Exploring the Influence of Toasting Levels, Grain Sizes, and Their Combination on the Volatile Profile of Tempranillo Red Wines Aged in Quercus petraea Barrels
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Percentage of Attributable Variance (%) of the Independent Effect of Toasting Level and Grain Size of the Barrel, and the Interaction of Both (Toasting Level × Grain Size)
2.2. Toasting Effect on Volatile Profile of Tempranillo Red Wine Aged in Quercus petraea Barrels
2.3. Grain Size Effect on Volatile Profile of Tempranillo Red Wine Aged in Quercus petraea Barrels
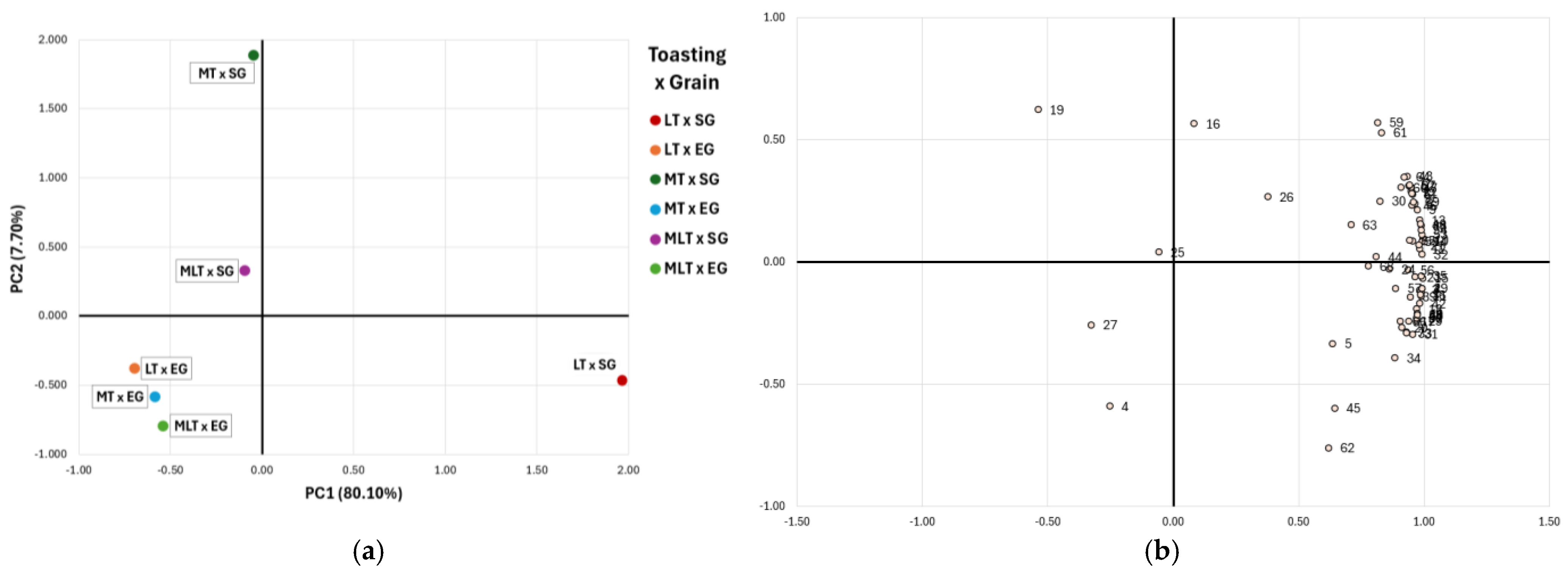
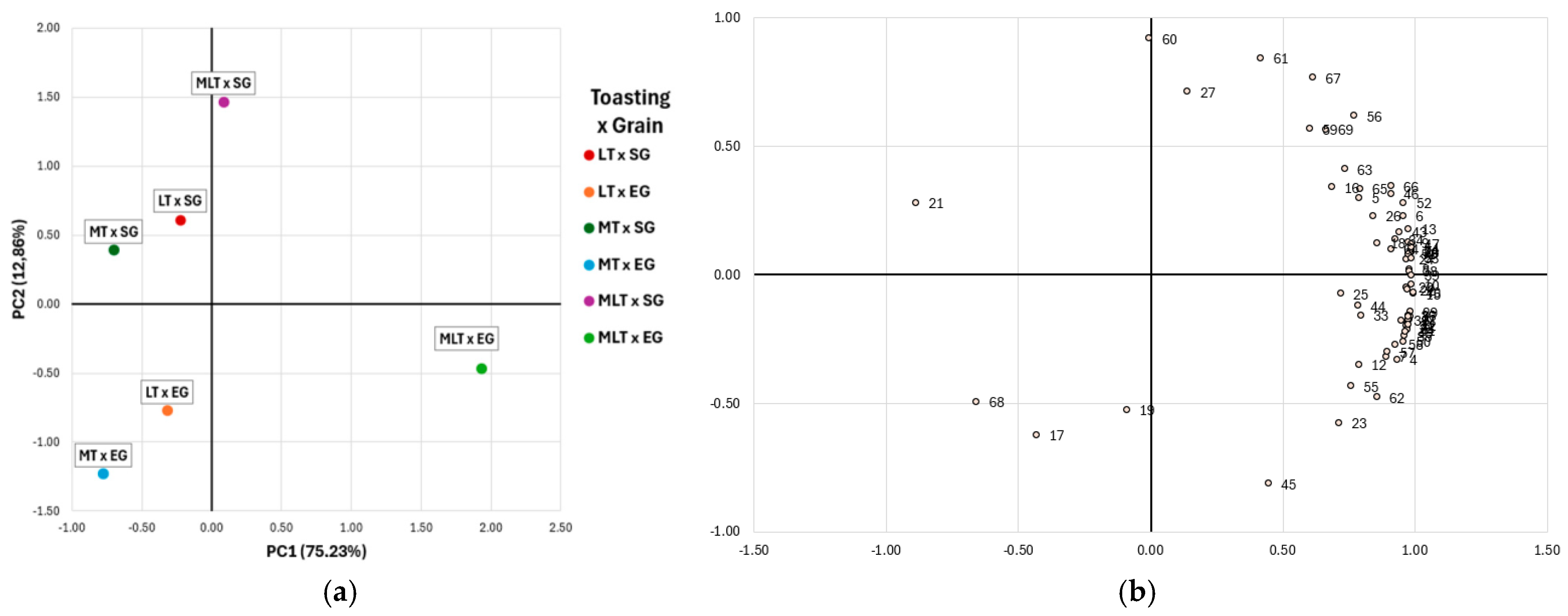
2.4. Combined Effect of Toasting Level and Grain Sizes on Volatile Profile of Tempranillo Red Wine Aged in Quercus petraea Barrels
3. Materials and Methods
3.1. Barrels
3.2. Wine Barrel Ageing and Sample Collection
3.3. Wine Volatile Composition
3.4. Odour Active Values (OAVs)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chira, K.; Teissedre, P. Relation between volatile composition, ellagitannin content and sensory perception of oak wood chips representing different toasting processes. Eur. Food Res. Technol. 2013, 236, 735–746. [Google Scholar] [CrossRef]
- Zamora, F. Barrel Aging; Types of Wood. In En Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–147. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Goñi, D.T.; Azpilicueta, C.A. Accumulation of volatile compounds during ageing of two red wines with different composition. J. Food Eng. 2004, 65, 349–356. [Google Scholar] [CrossRef]
- Prida, A.; Chatonnet, P. Impact of Oak-Derived Compounds on the Olfactory Perception of Barrel-Aged Wines. Am. J. Enol. Vitic. 2010, 61, 408–413. [Google Scholar] [CrossRef]
- Doussot, F.; De Jéso, B.; Quideau, S.; Pardon, P. Extractives Content in Cooperage Oak Wood during Natural Seasoning and Toasting; Influence of Tree Species, Geographic Location, and Single-Tree Effects. J. Agric. Food Chem. 2002, 50, 5955–5961. [Google Scholar] [CrossRef]
- Navarro, M.; Kontoudakis, N.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Influence of the botanical origin and toasting level on the ellagitannin content of wines aged in new and used oak barrels. Food Res. Int. 2016, 87, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.; Peinado, R.A.; Cotea, V.V.; De Lerma, N.L. Volatilome fingerprint of red wines aged with chips or staves: Influence of the aging time and toasting degree. Food Chem. 2019, 310, 125801. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.; Chira, K.; Teissedre, P. Ellagitannin content, volatile composition and sensory profile of wines from different countries matured in oak barrels subjected to different toasting methods. Food Chem. 2016, 210, 500–511. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Del Álamo, M.; Nevares, I. Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Anal. Chim. Acta 2009, 660, 211–220. [Google Scholar] [CrossRef]
- Bozalongo, R.; Carrillo, J.D.; Torroba, M.Á.F.; Tena, M.T. Analysis of French and American oak chips with different toasting degrees by headspace solid-phase microextraction-gas chromatography–mass spectrometry. J. Chromatogr. A 2007, 1173, 10–17. [Google Scholar] [CrossRef]
- Bosso, A.; Petrozziello, M.; Santini, D.; Motta, S.; Guaita, M.; Marulli, C. Effect of Grain Type and Toasting Conditions of Barrels on the Concentration of the Volatile Substances Released by the Wood and on the Sensory Characteristics of Montepulciano d’Abruzzo. J. Food Sci. 2008, 73, S373–S382. [Google Scholar] [CrossRef]
- Cadahía, E.; De Simón, B.F.; Jalocha, J. Volatile Compounds in Spanish, French, and American Oak Woods after Natural Seasoning and Toasting. J. Agric. Food Chem. 2003, 51, 5923–5932. [Google Scholar] [CrossRef] [PubMed]
- De Simón, B.F.; Esteruelas, E.; Muñoz, Á.M.; Cadahía, E.; Sanz, M. Volatile Compounds in Acacia, Chestnut, Cherry, Ash, and Oak Woods, with a View to Their Use in Cooperage. J. Agric. Food Chem. 2009, 57, 3217–3227. [Google Scholar] [CrossRef]
- Dumitriu, G.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; De Lerma, N.L. Evaluation of Aroma Compounds in the Process of Wine Ageing with Oak Chips. Foods 2019, 8, 662. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Muiño, I.; Del Álamo, M.; Nevares, I. Volatile Composition of Toasted Oak Chips and Staves and of Red Wine Aged with Them. Am. J. Enol. Vitic. 2010, 61, 157–165. [Google Scholar] [CrossRef]
- Feuillat, F.; Huber, F.; Keller, R. Mise Au Point Sur: “La Notion Du Grain Utiliseé Pour Le Classement Des Merrains de Chêne”. Rev. Fran. Œnol. 1992, 32, 65–69. [Google Scholar]
- Vivas, N. Sur La Notion de Grain en Tonnellerie. J. Sci. Technol. Tonnellerie 1995, 1, 17–32. [Google Scholar]
- Guillaume de Pracomtal, M.M.; Teissier Du Cros, R.; Monteau, A.-C. Types of Oak Grain, Wine Élevage in Barrel. Pract. Winery Vitic. 2014, 64–69. Available online: https://www.cantoncooperage.com/pdf/WV_July2014_Types-of-grain-elevage.pdf (accessed on 2 February 2025).
- Collins, T.S.; Miles, J.L.; Boulton, R.B.; Ebeler, S.E. Targeted volatile composition of oak wood samples taken during toasting at a commercial cooperage. Tetrahedron 2015, 71, 2971–2982. [Google Scholar] [CrossRef]
- Del Alamo-Sanza, M.; Cárcel, L.M.; Nevares, I. Characterization of the Oxygen Transmission Rate of Oak Wood Species Used in Cooperage. J. Agric. Food Chem. 2017, 65, 648–655. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Del Alamo-Sanza, M.; Nevares, I. Volatile composition of oak wood from different customised oxygenation wine barrels: Effect on red wine. Food Chem. 2020, 329, 127181. [Google Scholar] [CrossRef]
- Nevares, I.; Del Alamo-Sanza, M.; Martínez-Martínez, V.; Menéndez-Miguélez, M.; Van Den Bulcke, J.; Van Acker, J. Influence of Quercus petraea Liebl. wood structure on the permeation of oxygen through wine barrel staves. Holzforschung 2019, 73, 859–870. [Google Scholar] [CrossRef]
- Martínez-Martínez, V.; Del Alamo-Sanza, M.; Nevares, I. Application of image analysis and artificial neural networks to the prediction in-line of OTR in oak wood planks for cooperage. Mater. Des. 2019, 181, 107979. [Google Scholar] [CrossRef]
- Feng, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z. Volatile and sensory characterization of Tempranillo wines aged in Quercus alba oak barrels of different geographical origins in USA. LWT 2022, 173, 114328. [Google Scholar] [CrossRef]
- Pérez-Prieto, L.J.; López-Roca, J.M.; Martínez-Cutillas, A.; Mínguez, F.P.; Gómez-Plaza, E. Maturing Wines in Oak Barrels. Effects of Origin, Volume, and Age of the Barrel on the Wine Volatile Composition. J. Agric. Food Chem. 2002, 50, 3272–3276. [Google Scholar] [CrossRef]
- Del Barrio Galán, R.; Bueno-Herrera, M.; De la Cuesta, P.L.; Pérez-Magariño, S. Volatile composition of Spanish red wines: Effect of origin and aging time. Eur. Food Res. Technol. 2022, 248, 1903–1916. [Google Scholar] [CrossRef]
- Dumitriu, G.; De Lerma, N.L.; Zamfir, C.; Cotea, V.V.; Peinado, R.A. Volatile and phenolic composition of red wines subjected to aging in oak cask of different toast degree during two periods of time. LWT 2017, 86, 643–651. [Google Scholar] [CrossRef]
- Singleton, V.L. Maturation of Wines and Spirits: Comparisons, Facts, and Hypotheses. Am. J. Enol. Vitic. 1995, 46, 98–115. [Google Scholar] [CrossRef]
- Chira, K.; Teissedre, P. Chemical and sensory evaluation of wine matured in oak barrel: Effect of oak species involved and toasting process. Eur. Food Res. Technol. 2014, 240, 533–547. [Google Scholar] [CrossRef]
- Prida, A.; Heymann, H.; Balanuta, A.; Puech, J. Relation between chemical composition of oak wood used in cooperage and sensory perception of model extracts. J. Sci. Food Agric. 2009, 89, 765–773. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Henry, R.; Dubourdieu, D. Identification of Volatile Compounds with a “Toasty” Aroma in Heated Oak Used in Barrelmaking. J. Agric. Food Chem. 1997, 45, 2217–2224. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Teissedre, P.; Chira, K. Impact of oak wood modalities on the (non)-volatile composition and sensory attributes of red wines. OENO One 2021, 55, 285–299. [Google Scholar] [CrossRef]
- Chira, K.; Teissedre, P. Extraction of oak volatiles and ellagitannins compounds and sensory profile of wine aged with French winewoods subjected to different toasting methods: Behaviour during storage. Food Chem. 2013, 140, 168–177. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Escott, C.; Lepe, J.A.S. Evolution of the Phenolic Fraction and Aromatic Profile of Red Wines Aged in Oak Barrels. ACS Omega 2020, 5, 7235–7243. [Google Scholar] [CrossRef]
- Chatonnet, P.; Cutzach, I.; Pons, M.; Dubourdieu, D. Monitoring Toasting Intensity of Barrels by Chromatographic Analysis of Volatile Compounds from Toasted Oak Wood. J. Agric. Food Chem. 1999, 47, 4310–4318. [Google Scholar] [CrossRef]
- Navarro, M.; Kontoudakis, N.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Influence of the volatile substances released by oak barrels into a Cabernet Sauvignon red wine and a discolored Macabeo white wine on sensory appreciation by a trained panel. Eur. Food Res. Technol. 2017, 244, 245–258. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine Aging Technology: Fundamental Role of Wood Barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Mozaz, S.R.; Azpilicueta, C.A. Volatile composition of aged wine in used barrels of French oak and of American oak. Food Res. Int. 2002, 35, 603–610. [Google Scholar] [CrossRef]
- Coelho, E.; Lemos, M.; Genisheva, Z.; Domingues, L.; Vilanova, M.; Oliveira, J.M. Validation of a LLME/GC-MS Methodology for Quantification of Volatile Compounds in Fermented Beverages. Molecules 2020, 25, 621. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Naranjo, A.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z.; Pérez, I.; Canals, C.; Adell, E. Aromatic and Sensory Characterization of Maturana Blanca Wines Made with Different Technologies. Beverages 2021, 7, 10. [Google Scholar] [CrossRef]
- García-Carpintero, E.G.; Gallego, M.G.; Sánchez-Palomo, E.; Viñas, M.G. Impact of alternative technique to ageing using oak chips in alcoholic or in malolactic fermentation on volatile and sensory composition of red wines. Food Chem. 2012, 134, 851–863. [Google Scholar] [CrossRef]
- García-Carpintero, E.G.; Sánchez-Palomo, E.; Viñas, M.G. Volatile composition of Bobal red wines subjected to alcoholic/malolactic fermentation with oak chips. LWT 2013, 55, 586–594. [Google Scholar] [CrossRef]
- Mayr, C.M.; Geue, J.P.; Holt, H.E.; Pearson, W.P.; Jeffery, D.W.; Francis, I.L. Characterization of the Key Aroma Compounds in Shiraz Wine by Quantitation, Aroma Reconstitution, and Omission Studies. J. Agric. Food Chem. 2014, 62, 4528–4536. [Google Scholar] [CrossRef]
- Lu, H.; Cheng, B.; Lan, Y.; Duan, C.; He, F. Modifications in Aroma Characteristics of ‘Merlot’ Dry Red Wines Aged in American, French and Slovakian Oak Barrels with Different Toasting Degrees. Food Sci. Hum. Wellness 2023, 13, 381–391. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Wang, X.; Li, A.; Dizy, M.; Ullah, N.; Sun, W.; Tao, Y. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef]
- Tao, Y.; Li, H. Active volatiles of cabernet sauvignon wine from Changli County. Health 2009, 01, 176–182. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2003, 84, 585–590. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Trujillo, M.; Ruiz, A.G.; Viñas, M.G. Aroma profile of malbec red wines from La Mancha region: Chemical and sensory characterization. Food Res. Int. 2017, 100, 201–208. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, X.; Xiao, Z.; Niu, Y. Characterization of the differences in the aroma of cherry wines from different price segments using gas chromatography–mass spectrometry, odor activity values, sensory analysis, and aroma reconstitution. Food Sci. Biotechnol. 2017, 26, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Bescansa, L.; Masa, A.; Oliveira, J.M. Volatile composition of wines from cvs. Blanco lexítimo, Agudelo and Serradelo (Vitis vinifera) grown in Betanzos (NW Spain). J. Inst. Brew. 2009, 115, 35–40. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.; Martínez, M.; Simal-Gándara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2011, 132, 112–124. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas Chromatography−Olfactometry and Chemical Quantitative Study of the Aroma of Six Premium Quality Spanish Aged Red Wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of Aged Red Wine Aroma Properties from Aroma Chemical Composition. Partial Least Squares Regression Models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-Vine Grape Drying Effect on Volatile Compounds and Aromatic Series in Must from Pedro Ximénez Grape Variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
| 12 Months | 18 Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toasting Level (%) | Sig. | Grain Size (%) | Sig. | Interaction (%) | Sig. | Error (%) | Toasting Level (%) | Sig. | Grain Size (%) | Sig. | Interaction (%) | Sig. | Error (%) | |
| C6 Alcohols | 57.88 | ** | 5.63 | ns | 3.43 | ns | 33.05 | 36.85 | ** | 16.32 | ** | 26.40 | ** | 20.35 |
| 1-hexanol | 55.65 | ** | 5.92 | ns | 3.57 | ns | 34.87 | 36.50 | ** | 16.38 | ** | 26.63 | ** | 20.50 |
| Z-3-hexenol | 64.94 | ** | 2.47 | ns | 2.30 | ns | 30.29 | 39.72 | ** | 15.12 | * | 24.00 | * | 21.16 |
| E-3-hexenol | 68.71 | *** | 2.81 | ns | 1.78 | ns | 26.70 | 38.83 | ** | 15.64 | * | 25.11 | ** | 20.42 |
| E-2-hexenol | 24.69 | *** | 17.34 | *** | 54.70 | *** | 3.27 | 35.52 | *** | 33.08 | *** | 27.94 | *** | 3.45 |
| Z-2-hexenol | 98.84 | *** | 0.83 | *** | 0.22 | ** | 0.11 | 80.36 | *** | 0.03 | ns | 10.27 | * | 9.33 |
| Higher alcohols | 65.45 | *** | 4.74 | ns | 2.88 | ns | 26.92 | 50.86 | *** | 10.76 | * | 22.33 | ** | 16.05 |
| 1-propanol | 40.89 | *** | 25.05 | *** | 25.01 | *** | 9.05 | 72.15 | *** | 1.16 | ns | 11.83 | * | 14.86 |
| 2-octanol | 45.68 | *** | 7.69 | ns | 26.99 | ** | 19.64 | 21.70 | ns | 17.31 | * | 18.24 | ns | 42.75 |
| Isobutanol | 70.92 | *** | 8.39 | * | 5.06 | ns | 15.62 | 45.93 | *** | 4.79 | ns | 28.48 | ** | 20.80 |
| 1-butanol | 70.12 | *** | 8.92 | * | 5.73 | ns | 15.23 | 61.38 | *** | 2.79 | ns | 22.18 | ** | 13.66 |
| Isoamyl alcohol | 70.51 | *** | 1.71 | ns | 3.21 | ns | 24.56 | 45.62 | *** | 7.72 | * | 28.93 | ** | 17.73 |
| 3-methyl-1-pentanol | 56.45 | ** | 4.96 | ns | 4.68 | ns | 33.91 | 38.19 | ** | 15.41 | * | 23.46 | * | 22.94 |
| 2.3-butanediol | 29.54 | ** | 20.52 | ** | 36.22 | ns | 13.72 | 45.42 | *** | 47.66 | *** | 2.03 | ns | 4.89 |
| 3-methylthiopropanol | 80.51 | *** | 0.35 | ns | 3.14 | ns | 16.01 | 67.69 | *** | 1.87 | ns | 18.20 | ** | 12.25 |
| Benzyl alcohol | 74.29 | *** | 2.00 | ns | 2.65 | ns | 21.06 | 59.25 | *** | 3.77 | ns | 21.59 | ** | 15.39 |
| 2-phenylethanol | 54.82 | ** | 5.98 | ns | 5.08 | ns | 34.11 | 51.43 | *** | 12.45 | * | 18.52 | * | 17.60 |
| C13-norisoprenoids | 41.19 | *** | 42.75 | *** | 3.43 | ns | 12.63 | 68.65 | *** | 9.29 | * | 1.89 | ns | 20.17 |
| α-ionone | 32.67 | *** | 45.40 | *** | 18.58 | *** | 3.35 | 77.37 | *** | 0.51 | ns | 10.36 | * | 11.75 |
| β-ionone | 23.51 | *** | 58.54 | *** | 15.21 | *** | 2.74 | 18.00 | *** | 26.75 | *** | 51.31 | *** | 3.94 |
| β-damascenone | 62.63 | *** | 8.00 | ns | 3.14 | ns | 26.23 | 66.42 | *** | 5.83 | ns | 0.10 | ns | 27.65 |
| Terpenes | 70.95 | *** | 14.53 | ** | 4.06 | ns | 10.46 | 32.85 | ns | 0.36 | ns | 15.00 | ns | 51.79 |
| Terpinolene | 16.90 | *** | 55.47 | *** | 27.43 | *** | 0.20 | 41.27 | *** | 39.81 | *** | 18.20 | *** | 0.72 |
| α-terpineol | 42.51 | *** | 21.75 | ** | 21.48 | ** | 14.26 | 51.41 | *** | 9.53 | * | 26.43 | ** | 12.63 |
| E-geraniol | 44.42 | *** | 28.42 | *** | 13.25 | * | 13.91 | 40.01 | *** | 21.56 | *** | 29.48 | *** | 8.95 |
| Z-geraniol (Nerol) | 68.90 | *** | 0.08 | ns | 6.33 | ns | 24.70 | 46.01 | *** | 8.06 | * | 30.20 | ** | 15.73 |
| β-citronellol | 54.54 | *** | 3.55 | * | 36.77 | *** | 5.14 | 19.80 | *** | 55.40 | *** | 20.78 | *** | 4.02 |
| Linalool | 77.61 | *** | 4.04 | * | 10.51 | ** | 7.83 | 57.58 | *** | 4.49 | ns | 19.55 | * | 18.37 |
| β-pinene | 43.41 | *** | 6.86 | ** | 41.27 | *** | 8.46 | 23.37 | *** | 1.81 | * | 71.92 | *** | 2.90 |
| 4-carvomenthenol | 18.27 | ** | 4.23 | * | 69.03 | *** | 8.47 | 81.53 | *** | 2.54 | ns | 1.71 | ns | 14.22 |
| Citral | 26.45 | *** | 45.52 | *** | 21.02 | *** | 7.01 | 28.64 | *** | 37.85 | *** | 28.18 | *** | 5.32 |
| Ethyl esters and acetates | 71.29 | *** | 8.77 | ** | 9.53 | * | 10.41 | 50.10 | *** | 7.71 | * | 25.62 | ** | 16.56 |
| Ethyl butyrate | 56.96 | ** | 3.59 | ns | 3.63 | ns | 35.81 | 35.26 | ** | 17.34 | ** | 27.45 | ** | 19.95 |
| Ethyl 2-methylbutyrate | 64.26 | *** | 3.07 | ns | 3.23 | ns | 29.43 | 40.27 | ** | 13.96 | * | 24.66 | * | 21.11 |
| Ethyl decanoate | 43.56 | *** | 16.43 | ** | 23.81 | ** | 16.20 | 65.73 | *** | 4.73 | * | 18.33 | ** | 11.20 |
| Ethyl isovalerate | 62.92 | *** | 0.03 | ns | 8.56 | ns | 28.50 | 34.16 | ** | 15.91 | * | 25.32 | * | 24.61 |
| Ethyl myristate | 35.87 | *** | 19.61 | ** | 31.61 | *** | 12.91 | 38.04 | ** | 13.21 | * | 28.05 | ** | 20.71 |
| Methyl salicylate | 38.03 | *** | 23.21 | *** | 35.95 | *** | 2.82 | 60.25 | *** | 12.05 | ** | 17.36 | ** | 10.35 |
| Hexyl acetate | 74.56 | *** | 7.97 | ** | 7.76 | * | 9.72 | 56.72 | *** | 2.44 | ns | 31.09 | *** | 9.74 |
| Methyl vanillate | 68.04 | *** | 5.87 | ns | 0.65 | ns | 25.44 | 31.02 | ** | 17.65 | * | 26.24 | * | 25.09 |
| Ethyl vanillate | 64.63 | *** | 2.65 | ns | 4.91 | ns | 27.81 | 37.72 | ** | 14.13 | * | 29.10 | ** | 19.06 |
| Ethyl hexanoate | 59.90 | ** | 4.21 | ns | 2.73 | ns | 33.16 | 38.38 | ** | 14.58 | * | 28.30 | ** | 18.75 |
| Ethyl lactate | 66.56 | *** | 9.22 | * | 9.29 | ns | 14.93 | 45.34 | ** | 5.04 | ns | 29.13 | ** | 20.49 |
| Ethyl octanoate | 51.69 | ** | 6.91 | ns | 4.00 | ns | 37.40 | 49.11 | *** | 7.01 | * | 27.02 | ** | 16.86 |
| Diethyl succinate | 58.37 | ** | 3.87 | ns | 7.64 | ns | 30.13 | 51.89 | *** | 12.02 | * | 16.56 | * | 19.53 |
| Isoamyl acetate | 52.64 | ** | 6.02 | ns | 2.10 | ns | 39.25 | 35.48 | ** | 17.93 | ** | 24.07 | * | 22.52 |
| 2-phenylethyl acetate | 52.44 | ** | 3.69 | ns | 3.31 | ns | 40.56 | 37.24 | ** | 16.61 | ** | 25.33 | ** | 20.82 |
| Fatty acids | 61.06 | *** | 13.07 | ** | 10.63 | * | 15.24 | 46.71 | *** | 11.68 | ** | 27.74 | ** | 13.87 |
| Propanoic acid | 38.04 | *** | 18.90 | ** | 29.29 | ** | 13.77 | 63.22 | *** | 2.16 | ns | 16.91 | * | 17.70 |
| Geranic acid | 33.04 | *** | 3.77 | *** | 60.79 | *** | 2.40 | 63.02 | *** | 8.13 | ** | 23.41 | *** | 5.44 |
| Nerolic acid | 67.79 | *** | 1.64 | ns | 1.87 | ns | 28.70 | 0.44 | ns | 59.20 | *** | 11.34 | ns | 29.01 |
| Pentanoic acid | 83.46 | *** | 0.12 | ns | 6.48 | * | 9.93 | 78.17 | *** | 0.43 | ns | 3.15 | ns | 18.26 |
| Isobutyric acid | 57.74 | ** | 0.06 | ns | 13.06 | ns | 29.14 | 57.70 | *** | 2.74 | ns | 22.53 | ** | 17.03 |
| 2-methylbutyric acid | 55.07 | *** | 13.55 | ** | 14.46 | * | 16.92 | 57.59 | *** | 3.71 | ns | 24.32 | ** | 14.38 |
| Hexanoic acid | 52.00 | ** | 5.55 | ns | 4.80 | ns | 37.65 | 33.94 | ** | 17.92 | ** | 28.67 | ** | 19.47 |
| Octanoic acid | 55.46 | ** | 2.94 | ns | 5.59 | ns | 36.02 | 32.24 | ** | 21.01 | ** | 29.67 | ** | 17.08 |
| Butyric acid | 73.58 | *** | 6.01 | * | 7.63 | ns | 12.78 | 50.14 | *** | 2.97 | ns | 28.96 | ** | 17.93 |
| Acetic acid | 34.71 | *** | 29.67 | *** | 24.15 | ** | 11.47 | 72.26 | *** | 0.24 | ns | 15.92 | ** | 11.58 |
| Isovaleric acid | 62.45 | *** | 11.49 | * | 9.81 | ns | 16.25 | 54.06 | *** | 4.20 | ns | 25.57 | ** | 16.17 |
| γ-Lactones | 63.61 | *** | 9.33 | * | 10.29 | ns | 16.77 | 62.61 | *** | 3.72 | ns | 20.04 | ** | 13.63 |
| γ-butyrolactone | 63.61 | *** | 9.33 | * | 10.29 | ns | 16.77 | 62.61 | *** | 3.72 | ns | 20.04 | ** | 13.63 |
| Carbonyl compounds | 81.62 | *** | 4.08 | ns | 0.53 | ns | 13.77 | 23.13 | *** | 32.26 | *** | 35.62 | *** | 8.99 |
| Acetoin | 81.62 | *** | 4.08 | ns | 0.53 | ns | 13.77 | 23.13 | *** | 32.26 | *** | 35.62 | *** | 8.99 |
| Others | 64.72 | *** | 10.66 | ** | 12.37 | * | 12.25 | 35.98 | *** | 16.92 | *** | 40.01 | *** | 7.10 |
| Benzaldehyde | 69.78 | *** | 8.73 | ** | 11.65 | ** | 9.85 | 66.43 | *** | 7.22 | ns | 5.33 | ns | 21.02 |
| Hexanal | 44.93 | *** | 20.30 | ** | 20.66 | ** | 14.11 | 24.23 | ** | 21.76 | ** | 39.53 | *** | 14.48 |
| Vanillyl acetone | 43.67 | ** | 12.70 | * | 16.79 | ns | 26.84 | 28.57 | *** | 21.10 | *** | 40.35 | *** | 9.98 |
| Furanic compounds | 4.68 | ns | 76.78 | *** | 5.46 | *** | 13.08 | 25.01 | * | 38.46 | *** | 14.39 | * | 22.14 |
| Furfural | 4.68 | ns | 72.65 | *** | 7.55 | *** | 15.12 | 26.97 | * | 10.96 | * | 36.84 | ** | 25.23 |
| 5-methylfurfural | 5.94 | ns | 62.80 | *** | 7.26 | *** | 24.00 | 3.96 | ns | 72.84 | *** | 6.40 | ns | 16.80 |
| Furfuryl alcohol | 5.02 | ns | 82.23 | *** | 3.28 | *** | 9.46 | 47.89 | ** | 29.17 | ** | 0.77 | ns | 22.17 |
| Lactones | 51.54 | ** | 2.65 | ns | 14.14 | ns | 31.67 | 56.98 | *** | 17.66 | ** | 8.89 | ns | 16.47 |
| cis-whiskey-lactone | 47.47 | ** | 18.52 | ** | 11.84 | ns | 22.17 | 26.28 | ** | 45.56 | *** | 14.43 | * | 13.73 |
| trans-whiskey-lactone | 53.94 | *** | 13.29 | * | 10.51 | ns | 22.26 | 69.94 | *** | 0.24 | ns | 18.63 | ** | 11.20 |
| Volatile phenols | 63.16 | *** | 11.06 | * | 3.99 | ns | 21.80 | 76.88 | *** | 1.25 | ns | 6.93 | ns | 14.94 |
| 4-vinylphenol | 39.53 | ** | 27.79 | ** | 6.21 | ns | 26.47 | 68.56 | *** | 7.51 | * | 6.38 | ns | 17.55 |
| 4-vinylguaiacol | 71.32 | *** | 4.85 | ns | 0.36 | ns | 23.47 | 77.89 | *** | 0.46 | ns | 7.72 | ns | 13.93 |
| Eugenol | 63.67 | *** | 6.99 | ns | 7.53 | ns | 21.81 | 63.49 | ** | 0.07 | ns | 6.51 | ns | 29.93 |
| Guaiacol | 56.11 | ** | 3.58 | ns | 5.55 | ns | 34.76 | 60.57 | *** | 16.50 | ** | 2.27 | ns | 20.66 |
| 4-ethylphenol | 85.54 | *** | 0.72 | ns | 0.69 | ns | 13.05 | 85.66 | *** | 2.97 | ns | 1.94 | ns | 9.43 |
| Phenolic aldehydes | 65.38 | *** | 9.21 | * | 7.09 | ns | 18.31 | 41.14 | *** | 13.78 | ** | 34.22 | *** | 10.87 |
| Vanillin | 65.38 | *** | 9.21 | * | 7.09 | ns | 18.31 | 41.14 | *** | 13.78 | ** | 34.22 | *** | 10.87 |
| 12 Months | 18 Months | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LT | MT | MLT | LT | MT | MLT | |||||||||||||||
| µg/L | OAV | µg/L | OAV | µg/L | OAV | Sig. | µg/L | OAV | µg/L | OAV | µg/L | OAV | Sig. | |||||||
| C6 Alcohols | 1698 | b | 0.53 | 1117 | a | 0.32 | 1199 | a | 0.36 | ** | 1414 | a | 0.40 | 1085 | a | 0.31 | 1992 | b | 0.58 | ** |
| 1-hexanol | 1566 | b | 0.20 | 1043 | a | 0.13 | 1158 | a | 0.14 | * | 1320 | a | 0.16 | 1013 | a | 0.13 | 1854 | b | 0.23 | ** |
| Z-3-hexenol | 79.36 | b | 0.20 | 54.07 | a | 0.14 | 59.45 | a | 0.15 | ** | 67.61 | a | 0.17 | 51.91 | a | 0.13 | 96.57 | b | 0.24 | ** |
| E-3-hexenol | 27.27 | b | 0.07 | 18.10 | a | 0.05 | 20.79 | a | 0.05 | ** | 22.92 | a | 0.06 | 17.18 | a | 0.04 | 33.73 | b | 0.08 | ** |
| E-2-hexenol | 0.31 | a | 0.00 | 0.63 | b | 0.00 | 1.02 | c | 0.00 | *** | 1.40 | b | 0.00 | 0.54 | a | 0.00 | 3.01 | c | 0.01 | *** |
| Z-2-hexenol | 26.14 | b | 0.07 | 3.60 | a | 0.01 | 3.25 | a | 0.01 | *** | 2.25 | a | 0.01 | 3.02 | b | 0.01 | 5.44 | c | 0.01 | *** |
| Higher alcohols | 124,402 | b | 13.83 | 89,210 | a | 10.74 | 94,305 | a | 11.33 | * | 103,189 | a | 12.17 | 79,921 | a | 10.16 | 154,563 | b | 16.25 | *** |
| 1-propanol | 395.02 | a | 0.00 | 342.05 | a | 0.00 | 308.22 | a | 0.00 | ns | 350.51 | a | 0.00 | 262.79 | a | 0.00 | 607.19 | b | 0.00 | *** |
| 2-octanol | 488.11 | b | 4.07 | 464.82 | a | 3.87 | 473.12 | ab | 3.94 | * | 489.07 | ab | 4.08 | 468.43 | a | 3.90 | 498.19 | b | 4.15 | ns |
| Isobutanol | 2491 | b | 0.06 | 2158 | ab | 0.05 | 1899 | a | 0.05 | ns | 2190 | a | 0.05 | 1609 | a | 0.04 | 3264 | b | 0.08 | *** |
| 1-butanol | 119.07 | b | 0.00 | 99.91 | ab | 0.00 | 89.66 | a | 0.00 | ns | 104.01 | a | 0.00 | 79.56 | a | 0.00 | 166.85 | b | 0.00 | *** |
| Isoamyl alcohol | 38,361 | b | 1.28 | 28,973 | a | 0.97 | 28,978 | a | 0.97 | * | 31,367 | a | 1.05 | 24,377 | a | 0.81 | 48,100 | b | 1.60 | *** |
| 3-methyl-1-pentanol | 59.64 | b | 0.00 | 39.23 | a | 0.00 | 43.68 | a | 0.00 | ** | 49.33 | a | 0.00 | 37.24 | a | 0.00 | 67.39 | b | 0.00 | ** |
| 2.3-butanediol | 9.91 | c | 0.00 | 7.58 | b | 0.00 | 6.31 | a | 0.00 | *** | 5.76 | b | 0.00 | 2.84 | a | 0.00 | 9.55 | c | 0.00 | *** |
| 3-methylthiopropanol | 202.35 | b | 0.20 | 153.88 | a | 0.15 | 146.95 | a | 0.15 | * | 156.45 | b | 0.16 | 113.57 | a | 0.11 | 257.16 | c | 0.26 | *** |
| Benzyl alcohol | 117.09 | b | 0.00 | 84.06 | a | 0.00 | 83.83 | a | 0.00 | * | 85.35 | a | 0.00 | 69.58 | a | 0.00 | 130.75 | b | 0.00 | *** |
| 2-phenylethanol | 82,159 | b | 8.22 | 56,887 | a | 5.69 | 62,275 | a | 6.23 | * | 68,390 | a | 6.84 | 52,900 | a | 5.29 | 101,461 | b | 10.15 | *** |
| C13-norisoprenoids | 5.44 | b | 90.41 | 3.01 | a | 51.95 | 3.26 | a | 56.58 | *** | 3.19 | a | 54.00 | 3.08 | a | 51.49 | 4.81 | b | 82.50 | *** |
| α-ionone | 0.38 | a | 4.22 | 0.48 | ab | 5.35 | 0.58 | b | 6.40 | ** | 0.75 | a | 8.34 | 0.69 | a | 7.62 | 1.28 | b | 14.21 | *** |
| β-ionone | 1.69 | b | 18.77 | 0.45 | a | 5.01 | 0.40 | a | 4.44 | *** | 0.36 | b | 4.02 | 0.45 | c | 5.01 | 0.26 | a | 2.94 | *** |
| β-damascenone | 3.37 | b | 67.42 | 2.08 | a | 41.59 | 2.29 | a | 45.73 | ** | 2.08 | a | 41.64 | 1.94 | a | 38.86 | 3.27 | b | 65.35 | *** |
| Terpenes | 101.10 | b | 4.35 | 79.69 | a | 3.40 | 79.32 | a | 3.44 | * | 80.63 | ab | 3.43 | 70.65 | a | 3.06 | 85.72 | b | 3.42 | ns |
| Terpinolene | 0.40 | b | 0.01 | 0.51 | b | 0.01 | 0.31 | a | 0.01 | * | 0.84 | c | 0.02 | 0.40 | a | 0.01 | 0.46 | b | 0.01 | *** |
| α-terpineol | 6.62 | b | 0.01 | 4.34 | a | 0.00 | 4.57 | a | 0.00 | * | 4.69 | b | 0.00 | 3.59 | a | 0.00 | 5.90 | c | 0.01 | *** |
| E-geraniol | 48.55 | b | 2.43 | 35.15 | a | 1.76 | 39.72 | ab | 1.99 | * | 38.72 | b | 1.94 | 33.15 | b | 1.66 | 19.86 | a | 0.99 | *** |
| Z-geraniol (Nerol) | 35.26 | b | 1.76 | 30.05 | ab | 1.50 | 26.93 | a | 1.35 | * | 27.18 | a | 1.36 | 25.62 | a | 1.28 | 43.85 | b | 2.19 | *** |
| β-citronellol | 2.25 | c | 0.02 | 0.77 | a | 0.01 | 1.46 | b | 0.01 | *** | 1.28 | a | 0.01 | 1.77 | b | 0.02 | 2.55 | c | 0.03 | *** |
| Linalool | 2.97 | b | 0.12 | 2.80 | b | 0.11 | 1.95 | a | 0.08 | * | 2.48 | a | 0.10 | 2.31 | a | 0.09 | 4.73 | b | 0.19 | *** |
| β-pinene | 0.20 | a | 0.00 | 0.29 | b | 0.00 | 0.59 | c | 0.00 | *** | 0.81 | b | 0.00 | 0.41 | a | 0.00 | 0.78 | b | 0.00 | *** |
| 4-carvomenthenol | 4.31 | b | - | 5.25 | c | - | 1.93 | a | - | *** | 3.55 | a | - | 3.06 | a | - | 6.53 | b | - | *** |
| Citral | 0.55 | a | 0.02 | 0.54 | a | 0.02 | 1.86 | b | 0.07 | *** | 1.09 | b | 0.04 | 0.34 | a | 0.01 | 1.07 | b | 0.04 | *** |
| Ethyl esters and acetates | 74,018 | b | 244.83 | 51,968 | a | 164.92 | 53,376 | a | 158.64 | ** | 58,246 | a | 143.73 | 45,882 | a | 117.45 | 87,231 | b | 248.32 | *** |
| Ethyl butyrate | 305.61 | b | 15.28 | 198.54 | a | 9.93 | 232.57 | a | 11.63 | * | 253.47 | a | 12.67 | 203.94 | a | 10.20 | 352.51 | b | 17.63 | ** |
| Ethyl 2-methylbutyrate | 33.84 | b | 1.88 | 20.37 | a | 1.13 | 23.29 | a | 1.29 | ** | 26.29 | a | 1.46 | 19.69 | a | 1.09 | 36.97 | b | 2.05 | ** |
| Ethyl decanoate | 32.47 | a | 0.16 | 35.41 | a | 0.18 | 29.08 | a | 0.15 | ns | 13.30 | a | 0.07 | 10.81 | a | 0.05 | 29.04 | b | 0.15 | *** |
| Ethyl isovalerate | 63.45 | b | 21.15 | 36.18 | a | 12.06 | 42.95 | a | 14.32 | ** | 48.50 | a | 16.17 | 36.79 | a | 12.26 | 66.53 | b | 22.18 | ** |
| Ethyl myristate | 15.39 | b | 0.01 | 11.73 | a | 0.01 | 11.07 | a | 0.01 | ** | 9.20 | a | 0.00 | 9.93 | a | 0.00 | 17.19 | b | 0.01 | ** |
| Methyl salicylate | 4.42 | b | 44.18 | 2.59 | a | 25.90 | 2.24 | a | 22.42 | *** | 0.77 | a | 7.72 | 2.29 | b | 22.85 | 4.11 | c | 41.14 | *** |
| Hexyl acetate | 277.18 | b | 0.41 | 130.14 | a | 0.19 | 152.12 | a | 0.23 | *** | 188.54 | b | 0.28 | 93.60 | a | 0.14 | 296.08 | c | 0.44 | *** |
| Methyl vanillate | 8.76 | b | 0.00 | 6.50 | a | 0.00 | 6.73 | a | 0.00 | ns | 7.17 | a | 0.00 | 5.53 | a | 0.00 | 9.86 | b | 0.00 | ** |
| Ethyl vanillate | 530.84 | b | 0.54 | 331.76 | a | 0.34 | 391.69 | a | 0.40 | * | 400.93 | a | 0.40 | 324.03 | a | 0.33 | 532.11 | b | 0.54 | ** |
| Ethyl hexanoate | 677.39 | b | 48.38 | 417.45 | a | 29.82 | 459.03 | a | 32.79 | ** | 505.39 | a | 36.10 | 373.67 | a | 26.69 | 728.07 | b | 52.01 | ** |
| Ethyl lactate | 43,498 | b | 0.28 | 33,759 | a | 0.22 | 32,014 | a | 0.21 | * | 35,675 | a | 0.23 | 27,168 | a | 0.18 | 52,989 | b | 0.34 | *** |
| Ethyl octanoate | 468.97 | b | 93.79 | 365.64 | a | 73.13 | 306.42 | a | 61.28 | * | 270.77 | b | 54.15 | 158.35 | a | 31.67 | 452.13 | c | 90.43 | *** |
| Diethyl succinate | 27,578 | b | 4.60 | 16,310 | a | 2.72 | 19,311 | a | 3.22 | ** | 20,441 | a | 3.41 | 17,136 | a | 2.86 | 31,120 | b | 5.19 | *** |
| Isoamyl acetate | 411.34 | b | 13.71 | 270.32 | a | 9.01 | 311.29 | ab | 10.38 | * | 321.61 | a | 10.72 | 264.93 | a | 8.83 | 471.31 | b | 15.71 | ** |
| 2-phenylethyl acetate | 111.93 | b | 0.45 | 71.67 | a | 0.29 | 81.73 | a | 0.33 | * | 83.72 | a | 0.33 | 73.24 | a | 0.29 | 126.57 | b | 0.51 | ** |
| Fatty acids | 4856 | b | 9.78 | 3528 | a | 7.32 | 3711 | a | 7.24 | * | 3912 | a | 7.54 | 3115 | a | 6.37 | 5672 | b | 11.82 | *** |
| Propanoic acid | 21.49 | b | 0.00 | 18.83 | ab | 0.00 | 16.30 | a | 0.00 | ns | 21.23 | b | 0.00 | 11.83 | a | 0.00 | 28.86 | c | 0.00 | *** |
| Geranic acid | 22.63 | c | 0.57 | 15.52 | b | 0.39 | 9.82 | a | 0.25 | *** | 3.90 | a | 0.10 | 16.89 | b | 0.42 | 28.22 | c | 0.71 | *** |
| Nerolic acid | 11.85 | b | - | 6.87 | a | - | 12.74 | b | - | ** | 7.86 | a | - | 7.46 | a | - | 7.79 | a | - | ns |
| Pentanoic acid | 5.60 | a | 0.03 | 4.89 | a | 0.03 | 4.57 | a | 0.03 | ns | 4.33 | a | 0.03 | 3.34 | a | 0.02 | 7.52 | b | 0.05 | *** |
| Isobutyric acid | 71.46 | a | 0.00 | 62.27 | a | 0.00 | 56.79 | a | 0.00 | ns | 59.47 | a | 0.00 | 44.18 | a | 0.00 | 97.70 | b | 0.00 | *** |
| 2-methylbutyric acid | 129.05 | b | 0.04 | 103.28 | a | 0.03 | 100.53 | a | 0.03 | * | 101.59 | a | 0.03 | 82.43 | a | 0.03 | 157.91 | b | 0.05 | *** |
| Hexanoic acid | 1694 | b | 0.56 | 1172 | a | 0.39 | 1284 | a | 0.43 | * | 1404 | a | 0.47 | 1112 | a | 0.37 | 1895 | b | 0.63 | ** |
| Octanoic acid | 1583 | b | 1.58 | 1005 | a | 1.01 | 1188 | a | 1.19 | * | 1260 | a | 1.26 | 1064 | a | 1.06 | 1764 | b | 1.76 | ** |
| Butyric acid | 145.76 | b | 0.07 | 125.67 | ab | 0.06 | 113.69 | a | 0.05 | ns | 123.28 | a | 0.06 | 95.14 | a | 0.04 | 181.42 | b | 0.08 | *** |
| Acetic acid | 943.41 | a | 0.05 | 836.36 | a | 0.04 | 752.64 | a | 0.04 | ns | 742.72 | b | 0.04 | 532.18 | a | 0.03 | 1.223 | c | 0.06 | *** |
| Isovaleric acid | 226.82 | b | 6.87 | 177.23 | a | 5.37 | 172.51 | a | 5.23 | * | 183.38 | a | 5.56 | 145.08 | a | 4.40 | 279.65 | b | 8.47 | *** |
| γ-Lactones | 710.21 | b | 20.29 | 549.35 | a | 15.70 | 536.13 | a | 15.32 | * | 568.06 | a | 16.23 | 448.49 | a | 12.81 | 946.18 | b | 27.03 | *** |
| γ-butyrolactone | 710.21 | b | 20.29 | 549.35 | a | 15.70 | 536.13 | a | 15.32 | * | 568.06 | a | 16.23 | 448.49 | a | 12.81 | 946.18 | b | 27.03 | *** |
| Carbonyl compounds | 263.03 | a | 0.00 | 216.61 | a | 0.00 | 241.13 | a | 0.00 | ns | 260.67 | a | 0.00 | 308.83 | a | 0.00 | 467.76 | b | 0.00 | *** |
| Acetoin | 263.03 | a | 0.00 | 216.61 | a | 0.00 | 241.13 | a | 0.00 | ns | 260.67 | a | 0.00 | 308.83 | a | 0.00 | 467.76 | b | 0.00 | *** |
| Others | 33.10 | b | 0.01 | 19.74 | a | 0.01 | 21.21 | a | 0.01 | *** | 22.34 | b | 0.01 | 18.36 | a | 0.01 | 29.45 | c | 0.01 | *** |
| Benzaldehyde | 5.70 | b | 0.00 | 3.44 | a | 0.00 | 3.67 | a | 0.00 | *** | 2.49 | a | 0.00 | 2.07 | a | 0.00 | 3.81 | b | 0.00 | *** |
| Hexanal | 3.29 | b | 0.01 | 1.52 | a | 0.00 | 1.04 | a | 0.00 | *** | 1.50 | a | 0.00 | 1.38 | a | 0.00 | 2.65 | b | 0.01 | ** |
| Vanillyl acetone | 24.11 | b | - | 14.78 | a | - | 16.49 | a | - | *** | 18.36 | b | - | 14.92 | a | - | 22.99 | c | - | *** |
| Furanic compounds | 2222 | a | 0.13 | 1765 | a | 0.12 | 1599 | a | 0.10 | ns | 1363 | ab | 0.08 | 1043 | a | 0.06 | 1819 | b | 0.11 | * |
| Furfural | 907.69 | a | 0.06 | 818.98 | a | 0.06 | 665.66 | a | 0.05 | ns | 425.43 | a | 0.03 | 344.30 | a | 0.02 | 701.77 | b | 0.05 | * |
| 5-methylfurfural | 887.43 | a | 0.04 | 568.59 | a | 0.03 | 644.83 | a | 0.03 | ns | 567.19 | a | 0.03 | 544.98 | a | 0.03 | 650.74 | a | 0.03 | ns |
| Furfuryl alcohol | 427.80 | b | 0.03 | 377.29 | ab | 0.03 | 288.13 | a | 0.02 | ns | 370.22 | b | 0.02 | 153.37 | a | 0.01 | 466.33 | b | 0.03 | ** |
| Lactones | 850.69 | b | 11.80 | 559.24 | a | 7.61 | 767.89 | b | 9.37 | * | 601.85 | a | 8.23 | 510.70 | a | 6.60 | 980.79 | b | 12.04 | *** |
| cis-whiskey-lactone | 499.21 | b | 10.85 | 320.50 | a | 6.97 | 383.03 | a | 8.33 | ** | 346.68 | a | 7.54 | 274.00 | a | 5.96 | 493.27 | b | 10.72 | ** |
| trans-whiskey-lactone | 351.48 | b | 0.95 | 238.74 | a | 0.65 | 384.86 | b | 1.04 | ** | 255.18 | a | 0.69 | 236.71 | a | 0.64 | 487.52 | b | 1.32 | *** |
| Volatile phenols | 290.29 | b | 20.89 | 203.17 | a | 11.65 | 202.99 | a | 16.17 | * | 244.02 | b | 15.25 | 165.80 | a | 11.17 | 349.89 | c | 22.11 | *** |
| 4-vinylphenol | 80.43 | b | 0.45 | 69.97 | ab | 0.39 | 57.31 | a | 0.32 | ns | 76.87 | b | 0.43 | 52.55 | a | 0.29 | 119.22 | c | 0.66 | *** |
| 4-vinylguaiacol | 76.79 | b | 1.92 | 60.47 | ab | 1.51 | 49.91 | a | 1.25 | * | 73.19 | b | 1.83 | 41.13 | a | 1.03 | 104.61 | c | 2.62 | *** |
| Eugenol | 95.01 | b | 15.83 | 47.18 | a | 7.86 | 76.91 | b | 12.82 | ** | 65.40 | a | 10.90 | 49.85 | a | 8.31 | 96.17 | b | 16.03 | ** |
| Guaiacol | 25.31 | b | 2.66 | 17.83 | a | 1.88 | 16.92 | a | 1.78 | * | 19.72 | b | 2.08 | 14.52 | a | 1.53 | 26.57 | c | 2.80 | *** |
| 4-ethylphenol | 12.75 | c | 0.02 | 7.71 | b | 0.01 | 1.94 | a | 0.00 | *** | 8.84 | b | 0.01 | 7.76 | b | 0.01 | 3.30 | a | 0.01 | *** |
| Phenolic aldehydes | 277.41 | b | 1.39 | 197.33 | a | 0.99 | 200.28 | a | 1.00 | * | 200.49 | b | 1.00 | 114.35 | a | 0.57 | 242.19 | b | 1.21 | *** |
| Vanillin | 277.41 | b | 1.39 | 197.33 | a | 0.99 | 200.28 | a | 1.00 | * | 200.49 | b | 1.00 | 114.35 | a | 0.57 | 242.19 | b | 1.21 | *** |
| 12 Months | 18 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG | EG | SG | EG | |||||||
| µg/L | OAV | µg/L | OAV | Sig. | µg/L | OAV | µg/L | OAV | Sig. | |
| C6 Alcohols | 1613 | 0.48 | 1066 | 0.33 | ** | 1247 | 0.36 | 1747 | 0.50 | ** |
| 1-hexanol | 1498 | 0.19 | 1013 | 0.13 | ** | 1163 | 0.15 | 1628 | 0.20 | ** |
| Z-3-hexenol | 76.64 | 0.19 | 51.94 | 0.13 | ** | 60.62 | 0.15 | 83.45 | 0.21 | * |
| E-3-hexenol | 26.33 | 0.07 | 17.78 | 0.04 | *** | 20.26 | 0.05 | 28.96 | 0.07 | * |
| E-2-hexenol | 0.35 | 0.00 | 0.95 | 0.00 | *** | 0.66 | 0.00 | 2.64 | 0.01 | *** |
| Z-2-hexenol | 12.06 | 0.03 | 9.93 | 0.02 | ns | 3.60 | 0.01 | 3.54 | 0.01 | ns |
| Higher alcohols | 124,856 | 13.75 | 80,422 | 10.19 | *** | 98,213 | 11.62 | 126,902 | 14.10 | * |
| 1-propanol | 438.26 | 0.00 | 258.60 | 0.00 | *** | 388.34 | 0.00 | 425.33 | 0.00 | ns |
| 2-octanol | 481.81 | 4.02 | 468.88 | 3.91 | ns | 474.11 | 3.95 | 496.35 | 4.14 | * |
| Isobutanol | 2699 | 0.07 | 1666 | 0.04 | *** | 2133 | 0.05 | 2576 | 0.06 | ns |
| 1-butanol | 127.44 | 0.00 | 78.32 | 0.00 | *** | 108.98 | 0.00 | 124.64 | 0.00 | ns |
| Isoamyl alcohol | 39,253 | 1.31 | 24,955 | 0.83 | *** | 30,521 | 1.02 | 38,709 | 1.29 | * |
| 3-methyl-1-pentanol | 56.90 | 0.00 | 38.14 | 0.00 | *** | 43.45 | 0.00 | 59.19 | 0.00 | * |
| 2.3-butanediol | 10.17 | 0.00 | 5.69 | 0.00 | *** | 3.24 | 0.00 | 8.87 | 0.00 | *** |
| 3-methylthiopropanol | 206.89 | 0.21 | 128.56 | 0.13 | *** | 165.73 | 0.17 | 185.72 | 0.19 | ns |
| Benzyl alcohol | 116.64 | 0.00 | 73.35 | 0.00 | *** | 88.68 | 0.00 | 101.77 | 0.00 | ns |
| 2-phenylethanol | 81,465 | 8.15 | 52,749 | 5.27 | *** | 64,286 | 6.43 | 84,215 | 8.42 | * |
| C13-norisoprenoids | 4.77 | 79.93 | 3.04 | 52.69 | *** | 3.40 | 57.87 | 3.99 | 67.46 | * |
| α-ionone | 0.56 | 6.20 | 0.40 | 4.45 | ** | 0.88 | 9.82 | 0.93 | 10.30 | ns |
| β-ionone | 1.18 | 13.12 | 0.51 | 5.69 | *** | 0.27 | 2.95 | 0.45 | 5.02 | *** |
| β-damascenone | 3.03 | 60.61 | 2.13 | 42.55 | * | 2.25 | 45.10 | 2.61 | 52.14 | ns |
| Terpenes | 104.80 | 4.59 | 68.61 | 2.93 | *** | 79.66 | 3.44 | 78.35 | 3.23 | ns |
| Terpinolene | 0.40 | 0.01 | 0.41 | 0.01 | ns | 0.37 | 0.01 | 0.76 | 0.02 | *** |
| α-terpineol | 5.66 | 0.01 | 4.69 | 0.00 | ns | 4.32 | 0.00 | 5.13 | 0.01 | * |
| E-geraniol | 50.66 | 2.53 | 31.62 | 1.58 | *** | 36.38 | 1.82 | 24.77 | 1.24 | *** |
| Z-geraniol (Nerol) | 37.74 | 1.89 | 23.75 | 1.19 | *** | 28.76 | 1.44 | 35.67 | 1.78 | * |
| β-citronellol | 2.45 | 0.02 | 0.54 | 0.01 | *** | 1.00 | 0.01 | 2.74 | 0.03 | *** |
| Linalool | 2.97 | 0.12 | 2.18 | 0.09 | * | 2.87 | 0.11 | 3.48 | 0.14 | ns |
| β-pinene | 0.43 | 0.00 | 0.29 | 0.00 | *** | 0.62 | 0.00 | 0.72 | 0.00 | * |
| 4-carvomenthenol | 4.01 | - | 3.65 | - | ns | 4.11 | - | 4.65 | - | ns |
| Citral | 0.47 | 0.02 | 1.50 | 0.05 | *** | 1.23 | 0.04 | 0.43 | 0.02 | *** |
| Ethyl esters and acetates | 72,851 | 222.56 | 46,723 | 156.36 | *** | 56,987 | 140.24 | 70,586 | 199.43 | * |
| Ethyl butyrate | 288.68 | 14.43 | 202.47 | 10.12 | ** | 226.66 | 11.33 | 313.29 | 15.66 | ** |
| Ethyl 2-methylbutyrate | 30.54 | 1.70 | 21.13 | 1.17 | ** | 23.46 | 1.30 | 31.84 | 1.77 | * |
| Ethyl decanoate | 37.94 | 0.19 | 26.71 | 0.13 | ** | 15.55 | 0.08 | 19.88 | 0.10 | * |
| Ethyl isovalerate | 55.85 | 18.62 | 39.20 | 13.07 | ** | 42.25 | 14.08 | 58.96 | 19.65 | * |
| Ethyl myristate | 15.41 | 0.01 | 10.05 | 0.01 | *** | 9.98 | 0.00 | 14.23 | 0.01 | * |
| Methyl salicylate | 3.73 | 37.27 | 2.44 | 24.40 | *** | 1.78 | 17.80 | 3.00 | 30.02 | ** |
| Hexyl acetate | 217.48 | 0.32 | 155.48 | 0.23 | ** | 175.58 | 0.26 | 209.90 | 0.31 | ns |
| Methyl vanillate | 8.92 | 0.00 | 5.73 | 0.00 | ** | 6.18 | 0.00 | 8.87 | 0.00 | * |
| Ethyl vanillate | 519.11 | 0.52 | 317.08 | 0.32 | *** | 366.46 | 0.37 | 471.60 | 0.48 | * |
| Ethyl hexanoate | 608.42 | 43.46 | 427.49 | 30.53 | ** | 445.56 | 31.83 | 625.85 | 44.70 | * |
| Ethyl lactate | 44,811 | 0.29 | 28,037 | 0.18 | *** | 35,029 | 0.23 | 42,193 | 0.27 | ns |
| Ethyl octanoate | 440.43 | 88.09 | 320.26 | 64.05 | ** | 248.02 | 49.60 | 339.48 | 67.90 | * |
| Diethyl succinate | 25,317 | 4.22 | 16,816 | 2.80 | ** | 20,027 | 3.34 | 25,771 | 4.30 | * |
| Isoamyl acetate | 390.36 | 13.01 | 271.61 | 9.05 | * | 290.71 | 9.69 | 414.52 | 13.82 | ** |
| 2-phenylethyl acetate | 106.26 | 0.43 | 70.63 | 0.28 | ** | 79.10 | 0.32 | 109.92 | 0.44 | ** |
| Fatty acids | 4869 | 9.85 | 3194 | 6.38 | *** | 3699 | 7.69 | 4767 | 9.47 | ** |
| Propanoic acid | 24.48 | 0.00 | 13.27 | 0.00 | *** | 19.35 | 0.00 | 21.93 | 0.00 | ns |
| Geranic acid | 21.36 | 0.53 | 10.63 | 0.27 | *** | 12.77 | 0.32 | 19.91 | 0.50 | ** |
| Nerolic acid | 11.04 | - | 9.94 | - | ns | 5.69 | - | 9.72 | - | *** |
| Pentanoic acid | 6.38 | 0.04 | 3.66 | 0.02 | *** | 4.93 | 0.03 | 5.20 | 0.03 | ns |
| Isobutyric acid | 77.12 | 0.00 | 49.89 | 0.00 | *** | 62.21 | 0.00 | 72.03 | 0.00 | ns |
| 2-methylbutyric acid | 135.53 | 0.05 | 86.38 | 0.03 | *** | 105.85 | 0.04 | 122.10 | 0.04 | ns |
| Hexanoic acid | 1647 | 0.55 | 1119 | 0.37 | ** | 1236 | 0.41 | 1705 | 0.57 | ** |
| Octanoic acid | 1489 | 1.49 | 1029 | 1.03 | ** | 1125 | 1.12 | 1601 | 1.60 | ** |
| Butyric acid | 158.36 | 0.07 | 98.39 | 0.04 | *** | 124.53 | 0.06 | 142.02 | 0.06 | ns |
| Acetic acid | 1065 | 0.05 | 623.10 | 0.03 | *** | 816.03 | 0.04 | 849.53 | 0.04 | ns |
| Isovaleric acid | 233.22 | 7.07 | 151.15 | 4.58 | *** | 186.93 | 5.66 | 218.48 | 6.62 | ns |
| γ-Lactones | 737.27 | 21.06 | 459.85 | 13.14 | *** | 602.54 | 17.22 | 705.95 | 20.17 | ns |
| γ-butyrolactone | 737.27 | 21.06 | 459.85 | 13.14 | *** | 602.54 | 17.22 | 705.95 | 20.17 | ns |
| Carbonyl compounds | 281.31 | 0.00 | 199.20 | 0.00 | *** | 241.26 | 0.00 | 450.25 | 0.00 | *** |
| Acetoin | 281.31 | 0.00 | 199.20 | 0.00 | *** | 241.26 | 0.00 | 450.25 | 0.00 | *** |
| Others | 29.78 | 0.01 | 19.59 | 0.01 | *** | 20.24 | 0.01 | 26.53 | 0.01 | *** |
| Benzaldehyde | 5.30 | 0.00 | 3.24 | 0.00 | *** | 3.03 | 0.00 | 2.54 | 0.00 | ns |
| Hexanal | 2.42 | 0.01 | 1.48 | 0.00 | ** | 1.30 | 0.00 | 2.39 | 0.01 | ** |
| Vanillyl acetone | 22.06 | - | 14.86 | - | *** | 15.91 | - | 21.60 | - | *** |
| Furanic compounds | 2932 | 0.18 | 792.55 | 0.10 | *** | 1803 | 0.11 | 1013 | 0.07 | *** |
| Furfural | 1191 | 0.08 | 403.50 | 0.06 | *** | 588.03 | 0.04 | 392.97 | 0.03 | * |
| 5-methylfurfural | 1142 | 0.06 | 258.30 | 0.02 | *** | 782.92 | 0.04 | 392.36 | 0.02 | *** |
| Furfuryl alcohol | 598.06 | 0.04 | 130.75 | 0.02 | *** | 432.14 | 0.03 | 227.81 | 0.02 | ** |
| Lactones | 812.41 | 9.60 | 639.47 | 9.58 | * | 584.45 | 6.36 | 811.11 | 11.55 | ** |
| cis-whiskey-lactone | 389.19 | 8.46 | 412.64 | 8.97 | ns | 251.25 | 5.46 | 491.38 | 10.68 | *** |
| trans-whiskey-lactone | 423.23 | 1.14 | 226.82 | 0.61 | *** | 333.21 | 0.90 | 319.73 | 0.86 | ns |
| Volatile phenols | 288.21 | 20.85 | 176.09 | 11.62 | *** | 243.63 | 16.24 | 262.84 | 16.11 | ns |
| 4-vinylphenol | 85.09 | 0.47 | 53.38 | 0.30 | ** | 73.76 | 0.41 | 92.00 | 0.51 | * |
| 4-vinylguaiacol | 71.90 | 1.80 | 52.88 | 1.32 | * | 70.99 | 1.77 | 74.96 | 1.87 | ns |
| Eugenol | 93.00 | 15.50 | 53.07 | 8.85 | *** | 69.85 | 11.64 | 71.11 | 11.85 | ns |
| Guaiacol | 29.16 | 3.07 | 10.89 | 1.15 | *** | 22.84 | 2.40 | 17.69 | 1.86 | ** |
| 4-ethylphenol | 9.06 | 0.01 | 5.87 | 0.01 | ** | 6.19 | 0.01 | 7.08 | 0.01 | ns |
| Phenolic aldehydes | 330.15 | 1.65 | 119.86 | 0.60 | *** | 216.48 | 1.08 | 154.87 | 0.77 | ** |
| Vanillin | 330.15 | 1.65 | 119.86 | 0.60 | *** | 216.48 | 1.08 | 154.87 | 0.77 | ** |
| LT × SG | LT × EG | MT × SG | MT × EG | MLT × SG | MLT × EG | Sig. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C6 Alcohols | 2325 | b | 1073 | a | 1199 | a | 1040 | a | 1316 | a | 1083 | a | *** |
| 1-hexanol | 2148 | b | 983.53 | a | 1117 | a | 969.35 | a | 1229 | a | 1087 | a | ** |
| Z-3-hexenol | 108.86 | b | 49.85 | a | 58.35 | a | 49.79 | a | 62.70 | a | 56.19 | a | *** |
| E-3-hexenol | 37.67 | b | 16.87 | a | 20.03 | a | 16.17 | a | 21.28 | a | 20.29 | a | *** |
| E-2-hexenol | 0.47 | b | 0.16 | a | 0.18 | a | 1.07 | c | 0.40 | b | 1.64 | d | *** |
| Z-2-hexenol | 29.63 | c | 22.65 | b | 3.29 | a | 3.90 | a | 3.26 | a | 3.24 | a | *** |
| Higher alcohols | 172,903 | b | 75,902 | a | 99,426 | a | 78,993 | a | 102,238 | a | 86,372 | a | *** |
| 1-propanol | 557.22 | c | 232.82 | a | 407.42 | b | 276.86 | a | 350.14 | ab | 266.30 | a | ** |
| 2-octanol | 504.34 | b | 471.88 | a | 464.83 | a | 464.80 | a | 476.28 | a | 469.96 | a | * |
| Isobutanol | 3485 | c | 1498 | a | 2524 | b | 1791.32 | a | 2090 | ab | 1709 | a | *** |
| 1-butanol | 166.18 | c | 71.97 | a | 117.01 | b | 82.81 | ab | 99.12 | ab | 80.20 | ab | ** |
| Isoamyl alcohol | 52,613 | b | 24,109 | a | 33,434 | a | 24,512 | a | 31,713 | a | 26,244 | a | *** |
| 3-methyl-1-pentanol | 82.77 | b | 36.51 | a | 41.69 | a | 36.78 | a | 46.24 | a | 41.12 | a | *** |
| 2.3-butanediol | 15.02 | d | 4.80 | a | 8.32 | c | 6.85 | a | 7.19 | b | 5.43 | a | *** |
| 3-methylthiopropanol | 274.70 | b | 129.99 | a | 185.24 | a | 122.52 | a | 160.72 | a | 133.18 | a | ** |
| Benzyl alcohol | 160.55 | b | 73.62 | a | 100.16 | a | 67.96 | a | 89.19 | a | 78.47 | a | *** |
| 2-phenylethanol | 115,045 | b | 49,273 | a | 62,143 | a | 51,631 | a | 67,207 | a | 57,344 | a | *** |
| C13-norisoprenoids | 7.64 | b | 3.24 | a | 3.38 | a | 2.65 | a | 3.30 | a | 3.23 | a | *** |
| α-ionone | 0.45 | bc | 0.31 | ab | 0.76 | d | 0.20 | ab | 0.47 | c | 0.69 | d | *** |
| β-ionone | 2.71 | b | 0.67 | a | 0.46 | a | 0.44 | a | 0.37 | a | 0.43 | a | *** |
| β-damascenone | 4.48 | b | 2.26 | a | 2.15 | a | 2.01 | a | 2.46 | a | 2.11 | a | ** |
| Terpenes | 138.00 | c | 64.20 | a | 94.68 | b | 64.70 | a | 81.71 | ab | 76.93 | ab | *** |
| Terpinolene | 0.00 | a | 0.79 | d | 0.77 | d | 0.24 | a | 0.43 | c | 0.19 | b | *** |
| α-terpineol | 8.18 | b | 5.07 | a | 4.52 | a | 4.16 | a | 4.29 | a | 4.84 | a | * |
| E-geraniol | 67.26 | b | 29.84 | a | 41.74 | a | 28.56 | a | 42.98 | a | 36.45 | a | *** |
| Z-geraniol (Nerol) | 47.86 | c | 22.66 | a | 36.54 | b | 23.57 | a | 28.83 | ab | 25.04 | a | *** |
| β-citronellol | 4.27 | e | 0.24 | a | 1.00 | c | 0.53 | a | 2.09 | d | 0.84 | bc | *** |
| Linalool | 4.36 | c | 1.58 | a | 2.76 | b | 2.83 | a | 1.77 | ab | 2.13 | ab | *** |
| β-pinene | 0.26 | b | 0.13 | a | 0.26 | b | 0.32 | a | 0.77 | d | 0.42 | c | *** |
| 4-carvomenthenol | 5.52 | d | 3.11 | b | 6.22 | d | 4.28 | a | 0.30 | a | 3.55 | bc | *** |
| Citral | 0.29 | a | 0.80 | b | 0.86 | b | 0.22 | a | 0.26 | a | 3.47 | c | *** |
| Ethyl esters and acetates | 102,561 | b | 45,474 | a | 58,409 | a | 45,525 | a | 57,582 | a | 49,170 | a | *** |
| Ethyl butyrate | 421.71 | b | 189.51 | a | 203.16 | a | 193.92 | a | 241.17 | a | 223.98 | a | ** |
| Ethyl 2-methylbutyrate | 47.29 | b | 20.40 | a | 20.63 | a | 20.11 | a | 23.71 | a | 22.86 | a | ** |
| Ethyl decanoate | 45.05 | c | 19.90 | a | 36.65 | bc | 34.17 | a | 32.12 | abc | 26.05 | ab | * |
| Ethyl isovalerate | 88.71 | b | 38.18 | a | 35.03 | a | 37.33 | a | 43.82 | a | 42.09 | a | *** |
| Ethyl myristate | 21.62 | c | 9.16 | a | 13.21 | b | 10.25 | ab | 11.39 | ab | 10.74 | ab | *** |
| Methyl salicylate | 6.99 | b | 1.85 | a | 2.20 | a | 2.98 | a | 1.99 | a | 2.49 | a | *** |
| Hexyl acetate | 378.80 | c | 175.56 | b | 99.05 | a | 161.23 | b | 174.59 | b | 129.65 | ab | *** |
| Methyl vanillate | 12.77 | b | 4.75 | a | 7.01 | a | 5.98 | a | 6.98 | a | 6.47 | a | *** |
| Ethyl vanillate | 773.02 | b | 288.66 | a | 350.85 | a | 312.66 | a | 433.46 | a | 349.92 | a | *** |
| Ethyl hexanoate | 929.03 | b | 425.74 | a | 422.31 | a | 412.58 | a | 473.92 | a | 444.15 | a | ** |
| Ethyl lactate | 59,906 | b | 27,091 | a | 39,904 | a | 27,613 | a | 34,622 | a | 29,407 | a | *** |
| Ethyl octanoate | 646.61 | b | 291.34 | a | 346.72 | a | 384.56 | a | 327.95 | a | 284.88 | a | *** |
| Diethyl succinate | 38,554 | b | 16,601 | a | 16,620 | a | 16,001 | a | 20,778 | a | 17,845 | a | *** |
| Isoamyl acetate | 572.02 | b | 250.67 | a | 273.58 | a | 267.06 | a | 325.49 | a | 297.09 | a | ** |
| 2-phenylethyl acetate | 157.41 | b | 66.45 | a | 74.82 | a | 68.52 | a | 86.54 | a | 76.92 | a | ** |
| Fatty acids | 6774 | b | 2938 | a | 3898 | a | 3157 | a | 3936 | a | 3487 | a | *** |
| Propanoic acid | 31.04 | d | 11.95 | a | 23.71 | c | 13.96 | a | 18.70 | bc | 13.90 | ab | *** |
| Geranic acid | 31.88 | c | 13.38 | b | 13.69 | b | 17.35 | b | 18.50 | b | 1.14 | a | *** |
| Nerolic acid | 17.98 | d | 5.72 | ab | 4.58 | a | 9.16 | c | 10.55 | c | 14.92 | d | *** |
| Pentanoic acid | 8.11 | c | 3.09 | a | 5.62 | b | 4.17 | a | 5.42 | b | 3.71 | ab | *** |
| Isobutyric acid | 95.23 | c | 47.69 | a | 74.18 | bc | 50.36 | ab | 61.95 | ab | 51.62 | ab | ** |
| 2-methylbutyric acid | 177.35 | c | 80.75 | a | 120.85 | b | 85.70 | a | 108.37 | ab | 92.68 | ab | *** |
| Hexanoic acid | 2339 | b | 1050 | a | 1261 | a | 1082 | a | 1342 | a | 1225 | a | *** |
| Octanoic acid | 2218 | b | 948.38 | a | 1011 | a | 999.10 | a | 1238 | a | 1138 | a | ** |
| Butyric acid | 201.37 | c | 90.15 | a | 152.46 | b | 98.88 | a | 121.24 | ab | 106.14 | a | ** |
| Acetic acid | 1343 | d | 543.59 | a | 1025 | c | 648.01 | a | 827.58 | bc | 677.70 | ab | *** |
| Isovaleric acid | 311.17 | b | 142.47 | a | 205.70 | a | 148.76 | a | 182.79 | a | 162.22 | a | *** |
| γ-Lactones | 987.77 | c | 432.64 | a | 648.40 | b | 450.29 | ab | 575.64 | ab | 496.62 | ab | *** |
| γ-butyrolactone | 987.77 | c | 432.64 | a | 648.40 | b | 450.29 | ab | 575.64 | ab | 496.62 | ab | *** |
| Carbonyl compounds | 340.59 | c | 185.46 | a | 239.04 | ab | 194.18 | a | 264.31 | b | 217.95 | ab | ** |
| Acetoin | 340.59 | c | 185.46 | a | 239.04 | ab | 194.18 | a | 264.31 | b | 217.95 | ab | ** |
| Others | 46.56 | b | 19.63 | a | 20.70 | a | 18.79 | a | 22.07 | a | 20.34 | a | *** |
| Benzaldehyde | 7.45 | d | 3.95 | bc | 3.74 | abc | 3.14 | a | 4.70 | c | 2.65 | a | *** |
| Hexanal | 4.42 | c | 2.15 | b | 1.78 | ab | 1.25 | a | 1.06 | a | 1.03 | a | *** |
| Vanillyl acetone | 34.69 | b | 13.53 | a | 15.18 | a | 14.39 | a | 16.32 | a | 16.67 | a | *** |
| Furanic compounds | 3678 | c | 767.84 | a | 2745 | bc | 784.88 | a | 2372 | b | 824.92 | a | *** |
| Furfural | 1413 | c | 402.69 | a | 1279 | c | 358.37 | a | 881.88 | b | 449.43 | a | *** |
| 5-methylfurfural | 1539 | c | 235.81 | a | 875.82 | ab | 261.36 | a | 1012 | bc | 277.74 | a | ** |
| Furfuryl alcohol | 726.25 | c | 129.34 | a | 589.43 | bc | 165.16 | a | 478.50 | b | 97.75 | a | *** |
| Lactones | 1124 | c | 577.23 | b | 470.71 | a | 647.77 | ab | 842.38 | b | 693.39 | b | ** |
| cis-whiskey-lactone | 648.37 | c | 350.05 | b | 170.75 | a | 470.25 | b | 348.44 | b | 417.62 | b | *** |
| trans-whiskey-lactone | 475.78 | b | 227.18 | a | 299.96 | a | 177.52 | a | 493.94 | b | 275.77 | a | *** |
| Volatile phenols | 402.40 | b | 178.17 | a | 232.24 | a | 174.10 | a | 229.98 | a | 176.00 | a | *** |
| 4-vinylphenol | 107.50 | c | 53.36 | a | 86.09 | bc | 53.84 | a | 61.67 | ab | 52.95 | a | ** |
| 4-vinylguaiacol | 97.21 | b | 56.37 | a | 67.54 | a | 53.41 | a | 50.95 | a | 48.87 | a | * |
| Eugenol | 141.46 | c | 48.55 | a | 44.95 | a | 49.42 | a | 92.59 | b | 61.23 | a | *** |
| Guaiacol | 39.37 | c | 11.26 | a | 25.42 | b | 10.24 | a | 22.69 | b | 11.16 | a | *** |
| 4-ethylphenol | 16.85 | c | 8.64 | b | 8.24 | b | 7.19 | a | 2.09 | a | 1.79 | a | *** |
| Phenolic aldehydes | 459.12 | c | 95.71 | a | 272.76 | b | 121.89 | a | 258.58 | b | 141.98 | a | *** |
| Vanillin | 459.12 | c | 95.71 | a | 272.76 | b | 121.89 | a | 258.58 | b | 141.98 | a | *** |
| LT × SG | LT × EG | MT × SG | MT × EG | MLT × SG | MLT × EG | Sig. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C6 Alcohols | 1395 | a | 1433 | a | 1055 | a | 1116 | a | 1293 | a | 2691 | b | *** |
| 1-hexanol | 1302 | a | 1338 | a | 984.71 | a | 1041 | a | 1201 | a | 2506 | b | *** |
| Z-3-hexenol | 66.72 | a | 68.51 | a | 50.32 | a | 53.51 | a | 64.83 | a | 128.32 | b | *** |
| E-3-hexenol | 22.79 | a | 23.06 | a | 16.40 | a | 17.96 | a | 21.58 | a | 45.88 | b | *** |
| E-2-hexenol | 0.85 | a | 1.95 | b | 0.37 | a | 0.70 | a | 0.76 | a | 5.27 | c | *** |
| Z-2-hexenol | 2.93 | b | 1.57 | a | 2.89 | b | 3.14 | b | 4.96 | c | 5.91 | c | *** |
| Higher alcohols | 104,843 | a | 101,534 | a | 78,751 | a | 81,091 | a | 111,045 | a | 198,080 | b | *** |
| 1-propanol | 396.44 | ab | 304.58 | a | 258.35 | a | 267.24 | a | 510.22 | b | 704.16 | c | *** |
| 2-octanol | 485.83 | a | 492.31 | a | 465.56 | a | 471.29 | a | 470.93 | a | 525.45 | b | * |
| Isobutanol | 2499 | a | 1882 | a | 1599 | a | 1621 | a | 2302 | a | 4226 | b | *** |
| 1-butanol | 119.68 | bc | 88.34 | ab | 77.82 | a | 81.31 | ab | 129.43 | c | 204.28 | d | *** |
| Isoamyl alcohol | 34,429 | a | 28,306 | a | 24,177 | a | 24,578 | a | 32,957 | a | 63,243 | b | *** |
| 3-methyl-1-pentanol | 49.00 | a | 49.66 | a | 35.54 | a | 38.94 | a | 45.81 | a | 88.97 | b | ** |
| 2.3-butanediol | 2.39 | a | 9.13 | c | 0.83 | a | 4.85 | b | 6.49 | b | 12.61 | d | *** |
| 3-methylthiopropanol | 176.22 | bc | 136.68 | ab | 116.91 | a | 110.23 | a | 204.07 | c | 310.25 | d | *** |
| Benzyl alcohol | 94.35 | ab | 76.35 | ab | 68.92 | a | 70.24 | a | 102.78 | b | 158.71 | c | *** |
| 2-phenylethanol | 66,591 | a | 70,189 | a | 51,952 | a | 53,848 | a | 74,316 | a | 128,607 | b | *** |
| C13-norisoprenoids | 2.85 | a | 3.54 | a | 2.66 | a | 3.50 | a | 4.70 | b | 4.92 | b | *** |
| α-ionone | 0.59 | a | 0.91 | b | 0.73 | ab | 0.64 | a | 1.33 | c | 1.23 | c | *** |
| β-ionone | 0.32 | c | 0.40 | d | 0.18 | a | 0.72 | e | 0.30 | bc | 0.23 | ab | *** |
| β-damascenone | 1.94 | a | 2.23 | a | 1.75 | a | 2.14 | a | 3.08 | b | 3.46 | b | ** |
| Terpenes | 87.24 | b | 74.03 | ab | 67.78 | a | 73.52 | ab | 83.96 | ab | 87.49 | b | ns |
| Terpinolene | 0.54 | c | 1.13 | e | 0.12 | a | 0.67 | d | 0.45 | b | 0.47 | b | *** |
| α-terpineol | 4.99 | c | 4.39 | abc | 3.38 | a | 3.79 | ab | 4.58 | bc | 7.22 | d | *** |
| E-geraniol | 41.48 | b | 35.95 | b | 32.60 | b | 33.71 | b | 35.08 | b | 4.64 | a | *** |
| Z-geraniol (Nerol) | 30.42 | a | 23.93 | a | 24.59 | a | 26.65 | a | 31.27 | a | 56.42 | b | *** |
| β-citronellol | 0.34 | a | 2.22 | d | 1.59 | c | 1.96 | cd | 1.06 | b | 4.04 | e | *** |
| Linalool | 2.88 | a | 2.09 | a | 2.15 | a | 2.47 | a | 3.57 | a | 5.88 | b | *** |
| β-pinene | 1.08 | d | 0.53 | c | 0.47 | bc | 0.36 | ab | 0.30 | a | 1.26 | e | *** |
| 4-carvomenthenol | 3.56 | a | 3.53 | a | 2.54 | a | 3.59 | a | 6.23 | b | 6.84 | b | *** |
| Citral | 1.93 | d | 0.24 | a | 0.34 | a | 0.33 | a | 1.42 | c | 0.72 | b | *** |
| Ethyl esters and acetates | 62,428 | a | 54,064 | a | 45,421 | a | 46,342 | a | 63,111 | a | 111,351 | b | *** |
| Ethyl butyrate | 245.52 | a | 261.42 | a | 202.25 | a | 205.63 | a | 232.22 | a | 472.81 | b | *** |
| Ethyl 2-methylbutyrate | 25.95 | a | 26.64 | a | 19.53 | a | 19.85 | a | 24.90 | a | 49.04 | b | *** |
| Ethyl decanoate | 13.92 | ab | 12.69 | a | 11.88 | a | 9.73 | a | 20.85 | b | 37.23 | c | *** |
| Ethyl isovalerate | 47.14 | a | 49.86 | a | 36.33 | a | 37.24 | a | 43.30 | a | 89.77 | b | ** |
| Ethyl myristate | 9.66 | a | 8.75 | a | 9.58 | a | 10.28 | a | 10.71 | a | 23.67 | b | *** |
| Methyl salicylate | 1.07 | a | 0.48 | a | 1.65 | ab | 2.92 | b | 2.62 | b | 5.61 | c | *** |
| Hexyl acetate | 171.46 | b | 205.62 | b | 151.41 | b | 35.80 | a | 203.89 | b | 388.27 | c | *** |
| Methyl vanillate | 7.12 | a | 7.23 | a | 5.21 | a | 5.85 | a | 6.20 | a | 13.52 | b | ** |
| Ethyl vanillate | 396.37 | a | 405.50 | a | 329.98 | a | 318.08 | a | 373.02 | a | 691.21 | b | *** |
| Ethyl hexanoate | 504.43 | a | 506.35 | a | 371.95 | a | 375.38 | a | 460.31 | a | 995.83 | b | *** |
| Ethyl lactate | 40,519 | a | 30,832 | a | 26,989 | a | 27,348 | a | 37,578 | a | 68,399 | b | *** |
| Ethyl octanoate | 275.23 | a | 266.31 | a | 188.54 | a | 128.16 | a | 280.30 | a | 623.97 | b | *** |
| Diethyl succinate | 19,821 | a | 21,061 | a | 16,777 | a | 17,496 | a | 23,482 | a | 38,757 | b | *** |
| Isoamyl acetate | 309.61 | a | 333.60 | a | 254.53 | a | 275.33 | a | 308.00 | a | 634.63 | b | ** |
| 2-phenylethyl acetate | 81.04 | a | 86.40 | a | 72.00 | a | 74.49 | a | 84.27 | a | 168.88 | b | *** |
| Fatty acids | 3960 | a | 3865 | a | 3163 | a | 3066 | a | 3973 | a | 7370 | b | *** |
| Propanoic acid | 21.33 | b | 21.13 | b | 14.10 | ab | 9.57 | a | 22.63 | b | 35.09 | c | *** |
| Geranic acid | 5.65 | a | 2.15 | a | 16.47 | b | 17.31 | b | 16.18 | b | 40.27 | c | *** |
| Nerolic acid | 5.44 | a | 10.28 | b | 6.67 | a | 8.25 | ab | 4.95 | a | 10.62 | b | ** |
| Pentanoic acid | 4.41 | a | 4.25 | a | 3.50 | a | 3.18 | a | 6.89 | b | 8.16 | b | *** |
| Isobutyric acid | 66.95 | ab | 51.99 | ab | 46.56 | ab | 41.80 | a | 73.12 | b | 122.29 | c | *** |
| 2-methylbutyric acid | 114.24 | ab | 88.93 | ab | 81.97 | a | 82.89 | a | 121.33 | b | 194.49 | c | *** |
| Hexanoic acid | 1381 | a | 1427 | a | 1085 | a | 1139 | a | 1240 | a | 2549 | b | *** |
| Octanoic acid | 1195 | a | 1325 | a | 1052 | a | 1076 | a | 1127 | a | 2401 | b | *** |
| Butyric acid | 142.52 | a | 104.05 | a | 95.44 | a | 94.83 | a | 135.63 | a | 227.20 | b | *** |
| Acetic acid | 817.44 | bc | 668.00 | ab | 615.90 | ab | 448.45 | a | 1014 | c | 1432 | d | *** |
| Isovaleric acid | 205.44 | a | 161.31 | a | 145.03 | a | 145.13 | a | 210.32 | a | 348.98 | b | *** |
| γ-Lactones | 631.75 | ab | 504.38 | ab | 446.87 | a | 450.12 | a | 728.99 | b | 1163 | c | *** |
| γ-butyrolactone | 631.75 | ab | 504.38 | ab | 446.87 | a | 450.12 | a | 728.99 | b | 1163 | c | *** |
| Carbonyl compounds | 307.54 | b | 213.80 | ab | 158.74 | a | 458.91 | c | 257.50 | ab | 678.02 | d | *** |
| Acetoin | 307.54 | b | 213.80 | ab | 158.74 | a | 458.91 | c | 257.50 | ab | 678.02 | d | *** |
| Others | 21.63 | ab | 23.05 | b | 19.54 | ab | 17.19 | a | 19.55 | ab | 39.35 | c | *** |
| Benzaldehyde | 2.96 | bc | 2.02 | ab | 2.36 | ab | 1.78 | a | 3.77 | c | 3.84 | c | *** |
| Hexanal | 1.12 | a | 1.87 | a | 1.63 | a | 1.12 | a | 1.13 | a | 4.17 | b | *** |
| Vanillyl acetone | 17.54 | ab | 19.17 | b | 15.54 | ab | 14.29 | a | 14.65 | ab | 31.34 | c | *** |
| Furanic compounds | 1691 | bc | 1035 | b | 1761 | c | 324.13 | a | 1957 | c | 1681 | bc | ** |
| Furfural | 447.50 | bc | 403.37 | b | 688.60 | bc | 0.00 | a | 628.00 | bc | 775.54 | c | ** |
| 5-methylfurfural | 751.47 | c | 382.91 | ab | 816.02 | c | 273.93 | a | 781.25 | c | 520.23 | b | *** |
| Furfuryl alcohol | 492.22 | c | 248.21 | b | 256.54 | b | 50.20 | a | 547.65 | c | 385.01 | bc | ** |
| Lactones | 503.59 | a | 700.12 | ab | 487.42 | a | 533.99 | ab | 762.36 | b | 1199 | c | *** |
| cis-whiskey-lactone | 303.45 | ab | 389.90 | b | 164.70 | a | 383.30 | b | 285.59 | ab | 700.95 | c | *** |
| trans-whiskey-lactone | 200.14 | a | 310.22 | b | 322.72 | b | 150.69 | a | 476.77 | c | 498.28 | c | *** |
| Volatile phenols | 220.55 | bc | 267.49 | cd | 188.13 | ab | 143.47 | a | 322.21 | de | 377.56 | e | *** |
| 4-vinylphenol | 63.28 | ab | 90.46 | bc | 55.19 | a | 49.90 | a | 102.80 | c | 135.65 | d | *** |
| 4-vinylguaiacol | 62.50 | bc | 83.88 | cd | 50.06 | ab | 32.20 | a | 100.42 | d | 108.80 | d | *** |
| Eugenol | 64.85 | ab | 65.96 | ab | 56.74 | a | 42.97 | a | 87.96 | bc | 104.39 | c | ** |
| Guaiacol | 21.97 | bc | 17.46 | b | 18.39 | b | 10.64 | a | 28.17 | c | 24.97 | c | *** |
| 4-ethylphenol | 7.95 | b | 9.73 | c | 7.75 | b | 7.76 | b | 2.86 | a | 3.75 | a | *** |
| Phenolic aldehydes | 244.40 | cd | 156.58 | b | 196.97 | bc | 31.73 | a | 208.08 | bc | 276.30 | d | *** |
| Vanillin | 244.40 | cd | 156.58 | b | 196.97 | bc | 31.73 | a | 208.08 | bc | 276.30 | d | *** |
| Number of Barrels | Toasting Level 1 | Grain Size 1 | Toasting Level × Grain Size |
|---|---|---|---|
| 6 | LT | SG | LT × SG |
| 6 | LT | EG | LT × EG |
| 6 | MT | SG | MT × SG |
| 6 | MT | EG | MT × EG |
| 6 | MLT | SG | MLT × SG |
| 6 | MLT | EG | MLT × EG |
| Volatile Families and Compounds | Odour Threshold (µg/L) | Descriptor | Ref. |
|---|---|---|---|
| C6 Alcohols (Non-oak) | |||
| 1-hexanol | 8000 | Green, grass | [46] |
| Z-3-hexenol | 400 | Green, grass, bitter | [46] |
| E-3-hexenol | 400 | Green, floral | [47] |
| E-2-hexenol | 400 | Green grass, herb | [48] |
| Z-2-hexenol | 400 | Green grass, herb | [48] |
| Higher alcohols (Non-oak) | |||
| 1-propanol | 306,000 | Fresh, alcohol | [49] |
| 2-octanol | 120 | - | [50] |
| Isobutanol | 40,000 | Alcohol, solvent, bitter | [46] |
| 1-butanol | 150,000 | Medicinal, phenolic | [51] |
| Isoamyl alcohol | 30,000 | Whiskey, solvent, sweet | [46] |
| 3-methyl-1-pentanol | 50,000 | Herbaceous, cocoa | [51] |
| 2,3-butanediol | 150,000 | Fruity | [51] |
| 3-methylthiopropanol | 1000 | Cabbage, cooked potato | [46] |
| Benzyl alcohol | 200,000 | Sweet, fruity | [51] |
| 2-phenylethanol | 10,000 | Floral, roses, perfume | [50] |
| C13-norisoprenoids (Non-oak) | |||
| α-ionone | 0.09 | Raspberry, violet, sweet fruity | [48] |
| β-ionone | 0.09 | Raspberry, violet, sweet fruity | [48] |
| β-damascenone | 0.05 | Bark, canned peach, baked apple, dry plum | [48] |
| Terpenes (Non-oak) | |||
| Terpinolene | 41 | Pine, citrus, earthy | [52] |
| α-terpineol | 1000 | Lilac, floral, sweet | [49] |
| E-geraniol | 20 | Floral, geranium, rose | [53] |
| Z-geraniol (Nerol) | 20 | Floral, rose, lime | [53] |
| β-citronellol | 100 | Green lemon | [50] |
| Linalool | 25.2 | Fruity, citric | [54] |
| β-pinene | 1500 | Pine, hay, green | [52] |
| 4-carvomenthenol | - | Nutmeg | - |
| Citral | 28 | Lemon | - |
| Ethyl esters and acetates (Non-oak) | |||
| Ethyl butyrate | 20 | Papaya, apple | [55] |
| Ethyl 2-methylbutyrate | 18 | Fruity, strawberry, anise | [26] |
| Ethyl decanoate | 200 | Fruity, fatty, pleasant | [50] |
| Ethyl isovalerate | 3 | Banana, sweet fruity | [48] |
| Ethyl myristate | 2000 | Sweet fruity, butter, fatty odour | [48] |
| Methyl salicylate | 0.1 | Peppermint | [53] |
| Hexyl acetate | 670 | Fruity, herbs, apple | [53] |
| Methyl vanillate | 3000 | Sweet, vanilla-like | [26] |
| Ethyl vanillate | 990 | Sweet, vanilla-like | [26] |
| Ethyl hexanoate | 14 | Apple, fruity, sweetish | [51] |
| Ethyl lactate | 154,000 | Strawberry, raspberry | [51] |
| Ethyl octanoate | 5 | Apple, fruity, sweetish | [51] |
| Diethyl succinate | 6000 | Light fruity, wine | [51] |
| Isoamyl acetate | 30 | Banana | [51] |
| 2-phenylethyl acetate | 250 | Floral | [51] |
| Fatty acids (Non-oak) | |||
| Propanoic acid | 8100 | Vinegarish | [54] |
| Geranic acid | 40 | Green | [56] |
| Nerolic acid | - | Honey, floral | - |
| Pentanoic acid | 160 | Black wallnut | - |
| Isobutyric acid | 200,000 | Fatty | [50] |
| 2-methylbutyric acid | 3000 | Buttery, cheesy | [50] |
| Hexanoic acid | 3000 | Cheese, fatty | [57] |
| Octanoic acid | 1000 | Cheese, fatty, rancid | [51] |
| Butyric acid | 2200 | Rancid, cheese, sweat | [58] |
| Acetic acid | 20,000 | Vinegar | [51] |
| Isovaleric acid | 33 | Acid, rancid | [46] |
| γ-Lactones (Non-oak) | |||
| γ-butyrolactone | 35 | Caramel, sweet, fruity | [58] |
| Carbonyl compounds (Non-oak) | |||
| Acetoin | 150,000 | Buttery, cream | [59] |
| Others (Non-oak) | |||
| Benzaldehyde | 2000 | Bitter almond | [49] |
| Hexanal | 350 | Fatty, herbaceous, and green | [59] |
| Vanillylacetone | - | Ginger | - |
| Furanic compounds (oak) | |||
| Furfural | 14,100 | Burned almonds, incense | [46,50] |
| 5-methylfurfural | 20,000 | Bitter almond, spice | [46] |
| Furfuryl alcohol | 15,000 | Hay | [46] |
| Lactones (oak) | |||
| cis-whiskey-lactone | 46 | Woody, coconut, vanilla | [50] |
| trans-whiskey-lactone | 370 | Woody, coconut, vanilla | [50] |
| Volatile phenols (oak) | |||
| 4-vinylphenol | 180 | Medicine, phenolic, paint | [26] |
| 4-vinylguaiacol | 40 | Spices, clove, curry | [26] |
| Eugenol | 6 | Clove, honey, spicy | [50] |
| Guaiacol | 9.5 | Smoke, toasted, spicy | [51] |
| 4-ethylphenol | 620 | Leather, animal | [51] |
| Phenolic aldehydes (oak) | |||
| Vanillin | 200 | Vanilla | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross-Magahy, M.L.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z. Exploring the Influence of Toasting Levels, Grain Sizes, and Their Combination on the Volatile Profile of Tempranillo Red Wines Aged in Quercus petraea Barrels. Molecules 2025, 30, 1293. https://doi.org/10.3390/molecules30061293
Ross-Magahy ML, Martínez-Lapuente L, Ayestarán B, Guadalupe Z. Exploring the Influence of Toasting Levels, Grain Sizes, and Their Combination on the Volatile Profile of Tempranillo Red Wines Aged in Quercus petraea Barrels. Molecules. 2025; 30(6):1293. https://doi.org/10.3390/molecules30061293
Chicago/Turabian StyleRoss-Magahy, Mikel Landín, Leticia Martínez-Lapuente, Belén Ayestarán, and Zenaida Guadalupe. 2025. "Exploring the Influence of Toasting Levels, Grain Sizes, and Their Combination on the Volatile Profile of Tempranillo Red Wines Aged in Quercus petraea Barrels" Molecules 30, no. 6: 1293. https://doi.org/10.3390/molecules30061293
APA StyleRoss-Magahy, M. L., Martínez-Lapuente, L., Ayestarán, B., & Guadalupe, Z. (2025). Exploring the Influence of Toasting Levels, Grain Sizes, and Their Combination on the Volatile Profile of Tempranillo Red Wines Aged in Quercus petraea Barrels. Molecules, 30(6), 1293. https://doi.org/10.3390/molecules30061293








