Physicochemical Characteristics, Antioxidant Properties, and Identification of Bioactive Compounds in Australian Stingless Bee Honey Using High-Performance Thin-Layer Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Parameters
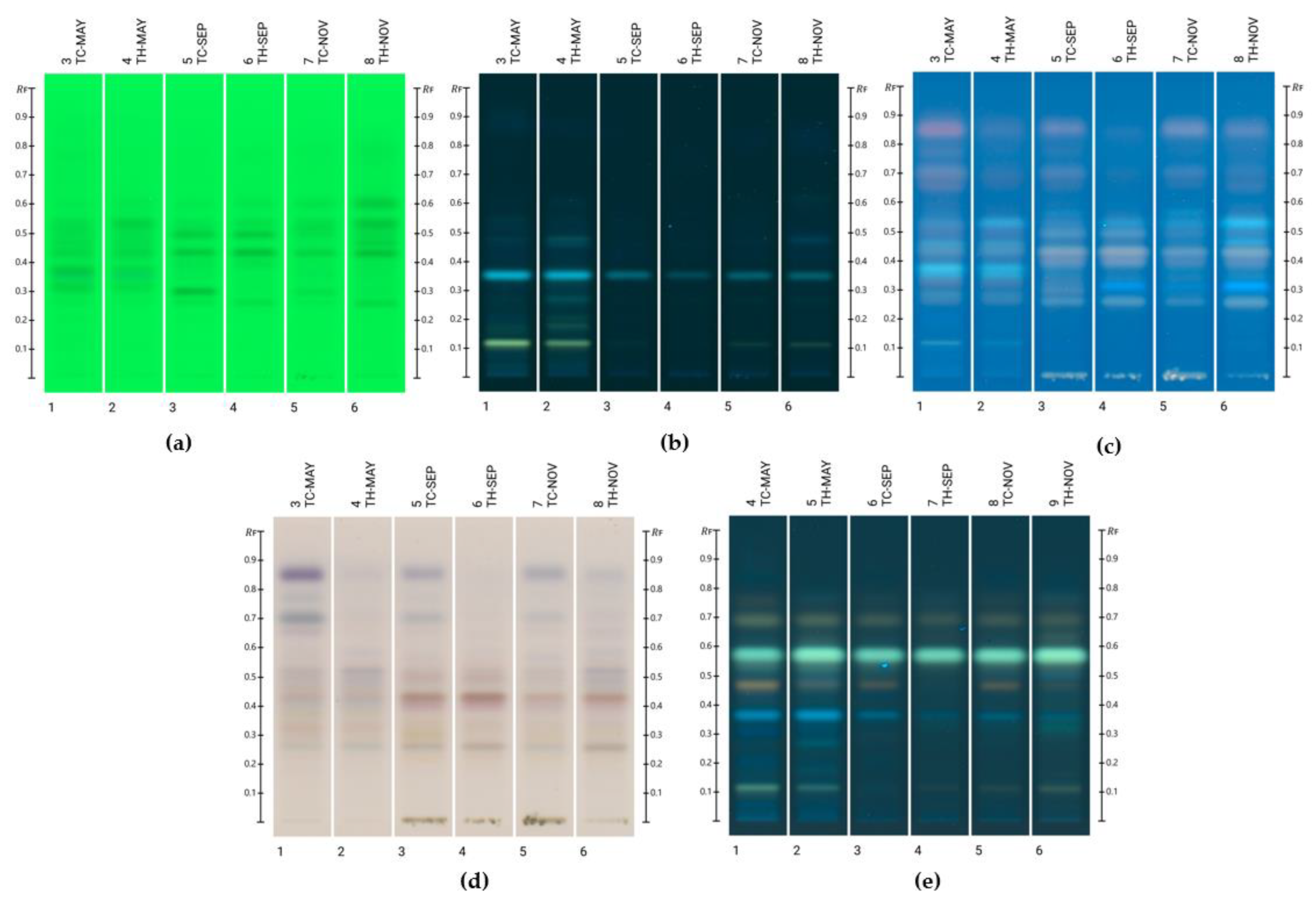
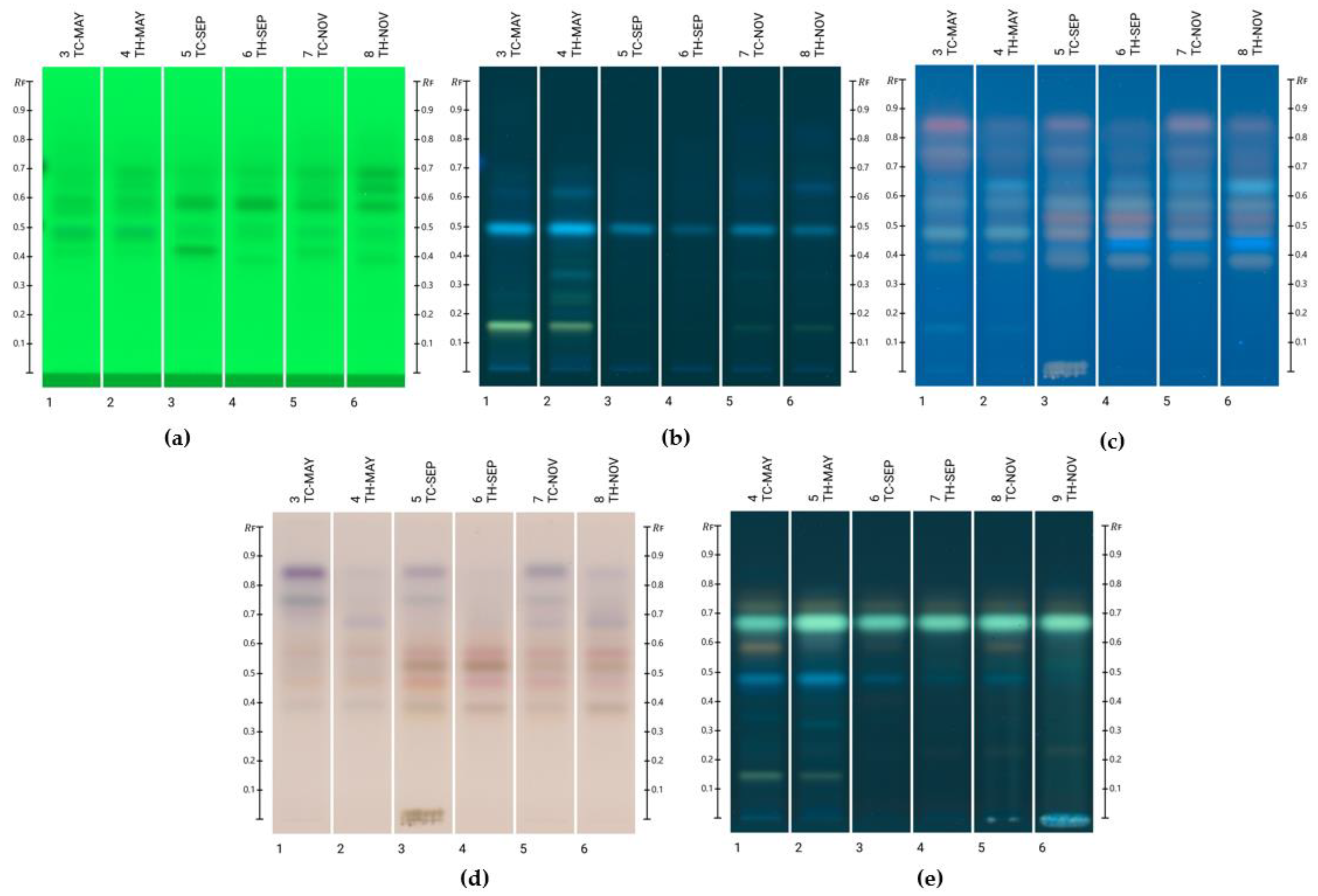
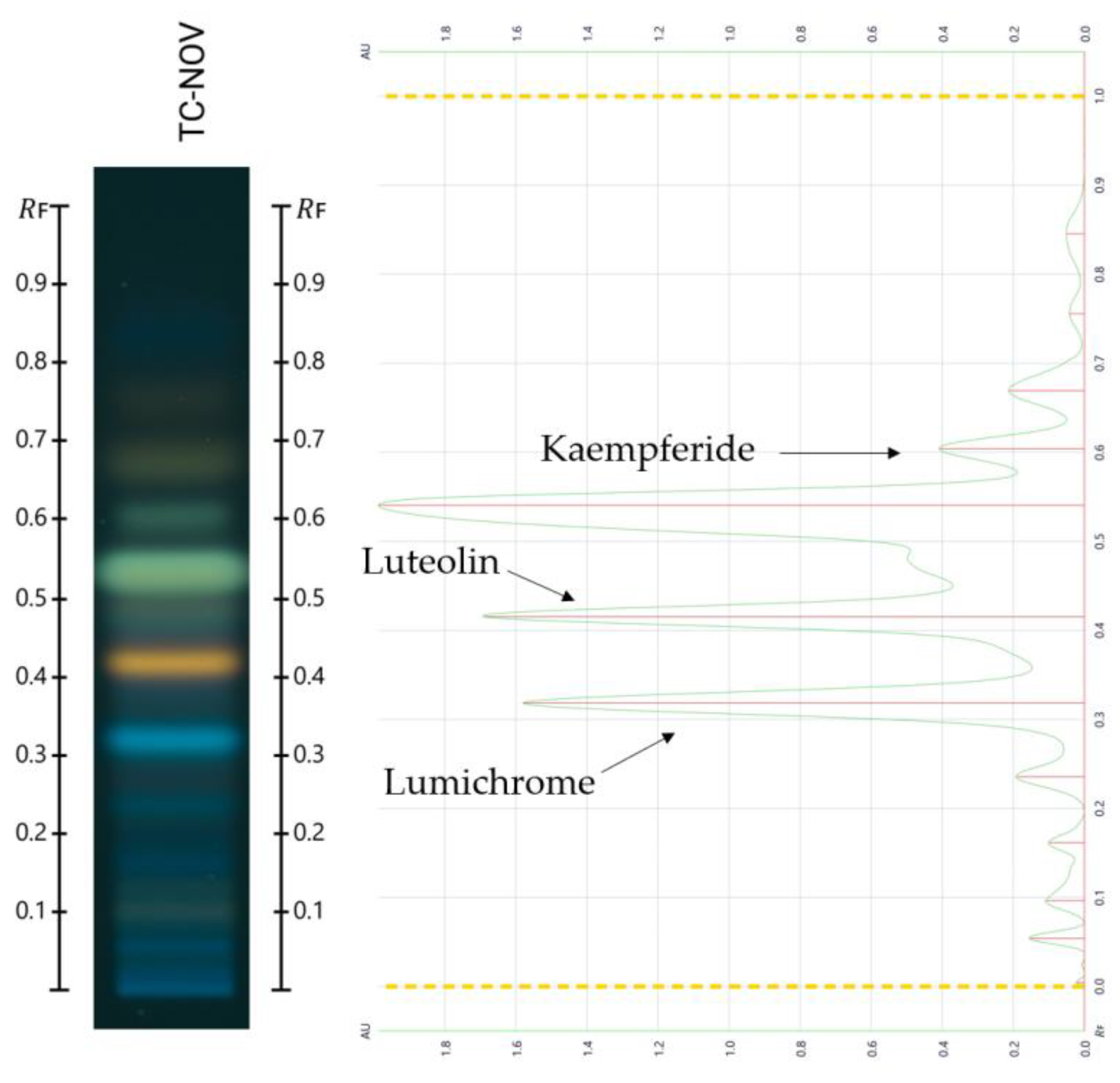
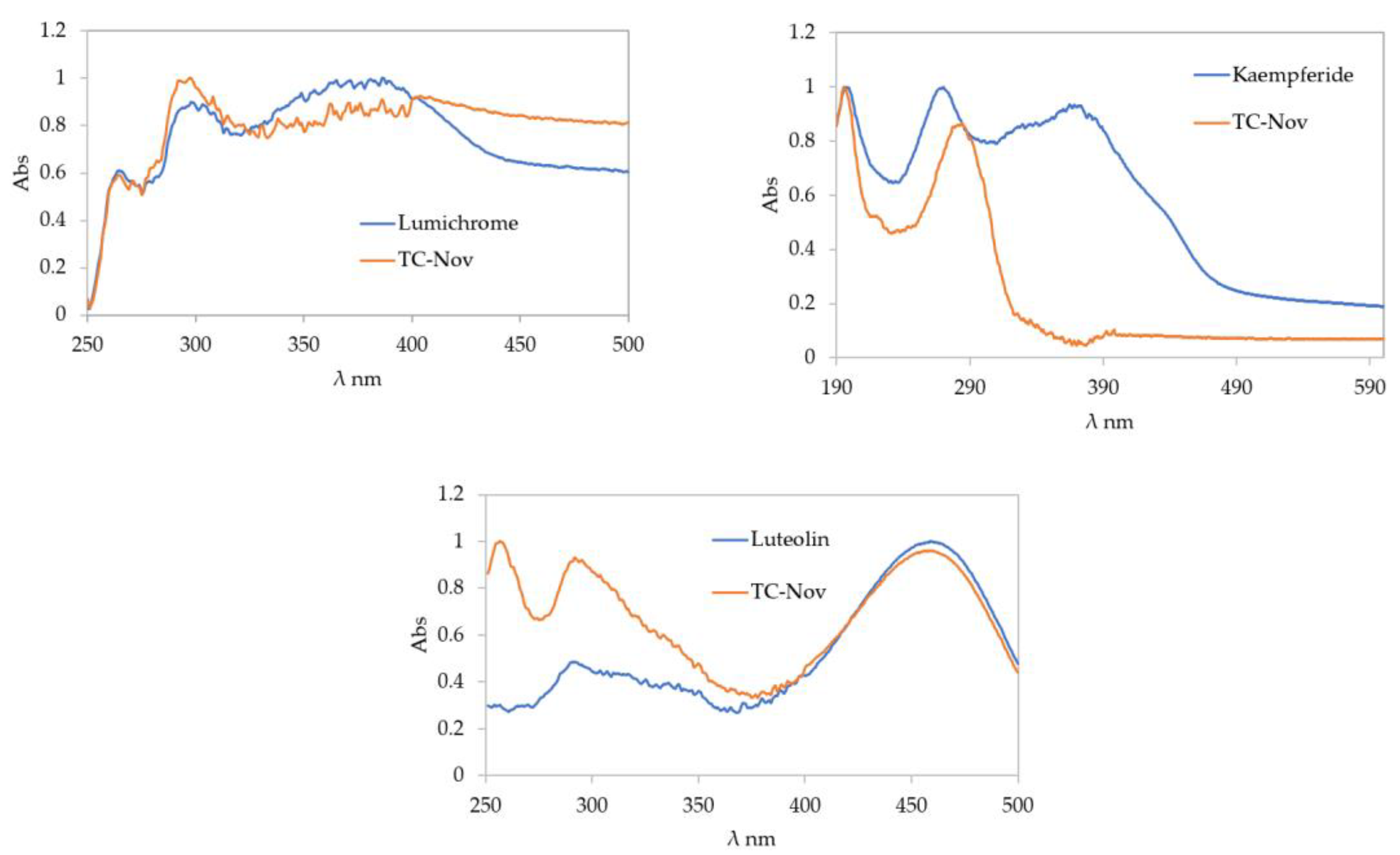
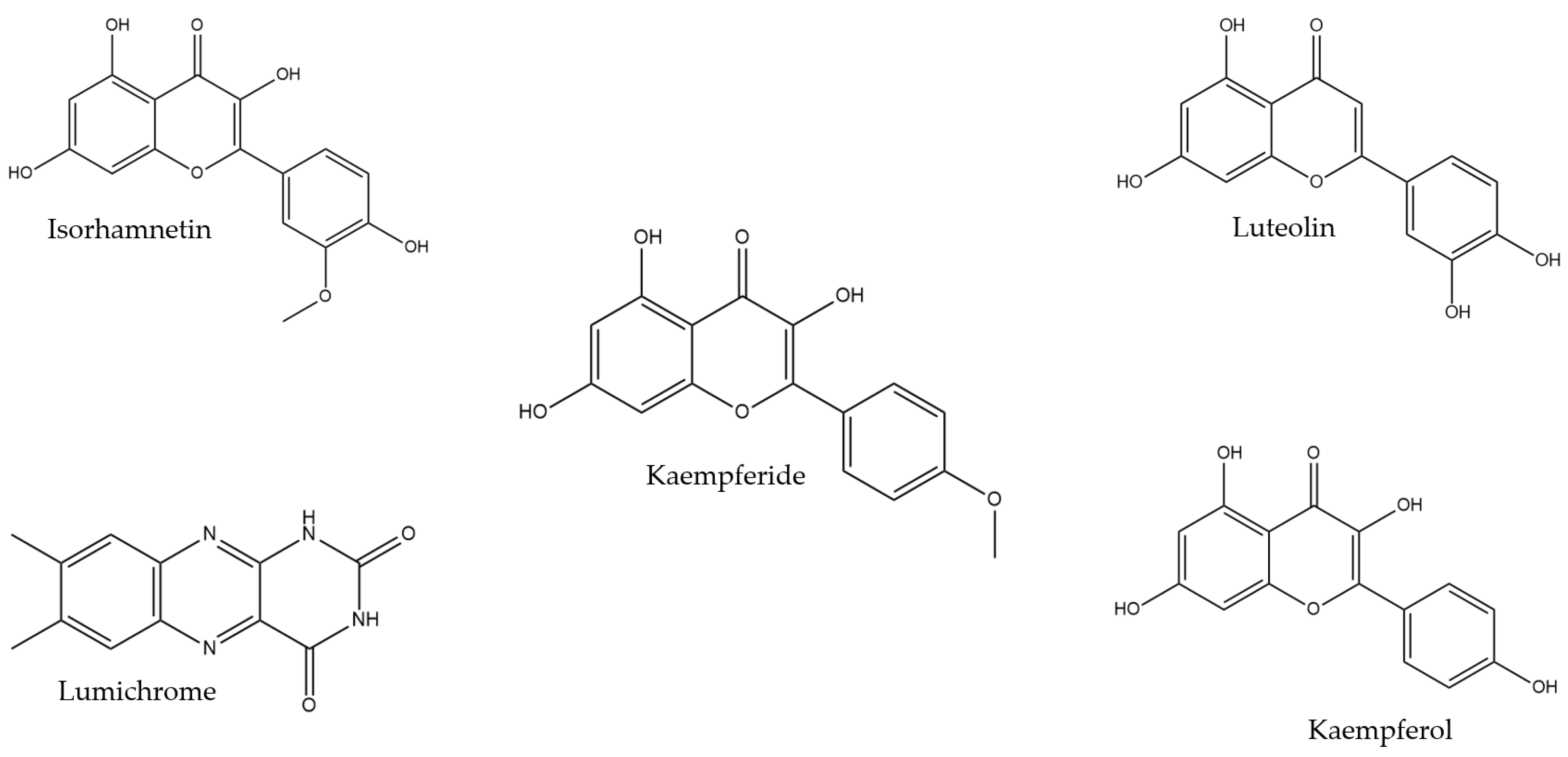
2.2. Compound Identification
2.3. Compound Quantification
2.4. Total Phenolic Content and Antioxidant Activity
2.5. HPTLC-DPPH Antioxidant Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Honey Samples
3.3. Sample Preparation
3.4. Physicochemical Analysis
3.4.1. Soluble Solids (°Brix) and Moisture Content
3.4.2. pH
3.4.3. Sugar Profile
3.5. Identification of Compounds in Honey
3.6. Quantification of Identified Compounds in Honey
3.7. Antioxidant Activity
3.7.1. Total Phenolic Content (TPC)
3.7.2. Total DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Assay
3.7.3. HPTLC-DPPH Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant activity and phenolic compound identification and quantification in western australian honeys. Antioxidants 2023, 12, 189. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Slaa, E.J.; Sánchez Chaves, L.A.; Malagodi-Braga, K.S.; Hofstede, F.E. Stingless bees in applied pollination: Practice and perspectives. Apidologie 2006, 37, 293–315. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-Based Medicinal Properties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef]
- Ávila, S.; Beux, M.R.; Ribani, R.H.; Zambiazi, R.C. Stingless bee honey: Quality parameters, bioactive compounds, health-promotion properties and modification detection strategies. Trends Food Sci. Technol. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and its role in relieving multiple facets of atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Lawag, I.L.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. The Development and Application of a HPTLC-Derived Database for the Identification of Phenolics in Honey. Molecules 2022, 27, 6651. [Google Scholar] [CrossRef]
- Mello dos Santos, M.; Jacobs, C.; Vinsen, K.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Locher, C. Profiling of Australian Stingless Bee Honey Using Multivariate Data Analysis of High-Performance Thin-Layer Chromatography Fingerprints. Chemosensors 2025, 13, 30. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Development of an HPTLC-based dynamic reference standard for the analysis of complex natural products using Jarrah honey as test sample. PLoS ONE 2021, 16, e0254857. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Codex. 2022. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/tr/ (accessed on 13 November 2024).
- Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J. Food Compos. Anal. 2016, 50, 61–69. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Morroni, G.; Simoni, S.; Forbes-Hernández, T.Y.; et al. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT Food Sci. Technol. 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Sousa, J.M.B.d.; Souza, E.L.d.; Marques, G.d.; Benassi, M.d.T.; Gullón, B.; Pintado, M.M.; Magnani, M. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT Food Sci. Technol. 2016, 65, 645–651. [Google Scholar] [CrossRef]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Bruni, R.; Maietti, S.; Poli, F.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Scalvenzi, L.; Sacchetti, G. Ecuadorian stingless bee (Meliponinae) honey: A chemical and functional profile of an ancient health product. Food Chem. 2009, 114, 1413–1420. [Google Scholar] [CrossRef]
- Biluca, F.C.; Braghini, F.; Campos Ferreira, G.; Costa dos Santos, A.; Baggio Ribeiro, D.H.; Valdemiro Gonzaga, L.; Vitali, L.; Amadeu Micke, G.; Carolina Oliveira Costa, A.; Fett, R. Physicochemical parameters, bioactive compounds, and antibacterial potential of stingless bee honey. J. Food Process. Preserv. 2021, 45, e15127. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Shamsudin, S.; Selamat, J.; Sanny, M.; Abd Razak, S.-B.; Jambari, N.N.; Mian, Z.; Khatib, A. Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int. J. Food Prop. 2019, 22, 239–264. [Google Scholar] [CrossRef]
- Nordin, A.; Sainik, N.Q.A.V.; Chowdhury, S.R.; Saim, A.B.; Idrus, R.B.H. Physicochemical properties of stingless bee honey from around the globe: A comprehensive review. J. Food Compos. Anal. 2018, 73, 91–102. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Comp. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.T.; Hungerford, N.L.; Webber, D.; Carpinelli de Jesus, M.; Zhang, J.; Stone, I.S.J.; Blanchfield, J.T.; Zawawi, N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020, 10, 12128. [Google Scholar] [CrossRef]
- Massaro, C.F.; Shelley, D.; Heard, T.A.; Brooks, P. In vitro antibacterial phenolic extracts from “sugarbag” pot-honeys of Australian stingless bees (Tetragonula carbonaria). J. Agric. Food Chem. 2014, 62, 12209–12217. [Google Scholar] [CrossRef]
- Chantarawong, W.; Kuncharoen, N.; Tanasupawat, S.; Chanvorachote, P. Lumichrome Inhibits Human Lung Cancer Cell Growth and Induces Apoptosis via a p53-Dependent Mechanism. Nutr. Cancer 2019, 71, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.A.A.; da Silva, T.M.S.; Camara, C.A.; Queiroz, N.; Magnani, M.; de Novais, J.S.; Soledade, L.E.B.; Lima, E.d.O.; de Souza, A.L.; de Souza, A.G. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013, 141, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.G.d.; Jain, S.; Luna, A.C.; Freitas, L.d.S.; Araújo, E.D.d. Screening for quality indicators and phenolic compounds of biotechnological interest in honey samples from six species of stingless bees (Hymenoptera: Apidae). Food Sci. Technol. 2017, 37, 552–557. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Wölfle, U.; Schempp, C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef]
- Rivera-Yañez, C.R.; Ruiz-Hurtado, P.A.; Mendoza-Ramos, M.I.; Reyes-Reali, J.; García-Romo, G.S.; Pozo-Molina, G.; Reséndiz-Albor, A.A.; Nieto-Yañez, O.; Méndez-Cruz, A.R.; Méndez-Catalá, C.F.; et al. Flavonoids Present in Propolis in the Battle against Photoaging and Psoriasis. Antioxidants 2021, 10, 2014. [Google Scholar] [CrossRef]
- Song, B.; Niu, W.; Zhang, S.; Hao, M.; Li, Y.; Chen, Q.; Li, S.; Tong, C. A mechanistic review of the pharmacological aspects of Kaempferide as a natural compound. Heliyon 2024, 10, e38243. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, Q.; Tang, T.; Wei, Y.; Pu, P. Kaempferide improves glycolipid metabolism disorder by activating PPARγ in high-fat-diet-fed mice. Life Sci. 2021, 270, 119133. [Google Scholar] [CrossRef] [PubMed]
- Chandrababu, G.; Varkey, M.; Devan, A.R.; Anjaly, M.V.; Unni, A.R.; Nath, L.R. Kaempferide exhibits an anticancer effect against hepatocellular carcinoma in vitro and in vivo. Naunyn Schmiedeberg’s Arch. Pharmacol. 2023, 396, 2461–2467. [Google Scholar] [CrossRef]
- Abd Jalil, M.A.; Kasmuri, A.R.; Hadi, H. Stingless bee honey, the natural wound healer: A review. Ski. Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Biluca, F.C.; de Gois, J.S.; Schulz, M.; Braghini, F.; Gonzaga, L.V.; Maltez, H.F.; Rodrigues, E.; Vitali, L.; Micke, G.A.; Borges, D.L.G.; et al. Phenolic compounds, antioxidant capacity and bioaccessibility of minerals of stingless bee honey (Meliponinae). J. Food Compos. Anal. 2017, 63, 89–97. [Google Scholar] [CrossRef]
- Ávila, S.; Hornung, P.S.; Teixeira, G.L.; Malunga, L.N.; Apea-Bah, F.B.; Beux, M.R.; Beta, T.; Ribani, R.H. Bioactive compounds and biological properties of Brazilian stingless bee honey have a strong relationship with the pollen floral origin. Food Res. Int. 2019, 123, 1–10. [Google Scholar] [CrossRef]
- Achenbach, J.; Deyerling, N.; Mello Dos Santos, M.; Sultana, S.; Islam, M.K.; Locher, C. Physicochemical Properties, Antioxidant Activity, and High-Performance Thin-Layer Chromatography Profiling of Propolis Samples from Western Australia. Plants 2024, 13, 1919. [Google Scholar] [CrossRef]
- Islam, M.K.; Lawag, I.L.; Sostaric, T.; Ulrich, E.; Ulrich, D.; Dewar, T.; Lim, L.Y.; Locher, C. Australian Honeypot Ant (Camponotus inflatus) Honey-A Comprehensive Analysis of the Physiochemical Characteristics, Bioactivity, and HPTLC Profile of a Traditional Indigenous Australian Food. Molecules 2022, 27, 2154. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. A validated method for the quantitative determination of sugars in honey using high-performance thin-layer chromatography. JPC J. Planar Chromatogr. 2020, 33, 489–499. [Google Scholar] [CrossRef]
- Mello dos Santos, M.; Jacobs, C.; Islam, M.K.; Lim, L.Y.; Locher, C. Validation of a high-performance thin-layer chromatography method for the quantitative determination of trehalulose. JPC J. Planar Chromatogr. 2023, 36, 201–210. [Google Scholar] [CrossRef]
- Liberato, M.d.C.C.; de Morais, S.M.; Siqueira, S.M.C.; de Menezes, J.E.S.A.; Ramos, D.N.; Machado, L.K.A.; Magalhães, I.L. Phenolic content and antioxidant and antiacetylcholinesterase properties of honeys from different floral origins. J. Med. Food 2011, 14, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant HPTLC-DPPH Fingerprinting of Honeys and Tracking of Antioxidant Constituents Upon Thermal Exposure. Foods 2021, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Development and validation of an HPTLC–DPPH assay and its application to the analysis of honey. JPC J. Planar Chromatogr. 2020, 33, 301–311. [Google Scholar] [CrossRef]
- Deyerling, N.; Achenbach, J.; dos Santos, M.M.; Locher, C. Physicochemical Properties, Antioxidant Activity and Phytochemical Profiling of Australian Tetragonula carbonaria Cerumen. J. Chromatogr. Open 2024, 100164. [Google Scholar] [CrossRef]

| Honey Blend | pH | Moisture (%w/w) | Soluble Solids (°Brix) | Trehalulose (g/100 g) | Fructose (g/100 g) | Glucose (g/100 g) |
|---|---|---|---|---|---|---|
| TC-May | 3.73 ± 0.0265 a | 27.0 ± 0.153 a | 73.0 ± 0.153 a | 30.3 ± 1.41 a | 12.9 ± 0.666 a | 4.80 ± 0.380 a |
| TH-May | 3.57 ± 0.0451 b | 30.0 ± 0.0577 b | 70.0 ± 0.0577 b | 24.3 ± 0.611 b | 19.9 ± 0.265 b | 7.29 ± 0.216 b |
| TC-Sep | 3.77 ± 0.00577 a | 28.0 ± 0.0577 c | 72.0 ± 0.0577 c | 18.8 ± 0.888c | 25.8 ± 0.961 c | 15.8 ± 0.231 c |
| TH-Sep | 4.14 ± 0.0100 c | 29.1 ± 0.0577 d | 70.9 ± 0.0577 d | 14.4 ± 0.395 d | 30.1 ± 0.650 d | 20.5 ± 0.420 d |
| TC-Nov | 3.93 ± 0.0208 d | 26.5 ± 0.000 e | 73.5 ± 0.000 e | 13.9 ± 0.784 d | 28.5 ± 0.309 d | 20.5 ± 0.307 d |
| TH-Nov | 4.19 ± 0.0265 c | 27.1 ± 0.0577 a | 72.9 ± 0.0577 a | 15.8 ± 1.21 d | 32.3 ± 0.530 e | 23.7 ± 1.18 e |
| Honey Sample | Lumichrome | Luteolin | Kaempferide |
|---|---|---|---|
| TC-May | 3.74 ± 0.479 a | 1.01 ± 0.159 a | ND |
| TH-May | 3.99 ± 0.659 a | 0.631 ± 0.0425 b,c | 1.97 ± 0.052 a |
| TC-Sep | 0.830 ± 0.0258 b | 0.576 ± 0.0635 b | ND |
| TH-Sep | 1.20 ± 0.166 b | ND | 0.387 ± 0.0211 b |
| TC-Nov | 0.786 ± 0.172 b | 0.804 ± 0.0827 c | 0.522 ± 0.0652 c |
| TH-Nov | 1.37 ± 0.170 b | 0.338 ± 0.0462 d | 0.875 ± 0.0795 d |
| Honey Blend | TPC (mg GAE/100 g) | DPPH (mmol TE/Kg) |
|---|---|---|
| TC-May | 44.7 ± 0.861 a | 4.57 ± 0.282 a |
| TH-May | 58.6 ± 3.47 b | 6.08 ± 0.105 b |
| TC-Sep | 29.5 ± 0.429 c,d | 2.49 ± 0.131 c |
| TH-Sep | 26.1 ± 0.662 c | 1.39 ± 0.282 d |
| TC-Nov | 33.8 ± 1.60 d | 3.59 ± 0.0849 e |
| TH-Nov | 33.2 ± 1.70 d | 2.73 ± 0.0366 c |
| Sample | Rf | Compound Match | Hue Value [°] | %RSA | Category |
|---|---|---|---|---|---|
| TC-May | 0.113 | - | 329.6 | 14.9 | + |
| 0.315 | Lumichrome | 335.1 | 21.5 | + | |
| 0.375 | - | 330.9 | 16.5 | + | |
| 0.477 | - | 333.4 | 19.5 | + | |
| 0.543 | - | 336.2 | 22.9 | + | |
| 0.683 | - | 338.6 | 25.8 | + | |
| TH-May | 0.115 | - | 323.4 | 7.41 | + |
| 0.311 | Lumichrome | 331.8 | 17.6 | + | |
| 0.374 | - | 325.3 | 9.70 | + | |
| 0.448 | - | 342.5 | 30.5 | + | |
| 0.480 | - | 352.4 | 42.5 | ++ | |
| 0.539 | - | 359.0 | 50.4 | ++ | |
| 0.580 | - | 332.5 | 18.4 | + | |
| 0.684 | - | 334.7 | 21.1 | + | |
| TC-Sep | 0.541 | - | 328.1 | 13.1 | + |
| 0.684 | - | 321.7 | 5.35 | + | |
| TH-Sep | 0.545 | - | 324.1 | 8.25 | + |
| TC-Nov | 0.546 | - | 330.5 | 16.0 | + |
| 0.681 | - | 326.3 | 10.9 | + | |
| TH-Nov | 0.121 | - | 323.7 | 7.77 | + |
| 0.479 | - | 332.5 | 18.4 | + | |
| 0.536 | - | 353.1 | 43.3 | ++ | |
| 0.683 | - | 332.5 | 18.4 | + | |
| 0.758 | - | 332.3 | 18.2 | + |
| Bee Species | Sample | Harvest Date |
|---|---|---|
| Tetragonula carbonaria | C1 | May 2022 |
| C2 | ||
| C3 | ||
| C4 | ||
| C5 | ||
| C6 | ||
| C7 | ||
| C8 | ||
| C9 | ||
| C10 | ||
| Tetragonula hockingsi | H1 | May 2022 |
| H2 | ||
| Tetragonula carbonaria | C13 | September 2022 |
| C14 | ||
| C15 | ||
| C16 | ||
| C17 | ||
| C18 | ||
| C19 | ||
| C20 | ||
| Tetragonula hockingsi | H5 | September 2022 |
| H6 | ||
| Tetragonula carbonaria | C21 | November 2022 |
| C22 | ||
| C23 | ||
| C24 | ||
| C25 | ||
| C26 | ||
| C27 | ||
| C28 | ||
| Tetragonula hockingsi | H7 | November 2022 |
| H8 |
| Honey Blend | Bee Species | Harvest Date |
|---|---|---|
| TC-May (n = 10) | Tetragonula carbonaria | May 2022 |
| TH-May (n = 2) | Tetragonula hockingsi | May 2022 |
| TC-Sep (n = 8) | Tetragonula carbonaria | September 2022 |
| TH-Sep (n = 2) | Tetragonula hockingsi | September 2022 |
| TC-Nov (n = 8) | Tetragonula carbonaria | November 2022 |
| TH-Nov (n = 2) | Tetragonula hockingsi | November 2022 |
| % DPPH RSA | Category | Inference |
|---|---|---|
| 0.0% | 0 | No activity |
| 1.0–33.3% | + | Low activity |
| 33.4–66.6% | ++ | Medium activity |
| 66.7–100.0% | +++ | High activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mello dos Santos, M.; Sostaric, T.; Lim, L.Y.; Locher, C. Physicochemical Characteristics, Antioxidant Properties, and Identification of Bioactive Compounds in Australian Stingless Bee Honey Using High-Performance Thin-Layer Chromatography. Molecules 2025, 30, 1223. https://doi.org/10.3390/molecules30061223
Mello dos Santos M, Sostaric T, Lim LY, Locher C. Physicochemical Characteristics, Antioxidant Properties, and Identification of Bioactive Compounds in Australian Stingless Bee Honey Using High-Performance Thin-Layer Chromatography. Molecules. 2025; 30(6):1223. https://doi.org/10.3390/molecules30061223
Chicago/Turabian StyleMello dos Santos, Mariana, Tomislav Sostaric, Lee Yong Lim, and Cornelia Locher. 2025. "Physicochemical Characteristics, Antioxidant Properties, and Identification of Bioactive Compounds in Australian Stingless Bee Honey Using High-Performance Thin-Layer Chromatography" Molecules 30, no. 6: 1223. https://doi.org/10.3390/molecules30061223
APA StyleMello dos Santos, M., Sostaric, T., Lim, L. Y., & Locher, C. (2025). Physicochemical Characteristics, Antioxidant Properties, and Identification of Bioactive Compounds in Australian Stingless Bee Honey Using High-Performance Thin-Layer Chromatography. Molecules, 30(6), 1223. https://doi.org/10.3390/molecules30061223









