Electrochromic Fabrics with Horizontal Patterning, Enhanced Strength, Comfort, High-Temperature Protection, and Long Coloring Retention Properties for Adaptive Camouflage
Abstract
:1. Introduction
2. Results and Discussion
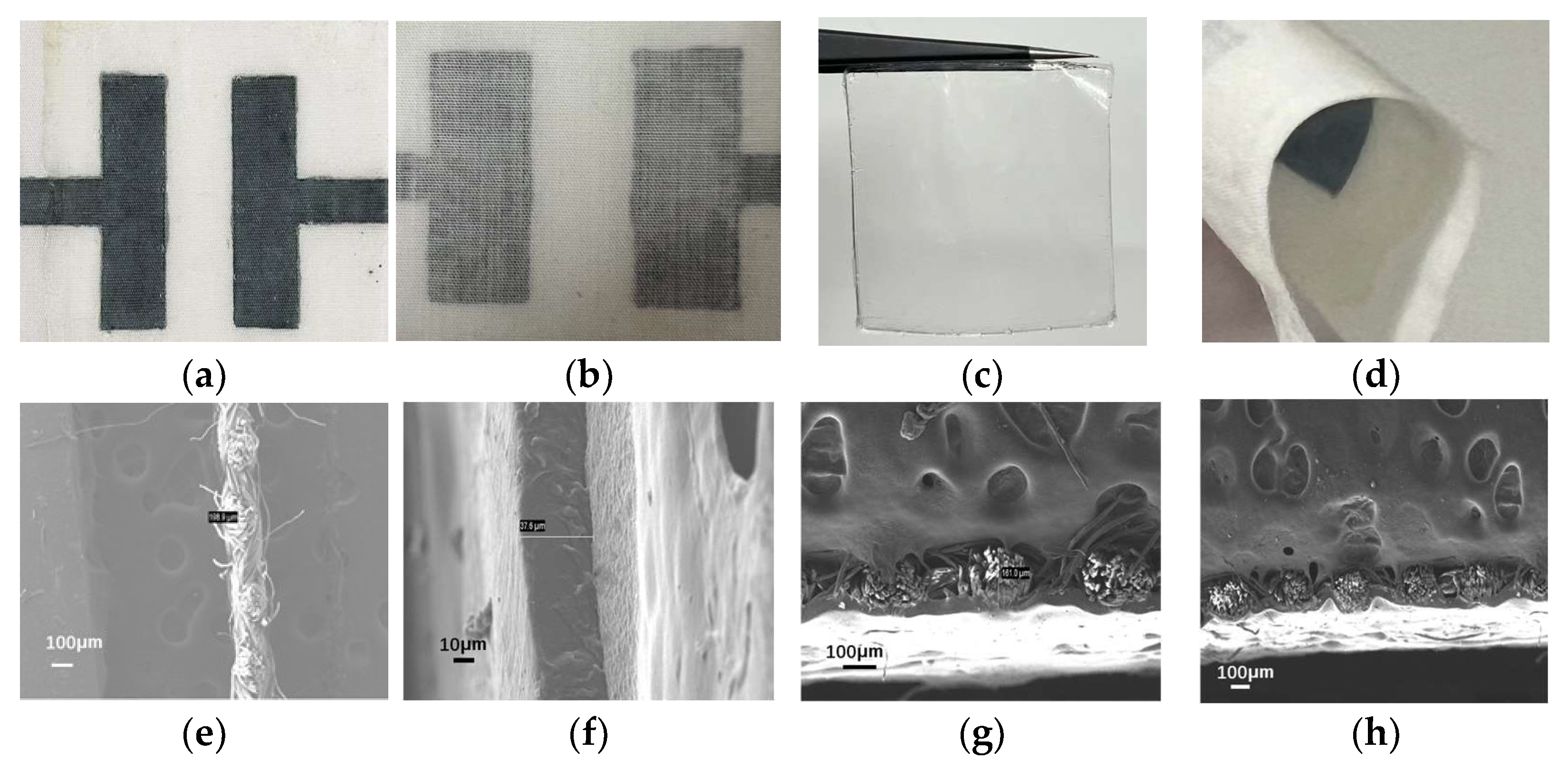
2.1. Performance Analysis of Electrochromic Fabrics
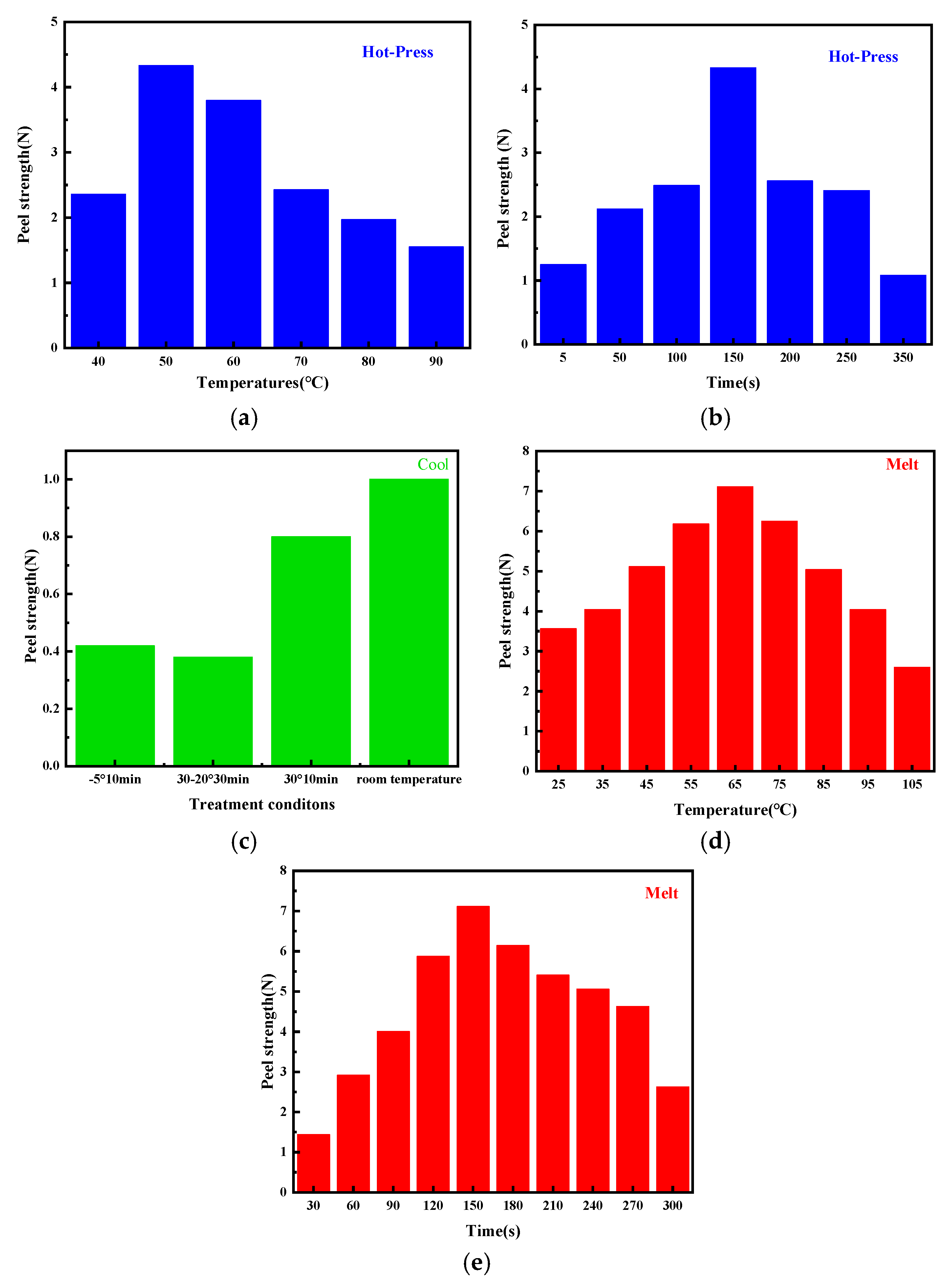
2.2. Enhanced Peel Strength by Refining the Hot-Pressing Process
2.3. Boosted Comfort by Fabricating Ultra-Thin Electrochromic Fabrics
3. Experimental Section
3.1. Materials
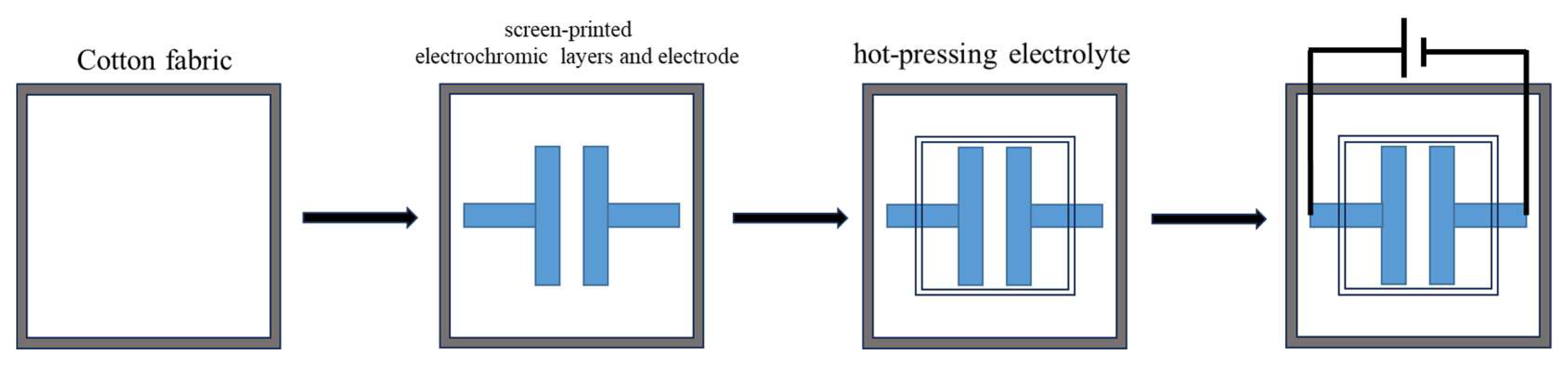
3.2. Electrochromic Cotton Fabrics Assembly
3.3. Sample Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Wang, Z.; Teng, C.; Kumar, S.; Li, X.; Min, R. Wearable Cardiorespiratory Sensor for Real-Time Monitoring with Smartphone Integration. IEEE Trans. Instrum. Meas. 2023, 73, 1–10. [Google Scholar] [CrossRef]
- Peng, Y.; Long, Z.; Liang, S.; Zhong, T.; Chen, M.; Xing, L.; Xue, X. A battery-free music-driven humidity sensor for intelligent wearable sensing system in smart diaper. Smart Mater. Struct. 2023, 32, 025016. [Google Scholar] [CrossRef]
- Usman, M.; Jamhour, N.; Hettinger, J.; Xue, W. Smart wearable flexible temperature sensor with compensation against bending and stretching effects. Sens. Actuators A Phys. 2023, 353, 114224. [Google Scholar] [CrossRef]
- Faisal, A.I.; Majumder, S.; Scott, R.; Mondal, T.; Cowan, D.; Deen, M.J. A Simple, Low-Cost Multi-Sensor-Based Smart Wearable Knee Monitoring System. IEEE Sens. J. 2020, 21, 8253–8266. [Google Scholar] [CrossRef]
- Homayounfar, S.Z.; Andrew, T.L. Wearable Sensors for Monitoring Human Motion: A Review on Mechanisms, Materials, and Challenges. SLAS Technol. 2020, 25, 9–24. [Google Scholar] [CrossRef]
- Xu, W.; Hong, L.; Zheng, J.; Li, M.; Hua, Y.; Zhao, X. Wearable Smart Sensor System for Monitoring and Intelligent Prediction of Sodium Ions in Human Perspiration. IEEE Internet Things J. 2023, 11, 8146–8155. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Zou, H.; Che, L.; Sun, P.; Yan, J.; Liu, W.; Xu, Z.; Yang, W.; Dong, L.; et al. Integrated wearable smart sensor system for real-time multi-parameter respiration health monitoring. Cell Rep. Phys. Sci. 2023, 4, 101191. [Google Scholar] [CrossRef]
- Cutrone, A.; Micera, S. Implantable Neural Interfaces and Wearable Tactile Systems for Bidirectional Neuroprosthetics Systems. Adv. Healthc. Mater. 2019, 8, e1801345. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. NeuroEng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef]
- Saikia, M.J. Smart Fabric and E-Textile Sensor Technology for Wearables to Measure High Pressure. IEEE Trans. Instrum. Meas. 2023, 72, 1–9. [Google Scholar] [CrossRef]
- Ma, J.; Shen, L.; Jiang, Y.; Ma, H.; Lv, F.; Liu, J.; Su, Y.; Zhu, N. Wearable Self-Powered Smart Sensors for Portable Nutrition Monitoring. Anal. Chem. 2022, 94, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-H.; Jeong, Y.; Ahn, J.; Hwang, S.; Jeon, S.; Kim, D.; Ko, J.; Kang, B.; Jung, Y.; Choi, J.; et al. A wearable colorimetric sweat pH sensor-based smart textile for health state diagnosis. Mater. Horiz. 2023, 10, 4163–4171. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, Q.; Ding, Y.; Zhang, C.; Song, X.; Chen, Z.; Zhang, Y.; Mei, Q.; Zhao, X.; Huang, Q.; et al. Wet-Adaptive Electronic Skin. Adv. Mater. 2023, 35, 2305630. [Google Scholar] [CrossRef]
- Xu, C.; Song, Y.; Sempionatto, J.R.; Solomon, S.A.; Yu, Y.; Nyein, H.Y.Y.; Tay, R.Y.; Li, J.; Heng, W.; Min, J.; et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 2024, 7, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; He, A.; Huang, Z.; Luo, Y.; Zhang, Y.; Wang, M.; Shi, Z.; Ke, G.; Bai, J.; Zhao, S.; et al. Silk-based flexible electronics and smart wearable Textiles: Progress and beyond. Chem. Eng. J. 2023, 474, 145534. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Tao, G.; Xia, L.; Xu, W.; Zhai, T. Smart Textile Optoelectronics for Human-Interfaced Logic Systems. Adv. Funct. Mater. 2023, 34, 2308136. [Google Scholar] [CrossRef]
- Chatterjee, K.; Tabor, J.; Ghosh, T.K. Electrically Conductive Coatings for Fiber-Based E-Textiles. Fibers 2019, 7, 51. [Google Scholar] [CrossRef]
- Meng, X.; Wu, Q. Design of an Interactive Device Based on e-Textile Material. In HCI International 2021—Late Breaking Papers: Multimodality, eXtended Reality, and Artificial Intelligence; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 68–81. ISBN 9783030909635. [Google Scholar]
- Xiao, Y.; Shen, M.; Li, J.; Wang, H.; Sun, H.; He, Y.; Huang, R.; Yu, T.; Huang, W. Thermally Activated Photochromism: Realizing Temperature-Gated Triphenylethylene Photochromic Materials. Adv. Funct. Mater. 2024, 34, 2312930. [Google Scholar] [CrossRef]
- Liu, B.; Rasines Mazo, A.; Gurr, P.A.; Qiao, G.G. Reversible Nontoxic Thermochromic Microcapsules. ACS Appl. Mater. Interfaces 2020, 12, 9782–9789. [Google Scholar] [CrossRef]
- Gu, C.; Jia, A.-B.; Zhang, Y.-M.; Zhang, S.X.-A. Emerging Electrochromic Materials and Devices for Future Displays. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef]
- Poh, W.C.; Eh, A.L.; Wu, W.; Guo, X.; Lee, P.S. Rapidly Photocurable Solid-State Poly(ionic liquid) Ionogels For Thermally Robust and Flexible Electrochromic Devices. Adv. Mater. 2022, 34, e2206952. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cao, M.; Kim, K.N.; Lin, M.; Moon, J.-M.; Sempionatto, J.R.; Yu, J.; Liu, R.; Wicker, C.; Trifonov, A.; et al. A stretchable epidermal sweat sensing platform with an integrated printed battery and electrochromic display. Nat. Electron. 2022, 5, 694–705. [Google Scholar] [CrossRef]
- Fan, H.; Wei, W.; Hou, C.; Zhang, Q.; Li, Y.; Li, K.; Wang, H. Wearable electrochromic materials and devices: From visible to infrared modulation. J. Mater. Chem. C 2023, 11, 7183–7210. [Google Scholar] [CrossRef]
- Ling, Y.; Li, L.; Liu, J.; Li, K.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. Air-Working Electrochromic Artificial Muscles. Adv. Mater. 2023, 36, e2305914. [Google Scholar] [CrossRef]
- Jo, M.-H.; Kim, K.-H.; Ahn, H.-J. P-doped carbon quantum dot graft-functionalized amorphous WO3 for stable and flexible electrochromic energy-storage devices. Chem. Eng. J. 2022, 445, 136826. [Google Scholar] [CrossRef]
- Banasz, R.; Wałęsa-Chorab, M. Polymeric complexes of transition metal ions as electrochromic materials: Synthesis and properties. Coord. Chem. Rev. 2019, 389, 1–18. [Google Scholar] [CrossRef]
- Lu, H.-C.; Kao, S.-Y.; Yu, H.-F.; Chang, T.-H.; Kung, C.-W.; Ho, K.-C. Achieving Low-Energy Driven Viologens-Based Electrochromic Devices Utilizing Polymeric Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 30351–30361. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.; Shen, D.E.; Reynolds, J.R. Fine-Tuning the Color Hue of π-Conjugated Black-to-Clear Electrochromic Random Copolymers. Macromolecules 2019, 52, 6773–6779. [Google Scholar] [CrossRef]
- Valiūnienė, A.; Virbickas, P.; Medvikytė, G.; Ramanavičius, A. Urea Biosensor Based on Electrochromic Properties of Prussian Blue. Electroanalysis 2019, 32, 503–509. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Ma, L.; Zhao, S.; Wu, N.; Xiao, D. Easy-to-make sulfonatoalkyl viologen/sodium carboxymethylcellulose hydrogel-based electrochromic devices with high coloration efficiency, fast response and excellent cycling stability. Dye. Pigment. 2020, 174, 108055. [Google Scholar] [CrossRef]
- Lang, A. Electrochromic Devices Incorporating Conjugated Polymers and Cellulose: New Opportunities for Organic Electronics; Georgia Institute of Technology: Atlanta, GA, USA, 2020. [Google Scholar]
- Wibowo, A.F.; Han, J.W.; Kim, J.H.; Prameswati, A.; Park, J.; Aisyah, S.; Entifar, N.; Lee, J.; Kim, S.; Lim, D.C.; et al. Multiple functionalities of highly conductive and flexible photo- and thermal-responsive colorimetric cellulose films. Mater. Res. Lett. 2022, 10, 36–44. [Google Scholar] [CrossRef]
- Phan, G.T.; Pham, D.V.; Patil, R.A.; Tsai, C.-H.; Lai, C.-C.; Yeh, W.-C.; Liou, Y.; Ma, Y.-R. Fast-switching electrochromic smart windows based on NiO-nanorods counter electrode. Sol. Energy Mater. Sol. Cells 2021, 231, 111306. [Google Scholar] [CrossRef]
- Wang, J.-L.; Sheng, S.-Z.; He, Z.; Wang, R.; Pan, Z.; Zhao, H.-Y.; Liu, J.-W.; Yu, S.-H. Self-Powered Flexible Electrochromic Smart Window. Nano Lett. 2021, 21, 9976–9982. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; He, J.; Zhang, D.; You, L.; Li, X.; Wang, H.; Mei, J. Bioinspired Dynamic Camouflage from Colloidal Nanocrystals Embedded Electrochromics. Nano Lett. 2021, 21, 4500–4507. [Google Scholar] [CrossRef]
- Yu, H.; Qi, M.; Wang, J.; Yin, Y.; He, Y.; Meng, H.; Huang, W. A feasible strategy for the fabrication of camouflage electrochromic fabric and unconventional devices. Electrochem. Commun. 2019, 102, 31–36. [Google Scholar] [CrossRef]
- Sinha, S.; Daniels, R.; Yassin, O.; Baczkowski, M.; Tefferi, M.; Deshmukh, A.; Cao, Y.; Sotzing, G. Electrochromic Fabric Displays from a Robust, Open-Air Fabrication Technique. Adv. Mater. Technol. 2021, 7, 2100548. [Google Scholar] [CrossRef]
- Yu, H.; Shao, S.; Yan, L.; Meng, H.; He, Y.; Yao, C.; Xu, P.; Zhang, X.; Hu, W.; Huang, W. Side-chain engineering of green color electrochromic polymer materials: Toward adaptive camouflage application. J. Mater. Chem. C 2016, 4, 2269–2273. [Google Scholar] [CrossRef]
- Gicevicius, M.; Cechanaviciute, I.A.; Ramanavicius, A. Electrochromic Textile Composites Based on Polyaniline-Coated Metallized Conductive Fabrics. J. Electrochem. Soc. 2020, 167, 155515. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Q.; Wang, H.; Li, Y. Red, Green, Blue (RGB) Electrochromic Fibers for the New Smart Color Change Fabrics. ACS Appl. Mater. Interfaces 2014, 6, 13043–13050. [Google Scholar] [CrossRef]
- Fan, H.; Li, K.; Liu, X.; Xu, K.; Su, Y.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. Continuously Processed, Long Electrochromic Fibers with Multi-Environmental Stability. ACS Appl. Mater. Interfaces 2020, 12, 28451–28460. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Wu, M.; Zhi, C.; Meng, J.; Zhang, L. Multicolor electrochromic fabric with a simple structure of PEDOT:PSS/DMSO. Dye. Pigment. 2023, 219, 111642. [Google Scholar] [CrossRef]
- Li, M.; Jiang, W.; Lin, Y.; Huang, C.; Hao, P.; Wang, W.; Yang, L.; Wang, Y.; Wang, D. Preparation of WO3-based flexible electrochromic fabrics and their near infrared shielding application. J. Mater. Chem. C 2024, 12, 5420–5430. [Google Scholar] [CrossRef]
- Yang, G.; Fan, J.; Zhang, K.; Gu, C.; Li, J.; Kang, K.; Xiang, C.; Qian, L.; Zhang, T. Electrochromic Reflective Displays Based on In Situ Photo-Crosslinked PEDOT: PSS Patterns. Adv. Funct. Mater. 2024, 34, 2314983. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Zhang, Z.; Wang, Y.; Piao, J.; Dai, Y.; Cai, B.; Shen, W.; Cao, K.; Chen, S. Photopatternable and Highly Conductive PEDOT:PSS Electrodes for Flexible Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2023, 15, 21344–21353. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.S.; Souza, V.H.; Schmidt, A.; Zarbin, A.J. PSS-free PEDOT and PEDOT/graphene transparent films: Synthesis, characterization and electrochromism. Synth. Met. 2023, 293, 117259. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, F.F.; Liu, D.D.; Han, G.-Z. In situ synthesis of PEDOT: PSS@ AgNPs nanocomposites. Synth. Met. 2017, 230, 1–6. [Google Scholar] [CrossRef]
- Huang, L.M.; Chen, C.H.; Wen, T.C. Development and characterization of flexible electrochromic devices based on polyaniline and poly (3, 4-ethylenedioxythiophene)-poly (styrene sulfonic acid). Electrochim. Acta 2006, 51, 5858–5863. [Google Scholar] [CrossRef]
- FZ/T81007-2022; Casual Wear. Ministry of Industry and Information Technology of the People’s Republic of China and Standards Press of China: Beijing, China, 2022.
- Fu, G.; Gong, H.; Xu, J.; Zhuang, B.; Rong, B.; Zhang, Q.; Chen, X.; Liu, J.; Wang, H. Highly integrated all-in-one electrochromic fabrics for unmanned environmental adaptive camouflage. J. Mater. Chem. A 2024, 12, 6351–6358. [Google Scholar] [CrossRef]
- FZ/T80007.1-2023; Test Method for Peeling Strength of Garments Used Adhesive Interlining. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2023.
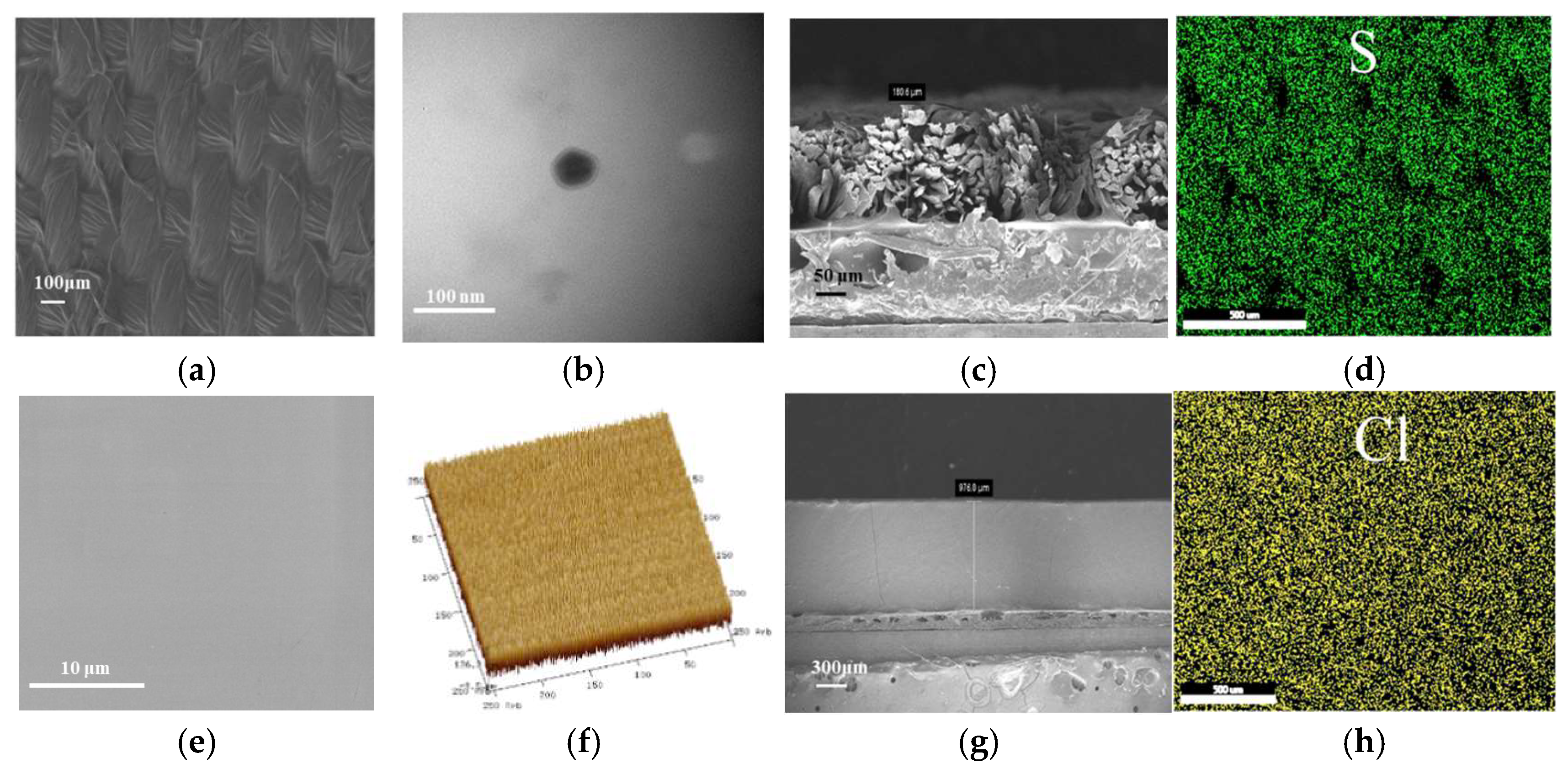
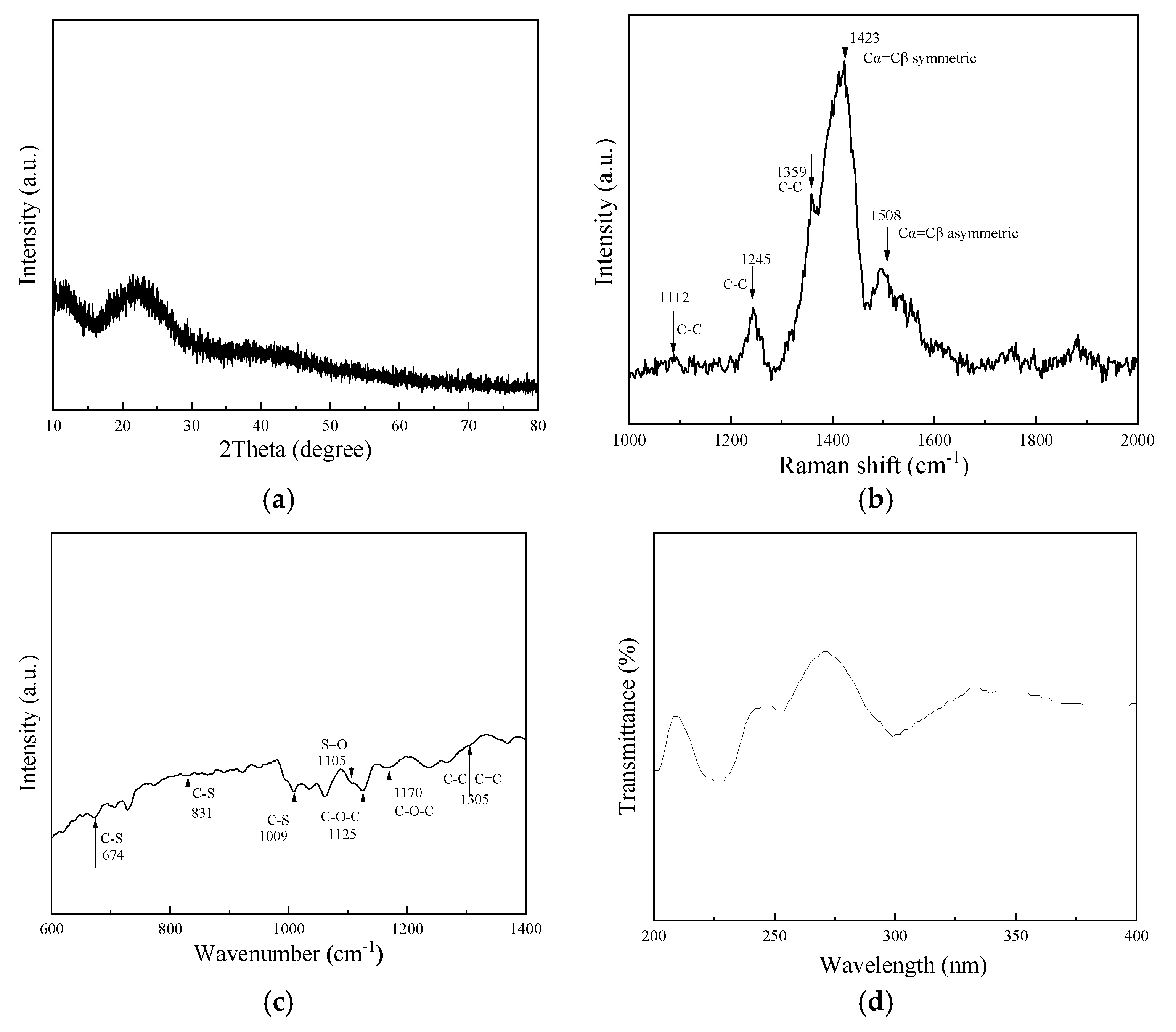
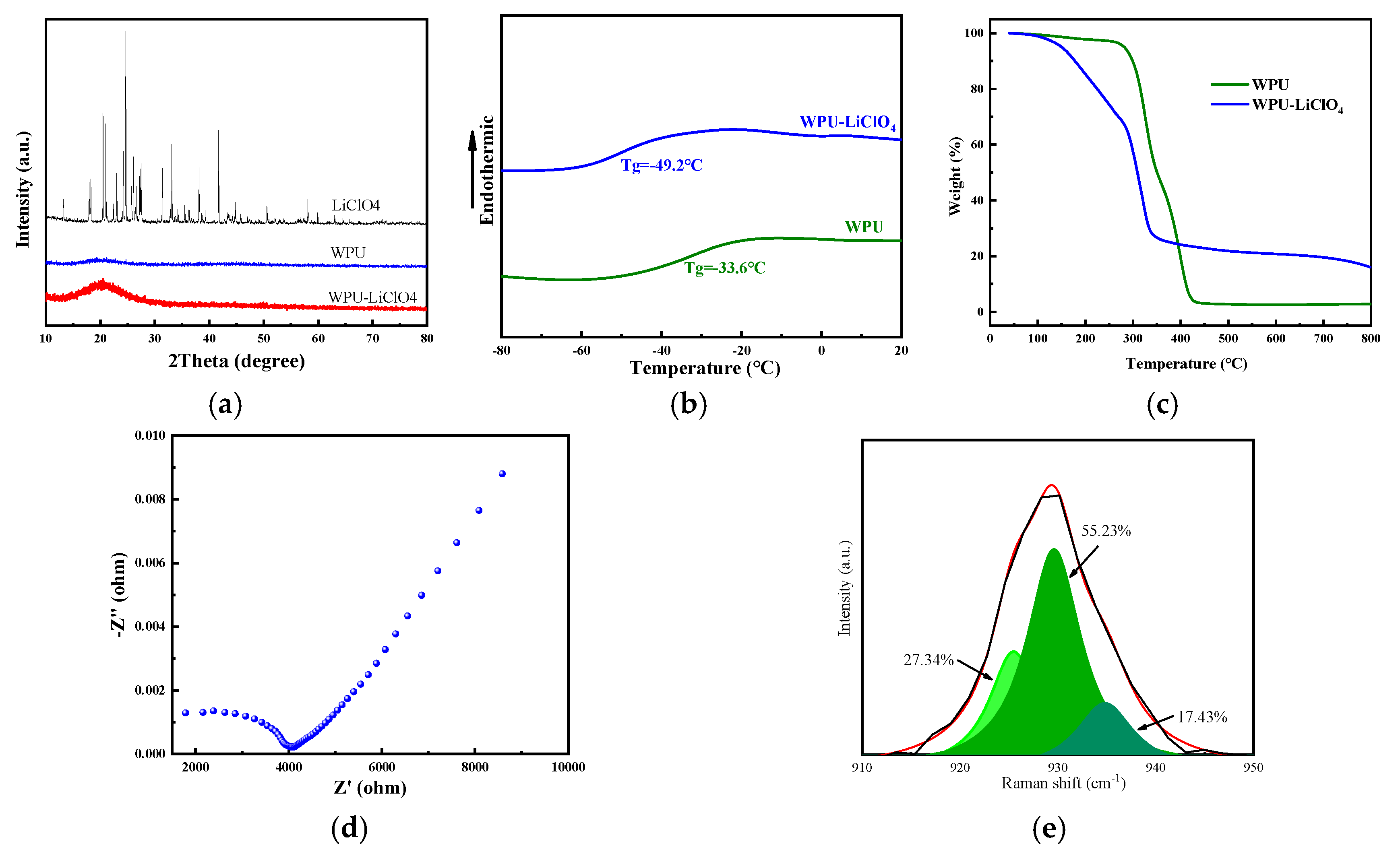
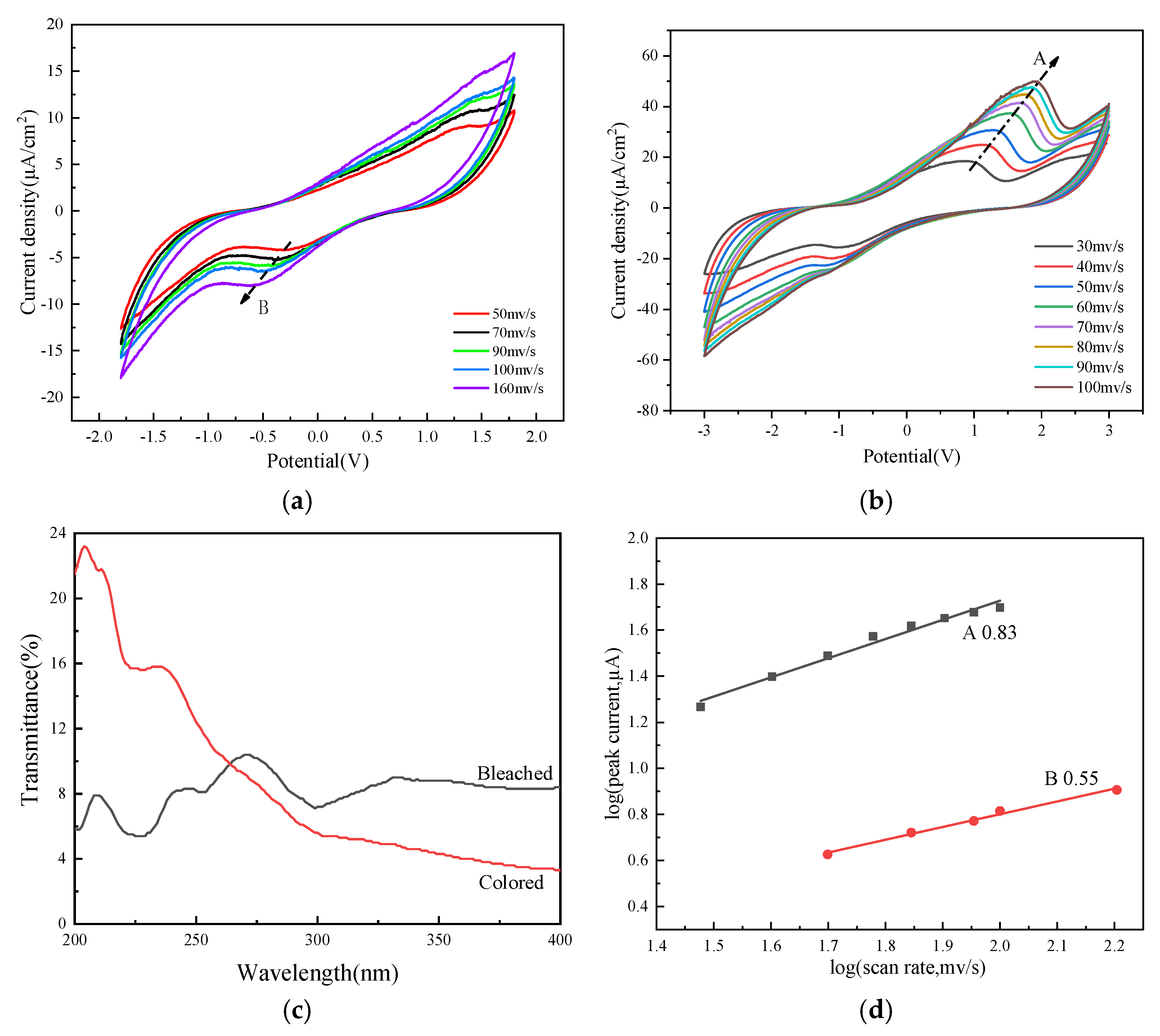
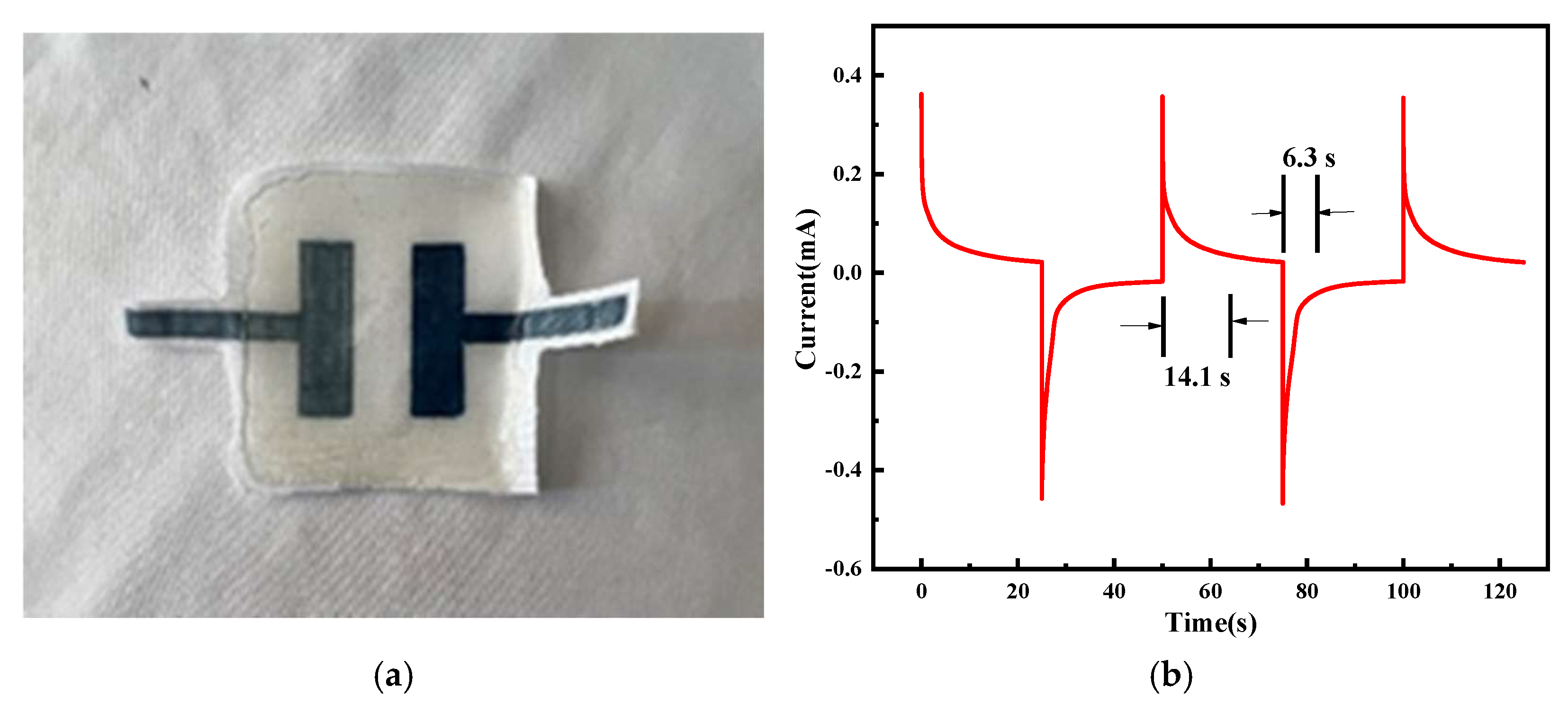
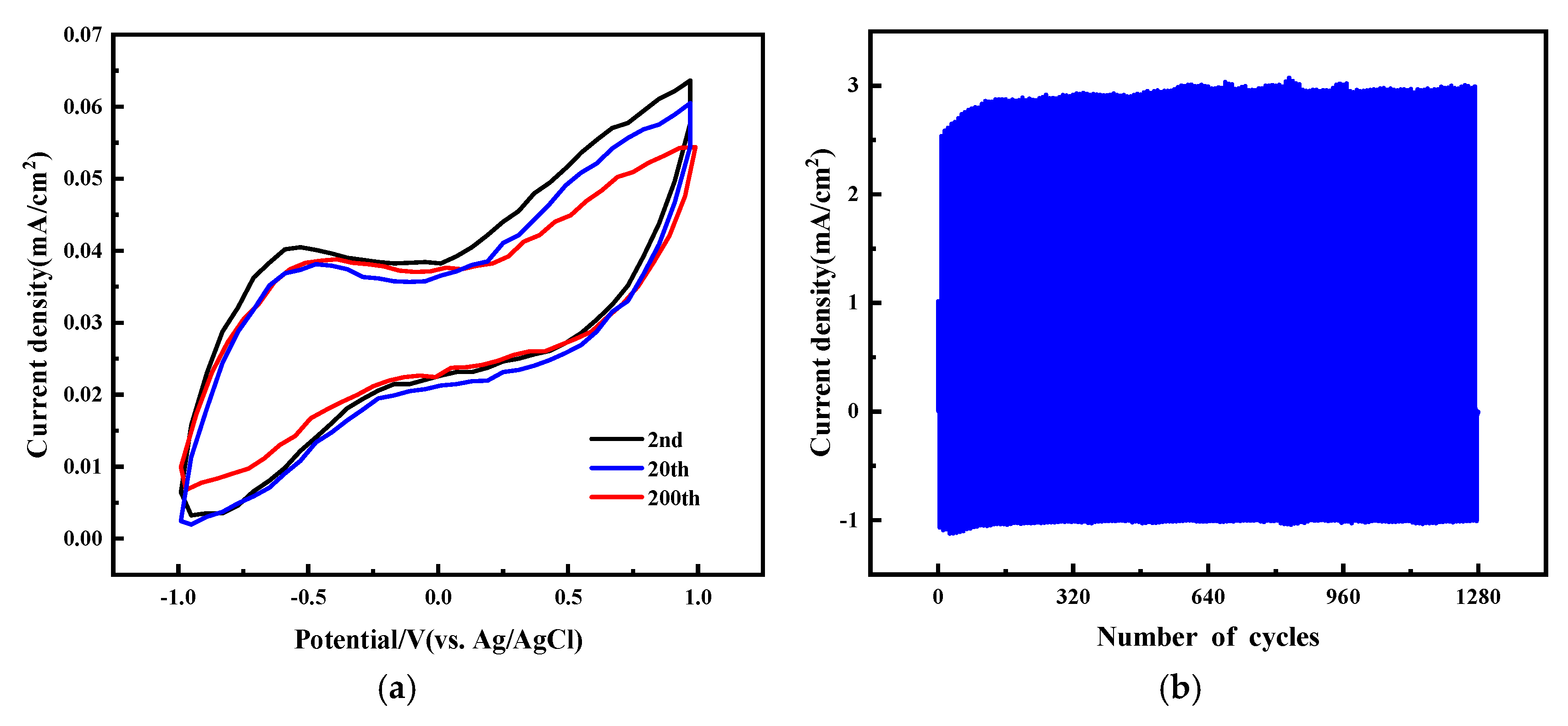
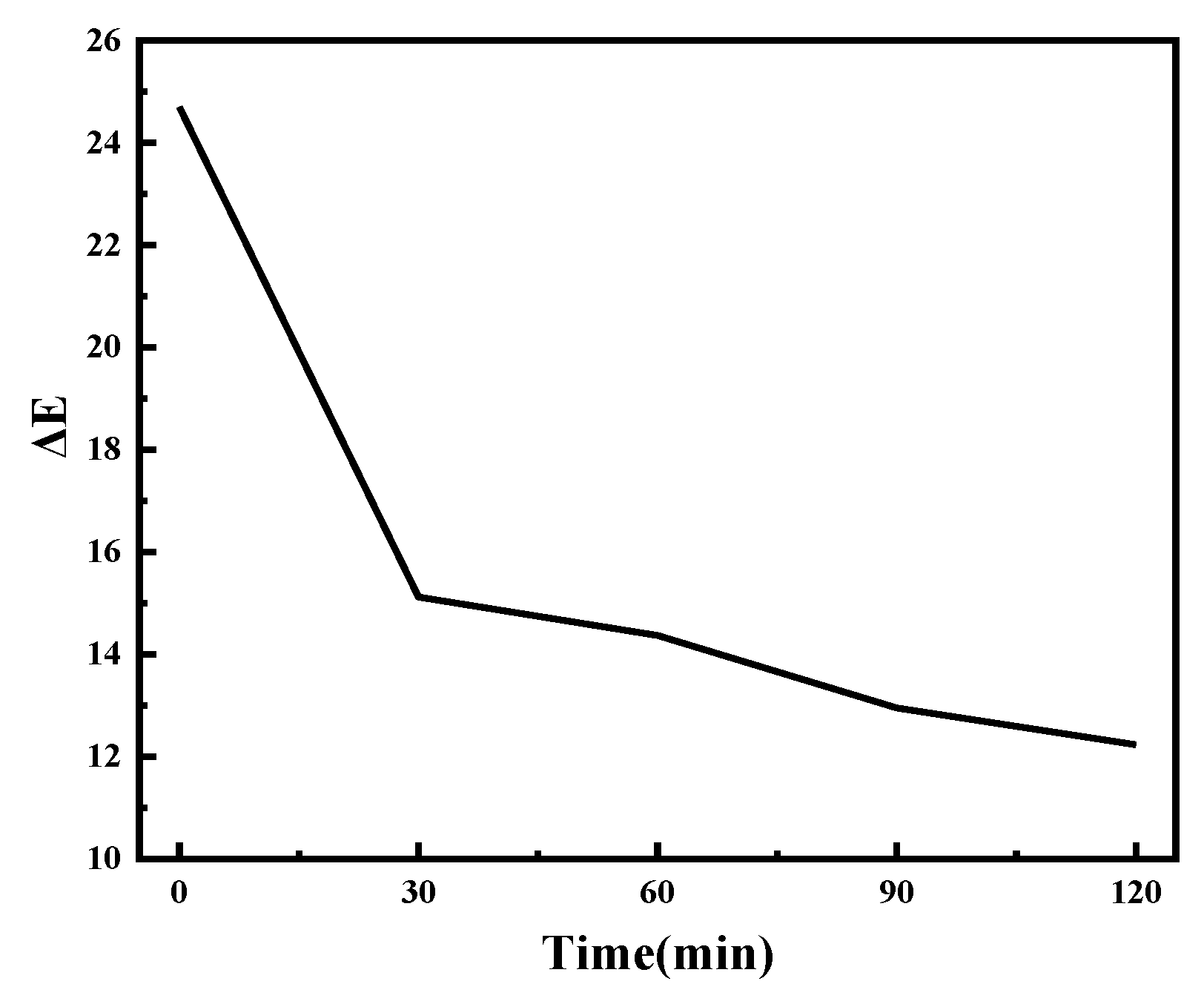
| Time/Min | L | a | b |
|---|---|---|---|
| colored | 22.92 | 2.21 | −23.07 |
| 30 | 30.86 | 1.31 | −17.63 |
| 60 | 31.19 | 1.07 | −16.60 |
| 90 | 32.57 | 0.96 | −16.21 |
| 120 | 33.26 | 0.75 | −16.05 |
| faded | 44.26 | −1.48 | −11.20 |
| Melt | Hot-Press | Cool | 180° Peeling Strength (N) | Peel Off State |
|---|---|---|---|---|
| Room temperature | 40° 150 s | Room temperature | 2.36 | film fracture |
| 50° 150 s | 4.33 | film fracture | ||
| 60° 150 s | 3.80 | film fracture | ||
| 70° 150 s | 2.43 | film fracture | ||
| 80° 150 s | 1.97 | film fracture | ||
| 90° 150 s | 1.55 | film fracture |
| Melt | Hot-Press | Cool | 180° Peeling Strength (N) | Peel Off State |
|---|---|---|---|---|
| Room temperature | 50° 5 s | Room temperature | 1.25 | complete peeling |
| 50° 50 s | 2.12 | film fracture | ||
| 50° 100 s | 2.49 | film fracture | ||
| 50° 150 s | 4.33 | film fracture | ||
| 50° 200 s | 2.56 | film fracture | ||
| 50° 250 s | 2.41 | complete peeling | ||
| 50° 350 s | 1.08 | complete peeling |
| Melt | Hot-Press | Cool | 180° Peeling Strength (N) | Peel Off State |
|---|---|---|---|---|
| 120° 150 s | 50° 150 s | −5° 10 min | 0.42 | complete peeling |
| 30-20° 30 min | 0.38 | complete peeling | ||
| 30° 10 min | 0.8 | complete peeling | ||
| Room temperature | 1.00 | film fracture |
| Melt | Hot-Press | Cool | 180° Peeling Strength (N) | Peel Off State |
|---|---|---|---|---|
| 25° 150 s | 50° 150 s | Room temperature | 3.56 | complete peeling |
| 35° 150 s | 4.04 | complete peeling | ||
| 45° 150 s | 5.11 | film fracture | ||
| 55° 150 s | 6.18 | film fracture | ||
| 65° 150 s | 7.11 | film fracture | ||
| 75° 150 s | 6.25 | film fracture | ||
| 85° 150 s | 5.04 | complete peeling | ||
| 95° 150 s | 4.04 | complete peeling | ||
| 105° 150 s | 2.60 | complete peeling |
| Melt | Hot-Press | Cool | 180° Peeling Strength (N) | Peel Off State |
|---|---|---|---|---|
| 65° 30 s | 50° 150 s | Room temperature | 1.44 | film fracture |
| 65° 60 s | 2.92 | film fracture | ||
| 65° 90 s | 4.01 | film fracture | ||
| 65° 120 s | 5.88 | film fracture | ||
| 65° 150 s | 7.11 | film fracture | ||
| 65° 180 s | 6.14 | film fracture | ||
| 65° 210 s | 5.41 | complete peeling | ||
| 65° 240 s | 5.06 | complete peeling | ||
| 65° 270 s | 4.63 | complete peeling | ||
| 65° 300 s | 2.63 | complete peeling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shi, H.; Gong, J.; Tian, G.; Yao, J. Electrochromic Fabrics with Horizontal Patterning, Enhanced Strength, Comfort, High-Temperature Protection, and Long Coloring Retention Properties for Adaptive Camouflage. Molecules 2025, 30, 1249. https://doi.org/10.3390/molecules30061249
Wang J, Shi H, Gong J, Tian G, Yao J. Electrochromic Fabrics with Horizontal Patterning, Enhanced Strength, Comfort, High-Temperature Protection, and Long Coloring Retention Properties for Adaptive Camouflage. Molecules. 2025; 30(6):1249. https://doi.org/10.3390/molecules30061249
Chicago/Turabian StyleWang, Jingjing, Haiting Shi, Jixian Gong, Geng Tian, and Jinbo Yao. 2025. "Electrochromic Fabrics with Horizontal Patterning, Enhanced Strength, Comfort, High-Temperature Protection, and Long Coloring Retention Properties for Adaptive Camouflage" Molecules 30, no. 6: 1249. https://doi.org/10.3390/molecules30061249
APA StyleWang, J., Shi, H., Gong, J., Tian, G., & Yao, J. (2025). Electrochromic Fabrics with Horizontal Patterning, Enhanced Strength, Comfort, High-Temperature Protection, and Long Coloring Retention Properties for Adaptive Camouflage. Molecules, 30(6), 1249. https://doi.org/10.3390/molecules30061249





