Phenolic Composition, Antioxidant, and Anti-Proliferative Activities Against Human Colorectal Cancer Cells of Amazonian Fruits Copoazú (Theobroma grandiflorum) and Buriti (Mauritia flexuosa)
Abstract
:1. Introduction
2. Results
2.1. Phenolic Compounds of T. grandiflorum and M. flexuosa
2.2. Antioxidant Activity of T. grandiflorum and M. flexuosa
2.3. Principal Component Analysis
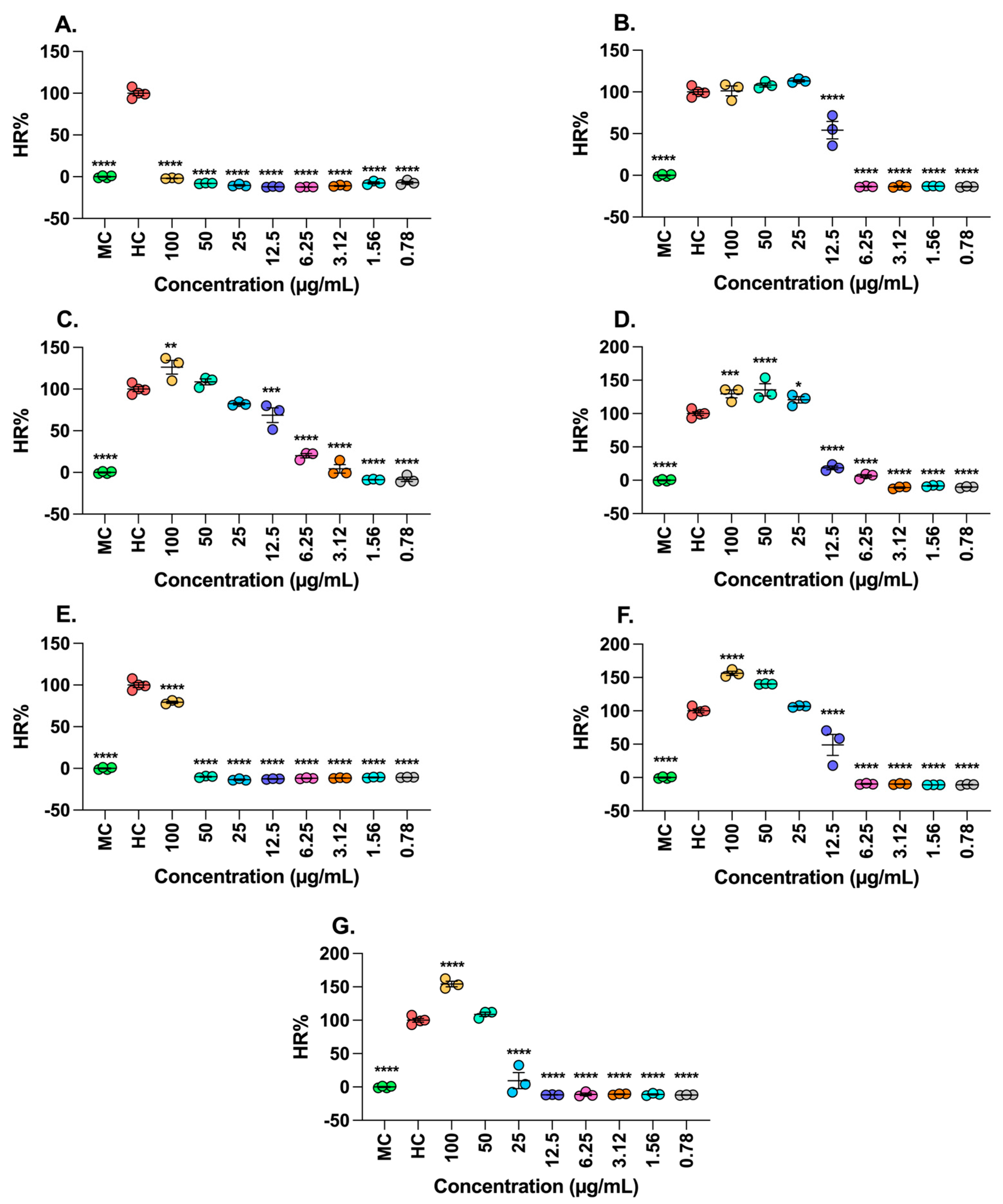
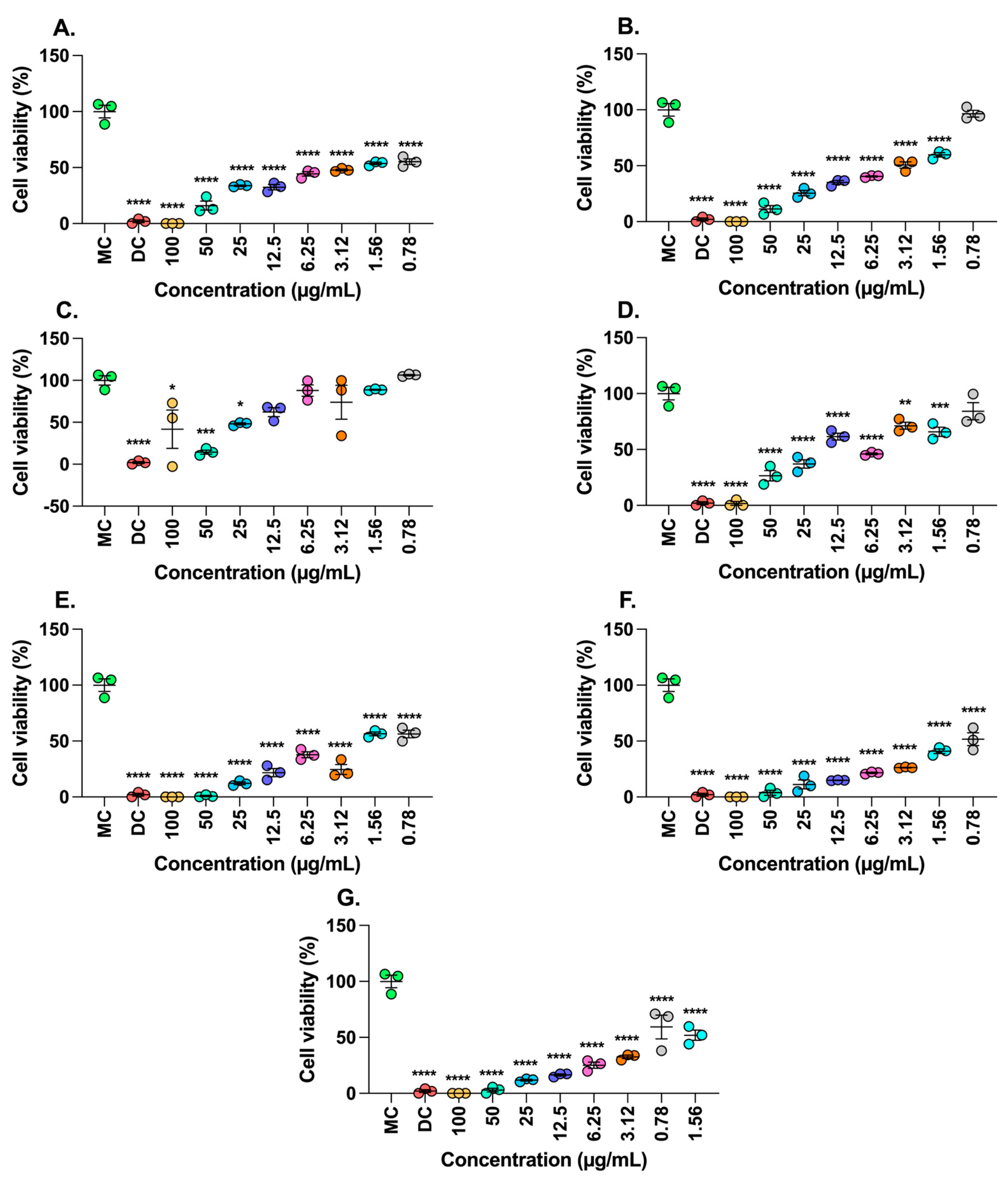
2.4. Antiproliferative Activity of Extracts T. grandiflorum and M. flexuose
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Sampling and Fruit Collection Area
4.3. Sample Preparation
4.4. Determination of Bioactive Compounds
4.4.1. Total Phenolic Content (TPC)
4.4.2. Total Flavonoid Content (TFC)
4.4.3. Total Carotenoid Content (TCC)
4.5. Quantification of Phenolic Compounds by (HPLC-UV)
4.6. Antioxidant Activity
4.6.1. Determination of Total Antioxidant Capacity by Oxygen Radical Absorption (ORAC)
- AUC: area under the fluorescence decay curve
- f0: the initial fluorescence reading at 0 min
- fn: the fluorescence reading at time n.
4.6.2. ABTS Assay
4.6.3. DPPH Radical Scavenging Capacity
4.7. Antiproliferative Activity
4.7.1. Hemolytic Activity
4.7.2. Cell Culture
4.7.3. Determination of Cell Viability by Incorporating Crystal Violet
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCI. Definición de Cancer—Diccionario de Cáncer del NCI—Instituto Nacional del Cáncer. Available online: https://www.cancer.gov/espanol/publicaciones/diccionarios/diccionario-cancer/def/cancer (accessed on 6 September 2024).
- Movahed, Z.G.; Yarani, R.; Mohammadi, P.; Mansouri, K. Sustained oxidative stress instigates differentiation of cancer stem cells into tumor endothelial cells: Pentose phosphate pathway, reactive oxygen species, and autophagy crosstalk. Biomed. Pharmacother. 2021, 139, 111643. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Bulbul, M.R.H.; Kabir, Y. Plant-based products in cancer prevention and treatment. In Functional Foods in Cancer Prevention and Therapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 237–259. [Google Scholar]
- Moloney, J.N.; Cotter, T.G. ROS signaling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Fidelis, M.; de Oliveira, S.M.; Santos, J.S.; Escher, G.B.; Rocha, R.S.; Cruz, A.G.; Carmo, M.A.V.D.; Azevedo, L.; Kaneshima, T.; Oh, W.Y.; et al. From byproduct to a functional ingredient: Camu-camu (Myrciaria dubia) seed extract as an antioxidant agent in a yogurt model. J. Dairy Sci. 2020, 103, 1131–1140. [Google Scholar] [CrossRef]
- Cavalcante, P.; Cedrim, A.S.; Marinho, E.; Barros, A.; Gomes Do Nascimento, T.; Amélio, P.C. Antioxidant properties of acai (Euterpe oleracea) in the metabolic syndrome. Braz. J. Food Technol. 2018, 21, e2017092. [Google Scholar]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, A.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Pardo, C.; Vries, E.D.E.; Buitrago, L. Atlas de Mortalidad por Cáncer en Colombia, 4th ed.; Instituto Nacional de Cancerología: Bogota, Colombia, 2017; 121p. [Google Scholar]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Maróstica, M.R., Jr.; Pastore, G.M. Evaluation of the antioxidant, antiproliferative and antimutagenic potential of araçá-boi fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Schulz, M.; Seraglio, S.K.T.; Brugnerotto, P.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Composition and potential health effects of dark-colored underutilized Brazilian fruits—A review. Food Res. Int. 2020, 137, 109744. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho-Silva, L.B.; Dionísio, A.P.; da Silva Pereira, A.C.; Wurlitzer, N.J.; de Brito, E.S.; Bataglion, G.A.; Brasil, I.M.; Eberlin, M.N.; Liu, R.H. Antiproliferative, antimutagenic, and antioxidant activities of a Brazilian tropical fruit juice. LWT Food Sci. Technol. 2014, 59, 1319–1324. [Google Scholar] [CrossRef]
- de Moraes Barros, H.R.; Garcia-Villalba, R.; Tomás-Barberán, F.A.; Genovese, M.I. Evaluation of the distribution and metabolism of polyphenols derived from cupuassu (Theobroma grandiflorum) in mice gastrointestinal tract by UPLC-ESI-QTOF. J. Funct. Foods 2016, 22, 477–489. [Google Scholar] [CrossRef]
- Silva da Costa, R.; de Farias Silva, N.; Gabbay Alves, T.V.; Fernandes da Silva, M.; do Socorro Barros Brasil, D.; Ribeiro-Costa, R.M.; Converti, A.; Silva, J.O.C. Antioxidant Activity of an Industrial Cupuassu Seed By-product: Molecular Modeling of Phenolic Compounds. Chem. Eng. Technol. 2019, 42, 397–406. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; González-Correa, C.H.; Le, M.; Idárraga-Mejía, A.M. Flavonoid/Polyphenol Ratio in Mauritia flexuosa and Theobroma grandiflorum as an Indicator of Effective Antioxidant Action. Molecules 2021, 26, 6431. [Google Scholar] [CrossRef] [PubMed]
- Fantinelli, J.C.; Cuéllar Álvarez, L.N.; González Arbeláez, L.F.; Ciocci Pardo, A.; Galeano García, P.L.; Schinella, G.R.; Mosca, S.M. Acute treatment with copoazú fermented extract ameliorates myocardial ischemia-reperfusion injury via eNOS activation. J. Funct. Foods 2017, 34, 470–477. [Google Scholar] [CrossRef]
- Arias, M.H.; Garavito, G.; Luengas, P.; Palacios, P. Plantas de Interés de la Chagra de la Comunidad Indígena Ziora-Amena Amazonia Colombiana; Universidad Nacional de Colombia: Bogota, Colombia, 2021; pp. 1–28. [Google Scholar]
- Pereira Freire, J.A.; Barros, K.B.N.T.; Lima, L.K.F.; Martins, J.M.; Araújo, Y.D.C.; da Silva Oliveira, G.L.; de Souza Aquino, J.; Ferreira, P.M.P. Phytochemistry Profile, Nutritional Properties and Pharmacological Activities of Mauritia flexuosa. J. Food Sci. 2016, 81, R2611–R2622. [Google Scholar] [CrossRef] [PubMed]
- Best, I.; Casimiro-Gonzales, S.; Portugal, A.; Olivera-Montenegro, L.; Aguilar, L.; Muñoz, A.M.; Ramos-Escudero, F. Phytochemical screening and DPPH radical scavenging activity of three morphotypes of Mauritia flexuosa L.f. from Peru, and thermal stability of a milk-based beverage enriched with carotenoids from these fruits. Heliyon 2020, 6, e05209. [Google Scholar] [CrossRef] [PubMed]
- Barboza, N.L.; dos Cruz, J.M.A.; Corrêa, R.F.; Lamarão, C.V.; Lima, A.R.; Inada, N.M.; Sanches, E.A.; de Araújo Bezerra, J.; Campelo, P.H. Buriti (Mauritia flexuosa L. f.): An Amazonian fruit with potential health benefits. Food Res. Int. 2022, 159, 111654. [Google Scholar] [CrossRef]
- Simas Frauches, N.; Montenegro, J.; Amaral, T.; Abreu, J.P.; Laiber, G.; Junior, J.; Borguini, R.; Santiago, M.; Pacheco, S.; Nakajima, V.M.; et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules 2021, 26, 564. [Google Scholar] [CrossRef]
- Do Carmo MA, V.; Fidelis, M.; Pressete, C.G.; Marques, M.J.; Castro-Gamero, A.M.; Myoda, T.; Granato, D.; Azevedo, L. Hydroalcoholic Myrciaria dubia (camu-camu) seed extracts prevent chromosome damage and act as antioxidant and cytotoxic agents. Food Res. Int. 2019, 125, 108551. [Google Scholar] [CrossRef]
- Sánchez-Capa, M.; Corell González, M.; Mestanza-Ramón, C. Edible Fruits from the Ecuadorian Amazon: Ethnobotany, Physicochemical Characteristics, and Bioactive Components. Plants 2023, 12, 3635. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, functional properties and health-promoting potential of native South American berries: A review. J. Sci. Food Agric. 2021, 101, 364–378. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- García-Chacón, J.M.; Marín-Loaiza, J.C.; Osorio, C. Camu Camu (Myrciaria dubia (Kunth) McVaugh): An Amazonian Fruit with Biofunctional Properties-A Review. ACS Omega 2023, 8, 5169–5183. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-B, C.; Castaño-R, D.; Hoyos, D.; Velasco-A, G.; Peña, J.L.; Sanín, D. Non-arboreal angiosperms of a humid tropical forest in the Colombian Andean-amazon piedmont. Bol. Cient. Cent. Mus. 2019, 23, 62–94. [Google Scholar]
- Pugliese, A.G.; Tomas-Barberan, F.A.; Truchado, P.; Genovese, M.I. Flavonoids, proanthocyanidins, vitamin C, and antioxidant activity of Theobroma grandiflorum (Cupuassu) pulp and seeds. J. Agric. Food Chem. 2013, 61, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef]
- Alvarez-B, C.; Castaño-R, D.; Hoyos, D.; Velasco-A, G.; Peña, J.L.; Sanín, D. Angiospermas no arbóreas de un bosque húmedo tropical en el piedemonte Andino-Amazónico colombiano. Boletín Científico Cent. Mus. Mus. Hist. Nat. 2019, 23, 62–94. [Google Scholar] [CrossRef]
- Abreu-Naranjo, R.; Paredes-Moreta, J.G.; Granda-Albuja, G.; Iturralde, G.; González-Paramás, A.M.; Alvarez-Suarez, J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 2020, 6, e05211. [Google Scholar] [CrossRef]
- Tauchen, J.; Bortl, L.; Huml, L.; Miksatkova, P.; Doskocil, I.; Marsik, P.; Marsik, P.; Villegas, P.P.P.; Flores, Y.B.; Van Damme, P.; et al. Phenolic composition, antioxidant and anti-proliferative activities of edible and medicinal plants from the Peruvian Amazon. Rev. Bras. Farmacogn. 2016, 26, 728–737. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; de Camargo, A.C.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Nobre, C.B.; Sousa, E.O.; Camilo, C.J.; Machado, J.F.; Silva, J.M.F.L.; Filho, J.R.; Coutinho, H.D.M.; Costa, J.G.M. Antioxidative effect and phytochemical profile of natural products from the fruits of “babaçu” (Orbignia speciose) and “buriti” (Mauritia flexuosa). Food Chem. Toxicol. 2018, 121, 423–429. [Google Scholar] [CrossRef]
- Fidelis, M.; do Carmo, M.A.V.; da Cruz, T.M.; Azevedo, L.; Myoda, T.; Furtado, M.M.; Marques, M.B.; Sant’Ana, A.S.; Genovese, M.I.; Oh, W.Y.; et al. Camu-camu seed (Myrciaria dubia)—From sidestream to an antioxidant, antihyperglycemic, antiproliferative, antimicrobial, antihemolytic, anti-inflammatory, and antihypertensive ingredient. Food Chem. 2020, 310, 125909. [Google Scholar] [CrossRef]
- Slima, S.B.; Trabelsi, I.; Ktari, N.; Bardaa, S.; Elkaroui, K.; Bouaziz, M.; Abdeslam, A.; Salah, R.B. Novel Sorghum bicolor (L.) seed polysaccharide structure, hemolytic and antioxidant activities, and laser burn wound healing effect. Int. J. Biol. Macromol. 2019, 132, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, A.A.; Piroski, C.S.; Daniel, T.G.; Cruz, T.M.; Escher, G.B.; Vieira do Carmo, M.A.; Azevedo, L.; Marques, M.B.; Granato, D.; Rosso, N.D. Red Chicory (Cichorium intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019, 84, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Villada Ramos, J.A.; Aguillón Osma, J.; Restrepo Cortes, B.; Loango Chamarro, N.; Maldonado Celis, M.E. Identification of potential bioactive compounds of Passiflora edulis leaf extract against colon adenocarcinoma cells. Biochem. Biophys. Rep. 2023, 34, 101453. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Hudaib, F.; Hourani, W.; Darwish, W.; Abu-Irmaileh, B.; Deb, P.K.; Venugopala, K.N.; Mohanlall, V.; Chandrashekharappa, S.; Abu-Dahab, R.; et al. Mass spectrometry-based metabolomics approach and in vitro assays revealed promising role of 2,3-dihydroquinazolin-4(1H)-one de-rivatives against colorectal cancer cell lines. Eur. J. Pharm. Sci. 2023, 1, 182. [Google Scholar]
- Sterling, A.; Rodríguez, N.; Quiceno, E.; Trujillo, F.; Clavijo, A.; Suárez-Salazar, J.C. Dynamics of photosynthetic responses in 10 rubber tree (Hevea brasiliensis) clones in Colombian Amazon: Implications for breeding strategies. PLoS ONE 2019, 14, e0226254. [Google Scholar] [CrossRef]
- Sánchez Olaya, D.M.; Rodríguez Pérez, W.; Castro Rojas, D.F.; Trujillo Trujillo, E.T. Respuesta agronómica de mucilago de cacao (Theobroma cacao L.) en cultivo de maíz (Zea mays L.). Cienc. En Desarro. 2019, 10, 43–58. [Google Scholar] [CrossRef]
- Olaya-Montes, A.; Llanos-Cabrera, M.P.; Cherubin, M.R.; Herrera-Valencia, W.; Ortiz-Morea, F.A.; Silva-Olaya, A.M. Restoring soil carbon and chemical properties through silvopastoral adoption in the Colombian Amazon region. Land Degrad. Dev. 2020, 32, 3720–3730. [Google Scholar] [CrossRef]
- Rodriguez-Pérez, W.; Forero-Doria, O.; Ortiz-Suárez, F.; Murillo-Palacios, Y.; Olate, V.R.; Guzman, L. Protein profile, morphometric analysis and toxicity of Bothropsatrox (viperidae) venom from Colombian Amazon. J. Chem. Pharm. Res. 2016, 8, 476–483. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Methods in Enzymology—Oxidants and Antioxidants Part, A. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Suárez, J.C.; Polanía-Hincapié, P.A.; Saldarriaga, S.; Ramón-Triana, V.Y.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Bioactive Compounds and Antioxidant Activity in Seeds of Bred Lines of Common Bean Developed from Interspecific Crosses. Foods 2023, 12, 2849. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Neves, L.C.; da Silva, V.X.; Pontis, J.A.; Flach, A.; Roberto, S.R. Bioactive compounds and antioxidant activity in pre-harvest camu-camu [Myrciaria dubia (H.B.K.) Mc Vaugh] fruits. Sci. Hortic. 2015, 186, 223–229. [Google Scholar]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First Action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Mancuello, C.; Pereira, C.; González, F.; Prieto, R.; Rolón, M.; Álvarez, S.; Benítez, B. Estudio espectrofotométrico de la actividad hemolítica del extracto crudo de Phoradendron bathyoryctum Eichler sobre eritrocitos humanos. Steviana 2021, 5, 114–121. [Google Scholar] [CrossRef]

| Fruto | F. P * | Place | TPC | TFC | TCC | Gallic Acid | Catechin | Quercetin |
|---|---|---|---|---|---|---|---|---|
| T. grandiflorum | Seed | Balcanes | 619.41 ± 12.05 a | 569.09 ± 4.51 a | 25.12 ± 0.16 b | 335.12 ± 10.87 a | 39.86 ± 0.29 a | 70.11 ± 0.48 b |
| Versalles | 524.25 ± 12.05 b | 502.85 ± 4.51 b | 30.25 ± 0.16 a | 26.81 ± 10.87 bc | 10.72 ± 0.29 e | 6.70 ± 0.48 c | ||
| Caraño | 361.17 ± 12.05 c | 404.68 ± 4.51 c | 22.78 ± 0.16 c | 17.92 ± 10.87 c | 26.42 ± 0.29 b | 79.27 ± 0.48 a | ||
| Pulp | Balcanes | 164.21 ± 12.0.5 d | 146.87 ± 4.51 d | 4.39 ± 0.16 d | 51.63 ± 10.87 b | 19.07 ± 0.29 c | 6.36 ± 0.48 cd | |
| Versalles | 154.12 ± 12.05 d | 142.59 ± 4.51 d | 1.61 ± 0.16 f | 47.31 ± 10.87 bc | 16.37 ± 0.29 d | 5.01 ± 0.48 d | ||
| Caraño | 170.58 ± 12.05 d | 141.91 ± 4.51 d | 3.03 ± 0.16 e | 46.60 ± 10.87 bc | 10.72 ± 0.29 d | 6.70 ± 0.48 d | ||
| M. flexuosa | Pulp | Macagual | 508.48 ± 10.06 a | 206.83 ± 3.92 b | 34.92 ± 0.28 c | 180.62 ± 0.68 d | 38.63 ± 0.56 c | 75.20 ± 2.80 b |
| Montañita | 475.55 ± 10.06 b | 206.89 ± 3.92 b | 37.24 ± 0.28 b | 195.80 ± 0.68 c | 45.76 ± 0.56 b | 76.00 ± 2.80 b | ||
| Florencia | 285.75 ± 10.06 d | 223.21 ± 3.92 a | 48.00 ± 0.28 a | 219.54 ± 0.68 a | 61.15 ± 0.56 a | 95.50 ± 2.80 a | ||
| Peel | Macagual | 354.00 ± 10.06 c | 177.23 ± 3.92 c | 25.12 ± 0.28 d | 175.72 ± 0.68 e | 5.68 ± 0.56 de | 17.06 ± 2.80 c | |
| Montañita | 367.55 ± 10.06 c | 183.00 ± 3.92 c | 18.21 ± 0.28 f | 125.43 ± 0.68 f | 4.27 ± 0.56 e | 12.17 ± 2.80 c | ||
| Florencia | 217.21 ± 10.06 e | 172.79 ± 3.92 c | 24.24 ± 0.28 e | 197.90 ± 0.68 b | 6.43 ± 0.56 d | 19.25 ± 2.80 c |
| Fruit | F. P * | Place | ABTS 1 | DPPH 1 | ORAC 1 |
|---|---|---|---|---|---|
| T. grandiflorum | Seed | Balcanes | 207.63 ± 1.64 a | 117.0 ± 1.24 a | 204.93 ± 0.41 a |
| Versalles | 192.05 ± 1.64 b | 98.3 ± 1.24 b | 170.86 ± 0.41 c | ||
| Caraño | 102.19 ± 1.64 c | 94.56 ± 1.24 b | 185.99 ± 0.41 b | ||
| Pulp | Balcanes | 96.89 ± 1.64 d | 4.11 ± 1.24 c | 67.97 ± 0.41 d | |
| Versalles | 91.34 ± 1.64 e | 4.09 ± 1.24 c | 50.22 ± 0.41 f | ||
| Caraño | 90.66 ± 1.64 e | 4.10 ± 1.24 c | 58.83 ± 0.41 e | ||
| M. flexuosa | Pulp | Macagual | 52.62 ± 0.65 c | 40.40 ± 0.59 c | 86.14 ± 2.08 f |
| Montañita | 59.19 ± 0.65 b | 44.28 ± 0.59 b | 195.48 ± 2.08 d | ||
| Florencia | 71.99 ± 0.65 a | 67.23 ± 0.59 a | 114.19 ± 2.08 e | ||
| Peel | Macagual | 41.98 ± 0.65 d | 15.78 ± 0.59 d | 256.49 ± 2.08 c | |
| Montañita | 30.17 ± 0.65 e | 17.37 ± 0.59 d | 526.23 ± 2.08 a | ||
| Florencia | 40.06 ± 0.65 d | 15.03 ± 0.59 d | 471.35 ± 2.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldarriaga, S.; Rodríguez-Salazar, C.A.; Recalde-Reyes, D.P.; Paladines Beltrán, G.M.; Cuéllar Álvarez, L.N.; Silva Ortíz, Y.L. Phenolic Composition, Antioxidant, and Anti-Proliferative Activities Against Human Colorectal Cancer Cells of Amazonian Fruits Copoazú (Theobroma grandiflorum) and Buriti (Mauritia flexuosa). Molecules 2025, 30, 1250. https://doi.org/10.3390/molecules30061250
Saldarriaga S, Rodríguez-Salazar CA, Recalde-Reyes DP, Paladines Beltrán GM, Cuéllar Álvarez LN, Silva Ortíz YL. Phenolic Composition, Antioxidant, and Anti-Proliferative Activities Against Human Colorectal Cancer Cells of Amazonian Fruits Copoazú (Theobroma grandiflorum) and Buriti (Mauritia flexuosa). Molecules. 2025; 30(6):1250. https://doi.org/10.3390/molecules30061250
Chicago/Turabian StyleSaldarriaga, Sebastián, Carlos Andrés Rodríguez-Salazar, Delia Piedad Recalde-Reyes, Gloria Magally Paladines Beltrán, Liceth N. Cuéllar Álvarez, and Yudy Lorena Silva Ortíz. 2025. "Phenolic Composition, Antioxidant, and Anti-Proliferative Activities Against Human Colorectal Cancer Cells of Amazonian Fruits Copoazú (Theobroma grandiflorum) and Buriti (Mauritia flexuosa)" Molecules 30, no. 6: 1250. https://doi.org/10.3390/molecules30061250
APA StyleSaldarriaga, S., Rodríguez-Salazar, C. A., Recalde-Reyes, D. P., Paladines Beltrán, G. M., Cuéllar Álvarez, L. N., & Silva Ortíz, Y. L. (2025). Phenolic Composition, Antioxidant, and Anti-Proliferative Activities Against Human Colorectal Cancer Cells of Amazonian Fruits Copoazú (Theobroma grandiflorum) and Buriti (Mauritia flexuosa). Molecules, 30(6), 1250. https://doi.org/10.3390/molecules30061250








