Flaxseed in Diet: A Comprehensive Look at Pros and Cons
Abstract
1. Introduction
2. Flaxseeds (Linum usitatissum L.)
3. Composition of Flaxseed
3.1. Nutrients
3.1.1. Alpha-Linolenic Acid
3.1.2. Proteins
3.1.3. Fiber
3.1.4. Vitamins
3.1.5. Minerals
3.2. Phenolic Compounds
3.2.1. Lignans
3.2.2. Phenolic Acids and Flavonoids
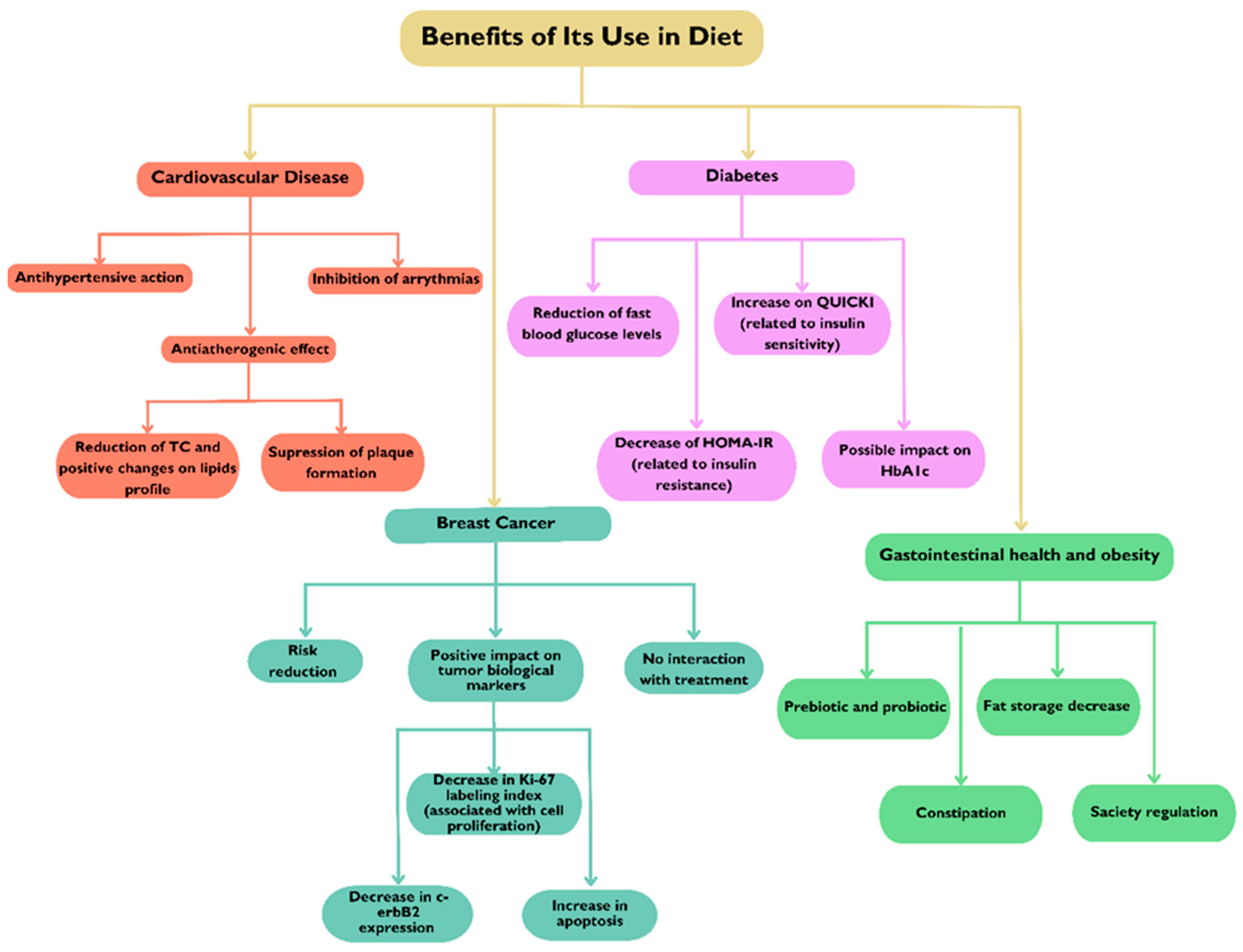
4. Benefits of Its Use in Diet
4.1. Cardiovascular Disease
4.2. Diabetes
4.3. Breast Cancer
4.4. Gastrointestinal Health and Obesity
5. Potential Toxicity
5.1. Phytic Acid
5.2. Trypsin Inhibitors
6. Cyanogenic Glycosides
6.1. Determination Techniques
| Samples | Cyanogenic Compounds | Extraction | Analytical Technique | LOD | LOQ | Recovery | Precision (RSD) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Flaxseed from a previous study, ethanol-extracted ground flax, flax pressed cake, spent flaxseed, and CO2-extracted flaxseed | Linamarin, linustatin, and neolinustatin | See Table 2. | HPLC-RI Analytical column: RP-18 (4 × 250 mm, 10 µm particle size). Mobile phase: 95/4.95/0.05 H2O/methanol/H3PO4. Injection volume: 20 µL. Flow rate: 0.7 mL/min. Temperature: 21 °C. Isocratic mode. | N.A. | N.A. | N.A. | 37.41% | 1996 | [63,64] |
| Mature oil-type flaxseed from 7 different cultivars grown in 2 locations in 3 different years | Linustatin | Extraction solvent: methanol/H2O 3:1 (v/v). Four successive extractions with a ratio of 6:1 of solvent volume to the mass of ground seed, with an ultrasonic bath for 30 min at 40 °C. Centrifugation between each extraction: 10 min, 4 °C, 3824× g. | qNMR Dried samples + 1.5 mL methanol/D2O 3:1 (v/v) + analysis standard: TMSP 0.1 mg/mL. Homogenization and centrifugation at 20,000× g and 4 °C for 10 min. Supernatant was collected in a 5 mm NMR tube. 1H and 13C assignments for linustatin, neolinustatin, and related compounds. | 149.4 μg/g | 249.1 μg/g | 95.41 ± 1.93% | <6% | 2017 | [61] |
| Neolinustatin | 230.8 μg/g | 384.6 μg/g | |||||||
| Linamarin | 7.1 μg/g | 11.8 μg/g | |||||||
| Lotaustralin | 7.6 μg/g | 12.7 μg/g | |||||||
| Epilotaustralin | 6.3 μg/g | 10.6 μg/g | |||||||
| Amygdalin | 193.8 μg/g | 323.0 μg/g | |||||||
| Linforce® (flaxseed coated with two herbal extracts—Senna alexandrina mill and Frangula alnus) and flaxseed | Linustatin | UAE: ultrasonic bath with 160 W; sonication cycle: 30 s ON, 10 s OFF at room temperature. Centrifugation: with conical rotor at 7000 rpm at room temperature. Cryogrounding: with a freezer/mill; duration time: 9 min (3 × 3 min); frequency: 10 impacts/s; stored at −20 °C until use. Three-step preparation method: 1. Aqueous methanol UAE (methanol/H2O (75/25, v/v)), resulting supernatant I (after centrifugation) and residue; 2. Residue submitted to alkaline UAE (0.08 M NaOH/methanol (25/75, v/v)), resulting supernatant II (after centrifugation); the combined extract solutions were hydrolyzed by 0.02 M NaOH, acidified (pH 2–3), diluted, and filtered. | UHPLC/ESI-HRMS Analytical column: BEH C18 column (2.1 × 100 mm, 1.7 um, 130 Å). ** Mobile phase A: Milli-Q water/formic acid (99.9/0.1, v/v), containing also 50 µM NaCl. Mobile phase B: acetonitrile/formic acid (99.9/0.1, v/v), containing also 50 µM NaCl. Gradient mode; flow rate: 0.2 mL/min. Temperature of the auto sampler: 6 °C. Volume of injection: 5 µL. ESI in positive mode; capillary potential: 4000 V; end-plate offset: 500 V; nebulizing gas: nitrogen, pressure at 276 K Pa; drying gas: nitrogen at 9.0 L/min; temperature: 200 °C. | 2.0 µg/g | 6.6 µg/g | 92.3–102.5% | Repeatability precision: 1.1–6.9%. Intermediate precision: 2.4–6.6%. | 2019 | [60] |
| Neolinustatin | |||||||||
| Field-grown flaxseed from 5 different cultivars and chamber-grown flaxseed from other 5 different cultivars | Linustatin | Derivatization of CNGs/preparation of TMS derivatives: Reaction: the defatted seed powder + HMDS + TMCS + IMD in a microcentrifuge tube for 15 min at 50 °C in a sonicating water bath. Centrifugation: 20,817× g for 5 min. Preparation for LC-MS/MS: an aliquot of the silylated extract + mobile phase was put in a microcentrifuge tube. Centrifugation: 14,000 rpm for 5 min. The diluted extract was transferred to a glass vial. | For quantification: GC–Flame ionization detector (FID) Analytical column: a capillary column (50% phenyl methylpolysiloxane, 30 m × 0.32 mm, 0.25 mm film thickness). Injection volume: 1 µL. Temperature gradient: increased from 190 °C to 280 °C at 50 °C/min. Held for 2.2 min, for an analysis of 4 min. Carrier gas: hydrogen. Flow rate: 5 mL/min. Injector temperature: 275 °C. Split ratio: 50:1. FID temperature: 300 °C. Nitrogen (make-up gas) + hydrogen + air flow rates: 30, 40, 430 mL/min, respectively. For characterization: HPLC-MS/MS Analytical column: 50 mm × 2.0 mm Fast Gradient RP-18e. Mobile phase: 100% acetonitrile. Injection volume: 3 µL. Flow rate: 0.4 mL/min. Temperature: ambient temperature. MS analysis: micrOTOF-Q II hybrid quadrupole time-of-flight MS/MS with ESI ion source operated in an MRM mode. | 6.43 µg/mL | 19.50 µg/mL | In 10 mg matrix: 79.9–107.4%. In 20 mg matrix: 94.2–112.7%. | <15% | 2019 | [65] |
| Neolinustatin | 4.72 µg/mL | 14.31 µg/mL | |||||||
| Flaxseed purchased from a supermarket | Linamarin | The samples were ground, dissolved in 80% methanol aqueous solution, placed in an ultrasonic bath, and centrifuged; this extraction was repeated twice. Solid-Phase Extraction (clean-up) Solid-phase extraction column: Prime HBL. Washing solution: 5 mL of water and 2 mL of 10% methanol aqueous solution. Elution solution: methanol/acetonitrile (30:70, v/v). The elution was repeated 3 times. Eluate was collected and evaporated at 40 °C to dryness under nitrogen. The resultant residue was redissolved in 10% methanol aqueous solution. | UHPLC-QqQ-MS/MS Analytical column: C18 RRHD (2.1 mm × 50 mm, 1.8 µm). *** Mobile phase A: water with 0.1% (v/v) formic acid. Mobile phase B: acetonitrile. Gradients used: isocratic and linear. Flow rate: 0.3 mL/min. Injection volume: 10 μL. Column temperature: 35 °C. MS analysis: QqQ MS equipped with ESI. | 5 ng/g | 20 ng/g | 89.88–125.69% | Intra-day: <10.38% Inter-day: <11.33% | 2020 | [59] |
| Lotaustralin | 1 ng/g | 5 ng/g | |||||||
| Linustatin | 5 ng/g | 20 ng/g | |||||||
| Neolinustatin | 5 ng/g | 20 ng/g | |||||||
| Taxiphyllin | 5 ng/g | 20 ng/g | |||||||
| Amygdalin | 2 ng/g | 10 ng/g | |||||||
| Dhurrin | 2.5 ng/g | 10 ng/g | |||||||
| Prunasin | 25 ng/g | 100 ng/g | |||||||
| 9 cold-pressed flaxseed oil from “Jingdong Mall” | Linustatin | Solid-Phase Extraction 1. SPE column filled with 120 g of cigarette filter fiber conditioned with 5% (v/v) isopropanol/n-hexane solution. 2. Loaded with the sample oil diluted in 5% (v/v) isopropanol/n-hexane solution. 3. Washed with 5% (v/v) isopropanol/n-hexane solution. 4. Desorption of the CNGs with methanol. 5. Blow-drying under nitrogen of the eluent, redissolution in 30% methanol aqueous solution. | UHPLC-MS/MS Analytical column: HSS T3 column (2.1 mm × 100 mm, 1.7 μm) **** Mobile phase A: water; Mobile phase B: acetonitrile. Temperature: 40 °C. Injection volume: 10 μL. Flow rate: 0.4 mL/min. Gradient mode. Detection with an ESI in the negative mode under selective detection mode (SDM). Voltage: 2800 V. Capillary temperature: 350 °C. Dry temperature: 300 °C. | 0.23 pg/g | 0.76 pg/g | 113–133% | 0.8–20.5% | 2022 | [62] |
| Neolinustatin | 0.13 pg/g | 0.44 pg/g | |||||||
| Linamarin | 0.43 pg/g | 1.43 pg/g | |||||||
| Lotaustralin | 0.44 pg/g | 1.48 pg/g | |||||||
| Flaxseed, flaxseed cake, and products containing these ingredients | Amygdalin | Extraction solvent: 1% formic acid in methanol/water (25/75) (v/v). Extraction: 30 min on a rotary tumbler. Centrifugation: 10 min at 3000 rpm. The supernatant is transferred to a filter vial and diluted. | UHPLC-MS/MS Analytical column: BEH C18 (100 × 2.1 mm, 1.7 µm). Mobile phase A: 0.1% formic acid in water. Mobile phase B: methanol (acetonitrile can also be used in small retention times). Column temp.: 50 °C. Flow rate: 0.4 mL/min. Injection volume: 2–5 µL. Gradient mode. ESI operated in positive mode; performing MRM: capillary voltage: 30 kV; cone voltage: 30 V; source temperature: 150 °C; desolvation temperature: 600 °C; cone gas flow: 150 L/h; desolvation gas flow: 800 L/h; CID gas: argon (0.0043 mbar); solvent discard: 0–2 and 10–11.5 min. | N.A. | 1 mg HCN eq./kg | N.A. | N.A. | 2023 | [66] |
| Linamarin | |||||||||
| Linustatin | |||||||||
| Lotaustralin | |||||||||
| Neolinustatin | |||||||||
| Prunasin |
| Sample | Hydrolysis (for HCN Determination) | Extraction | Analytical Technique | LOD | LOQ | Recovery | Precision (RSD) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Flaxseed from a previous study, ethanol-extracted ground flax, flax pressed cake, spent flaxseed, and CO2-extracted flaxseed. | Extraction of cyanogenic glycosides Extraction solvent: 70% ethanol aqueous solution. Extraction conditions: in a sonic water bath for 30 min at 30 °C. Filtration: with a 0.45 µm filter through glass wool. Extraction of the crude enzyme 1. From ground flaxseed with cold acetone (−20 °C) in a blender for 1 min. 2. Filtration under vacuum through a Whatman No. 1 paper and re-extraction of the residue 3 more times with acetone. 3. Solvent removal on a desiccator under vacuum at 4 °C. | Using a barbituric acid–pyridine reagent: 1. The flaxseed extract was transferred to a stoppered test tube and evaporated with a nitrogen stream. 2. Adding sodium acetate buffer 0.1 M (pH 6) and incubation of the solution with the crude enzyme extract for 1 h at 30 °C. 3. The reaction ends by adding 0.2 M NaOH, then it stands for 5 min at room temperature, and is neutralized with 0.2 M HCl. 4. Chloramine T 0.5% (w/v) + buffered extract + barbituric acid–pyridine reagent in a test tube that stands at room temperature for 4 min (pink complex). 5. Absorbance measured at 585 nm (using a blank solution). 6. The calibration curve was traced using potassium cyanide as a reference standard (concentration range of 0.1–3.5 µg of HCN). | N.A. | N.A. | 94 ± 3% | 36.27% | 1996 * | [63,64] | |
| _ | 4. Extract + cold sodium acetate buffer (pH 6) in a blender for 1 min. 5. Centrifugation: 12,000× g for 2 h. 6. The supernatant was put in a fume hood for 3 h at room temperature to allow hydrolysis of any residual cyanogenic glycosides to HCN. 7. The extract was freeze-dried and stored. | Using pyridine–pyrazolone reagent: 1. The flaxseed extract was transferred to a stoppered test tube and evaporated with a nitrogen stream. 2. Adding sodium phosphate buffer 0.1 M (pH 6) and incubation of the solution with the crude enzyme extract for 1 h at 30 °C. 3. The reaction ends by adding 0.1 M NaOH and 0.1 M sodium phosphate buffer (pH 6), to ensure total decomposition of the hydroxynitrites that result from enzymatic activity. 4. Chloramine T 0.5% (w/v) + buffered extract in a stoppered test tube, mixed, and put in an ice-water bath for 5 min. 5. Adding the pyridine–pyrazolone reagent to the mixture and let the tube stand for 1 h at room temperature (blue complex). 6. Absorbance measured at 620 nm (using a blank solution). 7. The calibration curve was traced using potassium cyanide as a reference standard (concentration range 0.1–3.5 µg of HCN). | N.A. | N.A. | 37.37% | [63,64] | |||
| Edible plants from 14 generas including Linum (flaxseed) from both South and North Korea, and China. | Acid Hydrolysis (50 min) 1. Centrifugation: sample + 0.1 M phosphoric acid for 20 min at 8000 rpm. 2. The supernatant + 4 M sulfuric acid added in a tightly capped vial. 3. Start hydrolysis with heating up to 100 °C. 4. The reaction mixture is cooled in ice. | Distillation Performed in a Micro-Dist tube. 1. The hydrolysis mixture + 0.79 M MgCl2 is heated in the tube for 45 min. 2. Cooling at ambient temperature. 3. Cyanide is collected in the 0.2 M NaOH solution. | Ion Chromatography Analytical column: IonPac AS7 (40 mm × 250 mm, 10 μm particle size). Guard column: IonPac AG7 (40 mm × 50 mm, 10 μm particle size). Detector: ED40 (electrochemical detector), DC Amperometry. Flow rate: 1.0 mL/min (isocratic). Injection volume: 200 μL. Electrode cell: silver working electrode (0.00 V vs. Ag/AgCl reference). Mobile phase: 0.5 M sodium acetate/0.1 M sodium hydroxide/0.5% (v/v) Ethylenediamine. | 0.005 mg/L | N.A. | N.A. | <10% | 2013 | [70] |
| 3 trademarks of each whole flaxseed type: brown and golden. 3 trademarks of bran of each flaxseed type: brown and golden. * All acquired in main supermarkets or natural products stores in Natal/Rio Grande do Norte. | Acid Hydrolysis (3 h) 1. The sample is transferred into a round bottom flask coupled to a distiller. 2. Closing of the distillation system by dipping the condenser’s end in an Erlenmeyer containing 2.5% NaOH solution. 3. Hydrolysis starts by adding distilled water and sulfuric acid 10%. | Distillation 1. Starts when sulfuric acid 10% is added. 2. First distillation: distillate 1 is collected into the Erlenmeyer containing the 2.5% NaOH solution. 3. Second distillation: begins when distilled water and 10% H2SO4 are added. The distillate 2 is collected in another Erlenmeyer with 2.5% NaOH solution. | Colorimetric Determination Sample tube: an aliquot of distillate + alkaline picrate 0.5% solution (red-orange compound). Blank tube: distilled water + alkaline picrate 0.5% solution. The tubes were shaken, closed, and put in a water bath at 70 °C for 10 min. Calibration curve: for its construction, a sodium cyanide solution (50 µg/mL) was used to prepare 5 standard solutions corresponding to 50, 100, 150, 200, and 250 µg CN−. Absorbance was measured at 490 nm. | N.A. | N.A. | N.A. | N.A. | 2023 | [71] |
6.2. Levels of Cyanogenic Glycosides in Flaxseeds
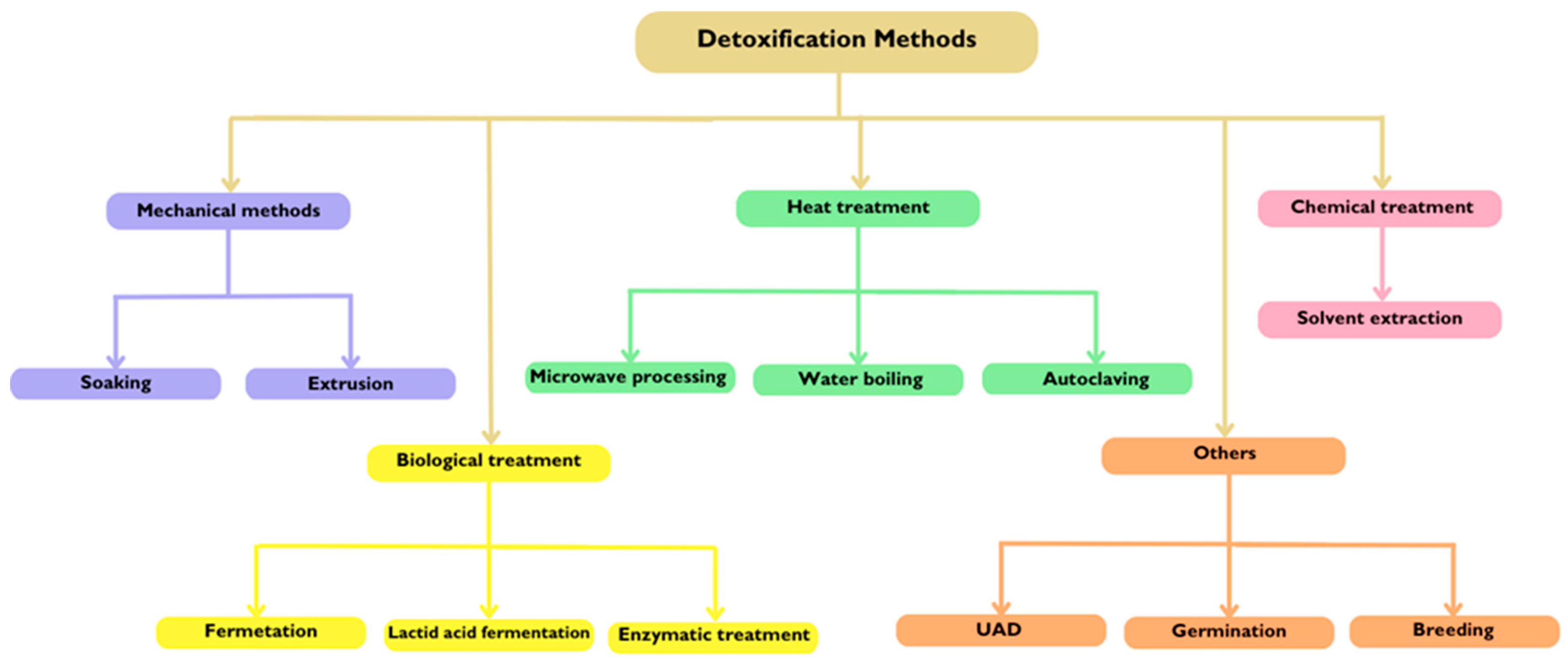
6.3. Detoxification Methods
| Sample | Detoxification Technique | Description | Removal Rate | Study Conclusion | Year | Ref. |
|---|---|---|---|---|---|---|
| Flaxseed, Nei Ya-3 Cultivar, northeast China | Extrusion | Equipment: co-rotating twin-screw extruder; length-to-diameter ratio of 27.9:1; screw diameter of 47 mm; circular die of 5.2 mm; equipped with two heating units. Optimal conditions obtained: temperature: 146.0 °C; feeding rate: 32.7 kg/h; crew speed: 152.5 rpm; moisture content: 12.5%. | 91.62% | The predicted value for the removal of HCN from flaxseed was 93.23% and the experimental result was 91.62%, constituting a relative error of only 1.76%. The feeding rate was increased to 60 kg/h to improve productivity. The experimental value was 83.32% (relative error of 1.27%). Both detoxification levels were within the required limits. | 2008 | [82] |
| Fresh flaxseed from the Lanxi County of Heilongjiang Province, China | Enzymatic treatment/fermentation | Enzymatic preparation: 12.5% human liver ꞵ-glucosidase and 8.9% (w/w) Bacillus sp. cyanide hydratase prepared in the laboratory. Standard fermentation medium: flaxseed power + water + MgCl2 + MnCl2; pH adjusted to 6.3; autoclaved at 115 °C for 1 h to inactivate the endogenous β-glucosidase. The fermentation medium and the enzymatic preparation were mixed and incubated at 46.8 °C for 48 h. After that, the residual cyanide and the CNGs were measured in the samples. | 99.3% (degradability) | The flaxseed power treated with the enzymatic preparation retained lignans and fatty acids. Although this method seems efficient, low-cost, and a protector of beneficial nutrients, it is necessary to prove that the detoxified flaxseed is safe for consumption. This method also provides new ways of removing CNGs from other edible plants. | 2012 | [83] |
| Flaxseed from 3 Polish high-α-linolenate flax varieties: 1 brown (Szafir variety) and 2 golden seeds (Oliwin and Jantarol varieties). | Solvent extraction method | Defatting previous double cold extraction with hexane. Aqueous extraction: DFF to water ratio of 1:15 (m/v), under constant stirring with a magnetic stirrer at ambient temperature for 1 h. Centrifugation: for 25 min, 1500× g. The supernatant was freeze-dried. | 95.7–97.7% | This was a comparative study of flaxseed extracts since they can be applied as food ingredients. Aqueous extracts demonstrated a significant reduction in CNGs but less antioxidant activity. Ethanolic extraction, although it resulted in higher antioxidant activity, also showed a higher CNG content, and if used, ethanolic extracts have to be controlled. For detoxifying purposes, flaxseed processing with water such as extraction, but also soaking and wet autoclaving, can decrease significantly CNG content. | 2015 | [84] |
| Ethanolic extraction: DFF was extracted twice with 60% aqueous ethanol with a flaxseed to solvent ratio of 1:7.5 (m/v), under constant vigorous shaking using a lab-scale orbital, at ambient temperature for 1 h. Filtration; centrifugation: 20 min, 1500× g. Removal of ethanol by evaporation of the supernatant using a rotary vacuum evaporator. The samples were freeze-dried. | 30.2–33.6% | |||||
| 5 samples of flaxseed intended for use in animal feed | Enzymatic hydrolysis followed by evaporation | Mixing, grinding, and extraction (with an acidic aqueous solution): adding one or more cellulose or starch materials (wheat bran and sunflower cake) helps disrupt the cells and facilitates the interaction between ꞵ-glycosidases (short-term vapor impregnation for activation) and the CNGs. The maturation: provides sufficient time for the hydrolytic reaction (pH 6 and 38 °C). Extrusion applies pressure and temperature that reduce the HCN and water content. Drying and cooling: decrease the humidity and increase the stability of the final detoxified material (most of the HCN is evaporated during the physical part). | 89–92% | The levels of hydrogen cyanide (HCN) in the 5 samples ranged from 86 to 127 mg/kg, which were reduced to <11 mg/kg after decontamination. An additional study on decontaminated samples containing flaxseed from various batches showed an average HCN content of 12 mg/kg. The method proved efficacy, meeting EU requirements for the quality of flaxseed concerning HCN after decontamination, without affecting the nutritional quality or leaving harmful residues. Emissions of HCN into the atmosphere must comply with national regulations. | 2017 | [85] |
| Brown and golden flaxseed | Germination | This method was carried out for 5 days in a dark and closed incubator at 25 °C. To avoid microbial growth seeds were washed twice a day and collected daily to measure the changes in root length. The samples were treated and oven-dried at 105 °C to constant weight to determine their content. The results correspond to the percentage of weight loss during drying. | Linustatin: 1309.09 ± 68.63 μg/g to 112.73 ± 7.44 μg/g (≈91.39%). Neolinustatin: 1144.91 ± 22.82 μg/g to 93.89 ± 5.77 μg/g (≈91.80%). Lotaustralin: 1138.46 ± 20.29 μg/g to 72.99 ± 7.26 μg/g (≈93.59%). | Even though germination significantly increased linamarin content, it reduced the other antinutritional compounds. This method also promotes active components, increasing SDG content, flaxseed oil, phenolic and vitamin E content, and thus flaxseed antioxidant activity. | 2019 | [86] |
| Flaxseed mixture of brown and golden seed varieties from local market in Beijing | Microwave processing | Samples were dispersed on glass plates. Equipment: household microwave oven. Frequency: 2450 MHz. Output power: 450 W. Heating up time: 12 min. | 70.9% | The water-boiling method eliminated the highest CNG content followed by UAD, solvent extraction method, microwave processing, autoclaving, and soaking. However, the analysis of this ranking needs to consider the disadvantages of the different methods. The water-boiling method requires a high temperature that may affect the functional properties of flaxseed since it causes vitamin loss and amino acid and protein denaturation. UAD, in turn, can be an effective method and not affect the beneficial nutrients under controlled conditions. | 2020 | [81] |
| Water-boiling method | DFF was poured into boiling water (100 °C) for 30 min with continuous stirring, then cooled down at room temperature, centrifuged (3000 rpm, 15 min, 25 °C), filtered, and freeze-dried. | 94.3% | ||||
| Autoclaving | Equipment: autoclave machine. Temperature: 120 °C. Time: 20 min. | 62.6% | ||||
| Ultrasound-assisted detoxification (UAD) | The samples were mixed with distilled water in a solid–liquid ratio of 1:20 (w/v) and stirred in a water bath for 30 min. Equipment: Sonics Vibracell Probe. Power: 300 W; frequency: 20 kHz; pulsed mode: 10 s ON and 3 s OFF; temperature: 50 °C; time interval: 20 min. | 92.1% | ||||
| Soaking | DFF was soaked in distilled water with a continuous change in water. Time interval: 48 h. Samples were submitted to a vacuum filtration and freeze-dried. | 48.2% | ||||
| Solvent extraction method | Extraction solvent: methanol/ammonia/water (90/5/5). The mixture was stirred (500 rpm) for 15 min, vacuum filtered, and freeze-dried. The extraction was repeated twice for each sample. | 82.4% | ||||
| Whole sorrel flaxseed from G.A & Robin Fenton Farm and G&G Edmunds Farm | Bench-scale fermentation | Equipment: square narrow-mouth polypropylene fermentation bottle with a gas trap. Inoculation of a mixed culture of Lactobacillaceae (i.e., Lactobacillus sp., Limosilactobacillus sp., and Lactiplantibacillus sp., Lacticaseibacillus sp., Levilactobacillus sp., and Lentilactobacillus sp.) in-house cultured previously isolated from wheat-based thin stillage in a solution containing whole flaxseed + commercial white vinegar (3% acetic acid v/v; pH ≈ 3) + distilled water. Conditions of incubation: water bath at 30 °C for 72 h. Preparation for analysis: the fermentation media was passed through a 12′′ 200 µm test sieve, rinsed with tap water, dried overnight at 60 °C, and ground in a coffee grinder. | After 48 h of fermentation, linustatin and neolinustatin were below the detection limits and, consequently, total HCN < 10 mg/kg. | Fermentation of flaxseed using Lactobacillaceae successfully removed cyanogenic glycosides and retained beneficial nutritional components such as oil, fatty acids, and SDG. The proposed method can be readily implemented in flaxseed processing based on a pilot-scale feasibility study but lacks further development to produce higher quality flaxseed that can be safely and readily used in food and other product production. | 2023 | [87] |
| Scale-up fermentation | These experiments were conducted at 2 volumes, 4 L and 8 L: sorrel flaxseed and bacterial inoculant. Fermentation took place in a mechanical oven incubator at 30 °C for 72 h. Flaxseed was degummed after being rinsed with tap water and dried, spread in silicone sheets, using a 160 L commercial-grade food dehydrator at 60 °C. | Linustatin: 2659.3 ± 45.7 mg/kg to <500 mg/kg (>81.2%). Neolinustatin: 2761.4 ± 16.8 mg/kg to <500 mg/kg (>81.9%). Total HCN: 351.8 ± 2.5 mg/kg to <10 mg/kg (>97.2%). |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, C., 3rd; Tulbek, M.C.; Xu, Y. Flaxseed. Adv. Food Nutr. Res. 2006, 51, 1–97. [Google Scholar] [PubMed]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency The Biology of Linum usitatissimum L. (Flax). Available online: https://inspection.canada.ca/en/plant-varieties/plants-novel-traits/applicants/directive-94-08/biology-documents/linum-usitatissimum-flax (accessed on 28 February 2024).
- Kauser, S.; Hussain, A.; Ashraf, S.; Fatima, G.; Ambreen; Javaria, S.; Abideen, Z.U.; Kabir, K.; Yaqub, S.; Akram, S.; et al. Flaxseed (Linum usitatissimum); Phytochemistry, Pharmacological Characteristics and Functional Food Applications. Food Chem. Adv. 2024, 4, 100573. [Google Scholar] [CrossRef]
- Fu, Y.-B. Genetic Evidence for Early Flax Domestication with Capsular Dehiscence. Genet. Resour. Crop Evol. 2011, 58, 1119–1128. [Google Scholar] [CrossRef]
- Cabi Digital Library. Linum usitatissimum (Flax). CABI Compendium; CABI Head Office: Wallingford, UK, 2019. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 5 August 2024).
- Olombrada, E.; Mesias, M.; Morales, F.J. Risk/Benefits of the Use of Chia, Quinoa, Sesame and Flax Seeds in Bakery Products. An Update Review. Food Rev. Int. 2024, 40, 1047–1068. [Google Scholar] [CrossRef]
- Nasiri, F.; Mohtarami, F.; Esmaiili, M.; Pirsa, S. Production of Gluten-Free Biscuits with Inulin and Flaxseed Powder: Investigation of Physicochemical Properties and Formulation Optimization. Biomass Convers. Biorefin. 2024, 14, 21443–21459. [Google Scholar] [CrossRef]
- Epaminondas, P.S.; Araújo, K.L.G.V.; de Souza, A.L.; Silva, M.C.D.; Queiroz, N.; Souza, A.L.; Soledade, L.E.B.; Santos, I.M.G.; Souza, A.G. Influence of Toasting on the Nutritious and Thermal Properties of Flaxseed. J. Therm. Anal. Calorim. 2011, 106, 551–555. [Google Scholar] [CrossRef]
- INSA_pt. Available online: https://jspapp.test.insa.foodcase-services.com/foodcomp/food?23745 (accessed on 2 August 2024).
- Melelli, A.; Durand, S.; Alvarado, C.; Kervoëlen, A.; Foucat, L.; Grégoire, M.; Arnould, O.; Falourd, X.; Callebert, F.; Ouagne, P.; et al. Anticipating Global Warming Effects: A Comprehensive Study of Drought Impact of Both Flax Plants and Fibres. Ind. Crops Prod. 2022, 184, 115011. [Google Scholar] [CrossRef]
- Zare, S.; Mirlohi, A.; Sabzalian, M.R.; Saeidi, G.; Koçak, M.Z.; Hano, C. Water Stress and Seed Color Interacting to Impact Seed and Oil Yield, Protein, Mucilage, and Secoisolariciresinol Diglucoside Content in Cultivated Flax (Linum usitatissimum L.). Plants 2023, 12, 1632. [Google Scholar] [CrossRef]
- Yaqoob, N.; Bhatti, I.A.; Anwar, F.; Mushtaq, M.; Artz, W. Variation in Physico-Chemical/Analytical Characteristics of Oil among Different Flaxseed (Linum usittatissimum L.) Cultivars. Ital. J. Food Sci. 2016, 28, 83–89. [Google Scholar]
- Qiu, C.; Wang, H.; Guo, Y.; Long, S.; Wang, Y.; Abbasi, A.M.; Guo, X.; Jarvis, D.I. Comparison of Fatty Acid Composition, Phytochemical Profile and Antioxidant Activity in Four Flax (Linum usitatissimum L.) Varieties. Oil Crop Sci. 2020, 5, 136–141. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and Functional Effects of Plant-Derived Omega-3 Fatty Acids in Humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Tufail, T.; Bader Ul Ain, H.; Ali, A.; Aadil, R.M.; Nemat, A.; Manzoor, M.F. Antioxidant Activity and Phytochemical Analysis of Fennel Seeds and Flaxseed. Food Sci. Nutr. 2023, 11, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Martinchik, A.N.; Baturin, A.K.; Zubtsov, V.V.; Molofeev, V.I. Nutritional value and functional properties of flaxseed. Vopr. Pitan. 2012, 81, 4–10. [Google Scholar] [PubMed]
- Plaha, N.S.; Awasthi, S.; Sharma, A.; Kaushik, N. Distribution, Biosynthesis and Therapeutic Potential of Lignans. 3 Biotech 2022, 12, 255. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and Flaxseed Oil: An Ancient Medicine & Modern Functional Food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [PubMed]
- Wang, Y.; Fofana, B.; Roy, M.; Ghose, K.; Yao, X.-H.; Nixon, M.-S.; Nair, S.; Nyomba, G.B.L. Flaxseed Lignan Secoisolariciresinol Diglucoside Improves Insulin Sensitivity through Upregulation of GLUT4 Expression in Diet-Induced Obese Mice. J. Funct. Foods 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Prasad, K. Antihypertensive Activity of Secoisolariciresinol Diglucoside (SDG) Isolated from Flaxseed: Role of Guanylate Cyclase. Int. J. Angiol. 2011, 13, 7–14. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, Detoxification, Utilization, and Opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Dave Oomah, B.; Mazza, G.; Kenaschuk, E.O. Flavonoid Content of Flaxseed. Influence of Cultivar and Environment. Euphytica 1996, 90, 163–167. [Google Scholar] [CrossRef]
- Associação Portuguesa de Nutrição—Dislipidemias: Caracterização e Tratamento Nutricional. Available online: https://www.apn.org.pt/documentos/ebooks/Ebook_Dislipidemias_CaracterizacaoETratamentoNutricional.pdf (accessed on 6 March 2024).
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its Bioactive Components and Their Cardiovascular Benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary Modulation of Oxylipins in Cardiovascular Disease and Aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Weighell, W.; Edel, A.L.; LaVallee, R.; Dibrov, E.; Pinneker, R.; Maddaford, T.G.; Ramjiawan, B.; Aliani, M.; Guzman, R.; et al. Potent Antihypertensive Action of Dietary Flaxseed in Hypertensive Patients. Hypertension 2013, 62, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Edel, A.L.; Rodriguez-Leyva, D.; Maddaford, T.G.; Caligiuri, S.P.; Austria, J.A.; Weighell, W.; Guzman, R.; Aliani, M.; Pierce, G.N. Dietary Flaxseed Independently Lowers Circulating Cholesterol and Lowers It beyond the Effects of Cholesterol-Lowering Medications Alone in Patients with Peripheral Artery Disease. J. Nutr. 2015, 145, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Yu, D.; Demark-Wahnefried, W.; Franco, O.H.; Lin, X. Meta-Analysis of the Effects of Flaxseed Interventions on Blood Lipids. Am. J. Clin. Nutr. 2009, 90, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Dupasquier, C.M.C.; Dibrov, E.; Kneesh, A.L.; Cheung, P.K.M.; Lee, K.G.Y.; Alexander, H.K.; Yeganeh, B.K.; Moghadasian, M.H.; Pierce, G.N. Dietary Flaxseed Inhibits Atherosclerosis in the LDL Receptor-Deficient Mouse in Part through Antiproliferative and Anti-Inflammatory Actions. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2394–H2402. [Google Scholar] [CrossRef]
- Francis, A.A.; Deniset, J.F.; Austria, J.A.; LaValleé, R.K.; Maddaford, G.G.; Hedley, T.E.; Dibrov, E.; Pierce, G.N. Effects of Dietary Flaxseed on Atherosclerotic Plaque Regression. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1743–H1751. [Google Scholar] [CrossRef] [PubMed]
- Dupasquier, C.M.C.; Weber, A.-M.; Ander, B.P.; Rampersad, P.P.; Steigerwald, S.; Wigle, J.T.; Mitchell, R.W.; Kroeger, E.A.; Gilchrist, J.S.C.; Moghadasian, M.M.; et al. Effects of Dietary Flaxseed on Vascular Contractile Function and Atherosclerosis during Prolonged Hypercholesterolemia in Rabbits. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2987–H2996. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Dietary Flaxseed Lignan Extract Lowers Plasma Cholesterol and Glucose Concentrations in Hypercholesterolaemic Subjects. Br. J. Nutr. 2008, 99, 1301–1309. [Google Scholar] [CrossRef]
- Almario, R.U.; Karakas, S.E. Lignan Content of the Flaxseed Influences Its Biological Effects in Healthy Men and Women. J. Am. Coll. Nutr. 2013, 32, 194–199. [Google Scholar] [CrossRef]
- Holy, E.W.; Forestier, M.; Richter, E.K.; Akhmedov, A.; Leiber, F.; Camici, G.G.; Mocharla, P.; Lüscher, T.F.; Beer, J.H.; Tanner, F.C. Dietary α-Linolenic Acid Inhibits Arterial Thrombus Formation, Tissue Factor Expression, and Platelet Activation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Weber, A.R.; Rampersad, P.P.; Gilchrist, J.S.C.; Pierce, G.N.; Lukas, A. Dietary Flaxseed Protects against Ventricular Fibrillation Induced by Ischemia-Reperfusion in Normal and Hypercholesterolemic Rabbits. J. Nutr. 2004, 134, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, W.; Li, J.; Liang, H.; Zhou, H.; Duan, W.; Xu, X.; Yu, S.; Zhang, H.; Yi, D. α-Linolenic Acid Intake Attenuates Myocardial Ischemia/Reperfusion Injury through Anti-Inflammatory and Anti-Oxidative Stress Effects in Diabetic but Not Normal Rats. Arch. Med. Res. 2011, 42, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective Mechanism of Omega-3 Polyunsaturated Fatty Acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, Z.; Pourfarziani, P.; Mohamad Jafari Kakhki, A.; Sedgh Ahrabi, S.; Hossein Moridpour, A.; Mollaghasemi, N.; Musazadeh, V.; Hossein Faghfouri, A. The Effect of Flaxseed Supplementation on Glycemic Control in Adults: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2023, 110, 105816. [Google Scholar] [CrossRef]
- Zhu, L.; Sha, L.; Li, K.; Wang, Z.; Wang, T.; Li, Y.; Liu, P.; Dong, X.; Dong, Y.; Zhang, X.; et al. Dietary Flaxseed Oil Rich in Omega-3 Suppresses Severity of Type 2 Diabetes Mellitus via Anti-Inflammation and Modulating Gut Microbiota in Rats. Lipids Health Dis. 2020, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Zhou, W.; Sohaib, M.; Niu, Y.; Zhu, R.; Guo, Y.; Wang, S.; Mao, J.; Wang, X.; Guo, L. Flaxseed Supplementation Significantly Reduces Hemoglobin A1c in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutr. Res. 2023, 110, 23–32. [Google Scholar] [CrossRef]
- Calado, A.; Neves, P.M.; Santos, T.; Ravasco, P. The Effect of Flaxseed in Breast Cancer: A Literature Review. Front. Nutr. 2018, 5, 4. [Google Scholar] [CrossRef]
- Haggans, C.J.; Hutchins, A.M.; Olson, B.A.; Thomas, W.; Martini, M.C.; Slavin, J.L. Effect of Flaxseed Consumption on Urinary Estrogen Metabolites in Postmenopausal Women. Nutr. Cancer 1999, 33, 188–195. [Google Scholar] [CrossRef]
- Thompson, L.U.; Chen, J.M.; Li, T.; Strasser-Weippl, K.; Goss, P.E. Dietary Flaxseed Alters Tumor Biological Markers in Postmenopausal Breast Cancer. Clin. Cancer Res. 2005, 11, 3828–3835. [Google Scholar] [CrossRef]
- McCann, S.E.; Edge, S.B.; Hicks, D.G.; Thompson, L.U.; Morrison, C.D.; Fetterly, G.; Andrews, C.; Clark, K.; Wilton, J.; Kulkarni, S. A Pilot Study Comparing the Effect of Flaxseed, Aromatase Inhibitor, and the Combination on Breast Tumor Biomarkers. Nutr. Cancer 2014, 66, 566–575. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, Y.; Mai, Y.; Gao, L.; Ou, S.; Wang, Y.; Liu, L.; Peng, X. Flaxseed Gum Reduces Body Weight by Regulating Gut Microbiota. J. Funct. Foods 2018, 47, 136–142. [Google Scholar] [CrossRef]
- Ma, J.; Sun, J.; Bai, H.; Ma, H.; Wang, K.; Wang, J.; Yu, X.; Pan, Y.; Yao, J. Influence of Flax Seeds on the Gut Microbiota of Elderly Patients with Constipation. J. Multidiscip. Healthc. 2022, 15, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Austria, J.A.; Richard, M.N.; Chahine, M.N.; Edel, A.L.; Malcolmson, L.J.; Dupasquier, C.M.C.; Pierce, G.N. Bioavailability of Alpha-Linolenic Acid in Subjects after Ingestion of Three Different Forms of Flaxseed. J. Am. Coll. Nutr. 2008, 27, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.; Kouakou, B.; Chen, J. Phytic Acid in Cereal Grains: Structure, Healthy or Harmful Ways to Reduce Phytic Acid in Cereal Grains and Their Effects on Nutritional Quality. Am. J. Plant Nutr. Fertil. Technol. 2010, 1, 1–22. [Google Scholar] [CrossRef]
- Nissar, J.; Ahad, T.; Naik, H.R.; Hussain, S.K. A Review Phytic Acid: As Antinutrient or Nutraceutical. Available online: https://www.phytojournal.com/archives/2017/vol6issue6/PartV/6-6-208-319.pdf (accessed on 15 May 2024).
- Khare, B.; Sangwan, V.; Rani, V. Influence of Sprouting on Proximate Composition, Dietary Fiber, Nutrient Availability, Antinutrient, and Antioxidant Activity of Flaxseed Varieties. J. Food Process. Preserv. 2021, 45, e15344. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation Methods of Trypsin Inhibitor in Legumes: A Review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef]
- Liener, I.E. Trypsin Inhibitors: Concern for Human Nutrition or Not? J. Nutr. 1986, 116, 920–923. [Google Scholar] [CrossRef]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of Functional Ingredients from Flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef]
- Vetter, J. Plant Cyanogenic Glycosides. In Plant Toxins; Springer: Dordrecht, The Netherlands, 2017; pp. 287–317. ISBN 9789400764637. [Google Scholar]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed-a Potential Functional Food Source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, T.; Chen, Q.; Li, K.; Zhang, Z.; Song, H.; Wang, M.; Wu, X.; Lu, B. Development and Validation of Eight Cyanogenic Glucosides via Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry in Agri-Food. Food Chem. 2020, 331, 127305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bergaentzlé, M.; Flieller, A.; Marchioni, E. Development and Validation of an Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry Method for Simultaneous Quantification of Cyanogenic Glycosides and Secoisolariciresinol Diglucoside in Flaxseed (Linum usitatissimum L.). J. Chromatogr. A 2019, 1601, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Roulard, R.; Fontaine, J.-X.; Jamali, A.; Cailleu, D.; Tavernier, R.; Guillot, X.; Rhazi, L.; Petit, E.; Molinie, R.; Mesnard, F. Use of qNMR for Speciation of Flaxseeds (Linum usitatissimum) and Quantification of Cyanogenic Glycosides. Anal. Bioanal. Chem. 2017, 409, 7011–7026. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.-D.; Wu, J.-Y.; Bai, Y.-L.; Feng, Y.-Q. Highly Sensitive Analysis of Cyanogenic Glycosides in Cold-Pressed Flaxseed Oil by Employing Cigarette Filter Fiber-Based SPE Coupled with Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Chem. 2022, 377, 131962. [Google Scholar] [CrossRef]
- Kobaisy, M.; Oomah, B.D.; Mazza, G. Determination of Cyanogenic Glycosides in Flaxseed by Barbituric Acid−Pyridine, Pyridine−Pyrazolone, and High-Performance Liquid Chromatography Methods. J. Agric. Food Chem. 1996, 44, 3178–3181. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G.; Kenaschuk, E.O. Cyanogenic Compounds in Flaxseed. J. Agric. Food Chem. 1992, 40, 1346–1348. [Google Scholar] [CrossRef]
- Shahwar, D.; Young, L.W.; Shim, Y.Y.; Reaney, M.J.T. Extractive Silylation Method for High Throughput GC Analysis of Flaxseed Cyanogenic Glycosides. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1132, 121816. [Google Scholar] [CrossRef]
- EURL-MP-Method_010 Cyanogenic Glucosides (CNGs) in Food and Feed by LC-MSMS. WFSR Wageningen University & Research, 2023, Version 1. pp. 1–20. Available online: https://www.wur.nl/en/show/eurlmp-method_010-cyanogenic-glucosides-cngs-in-food-and-feed-by-lc-msms-v1.htm (accessed on 12 July 2024).
- Water Corporation—Acquity UPLC Columns. Available online: https://imchem.fr/assets/files/Waters/ACQUITY_UPLC_%202012%20720001140en.pdf (accessed on 2 September 2024).
- Agilent—ZORBAX Part Number 959757-902. Available online: https://www.agilent.com/store/pt_BR/Prod-959757-902/959757-902 (accessed on 2 September 2024).
- Waters Corporation—How to Choose a Column. Available online: https://www.waters.com/webassets/cms/library/docs/lcHowToChooseAColumn.pdf (accessed on 2 September 2024).
- Cho, H.-J.; Do, B.-K.; Shim, S.-M.; Kwon, H.; Lee, D.-H.; Nah, A.-H.; Choi, Y.-J.; Lee, S.-Y. Determination of Cyanogenic Compounds in Edible Plants by Ion Chromatography. Toxicol. Res. 2013, 29, 143–147. [Google Scholar] [CrossRef]
- Pereira, M.L.d.S.; de Souza, R.d.C.P.; de Medeiros, J.V.F.; de Brito, G.Q.; Schwarz, A. Determination of Cyanide in Whole Seeds and Brans of Linseed (Linum usitatissimum Linn) by Molecular Spectrophotometry. Braz. J. Pharm. Sci. 2023, 59, e23059. [Google Scholar] [CrossRef]
- Nyirenda, K.K. Toxicity Potential of Cyanogenic Glycosides in Edible Plants. In Medical Toxicology; Erkekoglu, P., Ogawa, T., Eds.; IntechOpen: London, UK, 2021; ISBN 9781838802776. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Evaluation of the Health Risks Related to the Presence of Cyanogenic Glycosides in Foods Other than Raw Apricot Kernels. EFSA J. 2019, 17, e05662. [Google Scholar] [PubMed]
- Regulation-2023/915-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 21 July 2024).
- RASFF. Available online: https://food.ec.europa.eu/safety/rasff_en (accessed on 6 August 2024).
- European Commission—RASFF Window. Notification 2022.2641: Increased Cyanide Content in Organic Flaxseed from Germany. 2022. Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/547009 (accessed on 8 August 2024).
- European Commission—RASFF Window. Notification 2023.0750: Hydrocyanic Acid (HCN) in Organic Blond Flaxseed from Türkiye. 2023. Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/593771 (accessed on 8 August 2024).
- European Commission—RASFF Window. Notification 2024.2851: Hydrocyanic Acid in Flaxseed from Poland. 2024. Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/677295 (accessed on 8 August 2024).
- European Commission—RASFF Window. Notification 2024.3101: Hydrocyanic Acid in Flaxseed from Belgium. 2024. Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/679173 (accessed on 8 August 2024).
- European Commission—RASFF Window. Notification 2024.3166: Hydrocyanic Acid in Organic Brown Flaxseed from India. 2024. Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/679478 (accessed on 8 August 2024).
- Safdar, B.; Pang, Z.; Liu, X.; Rashid, M.T.; Jatoi, M.A. Structural and Functional Properties of Raw and Defatted Flaxseed Flour and Degradation of Cynogenic Contents Using Different Processing Methods. J. Food Process Eng. 2020, 43, e13406. [Google Scholar] [CrossRef]
- Wu, M.; Li, D.; Wang, L.-J.; Zhou, Y.-G.; Brooks, M.S.-L.; Chen, X.D.; Mao, Z.-H. Extrusion Detoxification Technique on Flaxseed by Uniform Design Optimization. Sep. Purif. Technol. 2008, 61, 51–59. [Google Scholar] [CrossRef]
- Wu, C.-F.; Xu, X.-M.; Huang, S.-H.; Deng, M.-C.; Feng, A.-J.; Peng, J.; Yuan, J.-P.; Wang, J.-H. An Efficient Fermentation Method for the Degradation of Cyanogenic Glycosides in Flaxseed. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of Extraction Method on the Phenolic and Cyanogenic Glucoside Profile of Flaxseed Extracts and Their Antioxidant Capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Assessment of a Decontamination Process for Hydrocyanic Acid in Linseed Intended for Use in Animal Feed. EFSA J. 2017, 15, e05004. [Google Scholar]
- Li, X.; Li, J.; Dong, S.; Li, Y.; Wei, L.; Zhao, C.; Li, J.; Liu, X.; Wang, Y. Effects of Germination on Tocopherol, Secoisolarlciresinol Diglucoside, Cyanogenic Glycosides and Antioxidant Activities in Flaxseed (Linum usitatissimum L.). Int. J. Food Sci. Technol. 2019, 54, 2346–2354. [Google Scholar] [CrossRef]
- Huang, C.; Tse, T.J.; Purdy, S.K.; Chicilo, F.; Shen, J.; Meda, V.; Reaney, M.J.T. Depletion of Cyanogenic Glycosides in Whole Flaxseed via Lactobacillaceae Fermentation. Food Chem. 2023, 403, 134441. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Reggiani, R. Variation in the Content of Cyanogenic Glycosides in Flaxseed Meal from Twenty-One Varieties. Food Nutr. Sci. 2014, 5, 1456–1462. [Google Scholar] [CrossRef]
- Pavelek, M.; Tejklová, E.; Bjelková, M. Results of Linseed Breeding in the Czech Republic. Tag. Ver. Pfl Anzenzüchter Saatgutkaufl Eute Osterr. 2011, 61, 127–129. [Google Scholar]
- Salem, M.A.; Ezzat, S.M.; Giavalisco, P.; Sattar, E.A.; El Tanbouly, N. Application of a Comprehensive Metabolomics Approach for the Selection of Flaxseed Varieties with the Highest Nutritional and Medicinal Attributes. J. Food Drug Anal. 2021, 29, 214–239. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, S.; Shah, M.A.; Sanches Silva, A. Flaxseed in Diet: A Comprehensive Look at Pros and Cons. Molecules 2025, 30, 1335. https://doi.org/10.3390/molecules30061335
Duarte S, Shah MA, Sanches Silva A. Flaxseed in Diet: A Comprehensive Look at Pros and Cons. Molecules. 2025; 30(6):1335. https://doi.org/10.3390/molecules30061335
Chicago/Turabian StyleDuarte, Sara, Muhammad Ajmal Shah, and Ana Sanches Silva. 2025. "Flaxseed in Diet: A Comprehensive Look at Pros and Cons" Molecules 30, no. 6: 1335. https://doi.org/10.3390/molecules30061335
APA StyleDuarte, S., Shah, M. A., & Sanches Silva, A. (2025). Flaxseed in Diet: A Comprehensive Look at Pros and Cons. Molecules, 30(6), 1335. https://doi.org/10.3390/molecules30061335









