Determination and Disposition of the Aromatase Inhibitor Exemestane in CYP3A-Deficient Mice
Abstract
1. Introduction
2. Results and Discussion
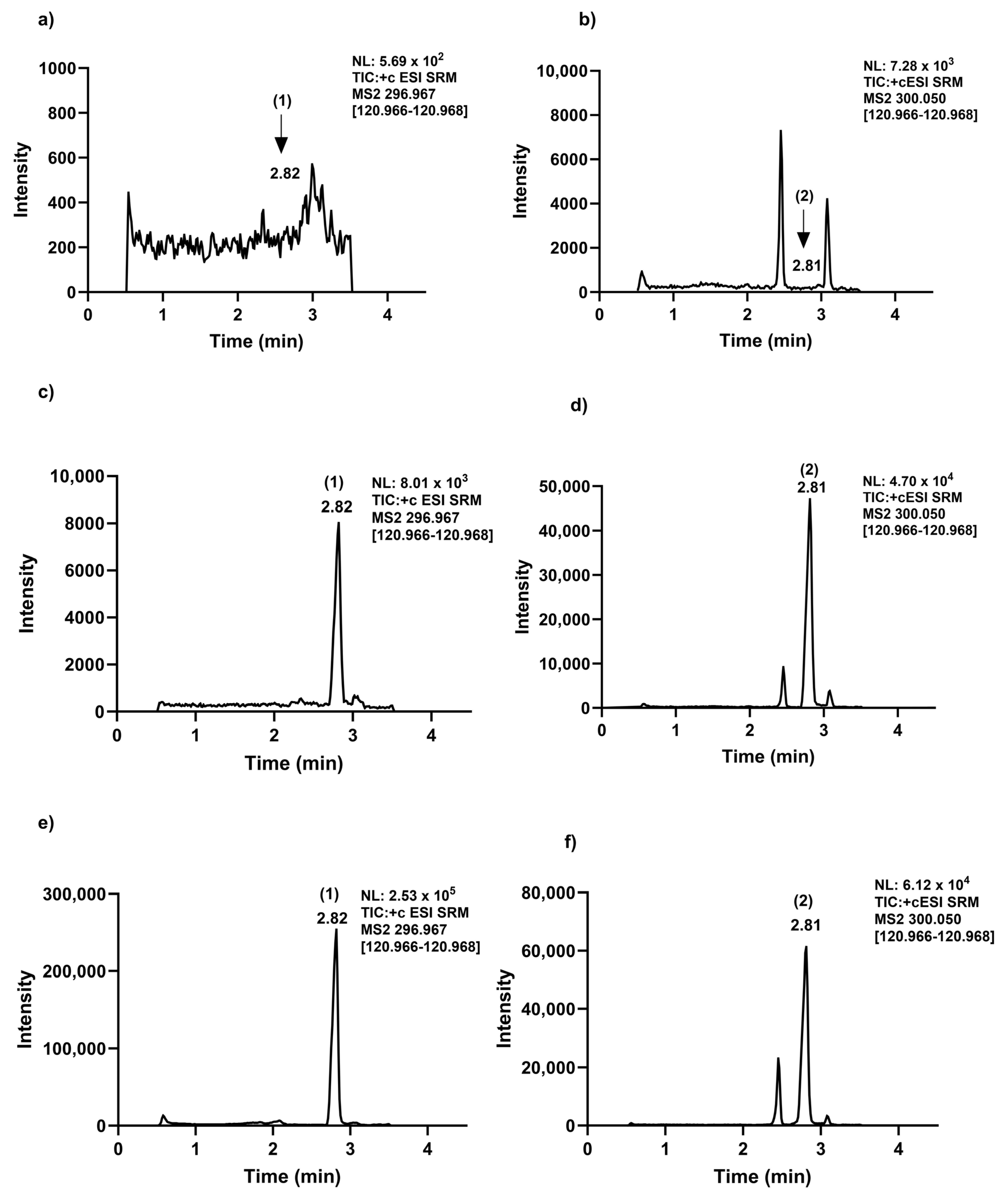
2.1. Chromatographic and Mass Spectrometric Conditions
2.2. Method Validation
2.2.1. Exemestane Selectivity and Linearity
2.2.2. Exemestane Precision and Accuracy
2.2.3. Matrix Effect, Extraction Recovery, and Carryover
2.2.4. Stability
Short-Term Stability
Freeze–Thaw Stability and Long-Term Stability
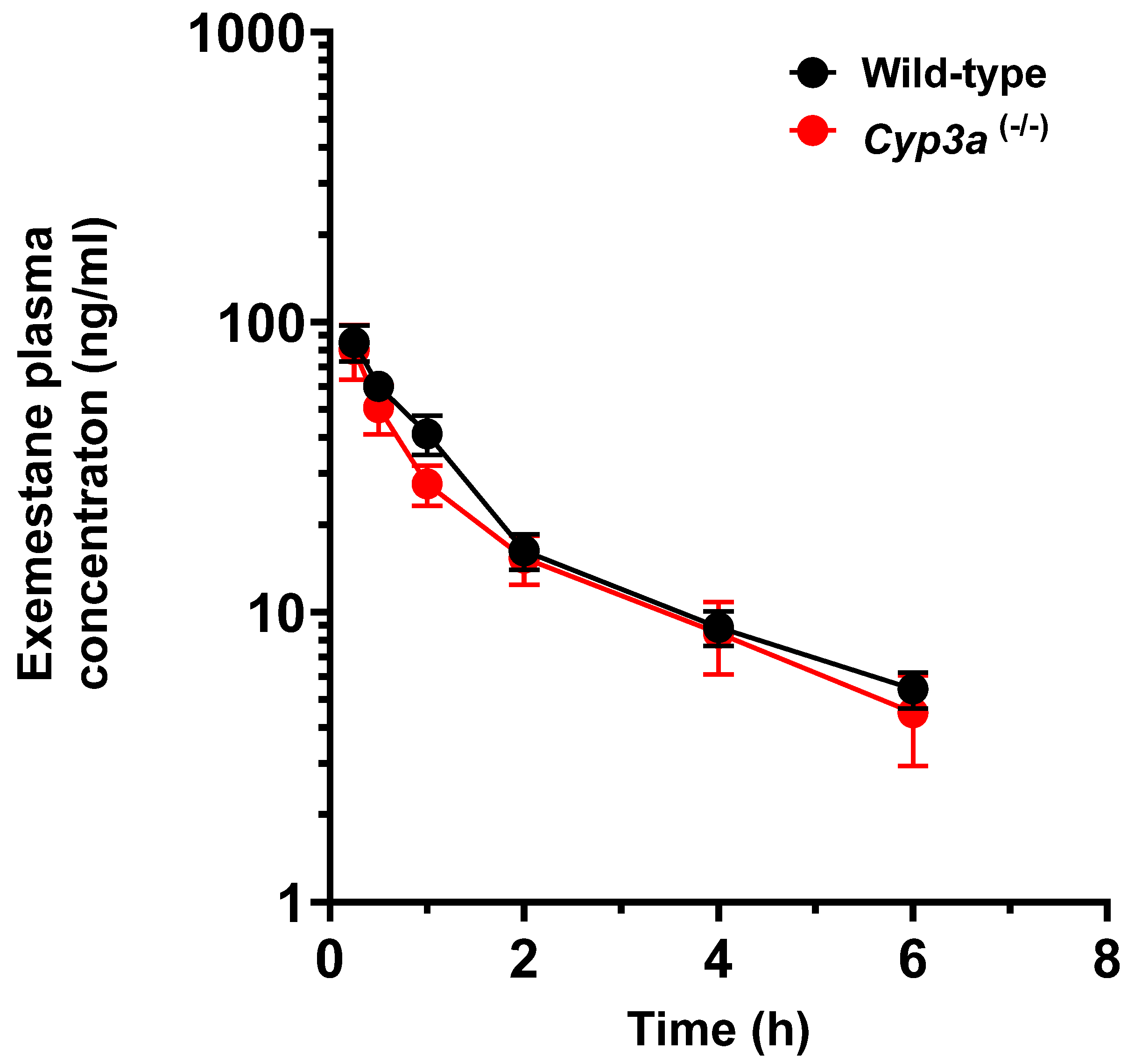
2.3. Pharmacokinetic Studies
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Instrumentation and Mass-Spectrometric Conditions
3.3. Calibration Standards and Quality Control Samples
3.4. Sample Extraction Procedure
3.5. Analytical Method Validation
3.5.1. Selectivity and Linearity
3.5.2. Precision and Accuracy Matrix Effect, Extraction Recovery, and Carryover
3.5.3. Matrix Effect, Extraction Recovery, and Carryover
3.5.4. Stability
Short-Term Stability
Freeze–Thaw Stability and Long-Term Stability
3.6. Application in In Vivo Pharmacokinetics Studies
3.6.1. Animal Studies
3.6.2. Pharmacokinetic Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LLOQ | lower limit of quantification |
| LQC | low-quality control |
| MQC | medium-quality control |
| HQC | high-quality control |
| AULQ | above upper limit of quantification |
| Cmax | peak plasma concentration |
| AUC | area under the plasma concentration–time curve between time zero and 6 h |
| N | number of mice per group |
References
- Deeks, E.D.; Scott, L.J. Exemestane: A review of its use in postmenopausal women with breast cancer. Drugs 2009, 69, 889–918. [Google Scholar] [CrossRef]
- Kharb, R.; Haider, K.; Neha, K.; Yar, M.S. Aromatase inhibitors: Role in postmenopausal breast cancer. Arch. Pharm. 2020, 353, 2000081. [Google Scholar] [CrossRef]
- Lønning, P. The potency and clinical efficacy of aromatase inhibitors across the breast cancer continuum. Ann. Oncol. 2011, 22, 503–514. [Google Scholar] [CrossRef]
- Rivera, E.; Valero, V.; Francis, D.; Asnis, A.G.; Schaaf, L.J.; Duncan, B.; Hortobagyi, G.N. Pilot study evaluating the pharmacokinetics, pharmacodynamics, and safety of the combination of exemestane and tamoxifen. Clin. Cancer Res. 2004, 10, 1943–1948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buzdar, A.U.; Robertson, J.F.; Eiermann, W.; Nabholtz, J.M. An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane. Cancer 2002, 95, 2006–2016. [Google Scholar] [CrossRef]
- Hyder, T.; Marino, C.C.; Ahmad, S.; Nasrazadani, A.; Brufsky, A.M. Aromatase inhibitor-associated musculoskeletal syndrome: Understanding mechanisms and management. Front. Endocrinol. 2021, 12, 713700. [Google Scholar] [CrossRef]
- Jelovac, D.; Macedo, L.; Handratta, V.; Long, B.J.; Goloubeva, O.G.; Ingle, J.N.; Brodie, A.M. Effects of exemestane and tamoxifen in a postmenopausal breast cancer model. Clin. Cancer Res. 2004, 10, 7375–7381. [Google Scholar] [CrossRef] [PubMed]
- Dudenkov, T.M.; Ingle, J.N.; Buzdar, A.U.; Robson, M.E.; Kubo, M.; Ibrahim-Zada, I.; Batzler, A.; Jenkins, G.D.; Pietrzak, T.L.; Carlson, E.E. SLCO1B1 polymorphisms and plasma estrone conjugates in postmenopausal women with ER+ breast cancer: Genome-wide association studies of the estrone pathway. Breast Cancer Res. Treat. 2017, 164, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Luthra, R.; Kirma, N.; Jones, J.; Tekmal, R.R. Use of letrozole as a chemopreventive agent in aromatase overexpressing transgenic mice. J. Steroid Biochem. Mol. Biol. 2003, 86, 461–467. [Google Scholar] [CrossRef]
- Geisler, J. Differences between the non-steroidal aromatase inhibitors anastrozole and letrozole–of clinical importance? Br. J. Cancer 2011, 104, 1059–1066. [Google Scholar] [CrossRef]
- Zhu, Y.; Koleck, T.A.; Bender, C.M.; Conley, Y.P. Genetic underpinnings of musculoskeletal pain during treatment with aromatase inhibitors for breast cancer: A biological pathway analysis. Biol. Res. Nurs. 2020, 22, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Grigorian, N.; Baumrucker, S.J. Aromatase inhibitor–associated musculoskeletal pain: An overview of pathophysiology and treatment modalities. SAGE Open Med. 2022, 10, 20503121221078722. [Google Scholar]
- Fields, J.; Richardson, A.; Hopkinson, J.; Fenlon, D. Nordic walking as an exercise intervention to reduce pain in women with aromatase inhibitor–associated arthralgia: A feasibility study. J. Pain Symptom Manag. 2016, 52, 548–559. [Google Scholar]
- Mauras, N.; Lima, J.; Patel, D.; Rini, A.; di Salle, E.; Kwok, A.; Lippe, B. Pharmacokinetics and dose finding of a potent aromatase inhibitor, aromasin (exemestane), in young males. J. Clin. Endocrinol. Metab. 2003, 88, 5951–5956. [Google Scholar] [CrossRef]
- Corona, G.; Elia, C.; Casetta, B.; Diana, C.; Rosalen, S.; Bari, M.; Toffoli, G. A liquid chromatography-tandem mass spectrometry method for the simultaneous determination of exemestane and its metabolite 17-dihydroexemestane in human plasma. J. Mass Spectrom. 2009, 44, 920–928. [Google Scholar]
- Gregory, B.J.; Chen, S.M.; Murphy, M.A.; Atchley, D.H.; Kamdem, L.K. Impact of the OATP 1B1 c. 521T> C single nucleotide polymorphism on the pharmacokinetics of exemestane in healthy post-menopausal female volunteers. J. Clin. Pharm. Ther. 2017, 42, 547–553. [Google Scholar]
- Jannuzzo, M.G.; Poggesi, I.; Spinelli, R.; Rocchetti, M.; Cicioni, P.; Buchan, P. The effects of degree of hepatic or renal impairment on the pharmacokinetics of exemestane in postmenopausal women. Cancer Chemother. Pharmacol. 2004, 53, 475–481. [Google Scholar] [PubMed]
- Kamdem, L.K.; Flockhart, D.A.; Desta, Z. In vitro cytochrome P450-mediated metabolism of exemestane. Drug Metab. Dispos. 2011, 39, 98–105. [Google Scholar] [CrossRef]
- Robinson, A. A review of the use of exemestane in early breast cancer. Ther. Clin. Risk Manag. 2009, 2009, 91–98. [Google Scholar]
- Landry, K.; David, F.; Zeruesenay, D. 17-Hydroexemestane: A potent inhibitor of CYP19 (aromatase) and substrate of CYP3A. J. Drug Metab. Toxicol. 2014, 5, 2. [Google Scholar]
- Sun, D.; Chen, G.; Dellinger, R.W.; Sharma, A.K.; Lazarus, P. Characterization of 17-dihydroexemestane glucuronidation: Potential role of the UGT2B17 deletion in exemestane pharmacogenetics. Pharmacogenetics Genom. 2010, 20, 575. [Google Scholar]
- Chen, S.M.; Atchley, D.H.; Murphy, M.A.; Gurley, B.J.; Kamdem, L.K. Impact of UGT2B17 gene deletion on the pharmacokinetics of 17-hydroexemestane in healthy volunteers. J. Clin. Pharmacol. 2016, 56, 875–884. [Google Scholar] [PubMed]
- Hertz, D.L.; Kidwell, K.M.; Seewald, N.J.; Gersch, C.L.; Desta, Z.; Flockhart, D.A.; Storniolo, A.-M.; Stearns, V.; Skaar, T.C.; Hayes, D.F. Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenomics J. 2017, 17, 521–527. [Google Scholar] [PubMed]
- Heery, M.; Farley, S.; Sparkman, R.; Healy, J.; Eighmy, W.; Zahrah, G.; Zelkowitz, R. Precautions for patients taking aromatase inhibitors. J. Adv. Pract. Oncol. 2020, 11, 184. [Google Scholar] [PubMed]
- Stolarczyk, E.U.; Rosa, A.; Kubiszewski, M.; Zagrodzka, J.; Cybulski, M.; Kaczmarek, Ł. Use of the hyphenated LC-MS/MS technique and NMR/IR spectroscopy for the identification of exemestane stress degradation products during the drug development. Eur. J. Pharm. Sci. 2017, 109, 389–401. [Google Scholar]
- Wang, L.-Z.; Goh, S.-H.; Wong, A.L.-A.; Thuya, W.-L.; Lau, J.-Y.A.; Wan, S.-C.; Lee, S.-C.; Ho, P.C.; Goh, B.-C. Validation of a rapid and sensitive LC-MS/MS method for determination of exemestane and its metabolites, 17β-hydroxyexemestane and 17β-hydroxyexemestane-17-O-β-D-glucuronide: Application to human pharmacokinetics study. PLoS ONE 2015, 10, e0118553. [Google Scholar]
- Ishii, T.; Nojiri, N.; Mano, Y. A simple UPLC-MS/MS assay with a core-shell column for the determination of exemestane in human plasma for clinical application. Eur. J. Mass Spectrom. 2022, 28, 94–103. [Google Scholar]
- Cenacchi, V.; Baratte, S.; Cicioni, P.; Frigerio, E.; Long, J.; James, C. LC–MS–MS determination of exemestane in human plasma with heated nebulizer interface following solid-phase extraction in the 96 well plate format. J. Pharm. Biomed. Anal. 2000, 22, 451–460. [Google Scholar]
- Luo, S.; Chen, G.; Truica, C.I.; Baird, C.C.; Xia, Z.; Lazarus, P. Identification and quantification of novel major metabolites of the steroidal aromatase inhibitor, exemestane. Drug Metab. Dispos. 2018, 46, 1867–1878. [Google Scholar]
- Ksycińska, H.; Buś-Kwaśnik, K.; Szlagowska, A.; Rudzki, P.J. Development and validation of a sensitive liquid chromatography/tandem mass spectrometry method for the determination of exemestane in human plasma. J. Chromatogr. B 2011, 879, 1905–1910. [Google Scholar]
- Singh, A.; Chaurasiya, A.; Warsi, M.H.; Chaurasiya, M.; Jain, G.K.; Asati, D.; Khar, R.K.; Mukherjee, R. Oral pharmacokinetic study of exemestane SMEDDS and suspension in rat plasma by liquid chromatography-mass spectrometric analysis. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2162–2174. [Google Scholar] [CrossRef]
- Li, Y.; Kazuki, Y.; Drabison, T.; Kobayashi, K.; Fujita, K.-i.; Xu, Y.; Jin, Y.; Ahmed, E.; Li, J.; Eisenmann, E.D. Vincristine Disposition and Neurotoxicity Are Unchanged in Humanized CYP3A5 Mice. Drug Metab. Dispos. 2024, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 23 June 2023).
- van Herwaarden, A.E.; Wagenaar, E.; van der Kruijssen, C.M.; van Waterschoot, R.A.; Smit, J.W.; Song, J.-Y.; van der Valk, M.A.; van Tellingen, O.; van der Hoorn, J.W.; Rosing, H. Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J. Clin. Investig. 2007, 117, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, E.D.; Fu, Q.; Muhowski, E.M.; Jin, Y.; Uddin, M.E.; Garrison, D.A.; Weber, R.H.; Woyach, J.A.; Byrd, J.C.; Sparreboom, A.; et al. Intentional modulation of ibrutinib pharmacokinetics through CYP3A inhibition. Cancer Res. Commun. 2021, 1, 79–89. [Google Scholar] [CrossRef]
- Leblanc, A.F.; Huang, K.M.; Uddin, M.E.; Anderson, J.T.; Chen, M.; Hu, S. Murine pharmacokinetic studies. Bio-Protocol 2018, 8, e3056. [Google Scholar] [CrossRef]

| List of LC Retention Time and MRM Transitions of Exmastane and [13C, D3]-Exemestane | |||||
|---|---|---|---|---|---|
| Analyte | Retention Time (min) | Mass Transition (m/z) | Collision Energy (V) | Min Dwell Time (ms) | |
| Exemestane | 2.82 | 297.0 → 121.0 | 20.6 | 198 | |
| 297.0 → 149.0 | 15.8 | 198 | |||
| [13C, D3]-exemestane | 2.81 | 300.1 → 121.0 | 19.5 | 198 | |
| 300.1 → 258.9 | 5.43 | 198 | |||
| LC parameters | |||||
| Mobile phase A | 0.1% acetic acid water | ||||
| Mobile Phase B | 0.1% Formic acid acetonitrile | ||||
| Gradient elution program | Time (min) | A% | B% | Elution change | |
| 0 | 70 | 30 | 5 | ||
| 0.5 | 70 | 30 | 5 | ||
| 3.0 | 5 | 95 | 5 | ||
| 4.0 | 5 | 95 | 5 | ||
| 4.1 | 70 | 30 | 5 | ||
| 4.5 | 70 | 30 | 5 | ||
| Column temperature | 40 °C | ||||
| Autosampler temperature | 4 °C | ||||
| Injection volume Run time | 10 μL 4.5 min | ||||
| Flow rate | 0.3 mL/min | ||||
| MS parameters | |||||
| Sheath gas | 25 Arb | ||||
| Auxiliary gas | 5 Arb | ||||
| Sweep gas | 1 Arb | ||||
| Ion transfer tube temperature | 340 °C | ||||
| vaporizer temperature | 360 °C | ||||
| The collision gas argon | 1.5 mTorr | ||||
| Positive ion spray voltage | 3600 V | ||||
| Q1 resolution number | 0.7 FWHM | ||||
| Q3 resolution number | 1.2 FWHM | ||||
| N | Conc. (ng/mL) | Intra-Assay (CV%) | Inter-Assay (CV%) | Accuracy (Bias%) | |
|---|---|---|---|---|---|
| LLOQ | 20 | 0.400 | 7.09 | 4.64 | 5.10 |
| LQC | 20 | 1.20 | 2.19 | 2.37 | 3.00 |
| MQC | 20 | 40.0 | 3.40 | 2.97 | −3.10 |
| HQC | 20 | 65.0 | 3.63 | 3.06 | −7.80 |
| AULQ (after 10X dilution) a | 20 | 65.0 | 3.67 | 4.93 | −2.50 |
| Nominal Con. (ng/mL) | Matrix Effect | Hemolysis Effect | Extraction Recovery | ||||
|---|---|---|---|---|---|---|---|
| N | Mean Matrix Effect (%) | CV (%) | Mean % Nominal | CV (%) | Mean Recovery (%) | CV (%) | |
| LQC (1.2) | 3 | 96.9 | 3.74 | 92.9 | 10.3 | 99.9 | 8.80 |
| MQC (40) | 3 | 104 | 1.93 | 96.4 | 5.08 | 88.4 | 3.79 |
| HQC (65) | 3 | 108 | 1.84 | 102 | 5.17 | 90.0 | 4.18 |
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Auto Sampler Stability a | Re-Injection Stability b | ||||||||
| Nominal Con. (ng/mL) | N | Mean Deviation (%) of t = 0 | CV (%) | Mean Deviation (%) of t = 0 | CV (%) | ||||
| LQC (1.2) | 5 | −5.4 | 4.61 | −2.18 | 2.87 | ||||
| MQC (40) | 5 | −6.99 | 3.38 | 2.11 | 4.07 | ||||
| HQC (65) | 5 | −0.19 | 3.9 | 0.35 | 2.16 | ||||
| (b) | |||||||||
| Bench-Top Stability a | |||||||||
| At 25 °C | At 37 °C | ||||||||
| Nominal Con. (ng/mL) | N | 3 h | 6 h | 3 h | 6h | ||||
| Mean (%) of t = 0 | CV (%) | Mean (%) of t = 0 | CV (%) | Mean (%) of t = 0 | CV (%) | Mean (%) of t = 0 | CV (%) | ||
| LQC (1.2) | 3 | 102 | 5.43 | 102 | 7.31 | 74.1 | 5.51 | ND | - |
| MQC (40) | 3 | 98.8 | 2.33 | 92.5 | 2.92 | 65.5 | 21.2 | 2.8 | 38.7 |
| HQC (65) | 3 | 96.8 | 2.57 | 92.5 | 1.23 | 62.5 | 23.9 | 3.76 | 49.9 |
| Nominal Con. (ng/mL) | Freeze–Thaw Stability (Cycles) a | Long-Term Stability b | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st Cycle | 2nd Cycle | 3rd Cycle | ||||||
| Mean % of t = 0 | CV (%) | Mean % of t = 0 | CV (%) | Mean % of t = 0 | CV (%) | Mean % of t = 0 | CV (%) | |
| LQC (1.2) | 98.1 | 4.19 | 95.5 | 3.13 | 99.8 | 4.15 | 94.8 | 2.11 |
| HQC (65) | 101 | 1.22 | 99.8 | 2.11 | 98.5 | 2.62 | 95.5 | 5.32 |
| Genotype | Sex | Dose (mg/kg) | N | Tmax (h) | Cmax (ng/mL) | T1/2(h) | AUC 0–6 h (ng × h/mL) |
|---|---|---|---|---|---|---|---|
| Wild-type | Female | 20.0 | 10 | 0.250 | 85.0 (±12.0) | 2.01 | 126 (±15.0) |
| Cyp3a (-/-) | Female | 20.0 | 10 | 0.250 | 80.0 (±17.0) | 2.90 | 113 (±22.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, H.; Ahmed, E.; Hu, P.; Sparreboom, A.; Hu, S. Determination and Disposition of the Aromatase Inhibitor Exemestane in CYP3A-Deficient Mice. Molecules 2025, 30, 1440. https://doi.org/10.3390/molecules30071440
Taheri H, Ahmed E, Hu P, Sparreboom A, Hu S. Determination and Disposition of the Aromatase Inhibitor Exemestane in CYP3A-Deficient Mice. Molecules. 2025; 30(7):1440. https://doi.org/10.3390/molecules30071440
Chicago/Turabian StyleTaheri, Hanieh, Eman Ahmed, Peng Hu, Alex Sparreboom, and Shuiying Hu. 2025. "Determination and Disposition of the Aromatase Inhibitor Exemestane in CYP3A-Deficient Mice" Molecules 30, no. 7: 1440. https://doi.org/10.3390/molecules30071440
APA StyleTaheri, H., Ahmed, E., Hu, P., Sparreboom, A., & Hu, S. (2025). Determination and Disposition of the Aromatase Inhibitor Exemestane in CYP3A-Deficient Mice. Molecules, 30(7), 1440. https://doi.org/10.3390/molecules30071440






