Detection of Selected Heavy Metal Ions Using Organic Chromofluorescent Chemosensors
Abstract
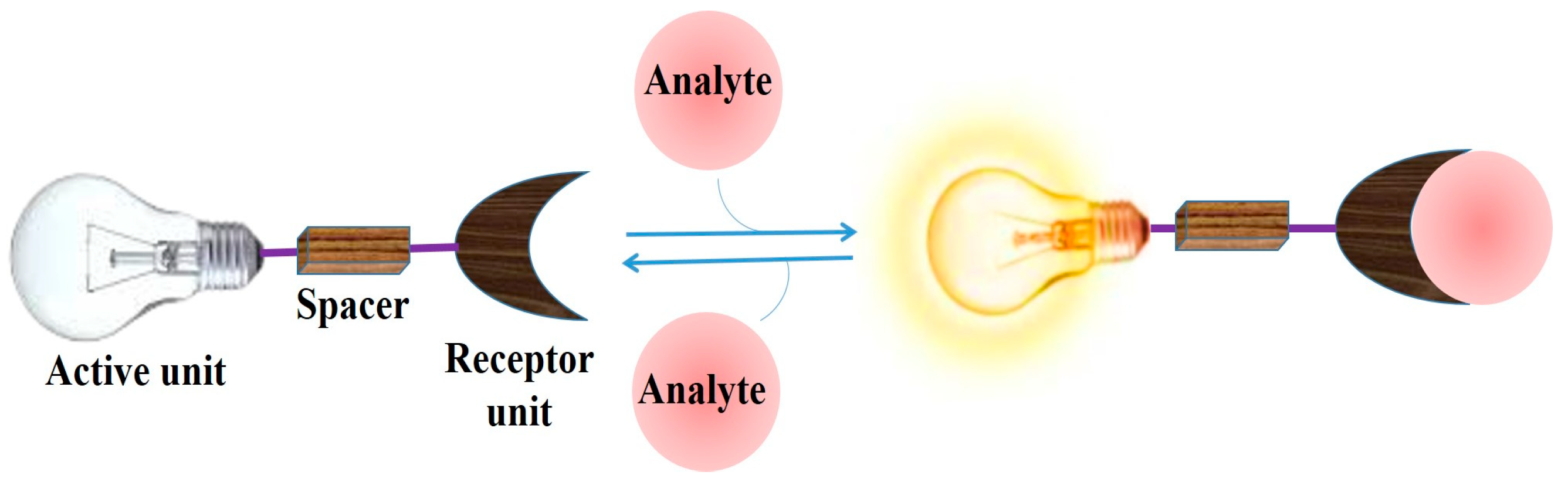
:1. Introduction
2. Detection of Heavy Metal Ions
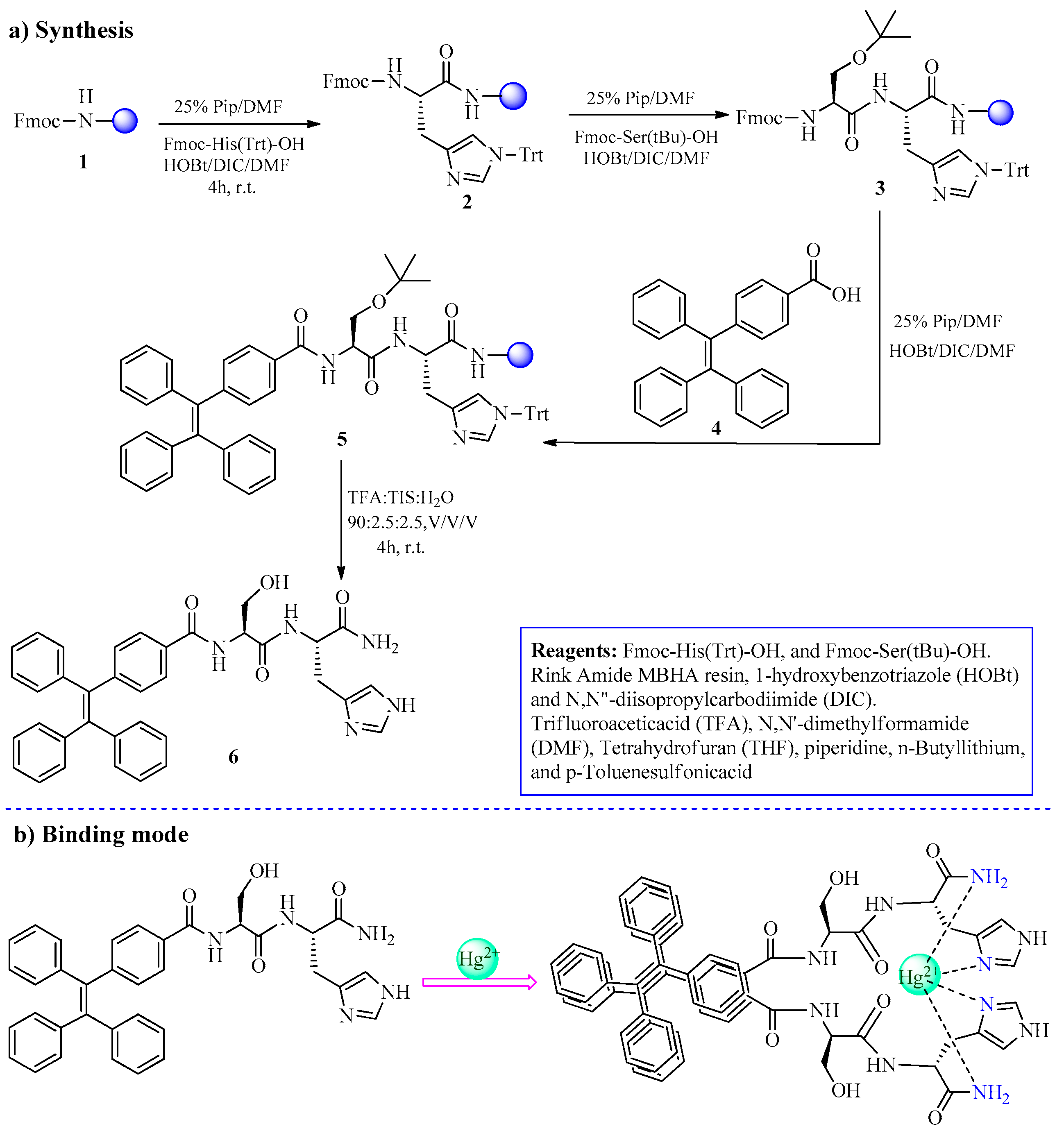
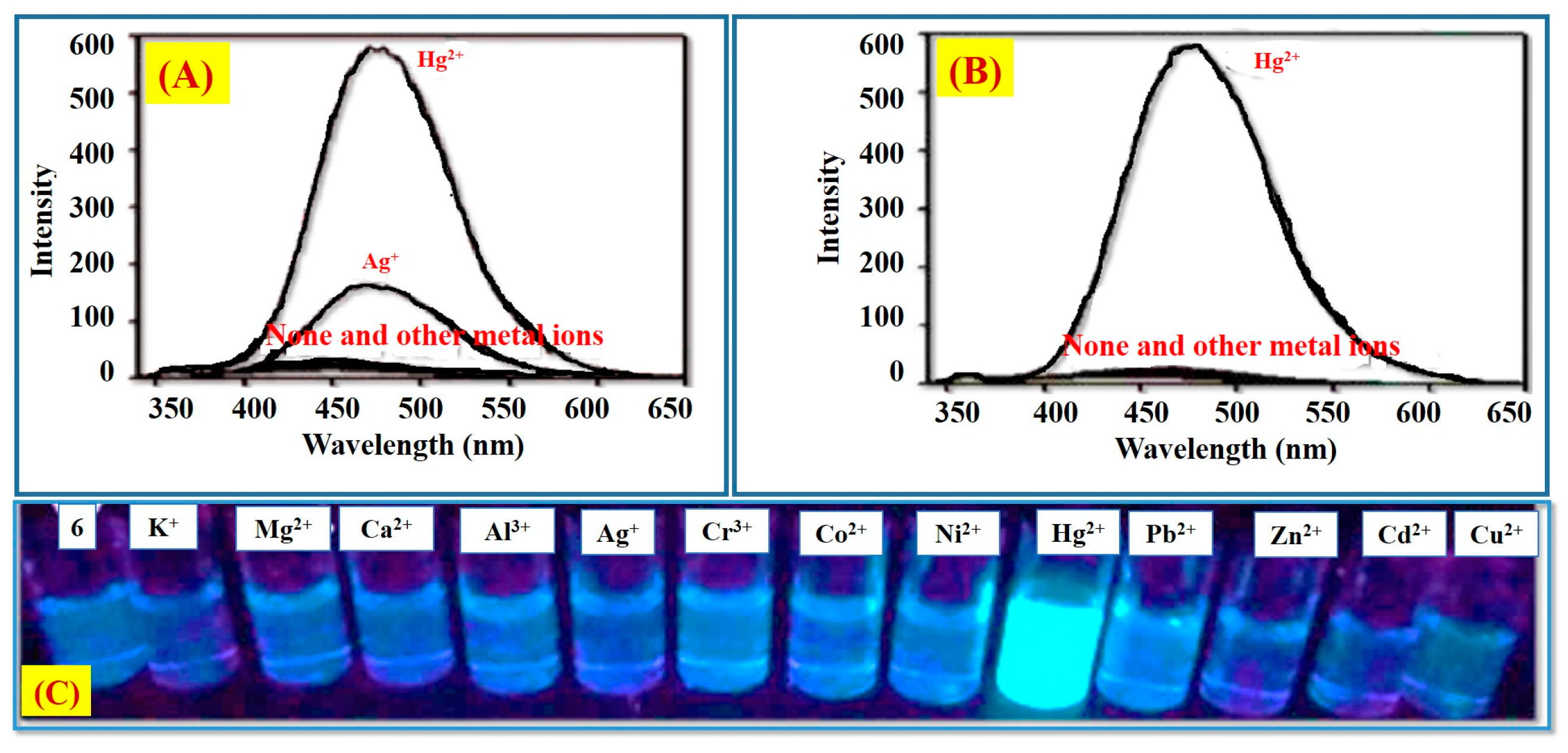
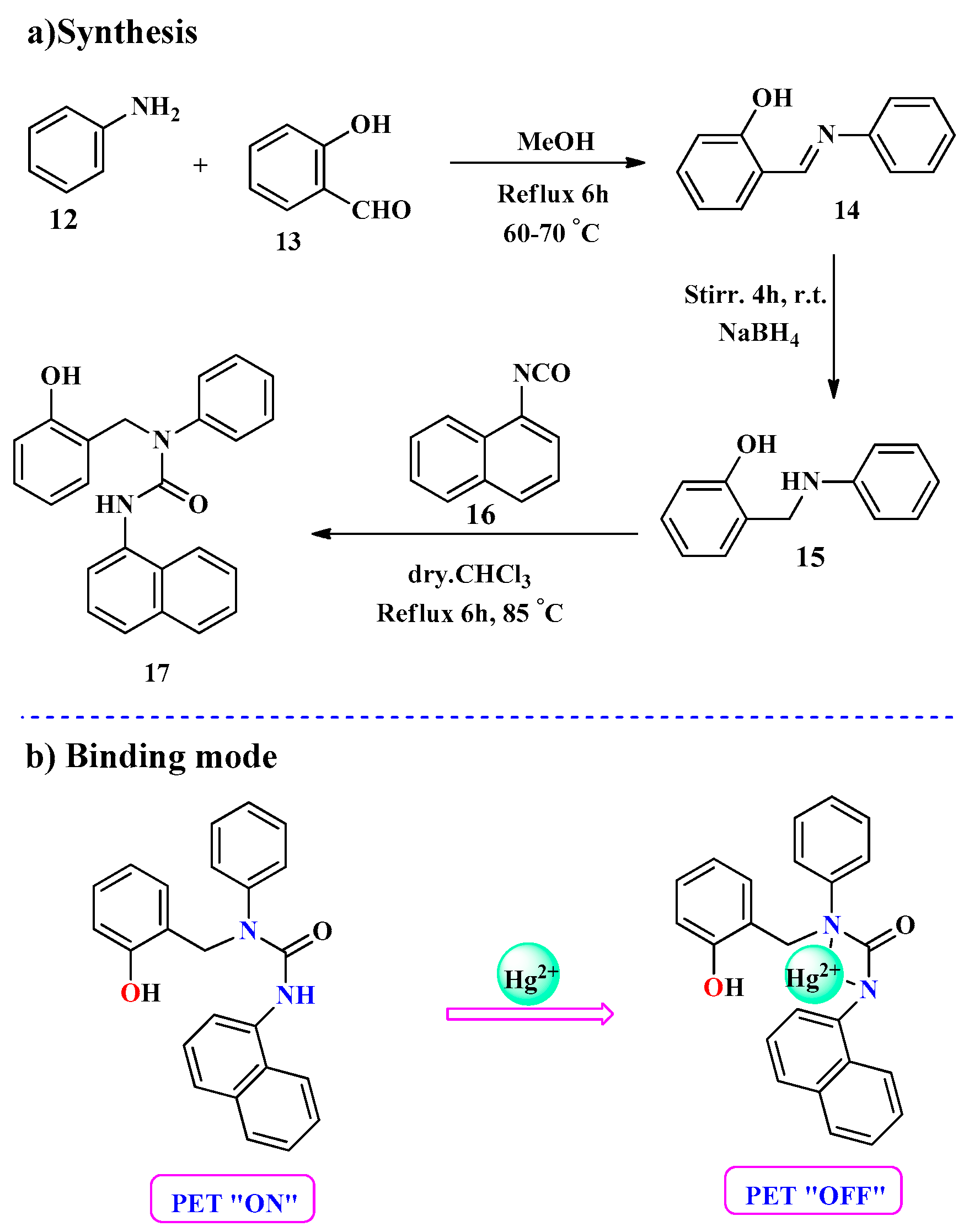
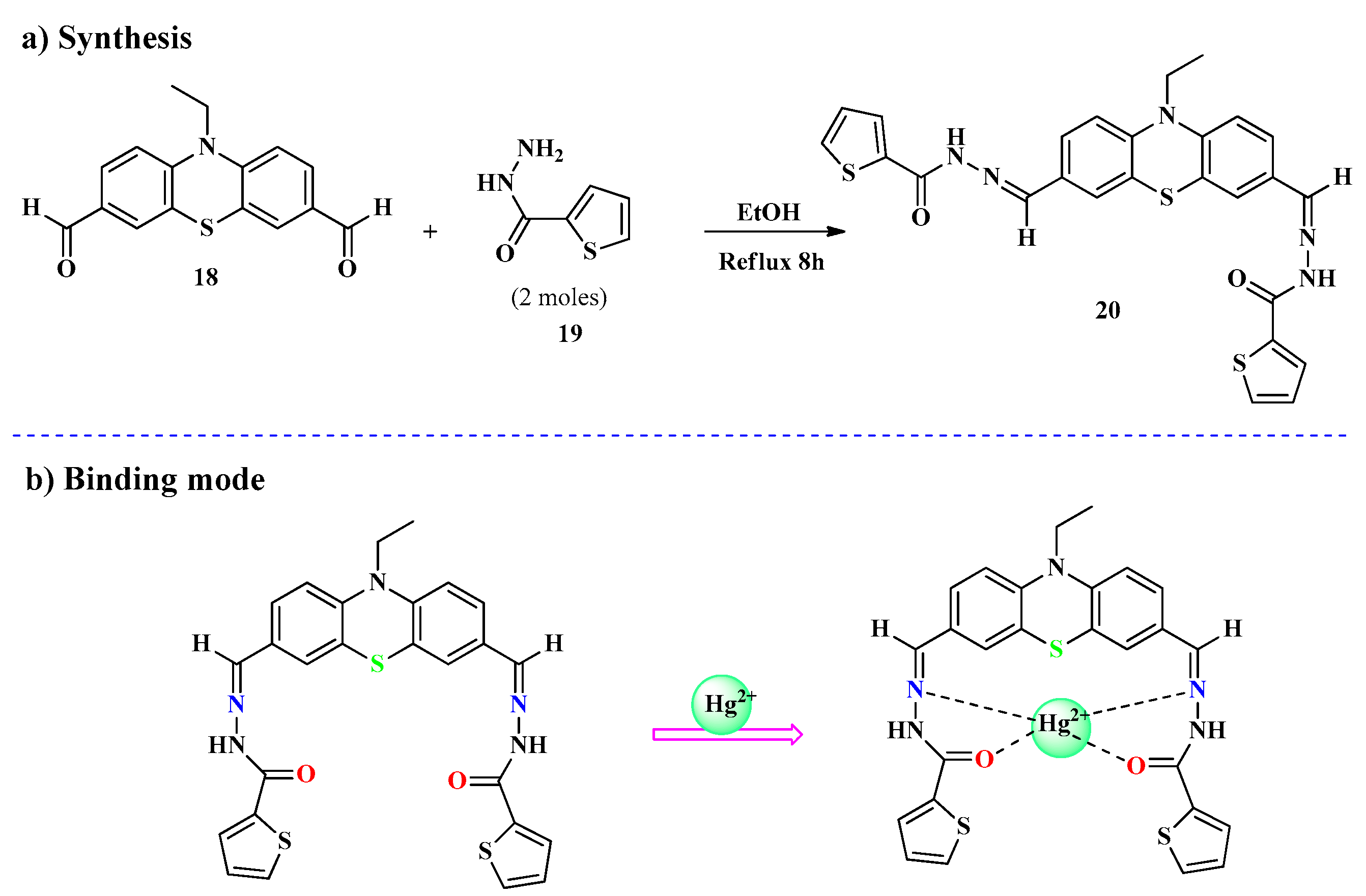
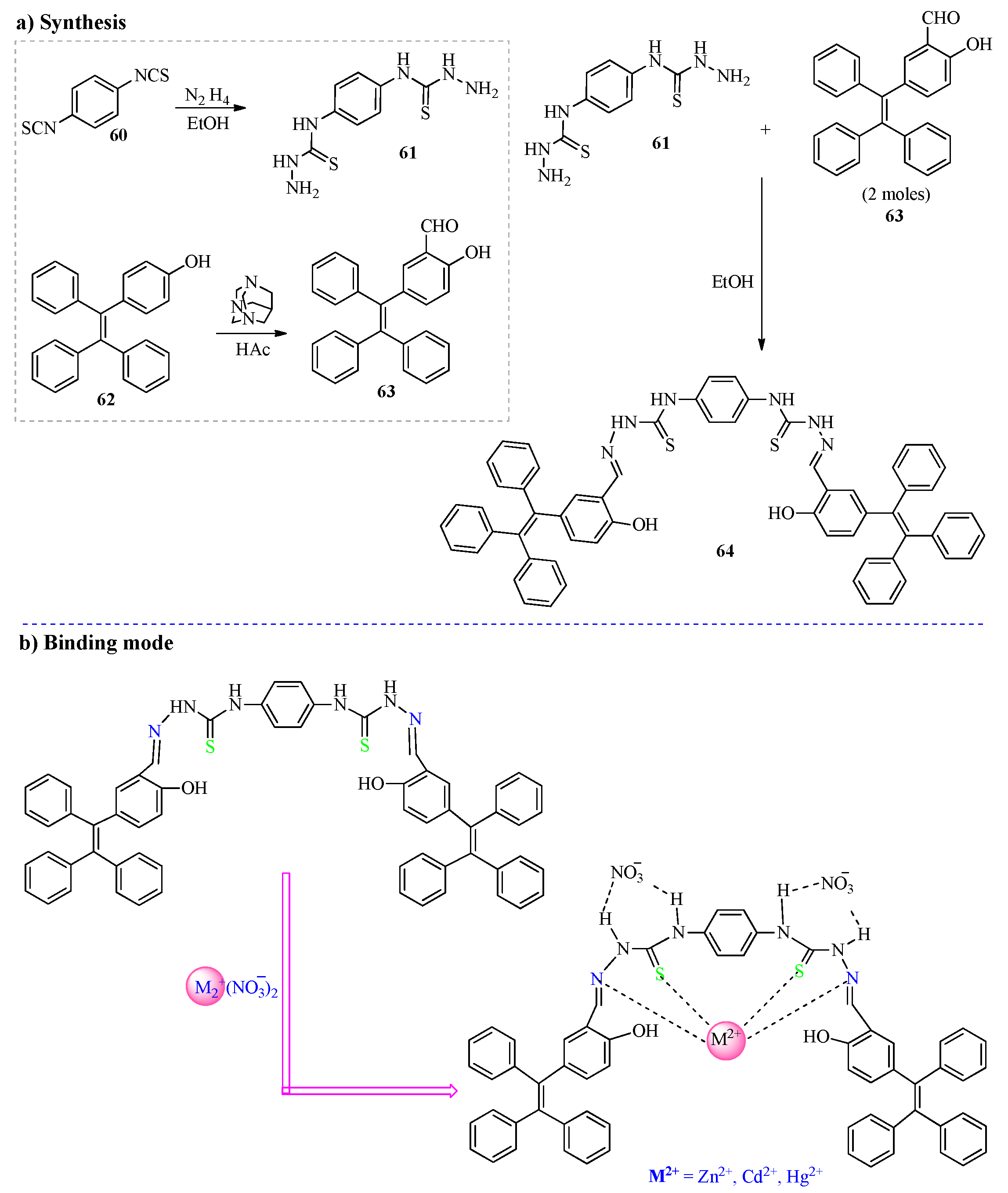
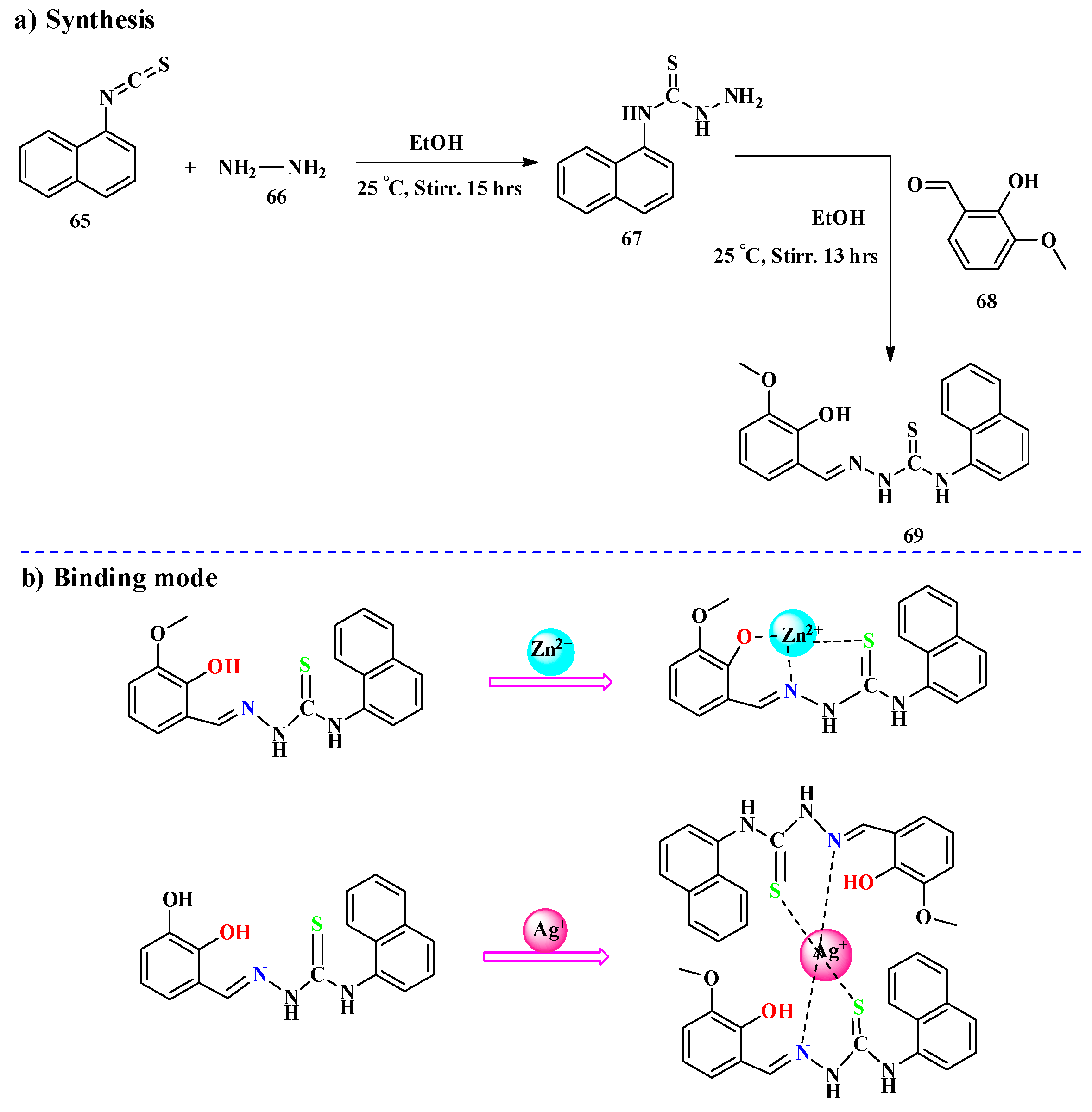
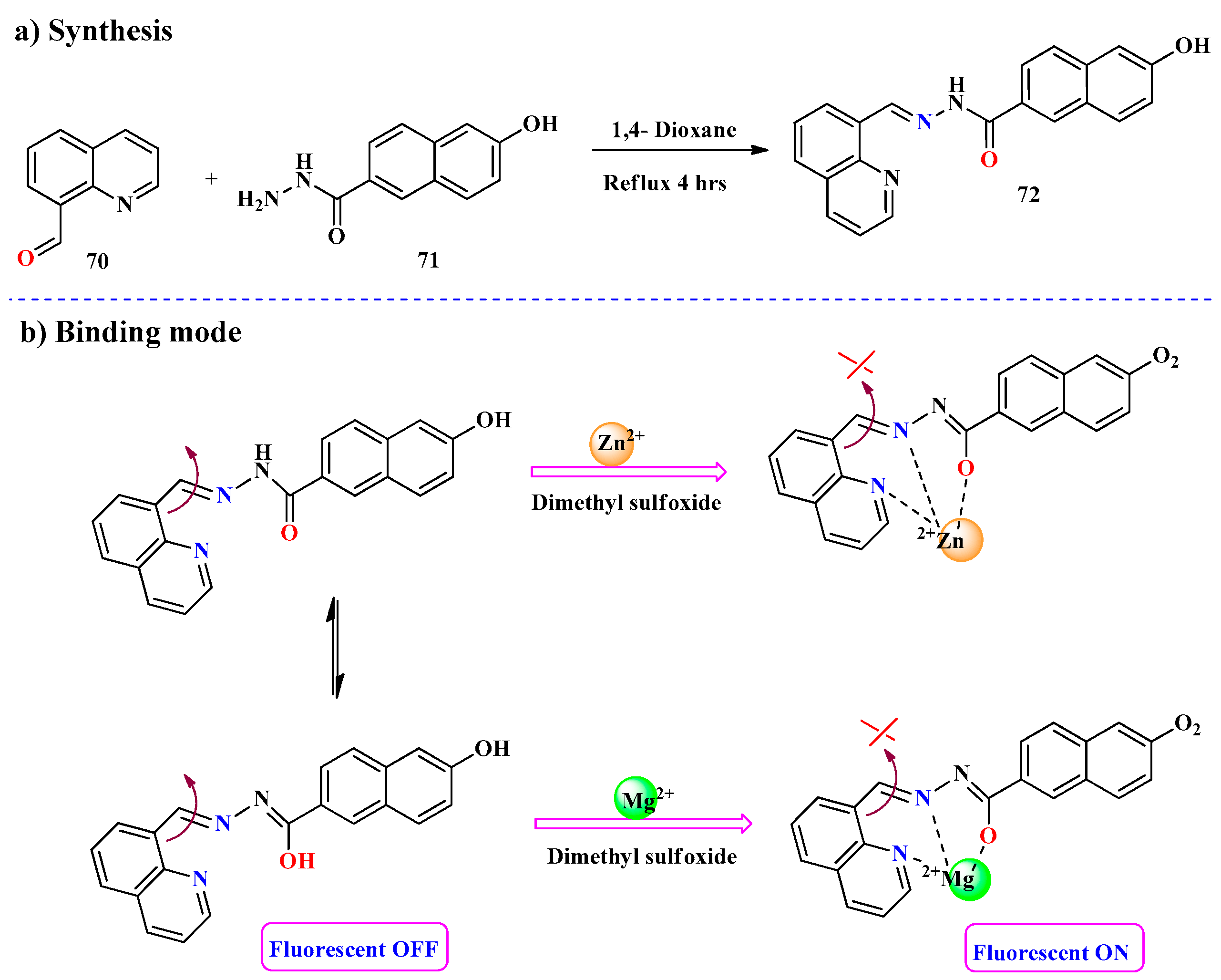
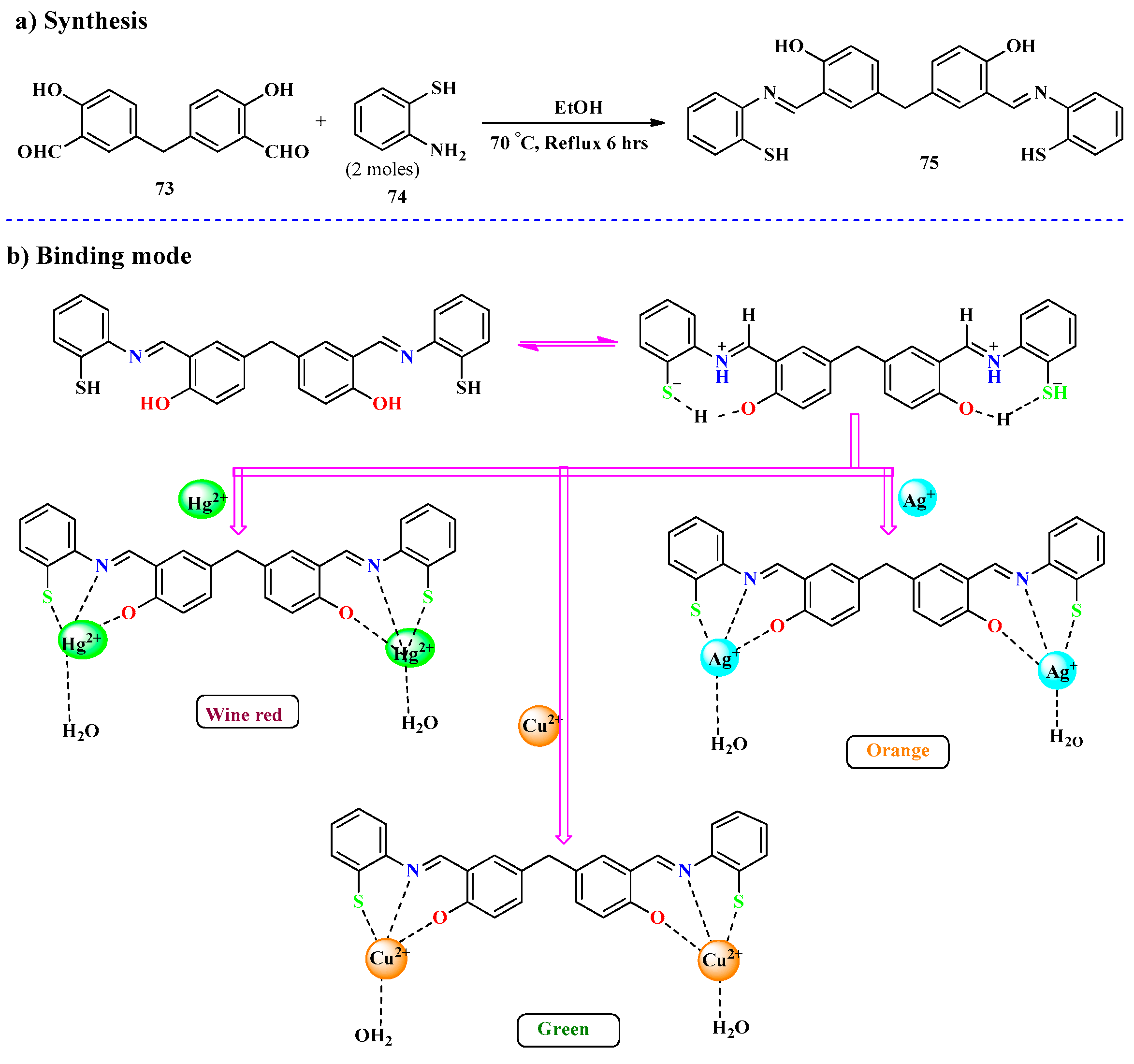
2.1. Hg2+ Ion Detection
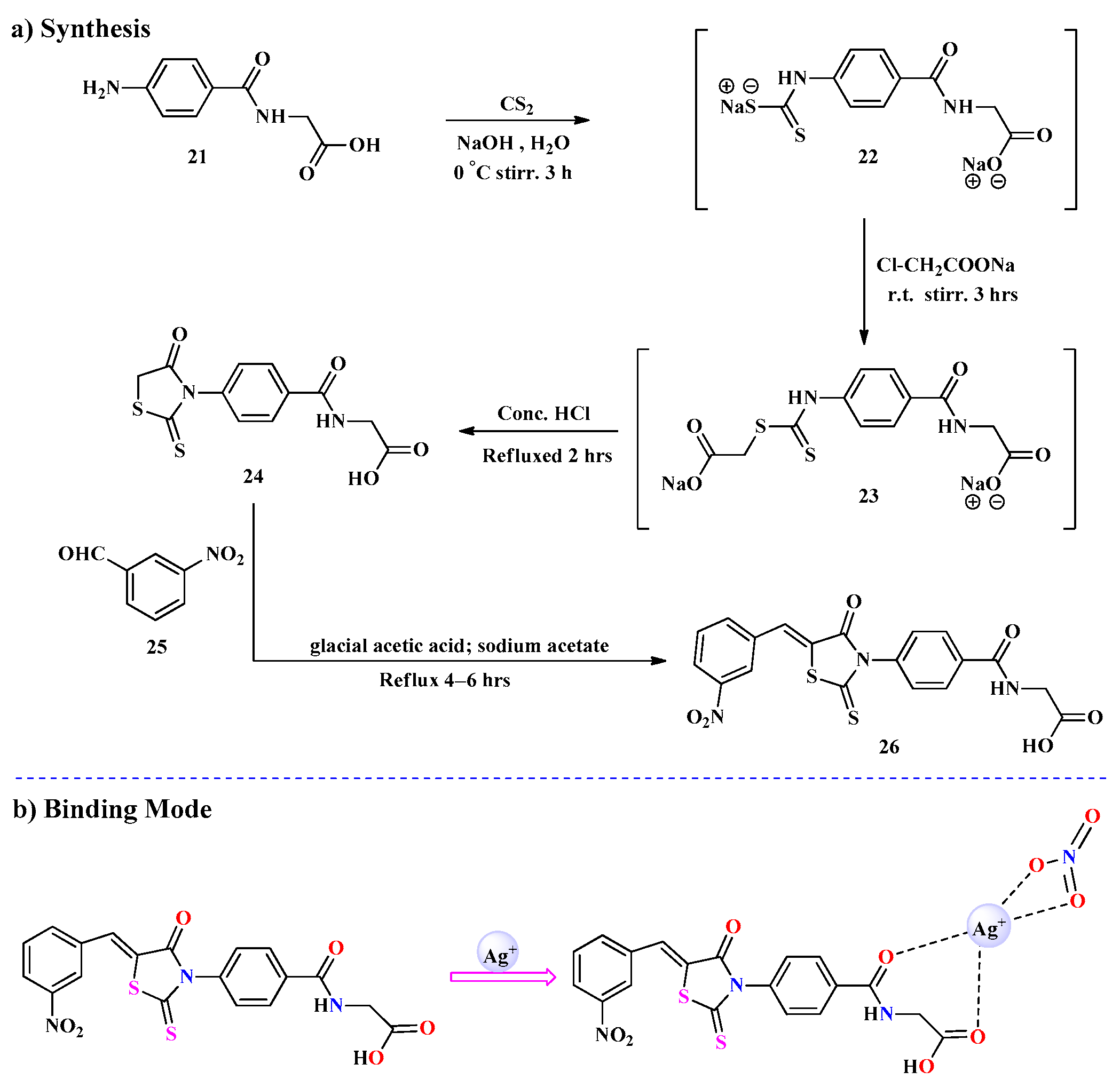
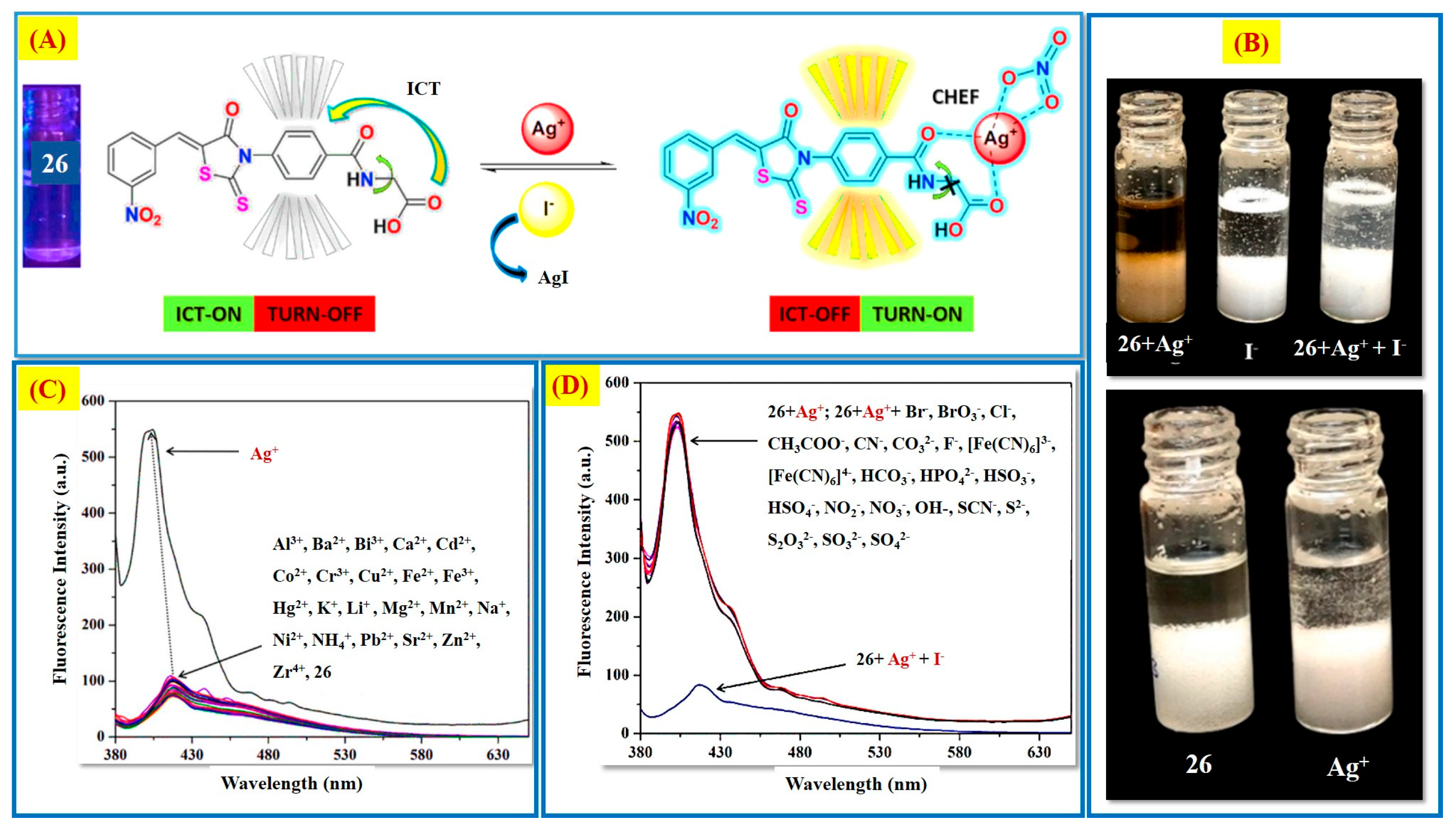
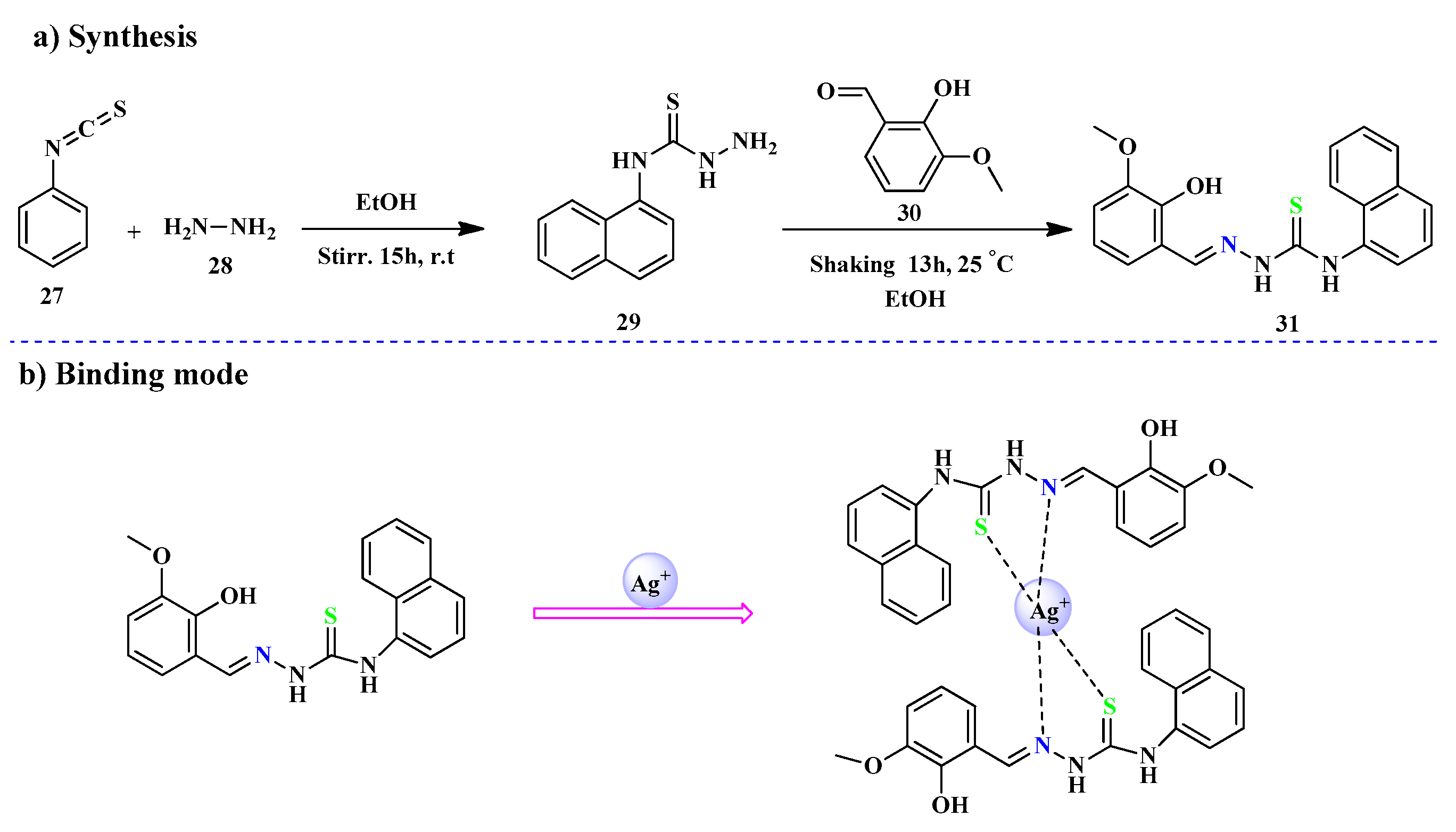
2.2. Ag+ Ions Detection
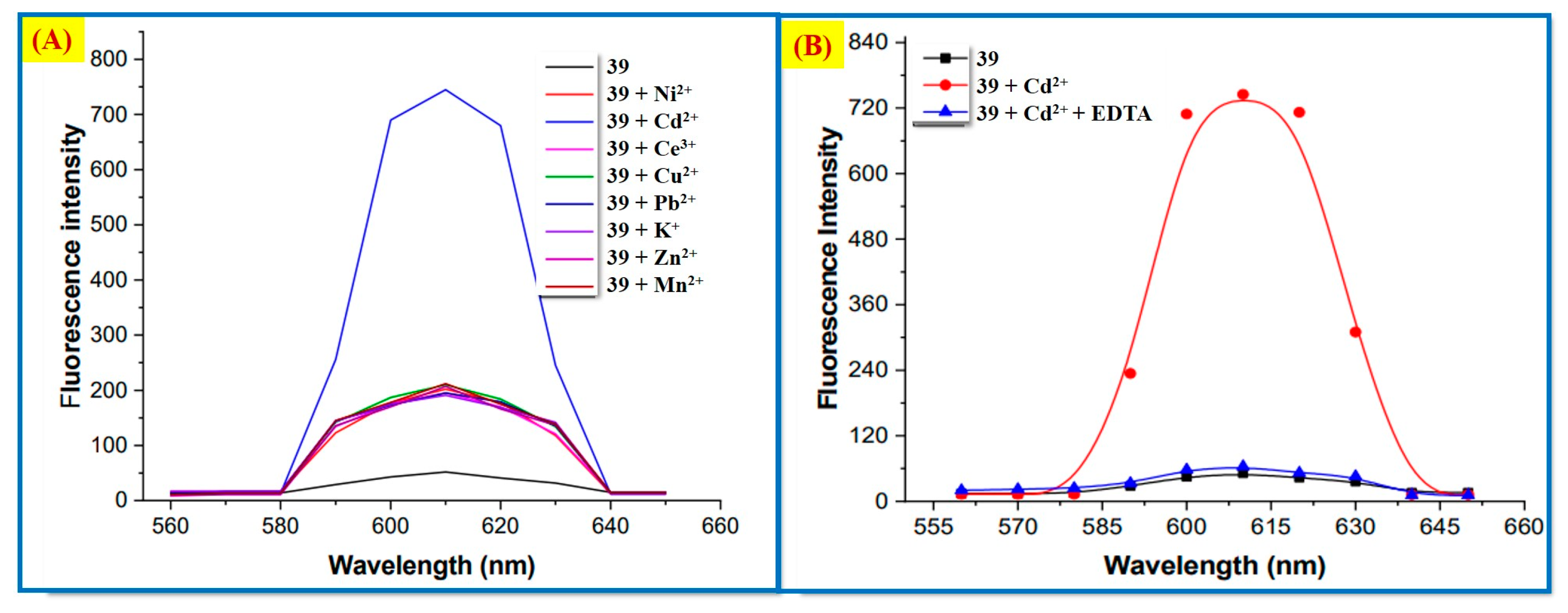
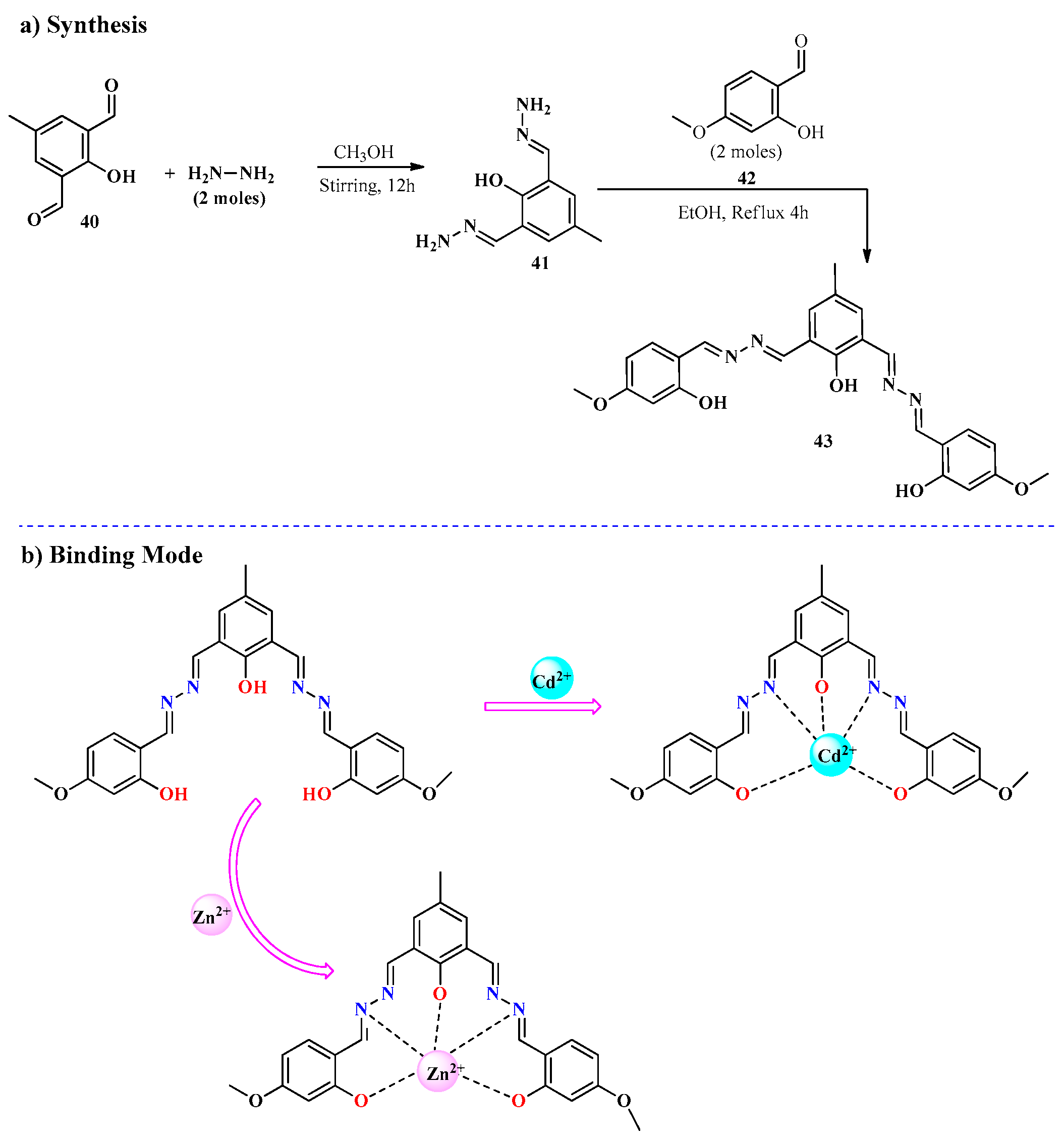
2.3. Cd2+ Ion Detection
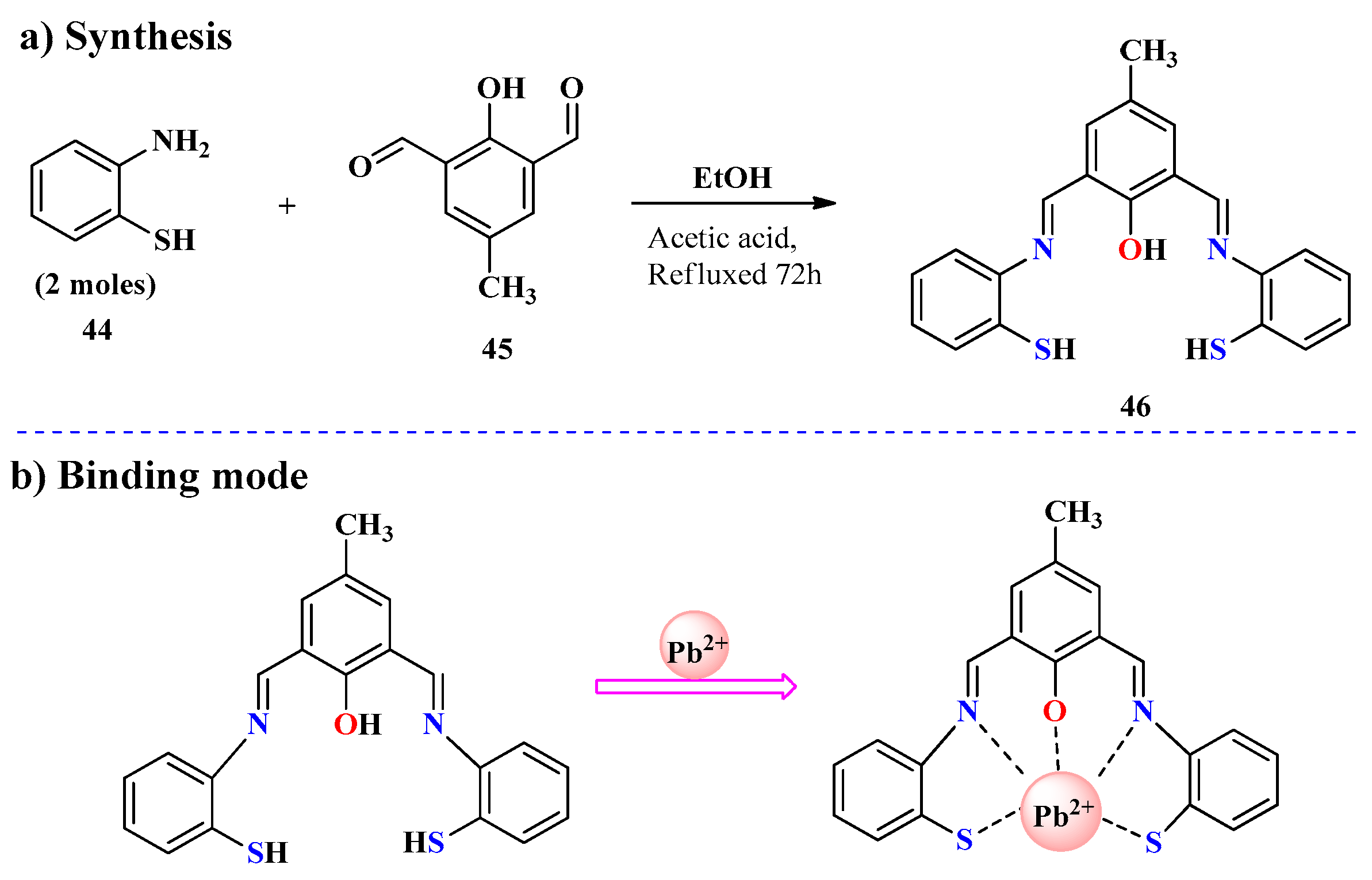
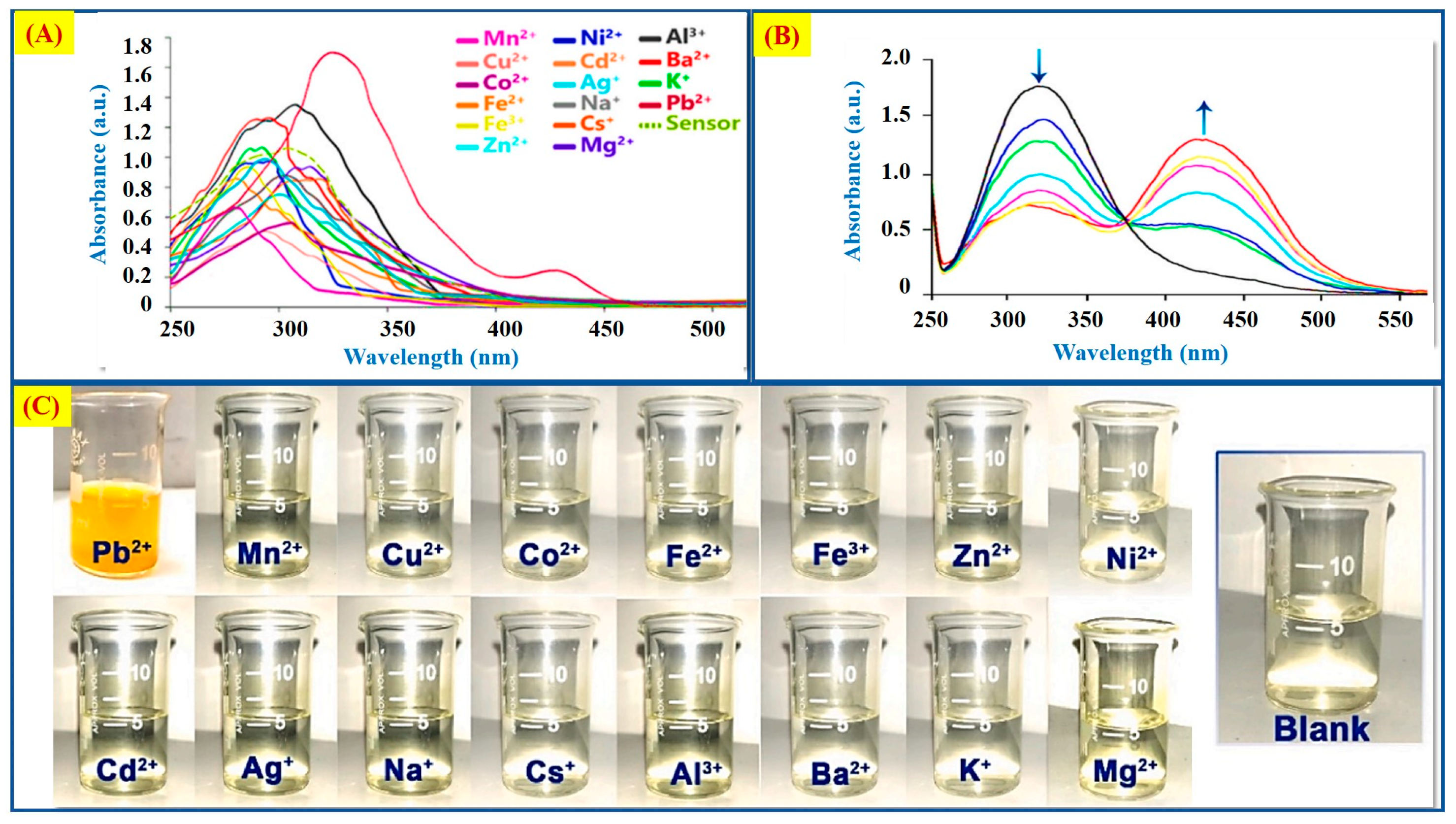
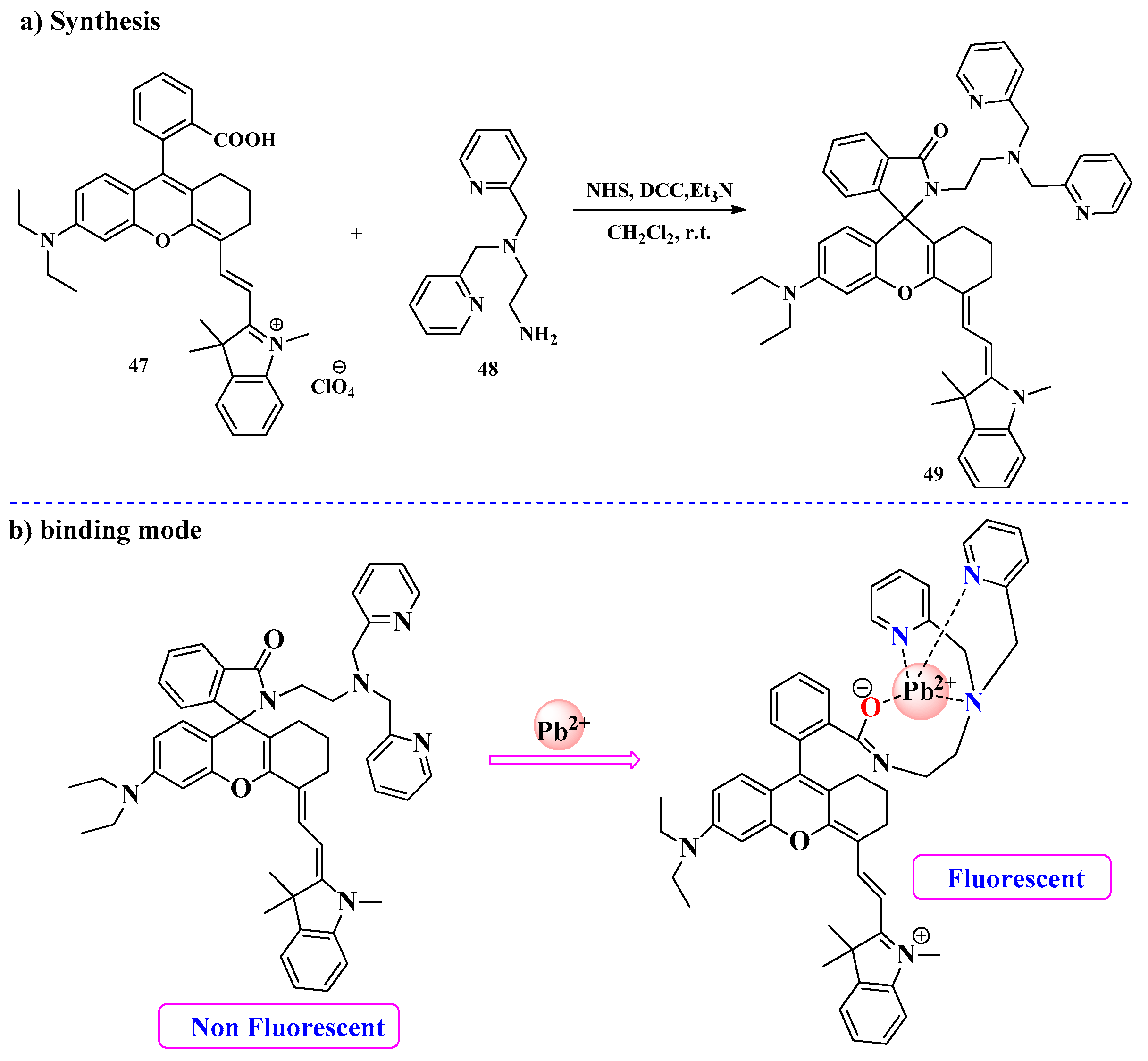
2.4. Pb2+ Ion Detection
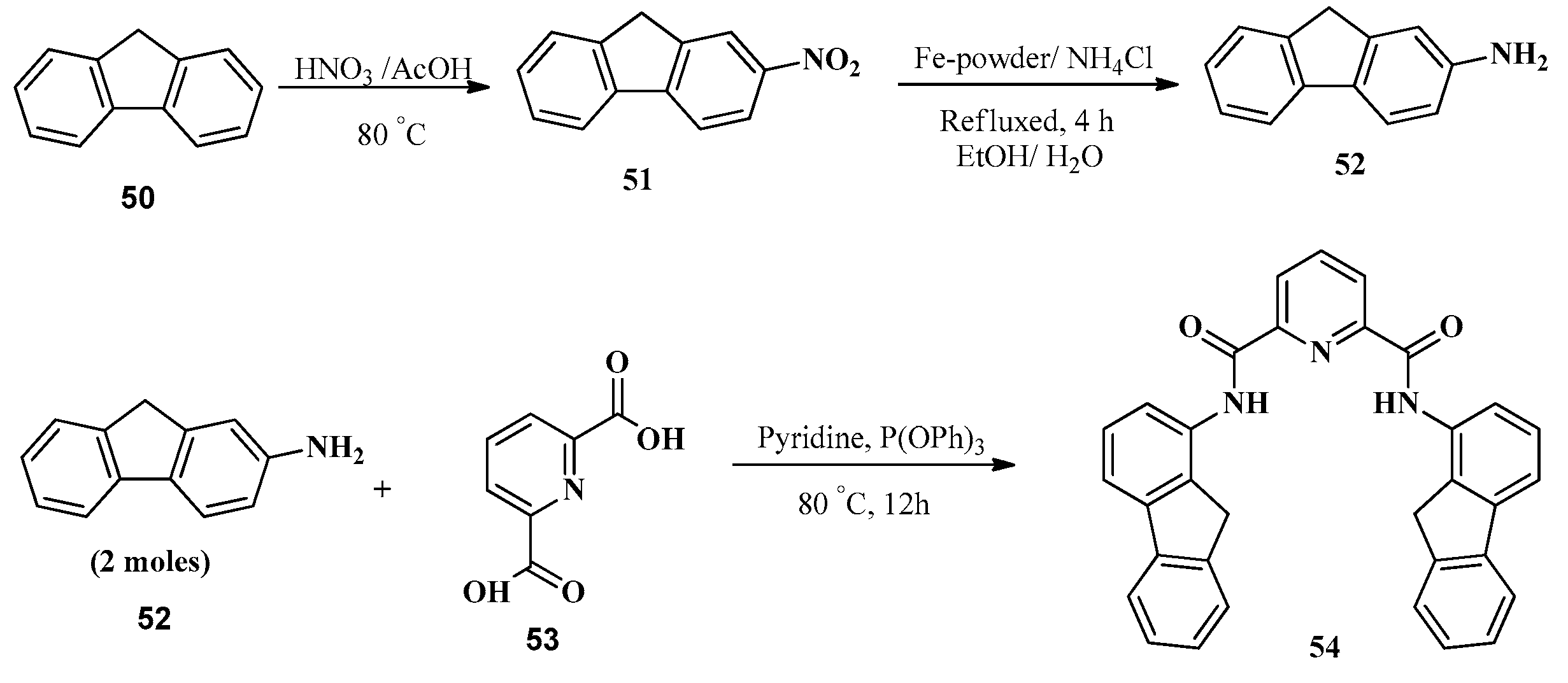
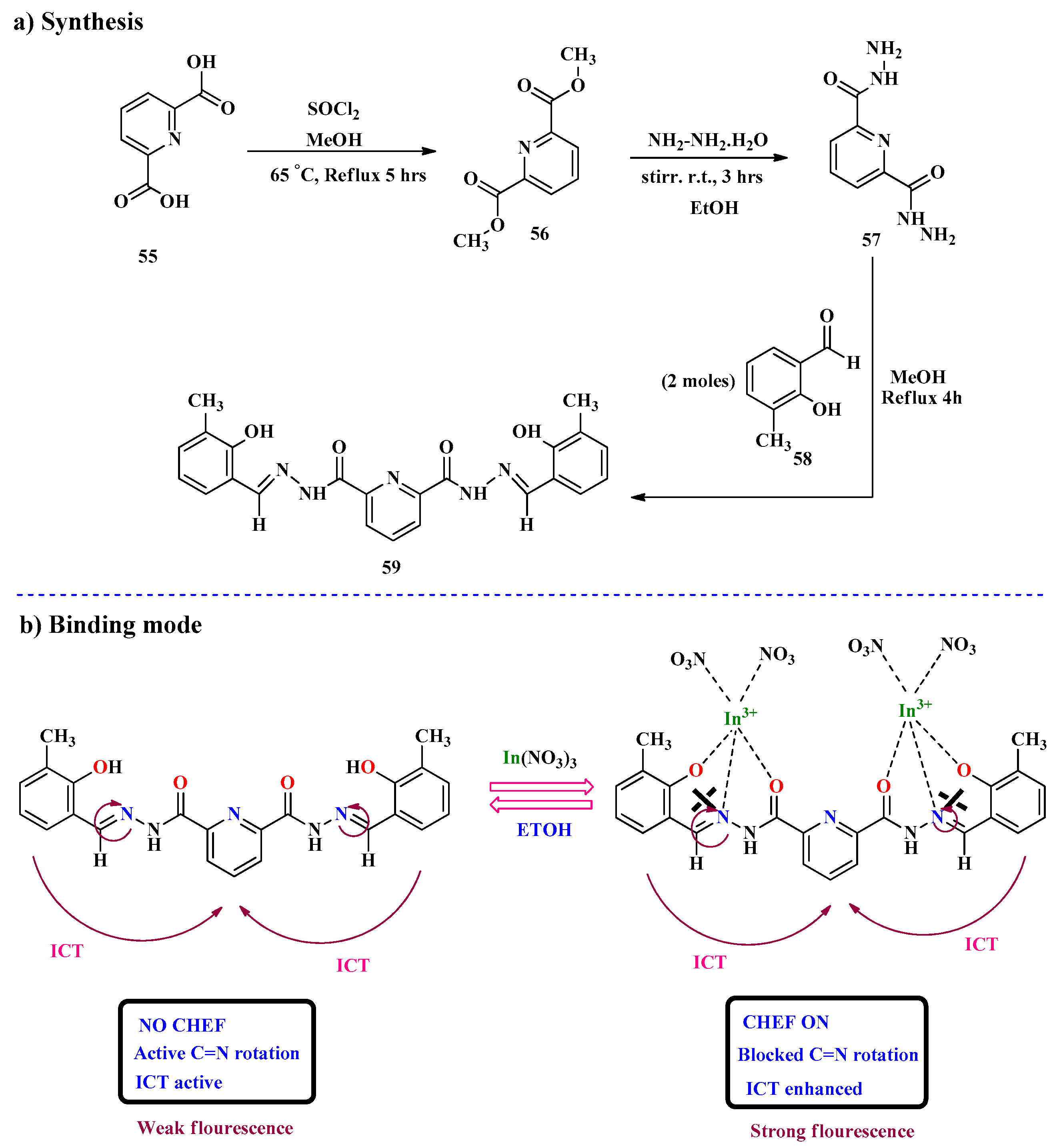
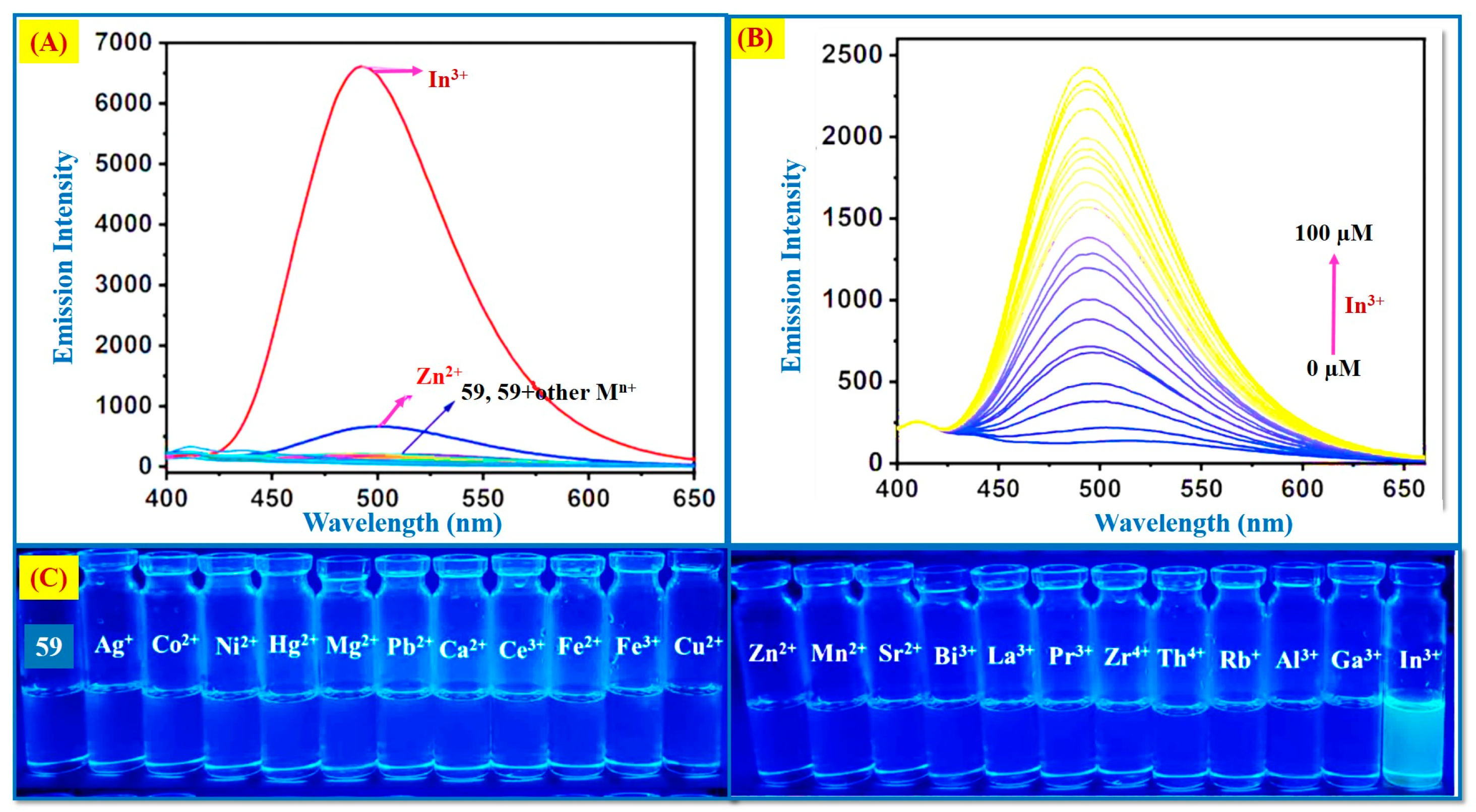
2.5. In3+ Ion Detection
2.6. Simultaneous Multiple-Ion Detection
3. Limitations and Challenges of the Current Chemosensors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar]
- Jonaghani, M.Z.; Zali-Boeini, H. Highly selective fluorescent and colorimetric chemosensor for detection of Hg2+ ion in aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 66–70. [Google Scholar]
- Xu, J.-F.; Chen, H.-H.; Chen, Y.-Z.; Li, Z.-J.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. A colorimetric and fluorometric dual-modal chemosensor for cyanide in water. Sens. Actuators B Chem. 2012, 168, 14–19. [Google Scholar]
- Upadhyay, S.; Singh, A.; Sinha, R.; Omer, S.; Negi, K. Colorimetric chemosensors for d-metal ions: A review in the past, present and future prospect. J. Mol. Struct. 2019, 1193, 89–102. [Google Scholar]
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar]
- Khan, B.; Hameed, A.; Minhaz, A.; Shah, M.R. Synthesis and characterisation of calix [4] arene based bis (triazole)-bis (hexahydroquinoline): Probing highly selective fluorescence quenching towards mercury (Hg2+) analyte. J. Hazard Mater. 2018, 347, 349–358. [Google Scholar]
- Amendola, V.; Fabbrizzi, L.; Foti, F.; Licchelli, M.; Mangano, C.; Pallavicini, P.; Poggi, A.; Sacchi, D.; Taglietti, A. Light-emitting molecular devices based on transition metals. Coord. Chem. Rev. 2006, 250, 273–299. [Google Scholar]
- Jonaghani, M.Z.; Zali-Boeini, H.; Moradi, H. A coumarin based highly sensitive fluorescent chemosensor for selective detection of zinc ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 16–22. [Google Scholar]
- Pundi, A.; Chang, C.-J. Recent developments in the preparation, characterization, and applications of chemosensors for environmental pollutants detection. J. Environ. Chem. Eng. 2023, 11, 110346. [Google Scholar]
- Liu, S.; Wang, Y.-M.; Han, J. Fluorescent chemosensors for copper (II) ion: Structure, mechanism and application. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 78–103. [Google Scholar]
- Bargossi, C.; Fiorini, M.C.; Montalti, M.; Prodi, L.; Zaccheroni, N. Recent developments in transition metal ion detection by luminescent chemosensors. Coord. Chem. Rev. 2000, 208, 17–32. [Google Scholar]
- Chong, S.Y. Performance of the Ortho, Meta and Para Electron-Donating Bearing Symmetrical Benzoylthuourea Act as Chemosensor for Heavy Metal Ion Detection; Tunku Abdul Rahman University of Management and Technology: Kuala Lumpur, Malaysia, 2023. [Google Scholar]
- Alharbi, K.H. A review on organic colorimetric and fluorescent chemosensors for the detection of Zn (II) ions. Crit. Rev. Anal. Chem. 2022, 53, 1472–1488. [Google Scholar]
- Keleş, E.; Aydıner, B.; Nural, Y.; Seferoğlu, N.; Şahin, E.; Seferoğlu, Z. A new mechanism for selective recognition of cyanide in organic and aqueous solution. Eur. J. Org. Chem. 2020, 2020, 4681–4692. [Google Scholar]
- Zhu, F.-L.; Yang, L.; Wang, Y.-H.; Tan, H.H. Preparation of Doped Carbon Quantum Dots and Its Application in Detection of Heavy Metal Ions. Chin. J. Anal. Chem. 2023, 51, 22–33. [Google Scholar]
- Iftikhar, R.; Parveen, I.; Mazhar, A.; Iqbal, M.S.; Kamal, G.M.; Hafeez, F.; Ling, P.A.; Ahmadipour, M. Small organic molecules as fluorescent sensors for the detection of highly toxic heavy metal cations in portable water. J. Environ. Chem. Eng. 2022, 11, 109030. [Google Scholar]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Kamaraj, E.; Seo, S.-Y.; Lee, K.H. Nano molar level chromogenic and fluorogenic sensing of heavy metal ions using multi-responsive novel Schiff base as a dual mode chemosensor. J. Photochem. Photobiol. A Chem. 2019, 385, 112089. [Google Scholar]
- Guo, S.; Fan, C.; Liu, G.; Pu, S. A colorimetric and fluorescent chemosensor for Hg2+ based on a photochromic diarylethene with a quinoline unit. RSC Adv. 2018, 8, 39854–39864. [Google Scholar] [PubMed]
- Xu, D.; Jia, H.; Niu, Y.; Yin, S. Fluorine-boron compound-based fluorescent chemosensors for heavy metal ion detection. Dye. Pigment. 2022, 200, 110185. [Google Scholar]
- Dos Santos, C.H.; Uchiyama, N.M.; Bagatin, I.A. Selective azo dye-based colorimetric chemosensor for F−, CH3COO− and PO43−. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 355–361. [Google Scholar]
- Yu, M.; Liu, B.; Guo, J.; Wu, F. A sustainable modificatory 1, 2-alternate thiacalix [4] arene for detection of silver ion. Dye. Pigment. 2023, 210, 110983. [Google Scholar]
- Asiri, A.M.; Al-Ghamdi, N.S.M.; Dzudzevic-Cancar, H.; Kumar, P.; Khan, S.A. Physicochemical and Photophysical investigation of newly synthesized carbazole containing pyrazoline-benzothiazole as fluorescent chemosensor for the detection of Cu2+, Fe3+ & Fe2+ metal ion. J. Mol. Struct. 2019, 1195, 670–680. [Google Scholar]
- Li, M.; Fang, H.; Ji, Y.; Chen, Y.; He, W.; Guo, Z. Rational Design of Ratiometric Fe3+ Fluorescent Probes Based on FRET Mechanism. Chem. Res. Chin. Univ. 2022, 38, 67–74. [Google Scholar]
- Lu, S.-Q.; Liu, Y.-Y.; Duan, Z.-M.; Wang, Z.-X.; Li, M.-X.; He, X. Improving water-stability and porosity of lanthanide metal–organic frameworks by stepwise synthesis for sensing and removal of heavy metal ions. Cryst. Growth Des. 2018, 18, 4602–4610. [Google Scholar]
- Wang, J.; Chu, S.; Kong, F.; Luo, L.; Wang, Y.; Zou, Z. Designing a smart fluorescence chemosensor within the tailored channel of mesoporous material for sensitively monitoring toxic heavy metal ions Pb (II). Sens. Actuators B Chem. 2010, 150, 25–35. [Google Scholar]
- Algethami, J.S. A Review on Recent Progress in Organic Fluorimetric and Colorimetric Chemosensors for the Detection of Cr3+/6+ Ions. Crit. Rev. Anal. Chem. 2022, 54, 487–507. [Google Scholar]
- Yang, H.; Qi, D.; Chen, Z.; Cao, M.; Deng, Y.; Liu, Z.; Shao, C.; Yang, L. A Zn-based metal–organic framework as bifunctional chemosensor for the detection of nitrobenzene and Fe3+. J. Solid State Chem. 2021, 296, 121970. [Google Scholar]
- Vanjare, B.D.; Mahajan, P.G.; Ryoo, H.-I.; Dige, N.C.; Choi, N.G.; Han, Y.; Kim, S.J.; Kim, C.-H.; Lee, K.H. Novel rhodamine based chemosensor for detection of Hg2+: Nanomolar detection, real water sample analysis, and intracellular cell imaging. Sens. Actuators B Chem. 2021, 330, 129308. [Google Scholar]
- Hu, Y.; Yin, J.; Yoon, J. A multi-responsive cyanine-based colorimetric chemosensor containing dipicolylamine moieties for the detection of Zn (II) and Cu (II) ions. Sens. Actuators B Chem. 2016, 230, 40–45. [Google Scholar]
- Mohammadi, A.; Yaghoubi, S. A new dual colorimetric chemosensor based on quinazolinone for CN−, AcO− and Cu2+ ions. Sens. Actuators B Chem. 2017, 241, 1069–1075. [Google Scholar]
- Zhou, Y.; You, X.-Y.; Fang, Y.; Li, J.-Y.; Liu, K.; Yao, C. A thiophen-thiooxorhodamine conjugate fluorescent probe for detecting mercury in aqueous media and living cells. Org. Biomol. Chem. 2010, 8, 4819–4822. [Google Scholar] [PubMed]
- Al-Saidi, H.M.; Khan, S. A review on organic fluorimetric and colorimetric chemosensors for the detection of Ag (I) ions. Crit. Rev. Anal. Chem. 2022, 54, 1810–1836. [Google Scholar]
- Aslam, S.; Kousar, I.; Rani, S.; Zainab, I.; Bristy, S.; Skouta, R. Modern Approaches in Organic Chromofluorescent Sensor Synthesis for the Detection of Considered First-Row Transition Metal Ions. Molecules 2025, 30, 1263. [Google Scholar] [CrossRef]
- Darwish, N.B.; Kurdi, A.; Alshihri, S.; Tabbakh, T. Organic heterocyclic-based colorimetric and fluorimetric chemosensors for the detection of different analytes: A review (from 2015 to 2022). Mater. Today Chem. 2023, 27, 101347. [Google Scholar]
- Jo, T.G.; Na, Y.J.; Lee, J.J.; Lee, M.M.; Lee, S.Y.; Kim, C. A multifunctional colorimetric chemosensor for cyanide and copper (II) ions. Sens. Actuators B Chem. 2015, 211, 498–506. [Google Scholar]
- Yang, M.; Chae, J.B.; Kim, C.; Harrison, R.G. A visible chemosensor based on carbohydrazide for Fe (II), Co (II) and Cu (II) in aqueous solution. Photochem. Photobiol. Sci. 2019, 18, 1249–1258. [Google Scholar]
- Hu, J.-H.; Li, J.-B.; Qi, J.; Sun, Y. Acylhydrazone based fluorescent chemosensor for zinc in aqueous solution with high selectivity and sensitivity. Sens. Actuators B Chem. 2015, 208, 581–587. [Google Scholar]
- Han, F.; Bao, Y.; Yang, Z.; Fyles, T.M.; Zhao, J.; Peng, X.; Fan, J.; Wu, Y.; Sun, S. Simple bisthiocarbonohydrazones as sensitive, selective, colorimetric, and switch-on fluorescent chemosensors for fluoride anions. Chem. Eur. J. 2007, 13, 2880–2892. [Google Scholar]
- Chen, Y.; Wu, L.; Chen, Y.; Bi, N.; Zheng, X.; Qi, H.; Qin, M.; Liao, X.; Zhang, H.; Tian, Y. Determination of mercury (II) by surface-enhanced Raman scattering spectroscopy based on thiol-functionalized silver nanoparticles. Microchim. Acta 2012, 177, 341–348. [Google Scholar]
- Bhaskar, R.; Sarveswari, S. Thiocarbohydrazide based Schiff Base as a Selective Colorimetric and Fluorescent Chemosensor for Hg2+ with “Turn-Off” Fluorescence Responses. Chem. Sel. 2020, 5, 4050–4057. [Google Scholar]
- Aliberti, A.; Vaiano, P.; Caporale, A.; Consales, M.; Ruvo, M.; Cusano, A. Fluorescent chemosensors for Hg2+ detection in aqueous environment. Sens. Actuators B Chem. 2017, 247, 727–735. [Google Scholar]
- Jia, H.; Yang, M.; Meng, Q.; He, G.; Wang, Y.; Hu, Z.; Zhang, R.; Zhang, Z. Synthesis and application of an aldazine-based fluorescence chemosensor for the sequential detection of Cu2+ and biological thiols in aqueous solution and living cells. Sensors 2016, 16, 79. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Cuadrado, I.; Pastor, C.; Souza, P. A Novel Sulfur-bridged Dimeric Zinc (II) Complex with 2, 6-Diacetylpyridine Bis (thiosemicarbazone). Z. Anorg. Allg. Chem. 2005, 631, 780–784. [Google Scholar]
- Kaur, K.; Saini, R.; Kumar, A.; Luxami, V.; Kaur, N.; Singh, P.; Kumar, S. Chemodosimeters: An approach for detection and estimation of biologically and medically relevant metal ions, anions and thiols. Coord. Chem. Rev. 2012, 256, 1992–2028. [Google Scholar]
- Casas, J.S.; Castellano, E.E.; Ellena, J.; García-Tasende, M.S.; Sánchez, A.; Sordo, J.; Vázquez-López, E.M.; Vidarte, M.J. Synthesis, Spectroscopic and Structural Aspects of Mono-and Diorganothallium (III) Complexes of 2,6-Diacetylpyridine Bis (thiosemicarbazone)(H2DAPTSC)—A New Coordination Mode of the HDAPTSC—Anion. Z. Anorg. Allg. Chem. 2003, 629, 261–267. [Google Scholar]
- Paswan, S.; Bharty, M.; Bharati, P.; Sonkar, P.K.; Ganesan, V.; Butcher, R. Square planar Ni (ii) complexes of acetone N 4-phenyl-thiosemicarbazone and in situ generated benzoyl thiosemicarbazide ligands: Synthesis, spectral and structural characterizations, thermal behaviour and electrochemical studies. New J. Chem. 2017, 41, 15466–15474. [Google Scholar]
- Kumar, A.; Kumar, D.; Chhibber, M. Determination of Mercury ions in Aqueous Medium and urine sample using Thiocarbohydrazide based Sensor. ChemistrySelect 2020, 5, 13738–13747. [Google Scholar]
- Ren, W.; Zhu, C.; Wang, E. Enhanced sensitivity of a direct SERS technique for Hg2+ detection based on the investigation of the interaction between silver nanoparticles and mercury ions. Nanoscale 2012, 4, 5902–5909. [Google Scholar]
- Fang, Y.; Li, X.; Li, J.-Y.; Wang, G.-Y.; Zhou, Y.; Xu, N.-Z.; Hu, Y.; Yao, C. Thiooxo-Rhodamine B hydrazone derivatives bearing bithiophene group as fluorescent chemosensors for detecting mercury (II) in aqueous media and living HeLa cells. Sens. Actuators B Chem. 2018, 255, 1182–1190. [Google Scholar]
- Sharma, S.; Dubey, G.; Sran, B.S.; Bharatam, P.V.; Hundal, G. Fabrication of a Hydrazone-based Al (III)-selective “turn-on” fluorescent chemosensor and ensuing potential recognition of picric acid. ACS Omega 2019, 4, 18520–18529. [Google Scholar]
- Pothulapadu, C.A.S.; Jayaraj, A.N.S.; Priyanka, R.N.; Sivaraman, G. Novel benzothiazole-based highly selective ratiometric fluorescent turn-on sensors for Zn2+ and colorimetric chemosensors for Zn2+, Cu2+, and Ni2+ ions. ACS Omega 2021, 6, 24473–24483. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mahapatra, A.K.; Gangopadhyay, A.; Maji, R.; Mondal, S.; Ali, S.S.; Das, S.; Sarkar, R.; Datta, P.; Mandal, D. Simple bisthiocarbonohydrazone as a sensitive, selective, colorimetric, and ratiometric fluorescent chemosensor for picric acids. ACS Omega 2017, 2, 1583–1593. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B.; Zhao, Y.; Du, J.; Xiao, D. A Hg2+ selective fluorescent chemosensor based on rhodamine B thiohydrazide and its application in bioimaging. Anal. Methods 2012, 4, 2369–2374. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Li, L.; Liang, X.; Li, S.; Fu, Y. A dual thiourea-appended perylenebisimide “turn-on” fluorescent chemosensor with high selectivity and sensitivity for Hg2+ in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118678. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, R.; Sankaran, K. Design, synthesis, computational calculation and biological evaluation of some novel 2-thiazolyl hydrazones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 984–993. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A. Synthesis and investigation of antimicrobial activities of nitrofurazone analogues containing hydrazide-hydrazone moiety. Saudi Pharm. J. 2017, 25, 1097–1102. [Google Scholar] [CrossRef]
- Gajendhiran, R.; Raees Ahmed, A.K.; Mithra, S.; Abdul Majeed, S.; Sahul Hameed, A.S.; Muthu, K.; Sarathkumar, S.; Nehru, S.; Rahiman, A.K. Carbohydrazone/Thiocarbohydrazone-Based Dual-Responsive Probes for Highly Selective Detection of Cu2+/Fe3+ Cations and F−/ClO4− Anions, and Their Application in Bioimaging of Live Cells and Zebrafish Larvae. ACS Omega 2024, 52, 50957–50977. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, W.; Fang, M.; Zhang, Q.; Li, C. A new carbazole-based Schiff-base as fluorescent chemosensor for selective detection of Fe3+ and Cu2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 186–192. [Google Scholar] [CrossRef]
- Moussa, Z.; Al-Mamary, M.; Al-Juhani, S.; Ahmed, S.A. Preparation and biological assessment of some aromatic hydrazones derived from hydrazides of phenolic acids and aromatic aldehydes. Heliyon 2020, 6, e05019. [Google Scholar] [CrossRef]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef]
- Kumari, N.; Singh, S.; Baral, M.; Kanungo, B. Schiff bases: A versatile fluorescence probe in sensing cations. J. Fluoresc. 2023, 33, 859–893. [Google Scholar] [CrossRef] [PubMed]
- Tamizhselvi, R.; Bhaskar, R.; Beena, M.; Palaniappan, A.; Kumar, S.A.; Napoleon, A.A. A dual responsive bis-thiophene affixed thiosemicarbazide based chemosensor for colorimetrically Hg2+ and fluorometrically Cu2+ ions and their applications in live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 322, 124766. [Google Scholar] [CrossRef]
- Santhiya, K.; Mathivanan, M.; Tharmalingam, B.; Anitha, O.; Ghorai, S.; Natarajan, R.; Murugesapandian, B. A new 7-(diethylamino) coumarin and 4-(diethylamino) phenol appended unsymmetrical thiocarbohydrazone: Detection of moisture in organic solvent and sequential fluorimetric detection of Cu2+ ions and cysteine. J. Photochem. Photobiol. A Chem. 2022, 432, 114105. [Google Scholar] [CrossRef]
- Duval, C.; Xuong, N.D. Emploi des thiocarbohydrazones en analyse organique fonctionelle. Microchim. Acta 1956, 44, 747–749. [Google Scholar] [CrossRef]
- Duval, C.; Xuong, N.D. Analyse organique fonctionnelle: Analyse thermogravimétrique des dinitro-2,4 phénylhydrazones. Anal. Chim. Acta 1954, 10, 520–522. [Google Scholar] [CrossRef]
- Esteban-Gómez, D.; Fabbrizzi, L.; Licchelli, M. Why, on interaction of urea-based receptors with fluoride, beautiful colors develop. J. Org. Chem. 2005, 70, 5717–5720. [Google Scholar] [CrossRef]
- Albert, A. The affinity of Iso nicotinic hydrazide for metals. Experientia 1953, 9, 370. [Google Scholar] [CrossRef]
- Rosales, D.; Gonzalez, G.; Ariza, J.L.G. Asymmetric derivatives of carbohydrazide and thiocarbohydrazide as analytical reagents. Talanta 1985, 32, 467–474. [Google Scholar] [CrossRef]
- Sigel, H.; McCormick, D.B. Discriminating behavior of metal ions and ligands with regard to their biological significance. Acc. Chem. Res. 1970, 3, 201–208. [Google Scholar] [CrossRef]
- Krivis, A.; Supp, G.; Doerr, R. Polarographic Study of Some Hydrazide Complexes of Cadmium. Anal. Chem. 1965, 37, 52–54. [Google Scholar] [CrossRef]
- Kurzer, F.; Wilkinson, M. Chemistry of carbohydrazide and thiocarbohydrazide. Chem. Rev. 1970, 70, 111–149. [Google Scholar] [CrossRef] [PubMed]
- Momidi, B.K.; Tekuri, V.; Trivedi, D.R. Multi-signaling thiocarbohydrazide based colorimetric sensors for the selective recognition of heavy metal ions in an aqueous medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Uahengo, V.; Zhang, Y.; Xiong, B.; Zhao, P.; Cai, P.; Rhyman, L.; Ramasami, P.; Hu, K.; Cheng, G. A Fluoro-Chromogenic Sensor Based on Organic Molecular Framework for Cu2+ and F− in Aqueous Soluble DMSO. J. Fluoresc. 2017, 27, 191–197. [Google Scholar] [CrossRef]
- Shao, J.; Qiao, Y.; Lin, H.; Lin, H. A C3-symmetric colorimetric anion sensor bearing hydrazone groups as binding sites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Tekuri, V. Design, Synthesis and Characterization of Chemosensors for Determination of Heavy Metal Ions. Ph.D. Thesis, National Institute of Technology Karnataka, Mangaluru, India, 2020. [Google Scholar]
- De Silva, A.P.; Gunaratne, H.N.; Gunnlaugsson, T.; Huxley, A.J.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- HeeáLee, M.; SeungáKim, J. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [PubMed]
- Coskun, A.; Yilmaz, M.D.; Akkaya, E.U. Bis (2-pyridyl)-substituted boratriazaindacene as an NIR-emitting chemosensor for Hg (II). Org. Lett. 2007, 9, 607–609. [Google Scholar] [CrossRef]
- Chen, P.; He, C. A general strategy to convert the MerR family proteins into highly sensitive and selective fluorescent biosensors for metal ions. J. Am. Chem. Soc. 2004, 126, 728–729. [Google Scholar] [CrossRef]
- Bhalla, V.; Tejpal, R.; Kumar, M.; Sethi, A. Terphenyl derivatives as “turn on” fluorescent sensors for mercury. Inorg. Chem. 2009, 48, 11677–11684. [Google Scholar] [CrossRef]
- Lee, M.H.; Kang, G.; Kim, J.W.; Ham, S.; Kim, J.S. Tren-spaced rhodamine and pyrene fluorophores: Excimer modulation with metal ion complexation. Supramol. Chem. 2009, 21, 135–141. [Google Scholar]
- Kumar, M.; Kumar, N.; Bhalla, V.; Singh, H.; Sharma, P.R.; Kaur, T. Naphthalimide appended rhodamine derivative: Through bond energy transfer for sensing of Hg2+ ions. Org. Lett. 2011, 13, 1422–1425. [Google Scholar] [PubMed]
- Li, C.-Y.; Zhang, X.-B.; Qiao, L.; Zhao, Y.; He, C.-M.; Huan, S.-Y.; Lu, L.-M.; Jian, L.-X.; Shen, G.-L.; Yu, R.-Q. Naphthalimide-porphyrin hybrid based ratiometric bioimaging probe for Hg2+: Well-resolved emission spectra and unique specificity. Anal. Chem. 2009, 81, 9993–10001. [Google Scholar]
- Ni, X.-L.; Wu, Y.; Redshaw, C.; Yamato, T. Direct evidence of a blocking heavy atom effect on the water-assisted fluorescence enhancement detection of Hg2+ based on a ratiometric chemosensor. Dalt. Trans. 2014, 43, 12633–12638. [Google Scholar]
- Hatai, J.; Pal, S.; Jose, G.P.; Bandyopadhyay, S. Histidine based fluorescence sensor detects Hg2+ in solution, paper strips, and in cells. Inorg. Chem. 2012, 51, 10129–10135. [Google Scholar] [PubMed]
- Suresh, M.; Mandal, A.K.; Saha, S.; Suresh, E.; Mandoli, A.; Di Liddo, R.; Parnigotto, P.P.; Das, A. Azine-based receptor for recognition of Hg2+ ion: Crystallographic evidence and imaging application in live cells. Org. Lett. 2010, 12, 5406–5409. [Google Scholar] [CrossRef]
- Srivastava, P.; Razi, S.S.; Ali, R.; Gupta, R.C.; Yadav, S.S.; Narayan, G.; Misra, A. Selective naked-eye detection of Hg2+ through an efficient turn-on photoinduced electron transfer fluorescent probe and its real applications. Anal. Chem. 2014, 86, 8693–8699. [Google Scholar]
- Shellaiah, M.; Rajan, Y.C.; Balu, P.; Murugan, A. A pyrene based Schiff base probe for selective fluorescence turn-on detection of Hg2+ ions with live cell application. New J. Chem. 2015, 39, 2523–2531. [Google Scholar]
- Yu, S.-Y.; Wu, S.-P. A highly selective turn-on fluorescence chemosensor for Hg (II) and its application in living cell imaging. Sens. Actuators B Chem. 2014, 201, 25–30. [Google Scholar]
- Chen, C.; Wang, R.; Guo, L.; Fu, N.; Dong, H.; Yuan, Y. A squaraine-based colorimetric and “turn on” fluorescent sensor for selective detection of Hg2+ in an aqueous medium. Org. Lett. 2011, 13, 1162–1165. [Google Scholar]
- Neupane, L.N.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Selective and sensitive detection of heavy metal ions in 100% aqueous solution and cells with a fluorescence chemosensor based on peptide using aggregation-induced emission. Anal. Chem. 2016, 88, 3333–3340. [Google Scholar] [PubMed]
- Fields, G.B.; Noble, R.L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 1990, 35, 161–214. [Google Scholar]
- Hosseinjani-Pirdehi, H.; Mahmoodi, N.O.; Nadamani, M.P.; Taheri, A. Novel synthesized azo-benzylidene-thiourea as dual naked-eye chemosensor for selective detection of Hg2+ and CN¯ ions. J. Photochem. Photobiol. A Chem. 2020, 391, 112365. [Google Scholar]
- Mishra, J.; Kaur, H.; Ganguli, A.K.; Kaur, N. Fluorescent chemosensor based on urea/thiourea moiety for sensing of Hg (II) ions in an aqueous medium with high sensitivity and selectivity: A comparative account on effect of molecular architecture on chemosensing. J. Mol. Struct. 2018, 1161, 34–43. [Google Scholar]
- Singh, H.; Bhargava, G.; Kumar, S.; Singh, P. Microstructural (self-assembly) and optical based discrimination of Hg2+, CN− and Hg(CN)2 ion-pair; Hg2+ promoted-ESIPT assisted guanylation of thiourea. Sens. Actuators B Chem. 2018, 272, 43–52. [Google Scholar]
- Zhang, Z.; Lu, S.; Sha, C.; Xu, D. A single thiourea-appended 1, 8-naphthalimide chemosensor for three heavy metal ions: Fe3+, Pb2+, and Hg2+. Sens. Actuators B Chem. 2015, 208, 258–266. [Google Scholar] [CrossRef]
- Govindasamy, V.; Perumal, S.; Sekar, I.; Madheswaran, B.; Karuppannan, S.; Kuppannan, S.B. Phenothiazine-thiophene hydrazide dyad: An efficient “on-off” chemosensor for highly selective and sensitive detection of Hg2+ ions. J. Fluoresc. 2021, 31, 667–674. [Google Scholar]
- Jang, S.; Thirupathi, P.; Neupane, L.N.; Seong, J.; Lee, H.; Lee, W.I.; Lee, K.-H. Highly sensitive ratiometric fluorescent chemosensor for silver ion and silver nanoparticles in aqueous solution. Org. Lett. 2012, 14, 4746–4749. [Google Scholar]
- Abu-Taweel, G.M.; Alharthi, S.S.; Al-Saidi, H.M.; Babalghith, A.O.; Ibrahim, M.M.; Khan, S. Heterocyclic Organic Compounds as a Fluorescent Chemosensor for Cell Imaging Applications: A Review. Crit. Rev. Anal. Chem. 2023, 54, 2538–2553. [Google Scholar]
- Cui, W.; Wang, L.; Xiang, G.; Zhou, L.; An, X.; Cao, D. A colorimetric and fluorescence “turn-off” chemosensor for the detection of silver ion based on a conjugated polymer containing 2, 3-di (pyridin-2-yl) quinoxaline. Sens. Actuators B Chem. 2015, 207, 281–290. [Google Scholar]
- Arulraj, A.D.; Devasenathipathy, R.; Chen, S.-M.; Vasantha, V.S.; Wang, S.-F. Highly selective and sensitive fluorescent chemosensor for femtomolar detection of silver ion in aqueous medium. Sens. Bio-Sens. Res. 2015, 6, 19–24. [Google Scholar]
- Velmurugan, K.; Suresh, S.; Santhoshkumar, S.; Saranya, M.; Nandhakumar, R. A simple Chalcone-based ratiometric chemosensor for silver ion. Luminescence 2016, 31, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Annaraj, B.; Neelakantan, M. Water-soluble pyridine-based colorimetric chemosensor for naked eye detection of silver ions: Design, synthesis, spectral and theoretical investigation. Anal. Methods 2014, 6, 9610–9615. [Google Scholar]
- Hussein, M.A.; Alamry, K.A.; Alsulami, Q.A.; Elshehy, E.A.; El-Said, W.A. Design and synthesis of a combined meso-adsorbent/chemo-sensor for extraction and detection of silver ions. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2022, 272, 120938. [Google Scholar]
- Hao, L.; Jiang, Z.; Wang, J.; Shen, D. Preparation of pyridine Schiff base functionalized mesoporous channels and its exploration as fluorescent detection of silver ions in aqueous medium. J. Porous Mater. 2023, 30, 351–361. [Google Scholar]
- Ziarani, G.M.; Moradi, R.; Mohajer, F.; Badiei, A. 2-Chloroquinoline-3-carbaldehyde modified nanoporous SBA-15-propylamine (SBA-Pr-NCQ) as a selective and sensitive Ag+ ion sensor in aqueous media. J. Phys. Chem. Solids 2022, 161, 110399. [Google Scholar]
- Jamasbi, N.; Mohammadi Ziarani, G.; Mohajer, F.; Badiei, A. Highly silver-selective fluorescent sensor based on functionalized nanoporous silica (SBA-Pr-NMP) in aqueous media. Res. Chem. Intermed. 2022, 48, 3957–3969. [Google Scholar]
- Hemmati Tirabadi, F.; Hajiaghababaei, L.; Tehrani, R.M.; Badiei, A.; Mollahosseini, A. Trithiocyanuric acid-functionalized nanoporous silica: Synthesis and application as an Ag+ selective optical probe. Chem. Pap. 2022, 76, 6629–6637. [Google Scholar]
- Celestina, S.K.; Sundaram, K.; Ravi, S. Novel Derivatives of Rhodanine-3-Hippuric Acid as Active Inhibitors of Aldose Reductase: Synthesis, Biological Evaluation, and Molecular Docking Analysis. Russ. J. Bioorg. Chem. 2019, 45, 405–415. [Google Scholar]
- David, C.I.; Prabakaran, G.; Sundaram, K.; Ravi, S.; Abiram, A.; Nandhakumar, R. Rhodanine-based fluorometric sequential monitoring of silver (I) and iodide ions: Experiment, DFT calculation and multifarious applications. J. Hazard Mater. 2021, 419, 126449. [Google Scholar]
- Wang, H.; Xue, L.; Jiang, H. Ratiometric fluorescent sensor for silver ion and its resultant complex for iodide anion in aqueous solution. Org. Lett. 2011, 13, 3844–3847. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Bok, K.H.; Kim, C. A fluorescence “turn-on” chemosensor for Hg2+ and Ag+ based on NBD (7-nitrobenzo-2-oxa-1,3-diazolyl). RSC Adv. 2017, 7, 290–299. [Google Scholar] [CrossRef]
- Seo, Y.; Park, S.; Kim, G.; Lee, M.; Kim, C. A naphthyl thiourea-based effective chemosensor for fluorescence detection of Ag+ and Zn2+. Luminescence 2021, 36, 1725–1732. [Google Scholar] [CrossRef]
- Lai, Q.; Liu, Q.; He, Y.; Zhao, K.; Wei, C.; Wojtas, L.; Shi, X.; Song, Z. Triazole-imidazole (TA-IM) derivatives as ultrafast fluorescent probes for selective Ag+ detection. Org. Biomol. Chem. 2018, 16, 7801–7805. [Google Scholar] [CrossRef]
- Inal, E. A fluorescent chemosensor based on schiff base for the determination of Zn2+, Cd2+ and Hg2+. J. Fluoresc. 2020, 30, 891–900. [Google Scholar] [CrossRef]
- Mohanasundaram, D.; Bhaskar, R.; Kumar, G.G.V.; Rajesh, J.; Rajagopal, G. A quinoline based Schiff base as a turn-on fluorescence chemosensor for selective and robust detection of Cd2+ ion in semi-aqueous medium. Microchem. J. 2021, 164, 106030. [Google Scholar] [CrossRef]
- Purkait, R.; Dey, S.; Sinhaa, C. A multi-analyte responsive chemosensor vanilinyl Schiff base: Fluorogenic sensing of Zn (ii), Cd (ii) and I−. New J. Chem. 2018, 42, 16653–16665. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, F.; Bai, Y.; Zhao, J.; Chen, X.; Ge, M.; Sun, W. Quinoline-based highly selective and sensitive fluorescent probe specific for Cd2+ detection in mixed aqueous media. Tetrahedron Lett. 2017, 58, 3868–3874. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, D.; Li, B.; Chen, X.; Wang, F.; Deng, Y.; Niu, Y.; Zhang, X. An exceptionally stable luminescent cadmium (ii) metal–organic framework as a dual-functional chemosensor for detecting Cr (vi) anions and nitro-containing antibiotics in aqueous media. CrystEngComm 2021, 23, 1218–1225. [Google Scholar] [CrossRef]
- Salek Maghsoudi, A.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent advances in nanotechnology-based biosensors development for detection of arsenic, lead, mercury, and cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, A.; Xu, Q.; Pandey, D.S. Zinc (II), copper (II) and cadmium (II) complexes as fluorescent chemosensors for cations. Dalt. Trans. 2020, 49, 542–568. [Google Scholar]
- Park, S.; Kim, H.-J. Reaction-based chemosensor for the reversible detection of cyanide and cadmium ions. Sens. Actuators B Chem. 2012, 168, 376–380. [Google Scholar] [CrossRef]
- Pham, T.C.; Kim, Y.K.; Park, J.B.; Jeon, S.; Ahn, J.; Yim, Y.; Yoon, J.; Lee, S. A selective colorimetric and fluorometric chemosensor based on conjugated polydiacetylenes for cadmium ion detection. ChemPhotoChem 2019, 3, 1133–1137. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Liu, L.; Zhou, P.; Ge, Y.; Liu, D.; Liu, W.; Tang, Y. A peptide-based fluorescent chemosensor for measuring cadmium ions in aqueous solutions and live cells. Dalt. Trans. 2015, 44, 18057–18064. [Google Scholar]
- Wang, Y.; Hu, X.; Wang, L.; Shang, Z.; Chao, J.; Jin, W. A new acridine derivative as a highly selective ‘off–on’fluorescence chemosensor for Cd2+ in aqueous media. Sens. Actuators B Chem. 2011, 156, 126–131. [Google Scholar]
- Prodi, L.; Bolletta, F.; Montalti, M.; Zaccheroni, N. Luminescent chemosensors for transition metal ions. Coord. Chem. Rev. 2000, 205, 59–83. [Google Scholar]
- Sadia, M.; Khan, J.; Khan, R.; Kamran, A.W.; Zahoor, M.; Ullah, R.; Bari, A.; Ali, E.A. Rapid Detection of Cd2+ Ions in the Aqueous Medium Using a Highly Sensitive and Selective Turn-On Fluorescent Chemosensor. Molecules 2023, 28, 3635. [Google Scholar] [CrossRef] [PubMed]
- Shaji, L.K.; Kumar, R.S.; Jose, J.; Bhaskar, R.; Vetriarasu, V.; Bhat, S.G.; Kumar, S.A. Selective chromogenic and fluorogenic signalling of Hg2+ ions using a benzothiazole-quinolinyl acrylate conjugate and its applications in the environmental water samples and living cells. J. Photochem. Photobiol. A Chem. 2023, 434, 114220. [Google Scholar]
- Wang, S.; Sun, J.; Gao, F. A turn-on near-infrared fluorescent chemosensor for selective detection of lead ions based on a fluorophore–gold nanoparticle assembly. Analyst 2015, 140, 4001–4006. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, B.; Ma, T.; Tian, Y.; Wang, G. A carbazole-grafted covalent organic framework as turn-on fluorescence chemosensor for recognition and detection of Pb2+ ions with high selectivity and sensitivity. J. Mater. Sci. 2021, 56, 11789–11800. [Google Scholar]
- Tayade, K.C.; Kuwar, A.S.; Fegade, U.A.; Sharma, H.; Singh, N.; Patil, U.D.; Attarde, S.B. Design and synthesis of a pyridine based chemosensor: Highly selective fluorescent probe for Pb2+. J. Fluoresc. 2014, 24, 19–26. [Google Scholar] [CrossRef]
- Sharma, V.; Savita, S.; Patra, G.K. A highly sensitive triazole-based perfectly water soluble novel bis-Schiff base reversible fluorescent-colorimetric chemosensor for fast detection of Pb 2+ ions. RSC Adv. 2024, 14, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Al-Saidi, H.M. Recent advancements in organic chemosensors for the detection of Pb2+: A review. Chem. Pap. 2023, 77, 4807–4822. [Google Scholar] [CrossRef]
- Karachi, N.; Azadi, O.; Razavi, R.; Tahvili, A.; Parsaee, Z. Combinatorial experimental and DFT theoretical evaluation of a nano novel thio-dicarboxaldehyde based Schiff base supported on a thin polymer film as a chemosensor for Pb2+ detection. J. Photochem. Photobiol. A Chem. 2018, 360, 152–165. [Google Scholar] [CrossRef]
- George, N.; Singh, G.; Singh, R.; Singh, G.; Singh, H.; Kaur, G.; Singh, J. Click modified bis-appended Schiff base 1, 2, 3-triazole chemosensor for detection of Pb (II) ion and computational studies. J. Mol. Struct. 2023, 1288, 135666. [Google Scholar] [CrossRef]
- Azadbakht, R.; Veisi, H.; Mohamadvand, H.; Khanabadi, J. A new fluorescent chemosensor for Pb2+ ions based on naphthalene derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, R.; Poongodi, M.; Vetriarasu, V.; Vivekanandan, K.; Selvakumar, K.; Al Obaid, S.; Pugazhendhi, A.; Venkatesan, G. Quinoline-quinoline schiff-base as an effective chromogenic, fluorogenic, and smartphone assisted RGB detection of Pb2+ ion in near aqueous medium. Environ. Res. 2024, 250, 118530. [Google Scholar]
- Ghorai, A.; Mondal, J.; Saha, R.; Bhattacharya, S.; Patra, G.K. A highly sensitive reversible fluorescent-colorimetric azino bis-Schiff base sensor for rapid detection of Pb2+ in aqueous media. Anal. Methods 2016, 8, 2032–2040. [Google Scholar] [CrossRef]
- Anbu Durai, W.; Ramu, A.; Dhakshinamoorthy, A. A visual and ratiometric chemosensor using thiophene functionalized hydrazone for the selective sensing of Pb2+ and F− ions. J. Fluoresc. 2021, 31, 465–474. [Google Scholar] [CrossRef]
- Rahimi, H.; Hosseinzadeh, R.; Tajbakhsh, M. A new and efficient pyridine-2,6-dicarboxamide-based fluorescent and colorimetric chemosensor for sensitive and selective recognition of Pb2+ and Cu2+. J. Photochem. Photobiol. A Chem. 2021, 407, 113049. [Google Scholar] [CrossRef]
- Xie, Y.; Li, H.; Liu, X.; Wang, Z.; Lv, H.; Cao, J.; Zhang, C.; Jia, Q.; Han, A. An aqueous fluorescent sensor for Pb2+ based on phenothiazine-polyamide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Aksuner, N. Development of a new fluorescent sensor based on a triazolo-thiadiazin derivative immobilized in polyvinyl chloride membrane for sensitive detection of lead (II) ions. Sens. Actuators B Chem. 2011, 157, 162–168. [Google Scholar] [CrossRef]
- Çubuk, S.; Taşci, N.; Kahraman, M.V.; Bayramoğlu, G.; Yetimoğlu, E.K. Reusable fluorescent photocrosslinked polymeric sensor for determining lead ions in aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 159, 106–112. [Google Scholar] [CrossRef]
- Ghorbanian, M.; Asghari, S.; Tajbakhsh, M. A new benzothiazole azo dye colorimetric chemosensor for detecting Pb2+ ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 296, 122652. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, L.; Li, P.; Liu, Y.; Li, S.; Fu, Y.; Ye, F. A simple and rapid fluorescent approach for Pb2+ determination and application in water samples and living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120168. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-Y.; Zhang, H.; Xiao, M.; Han, B.-X. A dual colorimetric and fluorescent sensor for lead ion based on naphthalene hydrazone derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Khurana, J.M. Phenazine containing indeno-furan based colorimetric and “on–off” fluorescent sensor for the detection of Cu2+ and Pb2+. J. Lumin. 2015, 167, 146–155. [Google Scholar] [CrossRef]
- Suguna, S.; Abiram, A.; Kumar, R.S.; Almansour, A.I.; Perumal, K.; Prabhu, J.; Nandhakumar, R. Symmetric and disulfide linked reversible fluorescent organic material: A chemosensor for Pb2+ ion and its applications in real world sample analysis. J. Photochem. Photobiol. A Chem. 2023, 442, 114777. [Google Scholar] [CrossRef]
- Hadi, H.; Bouzid, G.; Nasr, S.; Ghalla, H.; Chaabane, R.B.; Ayachi, S. Design, synthesis, and density functional theory studies of a new selective chemosensor for Pb2+. Heliyon 2023, 9, e20206. [Google Scholar] [CrossRef]
- Bi, J.; Fang, M.; Wang, J.; Xia, S.; Zhang, Y.; Zhang, J.; Vegesna, G.; Zhang, S.; Tanasova, M.; Luo, F.-T. Near-infrared fluorescent probe for sensitive detection of Pb (II) ions in living cells. Inorganica Chim. Acta 2017, 468, 140–145. [Google Scholar] [CrossRef]
- Wenqi, Q.; Yalei, C.; Jieshan, C. Indium and thallium background contents in soils in China. Int. J. Environ. Stud. 1992, 40, 311–315. [Google Scholar] [CrossRef]
- Gallagher, J.; Golduber, R.; Beck, T.; Benkechkache, A.; Tower, J.; Kargar, A.; Hong, H.; Gueorguiev, A.; Lukosi, E. In Neutron imaging with lithium indium diselenide semiconductors. In Proceedings of the 2020 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Boston, MA, USA, 31 October–7 November 2020; pp. 1–4. [Google Scholar]
- Karve, M.A.; Khopkar, S.M. Solvent extraction separation of indium with quaternary ammonium anion exchanger Aliquat 336S from an ascorbate solution. Anal. Sci. 1992, 8, 77–80. [Google Scholar]
- Qiu, W.; Huang, Y.; Tang, S.; Ji, H.; Tong, Y. Thin-layer indium oxide and cobalt oxyhydroxide cobalt-modified BiVO4 photoanode for solar-assisted water electrolysis. J. Phys. Chem. 2017, 121, 17150–17159. [Google Scholar]
- Dasari, S.G.; Nagaraju, P.; Yelsani, V.; Tirumala, S.; MV, R.R. Nanostructured indium oxide thin films as a room temperature toluene sensor. ACS Omega 2021, 6, 17442–17454. [Google Scholar]
- Hjiri, M.; Dhahri, R.; Omri, K.; El Mir, L.; Leonardi, S.; Donato, N.; Neri, G. Effect of indium doping on ZnO based-gas sensor for CO. Mater. Sci. Semicond. Process. 2014, 27, 319–325. [Google Scholar]
- Mehta, P.K.; Hwang, G.W.; Park, J.; Lee, K.-H. Highly sensitive ratiometric fluorescent detection of indium (III) using fluorescent probe based on phosphoserine as a receptor. Anal. Chem. 2018, 90, 11256–11264. [Google Scholar] [CrossRef]
- Shaji, L.K.; Selvam, P.K.; Bhaskar, R.; Majeed, S.A.; Hameed, A.S.; Kumar, S.A. A dual responsive novel bipyridyl carbohydrazide Schiff base as a colorimetric-fluorescent probe for In 3+ and Al 3+ ions and its potential applications. Sens. Diagn. 2024, 3, 455–467. [Google Scholar]
- Aatif, A.M.; Kumar, R.S.; Joseph, S.; Vetriarasu, V.; Majeed, S.A.; Kumar, S.A. Pyridinecarbohydrazide-based fluorescent chemosensor for In3+ ions and its applications in water samples, live cells, and zebrafish imaging. J. Photochem. Photobiol. A Chem. 2023, 434, 114257. [Google Scholar]
- Kim, C.; Chae, J.B. A highly selective fluorescent chemosensor for detecting indium (III) with a low detection limit and its application. J. Fluoresc. 2018, 28, 1363–1370. [Google Scholar]
- Zhu, S.; Yang, L.; Zhao, Y. Ethyl 3-aminobenzo [b] thiophene-2-carboxylate Derived Ratiometric Schiff Base Fluorescent Sensor for the Recognition of In3+ and Pb2+. J. Fluoresc. 2024, 35, 943–953. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, H.; Wei, K.; Kang, M.; Liu, P.; Yang, X.; Pei, M.; Zhang, G. A new Schiff base derived from 5-(thiophene-2-yl) oxazole as “off-on-off” fluorescence sensor for monitoring indium and ferric ions sequentially and its application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 292, 122376. [Google Scholar] [CrossRef] [PubMed]
- Huerta-José, D.S.; Hernández-Hernández, J.G.; Huerta-Aguilar, C.A.; Thangarasu, P. Novel insight of indium (III) complex of N, N-bis (salicylidene) ethylenediamine as chemo-sensor for selective recognition of HSO4− and hemolytic toxicity (Red Blood Cells) studies: Experimental and theoretical studies. Sens. Actuators B Chem. 2019, 293, 357–365. [Google Scholar] [CrossRef]
- Li, B.; Liu, Z.; Li, L.; Xing, Y.; Liu, Y.; Yang, X.; Pei, M.; Zhang, G. A Schiff base sensor for relay monitoring of In3+ and Fe3+ through “off–on–off” fluorescent signals. New J. Chem. 2021, 45, 6753–6759. [Google Scholar] [CrossRef]
- Lee, M.; Lee, J.J.; Kim, C. Sensitive fluorescent determination of indium (III) by a thiourea–quinoline-based chemosensor. Instrum. Sci. Technol. 2022, 50, 481–495. [Google Scholar] [CrossRef]
- Jayapriya, S.; Ebenazer, A.F.; Sampathkumar, N.; Rajesh, J.; Rajagopal, G. Chromene Carbohydrazide-Schiff Base as a Highly Selective Turn-Off Fluorescence Chemosensor for In3+ Ion and its Application. J. Fluoresc. 2024, 1–12. [Google Scholar] [CrossRef]
- Cho, H.; Chae, J.B.; Kim, C. A thiophene-based blue-fluorescent emitting chemosensor for detecting indium (III) ion. Inorg. Chem. Commun. 2018, 97, 171–175. [Google Scholar] [CrossRef]
- Ryu, K.Y.; Lee, S.Y.; Park, D.Y.; Kim, S.Y.; Kim, C. A novel colorimetric chemosensor for detection of Co2+ and S2− in an aqueous environment. Sens. Actuators B Chem. 2017, 242, 792–800. [Google Scholar] [CrossRef]
- Lee, S.A.; You, G.R.; Choi, Y.W.; Jo, H.Y.; Kim, A.R.; Noh, I.; Kim, S.-J.; Kim, Y.; Kim, C. A new multifunctional Schiff base as a fluorescence sensor for Al3+ and a colorimetric sensor for CN− in aqueous media: An application to bioimaging. Dalt. Trans. 2014, 43, 6650–6659. [Google Scholar] [CrossRef]
- Tang, X.-L.; Peng, X.-H.; Dou, W.; Mao, J.; Zheng, J.-R.; Qin, W.-W.; Liu, W.-S.; Chang, J.; Yao, X.-J. Design of a semirigid molecule as a selective fluorescent chemosensor for recognition of Cd (II). Org. Lett. 2008, 10, 3653–3656. [Google Scholar] [CrossRef]
- Chua, M.H.; Hui, B.; Chin, K.L.O.; Zhu, Q.; Liu, X.; Xu, J. Recent Advances in Aggregation-Induced Emission (AIE)-Based Chemosensors for the Detection of Organic Small Molecules. Mater. Chem. Front. 2023, 7, 5561–5660. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Zhu, Q.; Tang, B.Z.; Xu, J.W. Recent advances in cation sensing using aggregation-induced emission. Mater. Chem. Front. 2021, 5, 659–708. [Google Scholar]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Li, Y.; Yu, J. Solvatochromic AIE luminogens as supersensitive water detectors in organic solvents and highly efficient cyanide chemosensors in water. Chem. Sci. 2014, 5, 2710–2716. [Google Scholar]
- Majeed, S.; Waseem, M.T.; Khan, G.S.; Junaid, H.M.; Imran, M.; Nawazish, S.; Khan, T.A.; Mahmood, T.; Shahzad, S.A. Development of AIEE active fluorescent and colorimetric probe for the solid, solution, and vapor phase detection of cyanide: Smartphone and food applications. Analyst 2022, 147, 3885–3893. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, S.; Wang, Z.; Guo, H.; Yang, F. First fluorescence sensor for simultaneously detecting three kinds of IIB elements (Zn2+, Cd2+ and Hg2+) based on aggregation-induced emission. Sens. Actuators B Chem. 2020, 308, 127734. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, C.; Zhang, P.; Chen, Y.; Xie, J.; Song, W.; Liu, Z.; Liu, G.; Zheng, X. A dual-functional chemosensor based on acylhydrazone derivative for rapid detection of Zn (II) and Mg (II): Spectral properties, recognition mechanism and application studies. Arab. J. Chem. 2023, 16, 104603. [Google Scholar]
- Zhang, S.; Wu, X.; Niu, Q.; Guo, Z.; Li, T.; Liu, H. Highly selective and sensitive colorimetric and fluorescent chemosensor for rapid detection of Ag+, Cu2+ and Hg2+ based on a simple Schiff base. J. Fluoresc. 2017, 27, 729–737. [Google Scholar]
- Khalajiolyaie, A.; Jian, C. Advances in Graphene-Based Materials for Metal Ion Sensing and Wastewater Treatment: A Review. Environments 2025, 12, 43. [Google Scholar] [CrossRef]
- Kumar, A.; Seema, K.; Kumar, A. Sensors and Biosensors for Emerging Contaminants in Industrial Wastewater. In Emerging Contaminants in Water and Wastewater: Sources and Substances; Springer: Berlin/Heidelberg, Germany, 2025; pp. 11–41. [Google Scholar]
- Muhammad, M.; Khan, S.; Al-Saidi, H.M.; Algethami, J.S.; Alhamami, M.A.; Alshareef, M. Novel Thiourea-Based Chemosensor for Ultrasensitive and Selective Detection of Hg2+. J. Fluoresc. 2025, 1–12. [Google Scholar] [CrossRef]
- Abduljawad, M.M.; Hilal, R.H.; El-Sherif, A.A. Synthesis and Characterization of New Schiff Base Complex of Cadmium (II) Nanostructure: Assessing its Capability as a Cadmium Sensor with Low Detection Limits Using QCM-Based Sensing Method. Egypt. J. Chem. 2025, 68, 203–210. [Google Scholar]
- Ali, Y.S.; Shaw, I.; Liu, Y.; Chen, C. Perspective Chapter: Advanced Nanotechnology Approach for Heavy Metal Toxicity–Analysis. In Heavy Metals in the Environment—Contamination, Risk, and Remediation; IntechOpen Limited: London, UK, 2025; Volume 251. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, S.; Kousar, I.; Rani, S.; Altaf, W.; Bristy, S.; Skouta, R. Detection of Selected Heavy Metal Ions Using Organic Chromofluorescent Chemosensors. Molecules 2025, 30, 1450. https://doi.org/10.3390/molecules30071450
Aslam S, Kousar I, Rani S, Altaf W, Bristy S, Skouta R. Detection of Selected Heavy Metal Ions Using Organic Chromofluorescent Chemosensors. Molecules. 2025; 30(7):1450. https://doi.org/10.3390/molecules30071450
Chicago/Turabian StyleAslam, Samina, Iram Kousar, Sadia Rani, Wajiha Altaf, Sadia Bristy, and Rachid Skouta. 2025. "Detection of Selected Heavy Metal Ions Using Organic Chromofluorescent Chemosensors" Molecules 30, no. 7: 1450. https://doi.org/10.3390/molecules30071450
APA StyleAslam, S., Kousar, I., Rani, S., Altaf, W., Bristy, S., & Skouta, R. (2025). Detection of Selected Heavy Metal Ions Using Organic Chromofluorescent Chemosensors. Molecules, 30(7), 1450. https://doi.org/10.3390/molecules30071450







