A Facile, Sustainable One-Pot Synthesis of the Spiro-Dimers of α-Tocopheramine and Its N-Methyl Derivative
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preedy, V.R.; Watson, R.R. Encyclopedia of Vitamin E; CABI Publishing: Oxford, UK; Cambridge, UK, 2007. [Google Scholar]
- Smith, L.I.; Renfrow, W.B., Jr.; Opie, J.W. The Chemistry of Vitamin E. XXXVIII. 1,2 α-Tocopheramine, a New Vitamin E Factor. J. Am. Chem. Soc. 1942, 64, 1082–1084. [Google Scholar] [CrossRef]
- Mayer, H.; Isler, O. Tocopheramines and Tocopherthiols. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, London, 1971; Volume 18, part C; pp. 275–282, 334–342. [Google Scholar]
- Hettegger, H.; Zhang, J.; Koide, M.; Rinner, U.; Potthast, A.; Gotoh, Y.; Rosenau, T. Fiber Spinning from Cellulose Solutions in Imidazolium Ionic Liquids: Effects of Natural Antioxidants on Molecular Weight, Dope Discoloration, and Yellowing Behavior. Fibers 2022, 10, 50. [Google Scholar] [CrossRef]
- Zhang, J.; Kitayama, H.; Potthast, A.; Rosenau, T.; Gotoh, Y. Non-woven fabrics of fine regenerated cellulose fibers prepared from ionic-liquid solution via wet type solution blow spinning. Carbohydr. Polym. 2019, 226, 115258. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Adorjan, I.; Hofinger, A.; Sixta, H.; Firgo, H.; Kosma, P. Cellulose solutions in N-methylmorpholine-N-oxide (NMMO)—Degradation processes and stabilizers. Cellulose 2002, 9, 283–291. [Google Scholar] [CrossRef]
- McMasters, V.; Lewis, J.K.; Kinsell, L.W. Effect of supplementing the diet of man with tocopherol on the tocopherol levels of adipose tissue and plasma. Amer. J. Clin. Nutr. 1965, 17, 357–359. [Google Scholar] [CrossRef]
- Schlegel, W.; Schwieter, U.; Tamm, R. Non-toxic antioxidants, based on chromane derivatives. Chem. Abstr. 1969, 69, 21909. [Google Scholar]
- Søndergaard, E.; Dam, H. The vitamin E activity of 5 different tocopheramines on muscular dystrophy in chicks. Zeitschr. Ernährungswiss 1970, 10, 71–78. [Google Scholar] [CrossRef]
- Tokuwame, M. Synergistic antioxidant-heat stabilizer sys- tems for polyolefins. Chem. Abstr. 1991, 115, 73015. [Google Scholar]
- Lambert, K.J.; Lal, M. Preparation of aminobenzopyran derivatives as surfactants. Chem. Abstr. 2002, 137, 263202. [Google Scholar]
- Bieri, J.G.; Prival, E.L. Vitamin E Activity and Metabolism of N-Methyltocopheramines. Biochemistry 1967, 6, 2153–2158. [Google Scholar] [CrossRef]
- Itoh, S.; Nagaoka, S.; Mukai, K.; Ikesu, S.; Kaneko, Y. Kinetic study of quenching reactions of singlet oxygen and scavenging reactions of free radicals by alpha-, beta-, gamma- and delta-tocopheramines in ethanol solution and micellar dispersion. Lipids 1994, 29, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Tomic-Vatic, A.; Eytina, J.; Chapman, J.; Mahdavian, E.; Neuzil, J.; Salvatore, B.A. Vitamin E amides, a new class of vitamin E analogues with enhanced proapoptotic activity. Int. J. Cancer 2005, 117, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gille, L.; Stamberg, W.; Patel, A.; Böhmdorfer, S.; Rosenau, T. Isolated bc(1) complex as a screening system for potential anti-malaria drugs. Free Rad. Biol. Med. 2012, 53, 100–101. [Google Scholar]
- Zingg, J.M. Molecular and Cellular Activities of Vitamin E Analogues. Mini Rev. Med. Chem. 2007, 7, 545–560. [Google Scholar] [CrossRef]
- Neuzil, J.; Tomasetti, M.; Zhao, Y.; Dong, L.F.; Birringer, M.; Wang, X.F.; Low, P.; Wu, K.; Salvatore, B.A.; Ralph, S.J. Vitamin E analogs, a novel group of 'mitocans,' as anti-cancer agents: The importance of being redox-silent. Mol. Pharmacol. 2007, 71, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Yoshida, Y.; Kaidzu, S.; Chen, Z.H.; Cynshi, O.; Jishage, K.I.; Niki, E.; Ohira, A. Acceleration of age-related changes in the retina in α-tocopherol transfer protein null mice fed a vitamin E–deficient diet. Investig. Ophthalm. Visual. Sci. 2007, 48, 396–404. [Google Scholar] [CrossRef]
- Bieri, J.G.; Evarts, R.P. Tocopherols and fatty acids in American diets. The recommended allowance for vitamin E. J. Amer. Diet. Assoc. 1973, 62, 147–151. [Google Scholar] [CrossRef]
- Patel, A.; Hofinger, A.; Rosenau, T. Synthesis and analytical characterization of monomeric N-oxidized derivatives of alpha-tocopheramine. Chem. Monthly 2021, 152, 959–966. [Google Scholar] [CrossRef]
- Patel, A.; Liebner, F.; Netscher, T.; Mereiter, K.; Rosenau, T. Nitration of non-alpha tocopherols – products and mechanistic considerations. J. Org. Chem. 2007, 72, 6504–6512. [Google Scholar] [CrossRef]
- Patel, A.; Rosenau, T. Synthesis and analytical characterization of all N-N-coupled, dimeric oxidation products of α-tocopheramine: Hydrazino-, azo- and azoxy-tocopherol. Chem. Mon. 2021, 152, 1231–1239. [Google Scholar] [CrossRef]
- Schudel, P.; Mayer, H.; Metzger, J.; Rüegg, R.; Isler, O. Über die Chemie des Vitamins E. 2. Mitteilung. Die Struktur des Kaliumferricyanid-Oxydationsproduktes von α-Tocopherol. Helv. Chim. Acta 1963, 46, 636–649. [Google Scholar] [CrossRef]
- Schröder, H.; Netscher, T. Determination of the absolute stereochemistry of vitamin E derived oxa-spiro compounds by NMR spectroscopy. Magn. Reson. Chem. 2001, 39, 701–708. [Google Scholar] [CrossRef]
- Martinez, J.; Cortes, J.F.; Ruvalcaba, R.M. Green Chemistry Metrics, A Review. Processes 2022, 10, 1274. [Google Scholar] [CrossRef]
- Selka, A.; Abidli, A.; Schiavo, L.; Jeanmart, L.; Hanquet, G.; Lubell, W.D. Recent Advances in Sustainable Total Synthesis and Chiral Pool Strategies with Emphasis on (−)-Sclareol in Natural Products Synthesis. Eur. J. Org. Chem. 2024, 28, e202400983. [Google Scholar] [CrossRef]
- Rüegg, R.; Mayer, H.; Schudel, P.; Schwieter, U.; Tamm, R.; Isler, R. Über die Chemie des Vitamins E. 5. Mitteilung. Veröffentl. Deut. Ges. Ernährung 1967, 16, 22–27. [Google Scholar]
- Machlin, L.J. Vitamin E: A Comprehensive Treatise; Marcel Dekker Inc.: New York, NY, USA, 1980. [Google Scholar]
- Rosenau, T.; Böhmdorfer, S. Ortho-Quinone methides in tocopherol chemistry. In Wiley Series on Reactive Intermediates in Chemistry and Biology; Rokita, S., Ed.; Wiley: New York, NY, USA, 2009; Volume 1, pp. 163–216. [Google Scholar]
- Rosenau, T.; Ebner, G.; Stanger, A.; Perl, S.; Nuri, L. From a Theoretical Concept to Biochemical Reactions: Strain Induced Bond Localization (SIBL) in Oxidation of Vitamin E. Chem. Eur. J. 2005, 11, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, T.; Hofinger, A.; Potthast, A.; Kosma, P. A general, selective high-yield N-demethylation procedure for tertiary amines by solid reagents in a convenient column chromatography-like setup. Org. Lett. 2004, 6, 541–544. [Google Scholar] [CrossRef]
- Stolze, K.; Udilova, N.; Rosenau, T.; Hofinger, A.; Nohl, H. Synthesis and characterization of EMPO-derived 5,5-disubstituted 1-pyrroline N-oxides as spin traps forming exceptionally stable superoxide spin adducts. Biol. Chem. 2003, 384, 457–500. [Google Scholar] [CrossRef]
- Yokota, S.; Kitaoka, T.; Opietnik, M.; Rosenau, T.; Wariishi, H. Synthesis of Gold Nanoparticles for In Situ Conjugation with Structural Carbohydrates. Angew. Chem. Int. Ed. Engl. 2008, 47, 9866–9869. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Elder, T.; Kosma, P. Novel Tocopheryl Compounds XIII. Stabilization and first direct spectroscopic evidence of the ortho-quinone methide derived from vitamin E. Org. Lett. 2002, 4, 4285–4288. [Google Scholar] [CrossRef]
- Patel, A.; Netscher, T.; Rosenau, T. Stabilization of ortho-quinone methides by a bis(sulfonium ylide) derived from 2,5-dihydroxy-[1,4]benzoquinone. Tetrahedron Lett. 2008, 49, 2442–2445. [Google Scholar] [CrossRef]
- Rosenau, T.; Zhang, J.; Koide, M.; Rinner, U.; Hettegger, H.; Potthast, A.; Gotoh, Y. Chromophores in spinning dopes of cellulose and imidazolium ionic liquids. Cellulose 2024, 31, 4203–4215. [Google Scholar] [CrossRef]
- Lehrhofer, A.F.; Yoneda, Y.; Tran, T.H.; Melikhov, I.; Gille, L.; Hettegger, H.; Böhmdorfer, S.; Potthast, A.; Schottenberger, H.; Rosenau, T. Negative effect and removal of trace amounts of 1,3-dialkylimidazolium ionic liquids in samples from biorefineries. Cellulose 2025, 32, 147–163. [Google Scholar] [CrossRef]
- Barbini, S.; Jaxel, J.; Karlström, K.; Rosenau, T.; Potthast, A. Multistage fractionation of pine bark by liquid and supercritical CO2. Bioresour. Technol. 2021, 341, 125862. [Google Scholar] [CrossRef]
- Barbini, S.; Sriranganadane, D.; España Orozco, S.; Kabrelian, A.; Karlström, K.; Rosenau, T.; Potthast, A. Tools for bark biorefineries: Studies towards improved characterization of wood extractives by combining supercritical fluid chromatography and high-temperature GCMS. ACS Sust. Chem. Eng. 2021, 9, 1323–1332. [Google Scholar] [CrossRef]
- IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of tocopherols and related compounds. Recommendations 1981. Eur. J. Biochem. 1982, 123, 473. [Google Scholar]
- IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of quinones with isoprenoid side chains. Arch. Biochim. Biophys. 1974, 165, 1. [Google Scholar]
- Urano, S.; Hattori, Y.; Yamanoi, S.; Matsuo, M. 13C Nuclear Magnetic Resonance Studies on α-Tocopherol and Its Derivatives. Chem. Pharm. Bull. 1980, 28, 1992–1998. [Google Scholar] [CrossRef]
- Brownstein, S.; Burton, G.W.; Hughes, L.; Ingold, K.U. Chiral effects on the carbon-13 resonances of alpha-tocopherol and related compounds. A novel illustration of Newman’s “rule of six”. J. Org. Chem. 1989, 54, 560–569. [Google Scholar] [CrossRef]
- Mazzini, F.; Netscher, T.; Salvadori, P. Efficient Synthesis of Vitamin E Amines. Eur. J. Org. Chem. 2009, 13, 2063–2068. [Google Scholar] [CrossRef]
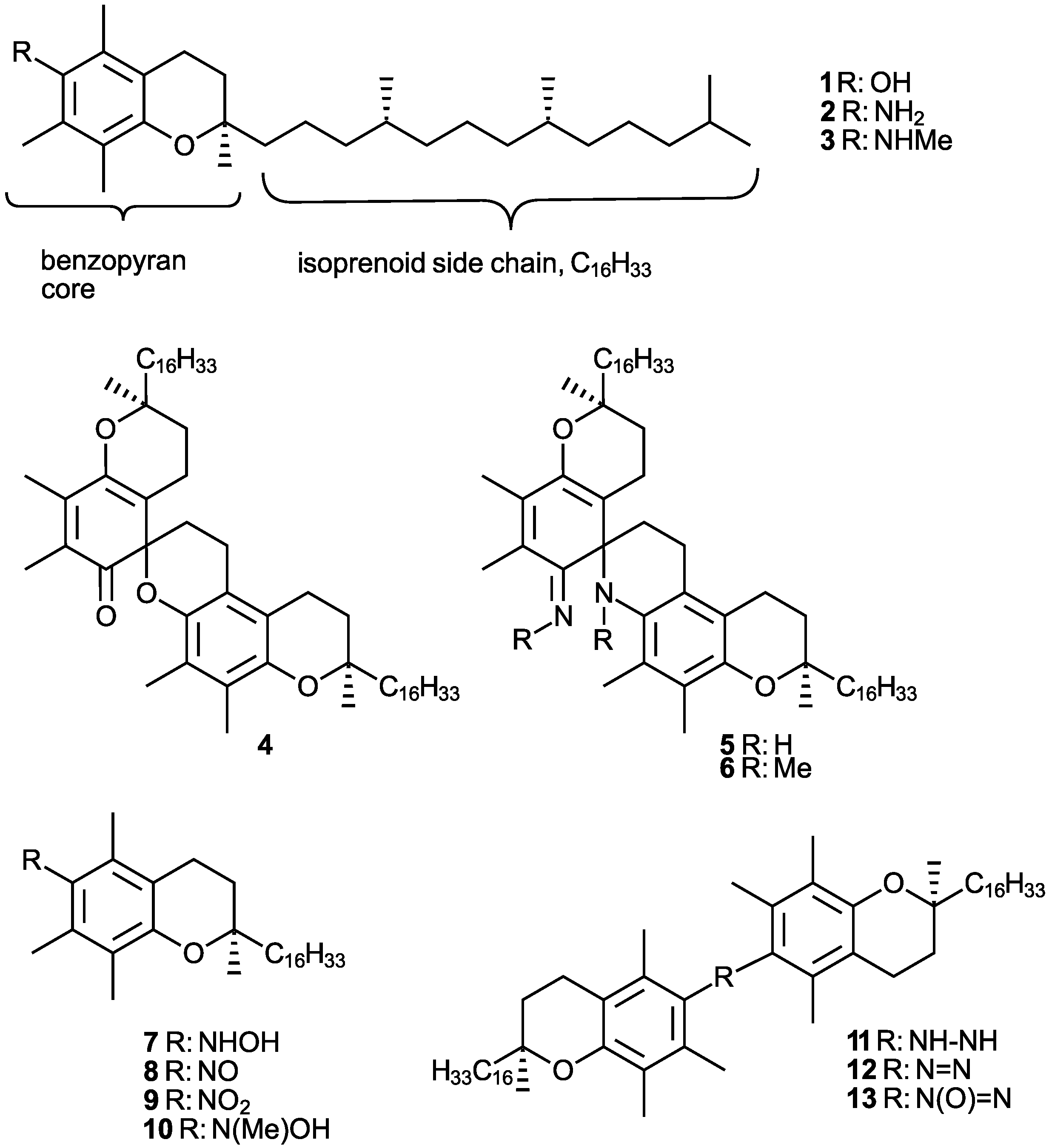
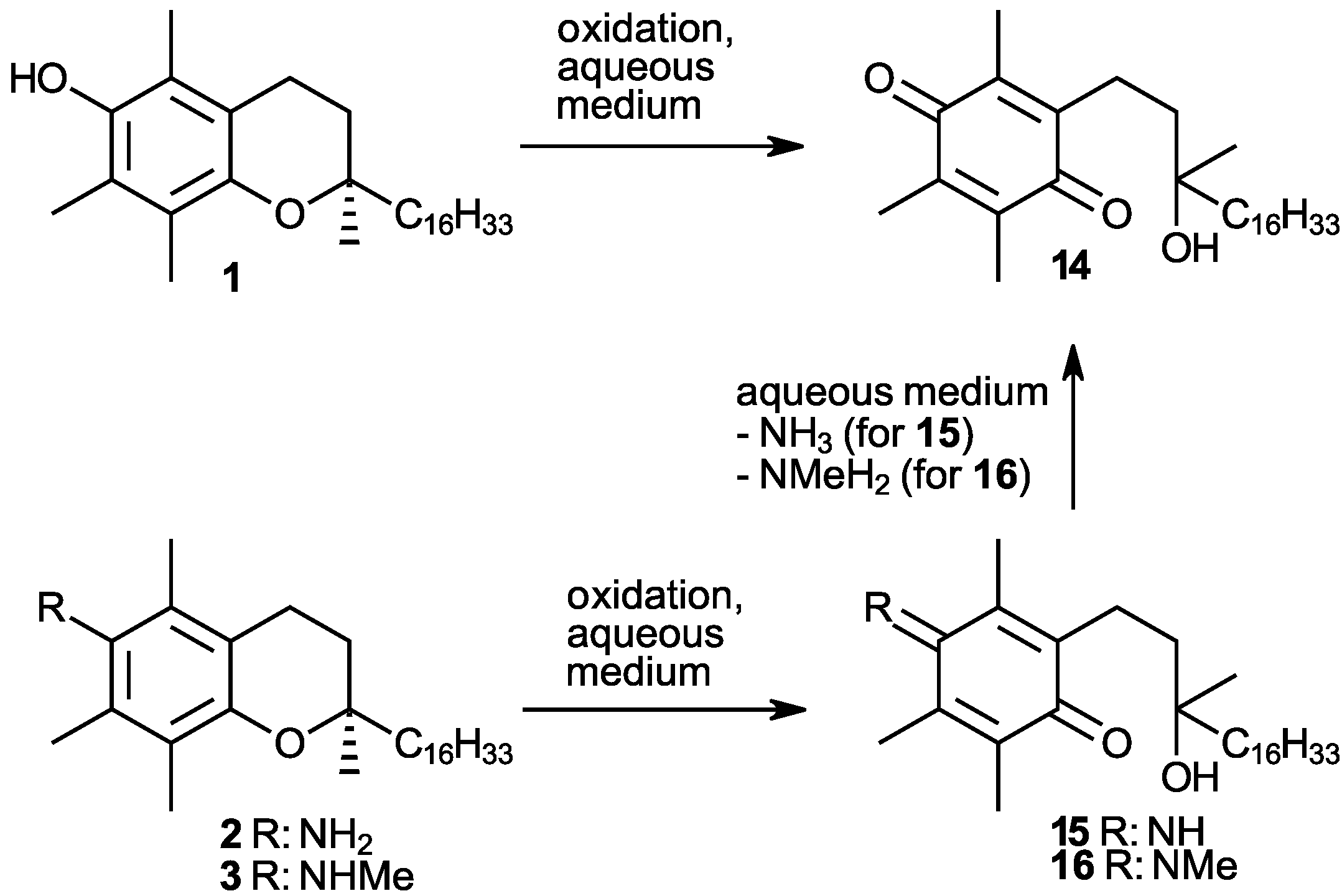

| Conditions (Solvent, Oxidant and Auxiliaries) 1 | Main Product (Yield%) 2 | Byproduct 14 (Yield%) 2 |
|---|---|---|
| EMIm-OAc, 2, H2O2 (aq. 30%, 1 eq.) | 46 | 33 |
| EMIm-Cl, 2, H2O2 (aq. 30%, 1 eq.) | 54 | 27 |
| BMIm-OAc, 2, H2O2 (aq. 30%, 1 eq.) | 33 | 35 |
| BMIm-Cl, 2, H2O2 (aq. 30%, 1 eq.) | 48 | 32 |
| EMIm-OAc, 2, H2O2*urea (1 eq.) | 82 | 8 |
| EMIm-Cl, 2, H2O2*urea (1 eq.) | 84 | 5 |
| BMIm-OAc, 2, H2O2*urea (1 eq.) | 74 | 13 |
| BMIm-Cl, 2, H2O2*urea (1 eq.) | 82 | 11 |
| EMIm-OAc, 2, H2O2*urea (2 eq.) | 83 | 17 |
| EMIm-Cl, 2, H2O2*urea (2 eq.) | 88 | 12 |
| BMIm-OAc, 2, H2O2*urea (2 eq.) | 74 | 26 |
| BMIm-Cl, 2, H2O2*urea (2 eq.) | 82 | 18 |
| EMIm-Cl, 2, H2O2*urea (2 eq.), alox 3 (10 eq.) | 92 | 8 |
| EMIm-Cl, 2, H2O2*urea (2 eq.), alox (30 eq.) | 98 | 2 |
| EMIm-Cl, 2, H2O2*urea (2 eq.), alox (50 eq.) | quant. | 0 |
| BMIm-Cl, 2, H2O2*urea (2 eq.), alox (10 eq.) | 85 | 15 |
| EMIm-Cl, 3, H2O2*urea (2 eq.), alox (50 eq.) | 92 | 8 |
| BMIm-Cl, 3, H2O2*urea (2 eq.), alox (50 eq.) | quant. | 0 |
| Dimcarb, 2, H2O2*urea (2 eq.), alox (50 eq.) | quant. | 0 |
| Dimcarb, 3, H2O2*urea (2 eq.), alox (50 eq.) | quant. | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Rosenau, T. A Facile, Sustainable One-Pot Synthesis of the Spiro-Dimers of α-Tocopheramine and Its N-Methyl Derivative. Molecules 2025, 30, 1269. https://doi.org/10.3390/molecules30061269
Patel A, Rosenau T. A Facile, Sustainable One-Pot Synthesis of the Spiro-Dimers of α-Tocopheramine and Its N-Methyl Derivative. Molecules. 2025; 30(6):1269. https://doi.org/10.3390/molecules30061269
Chicago/Turabian StylePatel, Anjan, and Thomas Rosenau. 2025. "A Facile, Sustainable One-Pot Synthesis of the Spiro-Dimers of α-Tocopheramine and Its N-Methyl Derivative" Molecules 30, no. 6: 1269. https://doi.org/10.3390/molecules30061269
APA StylePatel, A., & Rosenau, T. (2025). A Facile, Sustainable One-Pot Synthesis of the Spiro-Dimers of α-Tocopheramine and Its N-Methyl Derivative. Molecules, 30(6), 1269. https://doi.org/10.3390/molecules30061269








