Molecular Networking from Volatilome of Theobroma grandiflorum (Copoazu) at Different Stages of Maturation Analyzed by HS-SPME-GC-MS
Abstract
1. Introduction
2. Results and Discussions
2.1. Analysis of Volatile Metabolites Using HS-SPME-GC-MS
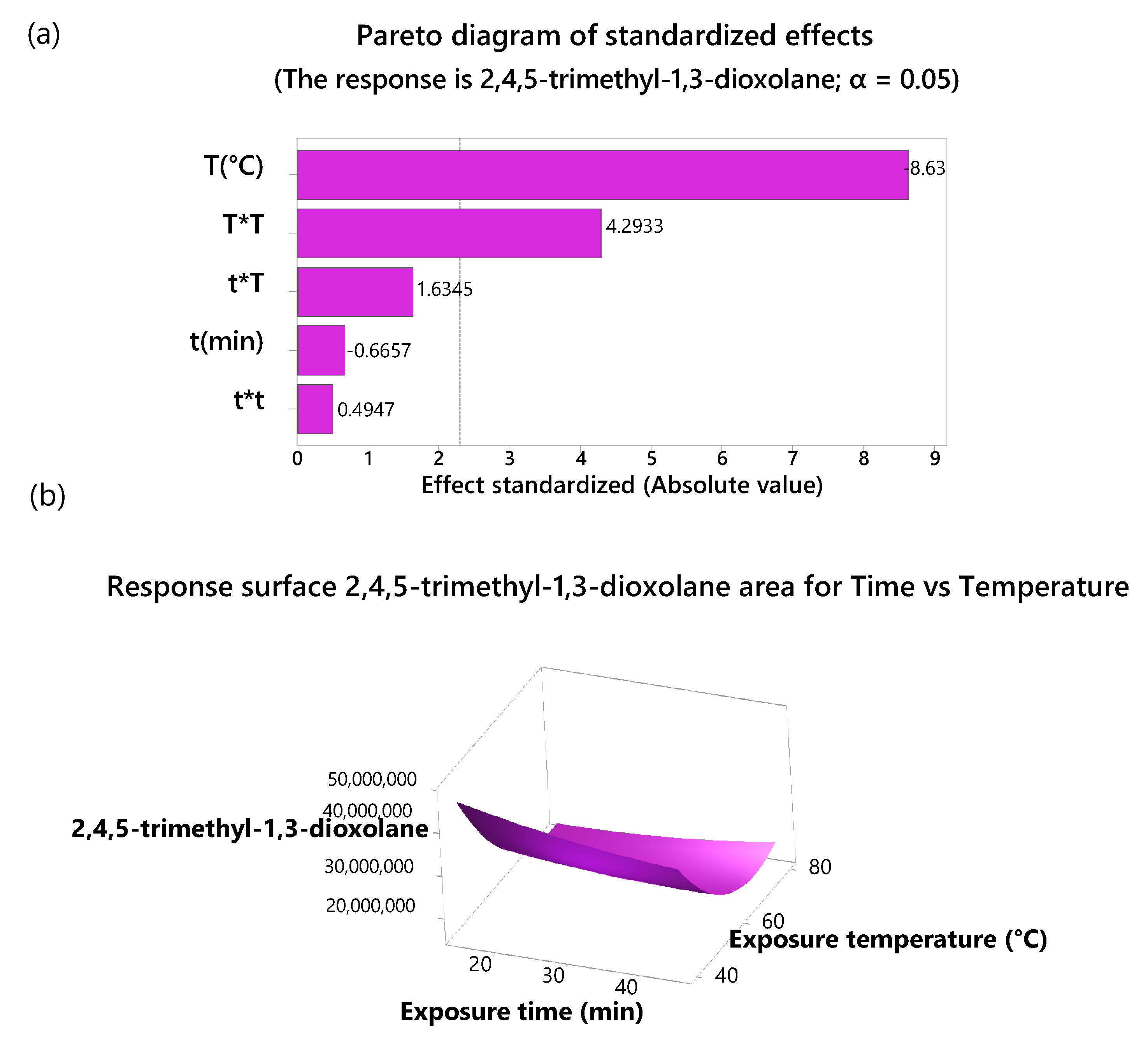
2.2. DoE
2.3. Maturation State Indices
2.3.1. Characterization of Copoazu Maturation States by ATR-FTIR
2.3.2. Carotenoid Analysis by HPLC-DAD
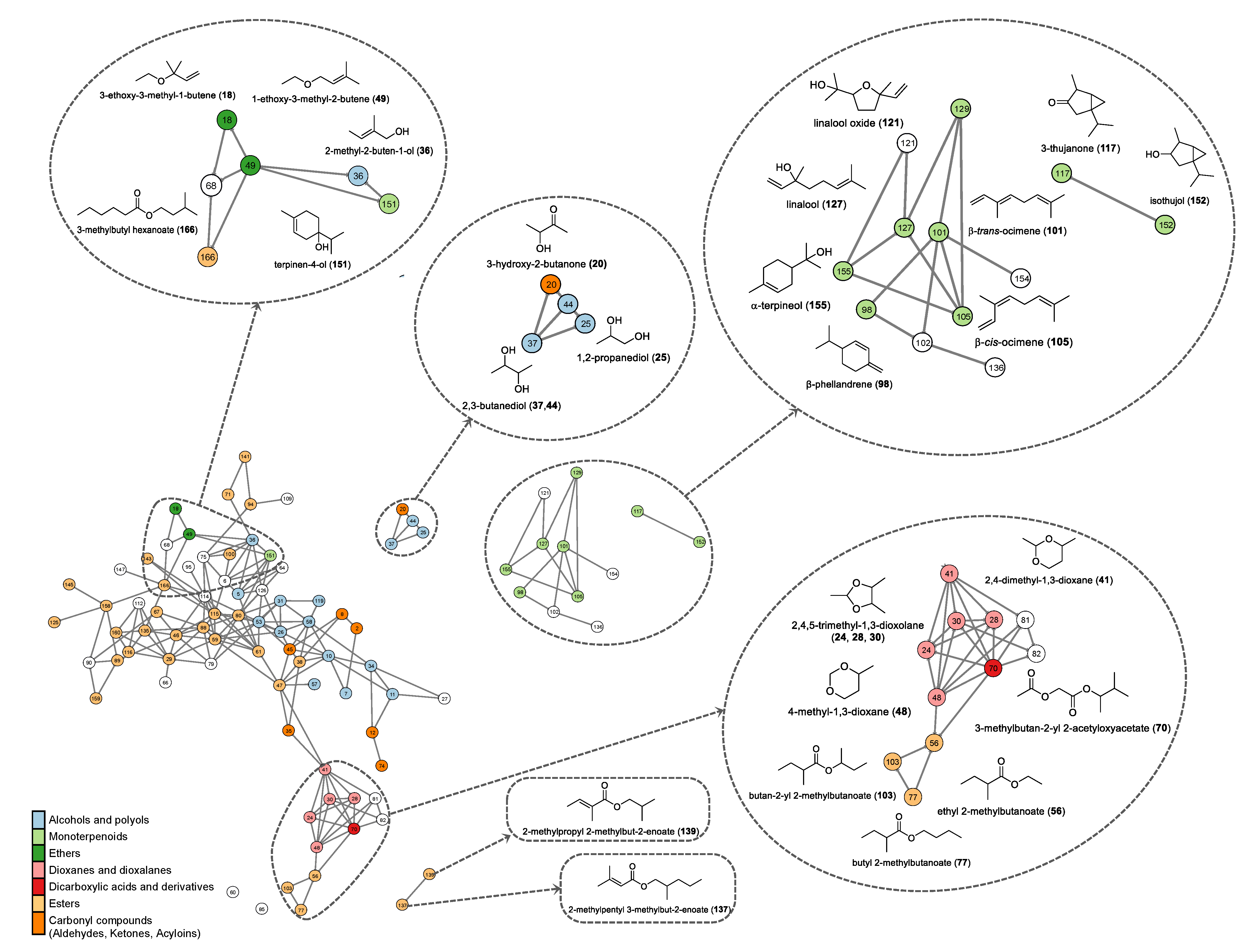
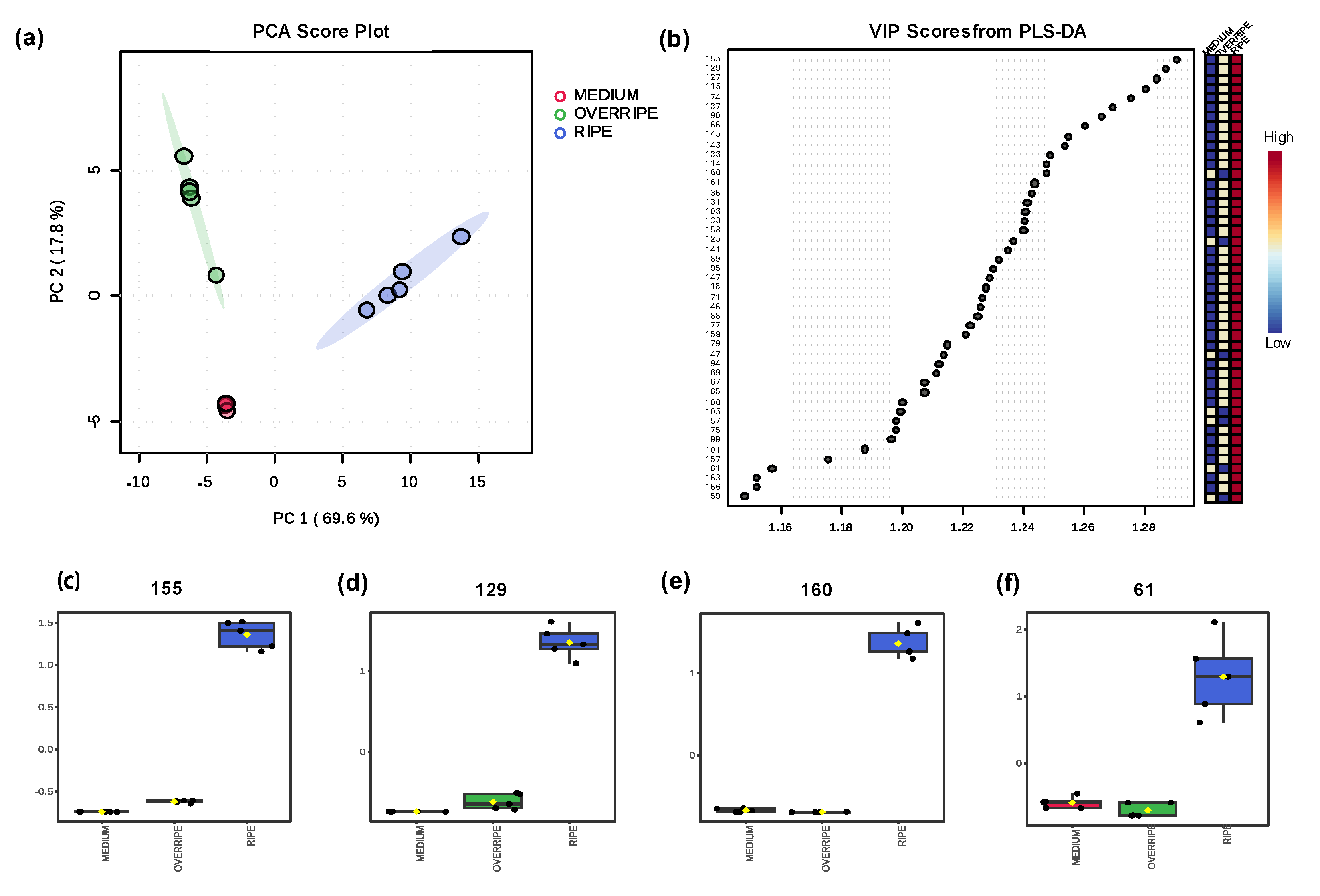
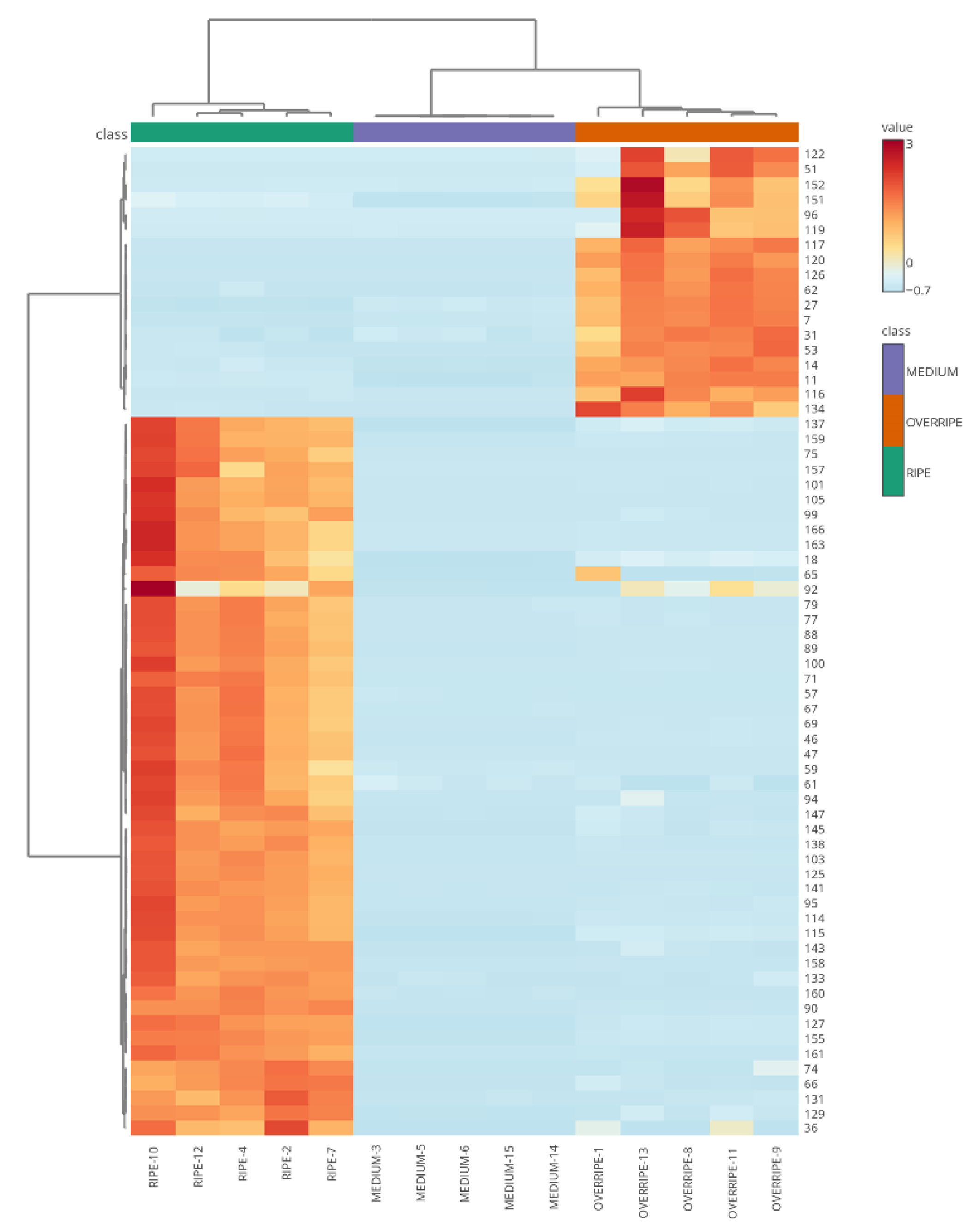
2.4. Volatilomics and Molecular Networking Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Copoazu Collection and Processing
3.3. VOCs Analysis and Optimization
3.3.1. Analysis of Volatile Metabolites Using HS-SPME-GC-MS
3.3.2. Optimization of Analysis Conditions Using Design of Experiments
3.3.3. Quality Control
3.4. Data Treatment, Metabolite Annotation, and Molecular Networking
3.5. Characterization of Copoazu Maturation Stages
3.5.1. pH and Total Soluble Solids (TSSs)
3.5.2. ATR-FTIR Analysis
3.5.3. Carotenoids Analysis by HPLC-DAD
3.5.4. Statistical Analysis for the Characterization of Maturation States of Copoazu
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lagneaux, E.; Andreotti, F.; Neher, C.M. Cacao, copoazu and macambo: Exploring Theobroma diversity in smallholder agroforestry systems of the Peruvian Amazon. Agrofor. Syst. 2021, 95, 1359–1368. [Google Scholar] [CrossRef]
- de Abreu, V.A.C.; Alves, R.M.; Silva, S.R.; Ferro, J.A.; Domingues, D.S.; Miranda, V.F.O.; Varani, A.M. Comparative analyses of Theobroma cacao and T. grandiflorum mitogenomes reveal conserved gene content embedded within complex and plastic structures. Gene 2023, 849, 146904. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.F.; Abreu, V.K.G.; Rodrigues, S. Cupuassu—Theobroma grandiflorum. In Exotic Fruits Reference Guide; Academic Press: Cambridge, MA, USA, 2018; pp. 159–162. [Google Scholar] [CrossRef]
- Albuquerque da Silva, D.; Manoel da Cruz Rodrigues, A.; Oliveira dos Santos, A.; Salvador-Reyes, R.; Meller da Silva, L.H. Physicochemical and technological properties of pracaxi oil, cupuassu fat and palm stearin blends enzymatically interesterified for food applications. LWT 2023, 184, 114961. [Google Scholar] [CrossRef]
- Orduz-Díaz, L.L.; Lozano-Garzón, K.; Quintero-Mendoza, W.; Díaz, R.; Cardona-Jaramillo, J.E.C.; Carrillo, M.P.; Guerrero, D.C.; Hernández, M.S. Effect of Fermentation and Extraction Techniques on the Physicochemical Composition of Copoazú Butter (Theobroma grandiflorum) as an Ingredient for the Cosmetic Industry. Cosmetics 2024, 11, 77. [Google Scholar] [CrossRef]
- Curimbaba, T.F.S.; Almeida-Junior, L.D.; Chagas, A.S.; Quaglio, A.E.V.; Herculano, A.M.; Di Stasi, L.C. Prebiotic, antioxidant and anti-inflammatory properties of edible Amazon fruits. Food Biosci. 2020, 36, 100599. [Google Scholar] [CrossRef]
- Costa, M.P.; Monteiro, M.L.G.; Frasao, B.S.; Silva, V.L.M.; Rodrigues, B.L.; Chiappini, C.C.J.; Conte-Junior, C.A. Consumer perception, health information, and instrumental parameters of cupuassu (Theobroma grandiflorum) goat milk yogurts. J. Dairy Sci. 2017, 100, 157–168. [Google Scholar] [CrossRef]
- de Oliveira, P.D.; da Silva, D.A.; Pires, W.P.; Bezerra, C.V.; da Silva, L.H.M.; da Cruz Rodrigues, A.M. Enzymatic interesterification effect on the physicochemical and technological properties of cupuassu seed fat and inaja pulp oil blends. Food Res. Int. 2021, 145, 110384. [Google Scholar] [CrossRef]
- Melo, S.; Weltman, J.; de Oliveira, A.; Herman, J.; Efraim, P. Cupuassu from bean to bar: Sensory and hedonic characterization of a chocolate-like product. Food Res. Int. 2022, 155, 111039. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Wang, X.; Yuan, Y.; Qi, K.; Zhang, S.; Yin, H. PusALDH1 gene confers high levels of volatile aroma accumulation in both pear and tomato fruits. J. Plant Physiol. 2023, 290, 154101. [Google Scholar] [CrossRef]
- Chen, X.; Fedrizzi, B.; Kilmartin, P.A.; Quek, S.Y. Development of volatile organic compounds and their glycosylated precursors in tamarillo (Solanum betaceum Cav.) during fruit ripening: A prediction of biochemical pathway. Food Chem. 2021, 339, 128046. [Google Scholar] [CrossRef]
- Souza, F.d.C.D.A.; Silva, E.P.; Aguiar, J.P.L. Vitamin characterization and volatile composition of camu-camu (Myrciaria dubia (HBK) McVaugh, Myrtaceae) at different maturation stages. Food Sci. Technol. 2021, 41, 961–966. [Google Scholar] [CrossRef]
- Quijano, C.E.; Pino, J.A. Volatile compounds of copoazú (Theobroma grandiflorum Schumann) fruit. Food Chem. 2007, 104, 1123–1126. [Google Scholar] [CrossRef]
- Boulanger, R.; Crouzet, J. Free and bound flavour components of Amazonian fruits: 3-glycosidically bound components of cupuacu. Food Chem. 2000, 70, 463–470. [Google Scholar] [CrossRef]
- Cabral, C.; Charles, D.J.; Simon, J.E. Volatile Fruit Constituents of Theobroma grandiflorum. HortScience 1991, 26, 608. [Google Scholar] [CrossRef]
- Augusto, F.; Valente, A.L.P.; Tada, E.D.S.; Rivellino, S.R. Screening of Brazilian fruit aromas using solid-phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2000, 873, 117–127. [Google Scholar] [CrossRef]
- Franco, M.R.B.; Shibamoto, T. Volatile Composition of Some Brazilian Fruits: Umbu-caja (Spondias citherea), Camu-camu (Myrciaria dubia), Araça-boi (Eugenia stipitata), and Cupuaçu (Theobroma grandiflorum). J. Agric. Food Chem. 2000, 48, 1263–1265. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Li, W.; Ding, H.; Yin, J.; Bie, S.; Li, F.; Tian, C.; Han, L.; Yang, W.; et al. Characterization of the key volatile organic components of different parts of fresh and dried perilla frutescens based on headspace-gas chromatography-ion mobility spectrometry and headspace solid phase microextraction-gas chromatography-mass spectrometry. Arab. J. Chem. 2023, 16, 104867. [Google Scholar] [CrossRef]
- Carazzone, C.; Rodríguez, J.P.G.; Gonzalez, M.; López, G.-D. Volatilomics of Natural Products: Whispers from Nature. In Metabolomics; Zhan, X., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 4. [Google Scholar] [CrossRef]
- Marinaki, M.; Sampsonidis, I.; Lioupi, A.; Arapitsas, P.; Thomaidis, N.; Zinoviadou, K.; Theodoridis, G. Development of two-level Design of Experiments for the optimization of a HS-SPME-GC-MS method to study Greek monovarietal PDO and PGI wines. Talanta 2023, 253, 123987. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Leardi, R.; Jalali-Heravi, M. Experimental design in analytical chemistry—Part I: Theory. J. AOAC Int. 2014, 97, 3–11. [Google Scholar] [CrossRef]
- Nunes, R.S.; Xavier, G.T.M.; Urzedo, A.L.; Fadini, P.S.; Romeiro, M.; Guimarães, T.G.S.; Labuto, G.; Carvalho, W.A. Cleaner production of iron-coated quartz sand composites for efficient phosphorus adsorption in sanitary wastewater: A design of experiments (DoE) approach. Sustain. Chem. Pharm. 2023, 35, 101206. [Google Scholar] [CrossRef]
- Galeano, P.G.; Zimmermann, B.H.; Carazzone, C. Hydrophilic interaction liquid chromatography coupled to mass spectrometry and multivariate analysis of the de novo pyrimidine pathway metabolites. Biomolecules 2019, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Gorbounov, M.; Taylor, J.; Petrovic, B.; Masoudi Soltani, S. To DoE or not to DoE? A Technical Review on & Roadmap for Optimisation of Carbonaceous Adsorbents and Adsorption Processes. S. Afr. J. Chem. Eng. 2022, 41, 111–128. [Google Scholar] [CrossRef]
- Chen, W.-H.; Uribe, M.C.; Kwon, E.E.; Lin, K.-Y.A.; Park, Y.-K.; Ding, L.; Saw, L.H. A comprehensive review of thermoelectric generation optimization by statistical approach: Taguchi method, analysis of variance (ANOVA), and response surface methodology (RSM). Renew. Sustain. Energy Rev. 2022, 169, 112917. [Google Scholar] [CrossRef]
- Baena, A.; Londoño, L.M.; Taborda, G. Volatilome study of the feijoa fruit [Acca sellowiana (O. Berg) Burret.] with headspace solid phase microextraction and gas chromatography coupled with mass spectrometry. Food Chem. 2020, 328, 127109. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Meghwal, M.; Prabhakar, P.K.; Kumar, N. Grinding characteristics and energy consumption in cryogenic and ambient grinding of ajwain seeds at varied moisture contents. Powder Technol. 2022, 405, 117531. [Google Scholar] [CrossRef]
- Gonzalez, M.; Palacios-Rodriguez, P.; Hernandez-Restrepo, J.; González-Santoro, M.; Amézquita, A.; Brunetti, A.E.; Carazzone, C. First characterization of toxic alkaloids and volatile organic compounds (VOCs) in the cryptic dendrobatid Silverstoneia punctiventris. Front. Zool. 2021, 18, 39. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of Solid-Phase Microextraction. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Elsevier: Oxford, UK, 2012; pp. 13–59. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Hosseini, S.V. Proteins, peptides, and amino acids. In Nutraceutical and Functional Food Components; Galanakis, C., Ed.; Elsevier: Chania, Greece, 2017; pp. 5–384. [Google Scholar] [CrossRef]
- Metrani, R.; Jayaprakasha, G.K.; Patil, B.S. Optimization of Experimental Parameters and Chemometrics Approach to Identify Potential Volatile Markers in Seven Cucumis melo Varieties Using HS–SPME–GC–MS. Food Anal. Methods 2022, 15, 607–624. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Kaewseejan, N. Quality, bioactive compounds and antioxidant capacity of selected climacteric fruits with relation to their maturity. Sci. Hortic. 2017, 221, 33–42. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Y.; Jia, C.; Zhang, H.; Gan, Z.; Li, X.; Yang, M.; Yin, Y.; Zhang, G.; Hao, J.; et al. Physiological and biochemical changes during fruit maturation and ripening in highbush blueberry (Vaccinium corymbosum L.). Food Chem. 2023, 410, 135299. [Google Scholar] [CrossRef]
- Macarena, L.S.; María, J.A.C.; Antonio, M.D. Olive fruit growth and ripening as seen by vibrational spectroscopy. J. Agric. Food Chem. 2010, 58, 82–87. [Google Scholar] [CrossRef]
- González, M.; Domínguez, A.; Ayora, M.J. Hyperspectral FTIR imaging of olive fruit for understanding ripening processes. Postharvest Biol. Technol. 2018, 145, 74–82. [Google Scholar] [CrossRef]
- Skolik, P.; Morais, C.L.M.; Martin, F.L.; McAinsh, M.R. Determination of developmental and ripening stages of whole tomato fruit using portable infrared spectroscopy and Chemometrics. BMC Plant Biol. 2019, 19, 236. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Yang, J.; Liu, J.; Jiang, Y.; Yang, B. The structure changes of water-soluble polysaccharides in papaya during ripening. Int. J. Biol. Macromol. 2018, 115, 152–156. [Google Scholar] [CrossRef]
- Naranjo, A.M.; Quintero, J.; Ciro, G.L.; Barona, M.J.; Contreras, J.d.C. Characterization of the antioxidant activity, carotenoid profile by HPLC-MS of exotic colombian fruits (goldenberry and purple passion fruit) and optimization of antioxidant activity of this fruit blend. Heliyon 2023, 9, e17819. [Google Scholar] [CrossRef] [PubMed]
- López, G.D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef] [PubMed]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DAD-APCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef]
- Schex, R.; Lieb, V.M.; Jiménez, V.M.; Esquivel, P.; Schweiggert, R.M.; Carle, R.; Steingass, C.B. HPLC-DAD-APCI/ESI-MSn analysis of carotenoids and α-tocopherol in Costa Rican Acrocomia aculeata fruits of varying maturity stages. Food Res. Int. 2018, 105, 645–653. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Y.; Yin, W.; Zhang, L.; Li, G.; Ma, J.; Xu, L.; Xiong, Y.; Liu, L.; Zhang, W.; et al. Neoxanthin alleviates the chronic renal failure-induced aging and fibrosis by regulating inflammatory process. Int. Immunopharmacol. 2023, 114, 109429. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef]
- Aksenov, A.A.; Laponogov, I.; Zhang, Z.; Doran, S.L.F.; Belluomo, I.; Veselkov, D.; Bittremieux, W.; Nothias, L.F.; Nothias-Esposito, M.; Maloney, K.N.; et al. Auto-deconvolution and molecular networking of gas chromatography–mass spectrometry data. Nat. Biotechnol. 2020, 39, 169–173. [Google Scholar] [CrossRef]
- Garg, N.; Sethupathy, A.; Tuwani, R.; Nk, R.; Dokania, S.; Iyer, A.; Gupta, A.; Agrawal, S.; Singh, N.; Shukla, S.; et al. FlavorDB: A database of flavor molecules. Nucleic Acids Res. 2018, 46, D1210–D1216. [Google Scholar] [CrossRef] [PubMed]
- Aragüez, I.; Valpuesta, V. Metabolic engineering of aroma components in fruits. Biotechnol. J. 2013, 8, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Giannone, R.J.; Yang, Y.H.; Trinh, C.T. Proteome reallocation enables the selective de novo biosynthesis of non-linear, branched-chain ethanoate esters. Metab. Eng. 2022, 73, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Klie, S.; Osorio, S.; Tohge, T.; Drincovich, M.F.; Fait, A.; Giovannoni, J.J.; Fernie, A.R.; Nikoloski, Z. Conserved changes in the dynamics of metabolic processes during fruit development and ripening across species. Plant Physiol. 2014, 164, 55–68. [Google Scholar] [CrossRef]
- Barrios, J.C.; Sinuco, D.C.; Morales, A.L. Compuestos volátiles libres y enlazados glicosídicamente en la pulpa de la uva Caimarona (Pourouma cecropiifolia Mart.). Acta Amaz. 2010, 40, 189–198. [Google Scholar] [CrossRef]
- Padilla-Jiménez, S.M.; Angoa-Pérez, M.V.; Mena-Violante, H.G.; Oyoque-Salcedo, G.; Montañez-Soto, J.L.; Oregel-Zamudio, E. Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits. Molecules 2021, 26, 504. [Google Scholar] [CrossRef]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef]
- Ferenczi, A.; Sugimoto, N.; Beaudry, R.M. Emission Patterns of Esters and Their Precursors Throughout Ripening and Senescence in ‘Redchief Delicious’ Apple Fruit and Implications Regarding Biosynthesis and Aroma Perception. J. Am. Soc. Hortic. Sci. 2021, 146, 297–328. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Eskin, N.A.M.; Hoehn, E. Fruits and Vegetables. In Biochemistry of Foods; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 49–126. [Google Scholar] [CrossRef]
- Liu, X.; Hao, N.; Feng, R.; Meng, Z.; Li, Y.; Zhao, Z. Transcriptome and metabolite profiling analyses provide insight into volatile compounds of the apple cultivar ‘Ruixue’ and its parents during fruit development. BMC Plant Biol. 2021, 21, 231. [Google Scholar] [CrossRef]
- Granell, A.; Rambla, J.L. Biosynthesis of Volatile Compounds. In The Molecular Biology and Biochemistry of Fruit Ripening; Seymour, G.B., Poole, M., Giovannoni, J.J., Tucker, G.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 135–161. [Google Scholar] [CrossRef]
- Yue, R.; Zhang, Z.; Shi, Q.; Duan, X.; Wen, C.; Shen, B.; Li, X. Identification of the key genes contributing to the LOX-HPL volatile aldehyde biosynthesis pathway in jujube fruit. Int. J. Biol. Macromol. 2022, 222, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Loviso, C.L.; Libkind, D. Síntesis y regulación de compuestos del aroma y el sabor derivados de la levadura en la cerveza: Ésteres. Rev. Argent. Microbiol. 2018, 50, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.N.; Yang, Y.N.; Yang, Y.; Liang, M.; Zheng, F.P.; Sun, B.G. Free and Bound Aroma Compounds of Turnjujube (Hovenia acerba Lindl.) during Low Temperature Storage. Foods 2020, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral Scents and Fruit Aromas: Functions, Compositions, Biosynthesis, and Regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, R.; Rodrigues, N.; Marx, Í.M.G.; Dias, L.G.; Veloso, A.C.A.; Ramos, A.C.; Peres, A.M.; Pereira, J.A. Sweet peppers discrimination according to agronomic production mode and maturation stage using a chemical-sensory approach and an electronic tongue. Microchem. J. 2020, 157, 105034. [Google Scholar] [CrossRef]
- Vazquez, L.; Celeiro, M.; Sergazina, M.; Dagnac, T.; Llompart, M. Optimization of a miniaturized solid-phase microextraction method followed by gas chromatography mass spectrometry for the determination of twenty four volatile and semivolatile compounds in honey from Galicia (NW Spain) and foreign countries. Sustain. Chem. Pharm. 2021, 21, 100451. [Google Scholar] [CrossRef]
- Chambers, M.C.; MacLean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Hackstadt, A.J.; Hess, A.M. Filtering for increased power for microarray data analysis. BMC Bioinform. 2009, 10, 11. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
- Liu, J.; Clarke, J.-A.; McCann, S.; Hillier, N.K.; Tahlan, K. Analysis of Streptomyces Volatilomes Using Global Molecular Networking Reveals the Presence of Metabolites with Diverse Biological Activities. Microbiol. Spectr. 2022, 10, e0055222. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Feunang, Y.D.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform 2016, 8, 61. [Google Scholar] [CrossRef]
- Lan, W.; Renard, C.M.G.C.; Jaillais, B.; Leca, A.; Bureau, S. Fresh, freeze-dried or cell wall samples: Which is the most appropriate to determine chemical, structural and rheological variations during apple processing using ATR-FTIR spectroscopy? Food Chem. 2020, 330, 127357. [Google Scholar] [CrossRef] [PubMed]
- López, G.D.; Suesca, E.; Álvarez-Rivera, G.; Rosato, A.E.; Ibáñez, E.; Cifuentes, A.; Leidy, C.; Carazzone, C. Carotenogenesis of Staphylococcus Aureus: New Insights and Impact on Membrane Biophysical Properties. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158941. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, L.; Suesca, E.; López, G.D.; Carazzone, C.; Manrique, M.; Leidy, C. Staphylococcus aureus Modulates Carotenoid and Phospholipid Content in Response to Oxygen-Restricted Growth Conditions, Triggering Changes in Membrane Biophysical Properties. Int. J. Mol. Sci. 2023, 24, 14906. [Google Scholar] [CrossRef]
- Agbangba, C.E.; Sacla Aide, E.; Honfo, H.; Glèlè Kakai, R. On the use of post-hoc tests in environmental and biological sciences: A critical review. Heliyon 2024, 10, e25131. [Google Scholar] [CrossRef]

| GNPS Scan Number | tR | Metabolite | Formula | Confidence Level * | Identification | ClassyFire (SubClass) |
|---|---|---|---|---|---|---|
| 5 | 2.30 | 2-methylbut-3-en-2-ol | C5H10O | 3 | NIST | Alcohols and polyols |
| 7 | 2.47 | 2-methylpropan-1-ol | C4H10O | 2 | GNPS, NIST | Alcohols and polyols |
| 10 | 2.93 | butan-1-ol | C4H10O | 2 | GNPS, NIST | Alcohols and polyols |
| 11 | 3.22 | pent-1-en-3-ol | C5H10O | 2 | GNPS, NIST | Alcohols and polyols |
| 14 | 3.53 | pentan-3-ol | C5H12O | 2 | GNPS, NIST | Alcohols and polyols |
| 25 | 4.26 | propane-1,2-diol | C3H8O2 | 3 | GNPS | Alcohols and polyols |
| 26 | 4.35 | 3-methylbutan-1-ol | C5H12O | 2 | GNPS, NIST | Alcohols and polyols |
| 27 | 4.44 | 2-methylbutan-1-ol | C5H12O | 2 | GNPS, NIST | Alcohols and polyols |
| 31 | 5.41 | pentan-1-ol | C5H12O | 3 | NIST | Alcohols and polyols |
| 34 | 5.53 | pent-2-en-1-ol | C5H10O | 2 | GNPS, NIST | Alcohols and polyols |
| 36 | 5.68 | 2-methylbut-2-en-1-ol | C5H10O | 2 | GNPS, NIST | Alcohols and polyols |
| 37 | 5.79 | 2,3-butanediol (Isomer I) | C4H10O2 | 2 | GNPS, NIST | Alcohols and polyols |
| 44 | 6.27 | 2,3-butanediol (Isomer II) | C4H10O2 | 3 | GNPS | Alcohols and polyols |
| 53 | 8.58 | 2-ethylbutan-1-ol | C6H14O | 2 | GNPS, NIST | Alcohols and polyols |
| 58 | 10.25 | hexan-1-ol | C6H14O | 2 | GNPS, NIST | Fatty alcohols |
| 119 | 24.12 | 2-ethyl-1-hexanol | C8H18O | 4 | GNPS | Alcohols and polyols |
| 2 | 2.15 | butanal | C4H8O | 3 | NIST | Carbonyl compounds |
| 8 | 2.78 | 3-methylbutanal | C5H10O | 2 | GNPS, NIST | Carbonyl compounds |
| 12 | 3.46 | pentan-3-one | C5H10O | 3 | NIST | Carbonyl compounds |
| 20 | 3.74 | 3-hydroxy-2-butanone | C4H8O2 | 3 | NIST | Carbonyl compounds |
| 35 | 5.60 | 3-methylpentan-2-one | C6H12O | 2 | GNPS, NIST | Carbonyl compounds |
| 45 | 6.50 | hexanal | C6H12O | 3 | GNPS | Carbonyl compounds |
| 74 | 15.11 | 5-hydroxy-2,7-dimethyl-4-octanone | C10H20O2 | 3 | GNPS | Carbonyl compounds |
| 84 | 18.77 | 6-methyl-5-hepten-2-one | C8H14O | 2 | GNPS, NIST | Carbonyl compounds |
| 131 | 25.49 | nonanal | C9H18O | 3 | NIST | Carbonyl compounds |
| 18 | 3.65 | 3-ethoxy-3-methylbut-1-ene | C7H14O | 3 | NIST | Ethers |
| 49 | 7.75 | 1-ethoxy-3-methylbut-2-ene | C7H14O | 2 | GNPS, NIST | Ethers |
| 64 | 11.30 | 3-butoxy-2-methylbut-1-ene | C9H18O | 4 | NIST | Ethers |
| 87 | 19.18 | β-myrcene | C10H16 | 1 | NIST, STD | Monoterpenoids |
| 98 | 21.45 | β-phellandrene | C10H16 | 3 | GNPS | Monoterpenoids |
| 99 | 21.45 | limonene | C10H16 | 1 | GNPS, NIST, STD | Monoterpenoids |
| 101 | 22.27 | β-trans-ocimene | C10H16 | 1 | GNPS, NIST, STD | Monoterpenoids |
| 105 | 22.82 | β-cis-ocimene | C10H16 | 1 | GNPS, NIST, STD | Monoterpenoids |
| 117 | 23.98 | 3-thujanone | C10H16O | 3 | GNPS | Monoterpenoids |
| 121 | 24.68 | cis-linalool oxide | C10H18O2 | 3 | GNPS, NIST | Tetrahydrofurans |
| 120 | 24.68 | trans-linalool oxide | C10H18O2 | 2 | NIST | Tetrahydrofurans |
| 127 | 25.35 | linalool | C10H18O | 1 | GNPS, NIST, STD | Monoterpenoids |
| 129 | 25.44 | trans-4-methoxythujane | C11H20O | 3 | GNPS | Monoterpenoids |
| 152 | 28.30 | isothujol | C10H18O | 3 | GNPS | Monoterpenoids |
| 155 | 28.75 | α-terpineol | C10H18O | 1 | GNPS, NIST, STD | Monoterpenoids |
| 24 | 4.15 | 2,4,5-trimethyl-1,3-dioxolane (Isomer I) | C6H12O2 | 2 | GNPS, NIST | 1,3-dioxolanes |
| 28 | 4.88 | 2,4,5-trimethyl-1,3-dioxolane (Isomer II) | C6H12O2 | 2 | GNPS, NIST | 1,3-dioxolanes |
| 30 | 5.33 | 2,4,5-trimethyl-1,3-dioxolane (Isomer III) | C6H12O2 | 2 | GNPS, NIST | 1,3-dioxolanes |
| 41 | 6.12 | 2,4-dimethyl-1,3-dioxane | C6H12O2 | 2 | GNPS, NIST | 1,3-dioxanes |
| 48 | 7.57 | 4-methyl-1,3-dioxane | C5H10O2 | 2 | GNPS, NIST | 1,3-dioxanes |
| 54 | 8.96 | 2,4,6-trimethyl-1,3-dioxane | C7H14O2 | 3 | NIST | 1,3-dioxanes |
| 70 | 13.38 | 3-methylbutan-2-yl 2-acetyloxyethanoate | C9H16O4 | 3 | GNPS | Dicarboxylic acids and derivatives |
| 29 | 5.10 | ethyl 2-methylpropanoate | C6H12O2 | 2 | GNPS, NIST | Carboxylic acid derivatives |
| 46 | 6.64 | ethyl butanoate | C6H12O2 | 2 | GNPS, NIST | Carboxylic acid derivatives |
| 47 | 7.25 | butyl ethanoate | C6H12O2 | 2 | GNPS, NIST | Fatty acid esters |
| 56 | 9.07 | ethyl 2-methylbutanoate | C7H14O2 | 2 | GNPS, NIST | Fatty acid esters |
| 59 | 10.67 | 3-methylbutyl ethanoate | C7H14O2 | 2 | GNPS, NIST | Carboxylic acid derivatives |
| 61 | 10.83 | 2-methylbutyl ethanoate | C7H14O2 | 2 | GNPS, NIST | Carboxylic acid derivatives |
| 69 | 12.26 | ethyl pentanoate | C7H14O2 | 3 | GNPS | Fatty acid esters |
| 71 | 13.87 | 3-methylbut-2-enyl ethanoate | C7H12O2 | 2 | GNPS, NIST | Carboxylic acid derivatives |
| 86 | 18.92 | ethyl 5-hexenoate | C8H14O2 | 2 | GNPS, NIST | Fatty acid esters |
| 88 | 19.62 | butyl butanoate | C8H16O2 | 2 | GNPS, NIST | Fatty acid esters |
| 89 | 19.95 | ethyl hexanoate | C8H16O2 | 2 | GNPS, NIST | Fatty acid esters |
| 94 | 20.67 | ethyl-4-hexenoate | C8H14O2 | 2 | GNPS, NIST | Fatty acid esters |
| 100 | 22.10 | pent-4-en-1-yl butanoate | C9H16O2 | 3 | GNPS | Fatty acid esters |
| 103 | 22.53 | butan-2-yl 2-methylbutanoate | C9H18O2 | 3 | GNPS, NIST | Fatty acid esters |
| 115 | 23.43 | 2-methylbutyl butanoate | C9H18O2 | 2 | GNPS, NIST | Fatty acid esters |
| 116 | 23.56 | pentan-2-yl propyl carbonate | C9H18O3 | 3 | GNPS | Carbonic acid diesters |
| 125 | 25.20 | propyl hexanoate | C9H18O2 | 2 | GNPS, NIST | Fatty acid esters |
| 137 | 26.26 | 2-methylpentyl 3-methylbut-2-enoate | C11H20O2 | 3 | GNPS | Fatty acid esters |
| 139 | 26.77 | 2-methylpropyl 2-methylbut-2-enoate | C9H16O2 | 3 | GNPS | Fatty acid esters |
| 141 | 27.05 | 3-methylbut-2-en-1-yl pivalate | C10H18O2 | 2 | GNPS, NIST | Carboxylic acid derivatives |
| 143 | 27.38 | 4-methylpentyl butanoate | C10H20O2 | 3 | GNPS | Fatty acid esters |
| 145 | 27.44 | 2-methylpropyl hexanoate | C10H20O2 | 2 | GNPS, NIST | Fatty acid esters |
| 157 | 28.81 | ethyl oct-4-enoate | C10H18O2 | 3 | NIST | Fatty acid esters |
| 158 | 28.88 | butyl hexanoate | C10H20O2 | 2 | GNPS, NIST | Fatty acid esters |
| 159 | 29.11 | ethyl octanoate | C10H20O2 | 2 | GNPS, NIST | Fatty acid esters |
| 160 | 30.24 | 1,2-dibutoxyethane | C10H18O4 | 2 | GNPS, NIST | Fatty acid esters |
| 161 | 30.56 | ethyl 2-phenylethanoate | C10H12O2 | 2 | GNPS, NIST | Benzene and substituted derivatives |
| 163 | 30.60 | 2-ethylphenyl ethanoate | C10H12O2 | 2 | GNPS, NIST | Phenol esters |
| 166 | 30.81 | 3-methylbutyl hexanoate | C11H22O2 | 2 | GNPS, NIST | Fatty acid esters |
| 57 | 9.27 | 2,2-dimethylpentanoic acid | C7H14O2 | 3 | GNPS | Fatty acids and conjugates |
| 63 | 11.20 | ethenylbenzene | C8H8 | 3 | GNPS | Styrenes |
| 109 | 23.00 | 2,7-dimethyl-2,6-octadiene | C10H18 | 2 | GNPS, NIST | Branched unsaturated hydrocarbons |
| 33 | 5.41 | unknown | - | 4 | - | unknown |
| 43 | 6.27 | unknown | - | 4 | - | unknown |
| 51 | 7.80 | unknown | - | 4 | - | unknown |
| 65 | 11.30 | unknown | - | 4 | - | unknown |
| 75 | 15.34 | unknown | - | 4 | - | unknown |
| 77 | 15.34 | unknown | - | 4 | - | unknown |
| 79 | 16.16 | unknown | - | 4 | - | unknown |
| 80 | 16.33 | unknown | - | 4 | - | unknown |
| 90 | 20.07 | unknown | - | 4 | - | unknown |
| 92 | 20.38 | unknown | - | 4 | - | unknown |
| 95 | 20.80 | unknown | - | 4 | - | unknown |
| 114 | 23.30 | unknown | - | 4 | - | unknown |
| 122 | 25.16 | unknown | - | 4 | - | unknown |
| 126 | 25.30 | unknown | - | 4 | - | unknown |
| 133 | 25.53 | unknown | - | 4 | - | unknown |
| 147 | 27.76 | unknown | - | 4 | - | unknown |
| 151 | 28.25 | unknown | - | 4 | - | unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia, M.; Pérez-Beltrán, M.; López, G.-D.; Carazzone, C.; Galeano Garcia, P. Molecular Networking from Volatilome of Theobroma grandiflorum (Copoazu) at Different Stages of Maturation Analyzed by HS-SPME-GC-MS. Molecules 2025, 30, 1209. https://doi.org/10.3390/molecules30061209
Valencia M, Pérez-Beltrán M, López G-D, Carazzone C, Galeano Garcia P. Molecular Networking from Volatilome of Theobroma grandiflorum (Copoazu) at Different Stages of Maturation Analyzed by HS-SPME-GC-MS. Molecules. 2025; 30(6):1209. https://doi.org/10.3390/molecules30061209
Chicago/Turabian StyleValencia, Mayrin, Mónica Pérez-Beltrán, Gerson-Dirceu López, Chiara Carazzone, and Paula Galeano Garcia. 2025. "Molecular Networking from Volatilome of Theobroma grandiflorum (Copoazu) at Different Stages of Maturation Analyzed by HS-SPME-GC-MS" Molecules 30, no. 6: 1209. https://doi.org/10.3390/molecules30061209
APA StyleValencia, M., Pérez-Beltrán, M., López, G.-D., Carazzone, C., & Galeano Garcia, P. (2025). Molecular Networking from Volatilome of Theobroma grandiflorum (Copoazu) at Different Stages of Maturation Analyzed by HS-SPME-GC-MS. Molecules, 30(6), 1209. https://doi.org/10.3390/molecules30061209







