Emerging Biochemical Conversion for Plastic Waste Management: A Review
Abstract
1. Introduction
2. Emerging Technologies
2.1. Chemical Methods
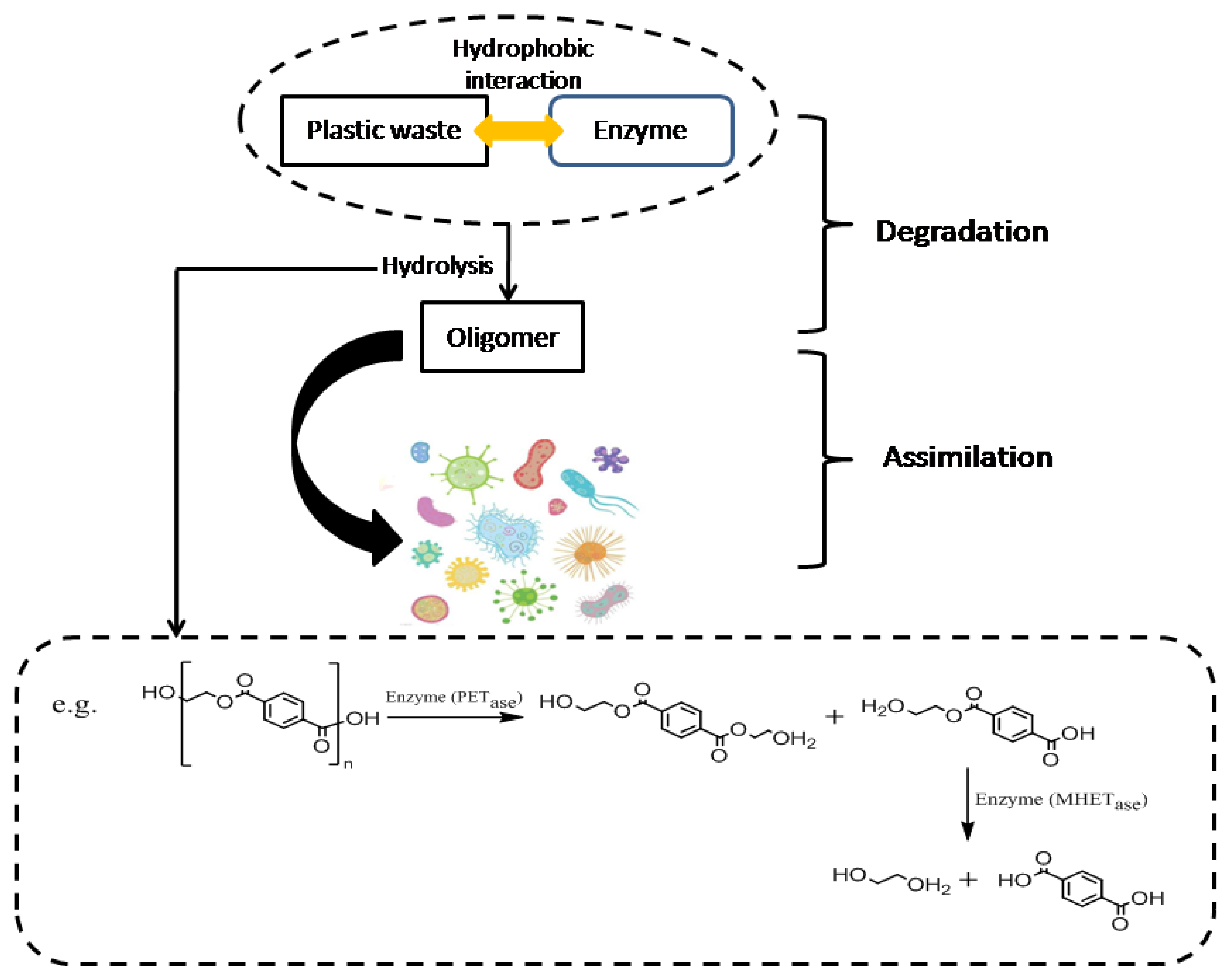
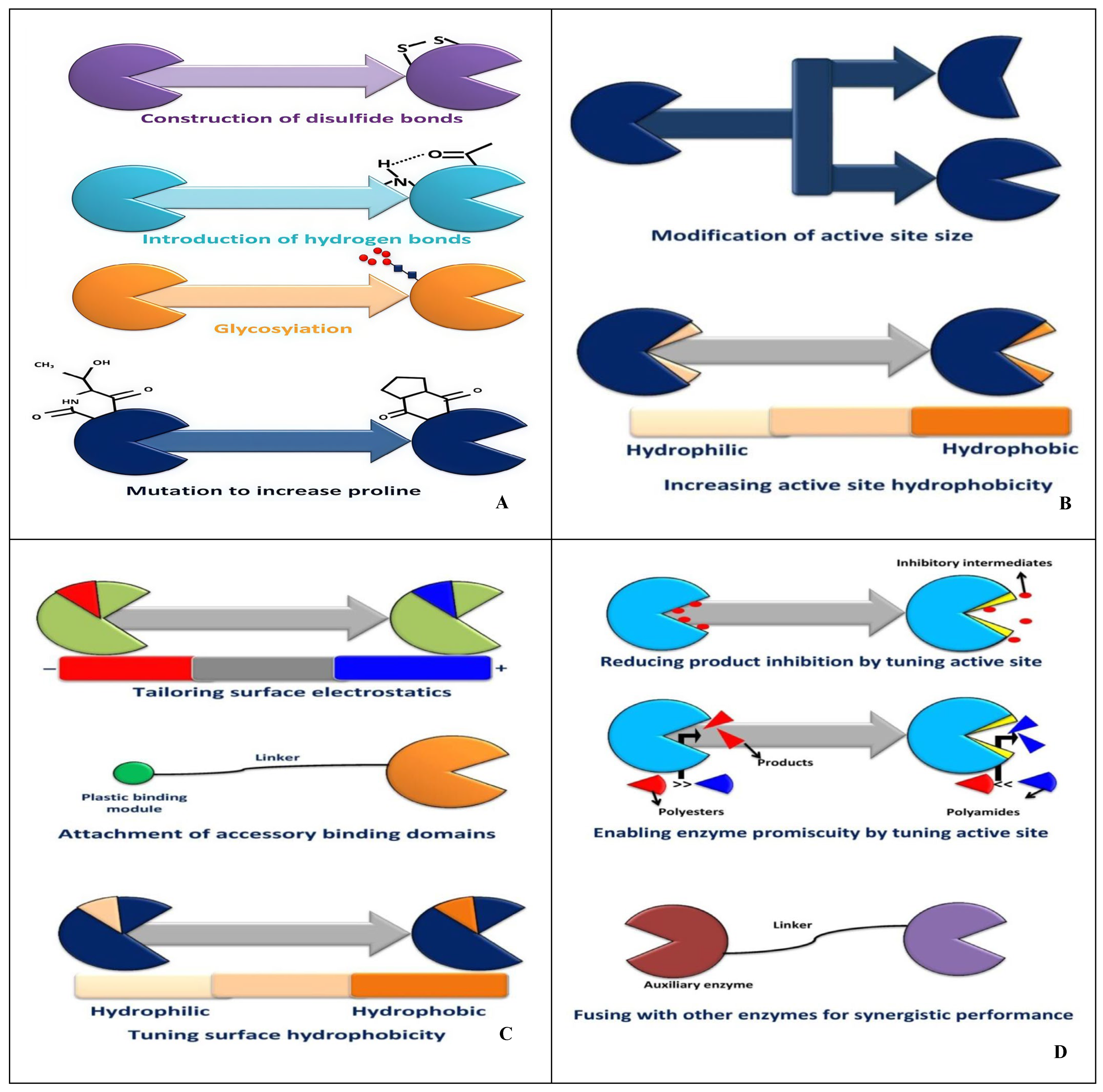
2.2. Biological Methods
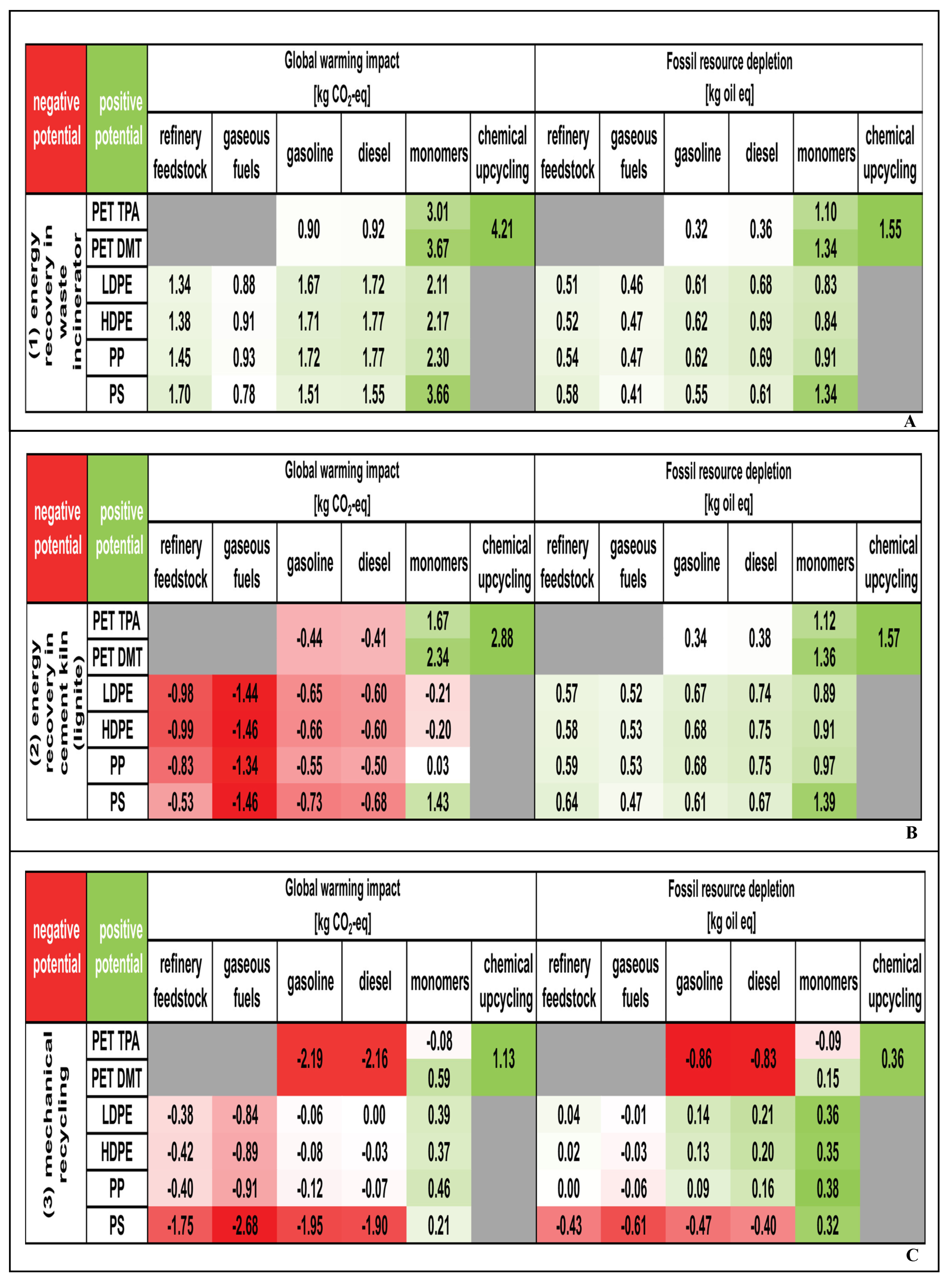
3. Economic Analysis and Life Cycle Assessment of Emerging Technologies
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, L.H.; Li, H.; Wang, F.F.; Deng, Y.X.; Xu, Q.J. International Governance Progress in Marine Plastic Litter Pollution and Policy Recommendations. Res. Environ. Sci. 2022, 35, 1334–1340. [Google Scholar]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Sorasan, C.; Edo, C.; González-Pleiter, M.; Fernández-Piñas, F.; Leganés, F.; Rodríguez, A.; Rosal, R. Ageing and fragmentation of marine microplastics. Sci. Total Environ. 2022, 827, 154438. [Google Scholar] [CrossRef]
- Liu, Z.C.; Bacha, A.U.R.; Yang, L. Control strategies for microplastic pollution in groundwater. Environ. Pollut. 2023, 335, 122323. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Marcos, R.; Hernández, A. Potential adverse health effects of ingested micro- and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J. Toxicol. Environ. Health Part B 2020, 23, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.G.; Takada, H.; Yamashita, R.; Okazaki, Y.; Uchida, K.; Tokai, T.; Tanaka, K.; Trenholm, N. PCBs and PBDEs in microplastic particles and zooplankton in open water in the Pacific Ocean and around the coast of Japan. Mar. Pollut. Bull. 2020, 151, 110806. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.H.; Zhang, W.; Shi, L.X.; Yi, K.X.; Yu, H.B.; Zhang, C.Y.; Li, S.Z.; Li, J.N. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manag. 2022, 302, 113995. [Google Scholar] [CrossRef]
- Hong, X.T.; Niu, B.X.; Sun, H.W.; Zhou, X. Insight into response characteristics and inhibition mechanism of anammox granular sludge to polyethylene terephthalate microplastics exposure. Bioresour. Technol. 2023, 385, 129355. [Google Scholar] [CrossRef]
- Kaur, K.; Reddy, S.; Barathe, P.; Oak, U.; Shriram, V.; Kharat, S.S.; Govarthanan, M.; Kumar, V. Microplastic-associated pathogens and antimicrobial resistance in environment. Chemosphere 2022, 291, 133005. [Google Scholar] [CrossRef]
- Harikrishnan, T.; Janardhanam, M.; Sivakumar, P.; Sivakumar, R.; Rajamanickam, K.; Raman, T.; Thangavelu, M.; Muthusamy, G.; Singaram, G. Microplastic contamination in commercial fish species in southern coastal region of India. Chemosphere 2023, 313, 137486. [Google Scholar] [CrossRef]
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Shao, L.M.; Ye, J.F.; He, P.J. Is incineration the terminator of plastics and microplastics? J. Hazard. Mater. 2021, 401, 123429. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Q.; Peng, S.Z.; Peng, C.; Hu, Y.K. Research progress in high value-added utilization technology of waste plastics. Chem. Ind. Eng. Prog. 2023, 42, 1020–1027. [Google Scholar]
- Hu, B.; Wang, S.; Yan, J.B.; Zhang, H.R.; Qiu, L.P.; Liu, W.J.; Guo, Y.; Shen, J.; Chen, B.; Shi, C.; et al. Review of waste plastics treatment and utilization: Efficient conversion and high value utilization. Process Saf. Environ. Prot. 2024, 183, 378–398. [Google Scholar]
- Su, K.Y.; Liu, H.F.; Zhang, C.F.; Wang, F. Photocatalytic conversion of waste plastics to low carbon number organic products. Chin. J. Catal. 2022, 43, 589–594. [Google Scholar] [CrossRef]
- Thew, C.X.E.; Lee, Z.S.; Srinophakun, P.; Ooi, C.W. Recent advances and challenges in sustainable management of plastic waste using biodegradation approach. Bioresour. Technol. 2023, 374, 128772. [Google Scholar] [CrossRef]
- Liu, Z.C.; Tran, K.Q. A review on disposal and utilization of phytoremediation plants containing heavy metals. Ecotoxicol. Environ. Saf. 2021, 226, 112821. [Google Scholar] [CrossRef]
- Wang, N.M.; Strong, G.; DaSilva, V.; Gao, L.J.; Huacuja, R.; Konstantinov, I.A.; Rosen, M.S.; Nett, A.J.; Ewart, S.; Geyer, R.; et al. Chemical Recycling of Polyethylene by Tandem Catalytic Conversionto Propylene. J. Am. Chem. Soc. 2022, 144, 18526–18531. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.H.; Xu, M.; Wang, Y.; Ge, R.X.; Kong, X.G.; Zheng, L.R.; Duan, H.H. Electrocatalytic upcycling of polyethyleneterephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021, 12, 4679. [Google Scholar] [CrossRef]
- Song, X.Y.; Hu, W.Y.; Huang, W.W.; Wang, H.; Yan, S.H.; Yu, S.T.; Liu, F.S. Methanolysis of polycarbonate into valuable product bisphenol A using choline chloride-based deep eutectic solvents as highly active catalysts. Chem. Eng. J. 2020, 388, 124324. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, L.Y.; Jeng, R.J.; Dai, S.A. 100% Atom-Economy Efficiency of Recycling Polycarbonate into Versatile Intermediates. ACS Sustain. Chem. Eng. 2018, 6, 8964–8975. [Google Scholar] [CrossRef]
- Li, L.; Luo, H.; Shao, Z.L.; Zhou, H.Z.; Lu, J.W.; Chen, J.J.; Huang, C.J.; Zhang, S.N.; Liu, X.F.; Xia, L.; et al. Converting Plastic Wastes to Naphtha for Closing the Plastic Loop. J. Am. Chem. Soc. 2023, 145, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Conk, R.J.; Hanna, S.; Shi, J.X.; Yang, J.; Ciccia, N.R.; Qi, L.; Bloomer, B.J.; Heuvel, S.; Wills, T.; Su, J.; et al. Catalytic deconstruction of waste polyethylene withethylene to form propylene. Science 2022, 377, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, M.H.; Yappert, R.D.; Sun, J.K.; Lee, Y.H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromaticsby tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef]
- Attique, S.; Batool, M.; Yaqub, M.; Goerke, O.; Gregory, D.H.; Shah, A.T. Highly efficient catalytic pyrolysis of polyethylene waste to derive fuel products by novel polyoxometalate/kaolin composites. Waste Manag. Res. 2020, 38, 689–695. [Google Scholar] [CrossRef]
- Yao, D.D.; Yang, H.P.; Hu, Q.; Chen, Y.Q.; Chen, H.P.; Williams, P.T. Carbon nanotubes from post-consumer waste plastics: Investigations intocatalyst metal and support material characteristics. Appl. Catal. B Environ. 2021, 280, 119413. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Duan, D.L.; Lei, H.W.; Villota, E.; Ruan, R. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl. Energy 2019, 251, 113337. [Google Scholar] [CrossRef]
- Huo, E.; Lei, H.W.; Liu, C.; Zhang, Y.Y.; Xin, L.Y.; Zhao, Y.F.; Qian, M.; Zhang, Q.F.; Lin, X.N.; Wang, C.X.; et al. Jet fuel and hydrogen produced from waste plastics catalytic pyrolysiswith activated carbon and MgO. Sci. Total Environ. 2020, 727, 138411. [Google Scholar] [CrossRef]
- Cai, N.; Li, X.Q.; Xia, S.W.; Sun, L.; Hu, J.H.; Bartocci, P.; Fantozzi, F.; Williams, P.T.; Yang, H.P.; Chen, H.P. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possiblereaction mechanisms. Energy Convers. Manag. 2021, 229, 113794. [Google Scholar] [CrossRef]
- Cao, B.; Sun, Y.K.; Guo, J.J.; Wang, S.; Yuan, J.P.; Esakkimuthu, S.; Uzoejinwa, B.B.; Yuan, C.; Abomohra, A.E.F.; Qian, L.L.; et al. Synergistic effects of co-pyrolysis of macroalgae and polyvinyl chloride onbio-oil/bio-char properties and transferring regularity of chlorine. Fuel 2019, 246, 319–329. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic Fuel Oils Produced from the Pyrolysis-Catalysis of PolyethylenePlastic with Metal-Impregnated Zeolite Catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; González, R.M.; Meirer, F.; Weckhuysen, B.M. Plastic Waste Conversion over a Refinery Waste Catalyst. Angew. Chem. Int. Ed. 2021, 60, 16101–16108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Z.; Cho, M.K.; Lee, J.G.; Choi, S.W.; Lee, K.B. Upcycling of Waste Polyethylene Terephthalate Plastic Bottles into Porous Carbon for CF4 Adsorption. Environ. Pollut. 2020, 265, 114868. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.W.; Liu, B.G.; Rong, Q.; Zhang, L.B.; Guo, S.H. Porous carbon materials derived from discarded COVID-19 masks via microwave solvothermal method for lithium-sulfur batteries. Sci. Total Environ. 2022, 817, 152995. [Google Scholar]
- Munir, D.; Amer, H.; Aslam, R.; Bououdina, M.; Usman, M.R. Composite zeolite beta catalysts for catalytic hydrocracking of plastic waste to liquid fuels. Mater. Renew. Sustain. Energy 2020, 9, 9. [Google Scholar] [CrossRef]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Low-temperature catalytic upgrading of waste polyolefinic plastics intoliquid fuels and waxes. Appl. Catal. B Environ. 2021, 285, 119805. [Google Scholar] [CrossRef]
- Lee, W.T.; Bobbink, F.D.; van Muyden, A.P.; Lin, K.H.; Corminboeuf, C.; Zamani, R.R.; Dyson, P.J. Catalytic hydrocracking of synthetic polymersinto grid-compatible gas streams. Cell Rep. Phys. Sci. 2021, 2, 100332. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Ok, Y.S.; Peng, W.X.; Chong, C.T.; Ma, N.L.; Chase, H.A.; Liew, Z.L.; Yusup, S.; Kwon, E.E.; et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sustain. Energy Rev. 2019, 115, 109359. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Xie, W.; Liu, Y.Y.; Cao, M.J.; Kong, X.H.; Chu, Q.L.; Mao, H.P. Hydrocarbon rich bio-oil production, thermal behavior analysis and kinetic study of microwave-assisted co-pyrolysis of microwave-torrefied lignin with low density polyethylene. Bioresour. Technol. 2019, 291, 121860. [Google Scholar] [CrossRef]
- Rex, P.; Msilamani, I.P.; Miranda, L.R. Microwave pyrolysis of polystyrene and polypropylene mixtures using different activated carbon from biomass. J. Energy Inst. 2020, 93, 1819–1832. [Google Scholar] [CrossRef]
- Zhou, N.; Dai, L.L.; Lv, Y.C.; Li, H.; Deng, W.Y.; Guo, F.Q.; Chen, P.; Lei, H.W.; Ruan, R. Catalytic pyrolysis of plastic wastes in a continuous microwave assistedpyrolysis system for fuel production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Cao, C.Q.; Bian, C.; Wang, G.Y.; Bai, B.; Xie, Y.P.; Jin, H. Co-gasification of plastic wastes and soda lignin in supercritical water. Chem. Eng. J. 2020, 388, 124277. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.G.; Wang, Q.X.; Zou, J.; Zhang, H.; Jin, H.; Li, X.W. Experimental investigation on gasification characteristics of plastic wastes in supercritical water. Renew. Energy 2019, 135, 32–40. [Google Scholar] [CrossRef]
- Bai, B.; Wang, W.Z.; Jin, H. Experimental study on gasification performance of polypropylene (PP) plastics in supercritical water. Energy 2020, 191, 116527. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Hu, C.S.; Peng, B.; Liu, C.; Li, Z.W.; Wu, K.; Zhang, H.Y.; Xiao, R. High H2/CO ratio syngas production from chemical looping co-gasificationof biomass and polyethylene with CaO/Fe2O3 oxygen carrier. Energy Convers. Manag. 2019, 199, 111951. [Google Scholar] [CrossRef]
- Jiao, X.C.; Zheng, K.; Chen, Q.X.; Li, X.D.; Li, Y.M.; Shao, W.W.; Xu, J.Q.; Zhu, J.F.; Pan, Y.; Sun, Y.F.; et al. Photocatalytic Conversion of Waste Plastics into C2 Fuels under Simulated Natural Environment Conditions. Angew. Chem. Int. Ed. 2020, 59, 15497–15501. [Google Scholar] [CrossRef]
- Zeng, M.H.; Lee, Y.H.; Strong, G.; LaPointe, A.M.; Kocen, A.L.; Qu, Z.Q.; Coates, G.W.; Scott, S.L.; Abu-Omar, M.M. Chemical Upcycling of Polyethylene to Value-Added α,ω-Divinyl-Functionalized Oligomers. ACS Sustain. Chem. Eng. 2021, 9, 13926–13936. [Google Scholar] [CrossRef]
- Zhao, D.T.; Wang, X.H.; Miller, J.B.; Huber, G.W. The Chemistry and Kinetics of Polyethylene Pyrolysis: A Process to Produce Fuels and Chemicals. ChemSusChem 2020, 13, 1764–1774. [Google Scholar] [CrossRef]
- Soliman, A.; Farag, H.A.; Nassef, E.; Amer, A.; ElTaweel, Y. Pyrolysis of low-density polyethylene waste plastics using mixtures of catalysts. J. Mater. Cycles Waste Manag. 2020, 22, 1399–1406. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yang, X.X.; Fu, Z.W.; Li, R.; Wu, Y.L. Synergistic effect of catalytic co-pyrolysis of cellulose and polyethylene over HZSM-5. J. Therm. Anal. Calorim. 2020, 140, 363–371. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R.; Shukla, A.; Haldar, S. Effective Deoxygenation for the Production of Liquid Biofuels via Microwave Assisted Co-pyrolysis of Agro Residues and Waste Plastics Combined withCatalytic Upgradation. Bioresour. Technol. 2020, 302, 122775. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Park, K.B.; Kim, J.S. Hydrogen production from steam gasification of polyethylene using a two-stage gasifier and active carbon. Appl. Energy 2020, 262, 114495. [Google Scholar] [CrossRef]
- Kong, S.N.; He, C.Z.; Dong, J.; Li, N.; Xu, C.R.; Pan, X.C. Sunlight-Mediated Degradation of Polyethylene under the Synergy of Photothermal C–H Activation and Modification. Macromol. Chem. Phys. 2022, 223, 2100322. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Zhang, X.M.; Guo, Y.; Qian, F.J.; Dong, H.P. Research Status and Prospect of Polycarbonate Chemical Depolymerization Technology. China Plast. Ind. 2023, 51, 30–37. [Google Scholar]
- Han, Y.Y.; Zhu, G.M.; Li, B. Research Progress in Photodegradable Polymers. China Plast. 2019, 33, 132–138. [Google Scholar]
- Wei, H.; Liu, M.J.; Zhou, H.; Su, B.G.; Yang, Y.W. Progress on catalytic cracking of waste polyolefin plastics to produce fuels. Ind. Catal. 2022, 30, 1–10. [Google Scholar]
- Susastriawan, A.A.P.; Purnomo; Sandria, A. Experimental study the influence of zeolite size on low-temperaturepyrolysis of low-density polyethylene plastic waste. Therm. Sci. Eng. Prog. 2020, 17, 100497. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, X.K.; Zhao, Z.G.; Li, Y.Q.; Wang, K.G. Progress of research on chemical upcycling of plastic waste based on pyrolysis. Energy Environ. Prot. 2023, 37, 98–108. [Google Scholar]
- Singh, M.V.; Kumar, S.; Sarker, M. Waste HD-PE Plastic Deformation into Liquid Hydrocarbons as Fuel by a Pyrolysis-Catalytic CrackingUsing the CuCO3 Catalyst. Sustain. Energy Fuels 2018, 2, 1057–1068. [Google Scholar] [CrossRef]
- Singh, M.V. Waste and virgin high-density poly(ethylene) into renewable hydrocarbons fuel by pyrolysis-catalytic cracking with a CoCO3 catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 150–161. [Google Scholar] [CrossRef]
- Kuang, T.R.; Jin, M.Y.; Lu, X.R.; Liu, T.; Vahabi, H.; Gu, Z.P.; Gong, X. Functional carbon dots derived from biomass and plastic wastes. Green Chem. 2023, 25, 6581. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhong, L.Q.; Gong, X. Robust Superhydrophobic Films Based on an Eco-Friendly Poly(L-lactic acid)/Cellulose Composite with Controllable Water Adhesion. Langmuir 2024, 40, 10362–10373. [Google Scholar] [CrossRef]
- Martín, A.J.; Mondelli, C.; Jaydev, S.D.; Pérez-Ramírez, J. Catalytic processing of plastic waste on the rise. Chem 2021, 7, 1487–1533. [Google Scholar] [CrossRef]
- Du, B.W.; Chen, X.; Ling, Y.; Niu, T.T.; Guan, W.X.; Meng, J.P.; Hu, H.Q.; Tsang, C.W.; Liang, C.H. Hydrogenolysis-Isomerization of Waste Polyolefin Plasticsto Multibranched Liquid Alkanes. ChemSusChe 2023, 16, e202202035. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.J.; Bansal, S.; Sonthalia, A.; Rai, A.K.; Singh, S.P. Biodegradation of plastics for sustainable environment. Bioresour. Technol. 2022, 347, 126697. [Google Scholar]
- Yang, S.S.; Wu, W.M.; Bertocchini, F.; Benbow, M.E.; Devipriya, S.P.; Cha, H.J.; Peng, B.Y.; Ding, M.Q.; He, L.; Li, M.X.; et al. Radical innovation breakthroughs of biodegradation of plastics by insects: History, present and future perspectives. Front. Environ. Sci. Eng. 2024, 18, 78. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.L.; Xia, M.L. Biodegradation and mineralization of polystyrene by plastic-eating superworms Zophobas atratus. Sci. Total Environ. 2020, 708, 135233. [Google Scholar] [CrossRef]
- Jia, Y.P.; Xing, J.M. Progress in biodegradation and upcycling of polyethylene terephthalate (PET). Chin. J. Bioprocess Eng. 2022, 20, 365–373. [Google Scholar]
- Rambabu, K.; Bharath, G.; Govarthanan, M.; Kumar, P.S.; Show, P.L.; Banat, F. Bioprocessing of plastics for sustainable environment: Progress, challenges, and prospects. Trends Anal. Chem. 2023, 166, 117189. [Google Scholar] [CrossRef]
- Dang, X.G.; Yu, Z.F.; Du, Y.M.; Wang, X.C.; Wang, C.H. Sustainable one-pot synthesis of novel soluble cellulose-based nonionic biopolymers for natural antimicrobial materials. Chem. Eng. J. 2023, 468, 143810. [Google Scholar] [CrossRef]
- Dang, X.G.; Yu, Z.F.; Wang, X.C.; Li, N. Eco-Friendly Cellulose-Based Nonionic Antimicrobial Polymers with Excellent Biocompatibility, Nonleachability, and Polymer Miscibility. ACS Appl. Mater. Interfaces 2023, 15, 50344–50359. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.G.; Fu, Y.T.; Wang, X.C. Versatile Biomass-Based Injectable Photothermal Hydrogel for Integrated Regenerative Wound Healing and Skin Bioelectronics. Adv. Funct. Mater. 2024, 34, 2405745. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.C.; Hao, D.Y.; Xie, L.; Yang, J.; Dang, X.G. Polysaccharides for sustainable leather production: A review. Environ. Chem. Lett. 2024, 22, 2553–2572. [Google Scholar] [CrossRef]
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ.—Sci. 2021, 336, 101538. [Google Scholar] [CrossRef]
- Bahl, S.; Dolma, J.; Singh, J.J.; Sehgal, S. Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2021, 39, 31–34. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Kosiorowska, K.E.; Miro_nczuk, A.M. Current knowledge on polyethylene terephthalate degradation by genetically modified microorganisms. Front. Bioeng. Biotechnol. 2021, 9, 771133. [Google Scholar] [CrossRef]
- Veluru, S.; Seeram, R. Biotechnological approaches: Degradation and valorization of waste plastic to promote the circular economy. Circ. Econ. 2024, 3, 100077. [Google Scholar] [CrossRef]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic Degradation Efficiency of PostconsumerPolyethylene Terephthalate Packaging Determinedby Their Polymer Microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Tylman-Mojżeszek, W.; Mierek-Adamska, A.; Richert, A.; Hrynkiewicz, K. Potential of Serratia plymuthica IV-11-34 strain for biodegradation of polylactide and poly(ethylene terephthalate). Int. J. Biol. Macromol. 2021, 193, 145–153. [Google Scholar] [CrossRef]
- Bollinger, A.; Thies, S.; Knieps-Grünhagen, E.; Gertzen, C.; Kobus, S.; Höppner, A.; Ferrer, M.; Gohlke, H.; Smits, S.H.J.; Jaeger, K.E. A Novel Polyester Hydrolase From the Marine Bacterium Pseudomonas aestusnigri—Structural and Functional Insights. Front. Microbiol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Wang, Y.Y.; Cheng, Y.Y.; Wang, X.; Tong, S.W.; Yang, H.T.; Wang, Z.F. Efficient biodegradation of highly crystallized polyethylene terephthalate through cell surface display of bacterial PETase. Sci. Total Environ. 2020, 709, 136138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Maitra, S.S.; Singh, R.; Burwal, D.K. Acclimatization of a newly isolated bacteria in monomer tere-phthalic acid (TPA) mayenable it to attack the polymer poly-ethylene tere-phthalate (PET). J. Environ. Chem. Eng. 2020, 8, 103977. [Google Scholar] [CrossRef]
- Jabloune, R.; Khalil, M.; Moussa, I.E.B.; Simao-Beaunoir, A.; Lerat, S.; Brzezinski, R.; Beaulieu, C. Enzymatic Degradation of p-Nitrophenyl Esters, Polyethylene Terephthalate, Cutin, and Suberin by Sub1, a Suberinase Encoded by the Plant Pathogen Streptomyces scabies. Microbes Environ. 2020, 35, ME19086. [Google Scholar] [CrossRef]
- Yan, F.; Wei, R.; Cui, Q.; Bornscheuer, U.T.; Liu, Y.J. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum. Microb. Biotechnol. 2021, 14, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Dehnad, A.; Fotouhi, A.F. Biocatalysis and agricultural biotechnology biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 2019, 17, 25–31. [Google Scholar] [CrossRef]
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.H.; Linne, U.; Erb, T.; Maier, U.G. Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb. Cell Factories 2019, 18, 171. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Xi, X.X.; Ni, K.F.; Hao, H.L.; Shang, Y.P.; Zhao, B.; Qian, Z. Secretory expression in Bacillus subtilis and biochemical characterization of a highly thermostable polyethylene terephthalate hydrolase from bacterium HR29. Enzym. Microb. Technol. 2021, 143, 109715. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019, 52, 35–41. [Google Scholar] [CrossRef]
- Vivi, V.K.; Martins-Franchetti, S.M.; Attili-Angelis, D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Biodegradation of polyvinyl chloride plastic films by enriched anaerobic marine consortia. Mar. Environ. Res. 2020, 158, 104949. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.A.; Barbosa, R.; Mesquita, A.B.S.; Ferreira, J.H.L.; de Carvalho, L.H.; Alves, T.S. Fungal degradation of reprocessed PP/PBAT/thermoplastic starch blends. J. Mater. Res. Technol. 2020, 9, 2338–2349. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Gao, D.L.; Li, Q.H.; Zhao, Y.X.; Li, L.; Lin, H.F.; Bi, Q.R.; Zhao, Y.C. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef] [PubMed]
- Khandare, S.D.; Chaudhary, D.R.; Jha, B. Marine bacterial biodegradation of low-density polyethylene (LDPE) plastic. Biodegradation 2021, 32, 127–143. [Google Scholar] [CrossRef]
- Spina, F.; Tummino, M.L.; Poli, A.; Prigione, V.; Ilieva, V.; Cocconcelli, P.; Puglisi, E.; Bracco, P.; Zanetti, M.; Varese, G.C. Low density polyethylene degradation by filamentous fungi. Environ. Pollut. 2021, 274, 116548. [Google Scholar] [CrossRef]
- Sanniyasi, E.; Gopal, R.K.; Gunasekar, D.K.; Raj, P.P. Biodegradation of low-density polyethylene (LDPE) sheet by microalga, Uronema africanum Borge. Sci. Rep. 2021, 11, 17233. [Google Scholar] [CrossRef]
- Dey, A.S.; Bose, H.; Mohapatra, B.; Sar, P. Biodegradation of Unpretreated Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., Isolated From Waste Dumpsite and Drilling Fluid. Front. Microbiol. 2020, 11, 603210. [Google Scholar] [CrossRef]
- Devi, R.S.; Ramya, R.; Kannan, K.; Antony, A.R.; Kannan, V.R. Investigation of biodegradation potentials of high-density polyethylene degrading marine bacteria isolated from the coastal regions of Tamil Nadu, India. Mar. Pollut. Bull. 2019, 138, 549–560. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef]
- Han, Y.N.; Wei, M.; Han, F.; Fang, C.; Wang, D.; Zhong, Y.J.; Guo, C.L.; Shi, X.Y.; Xie, Z.K.; Li, F.M. Greater Biofilm Formation and Increased Biodegradation of Polyethylene Film by a Microbial Consortium of Arthrobacter sp. and Streptomyces sp. Microorganisms 2020, 8, 1979. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.Z.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef]
- Sarker, R.K.; Chakraborty, P.; Paul, P.; Chatterjee, A.; Tribedi, P. Degradation of low-density poly ethylene (LDPE) by Enterobacter cloacae AKS7: A potential step towards sustainable environmental remediation. Arch. Microbiol. 2020, 2028, 2117–2125. [Google Scholar] [CrossRef]
- Montazer, Z.; Najafi, M.B.H.; Levin, D.B. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbiol. 2019, 653, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Taskeen, N.; Kishore, A.P.; Krishna, B.V.; Naidu, G. Novel consortia of Enterobacter and Pseudomonas formulated from cow dung exhibited enhanced biodegradation of polyethylene and polypropylene. J. Environ. Manag. 2021, 284, 112030. [Google Scholar] [CrossRef] [PubMed]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Hazard. Mater. 2019, 380, 120899. [Google Scholar] [CrossRef]
- Kumari, A.; Chaudhary, D.R.; Jha, B. Destabilization of polyethylene and polyvinylchloride structure by marine bacterial strain. Environ. Sci. Pollut. Res. Int. 2019, 26, 1507–1516. [Google Scholar] [CrossRef]
- Khoironi, A.; Anggoro, S.; Sudarno. Evaluation of the Interaction Among Microalgae Spirulina sp., Plastics Polyethylene Terephthalate and Polypropylene in Freshwater Environment. J. Ecol. Eng. 2019, 206, 161–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H.R.; Yang, D.C.; Zhang, G.Q.; Zhang, J.L.; Ju, F. Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat. Commun. 2022, 13, 5360. [Google Scholar] [CrossRef]
- Priya, K.L.; Renjith, K.R.; Cindrella, J.J.; Indu, M.S.; Reji, S.; Haddout, S. Fate, transport and degradation pathway of microplastics in aquatic environment—A critical review. Reg. Stud. Mar. Sci. 2022, 56, 102647. [Google Scholar]
- Skariyachan, S.; Setlur, A.S.; Naik, S.Y.; Naik, A.A.; Usharani, M.; Vasist, K.S. Enhanced biodegradation of low and high-density polyethylene by novel bacterial consortia formulated from plastic-contaminated cow dung under thermophilic conditions. Environ. Sci. Pollut. Res. 2017, 24, 8443–8457. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef]
- Liu, X.B.; Dong, X.S.; Xie, Z.H.; Ma, X.W.; Luo, Y.M. Ecological effects and biodegradation of microplastics in soils. Acta Pedol. Sin. 2022, 59, 349–363. [Google Scholar]
- Ali, S.S.; Al-Tohamy, R.; Koutra, E.; Kornaros, M.; Khalil, M.; Elsamahy, T.; El-Shetehy, M.; Sun, J.Z. Coupling azo dye degradation and biodiesel production by manganese-dependent peroxidase producing oleaginous yeasts isolated from wood-feeding termite gut symbionts. Biotechnol. Biofuels 2021, 14, 61. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal enzymes involved in plastics biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Zhong-Johnson, E.Z.L.; Voigt, C.A.; Sinskey, A.J. An absorbance method for analysis of enzymatic degradation kinetics of poly(ethylene terephthalate) films. Sci. Rep. 2021, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.L.; Chen, Y.C.; Liu, X.Y.; Dong, S.J.; Tian, Y.E.; Qiao, Y.X.; Mitra, R.; Han, J.; Li, C.L.; Han, X.; et al. Computational Redesign of a PETase for Plastic Biodegradation under Ambient Condition by the GRAPE Strategy. ACS Catal. 2021, 11, 1340–1350. [Google Scholar] [CrossRef]
- Zhu, B.T.; Wang, D.; Wei, N. Enzyme Discovery and Engineering for Sustainable Plastic Recycling. Trends Biotechnol. 2022, 40, 22–37. [Google Scholar] [CrossRef]
- Kim, N.K.; Lee, S.H.; Park, H.D. Current biotechnologies on depolymerization of polyethylene terephthalate (PET) and repolymerization of reclaimed monomers from PET for bio-upcycling: A critical review. Bioresour. Technol. 2022, 363, 127931. [Google Scholar] [CrossRef]
- Wang, B.; Song, Y.Y.; Wang, X.; Meng, Q.Q.; Zhang, B.; Zhao, L.P.; Wu, S.K. Hydrogen production from organic solid waste by thermochemical conversion process: A review. Chem. Ind. Eng. Prog. 2021, 40, 709–721. [Google Scholar]
- Chhabra, V.; Parashar, A.; Shastri, Y.; Bhattacharya, S. Techno-Economic and Life Cycle Assessment of Pyrolysis of Unsegregated Urban Municipal Solid Waste in India. Ind. Eng. Chem. Res. 2021, 60, 1473–1482. [Google Scholar] [CrossRef]
- Lin, X.J.; Boit, M.O.K.; Wu, K.; Jain, P.; Liu, E.J.; Hsieh, Y.F.; Zhou, Q.; Li, B.W.; Hung, H.C.; Jiang, S.Y. Zwitterionic carboxybetaine polymers extend the shelf-life of humanplatelets. Acta Biomater. 2020, 109, 51–60. [Google Scholar] [CrossRef]
- Dang, X.G.; Yu, Z.F.; Yang, M.; Woo, M.W.; Song, Y.Q.; Wang, X.C.; Zhang, H.J. Sustainable electrochemical synthesis of natural starch-based biomass adsorbent with ultrahigh adsorption capacity for Cr(VI) and dyes removal. Sep. Purif. Technol. 2022, 288, 120668. [Google Scholar] [CrossRef]
- Lin, X.J.; Tsao, C.T.; Kyomoto, M.; Zhang, M.Q. Injectable Natural Polymer Hydrogels for Treatment of Knee Osteoarthritis. Adv. Healthc. Mater. 2022, 11, 2101479. [Google Scholar] [CrossRef]
- Liu, Z.C. A review on the emerging conversion technology of cellulose, starch, lignin, protein and other organics from vegetable-fruit-based waste. Int. J. Biol. Macromol. 2023, 242, 124804. [Google Scholar] [CrossRef]
- Chang, S.H. Plastic waste as pyrolysis feedstock for plastic oil production: A review. Sci. Total Environ. 2023, 877, 162719. [Google Scholar] [CrossRef]
- Gluth, A.; Xu, Z.; Fifield, L.S.; Yang, B. Advancing biological processing for valorization of plastic wastes. Renew. Sustain. Energy Rev. 2022, 170, 112966. [Google Scholar] [CrossRef]
- Li, N.; Liu, H.X.; Cheng, Z.J.; Cheng, B.B.; Chen, G.Y.; Wang, S.B. Conversion of plastic waste into fuels: A critical review. J. Hazard. Mater. 2022, 424, 127460. [Google Scholar] [CrossRef]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes—The environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]

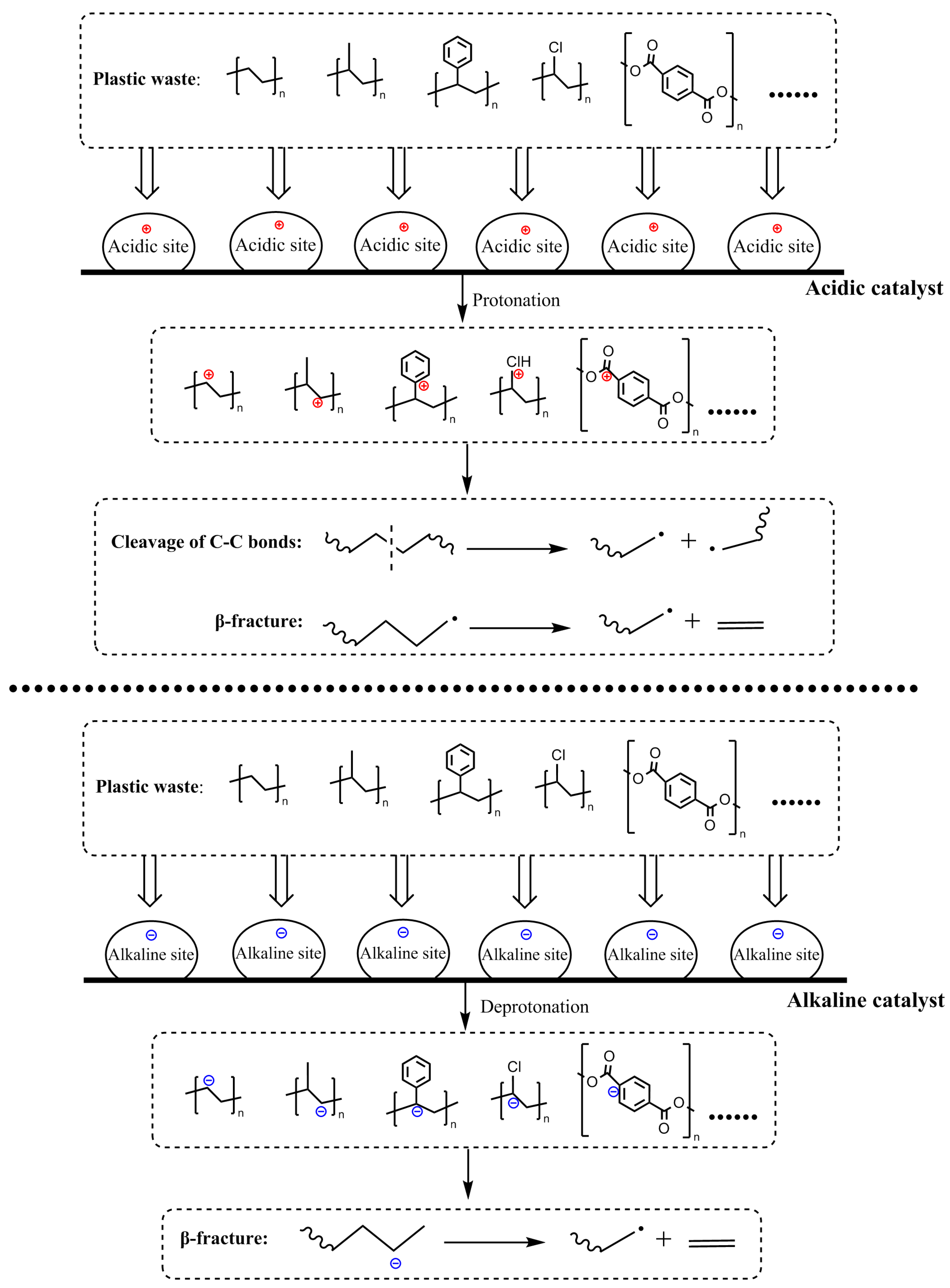
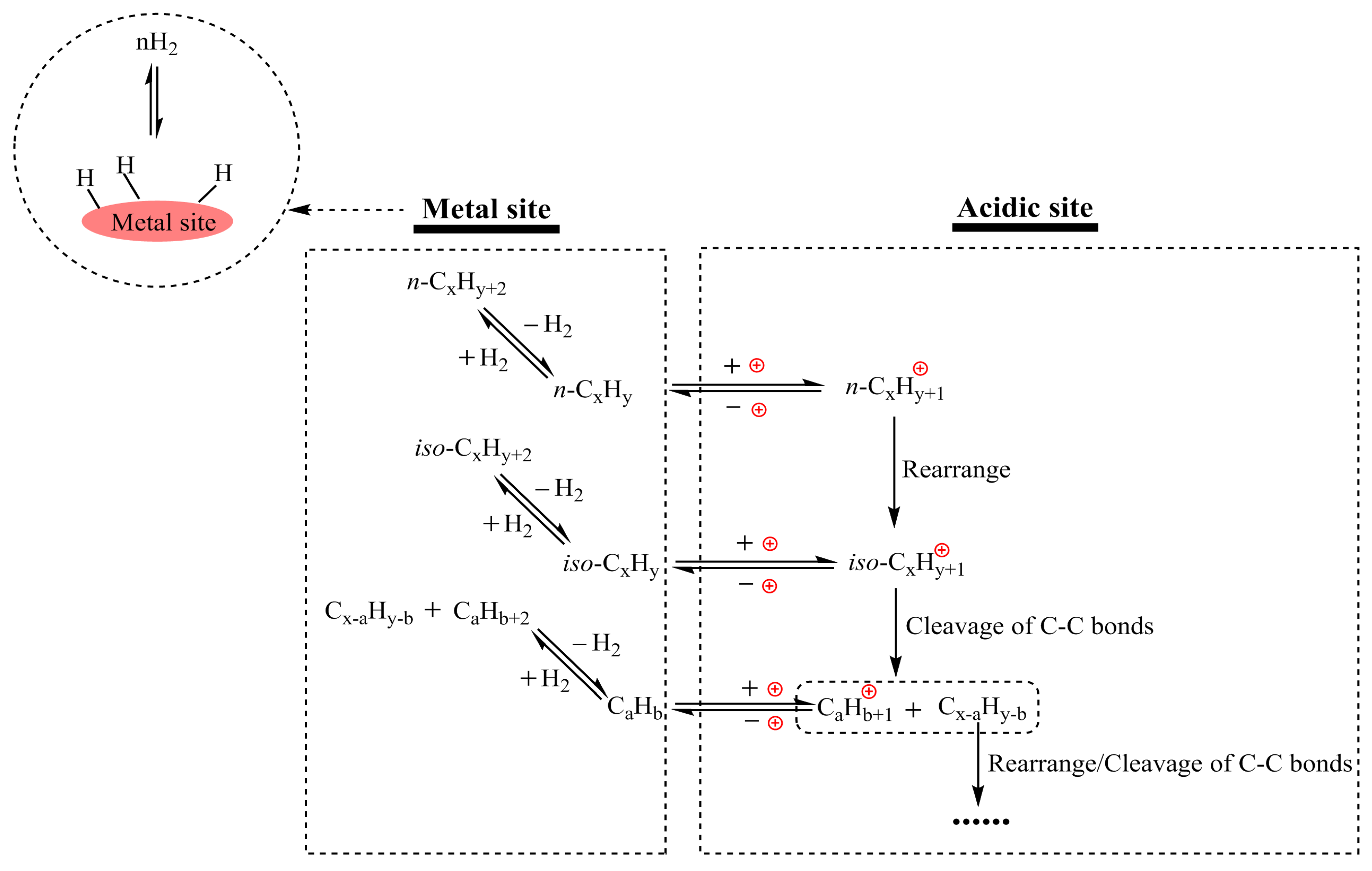
| Plastic Waste | Device | Reactant | Catalyst | Temperature | Reaction Medium | Illumination | Product | Reference |
|---|---|---|---|---|---|---|---|---|
| PE PET PC PC PE PE PE PE PE Plastic mixture Plastic mixture Low-density PE PP PE PS PVC High-density PE PP PET Medical masks Plastic mixture Low-density PE Plastic mixture Plastic mixture Low-density PE Plastic mixture PE, PP PE, PC, PP, ABS PS PP PE Plastic mixture PE PE Low-density PE PE PP, PS PE PE | Tank reactor Electrolyzer Autoclave Reaction vessel Autoclave Autoclave Reaction vessel Autoclave Furnace Fixed bed reactor Tube reactor Fixed bed reactor Fixed bed reactor Fixed bed reactor Fixed bed reactor Fixed bed reactor Fixed bed reactor Autoclave Horizontal furnace Tube furnace Autoclave Autoclave Autoclave Microwave oven Microwave oven Microwave oven Microwave oven Autoclave Tube reactor Tube reactor Fixed bed reactor Reaction vessel Autoclave Fluidized bed reactor Autoclave Pyrolyzer Microwave oven Fluidized bed gasifier, tar-cracking reactor Reaction vessel | C2H4 H2O Methanol C6HN, C8HN2O2 / / C2H4 / / / / / / / / EC / / / / / / / Cooking oil Lignin / / Soda lignin / / Pine wood / Br2, ethylene / / Cellulose Rice straw, sugarcane bagasse / DIAD | Pt/γ-Al2O3, MTO/Cl−Al2O3 Electrocatalyst ChCl-2Urea Stannous octoate Pt@S-1 Pt/SrTiO3 Ir-tBuPOCOP, [PdP(tBu)3(m-Br)]2 Pt/γ-Al2O3 KAB/kaolin composites Four Ni-Fe catalysts Activated carbon Activated carbon, MgO Fe/Al2O3 Fe/Al2O3 Fe/Al2O3 / Y-zeolite with transition metals Waste refinery catalyst / / Zeolite beta composite CeO2-supported Ru Ru-modified zeolite / / / ZSM-5 / / Seawater CaO/Fe2O3 oxygen carrier Nb2O5 Grubbs catalyst M202 / CB, kaolin, silica gel, activated charcoal HZSM-5 zeolite HZSM-5 Active carbon TBADT | 100 °C 60 °C 130 °C 70–75 °C 250 °C 300 °C 130–350 °C 280 °C 295 °C 500 °C 430–571 °C 450–600 °C 500 °C 500 °C 500 °C 550 °C 600 °C 100–450 °C 600–1000 °C 900 °C 360–400 °C 200 °C 300 °C 400–550 °C 550 °C 450–500 °C 500–740 °C 500–750 °C 500–800 °C 500–800 °C 750–850 °C RT 30–105 °C 500–600 °C 550–650 °C 650 °C 500 °C 790–840 °C 110 °C | Atmospheric C2H4 KOH aqueous solution Autogenous pressure Anisole 3 MPa of H2 170 Pa of H2 / / N2 N2 N2 N2 N2 N2 N2 N2 N2 / N2 Ar 20 bar of H2 2 MPa of of H2 50 bar of H2 Negative pressure N2 / / Supercritical water Supercritical water Supercritical water N2 / 2.7 bar of ethylene N2 / N2 N2 Air or oxygen / | / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Sunlight 400−410 nm UV / / / / / Sunlight | Propylene Potassium diformate, terephthalic acid, H2 Bisphenol A PU Naphtha hydrocarbons Fuel oil Propylene Alkylaromatics, alkylnaphthenes Fuel oil, syngas Carbon nanotubes Jet fuel, H2-enriched gases Jet fuel, H2-enriched gases H2, liquid fuels, carbon nanotubes H2, liquid fuels, carbon nanotubes H2, liquid fuels, carbon nanotubes Bio-oil, bio-char, non-condensable gas Aromatic fuel oils, H2 Methylbenzenes, alkanes Porous carbon Porous carbon materials Gasoline Liquid fuels, waxes CH4 Liquid fuel Hydrocarbon rich bio-oil Fuel oil Fuel oil Syngas H2, CH4, CO2 H2, CH4, CO2 Syngas with high H2/CO ratio C2 fuels α,ω-divinyl-functionalized oligomer H2, C1–C4 paraffins, C2–C4 olefins, 1,3-butadiene, C4–C60 n-paraffins, isoparaffins, mono-olefins, cycloalkanes/alkadienes, aromatics Paraffins, isoparaffins, olefins, naphthenes, aromatics, char, syngas Oxygenated chemicals, olefins, alkanes, aromatics Bio-oil, biochar, gas Syngas, tar Low molecular weight PE with tunable polarity | [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [30] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] [53] [54] |
| Plastic Waste | Microorganism/Enzyme | Reaction Condition | Product | Reference |
|---|---|---|---|---|
| PET PET PET PET PET PET PET PET PET PET PET PVC PVC PVC PP PE PE PE PE PE PE PE PE PE PE PE PE, PP PE, PET PE, PVC PP, PET | Thermobifida fusca/cutinase (TfCut2) Serratia plymuthica strain IV-11-34/synthase Pseudomonas aestusnigri/carboxylic ester hydrolase Pichia pastoris/PETase Rhococcus sp. SSM1/PETase Streptomyces scabies/protein sub1 Clostridium thermocellum/thermophilic cutinase Streptomyces sp. Phaeodactylum tricornutum/PETase LCC–ICCG variant/Depolymerase Bacillus subtilis HR29/BhrPETase Pseudomonas citronellolis, Bacillus flexus Chaetomium globosum Anaerobic marine consortia Aspergillus sp., Penicillium sp. Aspergillus flavus/AFLA_006190, AFLA_053930 Cobetia sp., Halomonas sp., Exiguobacterium sp., Alcanivorax sp. Aspergillus flavus, Fusarium falciforme, Fusarium oxysporum, Purpureocillium lilacinum Uronema africanum Borge Stenotrophomonas sp., Achromobacter sp./cutinase, lipase, esterase, alkane monooxygenase Bacillus spp., Pseudomonas spp. Paenibacillus sp., Bacillus sp. Arthrobacter sp., Streptomyces sp. Sterigmatomyces halophilus, Meyerozyma guilliermondii, Meyerozyma caribbica/MnP, Lac, LiP Enterobacter cloacae AKS7 PE-degrading bacteria, PHA-synthesizing bacteria Enterobacter, Pseudomonas Alcanivorax, Marinobacter, Arenibacter Bacillus spp. Spirulina sp. | 1000 r/min, 70 °C, 96 h 26 °C, 30 d 30 °C, 48 h 30 °C, 18 h 34 °C, pH 8.5 37 °C, 20 d Anaerobically, 60 °C, 14 d 120 rpm, 28 °C, 18 d 21–30 °C, 180 d 65 °C, 14 h, pH 8 37 °C, pH 7 Aerobically, 30 d 28 °C, 28 d Anaerobically, 20 °C, 2 a 29 °C, 30 d 28 d 30–90 d 30 d 30 d Aerobically, 150 rpm, 30 °C, 45 d 30 °C, 30 d 30 °C, 60 d 120 r/min, 25 °C, 90 d 30 °C, 45 d 30 °C, 45 d 30 °C, 21 d 37 °C, 160 d 30 °C, 80 d 180 rpm, 30 °C, 90 d 112 d | Ethylene glycol, terephthalic acid Small molecules Bis(2-hydroxyethyl) terephthalate, mono(2-hydroxyethyl) terephthalate Small molecules Small molecules Terephthalic acid Small molecules Small molecules Terephthalic acid, mono(2-hydroxyethyl) terephthalic acid Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules Small molecules | [79] [80] [81] [82] [83] [84] [85] [86] [87] [88] [89] [90] [91] [92] [93] [94] [95] [96] [97] [98] [99] [100] [101] [102] [103] [104] [105] [106] [107] [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chang, S.H.; Mailhot, G. Emerging Biochemical Conversion for Plastic Waste Management: A Review. Molecules 2025, 30, 1255. https://doi.org/10.3390/molecules30061255
Liu Z, Chang SH, Mailhot G. Emerging Biochemical Conversion for Plastic Waste Management: A Review. Molecules. 2025; 30(6):1255. https://doi.org/10.3390/molecules30061255
Chicago/Turabian StyleLiu, Zhongchuang, Siu Hua Chang, and Gilles Mailhot. 2025. "Emerging Biochemical Conversion for Plastic Waste Management: A Review" Molecules 30, no. 6: 1255. https://doi.org/10.3390/molecules30061255
APA StyleLiu, Z., Chang, S. H., & Mailhot, G. (2025). Emerging Biochemical Conversion for Plastic Waste Management: A Review. Molecules, 30(6), 1255. https://doi.org/10.3390/molecules30061255








