Effect of High-Pressure Homogenization and Wall Material Composition on the Encapsulation of Polyunsaturated Fatty Acids from Fish Processing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Homogenization Process Parameters (Pressure, Number of Cycles) on Encapsulation of Fish Oil
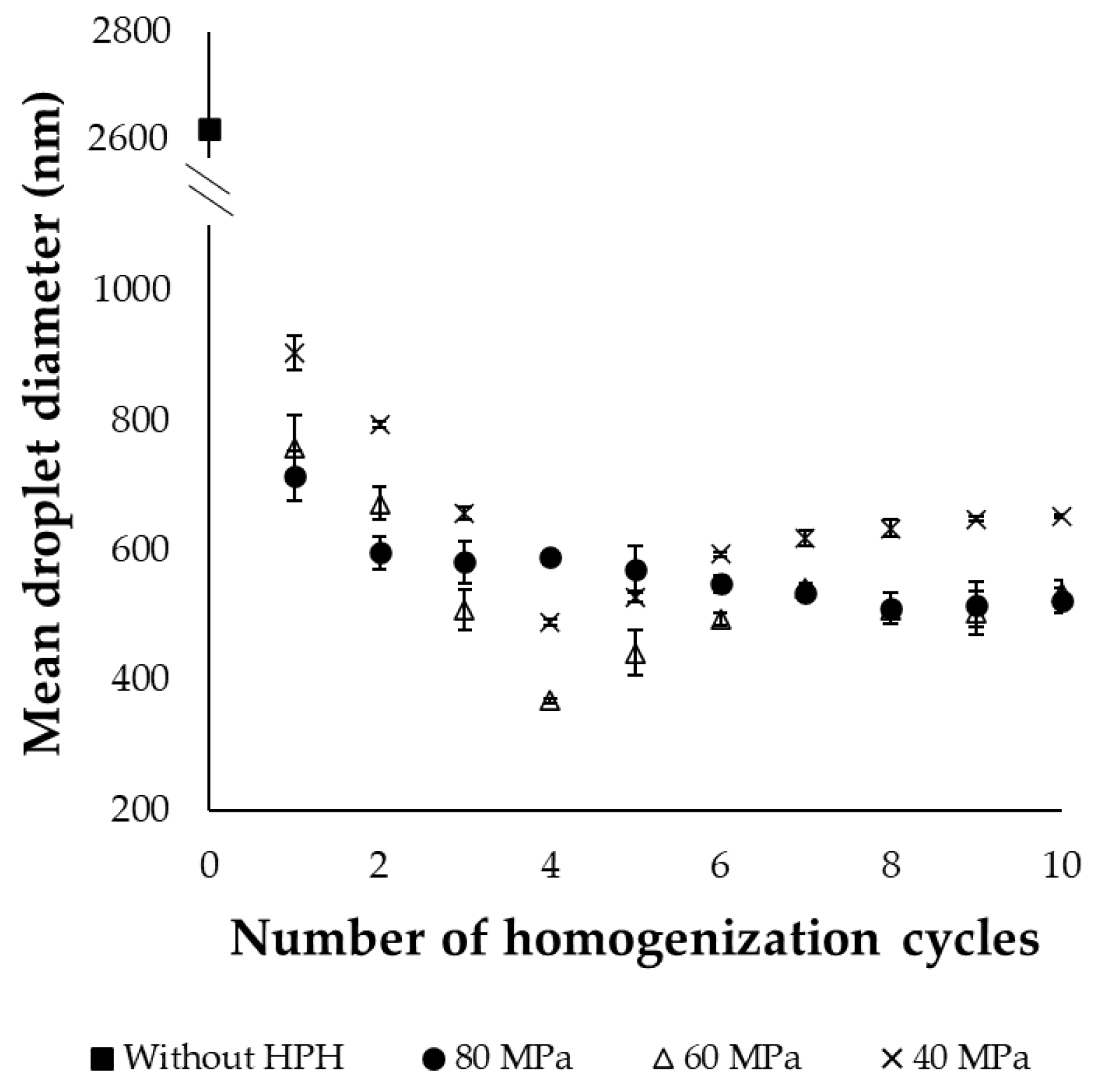
2.1.1. Droplet Size and Distribution of Emulsions
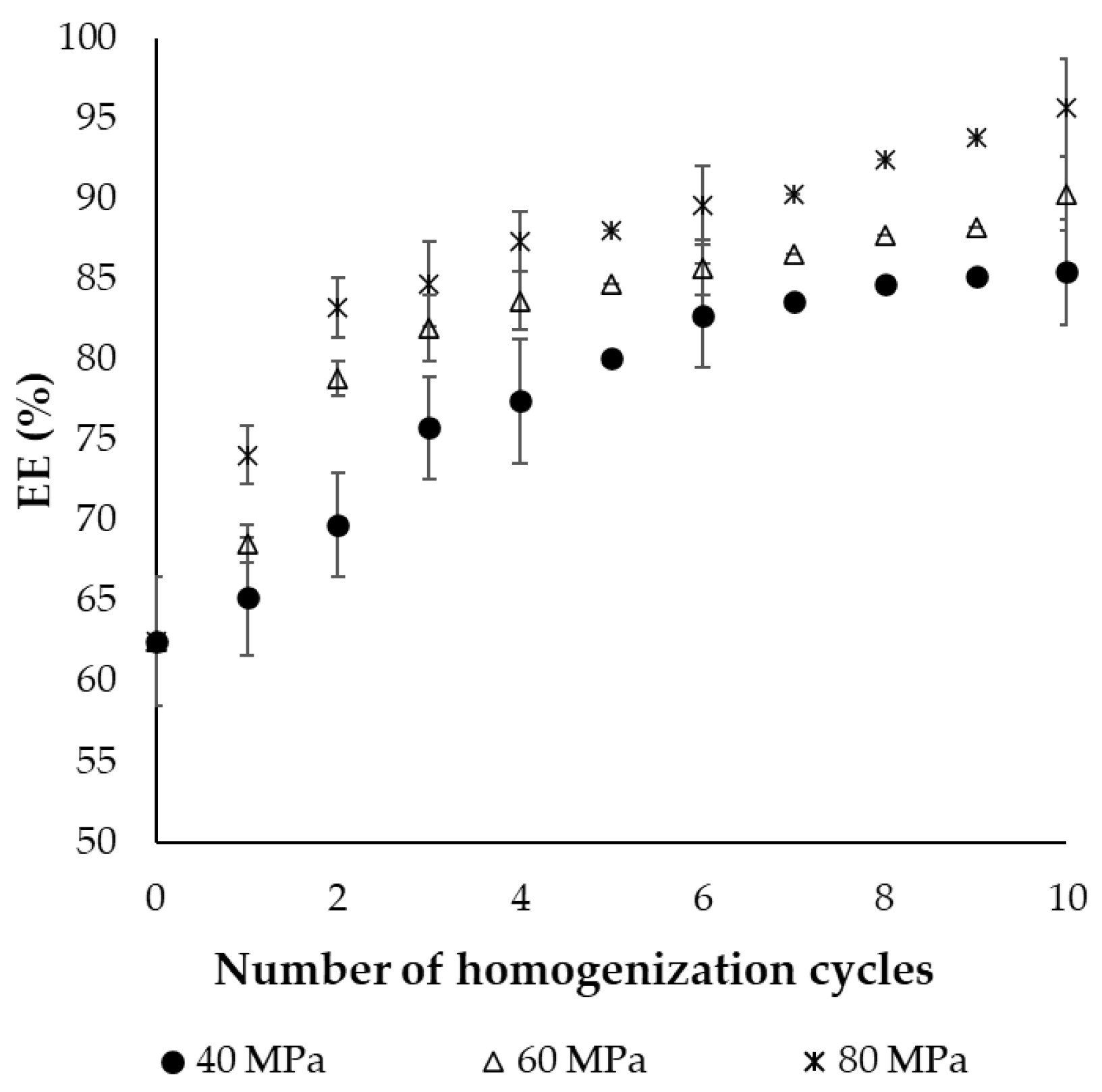
2.1.2. Encapsulation Efficiency of the Final Dried Product
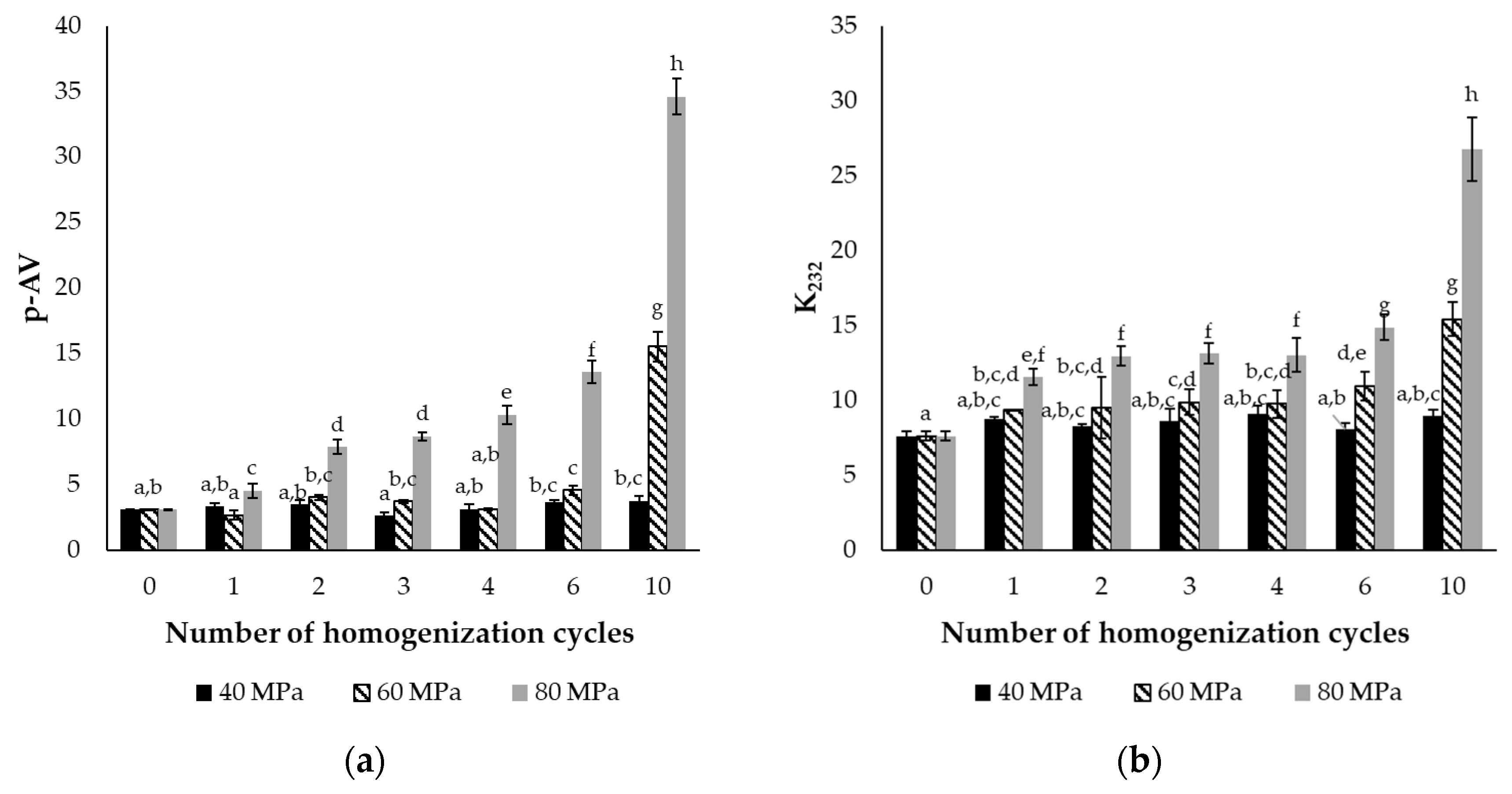
2.1.3. Lipid Oxidation of Encapsulated Oil
2.2. Effect of Wall Material on Encapsulation of Fish Oil
2.2.1. Encapsulation Efficiency
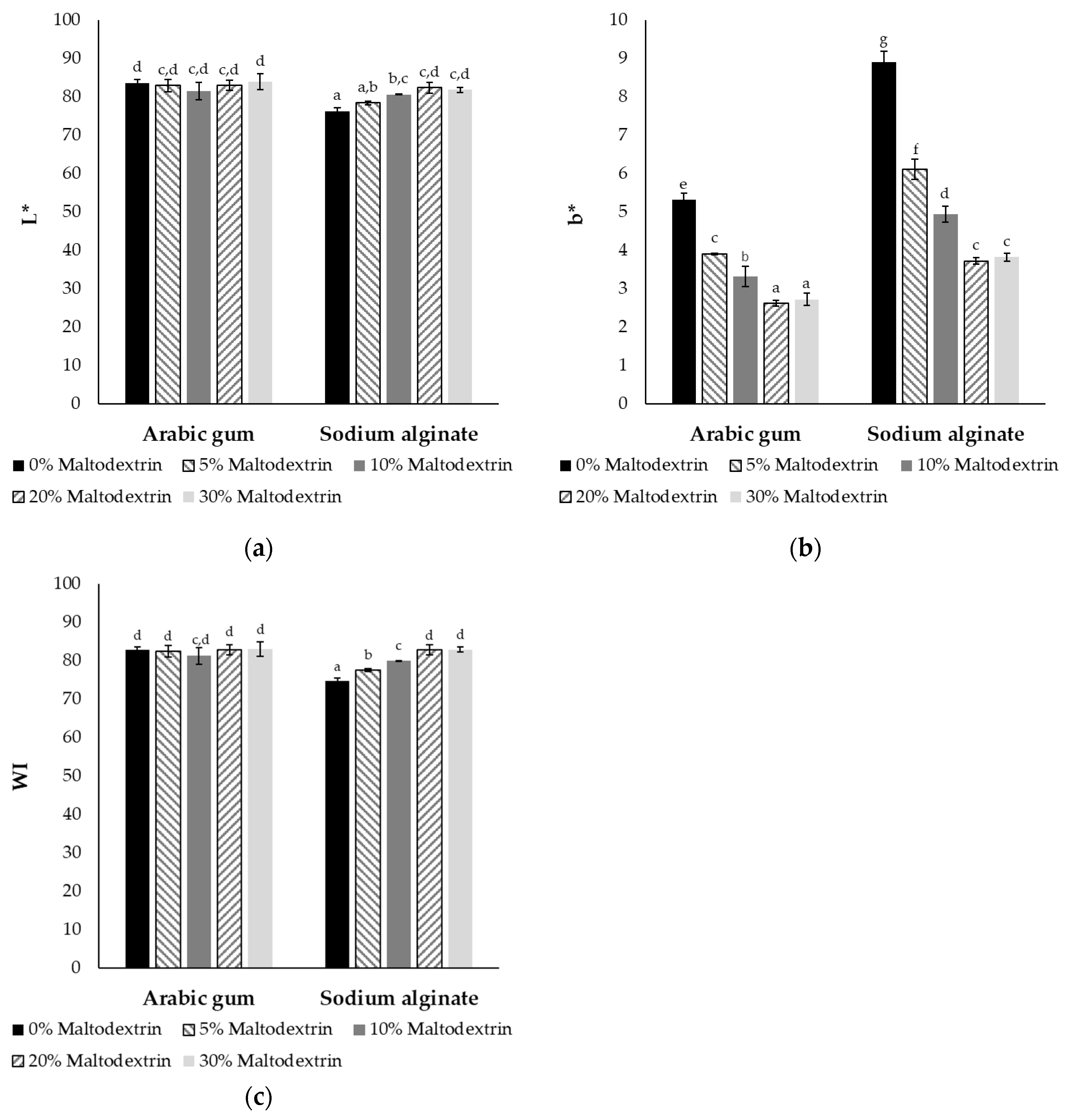
2.2.2. Color of Powders
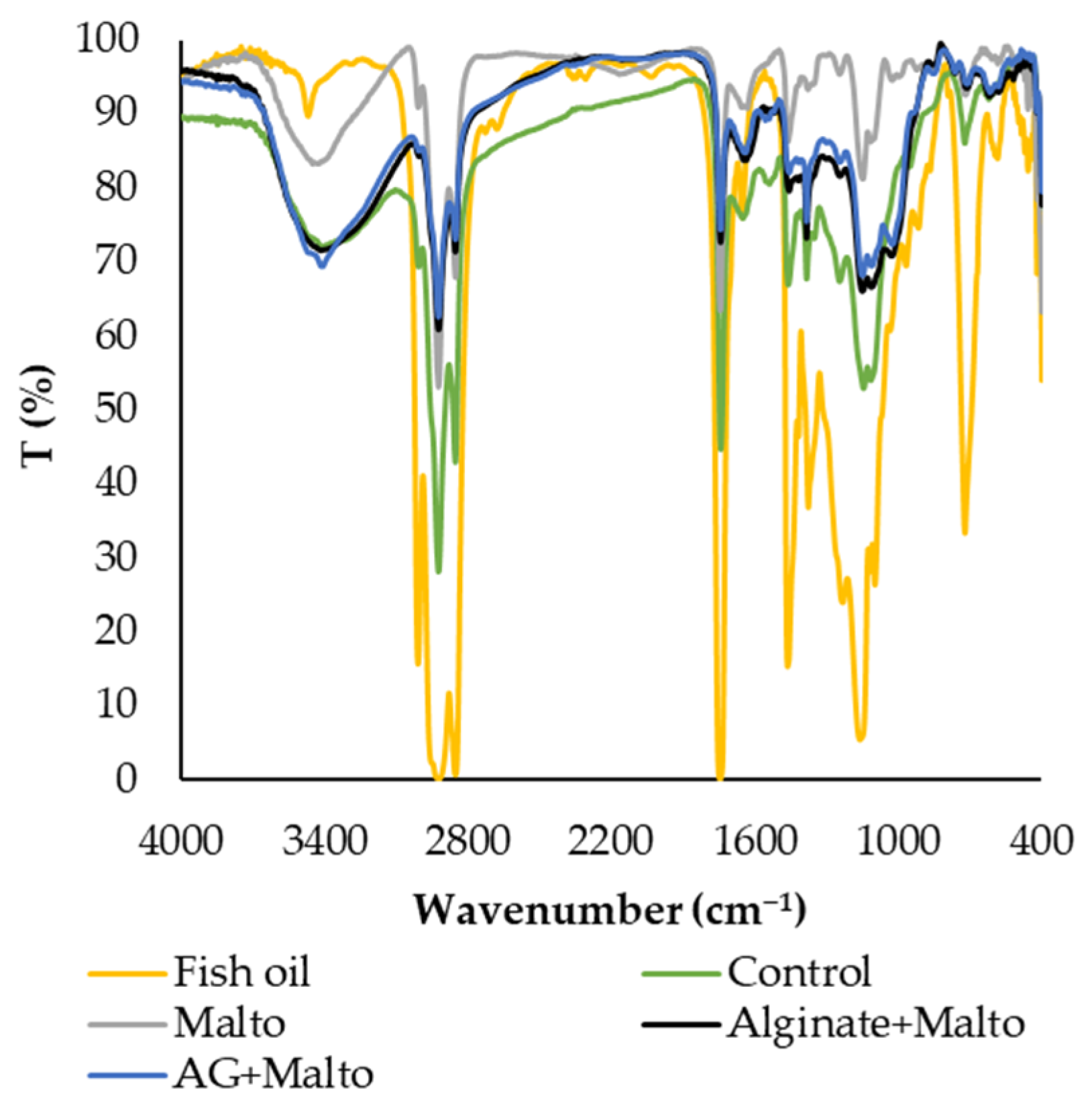
2.2.3. FTIR Spectra
3. Materials and Methods
3.1. Materials
3.2. Preparation of Oil-in-Water Nanoemulsion (o/w)
3.3. Freeze-Drying Process
3.4. Particle Size Distribution
3.5. Encapsulation Efficiency (EE)
3.6. Color Measurement
3.7. Oxidation of Fatty Acids
3.8. Fourier-Transform Infrared Spectroscopy (FTIR)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EE | Encapsulation efficiency |
| FTIR | Fourier-transform infrared spectroscopy |
| CAS | Sodium caseinate |
| SA | Sodium alginate |
| AG | Arabic gum |
| PUFA | Polyunsaturated fatty acids |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| PDI | Polydispersity Index |
| WI | Whiteness Index |
References
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A.; Norulaini, N.A. PUFAs in Fish: Extraction, Fractionation, Importance in Health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Tolerable Upper Intake Level of Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA) and Docosapentaenoic Acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar] [CrossRef]
- NIH Omega-3 Fatty Acids—Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/ (accessed on 20 March 2025).
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical Properties and Oxidative Stability of Fish Oil Nanoemulsions as Affected by Hydrophilic Lipophilic Balance, Surfactant to Oil Ratio and Storage Temperature. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 821–832. [Google Scholar] [CrossRef]
- Walker, R.M.; Gumus, C.E.; Decker, E.A.; McClements, D.J. Improvements in the Formation and Stability of Fish Oil-in-Water Nanoemulsions Using Carrier Oils: MCT, Thyme Oil, & Lemon Oil. J. Food Eng. 2017, 211, 60–68. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Vicente, A.A.; Monteiro, L.S.; Paiva-Martins, F. Influence of AO Chain Length, Droplet Size and Oil to Water Ratio on the Distribution and on the Activity of Gallates in Fish Oil-in-Water Emulsified Systems: Emulsion and Nanoemulsion Comparison. Food Chem. 2020, 310, 125716. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, P.; Kong, L.; Xu, B. Microencapsulation of Curcumin by Spray Drying and Freeze Drying. LWT 2020, 132, 109892. [Google Scholar] [CrossRef]
- Encina, C.; Márquez-Ruiz, G.; Holgado, F.; Giménez, B.; Vergara, C.; Robert, P. Effect of Spray-Drying with Organic Solvents on the Encapsulation, Release and Stability of Fish Oil. Food Chem. 2018, 263, 283–291. [Google Scholar] [CrossRef]
- Ramos, F.d.M.; Silveira Júnior, V.; Prata, A.S. Impact of Vacuum Spray Drying on Encapsulation of Fish Oil: Oxidative Stability and Encapsulation Efficiency. Food Res. Int. 2021, 143, 110283. [Google Scholar] [CrossRef]
- Kolanowski, W.; Jaworska, D.; Weißbrodt, J.; Kunz, B. Sensory Assessment of Microencapsulated Fish Oil Powder. J. Am. Oil Chem. Soc. 2007, 84, 37–45. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional Spray-Drying and Future Trends for the Microencapsulation of Fish Oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Kang, Y.; He, D.; Yang, B.; Wu, K. Cinnamon Essential Oil Nanoemulsions by High-Pressure Homogenization: Formulation, Stability, and Antimicrobial Activity. LWT 2021, 147, 111660. [Google Scholar] [CrossRef]
- Chang, M.; Guo, Y.; Jiang, Z.; Shi, L.; Zhang, T.; Wang, Y.; Gong, M.; Wang, T.; Lin, R.; Liu, R.; et al. Sea Buckthorn Pulp Oil Nanoemulsions Fabricated by Ultra-High Pressure Homogenization Process: A Promising Carrier for Nutraceutical. J. Food Eng. 2020, 287, 110129. [Google Scholar] [CrossRef]
- Selim, K.A.; Alharthi, S.S.; Abu El-Hassan, A.M.; Elneairy, N.A.; Rabee, L.A.; Abdel-Razek, A.G. The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils. Molecules 2021, 26, 6109. [Google Scholar] [CrossRef]
- Łozińska, N.; Głowacz-Różyńska, A.; Artichowicz, W.; Lu, Y.; Jungnickel, C. Microencapsulation of Fish Oil—Determination of Optimal Wall Material and Encapsulation Methodology. J. Food Eng. 2020, 268, 109730. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The Delivery of Sensitive Food Bioactive Ingredients: Absorption Mechanisms, Influencing Factors, Encapsulation Techniques and Evaluation Models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Heinzelmann, K.; Franke, K. Using Freezing and Drying Techniques of Emulsions for the Microencapsulation of Fish Oil to Improve Oxidation Stability. Colloids Surf. B Biointerfaces 1999, 12, 223–229. [Google Scholar]
- McClements, D.J.; Jafari, S.M. Improving Emulsion Formation, Stability and Performance Using Mixed Emulsifiers: A Review. Adv. Colloid. Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Yin, X.; Dong, H.; Cheng, H.; Ji, C.; Liang, L. Sodium Caseinate Particles with Co-Encapsulated Resveratrol and Epigallocatechin-3-Gallate for Inhibiting the Oxidation of Fish Oil Emulsions. Food Hydrocoll. 2022, 124, 107308. [Google Scholar] [CrossRef]
- Villiere, A.; Viau, M.; Bronnec, I.; Moreau, N.; Genot, C. Oxidative Stability of Bovine Serum Albumin- and Sodium Caseinate-Stabilized Emulsions Depends on Metal Availability. J. Agric. Food Chem. 2005, 53, 1514–1520. [Google Scholar] [CrossRef]
- Díaz, M.; Dunn, C.M.; McClements, D.J.; Decker, E.A. Use of Caseinophosphopeptides as Natural Antioxidants in Oil-in-Water Emulsions. J. Agric. Food Chem. 2003, 51, 2365–2370. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-Coalescence of Emulsion Droplets during High-Energy Emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Juttulapa, M.; Piriyaprasarth, S.; Takeuchi, H.; Sriamornsak, P. Effect of High-Pressure Homogenization on Stability of Emulsions Containing Zein and Pectin. Asian J. Pharm. Sci. 2017, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of High-Pressure Homogenization on Droplet Size Distributions and Rheological Properties of Model Oil-in-Water Emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective Droplet Size Reduction and Excellent Stability of Limonene Nanoemulsion Formed by High-Pressure Homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef]
- Lee, L.; Norton, I.T. Comparing Droplet Breakup for a High-Pressure Valve Homogeniser and a Microfluidizer for the Potential Production of Food-Grade Nanoemulsions. J. Food Eng. 2013, 114, 158–163. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of Nanoemulsions Stabilized by Model Food-Grade Emulsifiers Using High-Pressure Homogenization: Factors Affecting Particle Size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Keogh, M.K.; O’Kennedy, B.T.; Kelly, J.; Auty, M.A.; Kelly, P.M.; Fureby, A.; Haahr, A.M. Stability to Oxidation of Spray-Dried Fish Oil Powder Microencapsulated Using Milk Ingredients. J. Food Sci. 2001, 66, 217–224. [Google Scholar] [CrossRef]
- Drusch, S. Sugar Beet Pectin: A Novel Emulsifying Wall Component for Microencapsulation of Lipophilic Food Ingredients by Spray-Drying. Food Hydrocoll. 2007, 21, 1223–1228. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; Bhandari, B.; He, Y. Nano-Particle Encapsulation of Fish Oil by Spray Drying. Food Res. Int. 2008, 41, 172–183. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Ruiz-Carrascal, J.; Perez-Palacios, T. Improvement of Encapsulation and Stability of EPA and DHA from Monolayered and Multilayered Emulsions by High-Pressure Homogenization. J. Food Process Preserv. 2020, 44, e14290. [Google Scholar] [CrossRef]
- Semenoglou, I.; Katsouli, M.; Giannakourou, M.; Taoukis, P. Recovery of Omega-3-Rich Lipids: Toward the Sustainable Valorization of Sea-Bass Industry Side Streams. Separations 2024, 11, 101. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Evaluation of Food Grade Solvents for Lipid Extraction and Impact of Storage Temperature on Fatty Acid Composition of Edible Seaweeds Laminaria Digitata (Phaeophyceae) and Palmaria Palmata (Rhodophyta). Food Chem. 2016, 208, 161–168. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO Codex Alimentarius Commission. STANDARD FOR FISH OILS (CXS 329-2017); Adopted in 2017; Codex Alimentarius Commission: Rome, Italy, 2017. [Google Scholar]
- Jamshidi, A.; Antequera, T.; Solomando, J.C.; Perez-Palacios, T. Microencapsulation of Oil and Protein Hydrolysate from Fish within a High-Pressure Homogenized Double Emulsion. J. Food Sci. Technol. 2020, 57, 60–69. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Madadlou, A.; Rafiee, S. The Correlation of Wall Material Composition with Flow Characteristics and Encapsulation Behavior of Fish Oil Emulsion. Food Res. Int. 2012, 49, 379–388. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N. Protein-Polysaccharide Interactions for Stabilization of Food Emulsions. J. Dispers. Sci. Technol. 2002, 23, 93–123. [Google Scholar] [CrossRef]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the Molecular Assembly of Polysaccharide-Protein Systems as Encapsulation Materials. A Review. Adv. Colloid. Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef] [PubMed]
- Perez-Palacios, T.; Ruiz-Carrascal, J.; Solomando, J.C.; de-la-Haba, F.; Pajuelo, A.; Antequera, T. Recent Developments in the Microencapsulation of Fish Oil and Natural Extracts: Procedure, Quality Evaluation and Food Enrichment. Foods 2022, 11, 3291. [Google Scholar] [CrossRef]
- Pourashouri, P.; Shabanpour, B.; Razavi, S.H.; Jafari, S.M.; Shabani, A.; Aubourg, S.P. Impact of Wall Materials on Physicochemical Properties of Microencapsulated Fish Oil by Spray Drying. Food Bioproc. Tech. 2014, 7, 2354–2365. [Google Scholar] [CrossRef]
- Annamalai, J.; Aliyamveetil Abubacker, Z.; Lakshmi, N.M.; Unnikrishnan, P. Microencapsulation of Fish Oil Using Fish Protein Hydrolysate, Maltodextrin, and Gum Arabic: Effect on Structural and Oxidative Stability. J. Aquat. Food Prod. Technol. 2020, 29, 293–306. [Google Scholar] [CrossRef]
- Özyurt, G.; Sakarya, Y.; Durmuş, M. Chemical and Physical Characterization of Spray Dried Fish Oil with Different Combination Ratios of Wall Component. J. Food Process Preserv. 2022, 46, e17223. [Google Scholar] [CrossRef]
- Charles, A.L.; Abdillah, A.A.; Saraswati, Y.R.; Sridhar, K.; Balderamos, C.; Masithah, E.D.; Alamsjah, M.A. Characterization of Freeze-Dried Microencapsulation Tuna Fish Oil with Arrowroot Starch and Maltodextrin. Food Hydrocoll. 2021, 112, 106281. [Google Scholar] [CrossRef]
- Özyurt, G.; Durmuş, M.; Uçar, Y.; Özoğul, Y. The Potential Use of Recovered Fish Protein as Wall Material for Microencapsulated Anchovy Oil. LWT 2020, 129, 109554. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Miller, M.R.; McNaughton, D.; Heraud, P.; Barrow, C.J. Rapid Discrimination and Determination of Polyunsaturated Fatty Acid Composition in Marine Oils by FTIR Spectroscopy and Multivariate Data Analysis. Food Bioproc. Tech. 2014, 7, 2410–2422. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Heraud, P.; Zhang, W.; Kralovec, J.A.; McNaughton, D.; Barrow, C.J. Quantitative Determination of Fatty Acid Compositions in Micro-Encapsulated Fish-Oil Supplements Using Fourier Transform Infrared (FTIR) Spectroscopy. Food Chem. 2012, 135, 603–609. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The Role of Water in Protein’s Behavior: The Two Dynamical Crossovers Studied by NMR and FTIR Techniques. Comput. Struct. Biotechnol. J. 2015, 13, 33–37. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Cd 18-90. In Official Methods and Recommended Practices of the American Oil Chemists’ Society (AOCS); AOCS press: Champaign, IL, USA, 2004. [Google Scholar]
| Arabic Gum | Sodium Alginate | |
|---|---|---|
| 0% maltodextrin | 66.2 ± 2.3 | 64.8 ± 2.2 |
| 5% maltodextrin | 72.2 ± 2.1 | 70.9 ± 2.1 |
| 10% maltodextrin | 76.5 ± 0.8 | 75.4 ±0.6 |
| 20% maltodextrin | 82.4 ± 1.5 | 77.3 ± 1.7 |
| 30% maltodextrin | 82.9 ± 1.8 | 79.1 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenoglou, I.; Katsouli, M.; Giannakourou, M.; Taoukis, P. Effect of High-Pressure Homogenization and Wall Material Composition on the Encapsulation of Polyunsaturated Fatty Acids from Fish Processing. Molecules 2025, 30, 1434. https://doi.org/10.3390/molecules30071434
Semenoglou I, Katsouli M, Giannakourou M, Taoukis P. Effect of High-Pressure Homogenization and Wall Material Composition on the Encapsulation of Polyunsaturated Fatty Acids from Fish Processing. Molecules. 2025; 30(7):1434. https://doi.org/10.3390/molecules30071434
Chicago/Turabian StyleSemenoglou, Ioanna, Maria Katsouli, Maria Giannakourou, and Petros Taoukis. 2025. "Effect of High-Pressure Homogenization and Wall Material Composition on the Encapsulation of Polyunsaturated Fatty Acids from Fish Processing" Molecules 30, no. 7: 1434. https://doi.org/10.3390/molecules30071434
APA StyleSemenoglou, I., Katsouli, M., Giannakourou, M., & Taoukis, P. (2025). Effect of High-Pressure Homogenization and Wall Material Composition on the Encapsulation of Polyunsaturated Fatty Acids from Fish Processing. Molecules, 30(7), 1434. https://doi.org/10.3390/molecules30071434





