design | CHOL:Span 60 1:10, SDC 5 mg | Histopathological study (regenerative potential) | [65] |
| Terbutaline sulfate | bronchodilator | Soybean PC (3–5% w/v), CHOL (20 mg), SDC (5–15% w/v), Chitosan (0–0.3% w/v), HPMC K15M (2% w/w) | Thin-film hydration/ Face-centered central composite design, artificial neural network modeling | Lipid 5% w/v, SDC 8% w/v, Chitosan 0.06% | In vivo histopathological study (safe) | [66] |
| Fluticasone propionate | corticosteroid | PC90G (1–3%), SDC (0.02–0.1%), STC (0–0.1%), stearylamine (0–0.3%), Carbopol 940 1% (w/w) | Thin-film evaporation/ 43 Draper-Lin small composite design | PC90G 2.99%, SDC 0.04%, stearylamine 0.29% | Histopathological study (regenerative potential) | [67] |
| Tamoxifen | cytostatic | PL90G (2–4%), STC or SC (0.2–0.4%), ethanol (15–25%) | Cold method with ethanol and extrusion/ 24 full factorial design | PL90G 2%, STC 0.4%, ethanol 25%; PL90G 4%, SC 0.2%, ethanol 25% | / | [68] |
| Simvastatin | hypolipidemic | Soy PC (1–3 molar concentration) CHOL, Span 60 (30–60 mg), SDC (10–30 mg), HPMC (2% w/w) | Thin-film hydration/ Box-Behnken 33 statistical design (BBD) | Lipid 1.427%, Span 60 60 mg, SDC 30 mg | / | [69] |
| Tizanidine hydrochloride | myorelaxant | Span 20, Span 40, Span 60, CHOL (Span:,CHOL 3:7, 1:1, 7:3 molar ratio), SDC (5–20 mg), CMC (5% w/w) | Thin-film hydration/ 33 full factorial design | Span 60:CHOL 1:1 molar ratio, SDC 20 mg | / | [70] |
| Diclofenac sodium | non-steroidal anti-inflammatory drug | Lecithin, CHOL, Span 40, Span 60, SC, SDC, SGC (8 mg, 18 mg), Carbopol 971P (2% w/w) | Thin-film hydration/ 31∙22 full factorial design | Span 60, SDC 18 mg | Histopathological study (normal periarticular and soft tissues) | [71] |
| Lornoxicam | non-steroidal anti-inflammatory drug | Soybean PC (0.02–0.06 M), SDC (10–30 mg), limonene (0.25–0.75%), Carbopol (1% w/w) | Thin-film hydration/Box-Behnken 33 full factorial design | Lipid 0.02 M, SDC 10 mg, limonene 0.47% | Draize test (score 0), histopathological study (safe) | [31] |
| Niflumic acid | non-steroidal anti-inflammatory drug | Brij-93, Brij-35 (5 mg), Span 20 (100 mg), CHOL (25 mg), SC, STC, SGC (5 mg, 15 mg), HPMC (2%) | Ethanol injection/31∙22 full factorial design | Brij-93, STC 5 mg | Histopathological study (regenerative potential) | [72] |
| Tenoxicam | non-steroidal anti-inflammatory drug | Span 40, Span 60, Span 80, CHOL (Span:CHOL 5:1, 5:3 molar ratio), SDC (0.5 M, 0.25 M) | Thin-film hydration/ 31∙22 full factorial design | Span 60:CHOL 5:1 molar ratio, SDC 0.25 M | In vivo histopathological study (no obvious skin irritation) | [73] |
| Sildenafil citrate | PDE-5 inhibitor | Pure soybean PC (3% w/v), SDC, DCA, STGC (soybean PC:BA 4:1, 6:1, 8:1) | Thin-film hydration and Ethanol injection method/33 Box-Behnken design | Pure soybean PC:STGC 4:1 | Skin integrity test (no skin defects) | [74] |
| Diacerein | structural modifying osteoarthritic drug | CHOL, Span 40, Span 60, SC, SGC, STC (5 mg, 15 mg) | Thin-film hydration/ 31∙22 full factorial design | Span 60, STC 15 mg | In vivo histopathological study (safe) | [75] |
| Active Substance | Investigated Activity | Bilosome Composition | Formation Technique/Optimization | Optimal Formulation | Ref. |
|---|---|---|---|---|---|
| Bovine serum albumin | vaccine | Sorbitan tristearate:CHOL:modified dipalmytoil phosphatidyl ethanolamine (7:3:1), SDC (20 mg), modified cholera toxin B subunit | Thin-film hydration | / | [84] |
| Bovine serum albumin | vaccine | Span 80:CHOL:SDC (2:1:0.1–0.4 molar ratio), stearylamine, GM-OCM-DSPE (5–15% w/w) | Thin-film hydration | Span 80:CHOL:SDC 2:1:0.1 molar ratio, GM-OCM-DSPE 10% | [85] |
| Diphtheria toxoid | vaccine | Sorbitan tristearatae:CHOL:diacetyl phosphate (7:3:1 molar ratio), SDC (100 mg) | Thin-film hydration | / | [86] |
| Synthetic peptide TTB; A/Texas influenza antigen | vaccine | 1-monopalmitoyl glycerol:CHOL:diacetyl phosphate (5:4:1 molar ratio), DCA (100 mg) | Thin-film hydration | / | [87] |
| A/Panama influenza antigen | vaccine | 1-monopalmitoyl glycerol:CHOL:dicetyl phosphate (5:4:1 molar ratio), DCA 100 mg/mL | / | / | [88] |
| Influenza A antigen | vaccine | 1-monopalmitoyl glycerol:CHOL:diacetyl phosphate (5:4:1 molar ratio), SDC (100 mg) | Thin-film hydration | / | [89] |
| Influenza antigen | vaccine | 1-monopalmitoyl glycerol:CHOL:diacetyl phosphate (5:4:1 molar ratio), DCA (10 mM, 100 mM) | / | / | [90] |
| Influenza antigen | vaccine | Monopalmitoyl glycerol, CHOL, diacetyl phosphate, SDC (70–120 mM) | Design of Experiments | Monopalmitoyl glycerol:CHOL:diacetyl phosphate 5:4:1, SDC 100 mM | [91] |
| Hepatitis B surface antigen (HBsAg) | vaccine | Sorbitan tristearate:CHOL:diacetyl phosphate (7:3:1 molar ratio), SDC (100 mg) | Thin-film hydration | / | [92] |
| Hepatitis B surface antigen (HBsAg) | vaccine | Sorbitan tristearate:CHOL:modified dipalmitoyl phosphatidyl ethanolamine (7:3:1 molar ratio), SDC (100 mg), modified cholera toxin B | Thin film hydration | / | [93] |
| Tetanus Toxoid | vaccine | 1-monopalmitoylglycerol, CHOL, diacetyl phosphate (5:4:1 molar ratio), SDC (100 mg) | Thin-film hydration | / | [94] |
| Tetanus Toxoid | vaccine | Span 80:CHOL:SDC (2:1:0.1 molar ratio), GM-OCM-DSPE (10%) | Thin-film hydration | / | [95] |
| Tetanus Toxoid | vaccine | Monopalmitoyl glycerol:CHOL:diacetyl phosphate (5:4:1), SDC (100 mM), xanthan gum (0.1% w/v) | Heating method (microwave method) | / | [96] |
| Active Substance | Investigated Activity a | Bilosome Composition | Formation Technique/Optimization | Optimal Formulation | Ref. |
|---|---|---|---|---|---|
| Epigallocatechin-gallate (EGCG) | / | Tween 40, CHOL, SC, SDC, STC, SDTC, SGC (20–160 mg) | Ethanol injection | CHOL:Tween 40 1:4 (m/m), SC:Tween 40 1:8 (m/m), EGCG:Tween 40 1:4 (m/m) | [102] |
| Lycopene | antibiotic | Span 60, CHOL (1:0.25 w/w), SC (0.01–0.04 molar concentration) | / | Span 60:CHOL 1:0.25 w/w, SC 0.02 molar concentration | [103] |
| Levofloxacin and Doxycycline hyclate | antibiotic | 1-monopalmitoyl glycerol, CHOL, diacetyl phosphate (5:4:1), SDC | Melt method | / | [104] |
| Sertraline hydrochloride (SER) | antidepressive | Span 60, CHOL (Span 60:CHOL 1:1, 7:1 molar ratio), SDC (SER:SDC 0.25, 0.5 molar ratio) | Thin-film hydration/23 full factorial design | Span:CHOL 1 molar ratio, SER:SDC 0.5 molar ratio | [105] |
| Apigenin | antidiabetic | CHOL (10–30%), Span 60 (50–70%), SDC (10–20%) | Thin-film hydration/33 Box-Behnken design | CHOL 15.5%, Span 60 70.2%, SDC 12.4% | [106] |
| Berberine | antidiabetic | Soy PC (0.03–0.06 molar concentration), CHOL (10–20 mg), SDC (15–30 mg) | Thin-film hydration/Central composite design | Soy PC 0.06 molar concentration, CHOL 15.2 mg, SDC 25 mg | [107] |
| Insulin | antidiabetic | Soya beans seed extract:palmitic acid:CHOL (0.25:1:1 w/w), SDC 0.5%; palmitic acid:CHOL (1:1 w/w), SDC 0.5% | Thin-film hydration | / | [108] |
| Insulin | antidiabetic | Soy PC, CHOL, deoxycholic acid-glycine, deoxycholic acid-glytamil methylester, N α-deoxycholyl-L-lysyl-methylester | Reverse phase evaporation | / | [109] |
| Quercetin | antidiabetic | Soy PC:SDC (4:1), lactoferrin (5–40 mg/mL) | Thin-film hydration/ | Soy PC:SDC 4:1, lactoferrin 30 mg/mL | [110] |
| Eprosartan mesylate | antidiabetic (diabetic neuropathy) | Soy PC, SDC (3:1, 4:1, 5:1, 6:1, 7:1. 9:1 ratio) | Thin-film hydration | Soy PC:SDC 4:1 | [111] |
| Carvedilol | antihypertensive | Span 60:CHOL 4:1 molar ratio, SC, STC (20, 30%) | Thin-film hydration | SC 20%, STC 30% | [112] |
| Diclofenac sodium | anti-inflammatory | PC (0.5–1.5%), CHOL (0.1–0.5%), SDC (0.5–1.5%), Pluronic F127 (0.3–0.7%) | Thin-film hydration/34 Box-Behnken design | PC 1% w/v, CHOL 0.3% w/v, SDC 1% w/v, Pluronic F127 0.5% w/v | [113] |
| Apigenin | antibiotic, cytotoxic | CHOL, PC, SDC (5, 10% w/v), Tween 80 (5, 10% w/v), SDC:Tween 80 (5, 10 % w/v), Chitosan (0.25, 0.5 % w/v) | Solvent-evaporation | SDC:Tween 80 10 % w/v, Chitosan 0.25% | [114] |
| Risedronate | antiosteoporotic | Soy PC:BS (SDC, STC, SGC):CHOL 4:1:0, 4:1:1, staerylamine, 1,2-dioleoyl-3-trimethyl ammonium propane | Reversed-phase evaporation/Thin-film hydration | / | [28] |
| Resveratrol | antioxidant, anti-inflammatory | Soy PC:CHOL:SDC (4:1:0, 4:1:1 molar ratio) | Thin-film hydration | Soy PC:CHOL:SDC 4:1:1 molar ratio, 3 extrusion cycles, drug concentration 10 mg/mL, pH 3 | [77] |
| Torularhodin | antioxidant, anti-inflammatory | Lecithin, Tween 80, SDC | Thin-film hydration | / | [115] |
| Silymarin | antioxidant, hepatoprotective | Soy PC, SDC, SC, STC (Soy PC:BA 4:1) | Thin-film hydration | Soy PC:STC 4:1 | [116] |
| Luteolin | antioxidant, antibiotic, cytotoxic | CHOL (1–5%), Span 60 (100–300 mg), SDC (0.15–0.35 mg), PEG 2000 (1–4 mg) | Thin-film hydration/34 Box-Behnken design | CHOL (25 mg), Span 60 45 mg, SDC 12.5 mg, PEG 2000 30 mg | [117] |
| Quercetin | antioxidant, antibiotic, cytotoxic | Lipid (4–6%), Span 60 (3–7%), SDC (4–12%), Chitosan (0.2–0.3%) | Solvent evaporation method/33 Box-Behnken design | Lipid 4%, Span 60 7%, SDC 8%, Chitosan 0.2% | [118] |
| Acyclovir | antiviral | CHOL (7.5–12.5 mg), Span 60, Tween 60 (1:2 ratio, 40–60 mg), SGC (5–10 mg) | Thin-film hydration/33 Box-Behnken design | CHOL 10 mg, surfactant 50 mg, SGC 7.5 mg | [119] |
| Resveratrol | antiviral | Span 60, CHOL, SGC, SDC, Brij 20, Brij 72 (15 mg, 30 mg) | Ethanol injection/23 full factorial design | SDC, Brij 20 15 mg | [120] |
| Sofosbuvir | antiviral | PC, Span 60 (Span 60:drug 1–5 ratio) STC (STC:Span 60 1–10 ratio), galactose | Thin- film hydration/ Central composite design | Span 60:drug 1:1 w/w, STC:Span 60 10:1 w/w | [121] |
| Piperine | antiviral, anti-inflammatory | Span 65, Brij 72, Brij 78, CHOL (Surfactant:CHOL 9:1, 1:1) SDC (1–5%) | Thin-film hydration/32∙21 Full factorial design | Brij72:CHOL 9:1, SDC 1% | [122] |
| Curcumin | cytotoxic | STC, SC (1%, 5% w/w), Span 60:CHOL (1:1, 5:1, 9:1 ratio), D-alpha-tocopheryl polyethylene glycol succinate (10, 20, 40 and 80 mg) | Thin-film hydration/31∙22 factorial design | STC 5% w/w, Span 60:CHOL 5:1, D-alpha-tocopheryl polyethylene glycol succinate 40 mg | [123] |
| Icariin, Melittin (MEL) | cytotoxic | CHOL, Span 20 (CHOL:Span 20 2, 4 molar ratio), SDC (0.25, 0.5 mM), MEL (1, 5% w/w) | Thin-film hydration/Box-Behnken design | CHOL:Span 20 2 molar ratio, SDC 0.25 mM, MEL 1.14% w/w | [124] |
| Piceatannol | cytotoxic | CHOL, Span 20 (1:2, 1:3, 1:4 molar ratio), SDC (0.25–0.5 mM), zein (5–10% w/w) | Thin-film hydration/33 Box-Behnken design | CHOL:Span 1:3.977, SDC 0.435 mM, zein 7.052% w/w | [125] |
| Pitavastatin | cytotoxic | Soy PC, SDC, SC, STC (SPC:BS 2:1, 4:1, 6:1), lactoferrin (20–200 mg/mL) | Thin-film hydration/23 Asymmetrical factorial design | Soy PC:SDC 4:1, lactoferrin 30 mg/ml | [126] |
| Psoralidin | cytotoxic | PC:CHOL:Span 60 1:0.4:0.2 molar ratio, SDC (0.125–0.5 mM), Chitosan 0.125–0.25% w/v | Thin-film hydration | SDC 0.25 mM, Chitosan 0.25 % w/v | [127] |
| Rolipram | cytotoxic | Brij 97, STC (1:1, 1:4) | Thin-film hydration | / | [128] |
| Silymarin | cytotoxic | Tween 20, Span 60 (100–300 mg), CHOL (10–20 mg), SDC (50–150 mg), Dextrose 60 1%, Dextrose 40 1% | Thin-film hydration/33 Box Behnken design | / | [129] |
| Sulfated polysaccharide-protein complexes of Enteromorpha intestinalis | cytotoxic | CHOL, Span 40, Span 65 (CHOL:Span 1:5 molar ratio), SC, SDC, STDC | Thin-film hydration | Span 65, SC | [130] |
| Chrysin | hepatoprotective | CHOL (10–40 mg) Lecithin (100–200 mg), SDC (10–40 mg) | Thin-film hydration/Fractional factorial design | CHOL 20 mg, Lecithin 100 mg, SDC 20 mg | [131] |
| Progesterone | hormone | PC (0.75–1.25% w/v), CHOL (0.15–0.45% w/v), SDC (0.2–0.3% w/v) | Thin-film hydration/33 Box-Behnken design | PC 1.25% w/v, CHOL 0.15% w/v, SDC 0.29% w/v | [132] |
| Cyclosporin A (CyA) | immunosuppressive | CHOL, Soy PC (2–6%), SDC (Soy PC:SDC 3:1–9:1) gelatin (0–20%) | Thin-layer hydration with high-pressure homogenization | Soy PC 6%, Soy PC:SDC 3:1, CyA 2 mg/mL, gelatin 10% | [133] |
| Berberine and Curcumin (CUR) | NAFLD therapy | SPC, CHOL, octadecylamine, SDC, CUR (20:2:1:3:1 mass ratio), diethylaminoethyl dextran (0.13–0.52 mg/mL) | Thin-film hydration | diethylaminoethyl dextran 0.39 mg/mL | [134] |
| Bacopa monnieri | nootropic | Soy PC (400–1600 mg), SDC (5–10 mg) | Thin-film hydration | Soy PC 1600 mg, SDC 10 mg | [135] |
| Active Substance | Investigated Activity a | Bilosome Composition | Formation Technique/Optimization | Optimal Formulation | Ref. |
|---|---|---|---|---|---|
| Luteolin | anti-Alzheimer’s disease | Lipoid S100:CHOL (2:1, 4:1 molar ratio), Span 60 (100, 200 mg), SDC (10, 25 mg) | Thin-film hydration/23 factorial design | CHOL:Lipoid S100 2:1 molar ratio, Span 60 100 mg, SDC 25 mg | [140] |
| Resveratrol with paramagnetic iron oxide | anti-Alzheimer’s disease | Span 60:CHOL (2:1, 1:1, 1:2 molar ratio), SDC (10 mg), chitosan (0.05–0.2% w/v) | Thin-film hydration | Span 60:CHOL 1:1 molar ratio, SDC 10 mg, chitosan 0.1% w/v | [141] |
| Doxylamine Succinate, Pyridoxine hydrochloride | antiemetic (gestational nausea and vomiting) | Soy PC (1–5% w/v), CHOL (15–50 mg), SC (5–10% w/w), poloxamer 407:poloxamer 188:carbopol 971P (20:10:0.2% w/w) | Thin-film hydration/33 Box-Behnken design | Soy PC 2.09% w/v, CHOL 31.71 mg, SC 6.37% w/w | [142] |
| Budesonide | anti-inflammatory | CHOL, Phospholipoin 80H, L-α phosphatidylcholine, Lipoid S45 (0.0965 g), Span 60 (1.1965 g), SC, SDC (0.215 g) | Thin-film hydration/Mixed-factorial design | CHOL 0.0965 g, SC 0.215 g, budesonide 10 mg | [143] |
| Zolmitriptan (Migraine) | antimigraine | CHOL:Span 40 (1:1–1:9 molar ratio, 100–300 mg), SDC (5–15 mg), HPMC 0.5% w/v, poloxamer 407 17% w/v | Thin-film hydration/33 Box-Behnken design | CHOL:Span 40 1:7.7 molar ratio 255 mg, SDC 5 mg | [144] |
| Active Substance | Investigated Activity a | Bilosome Composition | Formation Technique/Optimization | Optimal Formulation | Ref. |
|---|---|---|---|---|---|
| Ciprofloxacin | antibiotic | CHOL (10 mg, 30 mg), Span 60 (40 mg, 60 mg), SDC (15 mg, 25 mg), HPMC K100 M (0.2–1% w/v), Carbopol-934P (1–1.8% w/v) | Thin-film hydration/Box-Behnken design | CHOL 35 mg, Span 60 65 mg, SDC 20 mg, HPMC K100M 0.6% w/v, Carbopol 934P 1.4% w/v | [149] |
| Moxifloxacin | antibiotic | Span 60 (30–60 mg), CHOL (15%), Cremophor EL (7–15 mg), SDC (15–30 mg), chitosan (0.35%), sodium alginate (0.1–0.5%) | Thin-film hydration/33 Box-Behnken design | CHOL 15%, Span 60 30 mg, Cremophor EL 13 mg, SDC 18 mg, CH 0.35%, SA 0.4% | [150] |
| Natamycin | antifungal | Span 60:CHOL (2:1, 1:1, 1:2 molar ratio), STC (10 mg, 21 mg), gellan gum (20–40 mg), xanthan gum (20–40 mg) | Thin-film hydration | Span 60:CHOL 1:1 molar ratio, STC 10 mg, gellan gum 30 mg | [151] |
| Terconazole | antifungal | Span 60, CHOL, STC (10 mg, 20 mg), Cremophor EL, Cremophor RH 40 (5 mg, 10 mg) | Ethanol injection/23 factorial design | Span 60, CHOL, STC 10 mg, Cremophor EL 5 mg | [152] |
| Terconazole | antifungal | Span 60 (60–100 mg), CHOL (0–20 mg), SDC (0–10 mg), Labrafil® M 2125 CS:Tween® 80:Transcutol® HP (20:50:30) | Thin-film hydration/Box-Behnken design | Span 60 73.59 mg, CHOL 1.28 mg, SDC 3.11 mg, Labrafil® M 2125 CS:Tween® 80:Transcutol® HP (20:50:30) | [153] |
| Acetazolamide | antiglaucoma | Span 60, CHOL, SC, SDC, STC, STGC (Span 60:CHOL:BA 1:1:0.1, 1:1:0.2 molar ratio) | Thin-film hydration | Span 60:CHOL:SDC 1:1:0.2 molar ratio | [154] |
| Agomelatine | antiglaucoma | PC, SC, SDC, STC (PC:BA 2:1, 4:1), hyaluronic acid (0%, 0.5%) | Ethanol injection/-optimal design | PC:SC 2:1 ratio, hyaluronic acid 0.26% | [155] |
| Betaxolol hydrochloride | antiglaucoma | Soy PC, Span 60, CHOL (4:1:1 ratio), Cremophor EL (5 mg, 10 mg) SDC, STC (0.25%, 0.75% w/v) | Ethanol injection/23 factorial design | Soy PC, Span 60, CHOL (4:1:1 ratio), Cremophor EL 10 mg, SDC 0.75% w/v | [156] |
| Brinzolamide | antiglaucoma | Span 60, CHOL, SDC, STC, Kolliphor RH40, Tween 80 | Ethanol injection | Span 60:CHOL:STC:Tween 80 5:5:2:1 ratio | [157] |
| Active Substance | Investigated Activity a | Bilosome Composition | Formation Technique/Optimization | Optimal Formulation | Ref. |
|---|---|---|---|---|---|
| Insulin | antidiabetic | Soy lecithin, SC, STC, SGC, SDGC, SDTC (85:15 w/w%) | Thin-film hydration | Soy lecithin, SDGC 85:15 w/w% | [162] |
| Carvedilol | Antihypertensive | Soy PC (1–3% w/w), CHOL (0–30% w/w), diacetyl phosphate (5% w/w of total lipid), SDC (25 mg), CMC:HPC (1:1) | Thin-film hydration | Soy PC 3% w/w, CHOL 30% w/w, diacetyl phosphate 5% of total lipid, SDC 25 mg, CMC:HPC 1:1 | [163] |
| Vardenafil | PDE-5 inhibitor | Soy PC (1–3 molar concentration), CHOL (0–30 molar concentration), SDC (10–30 mg), HPMC:CMC (50:50 ratio) | Thin-film hydration/33 Box-Behnken design | Soy PC 2.98%, CHOL 29.4%, SDC 17.25 mg | [164] |
| Active Substance | Investigated Activity | Bilosome Composition | Formation Technique/Optimization | Optimal Formulation | Ref. |
|---|---|---|---|---|---|
| Fenticonazole nitrate | antifungal | limonene:citral (1:1, 1:2, 1:3 w/w), SDC (10–30 mg), L-α-PC 100 mg, ethanol (5–15% v/v), HPMC | Thin-film hydration/33 Box-Behnken design | limonene:citric 1:3 w/w, SDC 10 mg, ethanol 11.26% v/v | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrović, D.; Zaklan, D.; Đanić, M.; Stanimirov, B.; Stankov, K.; Al-Salami, H.; Pavlović, N. The Pharmaceutical and Pharmacological Potential Applications of Bilosomes as Nanocarriers for Drug Delivery. Molecules 2025, 30, 1181. https://doi.org/10.3390/molecules30051181
Mitrović D, Zaklan D, Đanić M, Stanimirov B, Stankov K, Al-Salami H, Pavlović N. The Pharmaceutical and Pharmacological Potential Applications of Bilosomes as Nanocarriers for Drug Delivery. Molecules. 2025; 30(5):1181. https://doi.org/10.3390/molecules30051181
Chicago/Turabian StyleMitrović, Darko, Dragana Zaklan, Maja Đanić, Bojan Stanimirov, Karmen Stankov, Hani Al-Salami, and Nebojša Pavlović. 2025. "The Pharmaceutical and Pharmacological Potential Applications of Bilosomes as Nanocarriers for Drug Delivery" Molecules 30, no. 5: 1181. https://doi.org/10.3390/molecules30051181
APA StyleMitrović, D., Zaklan, D., Đanić, M., Stanimirov, B., Stankov, K., Al-Salami, H., & Pavlović, N. (2025). The Pharmaceutical and Pharmacological Potential Applications of Bilosomes as Nanocarriers for Drug Delivery. Molecules, 30(5), 1181. https://doi.org/10.3390/molecules30051181









