Lignin as a Bioactive Additive in Chlorzoxazone-Loaded Pharmaceutical Tablets
Abstract
:1. Introduction
- Improving the dissolution and bioavailability of drugs;
- Adsorbing and removing polyphenols and tannins from tinctures and herbal extracts and improving the taste of azithromycin, paracetamol, and vitamins [26].
2. Results
2.1. Pharmaco-Technical Parameter Values of Matrix Tablet Formulations
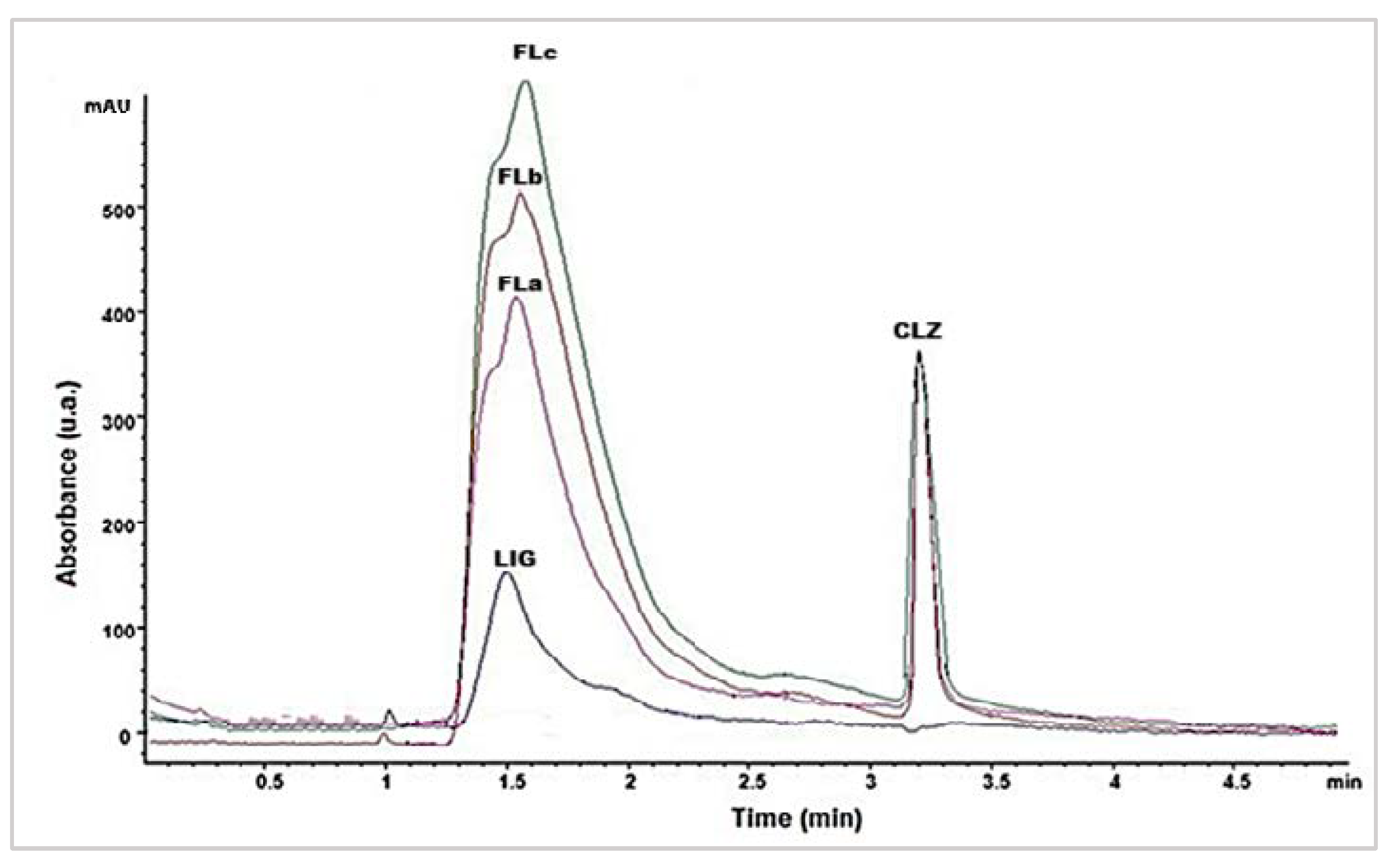
2.2. Drug-Excipients Compatibility Study
2.2.1. FTIR Spectroscopy
2.2.2. X-Ray Diffraction
2.2.3. Thermal Characterization
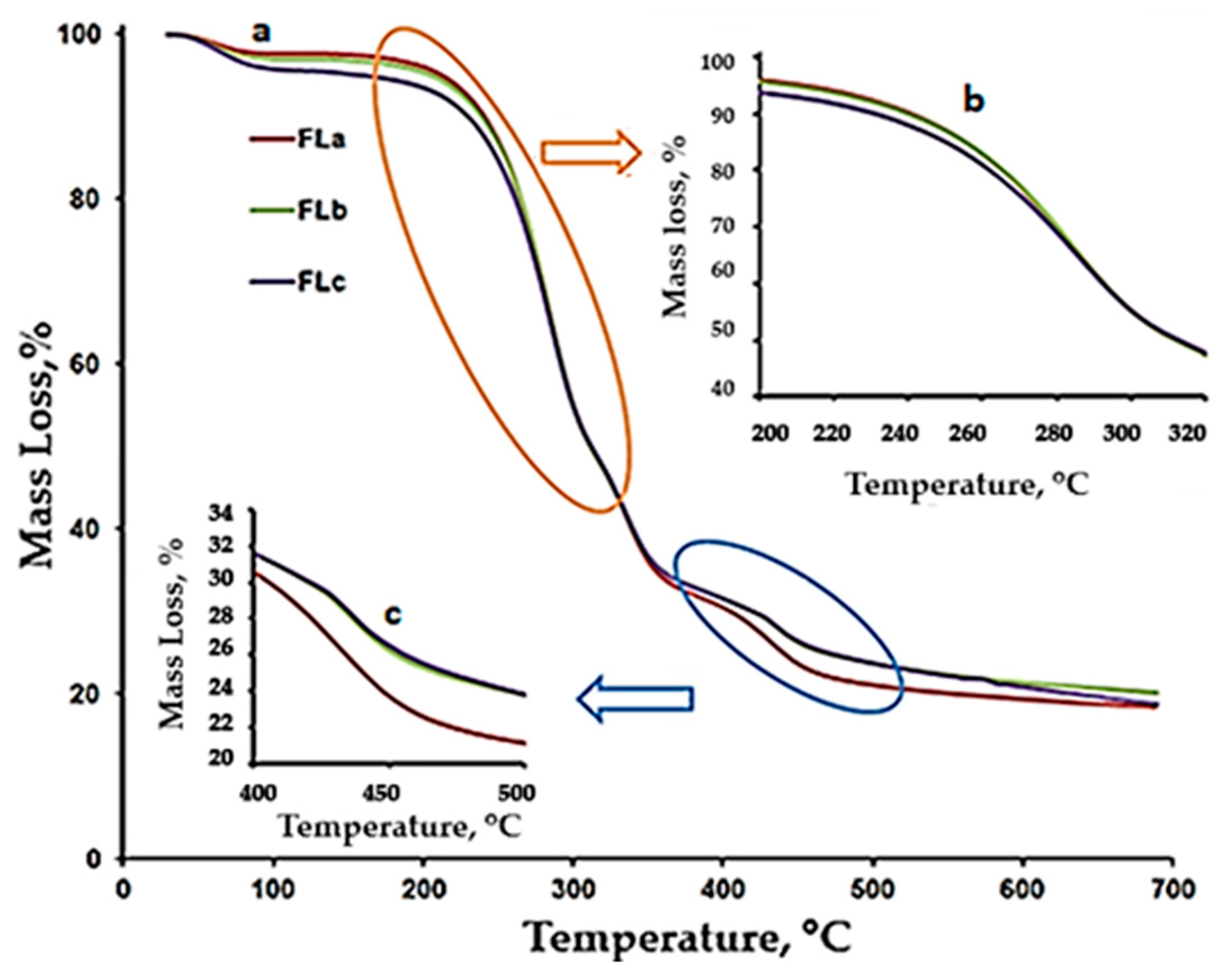
TG/DTG/DTA Characterization
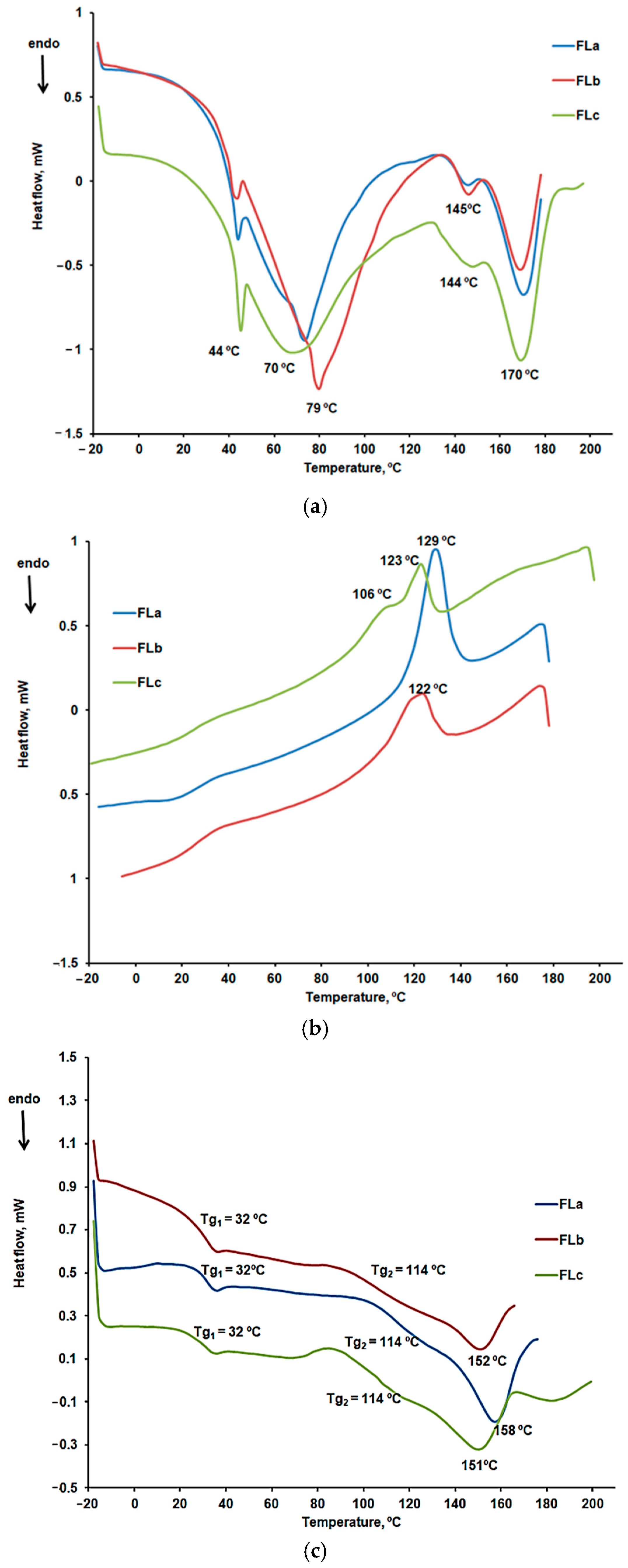
DSC Characterization
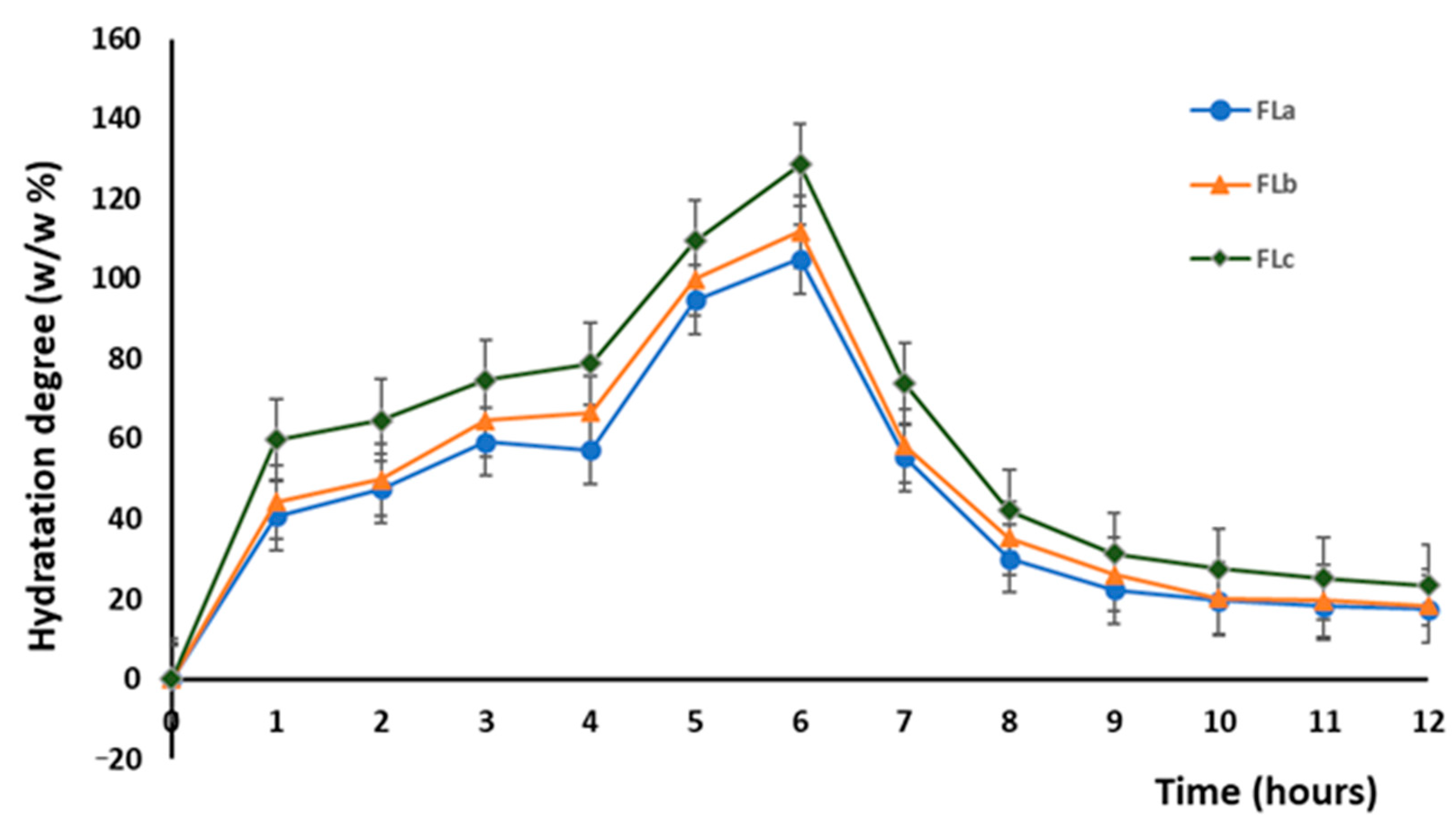
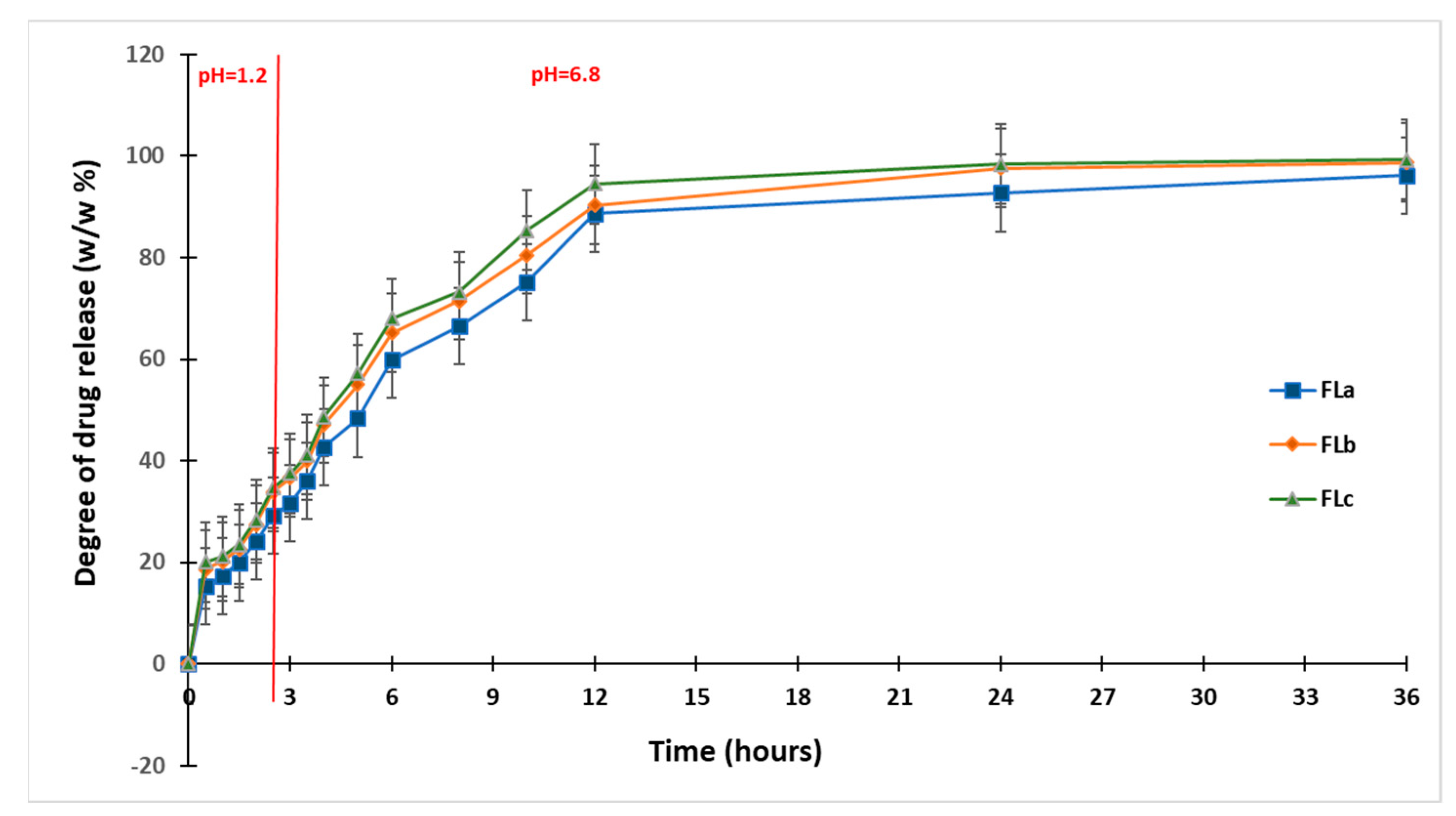
2.3. In Vitro Dissolution Studies
2.4. Drug Release Kinetics
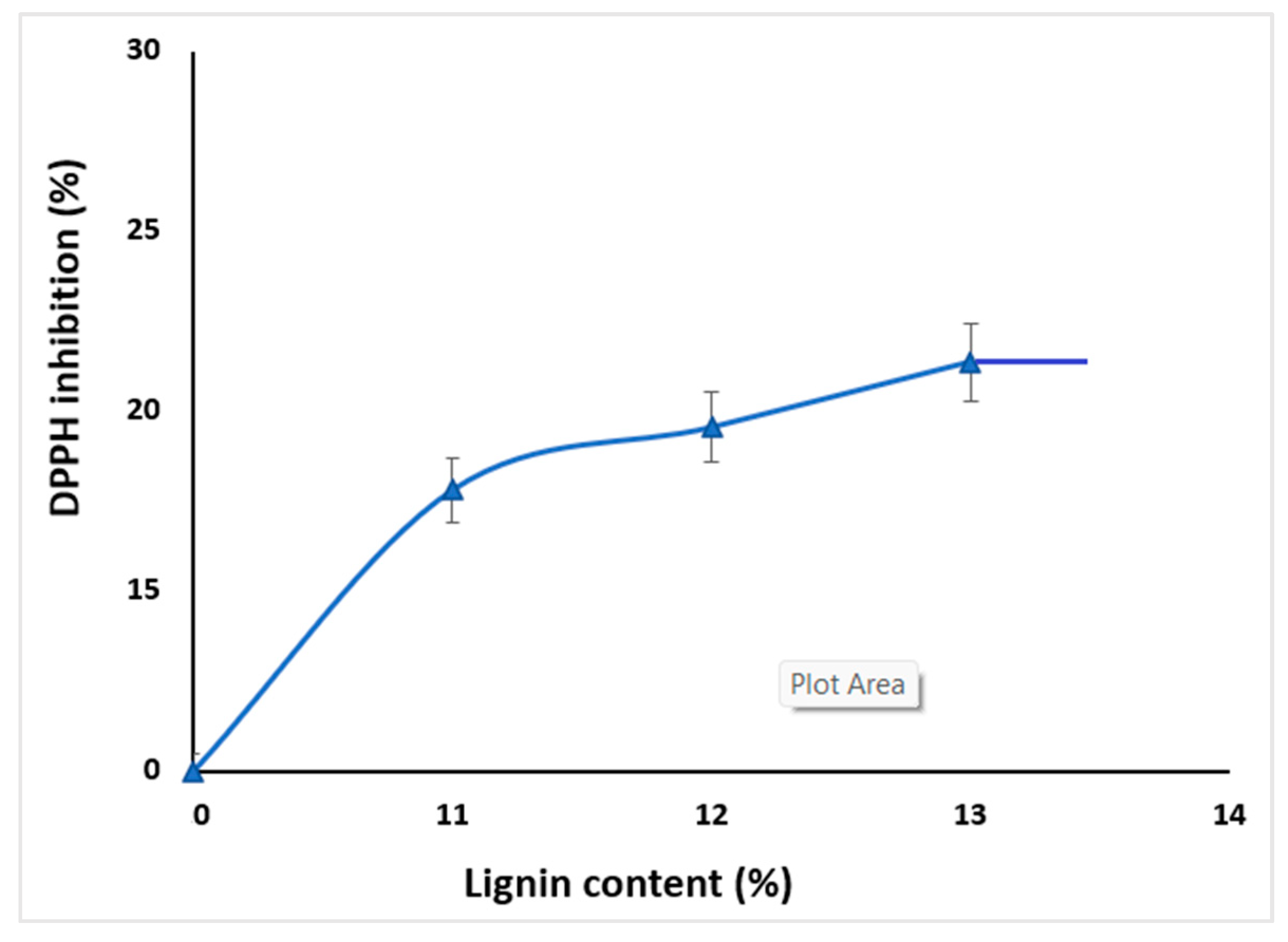
2.5. Antioxidant Activity
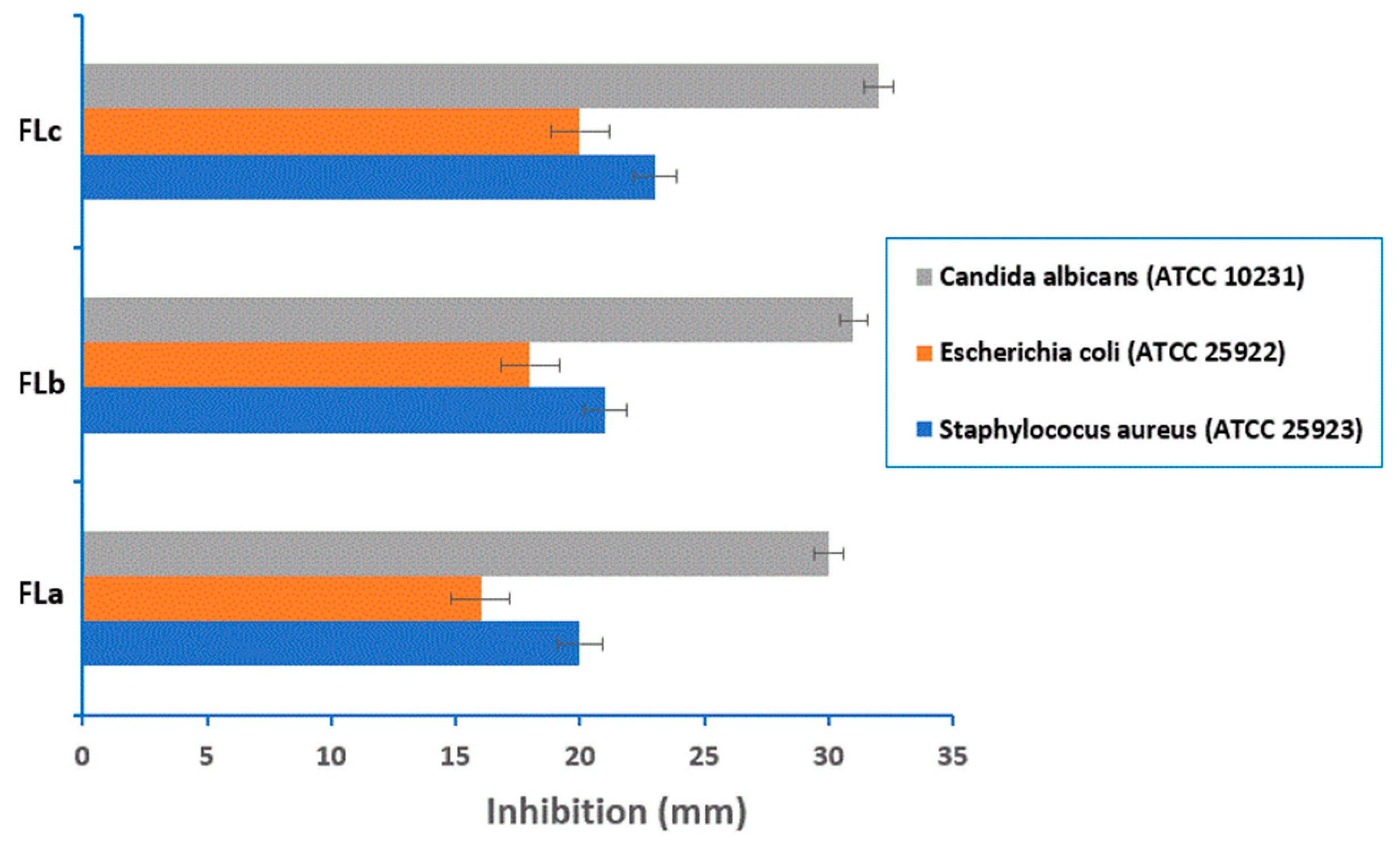
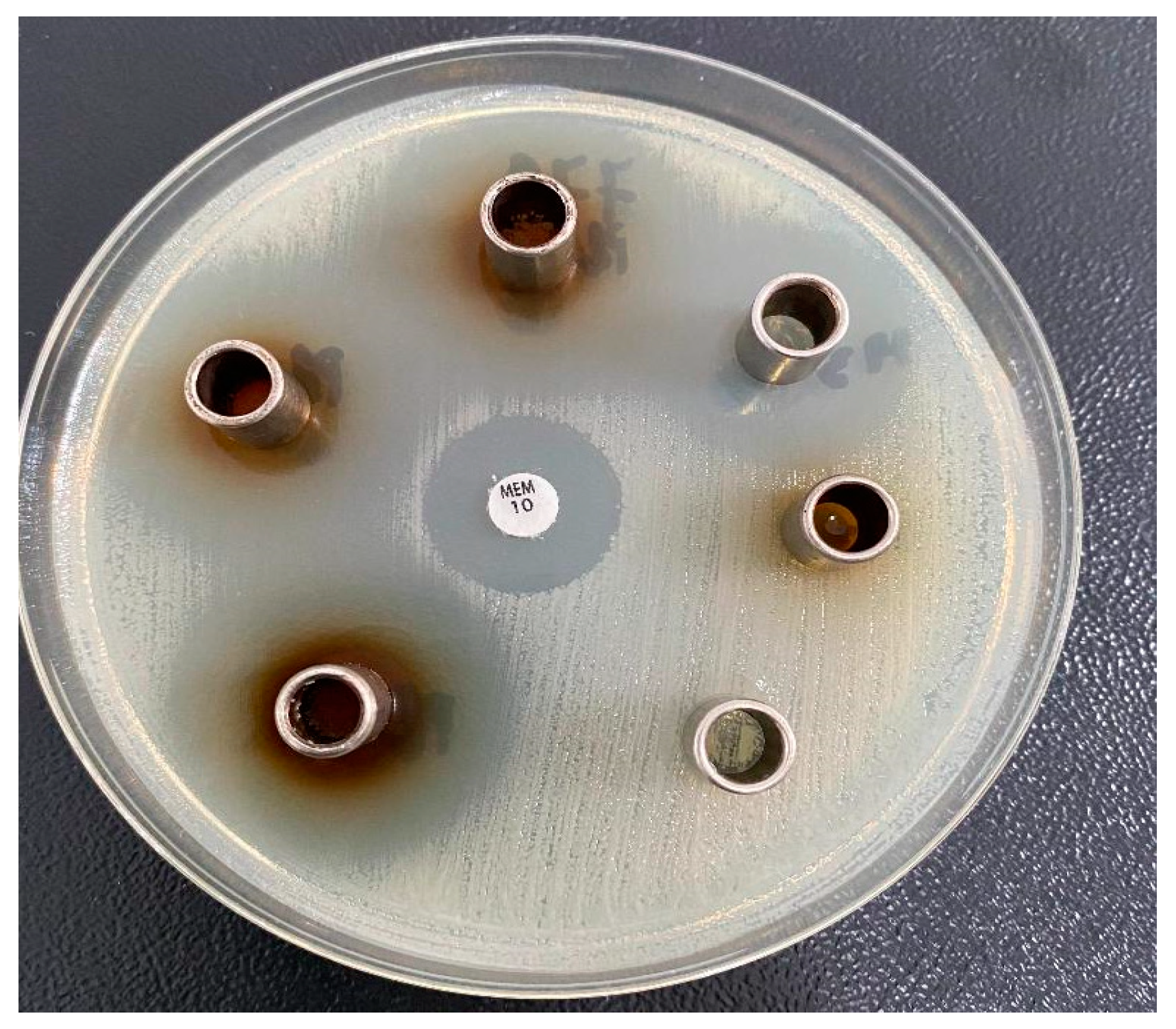
2.6. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Methods
Preparation of CLZ Matrix Tablets
3.3. Evaluation of the Prepared Tablets
3.3.1. Pharmaco-Technical Parameter of Matrix Tablets
3.3.2. Drug-Excipients Compatibility Study
Fourier-Transform Infrared Spectroscopy
X-Ray Diffraction
Thermal Characterization
3.3.3. In Vitro Dissolution Studies
3.3.4. Drug Release Kinetics
3.3.5. Antioxidant Activity
3.3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirani, A.G.; Patankar, S.P.; Borole, V.S.; Pawar, A.S.; Kadam, V.J. Direct Compression High Functionality Excipient using Coprocessing Technique: A Brief Review. Curr. Drug Deliv. 2011, 8, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.R.; Ibrahim, M.I.; Al-Haddad, M.S. The Influence of Consumers’ Preferences and Perceptions of Oral Solid Dosage Forms on their Treatment. Int. J. Clin. Pharm. 2012, 34, 728–732. [Google Scholar] [PubMed]
- Janssen, P.H.; Fathollahi, S.; Bekaert, B.; Vanderroost, D.; Roelofs, T.; Vanhoorne, V.; Vervaet, C.; Dickhoff, B.H.J. Impact of material properties and process parameters on tablet quality in a continuous direct compression line. Powder Technol. 2023, 424, 118520. [Google Scholar] [CrossRef]
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [PubMed]
- Chan, Y.; Goh, C.F.; Lau, J.Y.; Tiew, Y.C.; Balakrishnan, T.J.I. Rice starch thin films as a potential buccal delivery system: Effect of plasticiser and drug loading on drug release profile. Int. J. Pharm. 2019, 562, 203–211. [Google Scholar] [CrossRef]
- Singh, A.V. Biopolymers in drug delivery: A review. Pharmacol. Online 2011, 1, 666–674. [Google Scholar]
- Hsein, H.; Garrait, G.; Tamani, F.; Beyssac, E.; Hoffart, V. Denatured Whey Protein Powder as a New Matrix Excipient: Design and Evaluation of Mucoadhesive Tablets for Sustained Drug Release Applications. Pharm. Res. 2017, 34, 365–377. [Google Scholar] [CrossRef]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric Plant-Derived Excipients in Drug Delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef]
- Xavier, A.S. Skeletal Muscle Relaxants. In Introduction to Basics of Pharmacology and Toxicology; Paul, A., Anandabaskar, N., Mathaiyan, J., Raj, G.M., Eds.; Springer: Singapore, 2021; pp. 271–280. [Google Scholar]
- Chou, R.; Peterson, K.; Hefand, M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: A systemic review. J. Pain Symptom Manag. 2004, 28, 140–175. [Google Scholar] [CrossRef]
- Sweetman, S.C. Martindale. The Complete Drug Reference, 36th ed.; Sweetman, S.C., Ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Aronson, J.K. (Ed.) The International Encyclopedia of Adverse Drug Reactions an Interactions; Elsevier: Amsterdam, The Netherlands, 2016; p. 27. [Google Scholar]
- Xu, W.J.; Xie, H.J.; Cao, Q.R.; Shi, L.L.; Cao, Y.; Zhu, X.Y.; Cui, J.H. Enhaced dissolution and oral bioavaibility of valsartan solid dispersions prepared by freeze drying technique using hydrophilic polymers. Drug Deliv. 2016, 23, 41–48. [Google Scholar] [CrossRef]
- Creteanu, A.; Pamfil, D.; Vasile, C.; Ghilan, A.; Tantaru, G. The Influence of Amiodarone Complexation with 2-Hydroxypropyl-β- Cyclodextrin in Oral Matrix Tablets Delivery: In vitro and In vivo Evaluationon. Prog. Chem. Sci. Res. 2023, 9, 13–63. [Google Scholar]
- Creteanu, A.; Ochiuz, L.; Ghiciuc, C.-M.; Tantaru, G.; Vasile, C.; Popescu, C.M. Composition and Procedure to Obtain New Aminodarone Chlorohydrate-Loaded Matrix Tablets with Modified and Prolonged Release. Patent Nr. 131194, 30 August 2019. [Google Scholar]
- Aiswarya, A.; Suja, C. Formulation and Evaluation of Chlorzoxazone Microspheres. Eur. J. Pharm. Med. Res. 2021, 8, 379–389. [Google Scholar]
- Karagoz, P.; Khiawjan, S.; Marques, M.; Santzouk, S.; Bugg, T.D.H.; Lye, G.J. Pharmaceutical applications of lignin-derived chemicals and lignin-based materials: Linking lignin source and processing with clinical indication. Biomass Conv. Bioref. 2024, 14, 26553–26574. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of Lignin from residue of corn stover to ethanol production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Viksna, A.; Dobele, G.; Telysheva, G. Characterization of softwood and hardwood LignoBoost kraft lignins with emphasis on their antioxidant activity. BioResources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In Vitro Evaluation of Biodegradable Lignin-Based Nanoparticles for Drug Delivery and Enhanced Antiproliferation Effect in Cancer Cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Balasubramanian, V. A new approach to biopolymer composite synthesis using Lignin and bacterial biopolyester. J. Mater. Sci. 2013, 48, 7848–7855. [Google Scholar]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.; Kostiainen, M.A.; Santos, H.A.; Yli-Kauhaluoma, J. Properties and chemical modifications of Lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Materi. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar]
- Dow. Using Dow Excipients for Controlled Release of Drugs in Hydrophilic Matrix Systems; The Dow Chemical Company: Midland, TX, USA, 2006. [Google Scholar]
- Bühler, V. Kollidon: Polyvinylpyrrolidone Excipients for the Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2008; pp. 17–260. [Google Scholar]
- Alven, S.; Aderibigbe, B.A. Chitosan and cellulose-based hydrogels for wound management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Creteanu, A.; Lisa, G.; Vasile, C.; Popescu, M.-C.; Pamfil, D.; Lungu, C.N.; Panainte, A.D.; Tantaru, G. New Hydrophilic Matrix Tablets for the Controlled Release of Chlorzoxazone. Int. J. Mol. Sci. 2024, 25, 5137. [Google Scholar] [CrossRef]
- The European Pharmacopoeia. European Pharmacopoeia, (Ph. Eur.), 11th ed.; The European Pharmacopoeia: Strasbourg, France, 2023; pp. 348-–350+1005–1006. [Google Scholar]
- Liu, D.; Li, Y.; Qian, Y.; Xiao, Y.; Du, S.; Qiu, X. Synergistic Antioxidant Performance of Lignin and Quercetin Mixtures. ACS Sustain. Chem. Eng. 2017, 5, 8424–8428. [Google Scholar] [CrossRef]
- Yurdakul, S.; Yurdaku, M. FT-IR, FT-Raman spectra, and DFT computations of the vibrational spectra and molecular geometry of chlorzoxazone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 126, 339–348. [Google Scholar] [CrossRef]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and Improved Dissolution of Glycyrrhetinic Acid Solid Dispersion by Alkalizers. Pharmaceutics 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Spectroscopic studies on the temperature-dependent molecular arrangements in hybrid chitosan/1,3-β-D-glucan polymeric matrices. Int. J. Biol. Macromol. 2020, 159, 911–921. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Vasile, C.; Popescu, M.C.; Singurel, G.; Popa, V.I.; Munteanu, B.S. Analytical Methods for Lignin Characterization. II. Spectroscopic Studies. Cell Chem. Technol. 2006, 40, 597–621. [Google Scholar]
- Rojek, B.; Bartyzel, A.; Sawicki, W.; Plenis, A. DSC, TGA-FTIR and FTIR Assisted by Chemometric Factor Analysis and PXRD in Assessing the Incompatibility of the Antiviral Drug Arbidol Hydrochloride with Pharmaceutical Excipients. Molecules 2024, 29, 264. [Google Scholar] [CrossRef]
- Popescu, M.C.; Larsson, P.T.; Olaru, N.; Vasile, C. Spectroscopic study of acetylated kraft pulp fibers. Carbohydr. Polym. 2012, 288, 530–536. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Yang, Y.; Zhang, Y.; Zhao, C.; Yu, Y.; Wang, S. Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J. Anal. Appl. Pyrolysis. 2018, 134, 12–24. [Google Scholar] [CrossRef]
- Amit, T.A.; Roy, R.; Raynie, D.E. Thermal and structural characterization of two commercially available technical lignins for high-value applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100106. [Google Scholar]
- Damayanti; Wu, H.S. Pyrolysis kinetic of alkaline and dealkaline lignin using catalyst. J. Polym. Res. 2018, 25, 7. [Google Scholar]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efciency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar]
- Komisarz, K.; Majka, T.M.; Pielichowski, K. Chemical and Physical Modification of Lignin for Green Polymeric Composite Materials. Materials 2023, 16, 16. [Google Scholar]
- Ganesan, V.; Rosentrater, K.A.; Muthukumarappan, K. Flowability and handling characteristics of bulk solids and powders—A review with implications for DDGS. Biosyst. Eng. 2008, 101, 425–435. [Google Scholar] [CrossRef]
- Raval, M.; Patel, J.; Parikh, R.; Sheth, N. Dissolution enhancement of chlorzoxazone using cogrinding technique. Int. J. Pharm. Investig. 2015, 5, 247. [Google Scholar]
- Aadil, K.R.; Barapatre, A.; Sahu, S.; Jha, H.; Tiwary, B.N. Free radical scavenging activity and reducing power of Acacia nilotica wood lignin. Int. J. Biol. Macromol. 2014, 67, 220–227. [Google Scholar]
- Dominguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larraneta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, M.C.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 2016, 11, 5452–5481. [Google Scholar]
- Li, K.; Zhong, W.; Li, P.; Ren, J.; Jiang, K.; Wu, W. Recent advances in lignin antioxidant: Antioxidant mechanism, evaluation methods, influence factors and various applications. Int. J. Biol. Macromol. 2023, 251, 125992. [Google Scholar]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Shi, Q.S.; Zhong, X.O.; Guo, Y.; Xiao-Bao Xie, X.B. High value valorization of lignin as environmental benign antimicrobial. Mater. Today Bio 2023, 18, 100520. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial lignin-based Polyurethane/Ag composite foams for improving wound healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control Release 2012, 161, 351–362. [Google Scholar] [PubMed]
- Maderuelo, C.; Zarzuelo, A.; Lanoa, J. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control Release 2011, 154, 2–19. [Google Scholar]
- US Pharmacopoeia Convenction. United States Pharmacopoeia and the National Formulary, 26th ed.; US Pharmacopoeia Convenction, Inc.: Rockville, MD, USA, 2003. [Google Scholar]
- Vo, A.Q.; Kutz, G.; He, H.; Narala, S.; Bandari, S.; Repka, M.A. Continuous Manufacturing of Ketoprofen Delayed Release Pellets Using Melt Extrusion Technology: Application of QbD Design Space, Inline Near Infrared, and Inline Pellet Size Analysis. J. Pharm. Sci. 2020, 109, 3598–3607. [Google Scholar]
- Restrepo-Uribe, L.; Ioannidis, N.; del Pilar Noriega Escobar, M. Influence of screw configuration and processing parameters on the dissolution of ketoprofen in polymer blends. J. Appl. Polym. Sci. 2020, 137, 49407. [Google Scholar]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef]
- Muselík, J.; Komersová, A.; Kubová, K.; Matzick, K.; Skalická, B. A Critical Overview of FDA and EMA Statistical Methods to Compare In Vitro Drug Dissolution Profiles of Pharmaceutical Products. Pharmaceutics 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, M.; Esfandiari, N.; Honarvar, B.; Sajadian, S.A.; Amin Azdarpour, A. Kinetic Modeling to Explain the Release of Medicine from Drug Delivery Systems. Chem. Bio. Eng. 2023, 10, 1006–1049. [Google Scholar] [CrossRef]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Predicting Drug Release from 3D Printed Oral Medicines Based on the Surface Area to Volume Ratio of Tablet Geometry. Pharmaceutics 2021, 13, 1453. [Google Scholar] [CrossRef]
- Abhitul, P.; Aparna, J.; Kapil, K.; Ikram, I.; Vaishali, R. A comprehensive review on sustained release tablets. J. Integral Sci. 2023, 6, 8–12. [Google Scholar]
- Liu Dai, L.R.; Xu, C.; Wang, K.; Zheng, C.; Si, C. Lignin-based micro- and nanomaterials and their composites in biomedical applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef]
- CLSI. Performance Standard for Antimicrobial Susceptibility Testing, 26th ed.; CLSI: Malvern, PA, USA, 2016. [Google Scholar]







| Formulations | Without Lignin | FLa | FLb | FLc |
|---|---|---|---|---|
| Composition mg/cp; (w/w %) | ||||
| CLZ | 250 (50%) | 250 (50%) | 250 (50%) | 250 (50%) |
| KOL | 150 (30%) | 150 (30%) | 150 (30%) | 150 (30%) |
| CHT | 25 (5%) | 25 (5%) | 25 (5%) | 25 (5%) |
| LIG | 0 | 55 (11%) | 60 (12%) | 65 (13%) |
| ST | 2.5 (0.5%) | 2.5 (0.5%) | 2.5 (0.5%) | 2.5 (0.5%) |
| AV | 72.5 (14.5%) | 17.5(3.5%) | 12.5 (2.5%) | 7.5 (1.5%) |
| Parameters | ||||
| Diameter (mm) | 10.133 ± 0.025 | 10.121 ± 0.022 | 10.061 ± 0.015 | 10.053 ± 0.012 |
| Thickness (mm) | 2.908 ± 0.173 | 2.914 ± 0.185 | 2.802 ± 0.112 | 2.773 ± 0.117 |
| Average mass (g) | 0.501 ± 0.089 | 0.493 ± 0.061 | 0.486 ± 0.084 | 0.488 ± 0.070 |
| Dose uniformity (mg) | 249 ± 0.932 | 248 ± 0.912 | 249 ± 0.963 | 250 ± 0.028 |
| Mechanical strength (N) | 86.83 ± 2.422 | 85.73 ± 2.532 | 87.55 ± 2.283 | 89.62 ± 2.311 |
| Friability (%) | 1.309 ± 0.041 | 1.312 ± 0.033 | 1.253 ± 0.027 | 0.891 ± 0.023 |
| Compound | IR Band Position | Band Assignment | Reference |
|---|---|---|---|
| CLZ | 3468, 3204, and 3156 cm−1 | stretching vibration of NH groups | [32] |
| 3080 and 3055 cm−1 | stretching vibration of Ph-H groups | ||
| 2982 and 2827 cm−1 | stretching vibration of CH groups | ||
| 1773 cm−1 | stretching vibration of C=O groups | ||
| 1614 cm−1 | stretching vibration of C=C from aromatic rings | ||
| 1471 cm−1 | stretching vibration of C-C and CN groups and deformation vibration of CCH groups | ||
| 1359 and 1255 cm−1 | stretching vibration of CC groups and deformation vibration of CCH and CNH groups | ||
| 1100 cm−1 | stretching vibration of CN and CO groups and deformation vibration of CCH and CCC groups | ||
| 1061 cm−1 | stretching vibration of CC and CCl groups and deformation vibration of CCH groups | ||
| 958 cm−1 | stretching vibration of CN groups and deformation vibration of CCC groups | ||
| 591 cm−1 | deformation vibration of CNO, CCO, and CCN groups | ||
| 545 cm−1 | stretching vibration of CO and CCl groups and deformation vibration of CCC groups | ||
| KOL | 3447 cm−1 | stretching vibration of OH groups | [33] |
| 2965, 2933, and 2870 cm−1 | symmetric and asymmetric stretching vibration of CH groups | ||
| 1740 and 1658 cm−1 | stretching vibration of C=O groups from vinyl acetate and pyrrolidone ring | ||
| 1375 cm−1 | stretching vibration of COO groups | ||
| 1236 cm−1 | stretching vibration of CC groups and deformation vibration of CCH and CNH groups | ||
| 1119 cm−1 | stretching vibration of CN and CO groups and deformation vibration of CCH and CCC groups | ||
| 1024 cm−1 | stretching vibration of CC and CCl groups and deformation vibration of CCH groups | ||
| 944 cm−1 | stretching vibration of CN groups | ||
| CHT | 3444 cm−1 | stretching vibrations of OH bonds | [34] |
| 2960, 2923, 2886, and 2866 cm−1 | symmetric and asymmetric stretching vibrations of CH groups | ||
| 1651 cm−1, 1597 cm−1, 1424 cm−1, and 1381 cm−1 | stretching vibration of C=O groups of amide I, deformation vibrations of the NH (N-acetylated residues, amide II band), deformation vibration of CH2, CH3 groups and deformation vibration of OH groups | ||
| 1258 cm−1 | stretching vibration of NH, COC, and COH groups | ||
| 1158 cm−1 and 1079 cm−1 | stretching vibration of CO and COC groups | ||
| LIG | 3416 cm−1 | stretching vibration of OH groups in phenolic and aliphatic structures | [35] |
| 2934 cm−1 | stretching vibration of CH in aromatic methoxyl groups and aliphatic CH2 and CH3 groups | ||
| 2846 cm−1 | stretching vibration of CH in aromatic methoxyl groups and CH2 and CH3 groups | ||
| 1599 cm−1 | stretching vibration of C=C groups of the aromatic ring (S), and deformation vibration of CH groups | ||
| 1511 cm−1 | stretching vibration of C=C groups of the aromatic ring (G), CH deformation | ||
| 1457 cm−1 | asymmetric deformation C–H groups in CH2 and CH3 | ||
| 1422 cm−1 | asymmetric deformation of C–H groups in –OCH3 | ||
| 1364 cm−1 | symmetric bending of C–H from methoxyl group, O–H and C–O of phenol and tertiary alcohol | ||
| 1266 cm−1 | guaiacol ring breathing, C–O stretch in lignin, C–O linkage in guaiacyl aromatic methoxyl groups | ||
| 1222 cm−1 | syringyl ring breathing with C–O stretching | ||
| 1129 cm−1 | aromatic C–H in-plane deformation (typical for S units), secondary alcohols, C=O stretch | ||
| 1078 cm−1 | C–O deformation in secondary alcohols and aliphatic ethers | ||
| 1032 cm−1 | alkyl–O ether vibrations in methoxyl and β–O–4 in guaiacol | ||
| 845 cm−1 | CH out of plane vibrations in positions 2, 5, and 6 of guaiacyl units | ||
| ST | 3436 cm−1 | stretching vibrations of the associated water molecules | [36] |
| 2921 and 2852 cm−1 | stretching vibration of CH groups | ||
| 1576 and 1456 cm−1 | symmetric and asymmetric stretching vibration of (COO-) groups | ||
| AV | 3413, 3346, 1434, and 1324 cm−1 | stretching and in-plane deformation vibration of OH groups | [37] |
| 2901, 1371, and 1276 cm−1 | stretching and deformation vibration of CH groups | ||
| 1643 cm−1 | stretching vibration of absorbed OH and conjugated CO groups | ||
| 1243, 1163, 1113, 1061, and 1026 cm−1 | stretching vibration of CO groups | ||
| 901 cm−1 | stretching vibration of β-glucosidic linkage between the sugar units |
| Sample | CLZ | KOL | CHT | LIG | ST | AV | FLa | FLb | FLc |
|---|---|---|---|---|---|---|---|---|---|
| Degree of crystallinity % | 80.0 | 24.3 | 37.2 | 0.0 | 49.5 | 47.7 | 50.9 | 49.5 | 48.1 |
| Sample | Stage/ DTA Characteristic | Tonset, °C | Tpeak, °C | Tendset, °C | ΔW, % | Residue, % |
|---|---|---|---|---|---|---|
| LIG | I/endo | 49 | 62 | 93 | 6.68 | 60.08 |
| II/endo | 137 | 142 | 149 | 2.10 | ||
| III/exo | 212 | 288 | 327 | 13.14 | ||
| IV/exo | 327 | 350 | 378 | 7.39 | ||
| V/exo | 378 | 448 | 533 | 8.22 | ||
| VI/exo | 658 | - | - | 2.39 | ||
| FLa | I/endo | 53 | 62 | 91 | 2.36 | 18.47 |
| II/exo | 227 | 284 | 306 | 50.15 | ||
| III/exo | 327 | 336 | 357 | 15.18 | ||
| IV/exo | 408 | 434 | 465 | 13.84 | ||
| FLb | I/endo | 49 | 60 | 87 | 2.97 | 18.61 |
| II/exo | 234 | 285 | 305 | 44.77 | ||
| III/exo | 305 | 334 | 362 | 20.40 | ||
| IV/exo | 425 | 437 | 481 | 12.02 | ||
| FLc | I/endo | 47 | 64 | 96 | 4.55 | 19.84 |
| II/exo | 242 | 284 | 304 | 42.80 | ||
| III/exo | 304 | 335 | 354 | 21.00 | ||
| IV/exo | 424 | 438 | 511 | 13.04 |
| Reference Formulations | Test Formulations | Difference Factor f1 | Similarity Factor f2 |
|---|---|---|---|
| FLa | F1a | 47.7516 | 31.2235 |
| F2a | 41.8503 | 38.3546 | |
| FLb | F1b | 36.0455 | 58.0386 |
| F2b | 33.7543 | 57.9562 | |
| FLc | F1c | 51.6215 | 20.8511 |
| F2c | 52.1065 | 19.2135 |
| Formulation Name | Qmax [%] | T1/2 [h] |
|---|---|---|
| FLa | 96.17 | 4.96 |
| FLb | 98.32 | 4.25 |
| FLc | 99.30 | 4.15 |
| CLZ | 100 | 0.44 |
| Sample Name | n | R2n | k [h−n] | R2k |
|---|---|---|---|---|
| First step of kinetic release profile (0–2 h)—pH 1.2 | ||||
| FLa | 0.466 | 0.989 | 0.713 | 0.999 |
| FLb | 0.444 | 0.962 | 0.716 | 0.997 |
| FLc | 0.414 | 0.967 | 0.733 | 0.998 |
| CLZ | 0.403 | 0.983 | 0.745 | 0.996 |
| Second step of kinetic release profile (2.5 h–36 h)–pH 6.8 | ||||
| FLa | 0.684 | 0.990 | 0.162 | 0.996 |
| FLb | 0.645 | 0.991 | 0.184 | 0.994 |
| FLc | 0.656 | 0.991 | 0.187 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creteanu, A.; Lisa, G.; Vasile, C.; Popescu, M.-C.; Pamfil, D.; Panainte, A.-D.; Tantaru, G.; Vlad, M.-A.; Lungu, C.N. Lignin as a Bioactive Additive in Chlorzoxazone-Loaded Pharmaceutical Tablets. Molecules 2025, 30, 1426. https://doi.org/10.3390/molecules30071426
Creteanu A, Lisa G, Vasile C, Popescu M-C, Pamfil D, Panainte A-D, Tantaru G, Vlad M-A, Lungu CN. Lignin as a Bioactive Additive in Chlorzoxazone-Loaded Pharmaceutical Tablets. Molecules. 2025; 30(7):1426. https://doi.org/10.3390/molecules30071426
Chicago/Turabian StyleCreteanu, Andreea, Gabriela Lisa, Cornelia Vasile, Maria-Cristina Popescu, Daniela Pamfil, Alina-Diana Panainte, Gladiola Tantaru, Madalina-Alexandra Vlad, and Claudiu N. Lungu. 2025. "Lignin as a Bioactive Additive in Chlorzoxazone-Loaded Pharmaceutical Tablets" Molecules 30, no. 7: 1426. https://doi.org/10.3390/molecules30071426
APA StyleCreteanu, A., Lisa, G., Vasile, C., Popescu, M.-C., Pamfil, D., Panainte, A.-D., Tantaru, G., Vlad, M.-A., & Lungu, C. N. (2025). Lignin as a Bioactive Additive in Chlorzoxazone-Loaded Pharmaceutical Tablets. Molecules, 30(7), 1426. https://doi.org/10.3390/molecules30071426







