Chitosan and Its Derivatives as Nanocarriers for Drug Delivery
Abstract
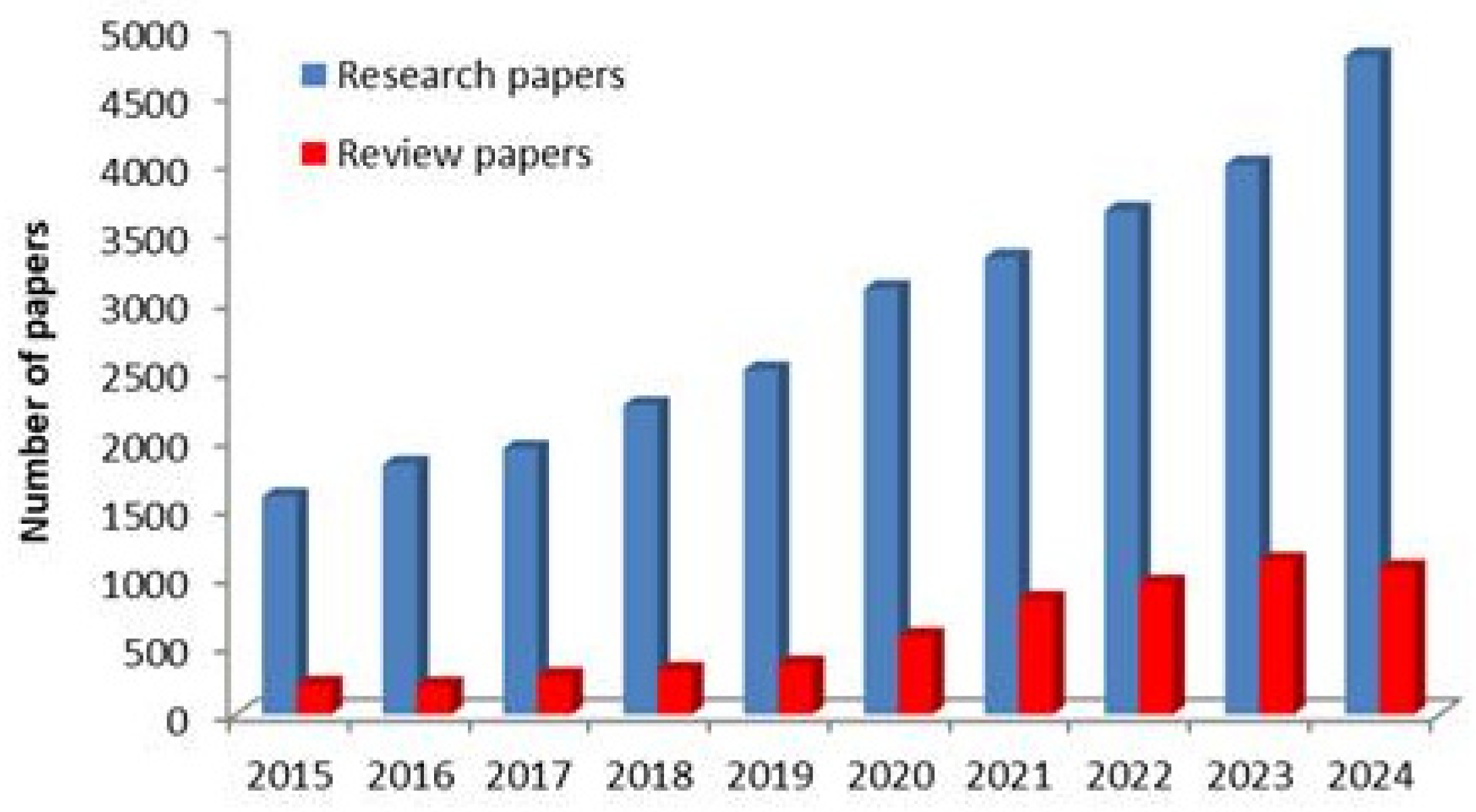
1. Introduction
2. Generalities
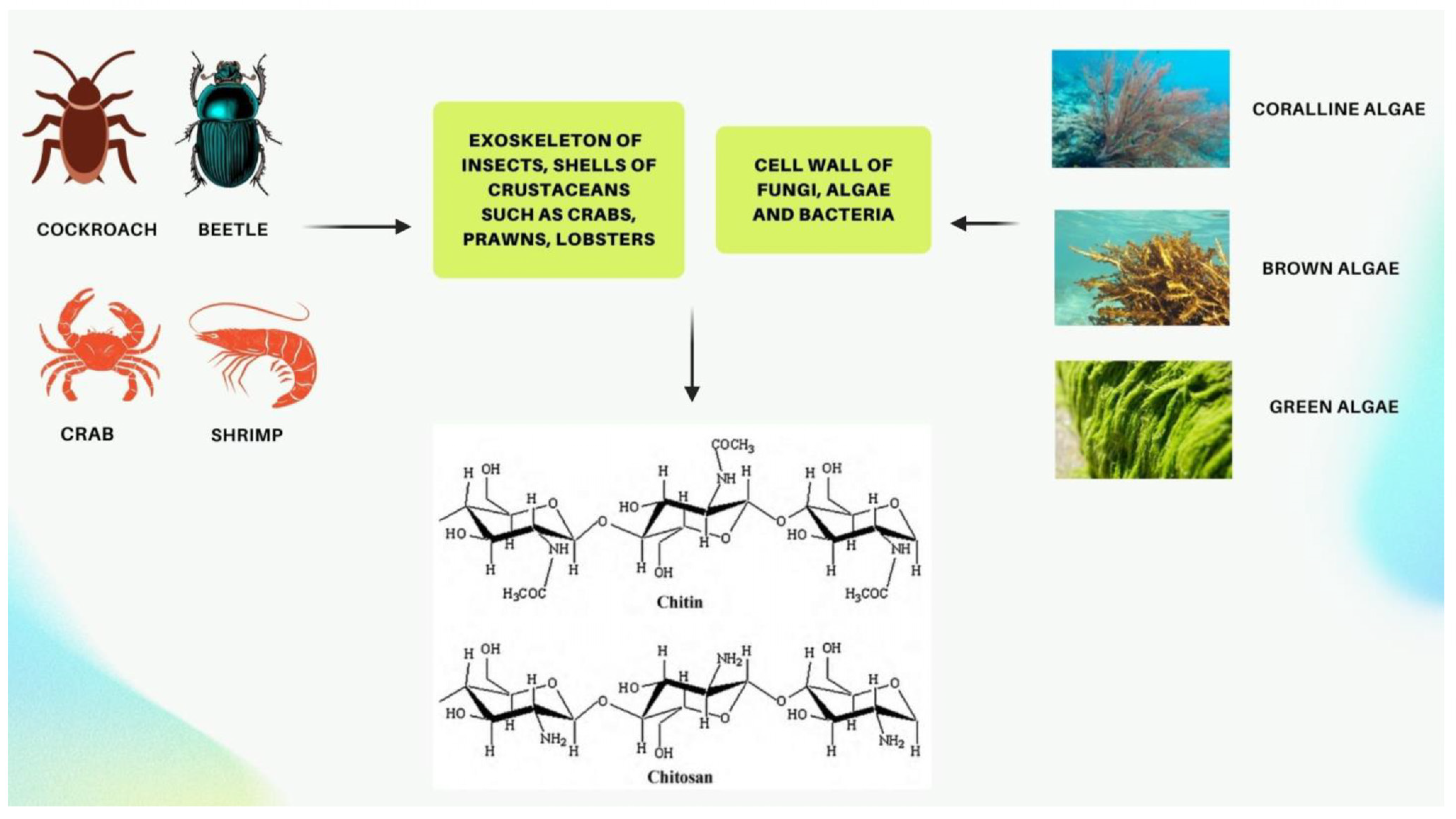
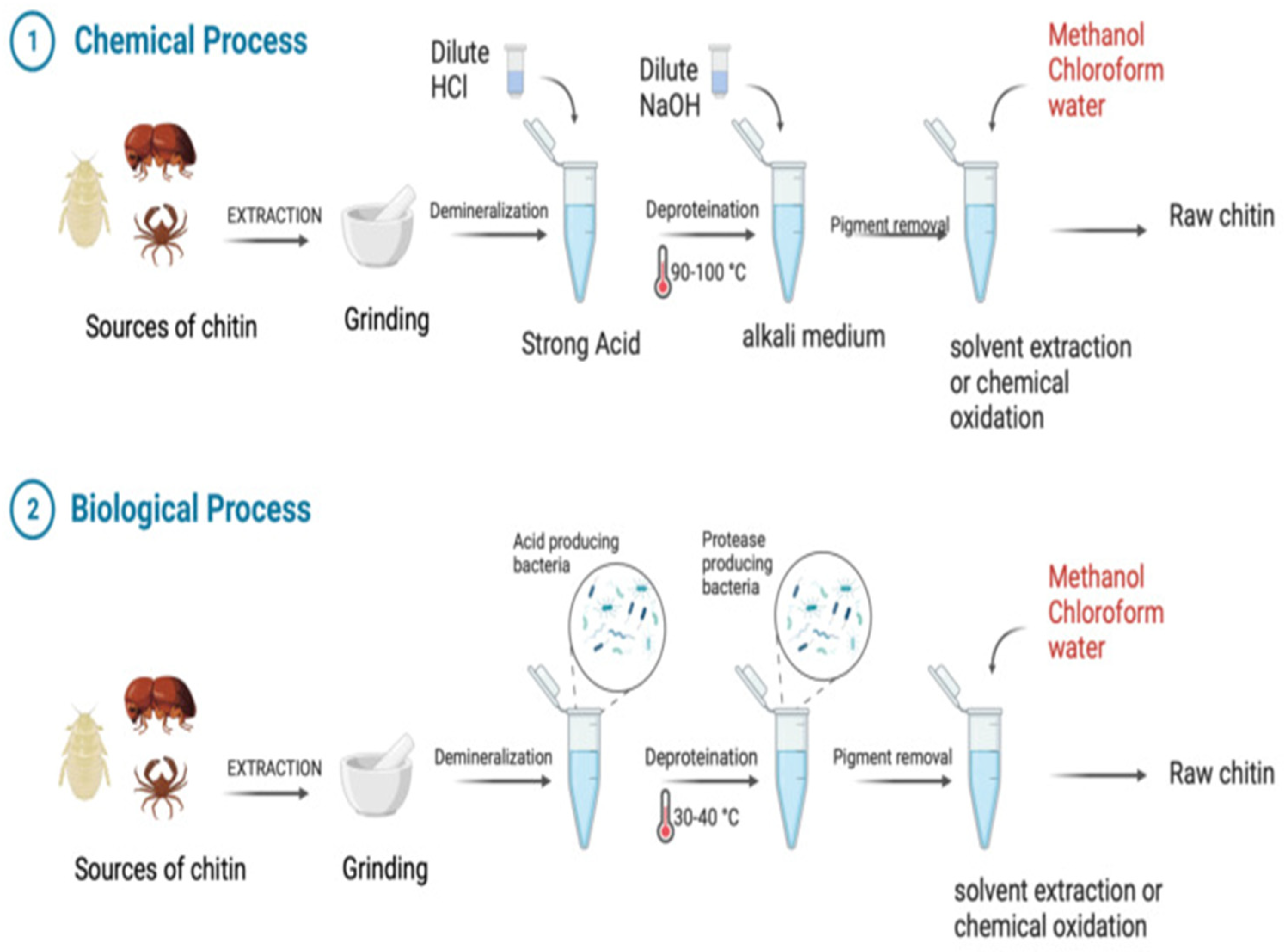
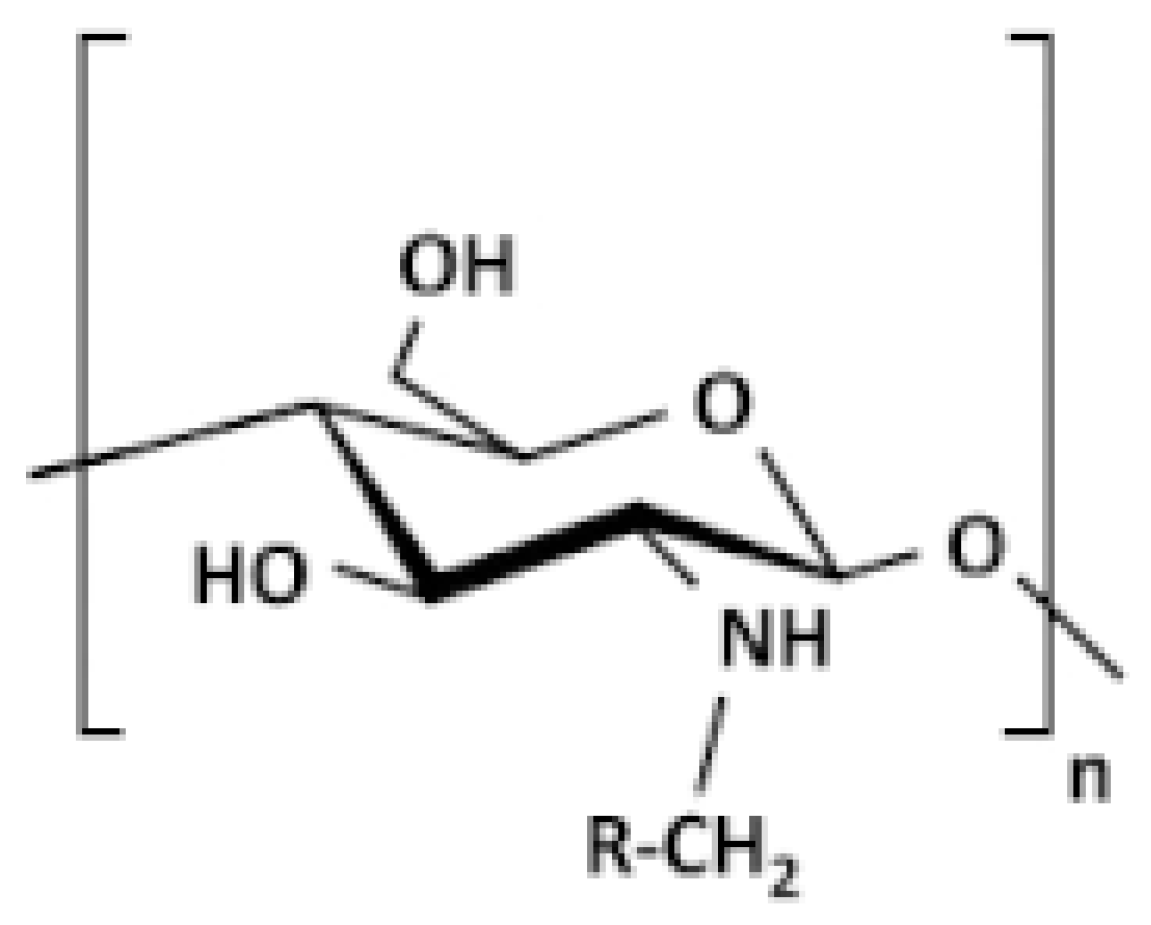
2.1. Sources and Structure of CS
2.2. Purity of Chitosan
2.3. Basic Characteristics of CS
2.3.1. Different Sizes and Types of Chitosan
2.3.2. Aqueous Solubility
2.3.3. Mucoadhesion
2.3.4. Controlled Release
2.3.5. Enhancement of Intestinal Permeability
2.3.6. Biodegradability and Safety
2.4. Physicochemical Properties and Preparation of CS
2.5. Drug Delivery Properties of CS
2.5.1. Mucoadhesive Properties
2.5.2. Drug Delivery Properties
2.5.3. Gelling Properties
2.5.4. Permeation Enhancing Properties
2.5.5. Gene Expression Properties
2.6. Enhancing CS’s Properties Through Chemical Modifications
2.6.1. Cationic and Anionic Substituents in Ionic CS Derivatives
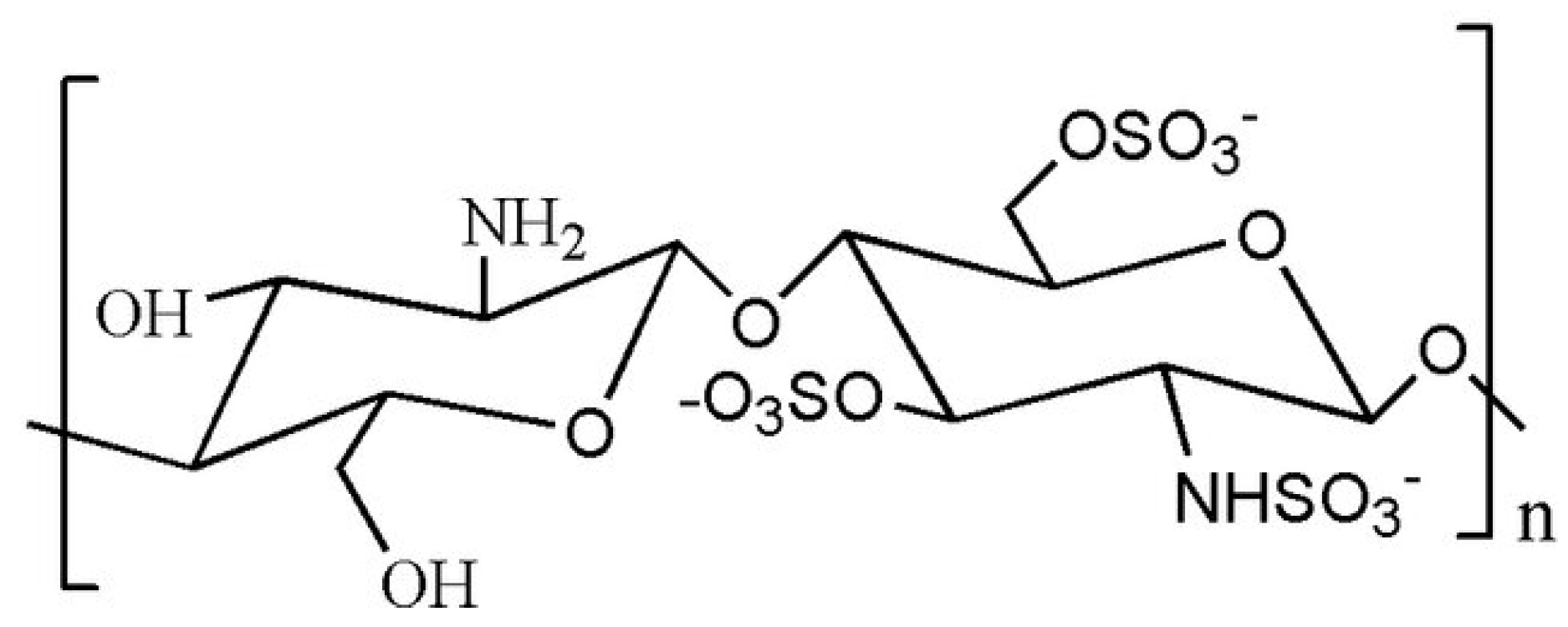
Sulfated CS Derivatives
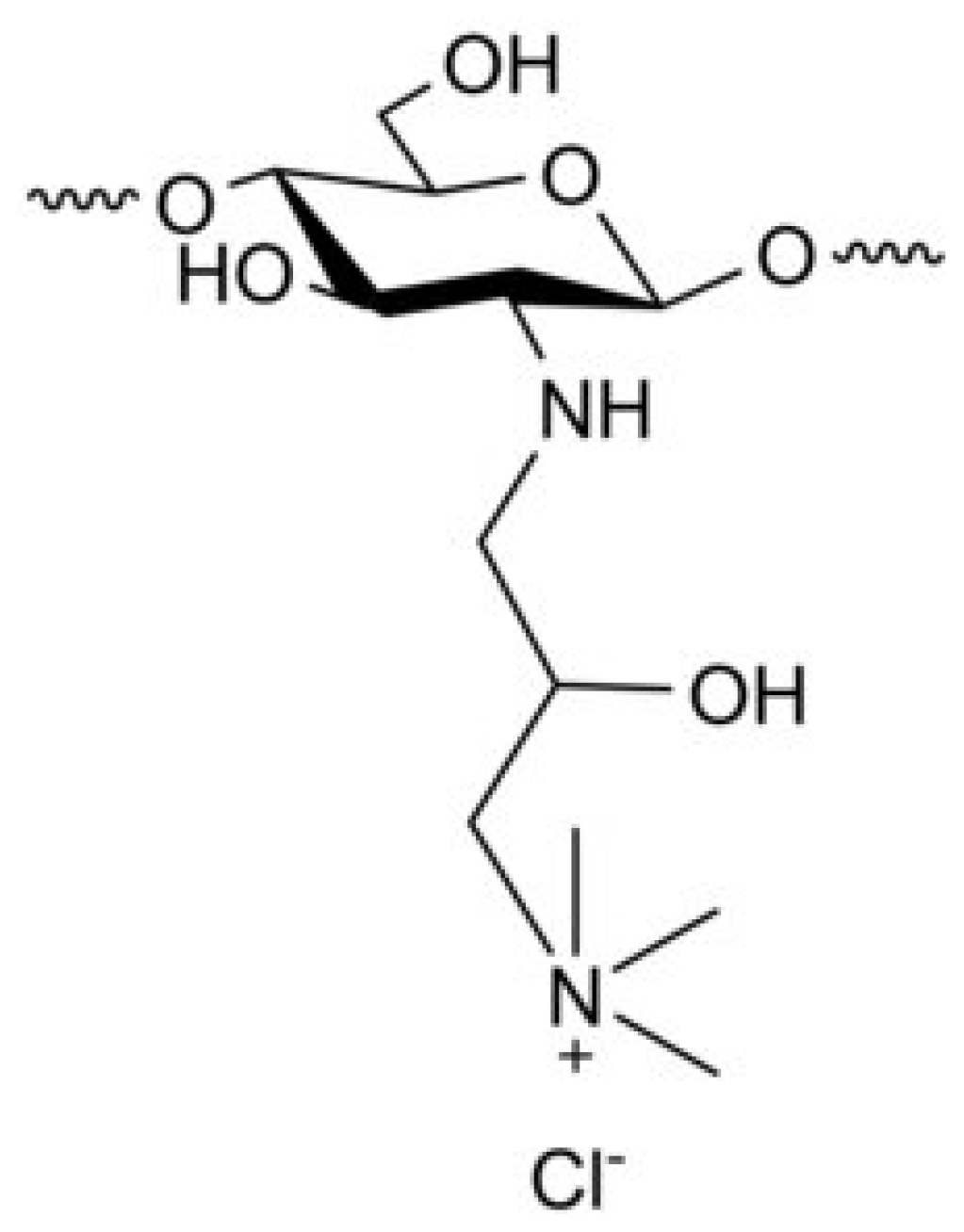
Derivatives of CS Modified with Quaternary Ammonium
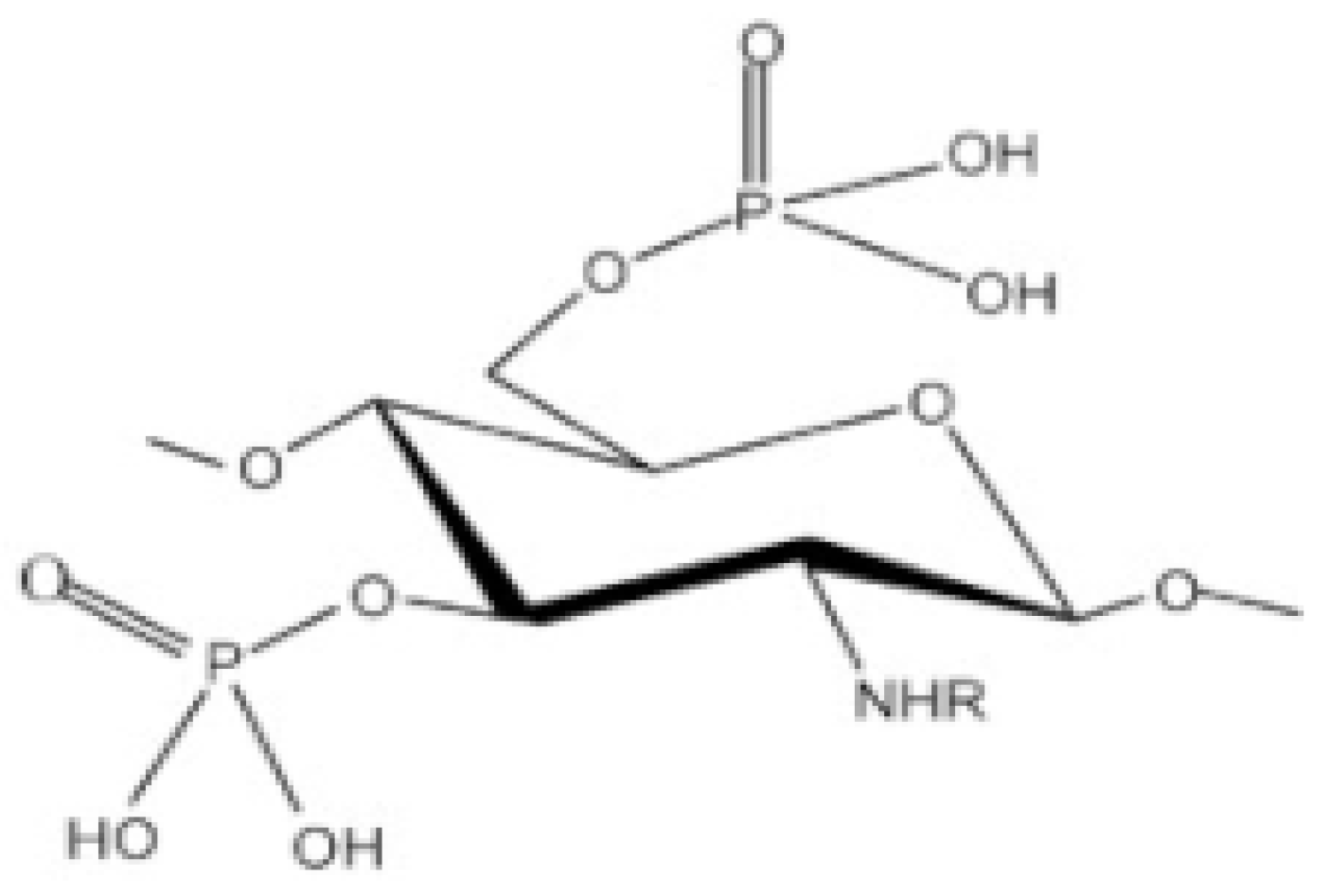
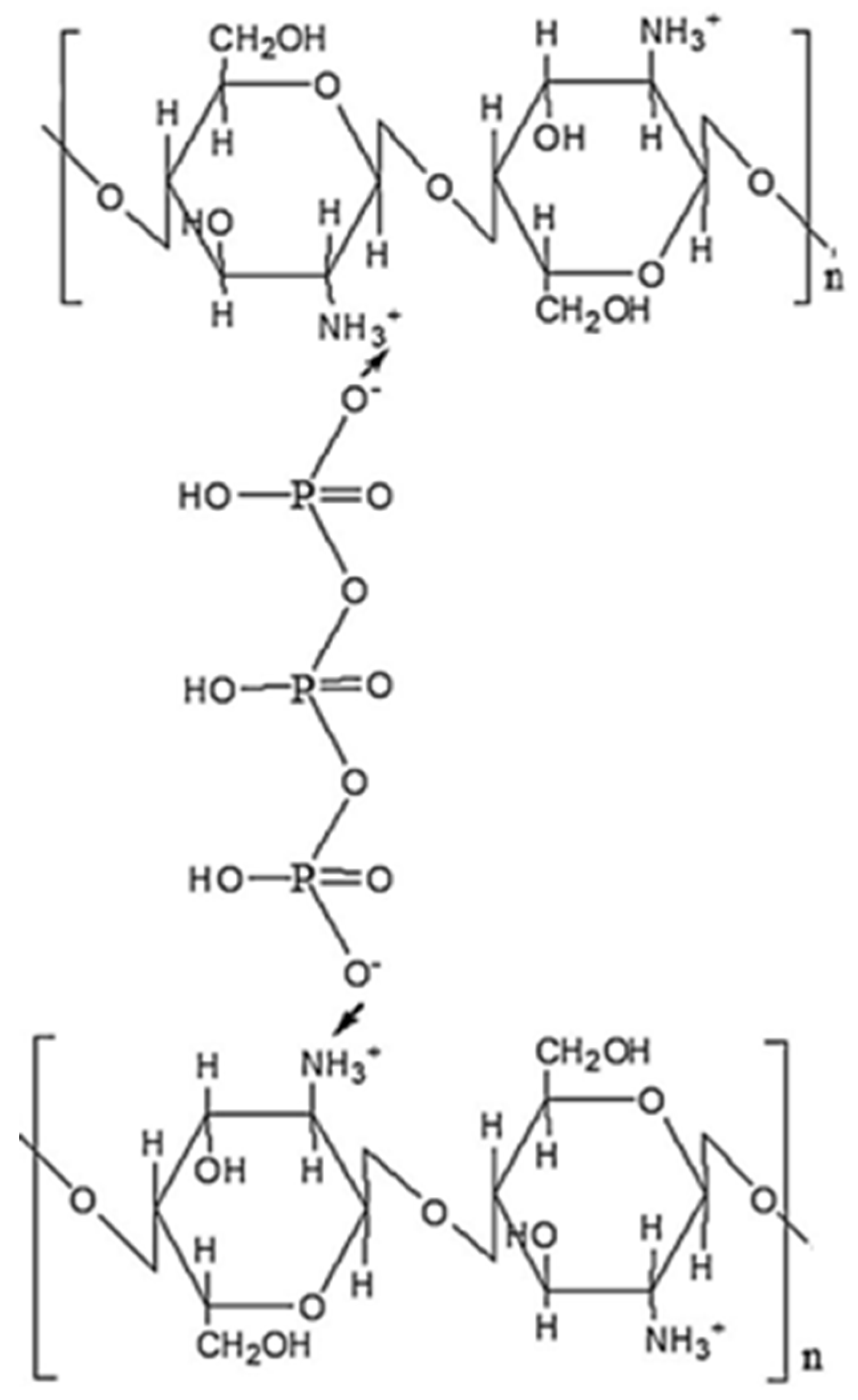
Phosphorylated CS
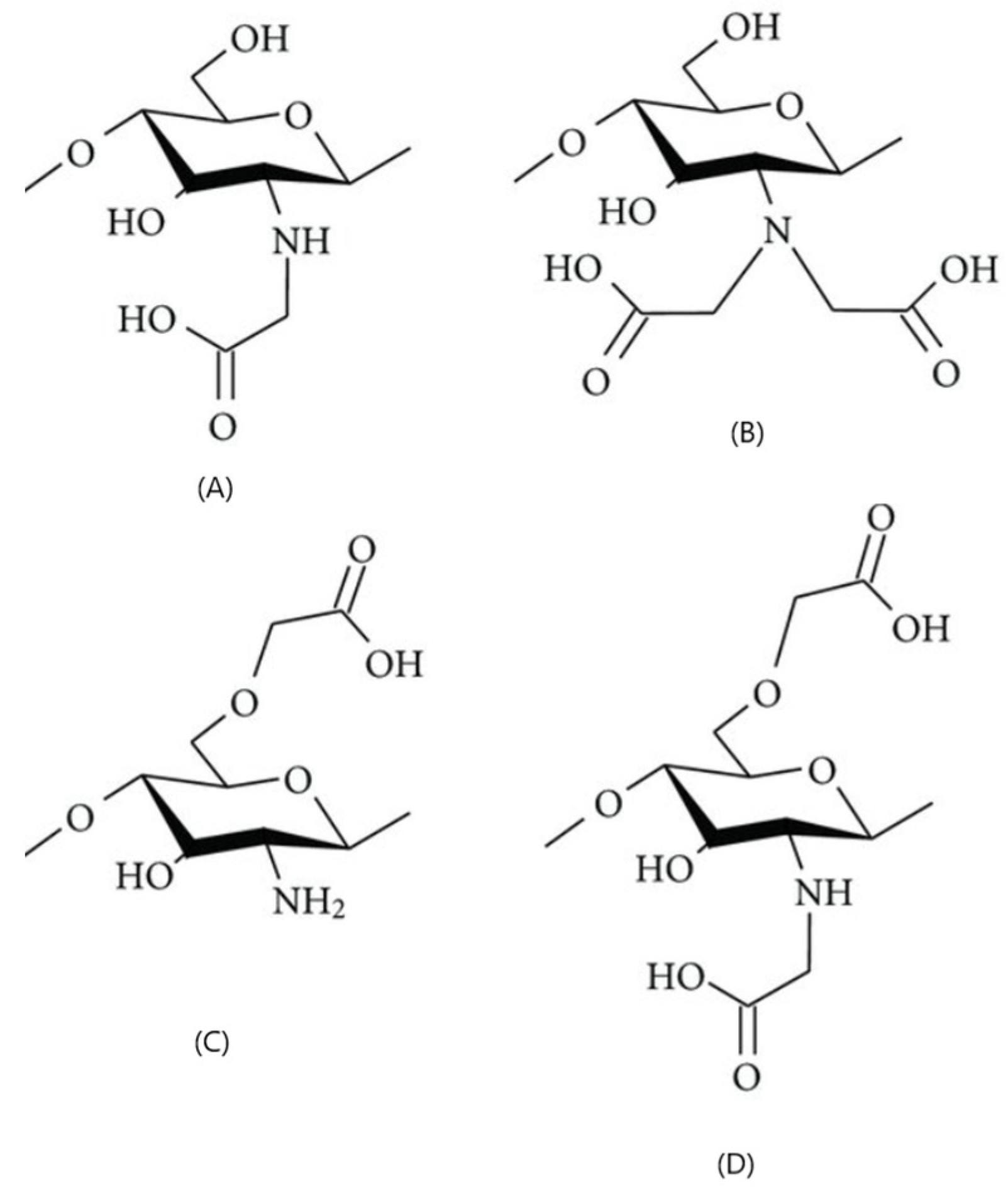
Succinylated CS
Carboxyalkylated CS
2.6.2. CS Derivatives Enhanced with Hydrophobic Substituents
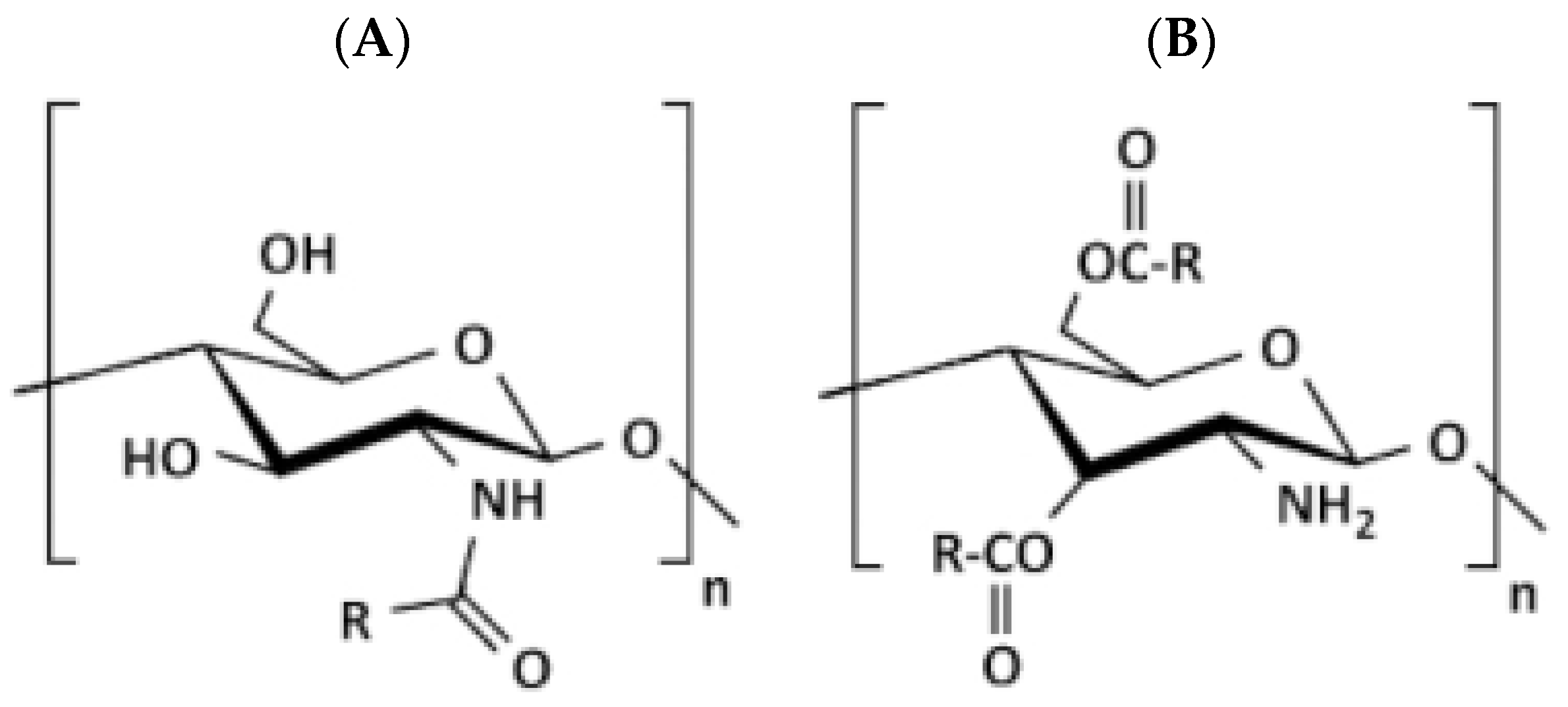
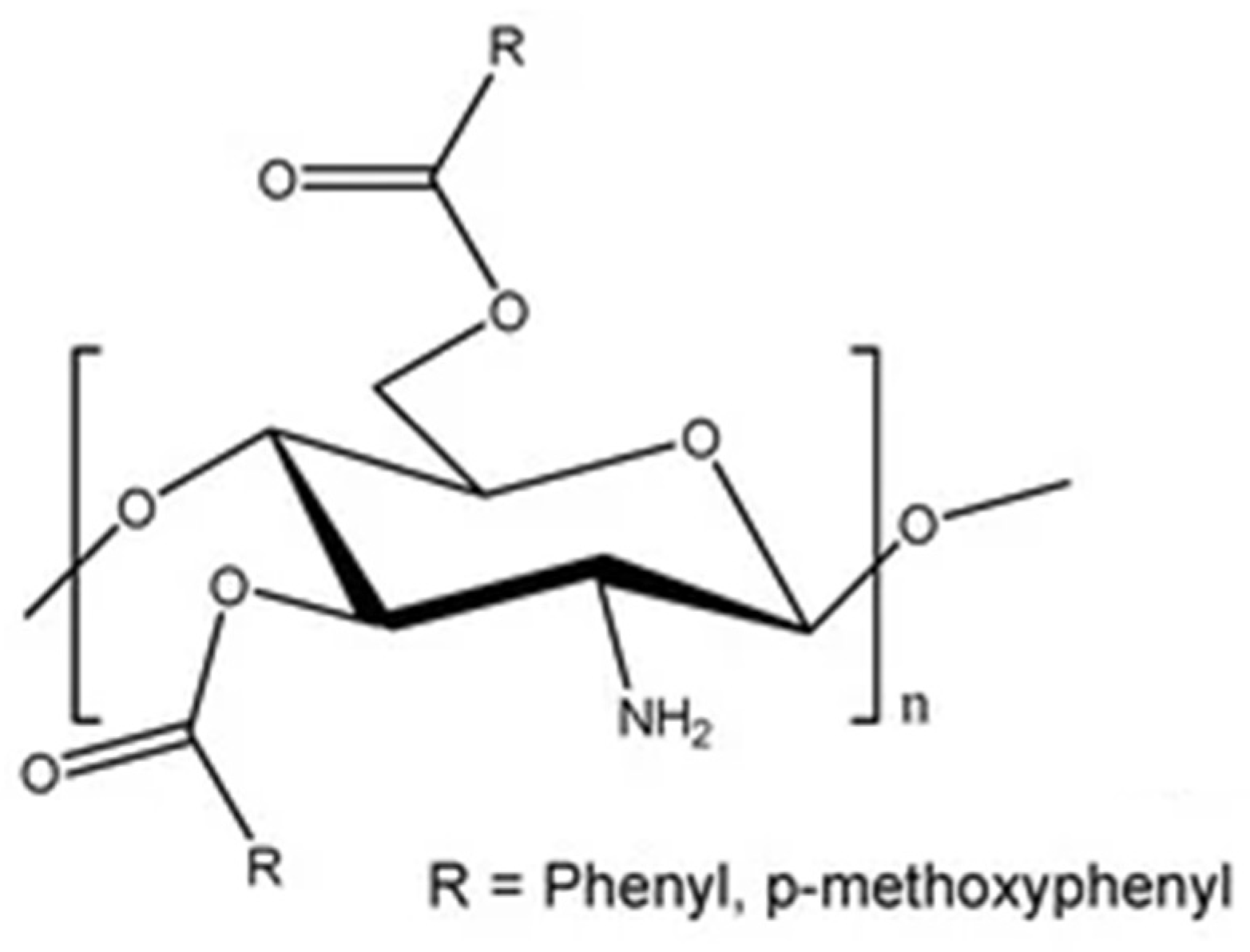
Acylated CS
Alkylated CS
Benzoylated CS
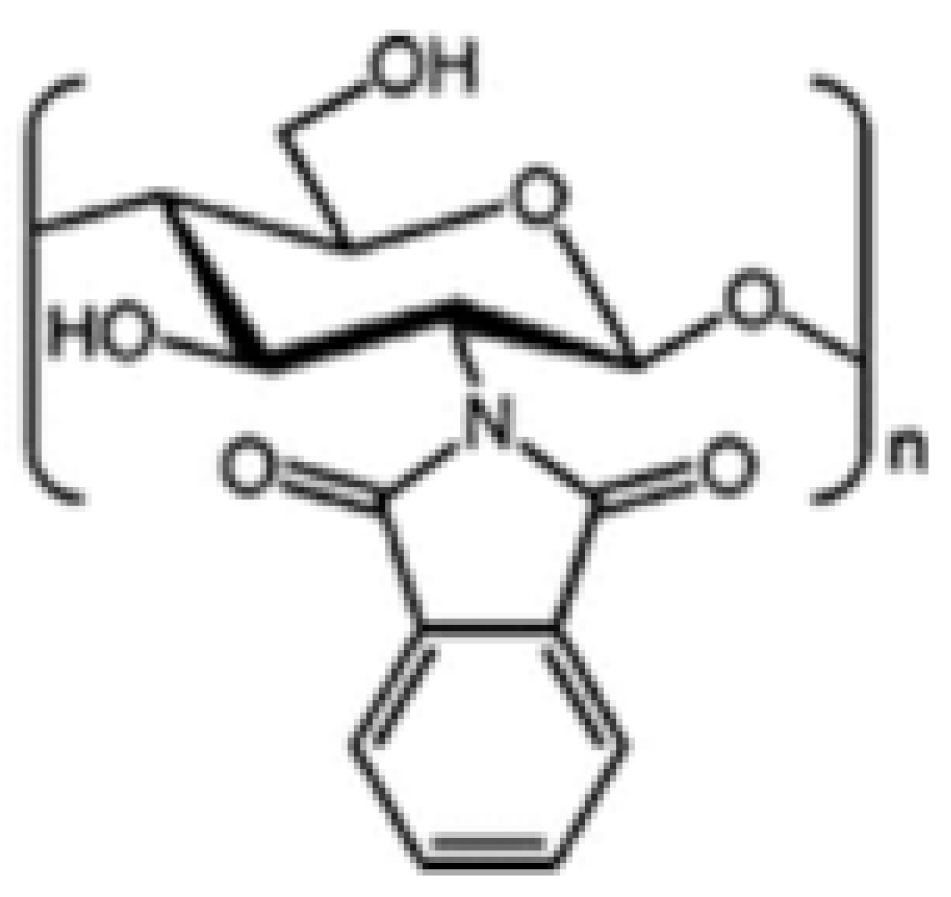
N-Phthaloylated CS
2.6.3. CS Derivatives with Amphiphilic Substituents
Cholic and Deoxycholic Acid-Modified CS
2.6.4. CS Copolymers (with Polymer Substituents)
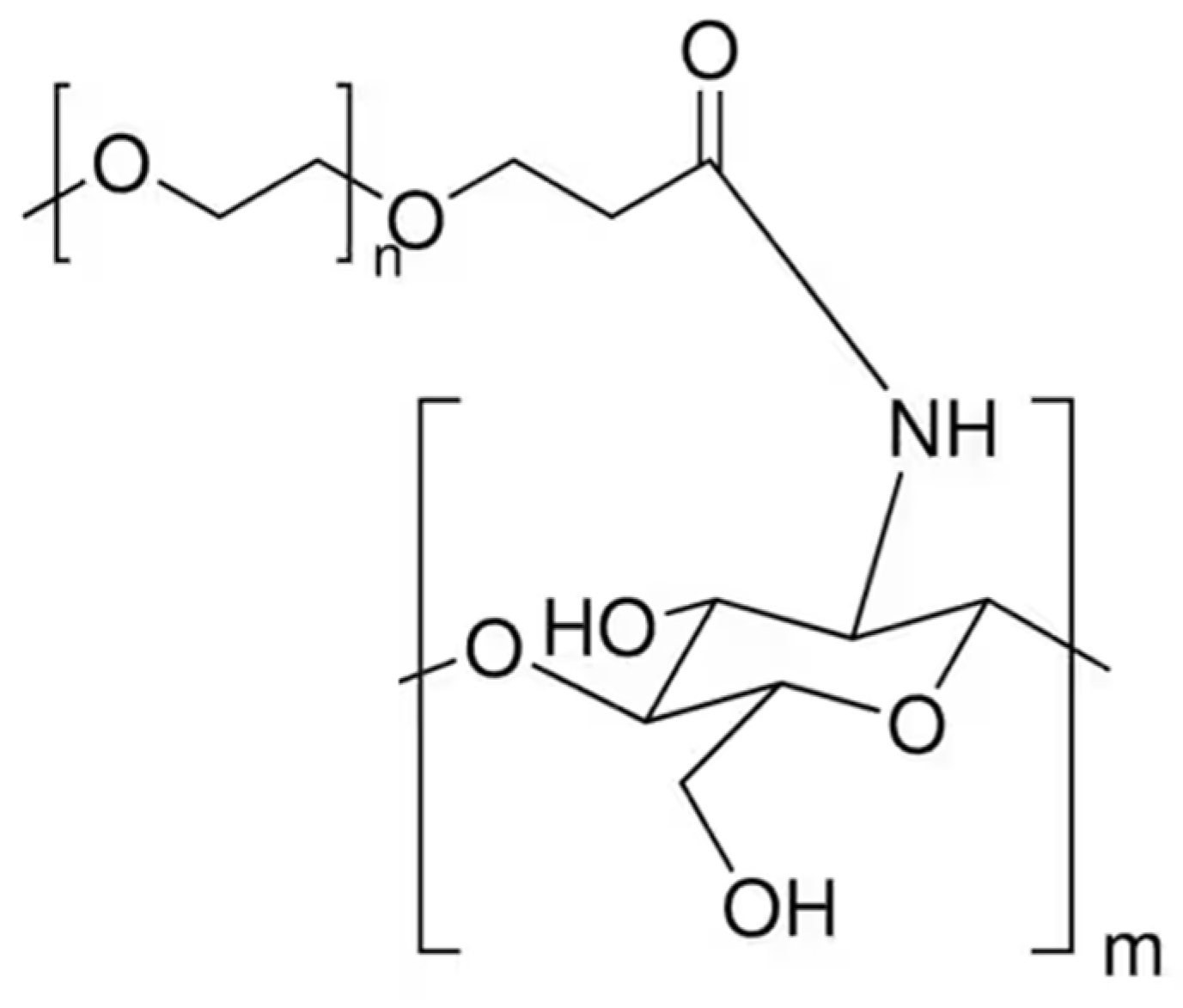
PEGylated CS
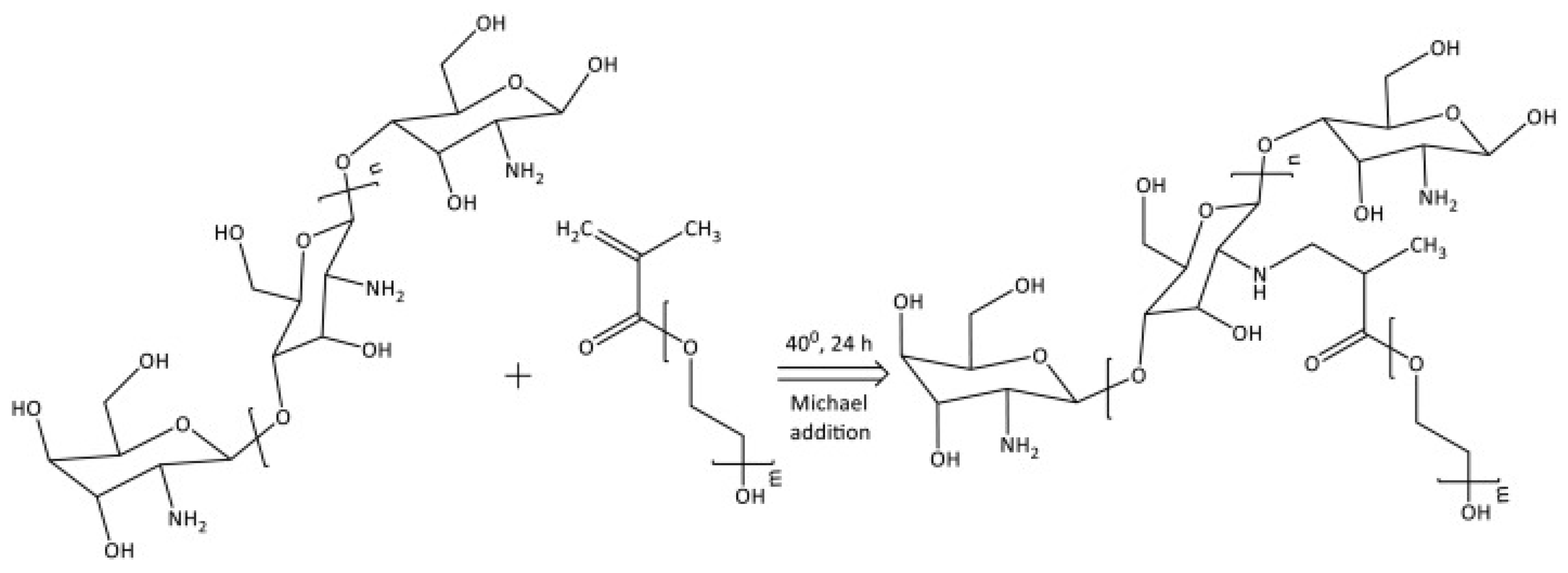
PEG-Methacrylate CS
2.6.5. Sugar Bound CS Derivatives
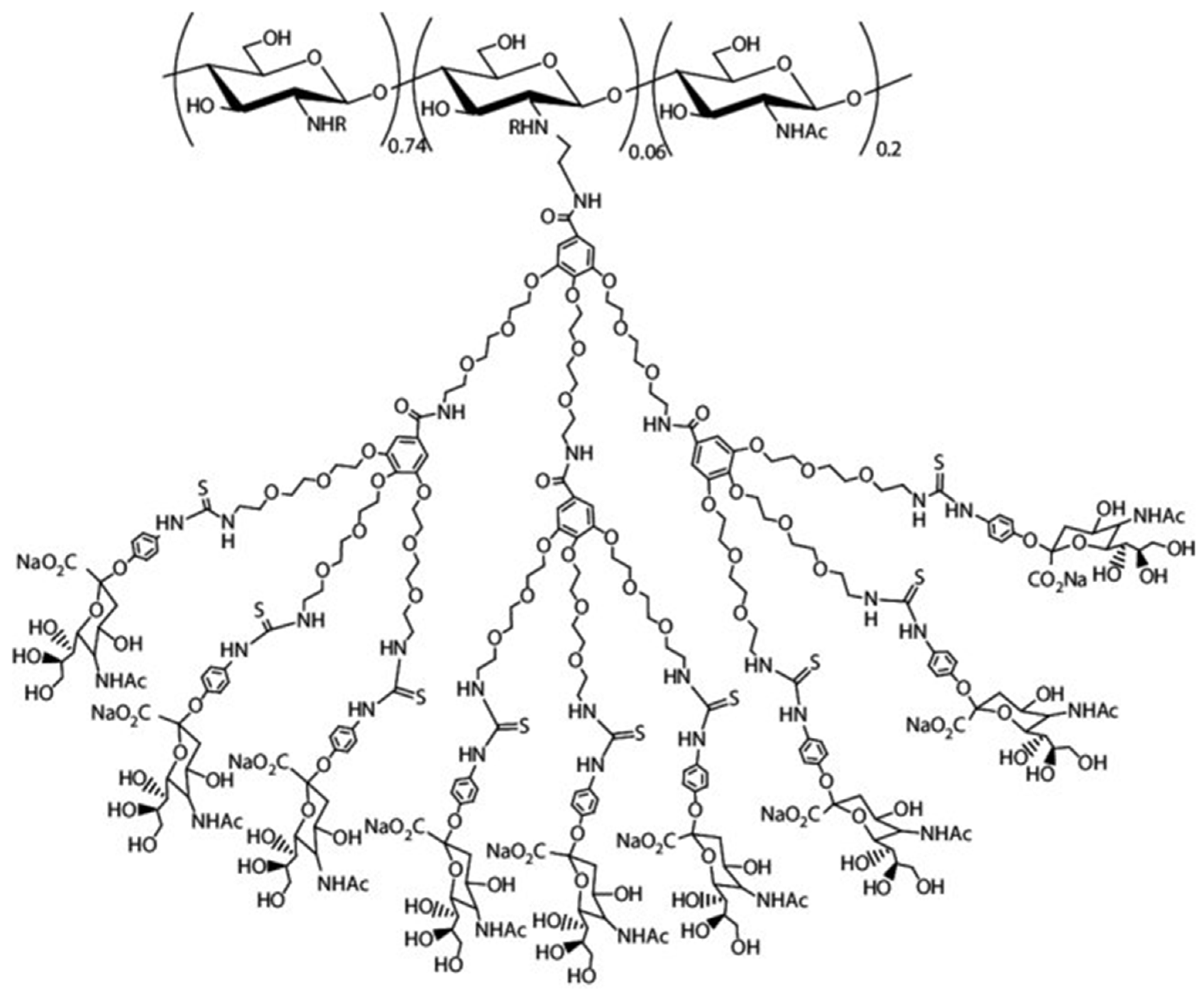
Dendrimer Hybrid CS
Galactosylated CS
2.6.6. Chitosan Derivatives with Cyclic Structures
Crown Ether-Linked CS
Cyclodextrin-Linked CS
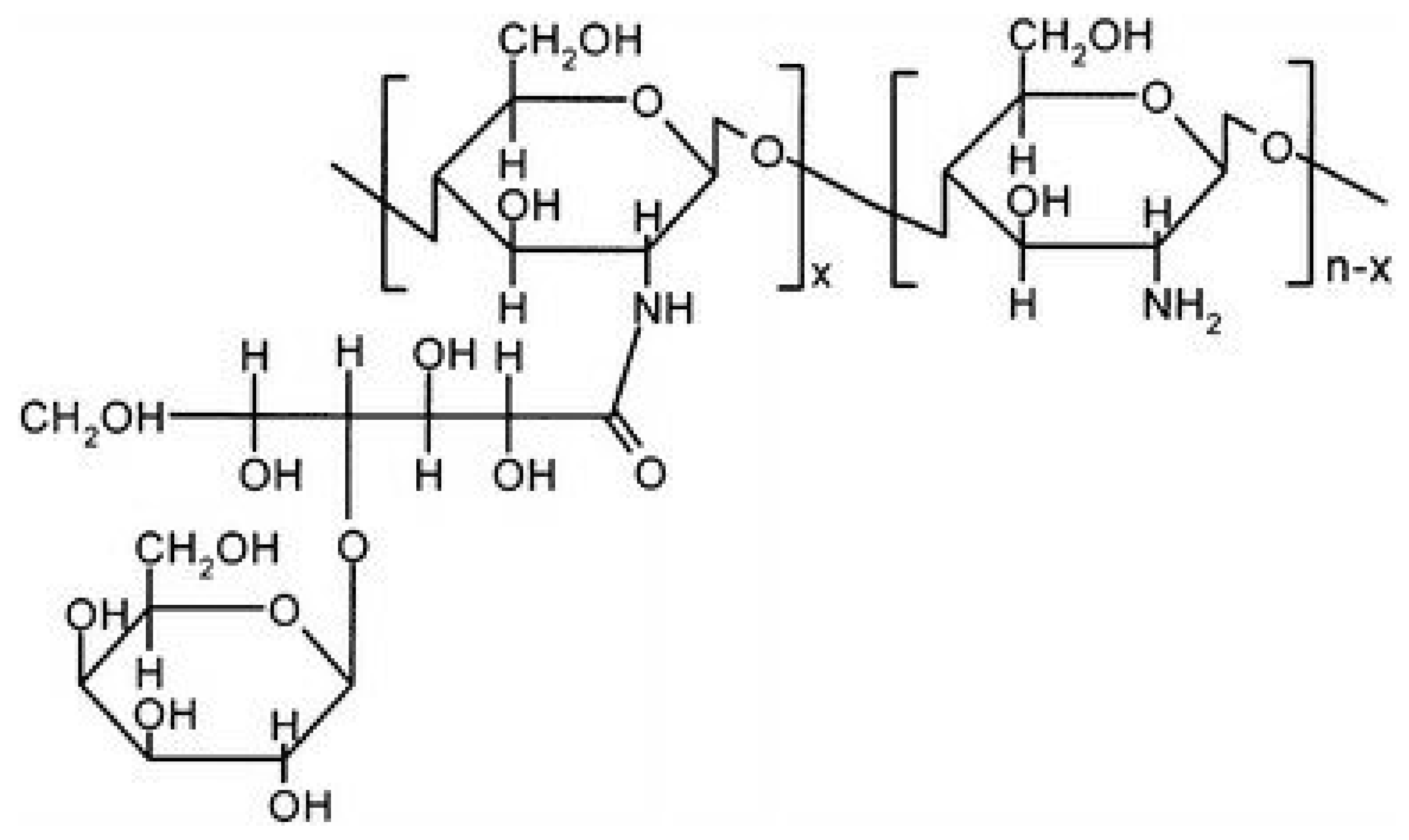
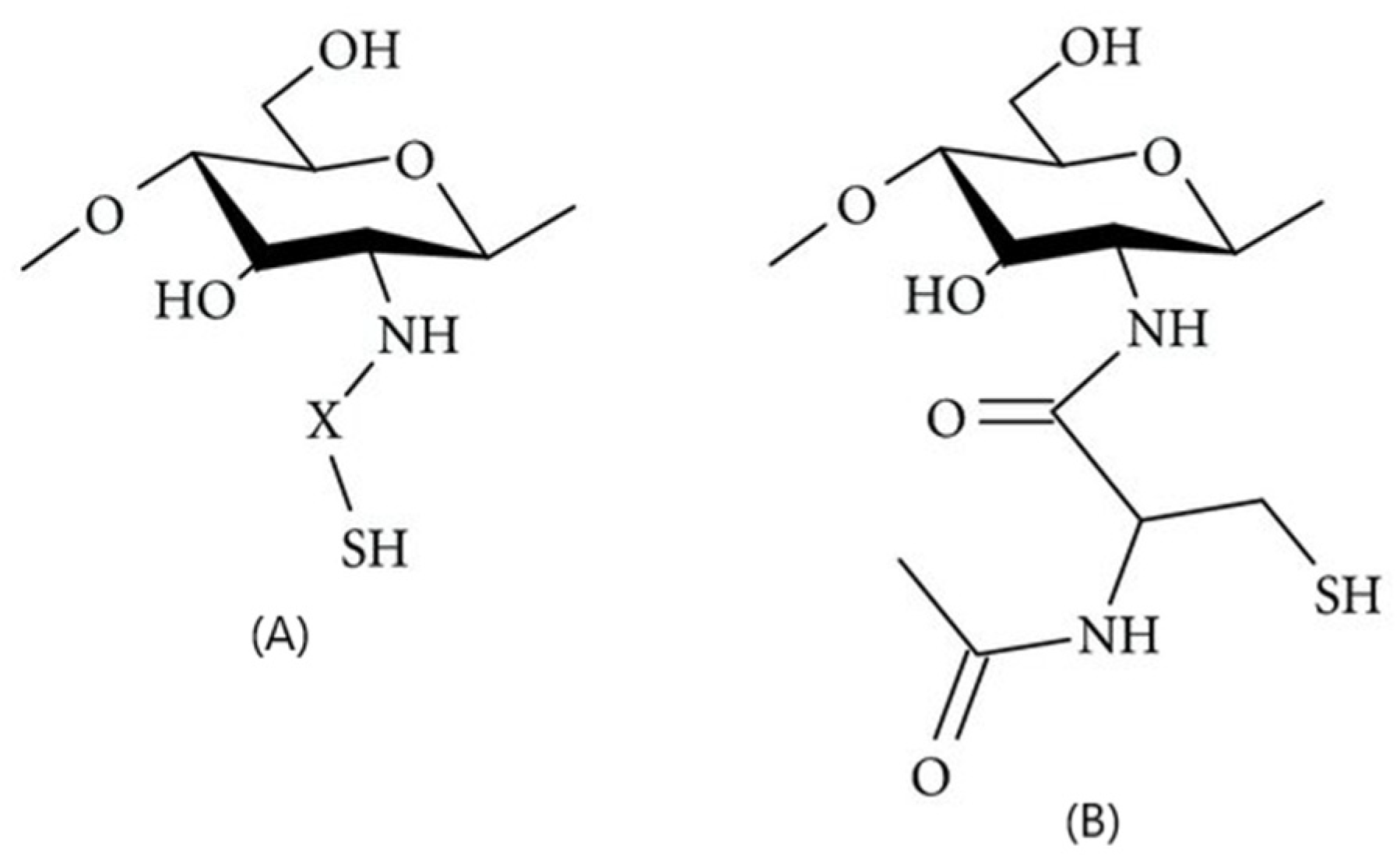
2.6.7. Thiol-Group Derivatives of CS
2.6.8. Crosslinked CS Derivatives
Chitosan–Tripolyphosphate Networks
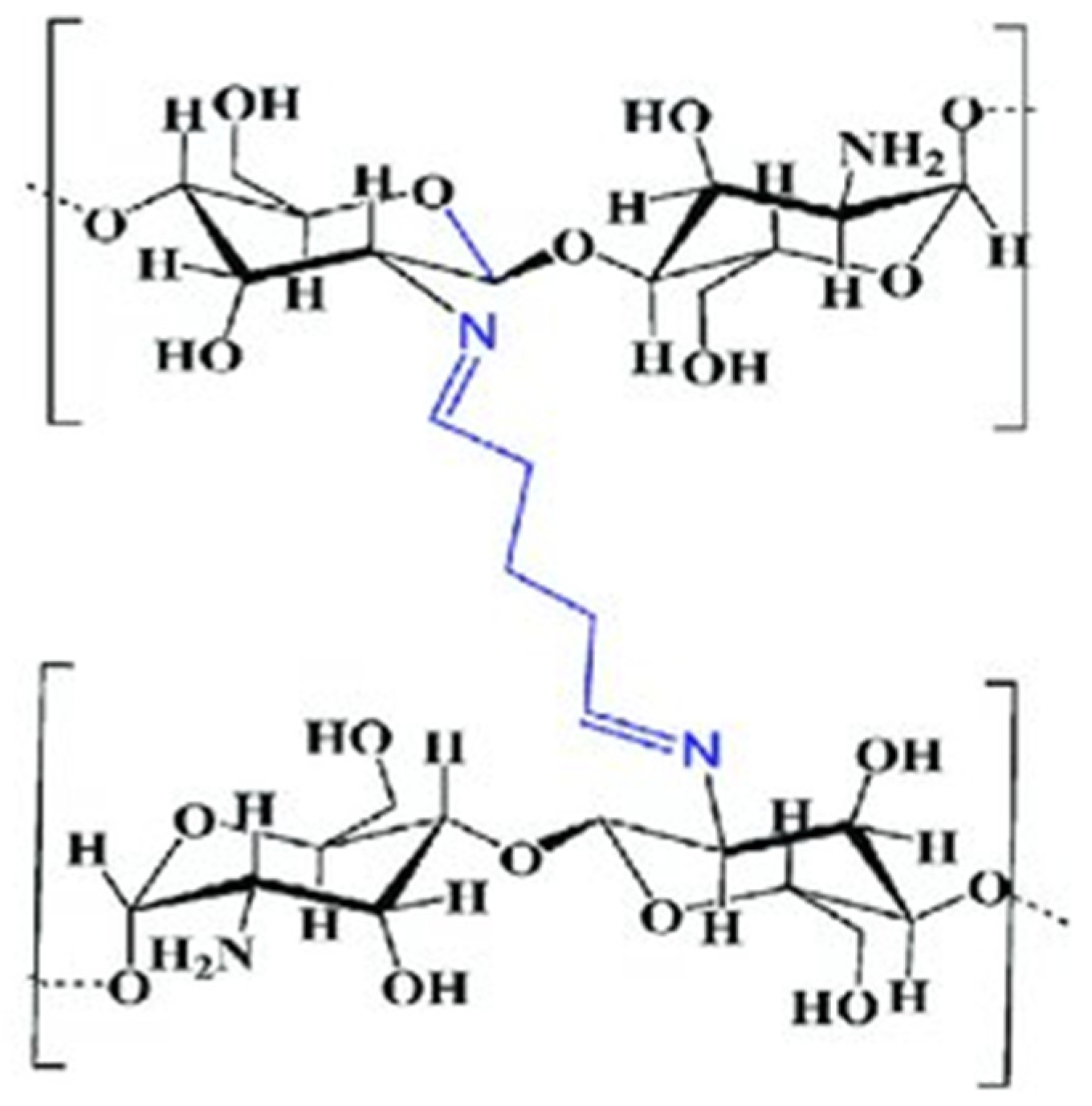
Chitosan–Glutaraldehyde Crosslinked Polymers
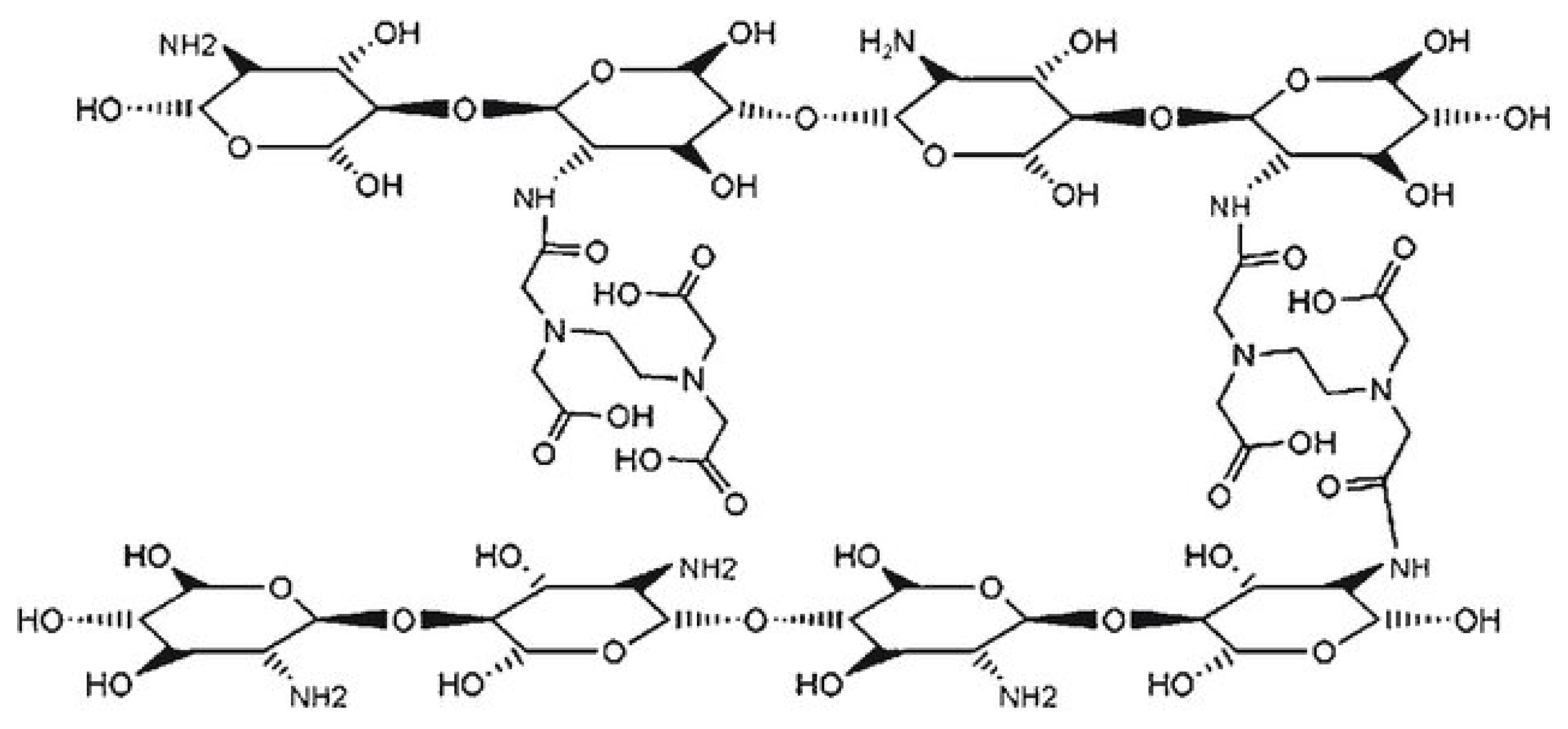
Chitosan–EDTA Conjugates
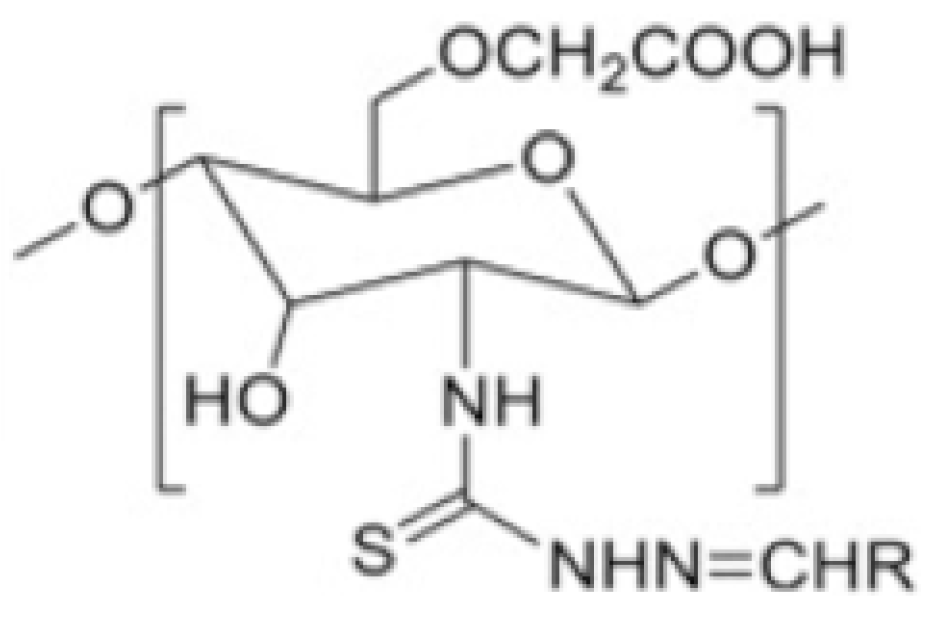
2.6.9. Chitosan Derivatives Based on Thiosemicarbazone
3. CS-Based Nanoparticles
3.1. Categorizing NPs Based on Their Structural Characteristics
3.2. Preparation Methods of CS-Based Nanoparticles
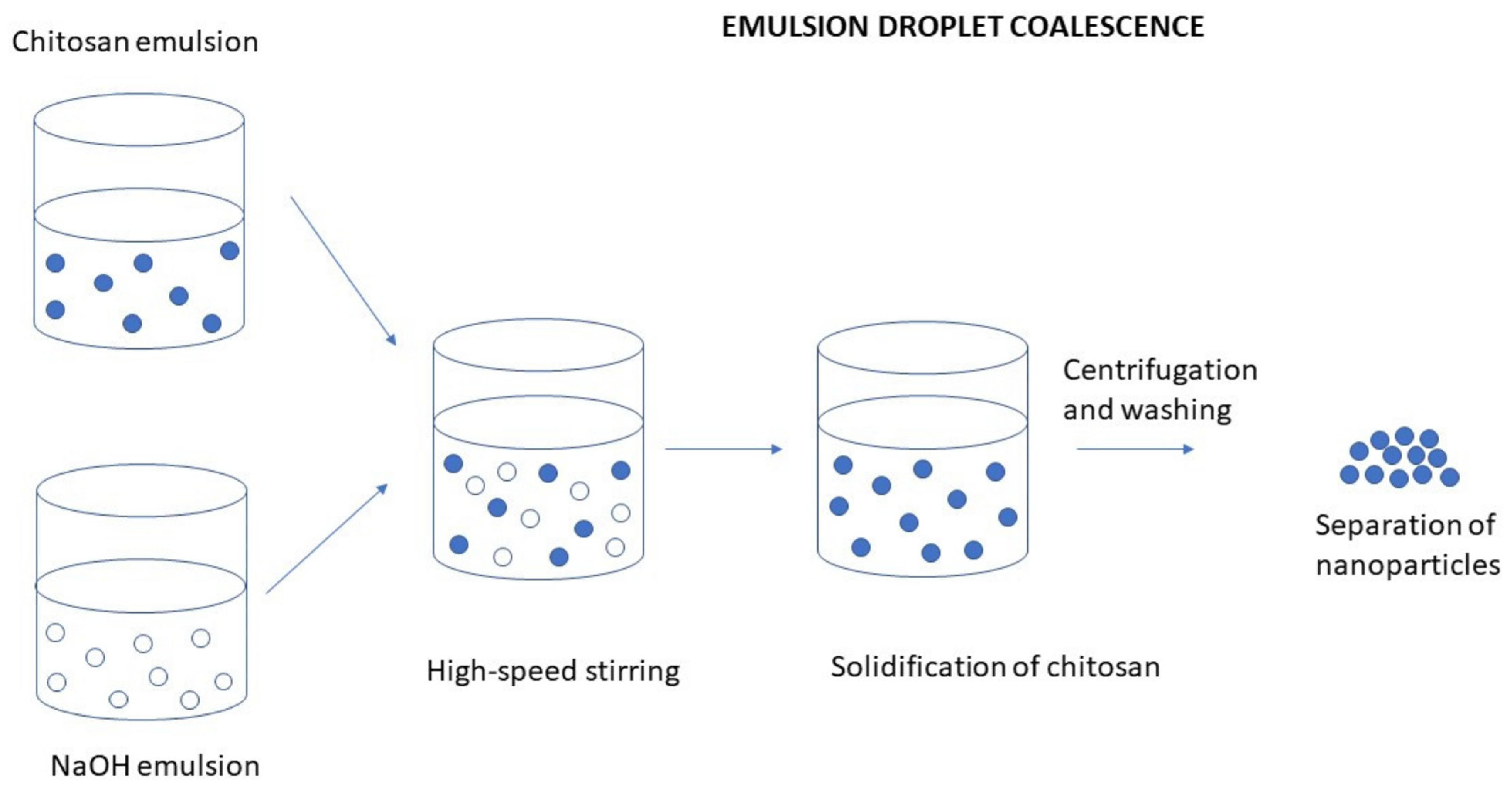
3.2.1. Emulsion Droplet Coalescence
3.2.2. Ionic Gelation/Polyelectrolyte Complexation
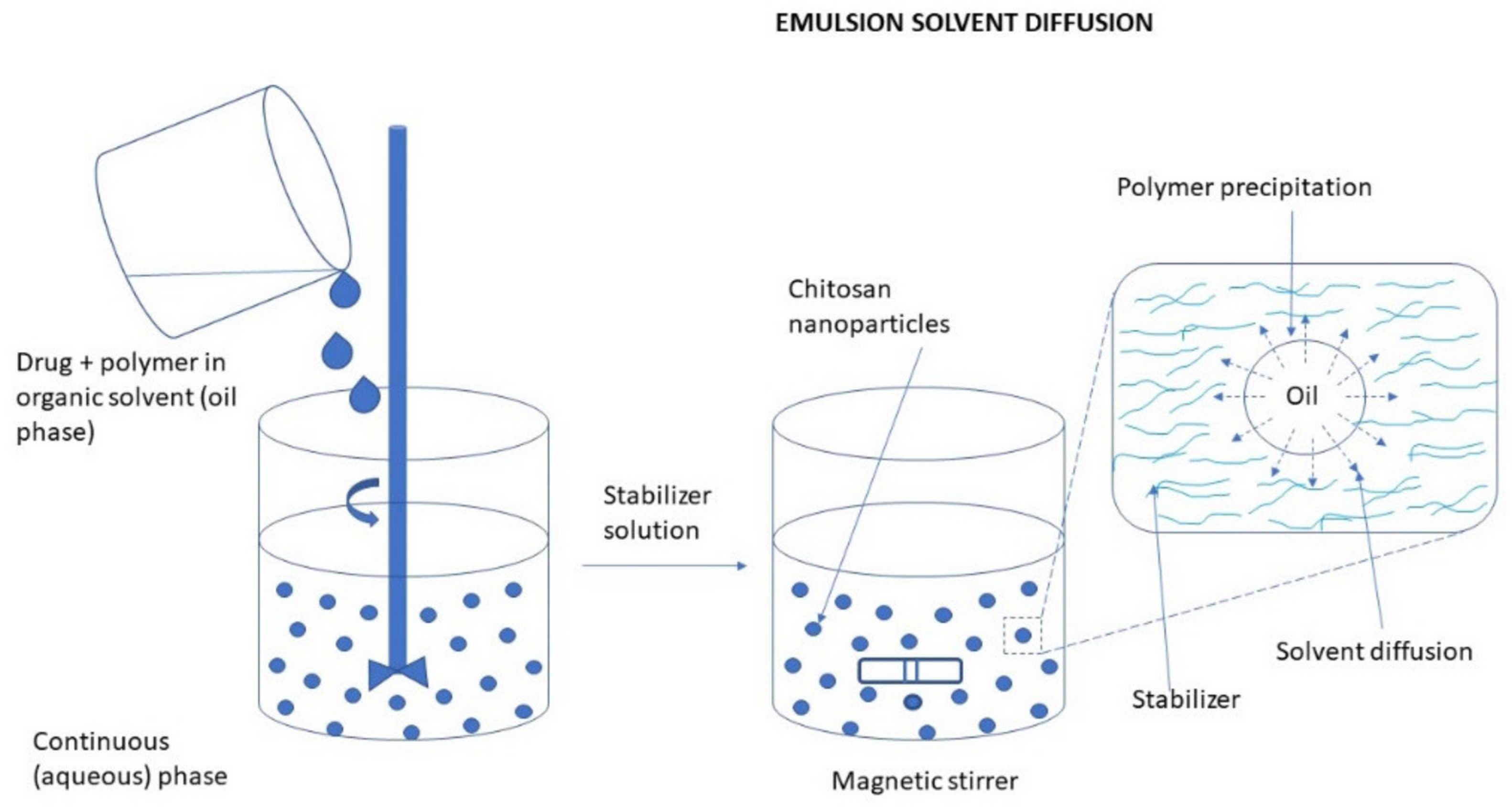
3.2.3. Diffusion of Solvent in Emulsion Systems
3.2.4. Desolvation
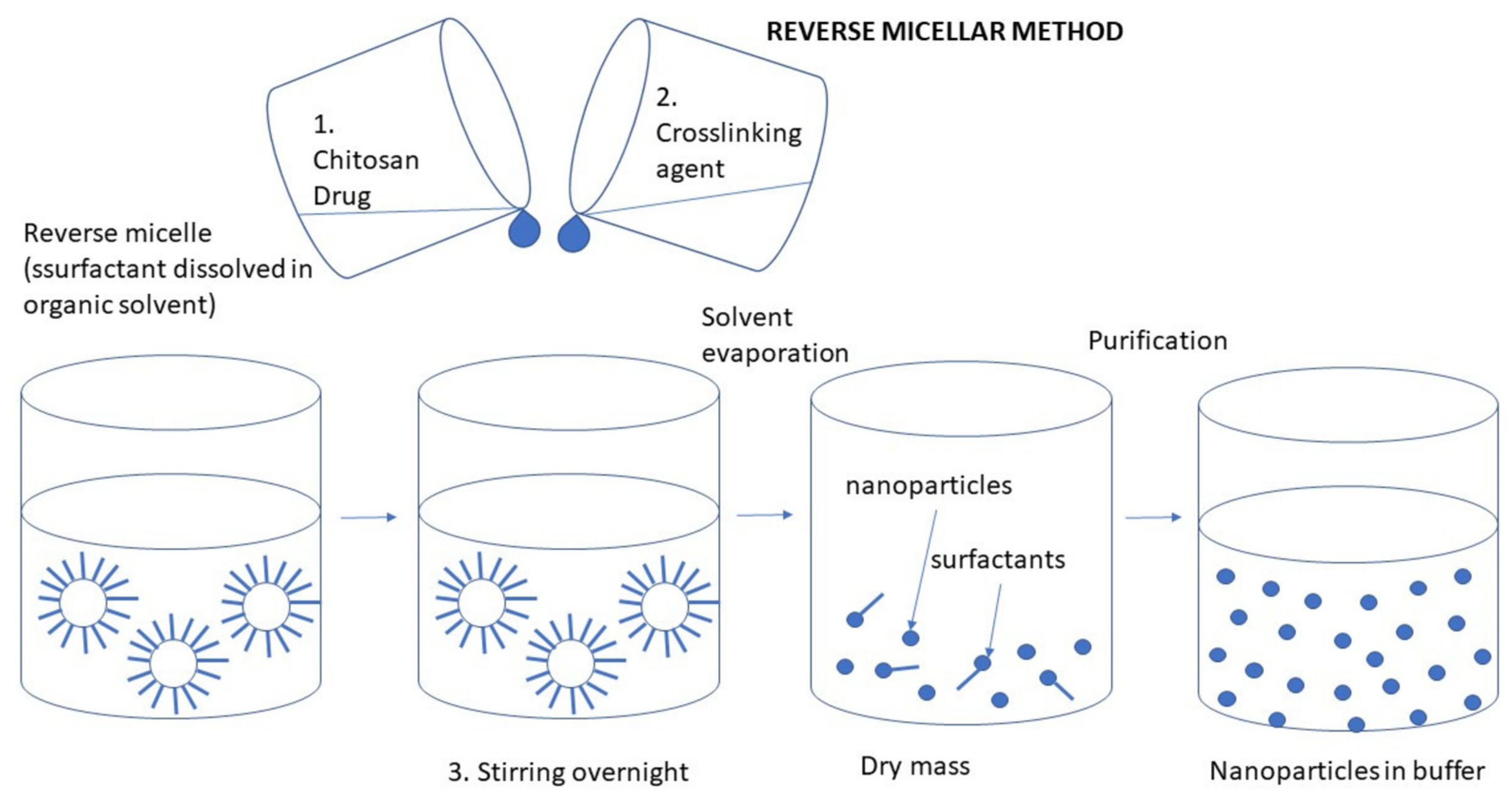
3.2.5. Reverse Micellization
3.2.6. Emulsification Cross-Inking
3.2.7. Combination of Ionic Gelation and Radical Polymerization
3.2.8. Spray-Drying
3.2.9. Nanoprecipitation
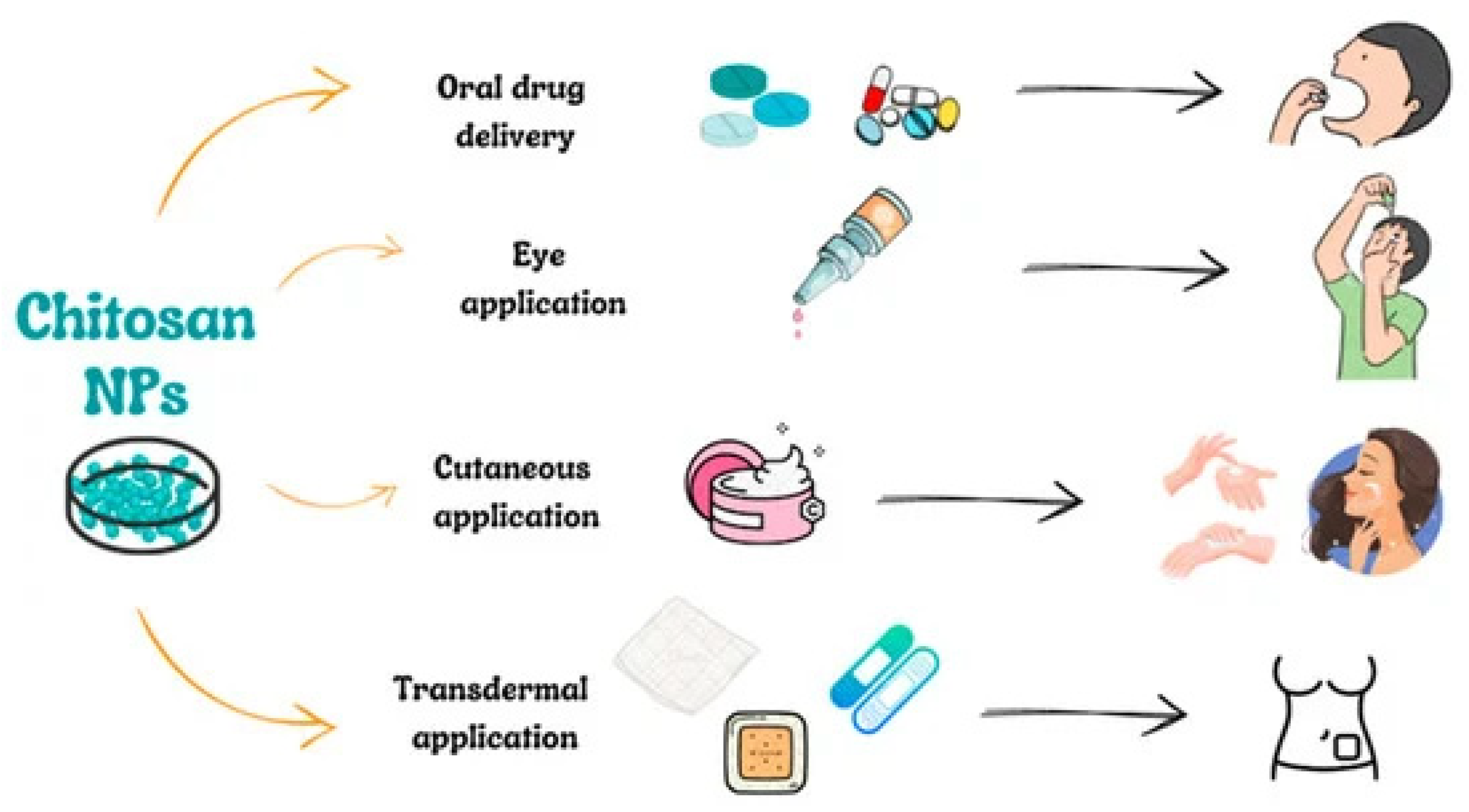
3.3. Administration Routes for CSNPs
3.3.1. Oral Administration of CSNPs
3.3.2. Ocular Administration Route of CSNPs
3.3.3. Cutaneous and Transdermal Administration of CSNPs
3.3.4. Vaccine Delivery
3.3.5. Targeting the Immune System
3.3.6. Immunotherapy of Cancer
3.4. The Protein Corona (Sometimes Called a “Protein Crown”)
- This protein corona is formed due to the following:
- Surface Charge and Composition: Chitosan is positively charged (due to amine groups) at physiological pH, making it highly interactive with negatively charged plasma proteins (e.g., albumin and fibrinogen). Modified chitosan (e.g., carboxymethylated chitosan) might have different interactions based on its charge and hydrophilicity.
- Hydrophilicity and Functional Groups: Chitosan has hydrophilic functional groups that can interact with proteins through hydrogen bonding, electrostatic forces, and van der Waals interactions.
- Type of Surrounding Medium: In serum-containing media, chitosan particles adsorb proteins like albumin, immunoglobulins, apolipoproteins, and fibrinogen.
- The exact protein composition depends on particle size, surface modifications, and media composition.
- Implications of protein corona/crown formation:
- Biological Fate and Circulation: The protein corona can alter cellular uptake, biodistribution, and immune response.
- Targeting Efficiency: Some proteins in the corona can block or enhance receptor interactions, affecting drug delivery efficiency.
- Stability and Aggregation: The corona may stabilize or destabilize chitosan particles, depending on the protein interactions.
- The protein corona formation can be controlled by the following:
- Surface Modification: PEGylation, acetylation, or grafting with other polysaccharides can reduce or modify protein adsorption.
- Charge Tuning: Adjusting chitosan derivatives (e.g., carboxymethylation and quaternization) can influence corona composition.
3.5. The Transcellular and Vesicular Permeability of Chitosan Nanocarrier
- 1.
- Transcellular permeability (direct passage through cells) refers to the movement of chitosan nanoparticles through the cell membrane and cytoplasm before being released on the other side. Chitosan enhances transcellular transport by the following:
- Mucoadhesive Properties: Chitosan interacts with negatively charged membrane components like sialic acid in mucins, improving retention at the absorption site.
- Opening Tight Junctions: Chitosan transiently reversibly disrupts tight junctions in epithelial barriers (like in the intestine or BBB), allowing nanoparticles and drugs to pass.
- Endocytosis Mechanisms: Chitosan nanoparticles are taken up via receptor-mediated, clathrin-dependent, or caveolae-mediated endocytosis, depending on their size and charge. e.g., chitosan enhances the absorption of poorly permeable drugs (e.g., insulin, heparin) via oral drug delivery, chitosan-based nanoparticles cross the blood–brain barrier (BBB) during brain delivery via receptor-mediated endocytosis, etc.
- 2.
- Vesicular permeability (transport via vesicles and endosomes) refers to the uptake of chitosan nanocarriers into endosomes, vesicles, or exosomes, allowing transport within cells before being exocytosed or degraded. Chitosan uses vesicular transport by endocytosis and intracellular trafficking (clathrin-mediated endocytosis for large or charged nanoparticles, Caveolae-mediated endocytosis for smaller, lipid-interacting particles), micropinocytosis for non-specific uptake and endosomal escape). Some chitosan formulations disrupt endosomal membranes (proton sponge effect), releasing the drug into the cytoplasm. Chitosan nanoparticles can be packed into exosomes, facilitating targeted intercellular transport, like in tumor targeting.Chitosan vesicular uptake is used for intracellular gene delivery (DNA/RNA transport into the cytoplasm), exosomal delivery of chitosan-based nanoparticles for drug transport into tumors, etc., are some examples of chitosan-based drug delivery. Chitosan nanoparticles use both transcellular and vesicular pathways to enhance drug permeability across biological barriers. Their ability to open tight junctions, facilitate endocytosis, and enable exosomal transport makes them highly effective for drug delivery applications [112,113].
3.6. The Photostability of Chitosan Nanocarrier
3.7. The Propensity of Nanoparticles to Aggregate
- Surface Charge (zeta potential): Nanoparticles with low surface charge (zeta potential near 0 mV) tend to aggregate due to weak repulsive forces. High positive (>+30 mV) or negative (<−30 mV) zeta potential creates strong electrostatic repulsion, preventing aggregation. Chitosan nanoparticles (positively charged) may aggregate in neutral or alkaline pH, where their charge is reduced.
- Van der Waals and Electrostatic Interactions: These forces naturally attract nanoparticles, promoting aggregation. Electrostatic repulsion (from charged surfaces) counteracts these forces. e.g., salt ions in biological fluids screen electrostatic repulsion, making aggregation more likely.
- pH and Ionic Strength of the Medium: At low pH, some nanoparticles lose their surface charge, reducing repulsion and increasing aggregation. High ionic strength (e.g., in blood or physiological fluids) reduces electrostatic repulsion, promoting aggregation. Chitosan nanoparticles aggregate in physiological pH (~7.4) due to charge reduction.
- Surface Coating and Stabilization: Hydrophilic polymers (e.g., PEGylation and polysaccharides) reduce aggregation by steric hindrance. Protein corona formation in biological fluids can sometimes stabilize or destabilize nanoparticles. E.g., PEG-coated chitosan nanoparticles resist aggregation better than uncoated ones.
- Hydrophobic vs. Hydrophilic Interactions: Hydrophobic nanoparticles (or hydrophobic regions) attract each other, leading to aggregation in aqueous environments. Hydrophilic nanoparticles (e.g., carboxylated chitosan NPs) remain better dispersed in water.
3.8. Thermodynamic Perspective of Chitosan Naoparticles
4. Physicochemical Properties of CSNPs
4.1. Zeta Potential and Particle Size
4.2. Stability
4.3. Cytotoxicity Study and Cellular Uptake
4.4. Drug Loading and Drug Release
4.5. Characteristics of Nanoparticles Used in Drug Delivery Systems
Key Morphological Features and Their Impact on the Application of Particles
4.6. Understanding the Mechanism of Intestinal Absorption for CSNPs
4.6.1. Transport Across Cellular Membranes
4.6.2. Paracellular Transport
5. CSNPs as Drug Delivery Systems
5.1. Enhancing Oral Absorption and Biological Activity of Phytochemical Compounds by Using CSNPs
5.1.1. Curcumin
5.1.2. Thymoquinone
5.1.3. Ferulic Acid
5.1.4. Berberine
5.1.5. Piperine
6. Limitations and Challenges
7. Future Prospects
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AbdElkodous, M.; El-Sayyad, G.S.; Abdelrahman, I.Y.; El-Bastawisy, H.S.; Mosallam, F.M.; Nasser, H.A.; Gobara, M.; Baraka, A.; Elsayed, M.A.; El-Batal, A.I. Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. B Biointerfaces 2019, 180, 411–428. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2019, 16, 5776. [Google Scholar] [CrossRef] [PubMed]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 11, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Hani, U.; Choudhary, V.T.; Ghazwani, M.; Alghazwani, Y.; Osmani, R.A.M.; Kulkarni, G.S.; Shivakumar, H.G.; Wani, S.U.D.; Paranthaman, S. Nanocarriers for Delivery of Anticancer Drugs: Current Developments, Challenges, and Perspectives. Pharmaceutics 2024, 16, 1527. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Haidar, M.K.; Demirbolat, G.M.; Timur, S.S.; Gürsoy, R.N.; Nemutlu, E.; Ulubayram, K.; Öner, L.; Eroğlu, H. Atorvastatin-loaded nanosprayed chitosan nanoparticles for peripheral nerve injury. Bioinspired Biomim. Nanobiomater. 2019, 21, 74–84. [Google Scholar] [CrossRef]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M. Nanocarriers-mediated drug delivery systems for anticancer agents: An overview and perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Mar. Drugs 2015, 9, 312–337. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the factors affecting the solubility of chitosan in water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Kurita, K.; Kamiya, M.; Nishimura, S.I. Solubilization of a rigid polysaccharide: Controlled partial N-acetylation of chitosan to develop solubility. Carbohydr. Polym. 1991, 16, 83–92. [Google Scholar] [CrossRef]
- Sánchez, L.F.; Cánepa, J.; Kim, S.; Nakamatsu, J. A Simple Approach to Produce Tailor-Made Chitosans with Specific Degrees of Acetylation and Molecular Weights. Polymers 2021, 13, 2415. [Google Scholar] [CrossRef]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and biomedical applications of chitosan-based nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 1, 1313. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.; Abd-Elaal, A.A.; Badr, E.A.; Abou Kana, M.T. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical and optical study of chitosan–terephthaldehyde derivative for biomedical applications. Int. J. Biol. Macromol. 2012, 51, 1167–1172. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Moraru, C.; Mincea, M.M.; Frandes, M.; Timar, B.; Ostafe, V. A meta-analysis on randomised controlled clinical trials evaluating the effect of the dietary supplement chitosan on weight loss, lipid parameters and blood pressure. Medicina 2018, 54, 109. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Sarmento, B.; Martins, S.; Ferreira, D.; Souto, E.B. Oral insulin delivery by means of solid lipid nanoparticles. Int. J. Nanomed. 2007, 2, 743–749. [Google Scholar]
- Grenha, A.; Seijo, B.; Remunán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent advancement in chitosan-based nanoparticles for improved oral bioavailability and bioactivity of phytochemicals: Challenges and perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and chitosan: Prospective biomedical applications in drug delivery, cancer treatment and wound healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef]
- Fu, S.; Xia, J.; Wu, J. Functional chitosan nanoparticles in cancer treatment. J. Biomed. Nanotechnol. 2016, 12, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Roy, S.G.; Shirsat, N.S.; Mishra, A.C.; Waghulde, S.O.; Kale, M.K. A review on chitosan nanoparticles applications in drug delivery. J. Pharmacogn. Phytochem. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef]
- Morgan, K.; Conway, C.; Faherty, S.; Quigley, C. AComparative Analysis of Conventional and Deep Eutectic Solvent (DES)-Mediated Strategies for the Extraction of Chitin from Marine Crustacean Shells. Molecules 2021, 26, 7603. [Google Scholar] [CrossRef] [PubMed]
- Román-Doval, R.; Gómez-Sánchez, A.; Millán-Casarrubias, E.J.; Prokhorov, E.; Montejo-Alvaro, F.; de Luna Bugallo, A.; Mendoza, S. Physicochemical properties of pullulan/chitosan/graphene oxide composite films. Polym. Int. 2022, 71, 959–965. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Jones, R.A.; Cheung, C.Y.; Black, F.E.; Zia, J.K.; Stayton, P.S.; Hoffman, A.S.; Wilson, M.R. Poly (2-alkylacrylic acid) polymers deliver molecules to the cytosol by pH-sensitive disruption of endosomal vesicles. Biochem. J. 2003, 372, 65–75. [Google Scholar] [CrossRef]
- McCarthy, P.C.; Zhang, Y.; Abebe, F. Recent applications of dual-stimuli responsive chitosan hydrogel nanocomposites as drug delivery tools. Molecules 2021, 26, 4735. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/media/154923/download (accessed on 1 February 2025).
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma Acute Care Surg. 2006, 60, 655–658. [Google Scholar] [CrossRef]
- Kean, T.; Roth, S.; Thanou, M. Trimethylatedchitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency. J. Control. Release 2005, 103, 643–653. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Kittur, F.S.; Kumar, A.B.; Tharanathan, R.N. Low molecular weight chitosans-preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr. Res. 2003, 338, 1283–1290. [Google Scholar] [CrossRef]
- Krajewska, B. Membrane-based processes performed with use of chitin/chitosan materials. Sep. Purif. Technol. 2005, 41, 305–312. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinämäki, J.; Räsänen, M.; Maunu, S.L.; Karjalainen, M.; Acosta, O.N.; Colarte, A.I.; Yliruusi, J. Solid-state characterization of chitosans derived from lobster chitin. Carbohydr. Polym. 2004, 58, 401–408. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, K.H.; Kittur, F.S.; Tharanathan, R.N. Solid state structure of chitosan prepared under different N-deacetylating conditions. Carbohydr. Polym. 2002, 50, 27–33. [Google Scholar] [CrossRef]
- Steenkamp, G.C.; Keizer, K.; Neomagus, H.W.; Krieg, H.M. Copper (II) removal from polluted water with alumina/chitosan composite membranes. J. Membr. Sci. 2002, 197, 147–156. [Google Scholar] [CrossRef]
- AbdElgadir, M.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.; Sarker, M.Z. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef]
- Nunthanid, J.; Puttipipatkhachorn, S.; Yamamoto, K.; Peck, G.E. Physical properties and molecular behavior of chitosan films. Drug Dev. Ind. Pharm. 2001, 27, 143–157. [Google Scholar] [CrossRef]
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef]
- Gao, X.; Dong, D.; Zhang, C.; Deng, Y.; Ding, J.; Niu, S.; Tan, S.; Sun, L. Chitosan-Functionalized Poly(β-Amino Ester) Hybrid System for Gene Delivery in Vaginal Mucosal Epithelial Cells. Pharmaceutics 2024, 16, 154. [Google Scholar] [CrossRef]
- Jintapattanakit, A.; Junyaprasert, V.B.; Kissel, T. The role of mucoadhesion of trimethyl chitosan and PEGylated trimethyl chitosan nanocomplexes in insulin uptake. J. Pharm. Sci. 2009, 98, 4818–4830. [Google Scholar] [CrossRef]
- Werle, M.; Bernkop-Schnürch, A. Thiolatedchitosans: Useful excipients for oral drug delivery. J. Pharm. Pharmacol. 2008, 60, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Bhise, K.S.; Dhumal, R.S.; Paradkar, A.R.; Kadam, S.S. Effect of drying methods on swelling, erosion and drug release from chitosan–naproxen sodium complexes. AAPS PharmSciTech 2008, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Mao, S.; Wang, Y.; Junyaprasert, V.B.; Zhang, T.; Na, L.; Wang, J. Bioadhesion and oral absorption of enoxaparin nanocomplexes. Int. J. Pharm. 2010, 386, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Vyas, S.P. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef]
- Osi, B.; Al-Kinani, A.A.; Al-Qaysi, Z.K.; Khoder, M.; Alany, R.G. Exploring the Ocular Absorption Pathway of Fasudil Hydrochloride towards Developing a Nanoparticulate Formulation with Improved Performance. Pharmaceutics 2024, 16, 112. [Google Scholar] [CrossRef]
- Metkar, S.P.; Fernandes, G.; Nikam, A.N.; Soman, S.; Birangal, S.; Seetharam, R.N.; Joshi, M.B.; Mutalik, S. Mannosylated-Chitosan-Coated Andrographolide Nanoliposomes for the Treatment of Hepatitis: In Vitro and In Vivo Evaluations. Membranes 2023, 13, 193. [Google Scholar] [CrossRef]
- Malmo, J.; Varum, K.M.; Strand, S.P. Effect of chitosan chain architecture on gene delivery: Comparison of self-branched and linear chitosans. Biomacromolecules 2011, 12, 721–729. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Yang, Y.; Zhang, L.; Liu, R.; Li, Z. Trimethyl chitosan nanoparticles for ocular baicalein delivery: Preparation, optimization, in vitro evaluation, in vivo pharmacokinetic study and molecular dynamics simulation. Int. J. Biol. Macromol. 2020, 156, 749–761. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Mitra, K.; Arumugam, S.K.; Doble, M. Preparation of Curdlan sulphate-Chitosan nanoparticles as a drug carrier to target Mycobacterium smegmatis infected macrophages. Carbohydr. Polym. 2021, 258, 117686. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, S.; Pan, Z.; Lin, Y.; Ding, S.; Li, L.; Luo, B.; Jiao, Y.; Zhou, C. Sulfonated chitosan and phosphorylated chitosan coated polylactide membrane by polydopamine-assisting for the growth and osteogenic differentiation of MC3T3-E1s. Carbohydr. Polym. 2020, 229, 115517. [Google Scholar] [CrossRef] [PubMed]
- Palacio, J.; Monsalve, Y.; Ramírez-Rodríguez, F.; López, B. Study of encapsulation of polyphenols on succinyl-chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101610. [Google Scholar] [CrossRef]
- Nanda, B.; Manjappa, A.S.; Chuttan, I.K.; Balasinor, N.H.; Mishra, A.K.; Murthy, R.S. Acylated chitosan anchored paclitaxel loaded liposomes: Pharmacokinetic and biodistribution study in Ehrlich ascites tumor bearing mice. Int. J. Biol. Macromol. 2019, 122, 367–379. [Google Scholar] [CrossRef]
- Chu, L.; Zhang, Y.; Feng, Z.; Yang, J.; Tian, Q.; Yao, X.; Zhao, X.; Tan, H.; Chen, Y. Synthesis and application of a series of amphipathic chitosan derivatives and the corresponding magnetic nanoparticle-embedded polymeric micelles. Carbohydr. Polym. 2019, 223, 114966. [Google Scholar] [CrossRef]
- Burr, S.J.; Williams, P.A.; Ratcliffe, I. Synthesis of cationic alkylated chitosans and an investigation of their rheological properties and interaction with anionic surfactant. Carbohydr. Polym. 2018, 201, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abd El-Ghany, N.A.; Abdel-Aziz, M.M. Synthesis, characterization, anti-inflammatory and anti-Helicobacter pylori activities of novel benzophenone tetracarboxylimide benzoyl thiourea cross-linked chitosan hydrogels. Int. J. Biol. Macromol. 2021, 181, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Permadi, R.; Suk, V.R.; Misran, M. Synthesis and Characterization of acylated low molecular weight chitosan and acylated low molecular weight phthaloyl chitosan. Sains Malays. 2020, 49, 2251–2260. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, Y.; Wang, X.; Kasparis, G.; Mao, S. Chitosan and its derivatives-based nano-formulations in drug delivery. Nanobiomaterials Drug Deliv. 2016, 9, 515–572. [Google Scholar]
- Hsu, C.W.; Hsieh, M.H.; Xiao, M.C.; Chou, Y.H.; Wang, T.H.; Chiang, W.H. pH-responsive polymeric micelles self-assembled from benzoic-imine-containing alkyl-modified PEGylated chitosan for delivery of amphiphilic drugs. Int. J. Bio. Macromol. 2020, 163, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Savin, C.L.; Popa, M.; Delaite, C.; Costuleanu, M.; Costin, D.; Peptu, C.A. Chitosan grafted-poly (ethylene glycol) methacrylate nanoparticles as carrier for controlled release of bevacizumab. Mater. Sci. Eng. C 2019, 98, 843–860. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, L.; Sahu, H.; Qayum, A.; Singh, S.K.; Nakhate, K.T.; Gupta, U. Chitosan engineered PAMAM dendrimers as nanoconstructs for the enhanced anti-cancer potential and improved in vivo brain pharmacokinetics of temozolomide. Pharm. Res. 2018, 35, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Z.; Chen, B.; Gu, L.; Li, Y.; Yu, S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 2018, 516, 332–341. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, Y.; Liu, H. Preparation of new crosslinked chitosan with crown ether and their adsorption for silver ion for antibacterial activities. Carbohydr. Polym. 2003, 53, 425–430. [Google Scholar] [CrossRef]
- Evangelista, T.F.; Andrade, G.R.; Nascimento, K.N.; Dos Santos, S.B.; Santos, M.D.; D’Oca, C.D.; Estevam, C.D.; Gimenez, I.F.; Almeida, L.E. Supramolecular polyelectrolyte complexes based on cyclodextrin-grafted chitosan and carrageenan for controlled drug release. Carbohydr. Polym. 2020, 245, 116592. [Google Scholar] [CrossRef]
- Tekade, M.; Maheshwari, N.; Youngren-Ortiz, S.R.; Pandey, V.; Chourasiya, Y.; Soni, V.; Deb, P.K.; Sharma, M.C. Thiolated-chitosan: A novel mucoadhesive polymer for better-targeted drug delivery. In Biomaterials and Bionanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 459–493. [Google Scholar]
- Guaresti, O.; Maiz–Fernández, S.; Palomares, T.; Alonso–Varona, A.; Eceiza, A.; Pérez–Álvarez, L.; Gabilondo, N. Dual charged folate labelled chitosan nanogels with enhanced mucoadhesion capacity for targeted drug delivery. Eur. Polym. J. 2020, 134, 109847. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano-and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhang, Q.; Wang, J. Adsorption of Co2+ and Sr2+ from aqueous solution by chitosan grafted with EDTA. J. Mol. Liq. 2021, 325, 115197. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Mariadoss, A.V.; Sathiyaseelan, A.; Wang, M.H. Synthesis and characterization of nano-chitosan capped gold nanoparticles with multifunctional bioactive properties. Int. J. Biol. Macromol. 2020, 165, 747–757. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.D.; Dhachinamoorthi, D.; Chandra, S.K. Formulation and in vitro evaluation of antineoplastic drug loaded nanoparticles as drug delivery system. Afr. J. Pharm. Pharmacol. 2013, 7, 1592–1604. [Google Scholar] [CrossRef]
- Barrera-Martínez, C.L.; Meléndez-Ortiz, H.I.; Padilla-Vaca, F.; Atanase, L.I.; Peralta-Rodríguez, R.D.; Liakos, I. Dual Loading of Trans-Cinnamaldehyde and Either Paclitaxel or Curcumin in Chitosan Nanoparticles: Physicochemical Characterization and Biological Evaluation Against MDCK and HeLa Cells. Polymers 2024, 16, 3087. [Google Scholar] [CrossRef]
- Adekiya, T.A.; Moore, M.; Thomas, M.; Lake, G.; Hudson, T.; Adesina, S.K. Preparation, Optimization, and In-Vitro Evaluation of Brusatol- and Docetaxel-Loaded Nanoparticles for the Treatment of Prostate Cancer. Pharmaceutics 2024, 16, 114. [Google Scholar] [CrossRef]
- Banerjee, T.; Mitra, S.; Singh, A.K.; Sharma, R.K.; Maitra, A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int. J. Pharm. 2002, 243, 93–105. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Khorram, M.; Mansouri, M.; Samimi, A.; Osfouri, S. Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran. Polym. J. 2012, 21, 99–107. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Luque-Alcaraz, A.G.; Lizardi-Mendoza, J.; Goycoolea, F.M.; Higuera-Ciapara, I.; Argüelles-Monal, W. Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. RSC Adv. 2016, 6, 59250–59256. [Google Scholar] [CrossRef]
- Mohanbhai, S.J.; Sardoiwala, M.N.; Gupta, S.; Shrimali, N.; Choudhury, S.R.; Sharma, S.S.; Guchhait, P.; Karmakar, S. Colon targeted chitosan-melatonin nanotherapy for preclinical Inflammatory Bowel Disease. Biomater. Adv. 2022, 136, 212796. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Mirza, M.A.; Aslam, M.; Mahmood, S.; Iqbal, Z. Doe guided chitosan based nano-ophthalmic preparation against fungal keratitis. Mater. Today Proc. 2021, 41, 19–29. [Google Scholar] [CrossRef]
- Yu, A.; Shi, H.; Liu, H.; Bao, Z.; Dai, M.; Lin, D.; Lin, D.; Xu, X.; Li, X.; Wang, Y. Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int. J. Pharm. 2020, 575, 118943. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, S.; Pereira, M.N.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Targeted clindamycin delivery to pilosebaceous units by chitosan or hyaluronic acid nanoparticles for improved topical treatment of acne vulgaris. Carbohydr. Polym. 2021, 253, 117295. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.U.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Caprifico, A.C.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the protein corona in chitosan-based nanoparticles. Drug Discov. Today 2021, 26, 1825–1840. [Google Scholar] [CrossRef]
- Taala, N.; Bagheri-Khoulenjani, S. Effect of initial particle size and surface charge on the formation of the plasma protein corona on chitosan-based nanoparticles. Polym. Adv. Technol. 2023, 34, 3792–3802. [Google Scholar] [CrossRef]
- Moraru, C.; Mincea, M.; Menghiu, G.; VasileOstafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef]
- Kesharwani, P.; KratikaHalwai, K.; Jha, S.K.; Mohammed, H.; Mughram, A.L.; Almujri, S.S.; Almalki, W.H.; Sahebkar, A. Folate-engineered chitosan nanoparticles: Next-generation anticancer nanocarriers. Mol. Cancer 2024, 23, 244. [Google Scholar] [CrossRef]
- Khalil, A.; Barras, A.; Boukherroub, R.; Tseng, C.; Devos, D.; Burnouf, T.; Neuhaus, W.; Sabine Szunerits, S. Enhancing paracellular and transcellular permeability using nanotechnological approaches for the treatment of brain and retinal diseases. Nanoscale Horiz. 2023, 9, 14–43. [Google Scholar] [CrossRef]
- Salem, D.S.; Hegazy, S.F.; Obayya, S.S. Nanogold-loaded chitosan nanocomposites for pH/light-responsive drug release and synergistic chemo-photothermal cancer therapy. Colloids Interface Sci. Commun. 2021, 41, 100361. [Google Scholar] [CrossRef]
- Mathew, S.A.; Prakash, P.A.; Jaabir, M.M.; Dhanavel, S.; Manikandan, R.; Stephen, A. Dopamine-conjugated CuS/chitosan nanocomposite for targeted photothermal drug delivery: In vitro cytotoxicity study to establish bio-compatibility. J. Drug Deliv. Sci. Technol. 2021, 61, 102193. [Google Scholar] [CrossRef]
- Bhatta, A.; Krishnamoorthy, G.; Marimuthu, N.; Dihingia, A.; Manna, P.; Biswal, H.T.; Das, M.; Krishnamoorthy, G. Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery. Int. J. Biol. Macromol. 2019, 136, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Chekanouskaya, L.; Kraskouski, A.; Hileuskaya, K.; Nikalaichuk, V.; Yuzhyk, L.; Ladutska, A.; Vasilkevich, V.; Bogdanov, R.; Grekova, N.; Yao, W.; et al. Antioxidant, Sun-Protective and Cytotoxic Effects of Chitosan–Glucose Derivatives: A Comparative Study. J. Polym. Environ. 2023, 31, 4875–4890. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, L.; Wang, H.; Hu, Z.; Dong, H.; Yu, Z.; Yuan, Y. A review of curcumin in food preservation: Delivery system and photosensitization. Food Chem. 2023, 30, 136464. [Google Scholar] [CrossRef]
- Alkhader, E.; Roberts, C.J.; Rosli, R.; Yuen, K.H.; Seow, E.K.; Lee, Y.Z.; Billa, N. Pharmacokinetic and anti-colon cancer properties of curcumin-containing chitosan-pectinate composite nanoparticles. J. Biomater. Sci. Polym. Ed. 2018, 29, 2281–2298. [Google Scholar] [CrossRef]
- Sewid, F.A.; Annas, K.I.; Dubavik, A.; Veniaminov, A.V.; Maslova, V.G.; Orlova, A.O. Chitosan nanocomposites with CdSe/ZnS quantum dots and porphyrin. RSC Adv. 2022, 12, 899–906. [Google Scholar] [CrossRef]
- Chachanidze, R.; Xie, K.; Massaad, H.; Roux, D.; Leonetti, M.; Loubens, C. Structural characterization of the interfacial self-assembly of chitosan with oppositely charged surfactant. J Colloid Interface Sci. 2022, 616, 911–920. [Google Scholar] [CrossRef]
- Fini, A.; Orienti, I. The role of chitosan in drug delivery: Current and potential applications. Am. J. Drug Deliv. 2023, 1, 43–59. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2024, 55, 77–93. [Google Scholar] [CrossRef]
- Chiappisi, L.; Gradzielski, M. Co-assembly in chitosan–surfactant mixtures: Thermodynamics, structures, interfacial properties and applications. Adv. Ccolloid Interface Sci. 2015, 220, 92–107. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Manzoor, K.; Venkatachalam, P.; Ikram, S. Kinetic and thermodynamic evaluation of adsorption of Cu (II) by thiosemicarbazide chitosan. Int. J. Biol. Macromol. 2016, 92, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Cellular uptake and cytotoxicity of chitosan–caseinophosphopeptidesnanocomplexes loaded with epigallocatechin gallate. Carbohydr. Polym. 2012, 89, 362–370. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef]
- Dmour, I.; Taha, M.O. Novel Nanoparticles Based on Chitosan-Dicarboxylate Conjugates via Tandem Ionotropic/Covalent Crosslinking with Tripolyphosphate and Subsequent Evaluation as Drug Delivery Vehicles. Int. J. Pharm. 2017, 529, 15–31. [Google Scholar] [CrossRef]
- Florence, A.T. Nanoparticle uptake by the oral route: Fulfilling its potential? Drug Discov. Today Technol. 2005, 2, 75–81. [Google Scholar] [CrossRef]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Drug permeability and mucoadhesion properties of thiolatedtrimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef]
- Lim, C.; Lee, D.W.; Israelachvili, J.N.; Jho, Y.; Hwang, D.S. Contact time-and pH-dependent adhesion and cohesion of low molecular weight chitosan coated surfaces. Carbohydr. Polym. 2015, 117, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.J.; Zhao, H.Y.; Zheng, J.M.; Xu, H.; Wei, G.; Hao, J.S. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2003, 249, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.M.; Dutta, G.; Biswas, R.; Sugumaran, A.; Salem, M.M.; Gamal, M.; AbdEIrahman, M.; Bekhit, M.M.S. Succinyl Curcumin Conjugated Chitosan Polymer-Prodrug Nanomicelles: A Potential Treatment for Type-II Diabetes in Diabetic Balb/C Mice. Acta Chim. Slov. 2024, 71, 421–435. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jerome, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Iurciuc, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef]
- Telange, D.R.; Jain, S.P.; Pethe, A.M.; Kharkar, P.S.; Rarokar, N.R. Use of combined nanocarrier system based on chitosan nanoparticles and phospholipids complex for improved delivery of ferulic acid. Int. J. Biol. Macromol. 2021, 171, 288–307. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef]
- Wu, S.J.; Don, T.M.; Lin, C.W.; Mi, F.L. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar. Drugs 2014, 12, 5677–5697. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alharbi, K.S.; Yasir, M.; Elmowafy, M.; Mohammed, E.F.; Al-Oanzi, Z.H. Formulation of chitosan-coated piperine NLCs: Optimization, in vitro characterization, and in vivo preclinical assessment. AAPS PharmSciTech 2021, 22, 231. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Uyanga, V.A.; Ejeromedoghene, O.; Lambo, M.T.; Alowakennu, M.; Alli, Y.A.; Ere-Richard, A.A.; Min, L.; Zhao, J.; Wang, X.; Jiao, H.; et al. Chitosan and chitosan-based composites as beneficial compounds for animal health: Impact on gastrointestinal functions and biocarrier application. J. Funct. Foods 2023, 104, 105520. [Google Scholar] [CrossRef]








| Marine Habitat | Chitin Source | CaCO3 (%) | Protein (%) | Other (%) | Purity (%) | Yield (%) |
|---|---|---|---|---|---|---|
| Chemical Method | ||||||
| All seas except polar | Lobster shells (Nephropidae) | 0.39 ± 0.23 | 2.22 ± 0.24 | 3.93 ± 0.09 | 93 | 17.21 ± 0.28 |
| All seas except polar | Lobster shells (Nephropidae) | 0.30 ± 0.20 | 2.90 ± 0.25 | 4.17 ± 0.03 | 93 | 16.53 ± 2.35 |
| Indian and North Pacific Ocean | Shrimp shells (Marsupenaeus japonicus) | 0.45 ± 0.10 | 1.13 ± 0.01 | 1.32 ± 0.00 | 97 | 16.08 ± 0.57 |
| DES—mediated method | ||||||
| Coastal mud shrimp | Shrimp shells (Solenocera crassicornis) | 0.3–0.4 | 0.5–0.6 | - | 99.1 ± 0.1 | 4.9v1 |
| Coastal mud shrimp | Shrimp shells (Solenocera crassicornis) | 0.6–0.7 | 07–0.8 | - | 98.6 ± 0.2 | 13.2 ± 1.1 |
| Indian and North Pacific Ocean | Japanese tiger prawn (Marsupenaeus japonicus) | 0.74 | 0.74 ± 0.02 | 1.53 ± 0.02 | 23.86 ± 0.07 | 3.72 ± 0.05 |
| North Atlantic Ocean | Shrimp shells (Solenocera crassicornis) | 0.56 | 0.98 | 0.46 | 98 ± 1 | 19–20 |
| All seas except polar | Lobster shells (Nephropidae) | 0.21 ± 0.31 | 1.81 ± 0.14 | 4.12 ± 0.21 | 93 | 22.21 ± 0.27 |
| All seas except polar | Lobster shells (Nephropidae) | 0.34 ± 0.22 | 1.77 ± 0.22 | 3.88 ± 0.11 | 93 | 21.01 ± 0.23 |
| All seas except polar | Lobster shells (Nephropidae) | 0.24 ± 0.16 | 1.75 ± 0.17 | 4.11 ± 0.23 | 93 | 19.01 ± 0.24 |
| Indian and North Pacific Ocean | Japanese tiger prawn (Marsupenaeus japonicus) | 1.44 ± 0.01 | 3.59 ± 0.02 | 1.92 ± 0.01 | 93 | 25.00 ± 0.60 |
| Indian and North Pacific Ocean | Japanese tiger prawn (Marsupenaeus japonicus) | 1.18 ± 0.01 | 8.37 ± 0.05 | 2.32 ± 0.06 | 88 | 25.18v0.38 |
| Indian and North Pacific Ocean | Japanese tiger prawn (Marsupenaeus japonicus) | 3.60 ± 0.14 | 13.05 ± 0.20 | 1.25 ± 0.04 | 82 | 25.22 ± 0.90 |
| Indian and North Pacific Ocean | Japanese tiger prawn (Marsupenaeus japonicus) | 41.01 ± 1.80 | 15.34 ± 0.18 | 4.56 ± 0.02 | 39 | 50.54 ± 1.07 |
| Indian and North Pacific Ocean | Japanese tiger prawn (Marsupenaeus japonicus) | 44.34 ± 3.40 | 13.50 ± 0.12 | 4.42 ± 0.04 | 38 | 52.45 ± 2.01 |
| Indian and North Pacific Ocean | Japanese tiger prawn (Marsupenaeus japonicus) | 46.19 ± 1.90 | 16.14 ± 0.10 | 6.42 ± 0.09 | 31 | 52.55 ± 0.70 |
| Types of Chitosan Biopolymer | Properties | |
|---|---|---|
| Physicochemical Properties | Biological Properties | |
| Low MW Chitosan (LMWC < 150 kDa) | LMWC possesses a higher solubility, permeability, and tensile strength, but lower viscosity than other melting points with reduced thermostability. It is widely used in food, agriculture, medical, and pharmaceutical fields. | Comparatively, LMWC is more bioactive than other types of chitosan due to the presence of short low-mw chains. Also, the higher degree of deacetylation is associated with increased bioactivity. As per studies, the relationship between chitosan’s mw and cytotoxicity is controversial. |
| Medium MW Chitosan (MMWC = 150–750 kDa) | The aqueous solubility of MMWC is less then LMWC but more than HMWC. Permeability and viscosity are in between the range of HMWC and LMWC, and they exhibit high tensile strength. | They have the best antioxidant properties among the three. The potential of MMWC film has been confirmed by several studies. |
| High MW Chitosan (HMWC > 750 kDa) | Viscosity of HMWC is high but solubility, permeability, and tensile strength are lower. | It can be used as an antibacterial coating in the food industry. It shows the least amount of antioxidant properties due to the presence of more intermolecular hydrogen bonds in the longer chain, resulting in less accessibility to the free radicals. |
| Important Values | Description |
|---|---|
| Sources and structure of CS: | CS is a mucopolysaccharide that occurs naturally, which resembles cellulose by chemical structure but differs in having a functional group of acetylamino. Depending on the origin of the chitin, its physicochemical properties are notably controlled by both its molecular weight and degree of deacetylation, including its solubility in various solvents and pH levels, hydrophobicity, and toxicity. |
| Basic characteristics of CS: | The biocompatible, non-toxic, mucoadhesive, and biodegradable chitosan biopolymer is soluble in an acidic aqueous solution, and its solubility is influenced by its molecular weight and degree of deacetylation. |
| Drug delivery properties of CS: | The positively charged characteristic of CS is likely responsible for its mucoadhesive characteristics. Chitosan can be used to deliver the therapeutics to the body in the form of film, hydrogel, composites, nanoparticles, nanocomposite, scaffolds, nanocarriers etc. |
| Enhancing CS’s properties through chemical modifications: | Chitosan molecules can be functionalized into different derivatives for desired and improved physicochemical and biological properties. |
| Preparation methods of CS-based nanoparticles: | Chitosan nanoparticles can be prepared by using different methods like ionic gelation, solvent diffusion, solvent evaporation methods, nanoprecipitation, the desolvation method, and so on. |
| Administration routes for CSNPs: | Chitosan nanoparticles can be delivered via many routes like ocular, oral, transdermal etc. They are also used for active targeting and vaccine delivery. |
| Enhancing oral absorption and biological activity of phytochemical compounds by using CSNPs: | Phytochemical encapsulation in CSNPs improves their solubility, oral bioavailability, controlled release, and gastrointestinal (GI) stability. Small-sized CS-NPs enhance bioactivity, target specific cells, and reduce extra-organ toxicity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, R.; Mondal, S.; Ansari, M.A.; Sarkar, T.; Condiuc, I.P.; Trifas, G.; Atanase, L.I. Chitosan and Its Derivatives as Nanocarriers for Drug Delivery. Molecules 2025, 30, 1297. https://doi.org/10.3390/molecules30061297
Biswas R, Mondal S, Ansari MA, Sarkar T, Condiuc IP, Trifas G, Atanase LI. Chitosan and Its Derivatives as Nanocarriers for Drug Delivery. Molecules. 2025; 30(6):1297. https://doi.org/10.3390/molecules30061297
Chicago/Turabian StyleBiswas, Ranu, Sourav Mondal, Md Ahesan Ansari, Tanima Sarkar, Iustina Petra Condiuc, Gisela Trifas, and Leonard Ionut Atanase. 2025. "Chitosan and Its Derivatives as Nanocarriers for Drug Delivery" Molecules 30, no. 6: 1297. https://doi.org/10.3390/molecules30061297
APA StyleBiswas, R., Mondal, S., Ansari, M. A., Sarkar, T., Condiuc, I. P., Trifas, G., & Atanase, L. I. (2025). Chitosan and Its Derivatives as Nanocarriers for Drug Delivery. Molecules, 30(6), 1297. https://doi.org/10.3390/molecules30061297






