The Co-Delivery of Natural Products and Small RNAs for Cancer Therapy: A Review
Abstract
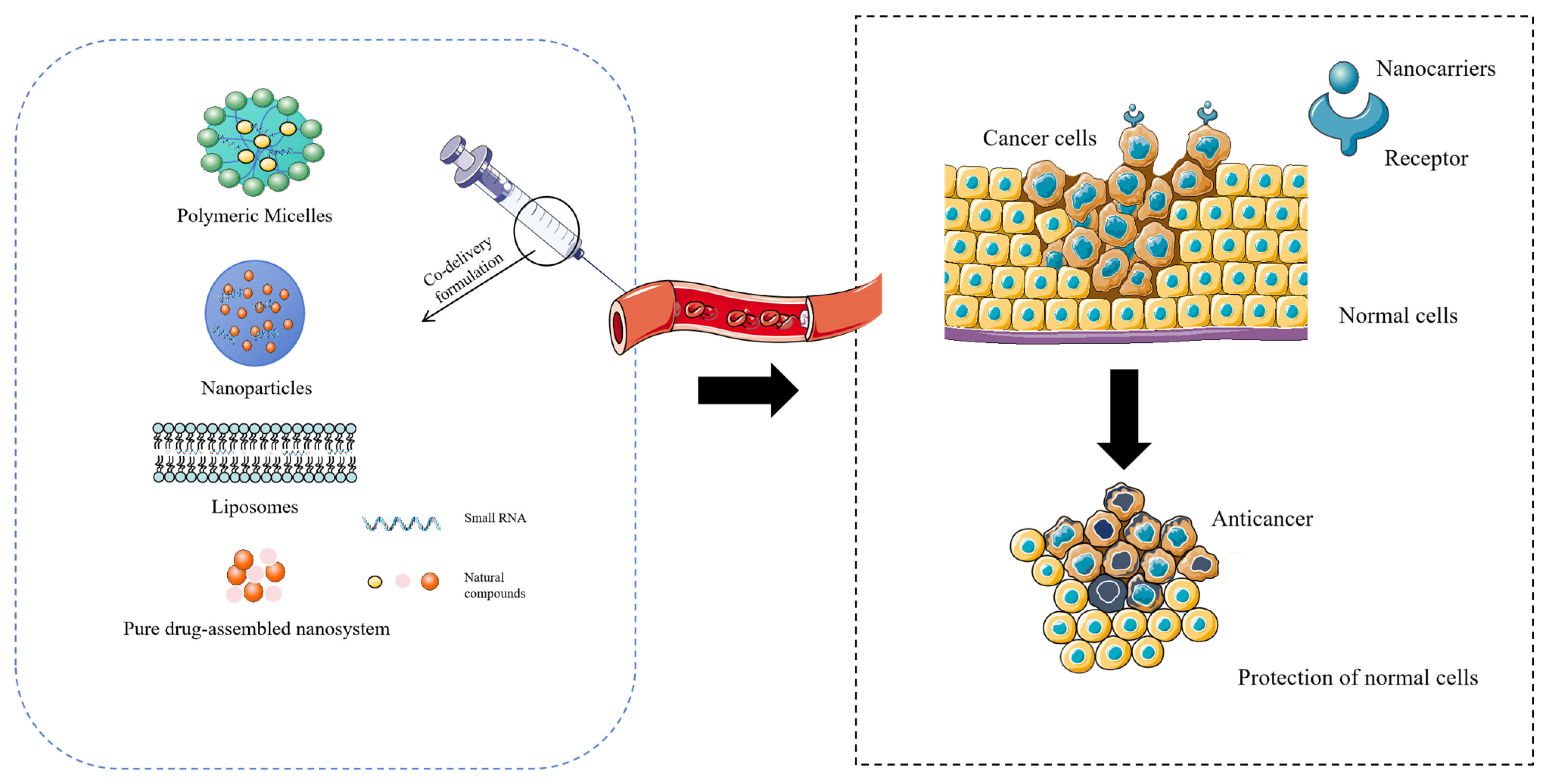
1. Introduction
2. Antitumor Effects of Natural Products
2.1. Paclitaxel (PTX)
2.2. Camptothecin (CPT)
2.3. Curcumin (Cur)
2.4. Resveratrol (RES)
3. Small-RNA Antitumor Effect Through Gene Regulation
3.1. The Biological Target of Small RNAs
3.2. Classification of Small RNAs
3.2.1. Small Interfering RNA (siRNA)
3.2.2. MicroRNAs (miRNAs)
3.2.3. Short Hairpin RNA (shRNA)
4. Co-Delivery of NPs-nc RNA by Nano-DDS
4.1. Co-Delivery of NPs-siRNA
4.2. Co-Delivery of NPs-miRNA
4.3. Co-Delivery of NPs-shRNA
4.4. Nanocarriers Can Enhance the Targeting of Drug Co-Delivery
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPT | Camptothecin |
| Cur | Curcumin |
| Dicer | Double-stranded RNA-specific ribonuclease |
| dsRNA | Double-stranded RNA |
| EPR | Enhanced permeability and retention |
| MDR | Multidrug resistance |
| miRNA | MicroRNA |
| PTX | Paclitaxel |
| Nano-DDS | Nanomaterial-based drug delivery system |
| NPs | Natural products |
| PDNA | Pure drug-assembled nanosystem |
| RES | Resveratrol |
| RISC | RNA-induced silencing complex |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| RNAi | RNA interference |
| ROS | Reactive oxygen species |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
References
- Ruberte, A.C.; Ramos-Inza, S.; Aydillo, C.; Talavera, I.; Encío, I.; Plano, D.; Sanmartín, C. Novel N,N’-Disubstituted Acylselenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2020, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jiang, Y.; Hao, L.; Yang, Y.; Gao, Y.; Zhang, N.; Zhang, X.; Song, Y. CD44/Folate Dual Targeting Receptor Reductive Response PLGA-Based Micelles for Cancer Therapy. Front. Pharmacol. 2022, 13, 829590. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Hsing, M.T.; Hsu, H.T.; Chang, C.H.; Chang, K.B.; Cheng, C.Y.; Lee, J.H.; Huang, C.-L.; Yang, M.-Y.; Yang, Y.-C.; Liu, S.-Y.; et al. Improved Delivery Performance of n-Butylidenephthalide-Polyethylene Glycol-Gold Nanoparticles Efficient for Enhanced Anti-Cancer Activity in Brain Tumor. Cells 2022, 11, 2172. [Google Scholar] [CrossRef]
- Zeng, Z.; Liao, S.; Zhou, H.; Liu, H.; Lin, J.; Huang, Y.; Zhou, C.; Xu, D. Novel Sigma-2 receptor ligand A011 overcomes MDR in adriamycin-resistant human breast cancer cells by modulating ABCB1 and ABCG2 transporter function. Front. Pharmacol. 2022, 13, 952980. [Google Scholar] [CrossRef]
- Fu, H.; Wu, Z.X.; Lei, Z.N.; Teng, Q.X.; Yang, Y.; Ashby, C.R.; Lei, Y.; Lian, Y.; Chen, Z.-S. The Resistance of Cancer Cells to Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, is Mediated by the ABCB1 Transporter. Front. Pharmacol. 2022, 13, 861642. [Google Scholar] [CrossRef]
- Andreani, C.; Bartolacci, C.; Wijnant, K.; Crinelli, R.; Bianchi, M.; Magnani, M.; Hysi, A.; Iezzi, M.; Amici, A.; Marchini, C. Resveratrol fuels HER2 and ERα-positive breast cancer behaving as proteasome inhibitor. Aging 2017, 9, 508–523. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.; Li, Z.; Zhang, X.; Li, X.; Hao, Y.; Su, X.; Zhu, J.; Zheng, C.; Xiao, W.; et al. ANovel Systems Pharmacology Method to Investigate Molecular Mechanisms of Scutellaria barbata, D. Don for Non-small Cell Lung Cancer. Front. Pharmacol. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Jia, Y.; He, T.; Wu, D.; Tong, J.; Zhu, J.; Li, Z.; Dong, J. The treatment of Qibai Pingfei Capsule on chronic obstructive pulmonary disease may be mediated by Th17/Treg balance and gut-lung axis microbiota. J. Transl. Med. 2022, 20, 281. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, M.; Liu, Z.; Liu, M.; Zhang, S.; Zhang, Y. Hesperidin Inhibits Lung Cancer In Vitro and In Vivo Through PinX1. Front. Pharmacol. 2022, 13, 918665. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Miller, C.R.; Sarkaria, J.N.; Jaeckle, K.; Buckner, J.C.; Ligon, K.L.; Ballman, K.V.; Moore, D.F.; Nebozhyn, M.; et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of Alliance N0874/ABTC 02. Neuro Oncol. 2018, 20, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Leon, A.; Ezponda, T.; Meydan, C.; Valcárcel, L.V.; Ordoñez, R.; Kulis, M.; Garate, L.; Miranda, E.; Segura, V.; Guruceaga, E.; et al. Characterization of complete lncRNAs transcriptome reveals the functional and clinical impact of lncRNAs in multiple myeloma. Leukemia 2021, 35, 1438–1450. [Google Scholar] [CrossRef]
- Wan, R.; Bai, L.; Cai, C.; Ya, W.; Jiang, J.; Hu, C.; Chen, Q.; Zhao, B.; Li, Y. Discovery of tumor immune infiltration-related snoRNAs for predicting tumor immune microenvironment status and prognosis in lung adenocarcinoma. Comput. Struct. Biotechnol. J. 2021, 19, 6386–6399. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, H.; Li, J.; Liu, L.; Zhang, X.; Sui, N. Comparative Transcriptome Analysis Reveals New lncRNAs Responding to Salt Stress in Sweet Sorghum. Front. Bioeng. Biotechnol. 2020, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Patterson, J. Small interfering RNA (siRNA)-based therapeutics. Drug Ther. Bull. 2023, 61, 72–76. [Google Scholar] [CrossRef]
- Mi, Z.; Song, Y.; Wang, J.; Liu, Z.; Cao, X.; Dang, L.; Lu, Y.; Sun, Y.; Xiong, H.; Zhang, L.; et al. cAMP-Induced Nuclear Condensation of CRTC2 Promotes Transcription Elongation and Cystogenesis in Autosomal Dominant Polycystic Kidney Disease. Adv. Sci. 2022, 9, e2104578. [Google Scholar] [CrossRef]
- Chen, X.; Guo, T.; Zhang, K.; Chen, J.; Wang, C.; Ren, X.; Wang, Q.; Yang, Y.; Liu, C.; Tan, W.; et al. Simultaneous improvement to solubility and bioavailability of active natural compound isosteviol using cyclodextrin metal-organic frameworks. Acta Pharm. Sin. B 2021, 11, 2914–2923. [Google Scholar] [CrossRef]
- Morais, E.S.; Silva, N.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sá, I.; Ventura, S.P.; Silvestre, A.J.; et al. Anti-inflammatory and antioxidant nanostructured cellulose membranes loaded with phenolic-based ionic liquids for cutaneous application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, X.; Jing, X.; Hu, H. PAMAM-Functionalized Cellulose Nanocrystals with Needle-Like Morphology for Effective Cancer Treatment. Nanomaterials 2021, 11, 1640. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, H.; Liu, T.; Tan, X.; Jiang, Q.; Sun, B.; Zheng, Y.; Wang, G.; Wang, Y.; Cheng, M.; et al. Structurally defined tandem-responsive nanoassemblies composed of dipeptide-based photosensitive derivatives and hypoxia-activated camptothecin prodrugs against primary and metastatic breast tumors. Acta Pharm. Sin. B 2022, 12, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Domiński, A.; Domińska, M.; Skonieczna, M.; Pastuch-Gawołek, G.; Kurcok, P. Shell-Sheddable Micelles Based on Poly(ethylene glycol)-hydrazone-poly[R,S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System. Pharmaceutics 2022, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Altay, Y.; Cao, S.; Che, H.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. Adaptive Polymeric Assemblies for Applications in Biomimicry and Nanomedicine. Biomacromolecules 2019, 20, 4053–4064. [Google Scholar] [CrossRef]
- Wang, W.; Xi, M.; Duan, X.; Wang, Y.; Kong, F. Delivery of baicalein and paclitaxel using self-assembled nanoparticles: Synergistic antitumor effect in vitro and in vivo. Int. J. Nanomed. 2015, 10, 3737–3750. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Chiou, Y.Y.; Hung, S.Y.; Lin, T.M.; Wang, H.K.; Lin, C.W.; Liou, H.-H.; Chang, M.-Y.; Lee, Y.-C. Novel Application of Magnetite Nanoparticle-Mediated Vitamin D3 Delivery for Peritoneal Dialysis-Related Peritoneal Damage. Int. J. Nanomed. 2021, 16, 2137–2146. [Google Scholar] [CrossRef]
- Cheng, J.; Fu, S.; Qin, Z.; Han, Y.; Yang, X. Self-assembled natural small molecule diterpene acids with favorable anticancer activity and biosafety for synergistically enhanced antitumor chemotherapy. J. Mater. Chem. B 2021, 9, 2674–2687. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, M.; Liu, J.; Yang, Y.; Yu, Y.; Li, J.; Pan, W.; Fan, L.; Li, G.; Li, X.; et al. Inhibition of tumor metastasis by targeted daunorubicin and dioscin codelivery liposomes modified with PFV for the treatment of non-small-cell lung cancer. Int. J. Nanomed. 2019, 14, 4071–4090. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Lee, J.M.; Bae, S.W.; Jang, K.J. Potential Antitumor Effects of 6-Gingerol in p53-Dependent Mitochondrial Apoptosis and Inhibition of Tumor Sphere Formation in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4660. [Google Scholar] [CrossRef]
- Lv, C.; Qu, H.; Zhu, W.; Xu, K.; Xu, A.; Jia, B.; Qing, Y.; Li, H.; Wei, H.-J.; Zhao, H.-Y. Low-Dose Paclitaxel Inhibits Tumor Cell Growth by Regulating Glutaminolysis in Colorectal Carcinoma Cells. Front. Pharmacol. 2017, 8, 244. [Google Scholar] [CrossRef]
- Han, Y.; Pan, J.; Liang, N.; Gong, X.; Sun, S. A pH-Sensitive Polymeric Micellar System Based on Chitosan Derivative for Efficient Delivery of Paclitaxel. Int. J. Mol. Sci. 2021, 22, 6659. [Google Scholar] [CrossRef] [PubMed]
- Falah, M.; Rayan, M.; Rayan, A. A Novel Paclitaxel Conjugate with Higher Efficiency and Lower Toxicity: A New Drug Candidate for Cancer Treatment. Int. J. Mol. Sci. 2019, 20, 4965. [Google Scholar] [CrossRef]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef]
- Yu, D.L.; Lou, Z.P.; Ma, F.Y.; Najafi, M. The interactions of paclitaxel with tumour microenvironment. Int. Immunopharmacol. 2022, 105, 108555. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Groysman, L.; Carlsen, L.; Huntington, K.E.; Shen, W.H.; Zhou, L.; El-Deiry, W.S. Chemotherapy-induced cytokines and prognostic gene signatures vary across breast and colorectal cancer. Am. J. Cancer Res. 2021, 11, 6086–6106. [Google Scholar] [PubMed]
- Lee, K.W.; Lee, K.H.; Zang, D.Y.; Park, Y.I.; Shin, D.B.; Kim, J.W.; Im, S.-A.; Koh, S.A.; Yu, K.-S.; Cho, J.-Y.; et al. Phase I/II Study of Weekly Oraxol for the Second-Line Treatment of Patients With Metastatic or Recurrent Gastric Cancer. Oncologist 2015, 20, 896–897. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, J.J.; Guo, N.H.; Chang, D.Y.; Wang, C.Y.; Chen, J.T.; Lin, W.-J.; Chi, K.-H.; Lee, Y.-J.; Liu, R.-S.; et al. Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration. Int. J. Mol. Sci. 2019, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Argenziano, M.; Gigliotti, C.L.; Ferrara, B.; Boggio, E.; Chiocchetti, A.; Caldera, F.; Trotta, F.; Benetti, E.; Annaratone, L.; et al. Paclitaxel-Loaded Nanosponges Inhibit Growth and Angiogenesis in Melanoma Cell Models. Front. Pharmacol. 2019, 10, 776. [Google Scholar] [CrossRef]
- Huang, S.T.; Wang, Y.P.; Chen, Y.H.; Lin, C.T.; Li, W.S.; Wu, H.C. Liposomal paclitaxel induces fewer hematopoietic and cardiovascular complications than bioequivalent doses of Taxol. Int. J. Oncol. 2018, 53, 1105–1117. [Google Scholar] [CrossRef]
- Yang, X.; Shen, J.; Gao, Y.; Feng, Y.; Guan, Y.; Zhang, Z.; Mankin, H.; Hornicek, F.J.; Duan, Z. Nsc23925 prevents the development of paclitaxel resistance by inhibiting the introduction of P-glycoprotein and enhancing apoptosis. Int. J. Cancer 2015, 137, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yan, J.; Chen, W.; Yang, J.; Liu, M.; Zhang, Y.; Shen, X.; Ma, Y.; Hu, X.; Wang, Y.; et al. Population Pharmacokinetics and Exposure-Safety Relationship of Paclitaxel Liposome in Patients With Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 1731. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Zhang, E.; Jiang, M.; Zhi, D.; Chen, H.; Cui, S.; Zhen, Y.; Cui, J.; Zhang, S. Co-delivery of paclitaxel and anti-VEGF siRNA by tripeptide lipid nanoparticle to enhance the anti-tumor activity for lung cancer therapy. Drug Deliv. 2020, 27, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Pandhi, S.; Mishra, S.; Barua, S.; Kurian, A.; Mahato, D.K.; Rasane, P.; Büsselberg, D.; Kumar, P.; Calina, D.; et al. Camptothecin and its derivatives: Advancements, mechanisms and clinical potential in cancer therapy. Med. Oncol. 2024, 41, 263. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Xin, L.T.; Liu, L.; Shao, C.L.; Yu, R.L.; Chen, F.L.; Yue, S.J.; Wang, M.; Guo, Z.-L.; Fan, Y.-C.; Guan, H.-S.; et al. Discovery of DNA Topoisomerase I Inhibitors with Low-Cytotoxicity Based on Virtual Screening from Natural Products. Mar. Drugs 2017, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Schütte LR, F.; Bücksteeg, D.; Alfke, J.; Uebel, T.; Esselen, M. Topoisomerase poisoning by the flavonoid nevadensin triggers DNA damage and apoptosis in human colon carcinoma HT29 cells. Arch. Toxicol. 2021, 95, 3787–3802. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Peng, M.Y.; Rong, L.; Li, B.; Wang, S.B.; Cheng, S.X.; Zhuo, R.-X.; Zhang, X.-Z. Self-defensive nano-assemblies from camptothecin-based antitumor drugs. Regen. Biomater. 2015, 2, 159–166. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, W.; Zheng, H.; Xiao, Z.; Hu, J.; Yang, L.; Chen, M.; Liang, G.; Zheng, S.; Zhao, C. Acid-responsive nanoparticles as a novel oxidative stress-inducing anticancer therapeutic agent for colon cancer. Int. J. Nanomed. 2019, 14, 1597–1618. [Google Scholar] [CrossRef]
- Di Paolo, A.; Bocci, G.; Polillo, M.; Del Re, M.; Di Desidero, T.; Lastella, M.; Danesi, R. Pharmacokinetic and pharmacogenetic predictive markers of irinotecan activity and toxicity. Curr. Drug Metab. 2011, 12, 932–943. [Google Scholar] [CrossRef]
- Thomas, A.; Pommier, Y. Targeting Topoisomerase I in the Era of Precision Medicine. Clin. Cancer Res. 2019, 25, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.V.; Kim, J.; Klinz, S.G.; Paz, N.; Cain, J.; Drummond, D.C.; Nielsen, U.B.; Fitzgerald, J.B. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014, 74, 7003–7013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, X.; Xu, H.; Yan, H.; Li, X.; Tang, H. The functional curcumin liposomes induce apoptosis in C6 glioblastoma cells and C6 glioblastoma stem cells in vitro and in animals. Int. J. Nanomed. 2017, 12, 1369–1384. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Olajide, T.M.; Xu, J.; Weng, X. Two Novel Lipophilic Antioxidants Derivatized from Curcumin. Antioxidants 2022, 11, 796. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Amer, S.A.; El-Araby, D.A.; Tartor, H.; Farahat, M.; Goda, N.I.A.; Farag, M.F.M.; Fahmy, E.M.; Hassan, A.M.; El-Maati, M.F.A.; Osman, A. Long-Term Feeding with Curcumin Affects the Growth, Antioxidant Capacity, Immune Status, Tissue Histoarchitecture, Immune Expression of Proinflammatory Cytokines, and Apoptosis Indicators in Nile Tilapia, Oreochromis niloticus. Antioxidants 2022, 11, 937. [Google Scholar] [CrossRef]
- Migliore, R.; D’Antona, N.; Sgarlata, C.; Consoli, G.M.L. Co-Loading of Temozolomide and Curcumin into a Calix [4]arene-Based Nanocontainer for Potential Combined Chemotherapy: Binding Features, Enhanced Drug Solubility and Stability in Aqueous Medium. Nanomaterials 2021, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Bolger, G.T.; Licollari, A.; Tan, A.; Greil, R.; Vcelar, B.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; et al. Pharmacokinetics of liposomal curcumin (Lipocurc™) infusion: Effect of co-medication in cancer patients and comparison with healthy individuals. Cancer Chemother. Pharmacol. 2019, 83, 265–275. [Google Scholar] [CrossRef]
- Wang, C.; Wang, N.; Li, N.; Yu, Q.; Wang, F. Combined Effects of Resveratrol and Vitamin E From Peanut Seeds and Sprouts on Colorectal Cancer Cells. Front. Pharmacol. 2021, 12, 760919. [Google Scholar] [CrossRef]
- Xu, J.; Dong, P.; Cui, Y.; Li, H.; Jiang, S.; Wang, Y.; Zhang, J. Comprehensive analysis of dihydromyricetin metabolites in rats using ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry. J. Sep. Sci. 2022, 45, 3930–3941. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, P.; Xie, L.; Chai, S.; Xu, Q.; Zeng, L.; Wang, X.; Jiang, N.; Sang, M. Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int. J. Mol. Med. 2021, 47, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Role of Resveratrol in Transmitochondrial AMD RPE Cells. Nutrients 2020, 12, 159. [Google Scholar] [CrossRef]
- Bai, L.; Ma, Y.; Wang, X.; Feng, Q.; Zhang, Z.; Wang, S.; Zhang, H.; Lu, X.; Xu, Y.; Zhao, E.; et al. Polydatin Inhibits Cell Viability, Migration, and Invasion Through Suppressing the c-Myc Expression in Human Cervical Cancer. Front. Cell Dev. Biol. 2021, 9, 587218. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, S.; Ma, M.; Zhang, Y. Fluorinated PEG-PEI Coated Magnetic Nanoparticles for siRNA Delivery and CXCR4 Knockdown. Nanomaterials 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, P.N.; Aarthi, J.J.; Manikandan, J.; Kumar, S.D. siRNA, miRNA, and shRNA: In vivo applications. J. Dent. Res. 2008, 87, 992–1003. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, X.J. Three kinds of treatment with Homoharringtonine, Hydroxychloroquine or shRNA and their combination against coronavirus PEDV in vitro. Virol. J. 2020, 17, 71. [Google Scholar] [CrossRef]
- Fan, Y.; Dhaliwal, H.K.; Menon, A.V.; Chang, J.; Choi, J.E.; Amiji, M.M.; Kim, J. Site-specific intestinal DMT1 silencing to mitigate iron absorption using pH-sensitive multi-compartmental nanoparticulate oral delivery system. Nanomedicine 2019, 22, 102091. [Google Scholar] [CrossRef]
- Valiulyte, I.; Pranckeviciene, A.; Bunevicius, A.; Tamasauskas, A.; Svitina, H.; Skrypkina, I.; Vaitkiene, P. Associations of miR-181a with Health-Related Quality of Life, Cognitive Functioning, and Clinical Data of Patients with Different Grade Glioma Tumors. Int. J. Mol. Sci. 2022, 23, 11149. [Google Scholar] [CrossRef]
- Li, J.; Ge, X.; Cui, C.; Zhang, Y.; Wang, Y.; Wang, X.; Sun, Q. Preparation and Characterization of Functionalized Graphene Oxide Carrier for siRNA Delivery. Int. J. Mol. Sci. 2018, 19, 3202. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, S.; Yu, W.; Rao, T.; Ruan, Y.; Zhu, S.; Xia, Y.; Song, H.; Cheng, F. SC66 inhibits the proliferation and induces apoptosis of human bladder cancer cells by targeting the AKT/β-catenin pathway. J. Cell Mol. Med. 2021, 25, 10684–10697. [Google Scholar] [CrossRef] [PubMed]
- Bandi, N.; Vassella, E. miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol. Cancer 2011, 10, 55. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Wang, C.; Zhao, Z.; Ma, W.; Wang, D.; Mao, H.; Liu, F.; Yang, Y.; Pan, W.; et al. DCE-MRI radiomics models predicting the expression of radioresistant-related factors of LRP-1 and survivin in locally advanced rectal cancer. Front. Oncol. 2022, 12, 881341. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, C.; Wang, L.; Xu, M.; Zang, Y.; Zhou, Y.; Liu, X.; Tao, W.; Xue, B.; Shan, Y.; et al. Targeting survivin using a combination of miR-494 and survivin shRNA has synergistic effects on the suppression of prostate cancer growth. Mol. Med. Rep. 2016, 13, 1602–1610. [Google Scholar] [CrossRef]
- Taylor, A.M.; Sun, J.M.; Yu, A.; Voicu, H.; Shen, J.; Barkauskas, D.A.; Triche, T.J.; Gastier-Foster, J.M.; Man, T.-K.; Lau, C.C. Integrated DNA Copy Number and Expression Profiling Identifies IGF1R as a Prognostic Biomarker in Pediatric Osteosarcoma. Int. J. Mol. Sci. 2022, 23, 8036. [Google Scholar] [CrossRef]
- Liu, P.Y.; Xu, N.; Malyukova, A.; Scarlett, C.J.; Sun, Y.T.; Zhang, X.D.; Ling, D.; Su, S.-P.; Nelson, C.; Chang, D.K.; et al. The histone deacetylase SIRT2 stabilizes Myc oncoproteins. Cell Death Differ. 2013, 20, 503–514. [Google Scholar] [CrossRef]
- Loginov, V.I.; Pronina, I.V.; Filippova, E.A.; Burdennyy, A.M.; Lukina, S.S.; Kazubskaya, T.P.; Uroshlev, L.A.; Fridman, M.V.; Brovkina, O.I.; Apanovich, N.V.; et al. Aberrant Methylation of 20 miRNA Genes Specifically Involved in Various Steps of Ovarian Carcinoma Spread: From Primary Tumors to Peritoneal Macroscopic Metastases. Int. J. Mol. Sci. 2022, 23, 1300. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Mihanfar, A.; Akbarzadeh, S.; Yousefi, B.; Majidinia, M. Crosstalk between miRNA and PI3K/AKT/mTOR signaling pathway in cancer. Life Sci. 2021, 285, 119984. [Google Scholar] [CrossRef]
- Cao, S.; Lin, C.; Li, X.; Liang, Y.; Saw, P.E. TME-Responsive Multistage Nanoplatform for siRNA Delivery and Effective Cancer Therapy. Int. J. Nanomed. 2021, 16, 5909–5921. [Google Scholar] [CrossRef]
- Zapletal, D.; Kubicek, K.; Svoboda, P.; Stefl, R. Dicer structure and function: Conserved and evolving features. EMBO Rep. 2023, 24, e57215. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef]
- Jiao, P.; Zhang, M.; Wang, Z.; Liang, G.; Xie, X.; Zhang, Y.; Chen, Z.; Jiang, Q.; Loor, J.J. Circ003429 Regulates Unsaturated Fatty Acid Synthesis in the Dairy Goat Mammary Gland by Interacting with miR-199a-3p, Targeting the YAP1 Gene. Int. J. Mol. Sci. 2022, 23, 4068. [Google Scholar] [CrossRef]
- Tang, Q.; Khvorova, A. RNAi-based drug design: Considerations and future directions. Nat. Rev. Drug Discov. 2024, 23, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Luo, W.; Yu, W.; Zhou, J.; Wang, X.; Sun, Y. Identification and Characterization of Csa-miR395s Reveal Their Involvements in Fruit Expansion and Abiotic Stresses in Cucumber. Front. Plant Sci. 2022, 13, 907364. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, H.E.; Jun, A.R.; Jung, M.G.; Jin, S.; Lee, J.H.; Ahn, J.H. Structural determinants of miR156a precursor processing in temperature-responsive flowering in Arabidopsis. J. Exp. Bot. 2016, 67, 4659–4670. [Google Scholar] [CrossRef]
- Campbell, A.M.; De La Cruz-Herrera, C.F.; Marcon, E.; Greenblatt, J.; Frappier, L. Epstein-Barr Virus BGLF2 commandeers RISC to interfere with cellular miRNA function. PLoS Pathog. 2022, 18, e1010235. [Google Scholar] [CrossRef]
- Dalmadi, Á.; Miloro, F.; Bálint, J.; Várallyay, É.; Havelda, Z. Controlled RISC loading efficiency of miR168 defined by miRNA duplex structure adjusts ARGONAUTE1 homeostasis. Nucleic Acids Res. 2021, 49, 12912–12928. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Yu, Y.; Jin, H.; Xie, Q.; Hua, X.; Wang, S.; Tian, Z.; Zhang, H.; Jiang, G.; et al. MicroRNA-3648 Is Upregulated to Suppress TCF21, Resulting in Promotion of Invasion and Metastasis of Human Bladder Cancer. Mol. Ther. Nucleic Acids 2019, 16, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, C.; Song, G.; Fan, X.; Peng, S.; Zhang, S.; Zhou, X.; Zhang, C.; Geng, X.; Wang, T.; et al. Comprehensive analysis of miRNA-mRNA regulatory pairs associated with colorectal cancer and the role in tumor immunity. BMC Genom. 2023, 24, 724. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Deng, W.J.; Luo, Z.Y.; Jing, J.; Pan, P.W.; Yao, Y.B.; Teng, J.-F.; Fang, Y.-B. Inhibition of microRNA-29b suppresses oxidative stress and reduces apoptosis in ischemic stroke. Neural Regen. Res. 2022, 17, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Mues, M.; Karra, L.; Romero-Moya, D.; Wandler, A.; Hangauer, M.J.; Ksionda, O.; Thus, Y.; Lindenbergh, M.; Shannon, K.; McManus, M.T.; et al. High-Complexity shRNA Libraries and PI3 Kinase Inhibition in Cancer: High-Fidelity Synthetic Lethality Predictions. Cell Rep. 2019, 27, 631–647.e5. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, Z.; Lang, J.; Perrett, R.M.; Elschami, M.; Hurry, M.E.; Kim, H.T.; Mazaraki, D.; Szabo, A.; Kessler, B.M.; Goldberg, A.L.; et al. Deubiquitinase Usp8 regulates α-synuclein clearance and modifies its toxicity in Lewy body disease. Proc. Natl. Acad. Sci. USA 2016, 113, E4688–E4697. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Itano, K.; Harada, Y.; Asada, K.; Oikawa, K.; Kashiwazako, M.; Okuyama, H.; Kumagai, K.; Takanashi, M.; Sudo, K.; et al. Development of Novel Small Hairpin RNAs That do not Require Processing by Dicer or AGO2. Mol. Ther. 2016, 24, 1278–1289. [Google Scholar] [CrossRef]
- Steinbacher, J.L.; Landry, C.C. Adsorption and release of siRNA from porous silica. Langmuir 2014, 30, 4396–4405. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.D.; Vorhies, J.S.; Senzer, N.; Nemunaitis, J. siRNA vs. shRNA: Similarities and differences. Adv. Drug Deliv. Rev. 2009, 61, 746–759. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, B.; Ling, L.; Xu, J.; You, L.; Aslam, A.F.; Tan, A.; Huang, Y. Enhancement of larval RNAi efficiency by over-expressing Argonaute2 in Bombyx mori. Int. J. Biol. Sci. 2015, 11, 176–185. [Google Scholar] [CrossRef]
- Bak, S.P.; Barnkob, M.S.; Wittrup, K.D.; Chen, J. CD8+ T-cell responses rapidly select for antigen-negative tumor cells in the prostate. Cancer Immunol. Res. 2013, 1, 393–401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Chinak, O.; Golubitskaya, E.; Pyshnaya, I.; Stepanov, G.; Zhuravlev, E.; Richter, V.; Koval, O. Nucleic Acids Delivery Into the Cells Using Pro-Apoptotic Protein Lactaptin. Front. Pharmacol. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Wu, F.; Qin, F.; Feng, P.; Xu, T.; Li, X.; Yang, L. A Novel Prostate-Specific Membrane-Antigen (PSMA) Targeted Micelle-Encapsulating Wogonin Inhibits Prostate Cancer Cell Proliferation via Inducing Intrinsic Apoptotic Pathway. Int. J. Mol. Sci. 2016, 17, 676. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, H.Q. Anti-liver cancer effects and mechanisms and its application in nano DDS of polysaccharides: A review. Int. J. Biol. Macromol. 2024, 279 Pt 2, 135181. [Google Scholar] [CrossRef]
- Cini, N.; Calisir, F. Layer-by-layer self-assembled emerging systems for nanosized drug delivery. Nanomedicine 2022, 17, 1961–1980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Zhang, S.; Sun, B.; Chen, Q.; He, Z.; Luo, C.; Sun, J. Emerging carrier-free nanosystems based on molecular self-assembly of pure drugs for cancer therapy. Med. Res. Rev. 2020, 40, 1754–1775. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, J.; An, X.; Wang, Z.; Zheng, X.; Zhang, Y. Fabrication of Ginsenoside-Based Nanodrugs for Enhanced Antitumor Efficacy on Triple-Negative Breast Cancer. Front. Bioeng. Biotechnol. 2022, 10, 945472. [Google Scholar] [CrossRef]
- Han, L.; Liang, S.; Mu, W.; Zhang, Z.; Wang, L.; Ouyang, S.; Yao, B.; Liu, Y.; Zhang, N. Amphiphilic small molecular mates match hydrophobic drugs to form nanoassemblies based on drug-mate strategy. Asian J. Pharm. Sci. 2022, 17, 129–138. [Google Scholar] [CrossRef]
- Wang, C.; Guan, W.; Peng, J.; Chen, Y.; Xu, G.; Dou, H. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020, 103, 247–258. [Google Scholar] [CrossRef]
- Kim, H.E.; Na, Y.G.; Jin, M.; Song, B.; Yun, T.S.; Hwang, Y.R.; Park, J.-S.; Lee, J.-Y.; Baek, J.-S.; Han, S.-C.; et al. Fabrication and evaluation of chitosan-coated nanostructured lipid carriers for co-delivery of paclitaxel and PD-L1 siRNA. Int. J. Pharm. 2024, 666, 124835. [Google Scholar] [CrossRef] [PubMed]
- Samson AA, S.; Park, S.; Kim, S.Y.; Min, D.H.; Jeon, N.L.; Song, J.M. Liposomal co-delivery-based quantitative evaluation of chemosensitivity enhancement in breast cancer stem cells by knockdown of GRP78/CLU. J. Liposome Res. 2019, 29, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Xiao, F.; Chen, M.; Gao, H. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy. Adv. Sci. 2022, 9, e2103836. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Li, Z.; Ren, L.; Ma, X.; Zhu, H.; Gong, Q.; Zhang, H.; Gu, Z.; Luo, K. A tumor cell membrane-coated self-amplified nanosystem as a nanovaccine to boost the therapeutic effect of anti-PD-L1 antibody. Bioact. Mater. 2023, 21, 299–312. [Google Scholar] [CrossRef]
- Jia, L.; Gao, Y.; Zhou, T.; Zhao, X.L.; Hu, H.Y.; Chen, D.W.; Qiao, M.-X. Enhanced response to PD-L1 silencing by modulation of TME via balancing glucose metabolism and robust co-delivery of siRNA/Resveratrol with dual-responsive polyplexes. Biomaterials 2021, 271, 120711. [Google Scholar] [CrossRef]
- Ripoll, M.; Pierdant, M.; Neuberg, P.; Bagnard, D.; Wagner, A.; Kichler, A.; Remy, J.-S. Co-delivery of anti-PLK-1 siRNA and camptothecin by nanometric polydiacetylenic micelles results in a synergistic cell killing. RSC Adv. 2018, 8, 20758–20763. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Venuganti, V.V. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2017, 25, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Kavya, K.V.; Vargheese, S.; Shukla, S.; Khan, I.; Dey, D.K.; Bajpai, V.K.; Thangavelu, K.; Vivek, R.; Kumar, R.R.; Han, Y.-K.; et al. A cationic amino acid polymer nanocarrier synthesized in supercritical CO2 for co-delivery of drug and gene to cervical cancer cells. Colloids Surf. B Biointerfaces 2022, 216, 112584. [Google Scholar] [CrossRef]
- Jia, F.; Li, Y.; Deng, X.; Wang, X.; Cui, X.; Lu, J.; Pan, Z.; Wu, Y. Self-assembled fluorescent hybrid nanoparticles-mediated collaborative lncRNA CCAT1 silencing and curcumin delivery for synchronous colorectal cancer theranostics. J. Nanobiotechnology 2021, 19, 238. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ellipilli, S.; Lee, W.J.; Li, X.; Vieweger, M.; Ho, Y.S.; Guo, P. Multivalent rubber-like RNA nanoparticles for targeted co-delivery of paclitaxel and MiRNA to silence the drug efflux transporter and liver cancer drug resistance. J. Control. Release 2021, 330, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, J.; Pang, T.; Zheng, H.; Liang, F.; He, X.; Tang, D.; Yu, T.; Xiong, J.; Chang, S. DNA Methylation Markers and Prediction Model for Depression and Their Contribution for Breast Cancer Risk. Front. Mol. Neurosci. 2022, 15, 845212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kennell, C.; Lee, J.Y.; Leung, Y.K.; Tarapore, P. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomedicine 2017, 13, 403–410. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Y.; Liu, Y.; Liu, Y.; Tu, J.; Shen, Y. EGFR Targeted Cetuximab-Valine-Citrulline (vc)-Doxorubicin Immunoconjugates- Loaded Bovine Serum Albumin (BSA) Nanoparticles for Colorectal Tumor Therapy. Int. J. Nanomed. 2021, 16, 2443–2459. [Google Scholar] [CrossRef]
- Rong, J.; Liu, T.; Yin, X.; Shao, M.; Zhu, K.; Li, B.; Wang, S.; Zhu, Y.; Zhang, S.; Yin, L.; et al. Co-delivery of camptothecin and MiR-145 by lipid nanoparticles for MRI-visible targeted therapy of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 247. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Xie, Y.; Lu, Z.; Sun, W.; Wang, K.; Liang, J.; Chen, X. Reactive oxygen species/glutathione dual sensitive nanoparticles with encapsulation of miR155 and curcumin for synergized cancer immunotherapy. J. Nanobiotechnol. 2024, 22, 400. [Google Scholar] [CrossRef]
- Deng, D.; Wang, L.; Chen, Y.; Li, B.; Xue, L.; Shao, N.; Wang, Q.; Xia, X.; Yang, Y.; Zhi, F. MicroRNA-124-3p regulates cell proliferation, invasion, apoptosis, and bioenergetics by targeting PIM1 in astrocytoma. Cancer Sci. 2016, 107, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Scalzone, A.; Tonda-Turo, C.; Girón-Hernández, J.; Gentile, P. Multimodal layer-by-layer nanoparticles: A breakthrough in gene and drug delivery for osteosarcoma. J. Mater. Chem. B 2024, 12, 12540–12552. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, Q.; Shu, T.; Xu, J.; Kong, J.; Mu, J.; Qiu, Y.; Zhou, X. Hepatitis C Virus NS2 Protein Suppresses RNA Interference in Cells. Virol. Sin. 2020, 35, 436–444. [Google Scholar] [CrossRef]
- Hu, Q.; Li, W.; Hu, X.; Hu, Q.; Shen, J.; Jin, X.; Zhou, J.; Tang, G.; Chu, P.K. Synergistic treatment of ovarian cancer by co-delivery of survivin shRNA and paclitaxel via supramolecular micellar assembly. Biomaterials 2012, 33, 6580–6591. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Abnous, K.; Taghdisi, S.M.; Taghavi, S.; Sh Saljooghi, A.; Ramezani, M.; Alibolandi, M. Targeted rod-shaped mesoporous silica nanoparticles for the co-delivery of camptothecin and survivin shRNA in to colon adenocarcinoma in vitro and in vivo. Eur. J. Pharm. Biopharm. 2020, 156, 84–96. [Google Scholar] [CrossRef]
- Sanati, S.; Taghavi, S.; Abnous, K.; Taghdisi, S.M.; Babaei, M.; Ramezani, M.; Alibolandi, M. Fabrication of anionic dextran-coated micelles for aptamer targeted delivery of camptothecin and survivin-shRNA to colon adenocarcinoma. Gene Ther. 2022, 29, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, R.; Dimitrov, I. Triblock Copolymer Micelles with Tunable Surface Charge as Drug Nanocarriers: Synthesis and Physico-Chemical Characterization. Nanomaterials 2022, 12, 434. [Google Scholar] [CrossRef]
- Li, L.; Liang, N.; Wang, D.; Yan, P.; Kawashima, Y.; Cui, F.; Sun, S. Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel. Int. J. Mol. Sci. 2018, 19, 3132. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Quarta, A.; Ferraro, M.M.; Dini, L.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Citti, C.; Gaballo, A.; Cannazza, G.; et al. Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery. Int. J. Mol. Sci. 2018, 19, 748. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, Z.; Zheng, D.; Li, Z.; Zhao, Z. A targeted and redox/pH-responsive chitosan oligosaccharide derivatives based nanohybrids for overcoming multidrug resistance of breast cancer cells. Carbohydr. Polym. 2021, 251, 117008. [Google Scholar] [CrossRef]
- Ma, Z.; Pi, J.; Zhang, Y.; Qin, H.; Zhang, B.; Li, N.; Li, Z.; Liu, Z. Enhanced Anticancer Efficacy of Dual Drug-Loaded Self-Assembled Nanostructured Lipid Carriers Mediated by pH-Responsive Folic Acid and Human-Derived Cell Penetrating Peptide dNP2. Pharmaceutics 2021, 13, 600. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, X.; Zhao, J.; Chen, S.; Liang, J. Cancer cell membrane-camouflaged curcumin nanoparticles trigger ferroptosis for accurate gastric cancer therapy. Eur. J. Pharm. Biopharm. 2024, 204, 114509. [Google Scholar] [CrossRef]
- An, L.; Wang, J.W.; Liu, J.D.; Zhao, Z.M.; Song, Y.J. Design, Preparation, and Characterization of Novel Calix [4]arene Bioactive Carrier for Antitumor Drug Delivery. Front. Chem. 2019, 7, 732. [Google Scholar] [CrossRef]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef] [PubMed]
- Papanota, A.M.; Karousi, P.; Kontos, C.K.; Artemaki, P.I.; Liacos, C.I.; Papadimitriou, M.A.; Bagratuni, T.; Eleutherakis-Papaiakovou, E.; Malandrakis, P.; Ntanasis-Stathopoulos, I.; et al. A Cancer-Related microRNA Signature Shows Biomarker Utility in Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 13144. [Google Scholar] [CrossRef] [PubMed]

| NP | Target Spot | Cancer | Characterization | Reference | ||
|---|---|---|---|---|---|---|
| Size (nm) | Zeta (mV) | EE (%) | ||||
| PTX | MDR1 | Ovarian cancer | 100 | - | 93 87.6 (RNA) | [111] |
| PTX | PD-L1 | Breast cancer | 181.97 | 18.66 | 99.96 | [112] |
| CPT | GRP78 clusterin | Breast cancer | 281.7 (CLU) and 242.7 (GRP78) | 48.89 (CLU) and 52.84 (GRP78) | - | [113] |
| CPT | PLK-1 | Cervical cancer and breast cancer | 60 | - | - | [117] |
| Cur | STAT3 | Skin cancer | 195.0 | 58.8 | 88.9 | [118] |
| Cur | Bcl2 | Cervical cancer | 160–180 | - | - | [119] |
| Cur | CCAT1 | Colon cancer | 180 | −10.48 | 97 | [120] |
| RES | PD-L1 | TME | 118.2 | ~10 | 8.80 (RNA) | [116] |
| NP | Target Spot | Cancer | Characterization | Reference | ||
|---|---|---|---|---|---|---|
| Size (nm) | Zeta (mV) | EE (%) | ||||
| PTX | ADAM10 | Liver cancer | 17.3 | - | - | [122] |
| PTX | p27kip1 and TIMP3 | Triple-negative breast cancer | 100 | - | - | [124] |
| CPT | HK2 and VDAC1 | Liver cancer | 160–170 | −3.5 | 85 (CPT) 81 (RNA) | [126] |
| Cur | Immunocyte | Breast cancer | 121.56 | 15.35 | 98.36 (RNA) | [127] |
| RES | Apoptosis-related gene | Osteosarcoma | 257 | 29.2 | ~85 (RNA) | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, S.; Wang, Z.; Kang, B.; Yan, H. The Co-Delivery of Natural Products and Small RNAs for Cancer Therapy: A Review. Molecules 2025, 30, 1495. https://doi.org/10.3390/molecules30071495
Wang X, Li S, Wang Z, Kang B, Yan H. The Co-Delivery of Natural Products and Small RNAs for Cancer Therapy: A Review. Molecules. 2025; 30(7):1495. https://doi.org/10.3390/molecules30071495
Chicago/Turabian StyleWang, Xuyi, Shuang Li, Zelong Wang, Baorong Kang, and Hong Yan. 2025. "The Co-Delivery of Natural Products and Small RNAs for Cancer Therapy: A Review" Molecules 30, no. 7: 1495. https://doi.org/10.3390/molecules30071495
APA StyleWang, X., Li, S., Wang, Z., Kang, B., & Yan, H. (2025). The Co-Delivery of Natural Products and Small RNAs for Cancer Therapy: A Review. Molecules, 30(7), 1495. https://doi.org/10.3390/molecules30071495







