Macro- and Microelement Composition, Antioxidant Activity, and Biological Effect of Cold-Pressed Edible Oils from Commercial and Amateur Companies
Abstract
1. Introduction
2. Results and Discussion
2.1. The Content of Macro- and Microelements in the Oils
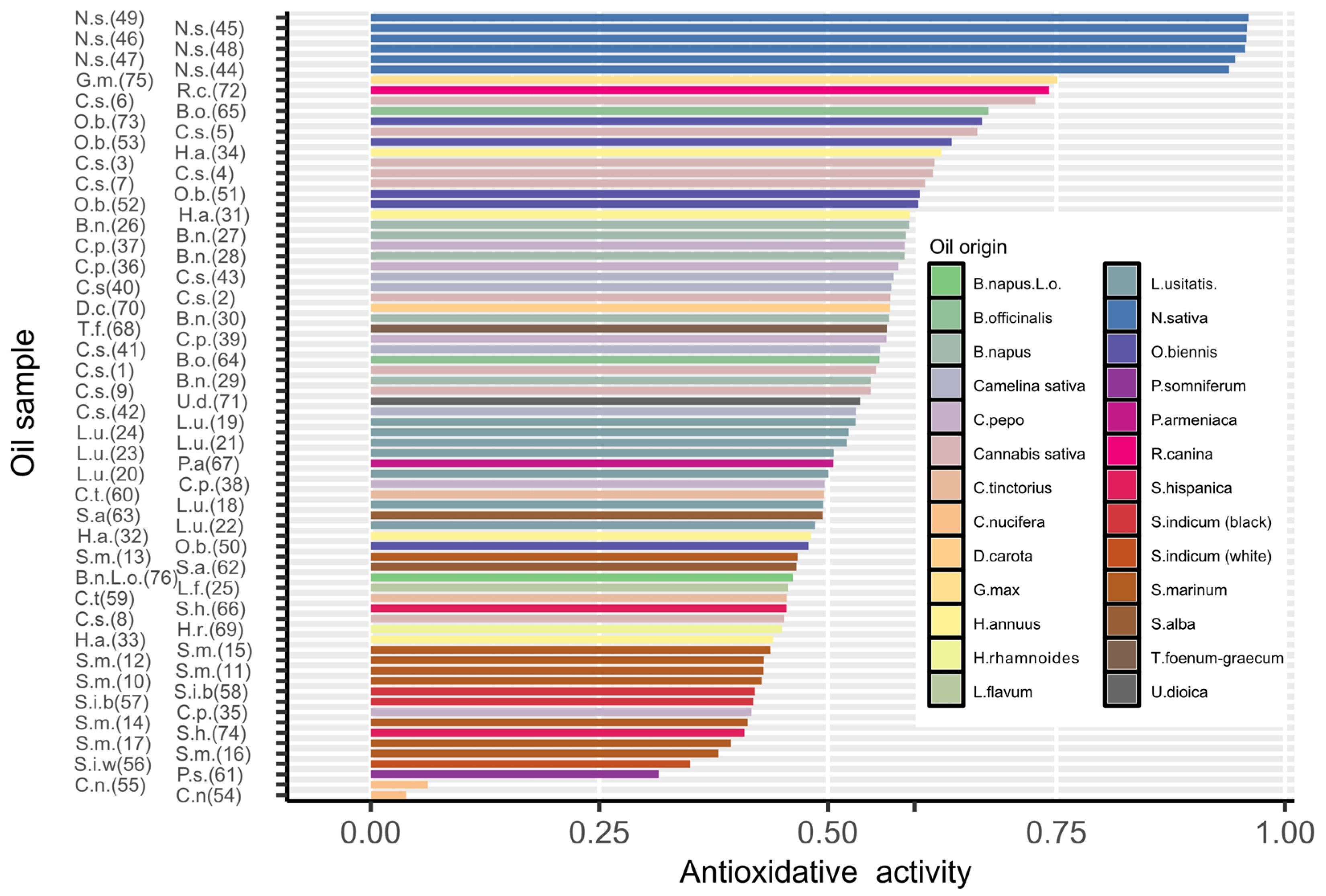
2.2. Total Antioxidant Potential (TAP)
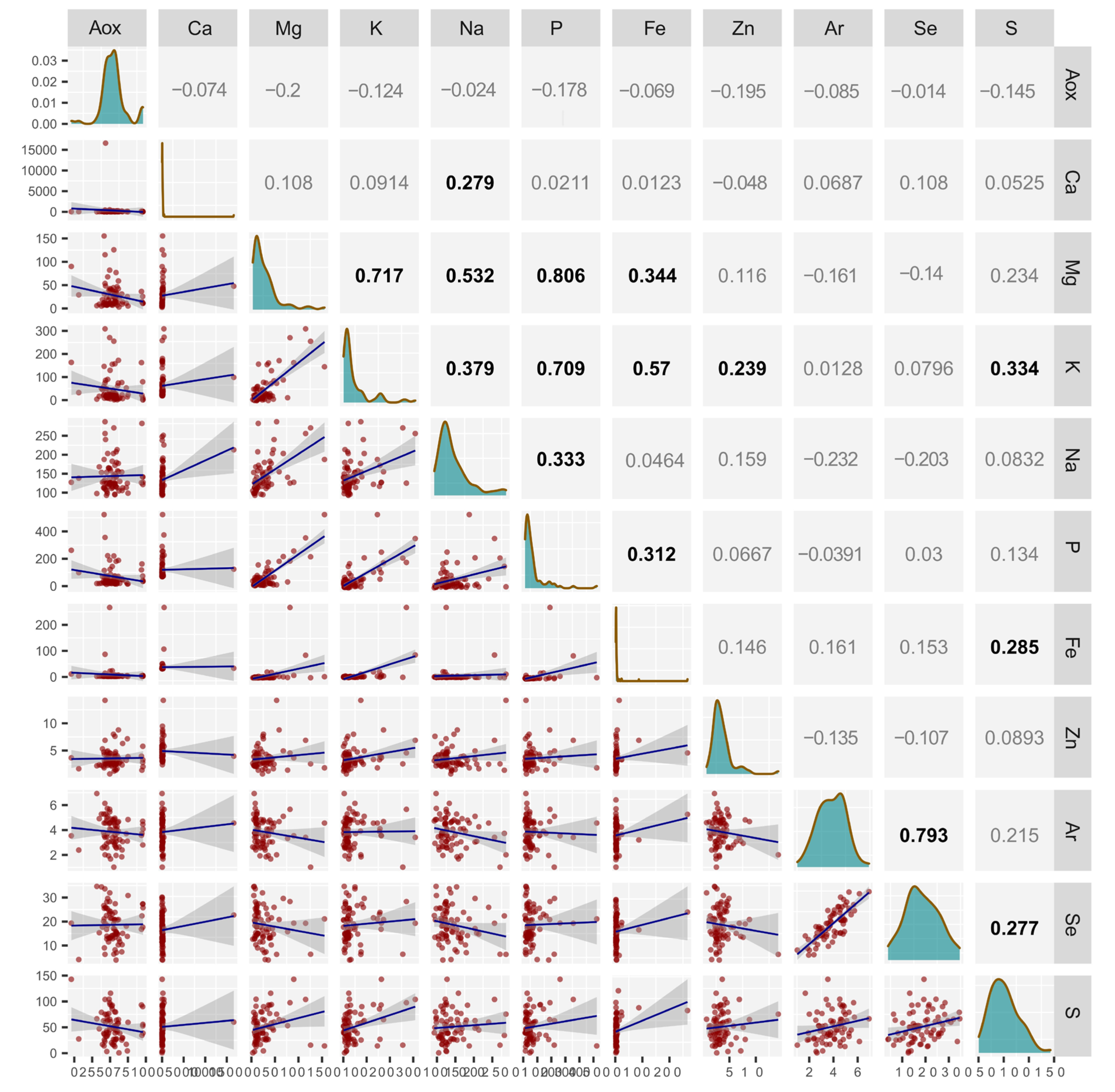
2.3. Correlations Between the Content of Macro- and Micronutrients and Total Antioxidant Potential (TAP)
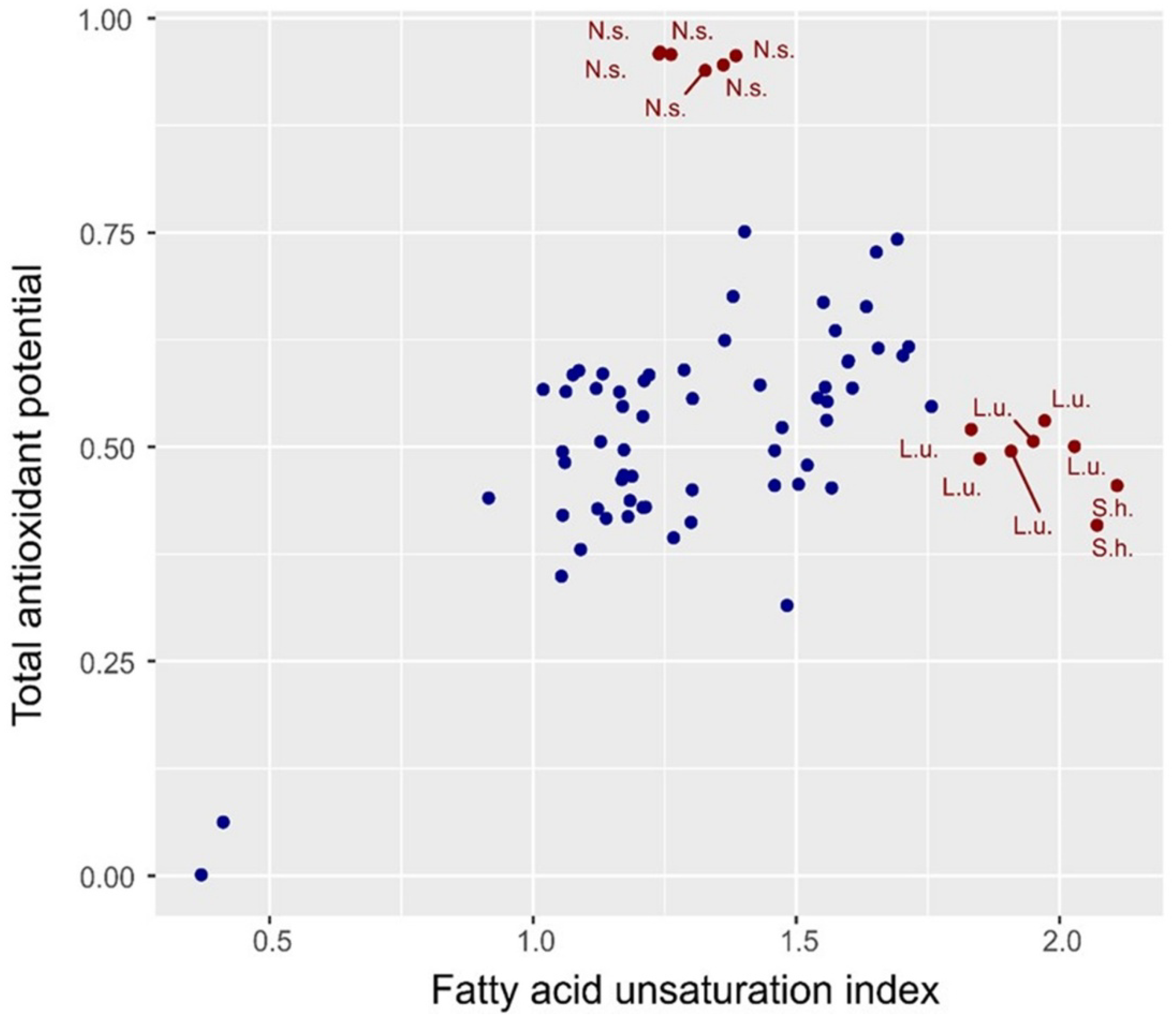
2.4. Spectral Index of the Degree of Oil Unsaturation
2.5. Antimicrobial Activity of the Oils
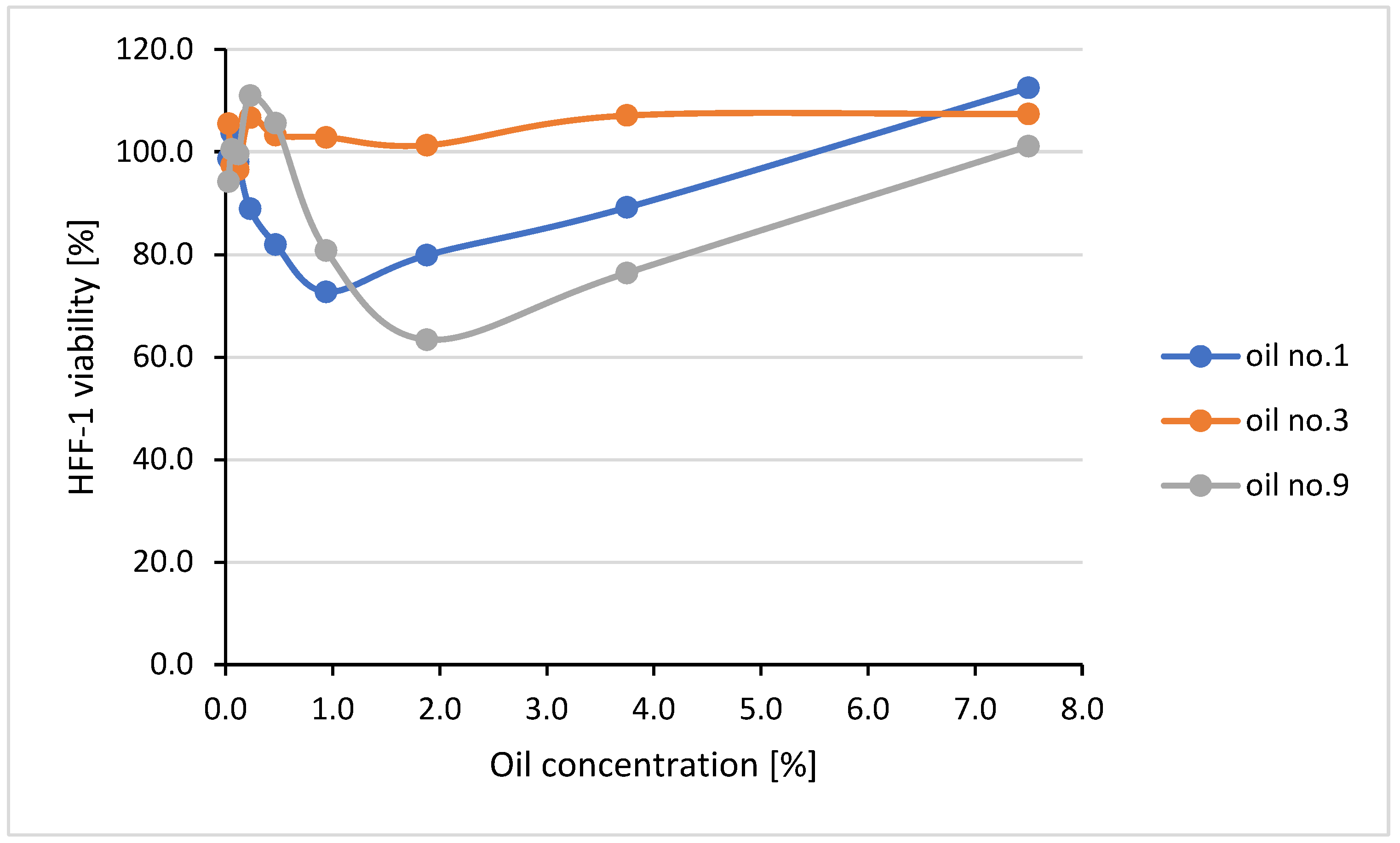
2.6. Cytotoxic Effect of the Oils
3. Materials and Methods
3.1. Tested Oils
3.2. Total Antioxidant Potential (TAP)
- Instrumentation
- Reagents
- Procedures
- Data Analysis
3.3. Macro- and Microelements’ Analysis
3.4. ATR-FTIR Spectroscopy
3.5. Microorganisms and Culture Conditions for Antimicrobial Activity Testing
3.6. Assessment of Minimum Inhibitory and Bactericidal/Fungicidal Concentration (MIC, MBC/MFC)
3.7. Evaluation of Cytotoxicity for Human Fibroblasts
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanimozhi, S.; Hariharan, P.A. Study on the Customer Preference and Purchase Decision of Refined Oil and Cold-PreQssed Oil among Households in India. Asian J. Manag. 2023, 14, 52–56. [Google Scholar] [CrossRef]
- Siger, A.; Nogalska-Kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Kachel-Jakubowska, M.; Kraszkiewicz, A.; Lorencowicz, E.; Koszel, M.; Przywara, A. Effects of thermal treatment of seeds on quality and oxidative stability of oils. Agric. Agric. Sci. Procedia 2015, 7, 255–259. [Google Scholar] [CrossRef][Green Version]
- Beardsell, D.; Francis, J.; Ridley, D.; Robards, K. Health promoting constituents in plant derived edible oils. J. Food Lipids 2002, 9, 1–34. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, X.; Yu, X.; Huyan, Z.; Wang, X. Rapid and simultaneous determination of the iodine value and saponification number of edible oils by FTIR spectroscopy. Eur. J. Lipid Sci. Technol. 2018, 120, 1700396. [Google Scholar] [CrossRef]
- de Souza, T.R.P.; Olenka, L.; Peternella, W.S. A study of degradation in vegetable oils by exposure to sunlight using fourier transform infrared spectroscopy. Mater. Sci. Appl. 2020, 11, 678–691. [Google Scholar] [CrossRef]
- Rohman, A. Infrared spectroscopy for quantitative analysis and oil parameters of olive oil and virgin coconut oil: A review. Int. J. Food Prop. 2017, 20, 1447–1456. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Huyan, Z.; Kou, Y.; Xu, L.; Yu, X.; Gao, J.M. Application of Fourier transform infrared spectroscopy for the quality and safety analysis of fats and oils: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3597–3611. [Google Scholar] [CrossRef]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Mousa, M.A.; Wang, Y.; Antora, S.A.; Al-Qurashi, A.D.; Ibrahim, O.H.; He, H.J.; Liu, S.; Kamruzzaman, M. An overview of recent advances and applications of FT-IR spectroscopy for quality, authenticity, and adulteration detection in edible oils. Crit. Rev. Food Sci. Nutr. 2022, 62, 8009–8027. [Google Scholar] [CrossRef]
- Rohman, A.; Ghazali, M.A.I.B.; Windarsih, A.; Riyanto, S.; Yusof, F.M.; Mustafa, S. Comprehensive review on application of FTIR spectroscopy coupled with chemometrics for authentication analysis of fats and oils in the food products. Molecules 2020, 25, 5485. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R.; Kumari, S.; Sharma, S.; Kelly, S.; Cannavan, A.; Singh, D.K. Recent trends in the use of FTIR spectroscopy integrated with chemometrics for the detection of edible oil adulteration. Vib. Spectrosc. 2021, 113, 103222. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Aguiar, L.M.; Geraldi, M.V.; Cazarin, C.B.B.; Junior, M.R.M. Functional Food Consumption and Its Physiological Effects. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead: Cambridge, UK, 2019; pp. 205–225. [Google Scholar]
- Zhu, F.; Fan, W.; Wang, X.; Qu, L.; Yao, S. Health risk assessment of eight heavy metals in nine varieties of edible vegetable oils consumed in China. Food Chem. Toxicol. 2011, 49, 3081–3085. [Google Scholar] [CrossRef]
- Shumoy, H.; Raes, K. Dissecting the facts about the impact of contaminant iron in human nutrition: A review. Trends Food Sci. Technol. 2021, 116, 918–927. [Google Scholar] [CrossRef]
- Özcan, M.M.; Chalchat, J.C. Chemical composition of carrot seeds (Daucus carota L.) cultivated in Turkey: Characterization of the seed oil and essential oil. Grasas Aceites 2007, 58, 359–365. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission: Fats, Oils & Related Products By Codex Alimentarius Commission - Codex Standard for Edible Fats and Oils not Covered by Individual Standards (Adopted in 1981. Revised in 1987, 1999. Amended in 2009, 2013, 2015, 2017, 2019, 2021, 2023). Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B19-1981%252FCXS_019e.pdf (accessed on 19 March 2025).
- Codex Alimentarius Commission: Codex General Standard for Contaminants and Toxins in Foods (Adopted in 1995. Revised in 1997, 2006, 2008, 2009. Amended in 2010, 2012, 2013, 2014, 2015, 2016, 2017, 2018, 2019, 2021, 2022, 2023). Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 19 March 2025).
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Valavanidis, A.; Nisiotou, C.; Papageorgiou, Y.; Kremli, I.; Satravelas, N.; Zinieris, N.; Zygalaki, H. Comparison of the Radical Scavenging Potential of Polar and Lipidic Fractions of Olive Oil and Other Vegetable Oils under Normal Conditions and after Thermal Treatment. J. Agric. Food Chem. 2004, 52, 2358–2365. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M.; Jóźwik-Dolęba, M. Importance of solvent association in the estimation of antioxidant properties of phenolic compounds by DPPH method. J. Food Sci. Technol. 2015, 52, 4523–4529. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS Assays to Assess the Antioxidant Profile of Untreated Oils. Food Anal. Methods 2014, 8, 1294–1302. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Zemour, K.; Labdelli, A.; Adda, A.; Dellal, A.; Talou, T.; Merah, O. Phenol content and antioxidant and antiaging activity of safflower seed oil (Carthamus tinctorius L.). Cosmetics 2019, 6, 55. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Antioxidant capacity and phenolic acids of virgin coconut oil. Int. J. Food Sci. Nutr. 2008, 60, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ghani, N.A.A.; Channip, A.A.; Chok Hwee Hwa, P.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Quality evaluation of light-and dark-colored Hungarian honeys, focusing on botanical origin, antioxidant capacity and mineral content. Molecules 2021, 26, 2825. [Google Scholar] [CrossRef]
- Batool, M.; Nadeem, M.; Imran, M.; Gulzar, N.; Shahid, M.Q.; Shahbaz, M.; Ajmal, M.; Khan, I.T. Impact of vitamin E and selenium on antioxidant capacity and lipid oxidation of cheddar cheese in accelerated ripening. Lipids Health Dis. 2018, 17, 79. [Google Scholar] [CrossRef]
- Hassan, F.; Mobarez, S.; Mohamed, M.; Attia, Y.; Mekawy, A.; Mahrose, K. Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals 2021, 11, 756. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Ziarati, P.; Mirmohammad Makki, F.; Vambol, S.; Vambol, V. Determination of toxic metals content in Iranian and Italian flavoured olive oil. Acta Technol. Agric. 2019, 22, 64–69. [Google Scholar] [CrossRef]
- Ferancová, A.; Heilerová, Ľ.; Korgová, E.; Šilhár, S.; Štepánek, I.; Labuda, J. Anti/pro-oxidative properties of selected standard chemicals and tea extracts investigated by DNA-based electrochemical biosensor. Eur. Food Res. Technol. 2004, 219, 416–420. [Google Scholar] [CrossRef]
- Kesić, A.; Crnkić, A.; Ibrišimović-Mehmedinović, N.; Šestan, A.; Ćatović, B. Changes of antioxidant activity in honey as a result of Haber-Wais reaction. Am. J. Appl. Chem. 2014, 2, 112–116. [Google Scholar] [CrossRef]
- Samuni, A.; Aronovitch, J.; Godinger, D.; Chevion, M.; Czapski, G. On the cytotoxicity of vitamic C and metal ions. A Site-specific Fenton mechanism. Eur. J. Biochem. 1983, 137, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Tjell, J.C.; Sloth, J.J.; Holm, P.E. Review of arsenic contamination, exposure through water and food and low cost mitigation options for rural areas. Appl. Geochem. 2014, 41, 11–33. [Google Scholar] [CrossRef]
- Mordukhovich, I.; Wright, R.O.; Amarasiriwardena, C.; Baja, E.; Baccarelli, A.; Suh, H.; Sparrow, D.; Vokonas, P.; Schwartz, J. Association Between Low-Level Environmental Arsenic Exposure and QT Interval Duration in a General Population Study. Am. J. Epidemiol. 2009, 170, 739–746. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on dietary reference values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar]
- Jabłońska, E.; Gromadzinska, J.; Klos, A.; Bertrandt, J.; Skibniewska, K.; Darago, A.; Wasowicz, W. Selenium, zinc and copper in the Polish diet. J. Food Compos. Anal. 2013, 31, 259–265. [Google Scholar] [CrossRef]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef]
- Van de Voort, F.R.; Sedman, J.; Emo, G.; Ismail, A.A. Rapid and direct lodine value and saponification number determination of fats and oils by attenuated total reflectance/fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1992, 69, 1118–1123. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Tutuncu, S.; Aydar, E.F.; Topkaya, C.; Mertdinc, Z.; Ozcelik, B.; Aital, M.; et al. Review of Recent Studies on the Antioxidant and Anti-Infectious Properties of Senna Plants. Oxid. Med. Cell. Longev. 2022, 2022, 6025900. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.P.; Poudel, D.K.; Satyal, P.; Khadayat, K.; Dhami, S.; Aryal, D.; Chaudhary, P.; Ghimire, A.; Parajuli, N. Volatile compounds and antioxidant and antimicrobial activities of selected citrus essential oils originated from Nepal. Molecules 2021, 26, 6683. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.Ł.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant characterization, antimicrobial activities, and genomic MicroRNA based marker fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Stochmal, A.; Skalski, B.; Pietukhov, R.; Sadowska, B.; Rywaniak, J.; Wójcik-Bojek, U.; Grabarczyk, Ł.; Żuchowski, J.; Olas, B. Evaluation of antimicrobial, antioxidant, and cytotoxic activity of phenolic preparations of diverse composition, obtained from Elaeagnus rhamnoides (L.) A. Nelson leaf and twig extracts. Molecules 2021, 26, 2835. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-value compounds in fruit, vegetable and cereal byproducts: An overview of potential sustainable reuse and exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Tsamo, D.L.F.; Tamokou, J.D.D.; Kengne, I.C.; Ngnokam, C.D.J.; Djamalladine, M.D.; Voutquenne-Nazabadioko, L.; Ngnokam, D. Antimicrobial and antioxidant secondary metabolites from Trifolium baccarinii Chiov. (Fabaceae) and their mechanisms of antibacterial action. BioMed Res. Int. 2021, 2021, 3099428. [Google Scholar] [CrossRef]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.H. From fighting critters to saving lives: Polyphenols in plant defense and human health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Wójcik-Bojek, U.; Rywaniak, J.; Bernat, P.; Podsędek, A.; Kajszczak, D.; Sadowska, B. An in vitro study of the effect of Viburnum opulus extracts on key processes in the development of staphylococcal infections. Molecules 2021, 26, 1758. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hanson, B.A. ChemoSpec: Exploratory Chemometrics for Spectroscopy. R Package. 2023. Available online: https://CRAN.R-project.org/package=ChemoSpec (accessed on 19 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2023. Available online: https://www.R-project.org/ (accessed on 19 March 2025).
- European Committee on Antimicrobial Susceptibility Testing—EUCAST. 2021. Available online: https://www.eucast.org/ (accessed on 19 March 2025).
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots, R Package Version 0.6.0. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 2 February 2023).
- Harrell, F., Jr. Hmisc: Harrell Miscellaneous, R Package Version 5.1-0. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 12 September 2023).


| Origin | I C | II C | III C | IV A | V A | VI C | VII A | VIII C | IX A | |
|---|---|---|---|---|---|---|---|---|---|---|
| Oil | ||||||||||
| Cannabis sativa L. (hemp oil) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Cannabis sativa L. ECO (hemp oil) | 9 | |||||||||
| Silybum marianum(L.) Gaertner. (milk thistle oil) | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |||
| Silybum marianum (L.) Gaertner ECO (milk thistle oil) | 17 | |||||||||
| Linum usitatissimum L. (linseed oil) | 18 | 19 | 20 | 21 | 22 | 23 | ||||
| Linum usitatissimum L. ECO (linseed oil) | 24 | |||||||||
| Linum flavum L. (golden linseed oil) | 25 | |||||||||
| Brassica napus L. (canola oil) | 26 | 27 | 28 | 29 | ||||||
| Brassica napus L. ECO (canola oil) | 30 | |||||||||
| Helianthus annus L. (sunflower oil) | 31 | 32 | 33 | 34 | ||||||
| Cucurbita pepo L. (pumpkin oil) | 35 | 36 | 37 | 38 | ||||||
| Cucurbita pepo L. ECO (pumpkin oil) | 39 | |||||||||
| Camelina sativa (L.) Crantz (camelina oil) | 40 | 41 | 42 | |||||||
| Camelina sativa (L.) Crantz ECO (camelina oil) | 43 | |||||||||
| Nigella sativa L. (black seed oil) | 44 | 45 | 46 | 47 | 48 | |||||
| Nigella sativa L. ECO (black seed oil) | 49 | |||||||||
| Oenothera biennis L. (evening primrose oil) | 50 | 51 | 73 | 52 | ||||||
| Oenothera biennis L. ECO | 53 | |||||||||
| Cocos nucifera L. (coconut oil) | 54 | 55 | ||||||||
| Sesamum indicum L. (sesame oil) | 56 | 57 | ||||||||
| Sesamum indicum L. black (black sesame oil) | 58 | |||||||||
| Carthamus tinctorius L. (safflower oil) | 59 | 60 | ||||||||
| Papaver somniferum L. (poppy seed oil) | 61 | |||||||||
| Sinapis alba L. (mustard oil) | 62 | 63 | ||||||||
| Borago officinalis L. (borage oil) | 64 | 65 | ||||||||
| Salvia hispanica L. (chia seed oil) | 66 | 74 | ||||||||
| Prunus armeniaca L. seeds (apricot kernel oil) | 67 | |||||||||
| Trigonella foenum-graecum L. (fenugreek oil) | 68 | |||||||||
| Hippophae rhamnoides L. (sea buckthorn oil) | 69 | |||||||||
| Daucus carota L. ECO (carrot seed oil) | 70 | |||||||||
| Urtica dioica L. ECO (nettle seed oil) | 71 | |||||||||
| Rosa canina L. ECO (rose seed oil) | 72 | |||||||||
| Glycine max (L.) Merr. ECO (soybean oil) | 75 | |||||||||
| Brassica napus L with Levisticum officinale W.D.J. Koch extract | 76 | |||||||||
| Oil No. | Oil Description | MIC [%] MBC/MFC [%] | ||||
|---|---|---|---|---|---|---|
| S. aureus ATCC 29213 | S. epidermidis ATCC 12228 | E. faecalis ATCC 29212 | E. coli ATCC 25922 | C. albicans ATCC 10231 | ||
| 1 | Cannabis sativa L. | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 3 | Cannabis sativa L. | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 9 | Cannabis sativa L. ECO | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 12 | Silybum marianum | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 17 | Silybum marianum ECO | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 25 | Linum flavum L. | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 27 | Brassica napus L. | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 39 | Cucurbita pepo L. ECO | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 44 | Nigella sativa L. | 0.09 | 0.19 | 0.19 | >25 | 6.25 |
| 0.39 | 0.19 | 12.5 | >25 | 25 | ||
| 46 | Nigella sativa L. | 0.78 | 0.19 | 0.39 | >12.5 | 6.25 |
| 0.78 | 0.78 | 0.78 | >12.5 | 12.5 | ||
| 48 | Nigella sativa L. | <0.09 | 0.02 | 1.56 | >12.5 | 0.78 |
| <0.09 | 0.09 | 1.56 | >12.5 | 3.12 | ||
| 49 | Nigella sativa L. ECO | 0.04 | 0.04 | 0.39 | >25 | 6.25 |
| 0.19 | 0.04 | 6.25 | >25 | 25 | ||
| 50 | Oenothera L. | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 53 | Oenothera L. | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 68 | Trigonella foenum-graecum L. | >25 | >25 | >25 | >25 | >25 |
| >25 | >25 | >25 | >25 | >25 | ||
| 69 | Hippophae rhamnoides L. | >25 | >25 | >25 | >25 | >25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciniuk, J.; Sadowska, B.; Więckowska-Szakiel, M.; Borkowski, M.; Zebrowski, J.; Głód, B.K.; Marciniuk, K.; Marciniuk, P. Macro- and Microelement Composition, Antioxidant Activity, and Biological Effect of Cold-Pressed Edible Oils from Commercial and Amateur Companies. Molecules 2025, 30, 1425. https://doi.org/10.3390/molecules30071425
Marciniuk J, Sadowska B, Więckowska-Szakiel M, Borkowski M, Zebrowski J, Głód BK, Marciniuk K, Marciniuk P. Macro- and Microelement Composition, Antioxidant Activity, and Biological Effect of Cold-Pressed Edible Oils from Commercial and Amateur Companies. Molecules. 2025; 30(7):1425. https://doi.org/10.3390/molecules30071425
Chicago/Turabian StyleMarciniuk, Jolanta, Beata Sadowska, Marzena Więckowska-Szakiel, Mateusz Borkowski, Jacek Zebrowski, Bronisław K. Głód, Kacper Marciniuk, and Paweł Marciniuk. 2025. "Macro- and Microelement Composition, Antioxidant Activity, and Biological Effect of Cold-Pressed Edible Oils from Commercial and Amateur Companies" Molecules 30, no. 7: 1425. https://doi.org/10.3390/molecules30071425
APA StyleMarciniuk, J., Sadowska, B., Więckowska-Szakiel, M., Borkowski, M., Zebrowski, J., Głód, B. K., Marciniuk, K., & Marciniuk, P. (2025). Macro- and Microelement Composition, Antioxidant Activity, and Biological Effect of Cold-Pressed Edible Oils from Commercial and Amateur Companies. Molecules, 30(7), 1425. https://doi.org/10.3390/molecules30071425







