An Alternative to Biliverdin, Mesobiliverdin IXα and Mesobiliverdin-Enriched Microalgae: A Review on the Production and Applications of Mesobiliverdin-Related Products
Abstract
:1. Introduction
1.1. Cytoprotective Activities of BV
1.2. Limitations of BV as a Therapeutic Anti-Inflammatory and Antioxidant
2. Mesobiliverdin IXα (MBV) as an Alternative to BV
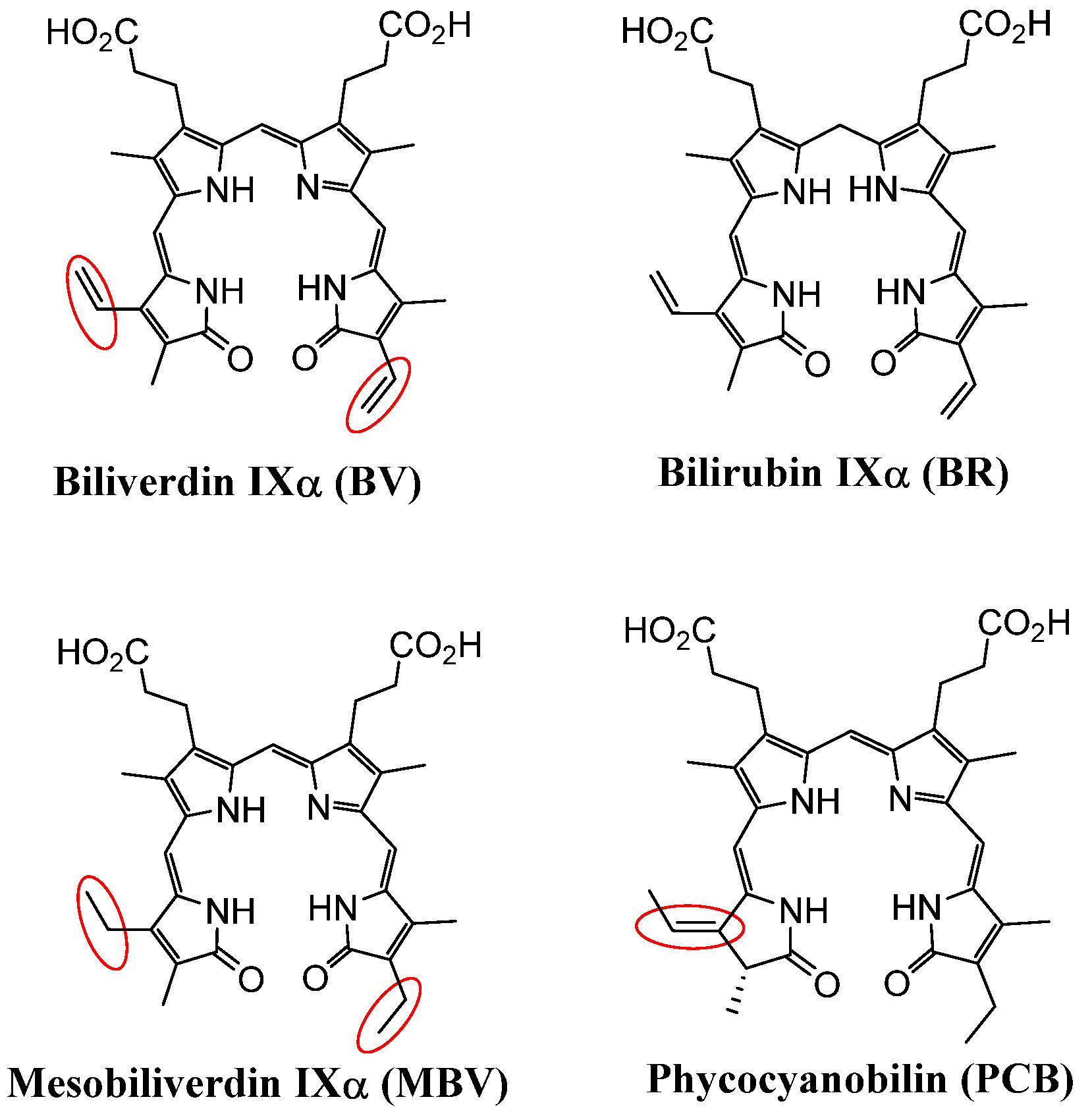
2.1. Structural Comparison of BV, Mesobiliverdin IXα (MBV), and Phycocyanobilin (PCB)
2.2. MBV Production from PCB
3. Investigations and Applications of MBV and MEM
3.1. MBV Cytoprotection Against Oxidative Stress in Pancreatic Islet Allograft Transplantation
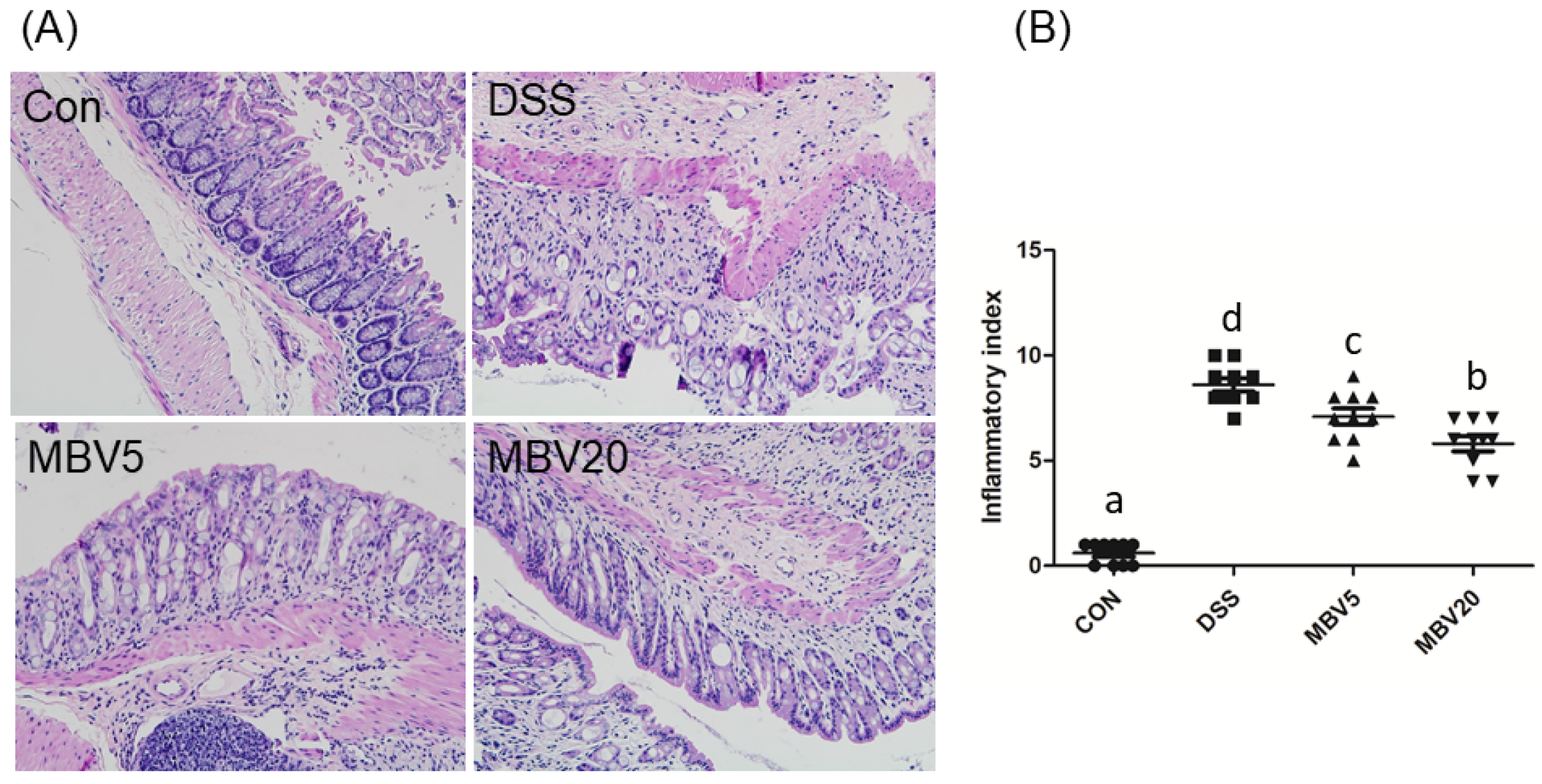
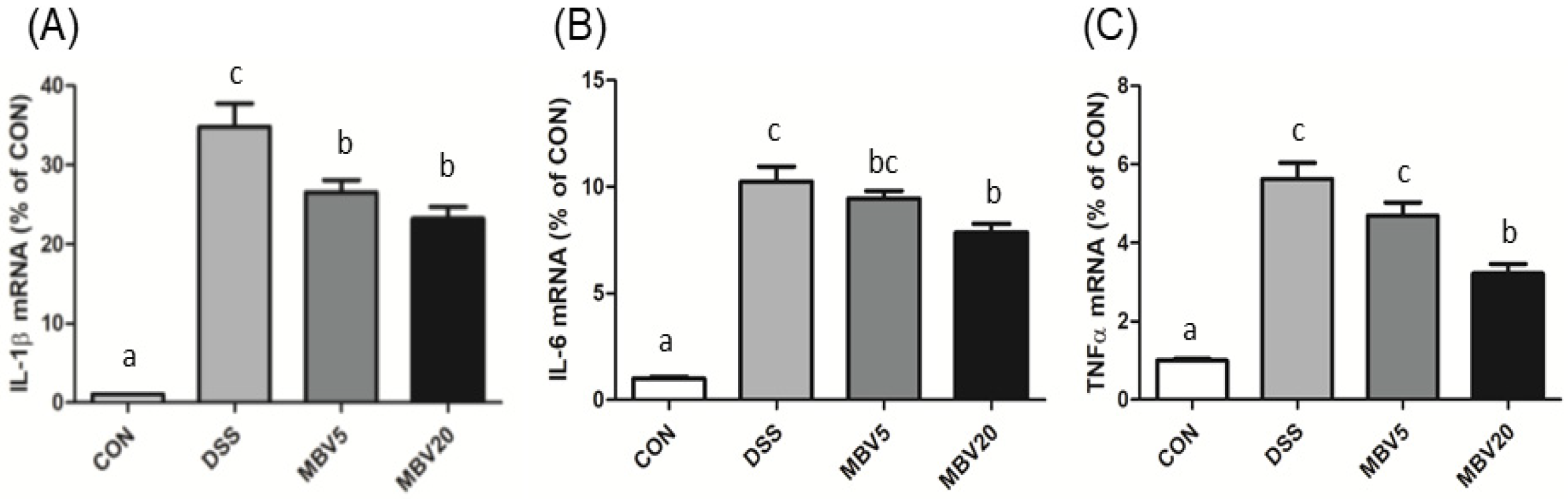
3.2. MBV Amelioration of DSS-Induced Colitis
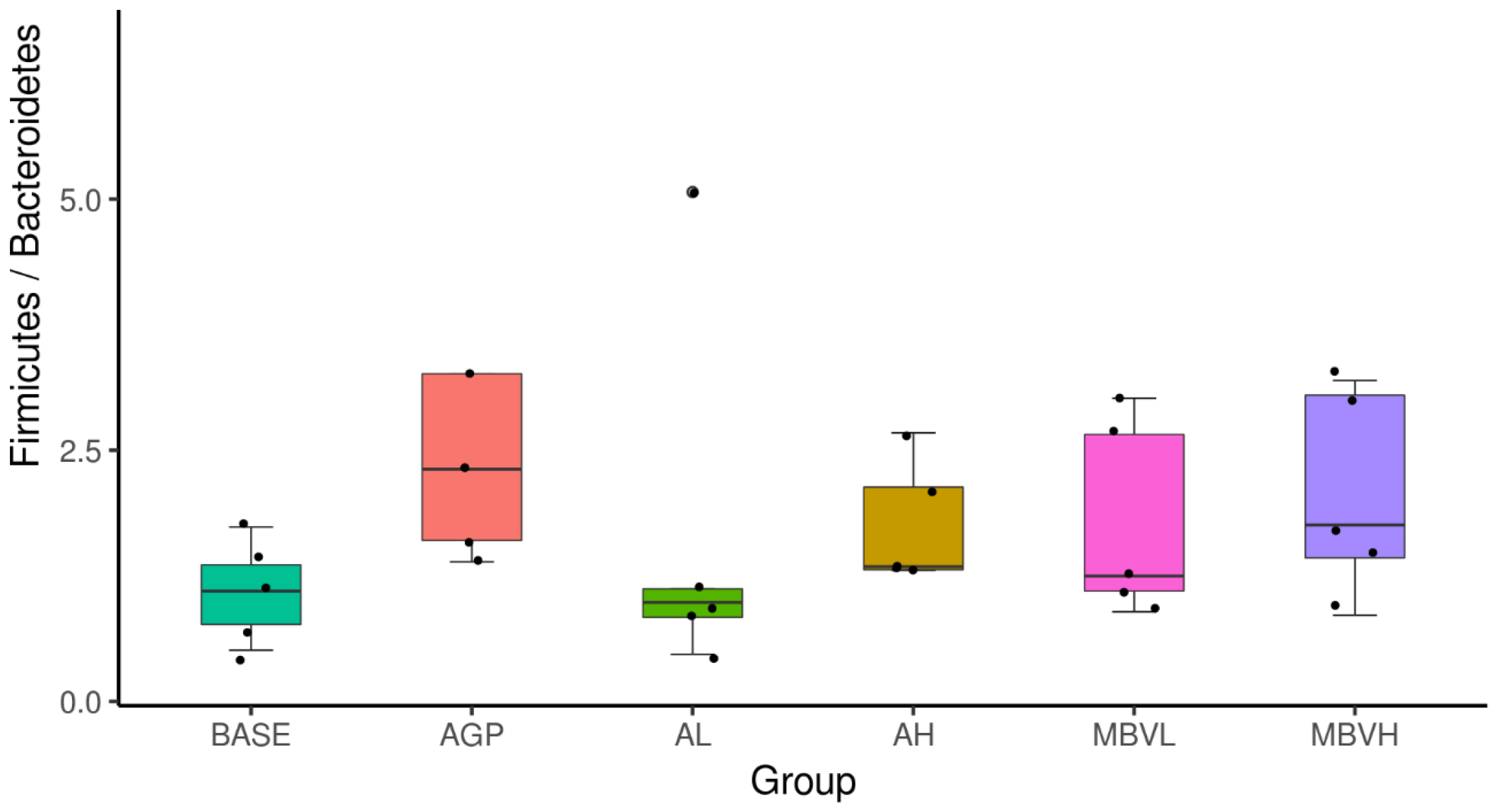
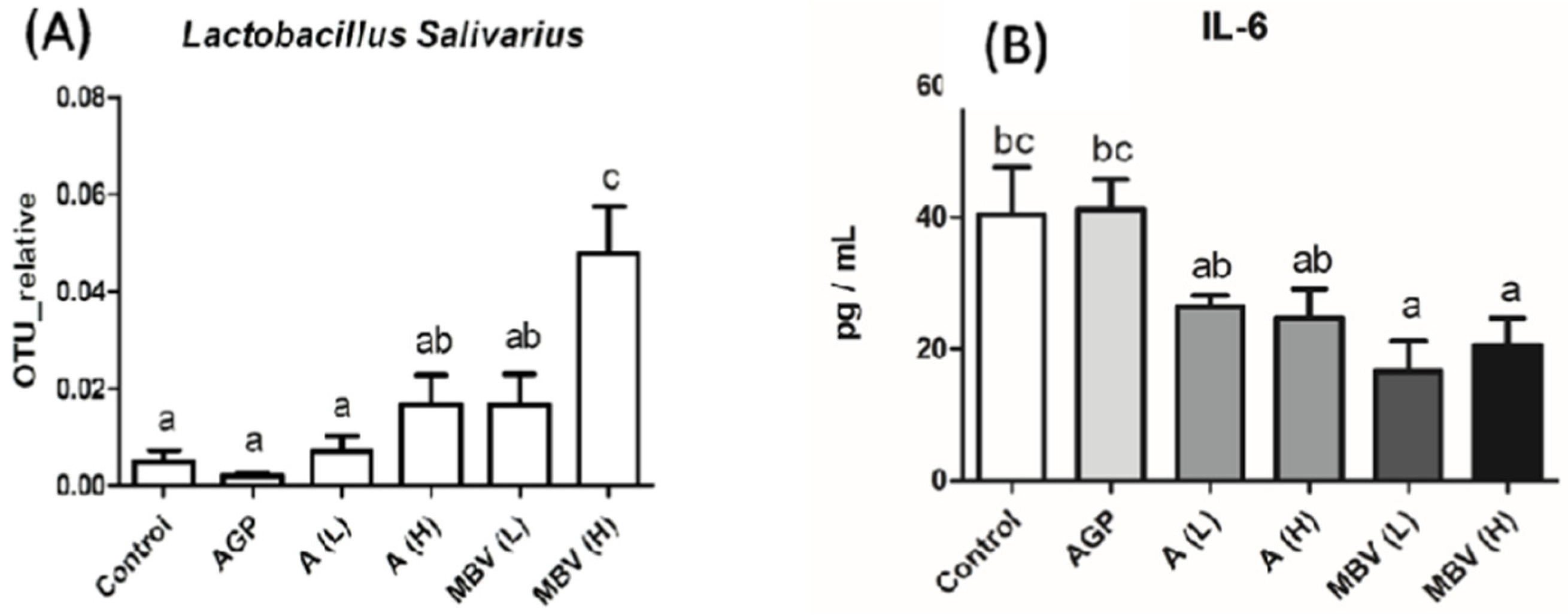
3.3. MEM Improved Chicken Gut Health and Growth Without Antibiotic Supplementation
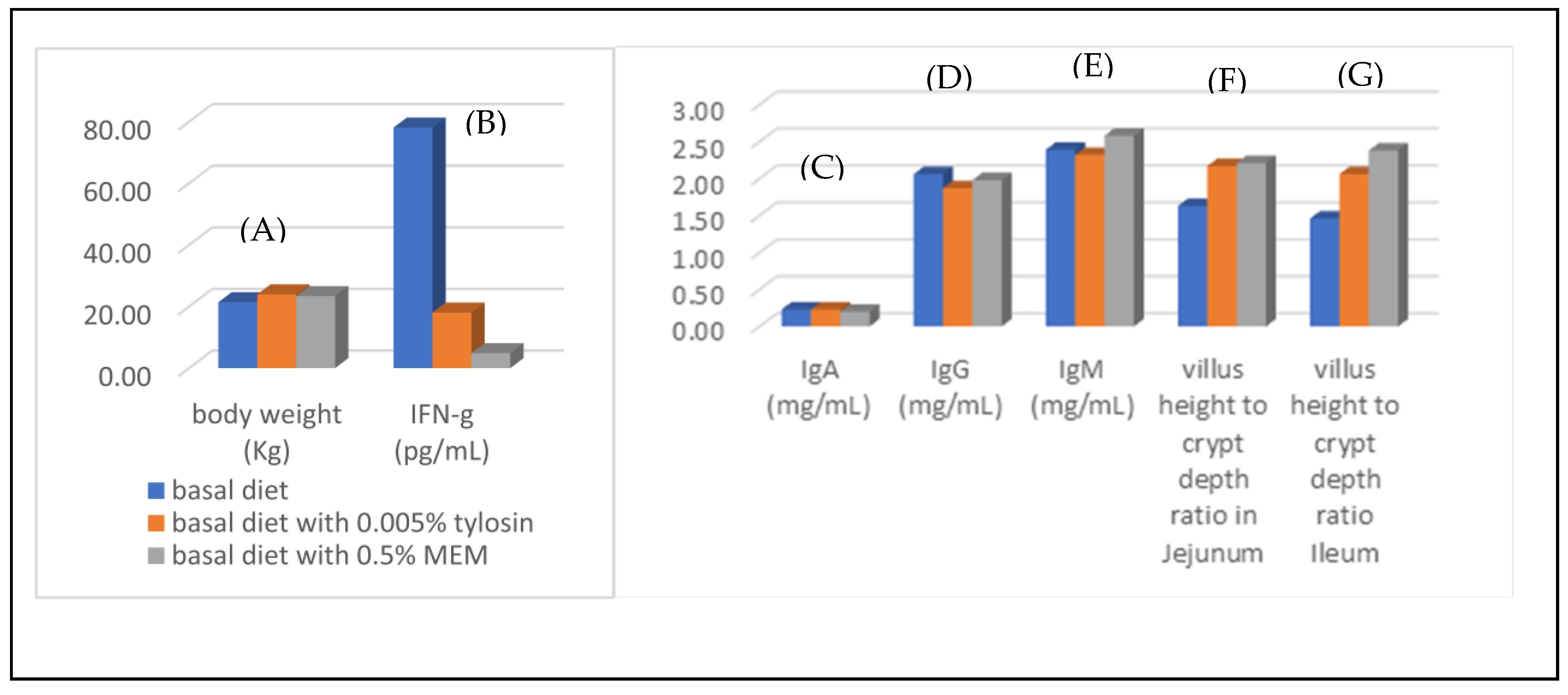
3.4. Gut Health Improvement of Weaning Piglets
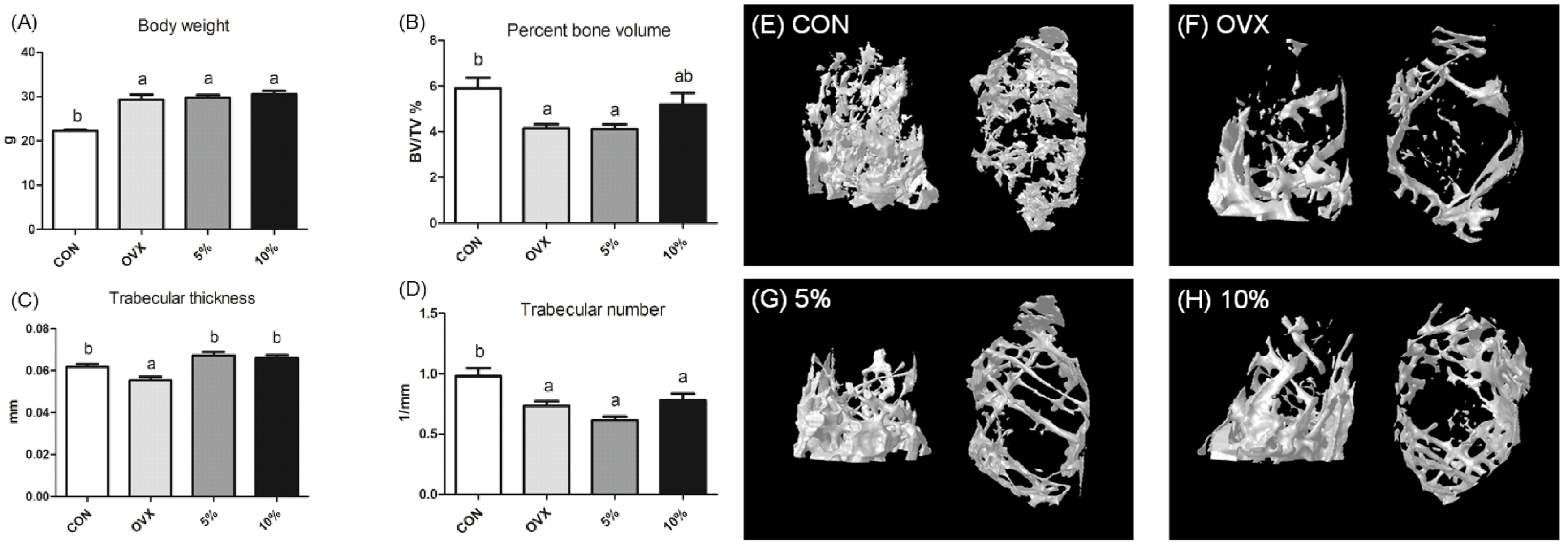
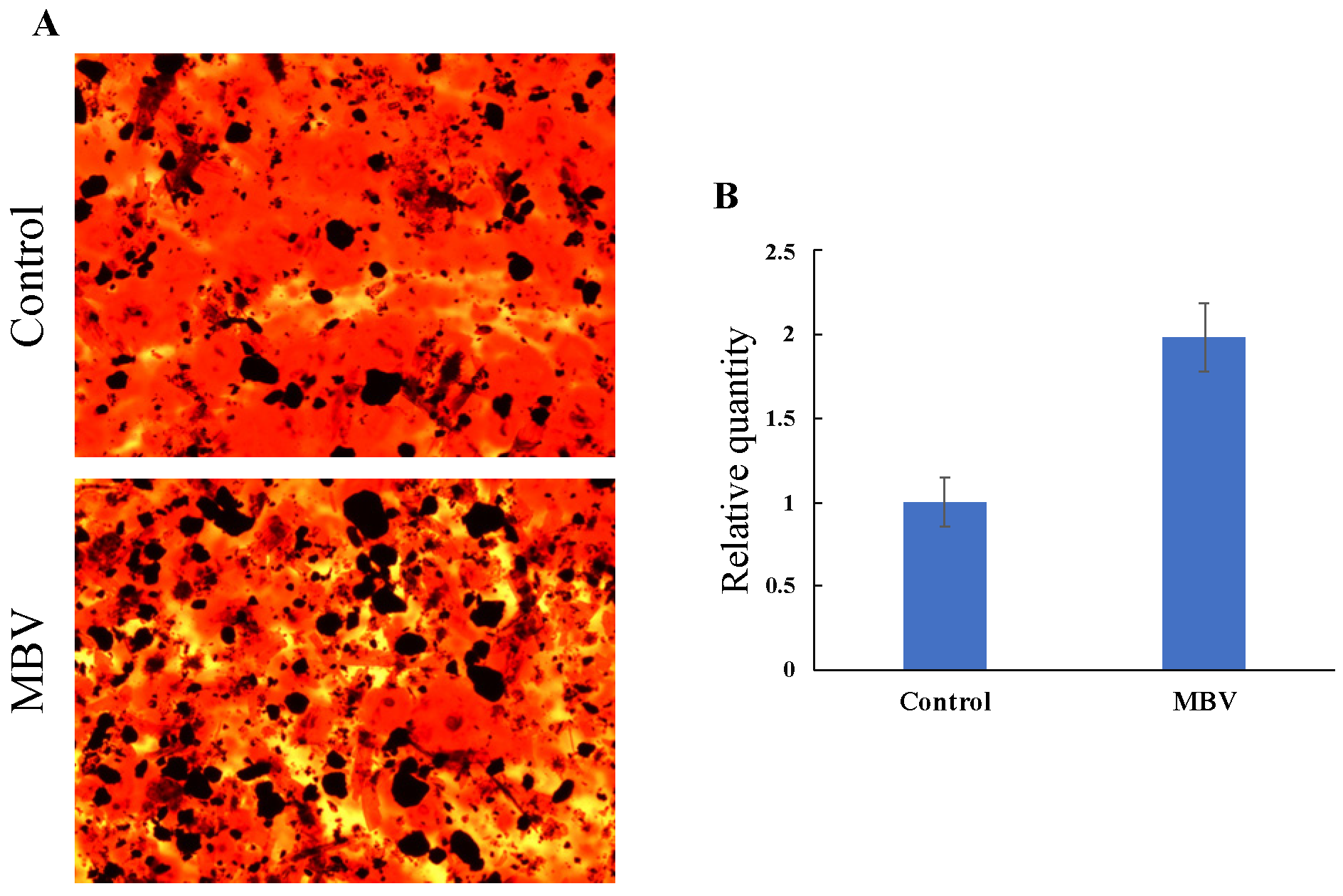
3.5. Amelioration of Osteoporosis via Promoting Osteogenic Differentiation of Mesenchymal Stem Cells
4. Conclusions and Future Research Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- McDonagh, A.F. Turning green to gold. Nat. Struct. Biol. 2001, 3, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Blanckaert, N.; Heirwegh, K.P.; Compernolle, F. Synthesis and separation by thin-layer chromatography of bilirubin-IX isomers. Their identification as tetrapyrroles and dipyrrolic ethyl anthranilate azo derivatives. Biochem. J. 1976, 155, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Cassey, P.; Thomas, G.H.; Portugal, S.J.; Maurer, G.; Hauber, M.E.; Grim, T.; Lovell, P.G.; Miksik, I. Why are birds’ eggs colourful? Eggshell pigments co-vary with life-history and nesting ecology among British breeding non-passerine birds. Biol. J. Linn. Soc. 2012, 106, 657–672. [Google Scholar] [CrossRef]
- Takemoto, J.Y.; Chen, D.; Chang, C.-W.T.; Wood, J. Therapeutic Meso-Biliverdin IXα Compositions and Associated Methods. U.S. Patent 9119842 B2, 1 September 2015. [Google Scholar]
- Chen, D.; Brown, J.D.; Kawasaki, Y.; Bommer, J.; Takemoto, J.Y. Scalable production of biliverdin IXα by Escherichia coli. BMC Biotechnol. 2012, 12, 89. [Google Scholar] [CrossRef]
- Kapitulnik, J.; Maines, M.D. Pleiotropic functions of biliverdin reductase: Cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009, 30, 129–137. [Google Scholar] [CrossRef]
- O’Brien, L.; Hosick, P.A.; John, K.; Stec, D.E.; Hinds, T.D., Jr. Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 2015, 4, 212–220. [Google Scholar] [CrossRef]
- Wegiel, B.; Otterbein, L.E. Go green: The anti-inflammatory effects of biliverdin reductase. Front. Pharmacol. 2012, 3, 47. [Google Scholar] [CrossRef]
- Florczyk, U.M.; Jozkowicz, A.; Dulak, J. Biliverdin reductase: New features of an old enzyme and its potential therapeutic significance. Pharmacol. Rep. 2008, 60, 38–48. [Google Scholar]
- Wang, H.; Ferran, C.; Attanasio, C.; Calise, F.; Otterbein, L.E. Induction of protective genes leads to islet survival and function. J. Transplant. 2011, 2011, 141898. [Google Scholar] [CrossRef]
- Gibbs, P.E.M.; Tudor, C.; Maines, M.D. Biliverdin reductase: More than a namesake—the reductase, its peptide fragments, and biliverdin regulate activity of the three classes of protein kinase C. Front. Pharmacol. 2012, 3, 31. [Google Scholar] [CrossRef]
- Fondevila, C.; Katori, M.; Lassman, C.; Carmody, I.; Busuttil, R.W.; Bach, F.H.; Kupiec-Weglinski, J.W. Biliverdin protects rat livers from ischemia/reperfusion injury. Transplant. Proc. 2003, 35, 1798–1799. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Otterbein, L.E.; Overhaus, M.; Sarady, J.K.; Tsung, A.; Kimizuka, K.; Nalesnik, M.A.; Kaizu, T.; Uchiyama, T.; Liu, F.; et al. Biliverdin protects the functional integrity of a transplanted syngeneic small bowel. Gastroenterology 2004, 127, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Murase, N.; Ho, C.; Toyokawa, H.; Billiar, T.R.; Kanno, S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation 2005, 112, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qian, H.; Liu, J.; Zhu, D.; Ding, W.; Pan, P.; Jin, D.; Wang, J.; Li, W. Protection against lung graft injury from brain-dead donors with carbon monoxide, biliverdin, or both. J. Heart Lung Transplant. 2011, 30, 460–466. [Google Scholar] [CrossRef]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; Mcdaid, J.; Tyagi, S.; Csizmadia, E.; Souza-Graça, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef]
- Sarady-Andrews, J.K.; Liu, F.; Gallo, D.; Nakao, A.; Overhaus, M.; Ollinger, R.; Choi, A.M.; Otterbein, L.E. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L1131–L1137. [Google Scholar] [CrossRef]
- Fujii, M.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; Zheng, J.; Kobayashi, K.; Takayanagi, R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int. 2010, 78, 905–919. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Dawson, J.H. Probing structure-function relations in heme-containing oxygenases and peroxidases. Science 1988, 240, 433–439. [Google Scholar] [CrossRef]
- Molzer, C.; Pfleger, B.; Putz, E.; Rossmann, A.; Schwarz, U.; Wallner, M.; Bulmer, A.C.; Wagner, K.H. In vitro DNA-damaging effects of intestinal and related tetrapyrroles in human cancer cells. Exp. Cell Res. 2013, 319, 536–545. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.; Glazer, A.; Ames, B. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science 1987, 235, 1043–1046. [Google Scholar] [PubMed]
- Stocker, R. Antioxidant activities of bile pigments. Antioxid. Redox Signal. 2004, 6, 841–849. [Google Scholar] [PubMed]
- Pachori, A.S.; Smith, A.; Mcdonald, P.; Zhang, L.; Dzau, V.J.; Melo, L.G. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J. Mol. Cell. Cardiol. 2007, 43, 580–592. [Google Scholar]
- Gibbs, P.E.M.; Maines, M.D. Biliverdin inhibits activation of NF-κB: Reversal of inhibition by human biliverdin reductase. Int. J. Cancer 2007, 121, 2567–2574. [Google Scholar]
- Wegiel, B.; Baty, C.J.; Gallo, D.; Csizmadia, E.; Scott, J.R.; Akhavan, A.; Chin, B.Y.; Kaczmarek, E.; Alam, J.; Bach, F.H.; et al. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 2009, 284, 21369–21378. [Google Scholar]
- Celis, A.I.; Dubois, J.L. Making and breaking heme. Curr. Opin. Struct. Biol. 2019, 59, 19–28. [Google Scholar] [PubMed]
- Ding, Z.K.; Xu, Y.Q. Purification and characterization of biliverdin IXα from Atlantic salmon (Salmo salar) bile. Biochemistry 2002, 67, 927–932. [Google Scholar]
- Halepas, S.; Hamchand, R.; Lindeyer, S.E.D.; Brückner, C. Isolation of biliverdin ixα, as its dimethyl ester, from emu eggshells. J. Chem. Educ. 2017, 94, 1533–1537. [Google Scholar] [CrossRef]
- Mei, J.; Wu, X.; Zheng, S.; Yi, Y.; Wang, X.; Ying, G. Production of bilirubin by biotransformation of biliverdin using recombinant Escherichia coli cells. Bioprocess Biosyst. Eng. 2022, 45, 563–571. [Google Scholar]
- Pendrak, M.L.; Roberts, D.D. Methods for the Production of Biliverdin. U.S. Patent 20050209305, 22 September 2005. [Google Scholar]
- Yan, S.; Shao, M.; Xu, M.; Zhang, X.; Yang, T.; Rao, Z. Efficient production of biliverdin through whole-cell biocatalysis using recombinant Escherichia coli. Chin. J. Biotechnol. 2002, 38, 2581–2593. [Google Scholar]
- Morales, J. Eggshell biliverdin as an antioxidant maternal effect: Biliverdin as an antioxidant resource in oviparous animals. BioEssays 2020, 42, e2000010. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, J.Y.; Chang, C.-W.T.; Chen, D.; Hinton, G. Heme-Derived Bilins. Isr. J. Chem. 2019, 59, 378–386. [Google Scholar] [CrossRef]
- Stadnichuk, I.N.; Kusnetsov, V.V. Phycobilisomes and Phycobiliproteins in the Pigment Apparatus of Oxygenic Photosynthetics: From Cyanobacteria to Tertiary Endosymbiosis. Int. J. Mol. Sci. 2023, 24, 2290. [Google Scholar] [CrossRef]
- Gantt, E. Structure and Function of Phycobilisomes: Light Harvesting Pigment Complexes in Red and Blue-Green Algae. Int. Rev. Cytol. 1980, 66, 45–80. [Google Scholar]
- Basheva, D.; Moten, D.; Stoyanov, P.; Belkinova, D.; Mladenov, R.; Teneva, I. Content of phycoerythrin, phycocyanin, alophycocyanin and phycoerythrocyanin in some cyanobacterial strains: Applications. Eng. Life Sci. 2018, 18, 861–866. [Google Scholar] [CrossRef]
- Li, Y. The Bioactivities of Phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, 4008991. [Google Scholar] [CrossRef]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 304, R110–R120. [Google Scholar] [CrossRef]
- Konickova, R.; Vankova, K.; Vanikova, J.; Vanova, K.; Muchova, L.; Subhanova, I.; Zadinova, M.; Zelenka, J.; Dvorak, A.; Kolar, M.; et al. Anti-cancer effects of blue-green alga Spirulina platensis, a natural source of bilirubin-like tetrapyrrolic compounds. Ann. Hepatol. 2014, 13, 273–283. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Z.; Yang, J.; Shi, X.; Zheng, Y. Phycocyanobilin from Arthrospira platensis: A potential photodynamic anticancer agent. Dyes Pigments 2023, 219, 111516. [Google Scholar] [CrossRef]
- Chang, C.-W.T.; Takemoto, J.Y.; Chang, P.-E.; AlFindee, M.N.; Lin, Y.-Y. Effects of Mesobiliverdin IXα-Enriched Microalgae Feed on Gut Health and Microbiota of Broilers. Front. Vet. Sci. 2021, 7, 586813. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Takemoto, J.Y.; Chang, C.-W.T.; Peng, C.-A. Mesobiliverdin IXα ameliorates osteoporosis via promoting osteogenic differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2022, 619, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.S.; Chen, C.Y.; Lin, C.S.; Chang, C.-W.T.; Takemoto, J.Y.; Lin, Y.Y. Mesobiliverdin IXα-enriched microalgae feed additive eliminates reliance on antibiotic tylosin to promote intestinal health of weaning piglets. J. Anim. Physiol. Anim. Nutr. 2023, 107, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Najarian, J.S.; Sutherland, D.E.; Matas, A.J.; Steffes, M.W.; Simmons, R.L.; Goetz, F.C. Human islet transplantation: A preliminary report. Transplant. Proc. 1977, 9, 233–236. [Google Scholar]
- Matsumoto, S.; Noguchi, H.; Naziruddin, B.; Onaca, N.; Jackson, A.; Nobuyo, H.; Teru, O.; Naoya, K.; Klintmalm, G.; Levy, M. Improvement of pancreatic islet cell isolation for transplantation. Bayl. Univ. Med. Cent. Proc. 2007, 20, 357–362. [Google Scholar] [CrossRef]
- Ito, T.; Chen, D.; Chang, C.-W.T.; Kenmochi, T.; Saito, T.; Suzuki, S.; Takemoto, J.Y. Mesobiliverdin IXα enhances rat pancreatic islet yield and function. Front. Pharmacol. 2013, 4, 50. [Google Scholar] [CrossRef]
- Kirsner, J.B. Inflammatory bowel diseases at the university of Chicago-early experiences: A personal historical account. Inflamm. Bowel Dis. 2005, 11, 407–416. [Google Scholar] [CrossRef]
- Sekyere, O.J. Antibiotic types and handling practices in disease management among pig farms in Ashanti Region, Ghana. J. Vet. Med. 2014, 2014, 531952. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Li, Y.; Bell, K.; Doig, K.; Potter, A.; Griebel, P.J.; Kusalik, A.; Napper, S. Mycobacterium avium subsp. paratuberculosis Inhibits gamma interferon-induced signaling in bovine monocytes: Insights into the cellular mechanisms of Johne’s Disease. Infect. Immun. 2012, 80, 3039–3048. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Neuroinflammation in inflammatory bowel disease. J. Neuroinflamm. 2010, 7, 37. [Google Scholar] [CrossRef]
- Reddy, J.G.; Loftus, E.V., Jr. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol. Clin. N. Am. 2006, 35, 837–855. [Google Scholar] [CrossRef]
- M’Koma, A.E. Inflammatory Bowel Disease: Clinical Diagnosis and Surgical Treatment-Overview. Medicina 2022, 58, 567. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Atreya, R.; Grunert, P.C.; Stallhofer, J.; de Laffolie, J.; Schmidt, C. Treatment Strategies in Inflammatory Bowel Diseases. Dtsch. Arztebl. Int. 2023, 120, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Cai1, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 2021. [Google Scholar]
- Okobi, O.E.; Udoete, I.O.; Fasehun, O.O.; Okobi, T.; Evbayekha, E.O.; Ekabua, J.J.; Elukeme, H.; Ebong, I.L.; Ajayi, O.O.; Olateju, I.V.; et al. A Review of Four Practice Guidelines of Inflammatory Bowel Disease. Cureus 2021, 13, e16859. [Google Scholar] [CrossRef]
- Toden, S.; Theiss, A.L.; Wang, X.; Goel, A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci. Rep. 2017, 7, 814. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chang, C.-W.T.; Takemoto, J.Y. Mesobiliverdin IXα ameliorate DSS-induced colitis by inhibiting inflammation and oxidative stress in mice. IOP Conf. Ser. Earth Environ. Sci. 2023, 1286, 012018. [Google Scholar]
- Alizadeh, M.; Shojadoost, B.; Boodhoo, N.; Astill, J.; Taha-Abdelaziz, K.; Hodgins, D.C.; Kulkarni, R.R.; Sharif, S. Necrotic enteritis in chickens: A review of pathogenesis, immune responses and prevention, focusing on probiotics and vaccination. Anim. Health Res. Rev. 2021, 22, 147–162. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar]
- Lum, K.K.; Kim, J.; Lei, X.G. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotechnol. 2013, 4, 53. [Google Scholar]
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar]
- El-Moataaz, S.; Ismael, H.; Aborhyem, S. Assessment of chemical composition of Spirulina platensis and its effect on fasting blood glucose and lipid profile in diabetic Rats. J. High Inst. Public Health 2019, 49, 199–211. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxid. Med. Cell Longev. 2017, 2017, 3247528. [Google Scholar] [PubMed]
- Bensch, H.M.; Tolf, C.; Waldenström, J.; Lundin, D.; Zöttl, M. Bacteroidetes to Firmicutes: Captivity changes the gut microbiota composition and diversity in a social subterranean rodent. Anim. Microbiome 2023, 5, 9. [Google Scholar]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Neville, B.A.; O’Toole, P.W. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Future Microbiol. 2010, 5, 759–774. [Google Scholar]
- Martín, R.; Miquel, S.; Ulmer, J.; Kechaou, N.; Langella, P.; Bermúdez-Humarán, L.G. Role of commensal and probiotic bacteria in human health: A focus on inflammatory bowel disease. Microb. Cell Factories 2013, 12, 71. [Google Scholar]
- O’Shea, E.F.; O’Connor, P.M.; Raftis, E.J.; O’Toole, P.W.; Stanton, C.; Cotter, P.D.; Ross, P.R.; Hill, C. Production of multiple bacteriocins from a single locus by gastrointestinal strains of Lactobacillus salivarius. J. Bacteriol. 2011, 193, 6973–6982. [Google Scholar]
- Stern, N.J.; Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Pokhilenko, V.D.; Levchuk, V.P.; Svetoch, O.E.; Seal, B.S. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. 2006, 50, 3111–3116. [Google Scholar]
- Zhang, J.; Deng, J.; Wang, Z.; Che, C.; Li, Y.F.; Yang, Q. Modulatory effects of Lactobacillus salivarius on intestinal mucosal immunity of piglets. Curr. Microbiol. 2011, 62, 1623–3147. [Google Scholar]
- Shokryazdan, P.; Faseleh, J.M.; Liang, J.B.; Ramasamy, K.; Sieo, C.C.; Ho, Y.W. Effects of Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS ONE 2017, 12, 0175959. [Google Scholar]
- Miller, B.G.; Whittemore, C.T.; Stokes, C.R.; Telemo, E. The effect of delayed weaning on the development of oral tolerance to soya-bean protein in pigs. Br. J. Nutr. 1994, 71, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2013, 14, 2765–2778. [Google Scholar] [CrossRef]
- Drewe, J.; Beglinger, C.; Fricker, G. Effect of ischemia on intestinal permeability of lipopolysaccharides. Eur. J. Clin. Investig. 2001, 31, 138–144. [Google Scholar] [CrossRef]
- Chen, T.; Yang, T.; Zhang, W.; Shao, J. The therapeutic potential of mesenchymal stem cells in treating osteoporosis. Biol. Res. 2021, 54, 42. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Vanella, L.; Kim, D.H.; Asprinio, D.; Peterson, S.J.; Barbagallo, I.; Vanella, A.; Goldstein, D.; Ikehara, S.; Kappas, A.; Abraham, N.G. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone 2010, 46, 236–243. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, C.A. Enhanced proliferation and differentiation of mesenchymal stem cells by astaxanthin-encapsulated polymeric micelles. PLoS ONE 2019, 14, e0216755. [Google Scholar] [CrossRef]
- Choi, K.M.; Seo, Y.K.; Yoon, H.H.; Song, K.Y.; Kwo, S.Y.; Lee, H.S.; Park, J.K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Melatonin plays critical role in mesenchymal stem cell-based regenerative medicine in vitro and in vivo. Stem Cell Res. Ther. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar] [PubMed]
- Schett, G.; Kiechl, S.; Weger, S.; Pederiva, A.; Mayr, A.; Petrangeli, M.; Oberhollenzer, F.; Lorenzini, R.; Redlich, K.; Axmann, R.; et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch. Intern. Med. 2006, 166, 2495–2501. [Google Scholar] [CrossRef]
- Ding, C.; Parameswaran, V.; Udayan, R.; Burgess, J.; Jones, G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: A longitudinal study. J. Clin. Endocrinol. Metab. 2008, 93, 1952–1958. [Google Scholar] [CrossRef]
- Eriksson, A.L.; Moverare-Skrtic, S.; Ljunggren, O.; Karlsson, M.; Mellstrom, D.; Ohlsson, C. High-sensitivity CRP is an independent risk factor for all fractures and vertebral fractures in elderly men: The MrOS Sweden study. J. Bone Miner. Res. 2014, 29, 418–423. [Google Scholar] [CrossRef]
- Pasco, J.A.; Kotowicz, M.A.; Henry, M.J.; Nicholson, G.C.; Spilsbury, H.J.; Box, J.D.; Schneider, H.G. High-sensitivity C-reactive protein and fracture risk in elderly women. JAMA 2006, 296, 1353–1355. [Google Scholar] [CrossRef]
- Florczyk-Soluch, U.; Józefczuk, E.; Stępniewski, J.; Bukowska-Strakova, K.; Mendel, M.; Viscardi, M.; Nowak, W.N.; Józkowicz, A.; Dulak, J. Various roles of heme oxygenase-1 in response of bone marrow macrophages to RANKL and in the early stage of osteoclastogenesis. Sci. Rep. 2018, 8, 10797. [Google Scholar] [CrossRef]
- Ke, K.; Safder, M.A.; Sul, O.J.; Kim, W.K.; Suh, J.H.; Joe, Y.; Chung, H.T.; Choi, H.S. Hemeoxygenase-1 maintains bone mass via attenuating a redox imbalance in osteoclast. Mol. Cell. Endocrinol. 2015, 409, 11–20. [Google Scholar] [CrossRef]
- Barbagallo, I.; Vanella, A.; Peterson, S.J.; Kim, D.H.; Tibullo, D.; Giallongo, C.; Vanella, L.; Parrinello, N.; Palumbo, G.A.; Raimondo, F.D.; et al. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J. Bone Miner. Metab. 2010, 28, 276–288. [Google Scholar] [CrossRef]
- Altmann, B.A.; Rosenau, S. Spirulina as Animal Feed: Opportunities and Challenges. Foods 2022, 11, 965. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Benemann, J.R.; Zhang, X.; Hu, H.; Qin, S. Microalgal industry in China: Challenges and prospects. J. Appl. Phycol. 2016, 28, 715–725. [Google Scholar]
- Ross, E.; Dominy, W. The nutritional value of dehydrated, blue-green algae (Spirulina plantensis) for poultry. Poult. Sci. 1990, 69, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Toyomizu, M.; Sato, K.; Taroda, H.; Kato, T.; Akiba, Y. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Br. Poult. Sci. 2001, 42, 197–202. [Google Scholar]
- Al-Batshan, H.A.; Al-Mufarrej, S.I.; Al-Homaidan, A.A.; Qureshi, M.A. Enhancement of chicken macrophage phagocytic function and nitrite production by dietary Spirulina platensis. Immunopharmacol. Immunotoxicol. 2001, 23, 281–289. [Google Scholar]
- Grinstead, G.S.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Sawyer, J.; Maxwell, K.; Stott, R.; Moser, A. Influence of Spirulina platensis on Growth Performance of Weanling Pigs; Kansas State University Swine Day 1998, Report of Progress 819; Kansas State University: Manhattan, KS, USA, 1998; pp. 67–74. [Google Scholar]
- Grinstead, G.S.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Nelssen, J.L. Effects of Spirulina platensis on growth performance of weanling pigs. Anim. Feed Sci. Technol. 2000, 83, 237–247. [Google Scholar]
- Peiretti, P.G.; Meineri, G. Effects of diets with increasing levels of Spirulina platensis on the performance and apparent digestibility in growing rabbits. Livest. Sci. 2008, 118, 173–177. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Meineri, G. Effects of diets with increasing levels of Spirulina platensis on the carcass characteristics, meat quality and fatty acid composition of growing rabbits. Livest. Sci. 2011, 140, 218–224. [Google Scholar]
- Belay, A.; Ota, Y.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liang, Z.P.; Chang, X.Y.; Li, F.T.; Wang, X.Q.; Lian, X.J. Progress of microencapsulated phycocyanin in food and pharma industries: A review. Molecules 2022, 27, 5854. [Google Scholar] [CrossRef]



| Compound | Concentration (mM) | # Islet Cells (IEQ) | % Increase over Control |
|---|---|---|---|
| BV | 1 | 1345 ± 629 | 4.3 |
| 10 | 1603 ± 1073 | 24.4 | |
| 100 | 1759 ± 703 | 35.5 | |
| control | 1289 ± 559 | ||
| MBV | 1 | 1599 ± 475 | 86.7 |
| 10 | 1318 ± 805 | 54.0 | |
| 100 | 1535 ± 287 | 79.3 | |
| control | 856 ± 229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudyal, N.; Takemoto, J.Y.; Lin, Y.-Y.; Chang, C.-W.T. An Alternative to Biliverdin, Mesobiliverdin IXα and Mesobiliverdin-Enriched Microalgae: A Review on the Production and Applications of Mesobiliverdin-Related Products. Molecules 2025, 30, 1379. https://doi.org/10.3390/molecules30061379
Poudyal N, Takemoto JY, Lin Y-Y, Chang C-WT. An Alternative to Biliverdin, Mesobiliverdin IXα and Mesobiliverdin-Enriched Microalgae: A Review on the Production and Applications of Mesobiliverdin-Related Products. Molecules. 2025; 30(6):1379. https://doi.org/10.3390/molecules30061379
Chicago/Turabian StylePoudyal, Naveena, Jon Y. Takemoto, Yuan-Yu Lin, and Cheng-Wei T. Chang. 2025. "An Alternative to Biliverdin, Mesobiliverdin IXα and Mesobiliverdin-Enriched Microalgae: A Review on the Production and Applications of Mesobiliverdin-Related Products" Molecules 30, no. 6: 1379. https://doi.org/10.3390/molecules30061379
APA StylePoudyal, N., Takemoto, J. Y., Lin, Y.-Y., & Chang, C.-W. T. (2025). An Alternative to Biliverdin, Mesobiliverdin IXα and Mesobiliverdin-Enriched Microalgae: A Review on the Production and Applications of Mesobiliverdin-Related Products. Molecules, 30(6), 1379. https://doi.org/10.3390/molecules30061379







