Sugarcane Straw Hemicellulose Extraction by Autohydrolysis for Cosmetic Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimal Autohydrolysis Conditions of Hemicellulose from Sugarcane Straw
2.1.1. Sugarcane Biomass Characterization
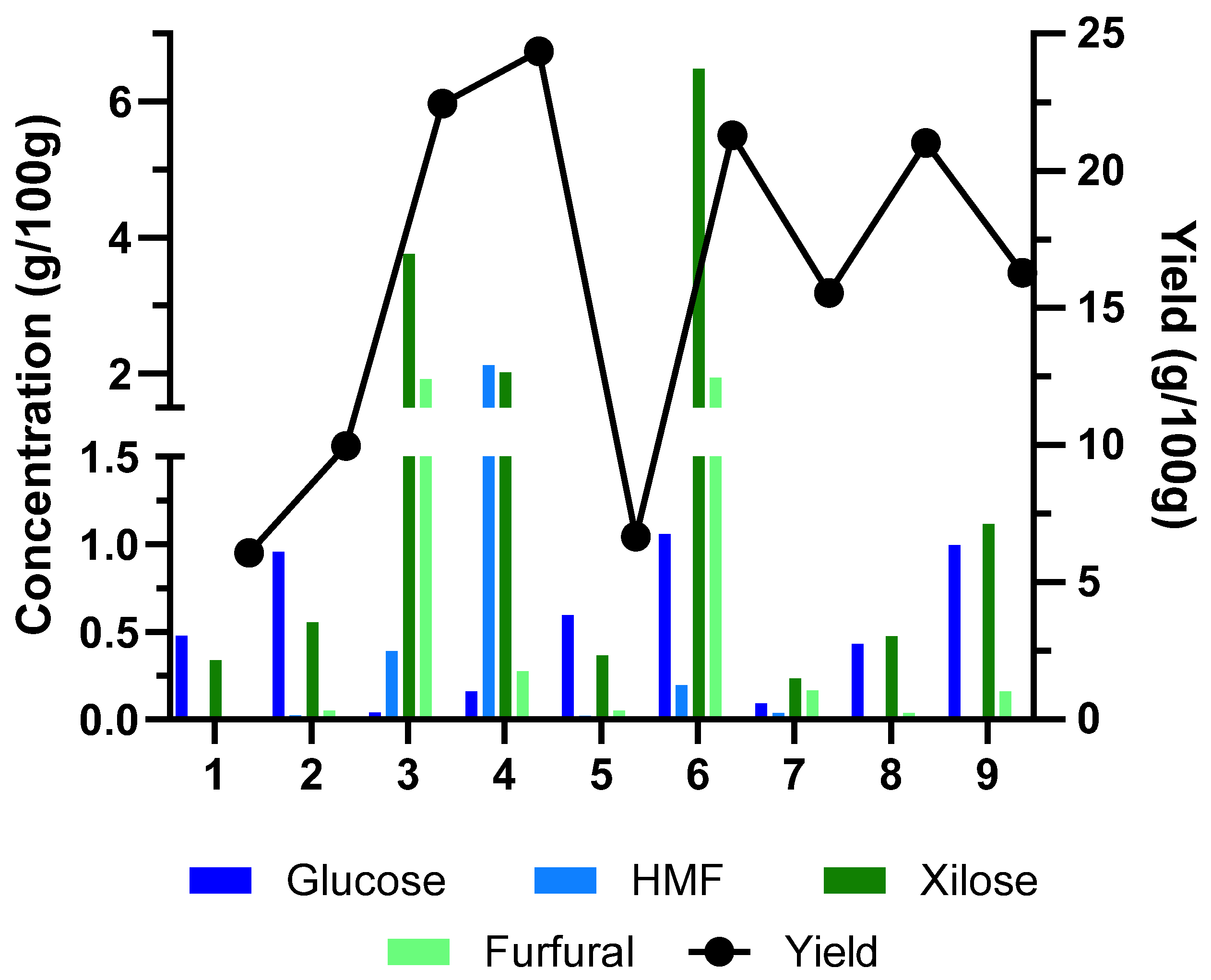
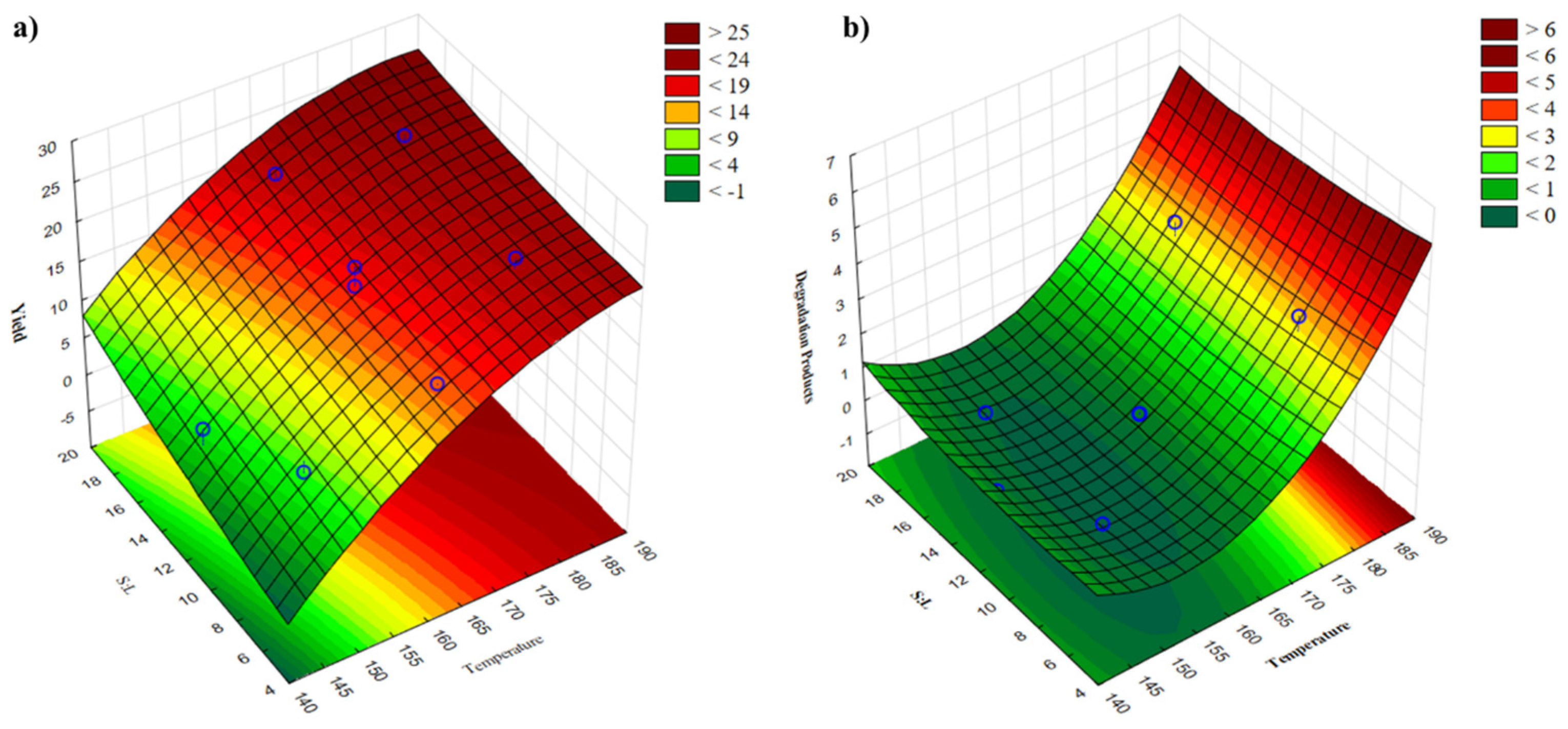
2.1.2. Solid/Liquid Ratio and Temperature
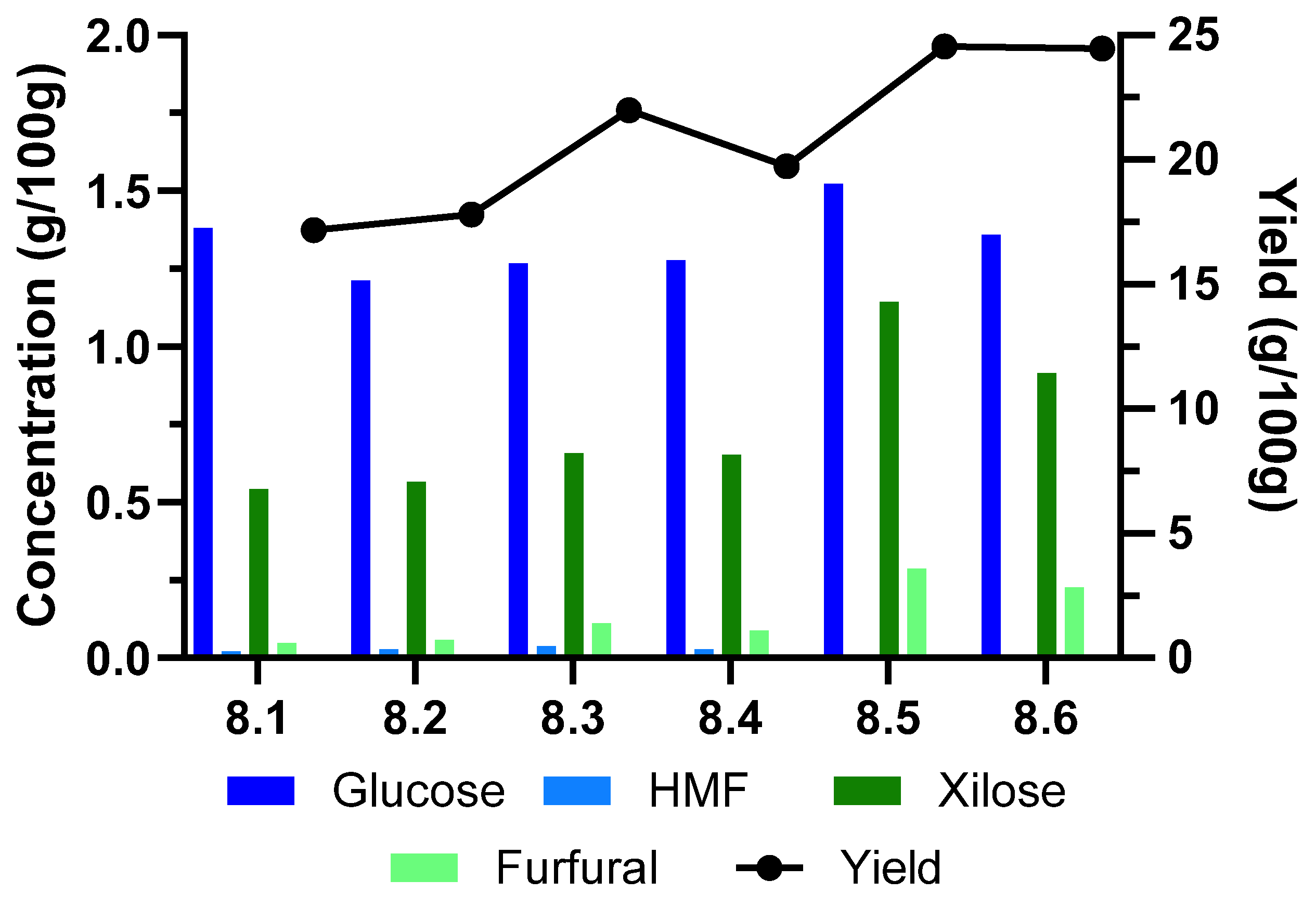
2.1.3. Hemicellulose Extraction Time
2.2. Hemicellulose Liquor and Purified XOS Extract from Sugarcane Straw Physicochemical Properties
Organic Acids and Phenolic Compounds Profile in XOS-Enriched Extracts
2.3. Antioxidant Activity
2.4. Antimicrobial Activity in Skin Microorganisms
2.5. Cosmetic Potential
2.5.1. Cytocompatibility
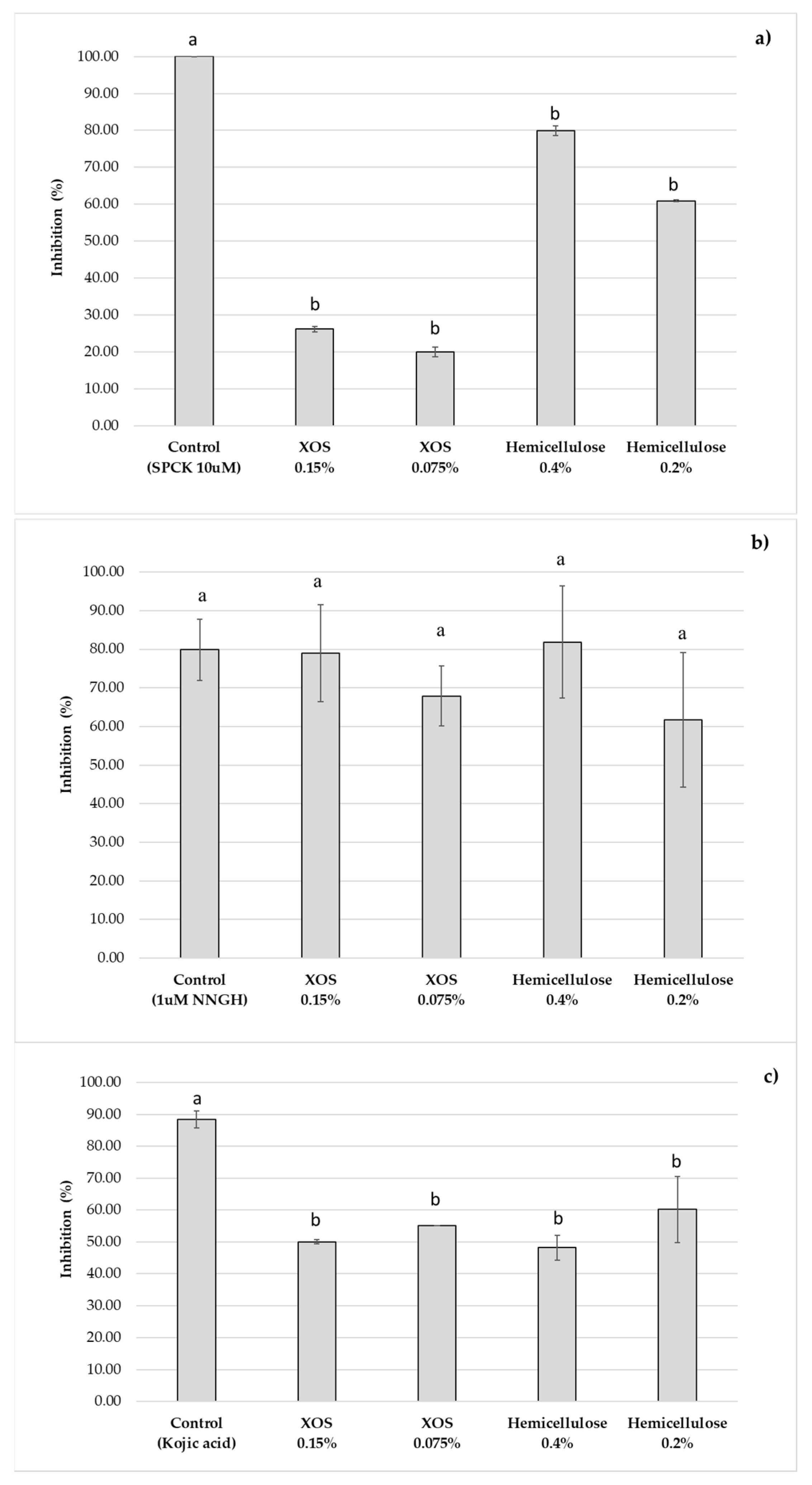
2.5.2. Skin Enzymes Inhibition
2.5.3. Compatibility with Cosmetic Ingredients
2.6. Economic Viability
3. Materials and Methods
3.1. Raw Material and Materials
3.2. Experimental Procedure
3.2.1. Autohydrolysis Process
Experimental Design
| Run | ϰ1—Temperature (°C) | ϰ2—S:L Ratio | Initial Weight (g) | Time (min) |
|---|---|---|---|---|
| 1 | 150 (−1.0) | 1:8 (−1.0) | 45 | 15 |
| 2 | 150 (−1.0) | 1:16 (+1.0) | 22 | |
| 3 | 180 (+1.0) | 1:8 (−1.0) | 45 | |
| 4 | 180 (+1.0) | 1:16 (+1.0) | 22 | |
| 5 | 143 (+1.41) | 1:12 (0) | 30 | |
| 6 | 186 (+1.41) | 1:12 (0) | 30 | |
| 7 | 165 (0) | 1:6 (−1.41) | 60 | |
| 8 | 165 (0) | 1:18 (+1.41) | 20 | |
| 9 | 165 (0) | 1:12 (0) | 30 | |
| 10 | 165 (0) | 1:12 (0) | 30 | |
| 11 | 165 (0) | 1:12 (0) | 30 |
3.2.2. Purification of XOS Fractions
3.3. Analytical Characterization
3.3.1. Quantification of Lignin, Cellulose, and Hemicellulose by Nrel Protocol
3.3.2. Modified NREL Protocol—Post Hydrolysis
3.3.3. Analysis of Degradation Products by HPLC
3.3.4. Size Exclusion Chromatography (SEC)
3.3.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3.6. Phenolic Compounds and Organic Acids Analysis by LC-ESI-QqTOF-HRMS
3.4. Antioxidant Potential Evaluation
3.4.1. ABTS Radical Cation Decolorization Assay
3.4.2. DPPH Radical Cation Decolorization Assay
3.5. Antimicrobial Activity Determination in Skin Microorganisms
3.5.1. Microorganisms
3.5.2. Minimal Inhibitory and Bactericidal Concentrations Evaluation
3.6. Cosmetic Potential Assessment
3.6.1. Cytocompatibility Evaluation Protocol
3.6.2. Skin Enzyme Inhibition
3.7. Statistical Analysis
3.8. Economic Viability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinales-Márquez, C.D.; Rodríguez-Jasso, R.M.; Araújo, R.G.; Loredo-Treviño, A.; Nabarlatz, D.; Gullón, B.; Ruiz, H.A. Circular Bioeconomy and Integrated Biorefinery in the Production of Xylooligosaccharides from Lignocellulosic Biomass: A Review. Ind. Crops Prod. 2021, 162, 113274. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Tompsett, G.A.; Guerra, P.; Timko, M.T.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Sugars and Char Formation on Subcritical Water Hydrolysis of Sugarcane Straw. Bioresour. Technol. 2017, 243, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Szczerbowski, D.; Pitarelo, A.P.; Zandoná Filho, A.; Ramos, L.P. Sugarcane Biomass for Biorefineries: Comparative Composition of Carbohydrate and Non-Carbohydrate Components of Bagasse and Straw. Carbohydr. Polym. 2014, 114, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Brenelli, L.B.; Bhatia, R.; Djajadi, D.T.; Thygesen, L.G.; Rabelo, S.C.; Leak, D.J.; Franco, T.T.; Gallagher, J.A. Xylo-Oligosaccharides, Fermentable Sugars, and Bioenergy Production from Sugarcane Straw Using Steam Explosion Pretreatment at Pilot-Scale. Bioresour. Technol. 2022, 357, 127093. [Google Scholar] [CrossRef]
- Conab-Página Inicial. Available online: https://www.conab.gov.br/ (accessed on 7 December 2023).
- Aguiar, A.; Milessi, T.S.; Mulinari, D.R.; Lopes, M.S.; da Costa, S.M.; Candido, R.G. Sugarcane Straw as a Potential Second Generation Feedstock for Biorefinery and White Biotechnology Applications. Biomass Bioenergy 2021, 144, 105896. [Google Scholar] [CrossRef]
- Egüés, I.; Sanchez, C.; Mondragon, I.; Labidi, J. Effect of Alkaline and Autohydrolysis Processes on the Purity of Obtained Hemicelluloses from Corn Stalks. Bioresour. Technol. 2012, 103, 239–248. [Google Scholar] [CrossRef]
- Scapini, T.; dos Santos, M.S.N.; Bonatto, C.; Wancura, J.H.C.; Mulinari, J.; Camargo, A.F.; Klanovicz, N.; Zabot, G.L.; Tres, M.V.; Fongaro, G.; et al. Hydrothermal Pretreatment of Lignocellulosic Biomass for Hemicellulose Recovery. Bioresour. Technol. 2021, 342, 126033. [Google Scholar] [CrossRef]
- Sun, D.; Lv, Z.W.; Rao, J.; Tian, R.; Sun, S.N.; Peng, F. Effects of Hydrothermal Pretreatment on the Dissolution and Structural Evolution of Hemicelluloses and Lignin: A Review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef]
- Kumar, V.; Bahuguna, A.; Ramalingam, S.; Kim, M. Developing a Sustainable Bioprocess for the Cleaner Production of Xylooligosaccharides: An Approach towards Lignocellulosic Waste Management. J. Clean. Prod. 2021, 316, 128332. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; Porto de Souza Vandenberghe, L.; Soccol, C.R. A Biorefinery Approach for Enzymatic Complex Production for the Synthesis of Xylooligosaccharides from Sugarcane Bagasse. Bioresour. Technol. 2021, 333, 125174. [Google Scholar] [CrossRef]
- Kaprelyants, L.; Zhurlova, O.; Shpyrko, T.; Pozhitkova, L. Xylooligosaccharides from Agricultural By-Products: Characterization, Production and Physiological Effects. Food Sci. Technol. 2017, 11, 25–34. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ahring, B.K. The Potential for Oligosaccharide Production from the Hemicellulose Fraction of Biomasses through Pretreatment Processes: Xylooligosaccharides (XOS), Arabinooligosaccharides (AOS), and Mannooligosaccharides (MOS). Carbohydr. Res. 2012, 360, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rigual, V.; Rodriguez, F. Effect of Autohydrolysis on Pinus Radiata Wood for Hemicellulose Extraction. Carbohydr. Polym. 2018, 194, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; Falqué, E.; Domínguez, H.; Parajó, J.C. Autohydrolysis of Agricultural Residues: Study of Reaction Byproducts. Bioresour. Technol. 2007, 98, 1951–1957. [Google Scholar] [CrossRef]
- Mokhena, T.C. Sugarcane Bagasse and Cellulose Polymer Composites; Mochane, M.J., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 12; ISBN 978-1-78923-151-9. [Google Scholar]
- Costa, J.R.; Pereira, M.J.; Pedrosa, S.S.; Gullón, B.; de Carvalho, N.M.; Pintado, M.E.; Madureira, A.R. Sugarcane Straw as a Source of Arabinoxylans: Optimization and Economic Viability of a Two-Step Alkaline Extraction. Foods 2023, 12, 2280. [Google Scholar] [CrossRef]
- Melati, R.B.; Shimizu, F.L.; Oliveira, G.; Pagnocca, F.C.; De Souza, W.; Anna, C.S.; Brienzo, M. Key Factors Affecting the Recalcitrance and Conversion Process of Biomass. BioEnergy Res. 2019, 12, 1–20. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Ruzene, D.S.; Silva, D.P.; Quintas, M.A.C.; Vicente, A.A.; Teixeira, J.A. Evaluation of a Hydrothermal Process for Pretreatment of Wheat Straw-Effect of Particle Size and Process Conditions. J. Chem. Technol. Biotechnol. 2011, 86, 88–94. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajo, J.C. Kinetic Modelling of Corncob Autohydrolysis. Process biochemistry 2001, 36, 571–578. [Google Scholar] [CrossRef]
- Gullón, B.; Yáñez, R.; Alonso, J.L.; Parajó, J.C. Production of Oligosaccharides and Sugars from Rye Straw: A Kinetic Approach. Bioresour. Technol. 2010, 101, 6676–6684. [Google Scholar] [CrossRef]
- Sun, X.F.; Sun, R.C.; Fowler, P.; Baird, M.S. Extraction and Characterization of Original Lignin and Hemicelluloses from Wheat Straw. J. Agric. Food Chem. 2005, 53, 860–870. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery Valorization of Autohydrolysis Wheat Straw Hemicellulose to Be Applied in a Polymer-Blend Film. Carbohydr. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C. Comparative Study of Hemicelluloses Obtained by Graded Ethanol Precipitation from Sugarcane Bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef] [PubMed]
- Montané, D.; Nabarlatz, D.; Martorell, A.; Torné-Fernández, V.; Fierro, V. Removal of Lignin and Associated Impurities from Xylo-Oligosaccharides by Activated Carbon Adsorption. Ind. Eng. Chem. Res. 2006, 45, 2294–2302. [Google Scholar] [CrossRef]
- Brienzo, M.; Carvalho, A.F.A.; de Figueiredo, F.C.; Neto, P.O. Sugarcane Bagasse Hemicellulose Properties, Extraction Technologies and Xylooligosaccharides Production. In Food Waste; Riley, G.L., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 156–188. ISBN 9781634850254. [Google Scholar]
- Alokika; Anu; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and Hemicellulosic Fractions of Sugarcane Bagasse: Potential, Challenges and Future Perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef]
- Mandelli, F.; Brenelli, L.B.; Almeida, R.F.; Goldbeck, R.; Wolf, L.D.; Hoffmam, Z.B.; Ruller, R.; Rocha, G.J.M.; Mercadante, A.Z.; Squina, F.M. Simultaneous Production of Xylooligosaccharides and Antioxidant Compounds from Sugarcane Bagasse via Enzymatic Hydrolysis. Ind. Crops. Prod. 2014, 52, 770–775. [Google Scholar] [CrossRef]
- Kabel, M.A.; Carvalheiro, F.; Garrote, G.; Avgerinos, E.; Koukios, E.; Parajó, J.C.; Gírio, F.M.; Schols, H.A.; Voragen, A.G.J. Hydrothermally Treated Xylan Rich By-Products Yield Different Classes of Xylo-Oligosaccharides. Carbohydr. Polym. 2002, 50, 47–56. [Google Scholar] [CrossRef]
- de Carvalho, D.M.; Sevastyanova, O.; Penna, L.S.; da Silva, B.P.; Lindström, M.E.; Colodette, J.L. Assessment of Chemical Transformations in Eucalyptus, Sugarcane Bagasse and Straw during Hydrothermal, Dilute Acid, and Alkaline Pretreatments. Ind. Crops. Prod. 2015, 73, 118–126. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Gomez-Molina, M.; Albaladejo-Marico, L.; Yepes-Molina, L.; Nicolas-Espinosa, J.; Navarro-León, E.; Garcia-Ibañez, P.; Carvajal, M. Exploring Phenolic Compounds in Crop By-Products for Cosmetic Efficacy. Int. J. Mol. Sci. 2024, 25, 5884. [Google Scholar] [CrossRef]
- Lima, M.D.S.; Silani, I.D.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic Compounds, Organic Acids and Antioxidant Activity of Grape Juices Produced from New Brazilian Varieties Planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef]
- Panzella, L. Natural Phenolic Compounds for Health, Food and Cosmetic Applications. Antioxidants 2020, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cortés, A.; Sánchez-Motta, T.; Tobar-Tosse, F.; Quimbaya, M. Spectrophotometric Estimation of Total Phenolic Content and Antioxidant Capacity of Molasses and Vinasses Generated from the Sugarcane Industry. Waste Biomass Valorization 2020, 11, 3453–3463. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Carvalho, M.J.; Oliveira, D.L.; Costa, E.; Pintado, M.; Madureira, A.R. Sugarcane Straw Polyphenols as Potential Food and Nutraceutical Ingredient. Foods 2022, 11, 4025. [Google Scholar] [CrossRef] [PubMed]
- Kallel, F.; Driss, D.; Chaabouni, S.E.; Ghorbel, R. Biological Activities of Xylooligosaccharides Generated from Garlic Straw Xylan by Purified Xylanase from Bacillus Mojavensis UEB-FK. Appl. Biochem. Biotechnol. 2015, 175, 950–964. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The Antibiotic Activity and Mechanisms of Sugarcane (Saccharum officinarum L.) Bagasse Extract against Food-Borne Pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef]
- Bratcher, D.F. Other Corynebacteria. In Principles and Practice of Pediatric Infectious Disease, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 759–761. [Google Scholar] [CrossRef]
- Yang, X. Moraxellaceae. In Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 826–833. [Google Scholar] [CrossRef]
- Prieto-Granada, C.N.; Lobo, A.Z.C.; Mihm, M.C. Skin Infections. In Diagnostic Pathology of Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2010; pp. 519–616. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus Epidermidis and Cutibacterium Acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Oliveira, A.L.; Pedrosa, S.S.; Pintado, M.; Madureira, A.R. Potential of Sugarcane Extracts as Cosmetic and Skincare Ingredients. Ind. Crops. Prod. 2021, 169, 113625. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. LAP 2008, 1617, 1–16. [Google Scholar]
- Dávila, I.; Gordobil, O.; Labidi, J.; Gullón, P. Assessment of Suitability of Vine Shoots for Hemicellulosic Oligosaccharides Production through Aqueous Processing. Bioresour. Technol. 2016, 211, 636–644. [Google Scholar] [CrossRef]
- Pablo, G.; Gullón, B.; Pérez-Pérez, A.; Romaní, A.; Garrote, G. Microwave Hydrothermal Processing of the Invasive Macroalgae Sargassum Muticum within a Green Biorefinery Scheme. Bioresour. Technol. 2021, 340, 125733. [Google Scholar]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of Elevated CO2 on Grapevine (Vitis vinifera L.): Volatile Composition, Phenolic Content, and in Vitro Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Wikler, M.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. Clsi Nccls 2006, 26, M7-A7. [Google Scholar]
- Hecht, D.W.; Citron, D.M.; Dzink-Fox, J.; Gregory, W.W.; Jacobus, N.V.; Jenkins, S.G.; Rosenblatt, J.E.; Schuetz, A.N.; Wexler, H. M11-A8 Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard-Eighth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, Number 5. [Google Scholar]
- Pfaller, M.A.; National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard; National Committee for Clinical Laboratory Standards: Villanova, PA, USA, 2002; ISBN 1562384694. [Google Scholar]
- Oliveira, A.L.S.; Gondim, S.; Gómez-García, R.; Ribeiro, T.; Pintado, M. Olive Leaf Phenolic Extract from Two Portuguese Cultivars –Bioactivities for Potential Food and Cosmetic Application. J. Environ. Chem. Eng. 2021, 9, 106175. [Google Scholar] [CrossRef]



| Run # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Ratio S/L | 1/8 | 1/8 | 1/8 | 1/8 | 1/8 | 1/8 | 1/8 | 1/8 | 1/8 |
| Temp (°C) | 150 | 150 | 180 | 180 | 143 | 186 | 165 | 165 | 165 |
| Yield liquid fraction (%) | 6.07 | 9.96 | 22.44 | 24.34 | 6.66 | 21.29 | 15.54 | 21.01 | 16.27 |
| Sugars (g/100 g BM) | 1.355 | 2.372 | 4.515 | 3.094 | 1.534 | 9.146 | 0.842 | 2.695 | 3.580 |
| Contaminants (g/100 g BM) | 0.119 | 0.237 | 3.384 | 3.241 | 0.175 | 3.842 | 0.272 | 0.274 | 0.371 |
| Factors | Estimated Effect of | |
|---|---|---|
| Yield | Degradation Products | |
| Intercept | 17.553 * | 0.395 |
| X1 | 12.635 * | 2.913 * |
| X2 | 3.293 | −0.005 |
| X21 | −3.482 | 1.924 * |
| X22 | 0.399 | 0.142 |
| X1X2 | −0.995 | −0.131 |
| Molecular Weight | |||
|---|---|---|---|
| Run | MW (kDa) | MP (kDa) | Mn (kDa) |
| 1 | 120.06 | 5.81 | 98.78 |
| 2 | 170.23 | 75.92 | 64.41 |
| 3 | 118.17 | 5.84 | 17.05 |
| 4 | 119.23 | 6.56 | 22.39 |
| 5 | 126.29 | 5.99 | 90.62 |
| 6 | 20.96 | 5.25 | 5.92 |
| 7 | 119.14 | 6.18 | 74.43 |
| 8 | 94.63 | 6.02 | 63.00 |
| 9 | 120.64 | 5.93 | 66.28 |
| 10 | 94.85 | 6.14 | 68.48 |
| 11 | 94.40 | 5.82 | 66.54 |
| Run # | 8.1 | 8.2 | 8.3 | 8.4 | 8.5 | 8.6 |
|---|---|---|---|---|---|---|
| Time (min) | 20 | 20 | 30 | 30 | 60 | 45 |
| Yield liquid fraction (%) | 17.17 | 17.80 | 21.98 | 19.73 | 24.55 | 24.46 |
| Sugars (g/100 g BM) | 3.315 | 3.029 | 3.289 | 3.605 | 4.584 | 3.976 |
| Contaminants (g/100 g BM) | 0.241 | 0.247 | 0.347 | 0.274 | 0.991 | 0.733 |
| Hemicellulose Characterization | ||||
|---|---|---|---|---|
| Composition | Molecular Weight | Yield (%) | ||
| Glucose (g/100 g) | 12.46 ± 0.25 | MW (kDa) | 38.44 | 18.80 ± 0.08 |
| Xylose (g/100 g) | 40.33 ± 1.35 | MP (kDa) | 2.57 | |
| Arabinose (g/100 g) | 10.71 ± 0.22 | Mn (kDa) | 17.49 | |
| Sol. Lignin (g/100 g) | 8.35 ± 0.18 | DP | 116.6 | |
| Acetic Acid | 5.34 ± 0.17 | |||
| XOS Characterization | ||||
|---|---|---|---|---|
| Composition | Molecular Weight | Yield (%) | ||
| Glucose (g/100 g) | 9.54 ± 0.05 | MW (kDa) | 14.11 | 9.36 ± 0.02 |
| Xylose (g/100 g) | 38.46 ± 0.28 | MP (kDa) | 0.17 | |
| Arabinose (g/100 g) | 23.00 ± 0.42 | Mn (kDa) | 2.58 | |
| Sol. Lignin (g/100 g) | 10.60 ± 0.40 | DP | 17.20 | |
| Compound | TR (min) | Molecular Formula | m/z Measured [M-H] | MS/MS Fragments (m/z,) | Hemi. Extract (µg/mg DW Extract) | XOS Extract (µg/mg DW Extract) |
|---|---|---|---|---|---|---|
| Organic acids | ||||||
| Quinic acid | 1.4 | C7H11O6 | 191.00 | 85 | 39.84 ± 1.92 | 63.73 ± 0.06 |
| Malic acid | 1.5 | C4H6O5 | 133.01 | 71, 115 | 27.17 ± 0.01 | 48.28 ± 3.35 |
| Dehydroascorbate/Aconitic acid | 1.7 | C6H5O6 | 173.01 | 111 | 10.97 ± 2.04 | 14.03 ± 1.31 |
| Azelaic acid | 13.3 | C9H16O4 | 187.10 | 125, 169, 187 | 10.39 ± 0.53 | 20.70 ± 2.06 |
| ∑ | 88.37 | 146.74 | ||||
| Hydroxybenzoic acids | ||||||
| 2,6-Dihydroxyphenylacetic acid | 6.2 | C8H7O4 | 167.04 | 108, 119, 152 | 1.02 ± 0.19 | 1.52 ± 0.03 |
| 2,3-Dihydroxybenzoic acid | 6.6 | C7H5O4 | 153.02 | 109, 153 | 0.95 ± 0.04 | 1.65 ± 0.02 |
| 4-Hydroxybenzoic acid | 7.9 | C7H5O3 | 137.02 | 137 | 1.96 ± 0.12 | 4.16 ± 0.05 |
| 3,4-Dihydroxybenzaldehyde | 8.1 | C7H5O3 | 137.02 | 93, 137 | 1.77 ± 0.01 | 4.29 ± 0.02 |
| Vanillaldehyde | 8.9 | C8H7O3 | 151.04 | 108 | 1.85 ± 0.32 | 1.68 ± 0.05 |
| 4-Hydroxybenzaldehyde | 9.3 | C7H5O2 | 121.03 | 92 | 18.14 ± 0.03 | 36.72 ± 0.79 |
| ∑ | 25.69 | 50.02 | ||||
| Hydroxycinnamic acids | ||||||
| p-Coumaric acid | 11.1 | C10H9O4 | 163.04 | 119 | 21.45 ± 0.14 | 31.15 ± 0.26 |
| Ferulic acid | 12.0 | C10H9O4 | 193.05 | 134 | 2.49 ± 0.00 | 3.57 ± 0.24 |
| 4-O-Feruloylquinic acid | 14.5 | C9H7O3 | 367.10 | 134, 193 | 0.46 ± 0.02 | 0.83 ± 0.03 |
| ∑ | 24.41 | 35.55 |
| Microorganism | MIC | MBC |
|---|---|---|
| S. aureus | - | - |
| S. epidermis | 1% | - |
| P. aeruginosa | 2% | - |
| E. coli | 2% | - |
| C. jeikeium | 2% | - |
| A. johnsonii | 1% | 3% |
| C. acnes | - | - |
| C. albicans | - | - |
| T. cutaneum | 1% | - |
| R. mucilaginosa | - | - |
| M. furfur | - | - |
| Equipment | Power (kW) | Time (h) | Electricity Consumption (kW.h) | Costs (EUR) |
|---|---|---|---|---|
| Parr reactor | 0.5 | 19.83 | 9.92 | 1.40 |
| Chiller Smart H150-2100 | 2.1 | 19.83 | 41.65 | 5.87 |
| Freeze-dryer | 1.78 | 72 | 128.16 | 18.058 |
| Reagents | Quantity (kg) | Price (EUR/kg) | Cost (EUR) | |
| dH2O (solvent) | 38.993 | 0.0024 | 0.0942 | |
| Total Costs (EUR/kg Biomass) | 25.42 | |||
| Overall costs | Yield (g hemi/kg straw) | Cost (EUR/g Hemicellulose liquor) | Cost (EUR/kg Hemi) | |
| Hemicellulose liquor | 194.66 | 0.130 | 130.57 | |
| Equipment | Power (kW) | Time (h) | Electricity Consumption (kW.h) | Costs (EUR) |
|---|---|---|---|---|
| Parr reactor | 0.5 | 19.83 | 9.92 | 1.40 |
| Chiller Smart H150-2100 | 2.1 | 19.83 | 41.65 | 5.87 |
| Freeze-dryer | 1.78 | 72 | 128.16 | 18.058 |
| Reagents | Quantity (kg) | Price (EUR/kg) | Cost (EUR) | |
| dH2O (solvent) | 76.493 | 0.0024 | 0.1848 | |
| Total Costs (EUR/kg Biomass) | 25.51 | |||
| Overall costs | Yield (g /kg straw) | Cost (EUR/g ingredient) | Cost (EUR/kg ingredient) | |
| XOS | 93.6 | 0.273 | 272.57 | |
| Run # | S/L Ratio | Temp (°C) | Time (min) |
|---|---|---|---|
| 8.1 | 1/18 | 165 | 20 |
| 8.2 | 1/18 | 165 | 20 |
| 8.3 | 1/18 | 165 | 30 |
| 8.4 | 1/18 | 165 | 30 |
| 8.5 | 1/18 | 165 | 60 |
| 8.6 | 1/18 | 165 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.J.; Pedrosa, S.S.; Costa, J.R.; Carvalho, M.J.; Neto, T.; Oliveira, A.L.; Pintado, M.; Madureira, A.R. Sugarcane Straw Hemicellulose Extraction by Autohydrolysis for Cosmetic Applications. Molecules 2025, 30, 1208. https://doi.org/10.3390/molecules30061208
Pereira MJ, Pedrosa SS, Costa JR, Carvalho MJ, Neto T, Oliveira AL, Pintado M, Madureira AR. Sugarcane Straw Hemicellulose Extraction by Autohydrolysis for Cosmetic Applications. Molecules. 2025; 30(6):1208. https://doi.org/10.3390/molecules30061208
Chicago/Turabian StylePereira, Maria João, Sílvia S. Pedrosa, Joana R. Costa, Maria João Carvalho, Tânia Neto, Ana L. Oliveira, Manuela Pintado, and Ana Raquel Madureira. 2025. "Sugarcane Straw Hemicellulose Extraction by Autohydrolysis for Cosmetic Applications" Molecules 30, no. 6: 1208. https://doi.org/10.3390/molecules30061208
APA StylePereira, M. J., Pedrosa, S. S., Costa, J. R., Carvalho, M. J., Neto, T., Oliveira, A. L., Pintado, M., & Madureira, A. R. (2025). Sugarcane Straw Hemicellulose Extraction by Autohydrolysis for Cosmetic Applications. Molecules, 30(6), 1208. https://doi.org/10.3390/molecules30061208










