Lactoferrin—A Regulator of Iron Homeostasis and Its Implications in Cancer
Abstract
:1. Introduction
2. Materials and Methods
3. Iron in the Human Body
3.1. Systemic Iron Turnover
3.2. Iron Turnover at the Cellular Level
4. Anemia and Iron Overload in Oncology Patients
5. Lactoferrin
5.1. Characteristics of Lactoferrin
| Biological Fluids | Concentration | References |
|---|---|---|
| Colostrum | 6–8 g/L | [19,82,83] |
| Human milk | 1–4 g/L | [84] |
| Seminal plasma | >400–1900 µg/mL | [83,85] |
| Gastric | 500–1000 µg/mL | [85] |
| Pancreatic | 500 µg/mL | [85] |
| Nasal | 100 µg/mL | [85] |
| Synoviral fluid | >10–80 μg/mL | [83,85] |
| Hepatic bile | 10–40 μg/mL | [85] |
| Saliva | 7–10 μg/mL | [83,85] |
| Vaginal secretion | 8 µg/mL | [19] |
| Urine | 1 ug/mL | [85] |
| Joint fluid | 1 µg/mL | [19] |
| Blood | 0.1–2.5 μg/mL | [85] |
5.2. Properties of Lactoferrin
5.3. Efficacy of LF in Cancer Linked to Iron Chelation Capacity
5.4. Anti-Inflammatory Properties of Lactoferrin
5.5. Bacteriostatic and Antiviral Effects of Lactoferrin
5.6. Lactoferrin Versus Iron-Driven ROS
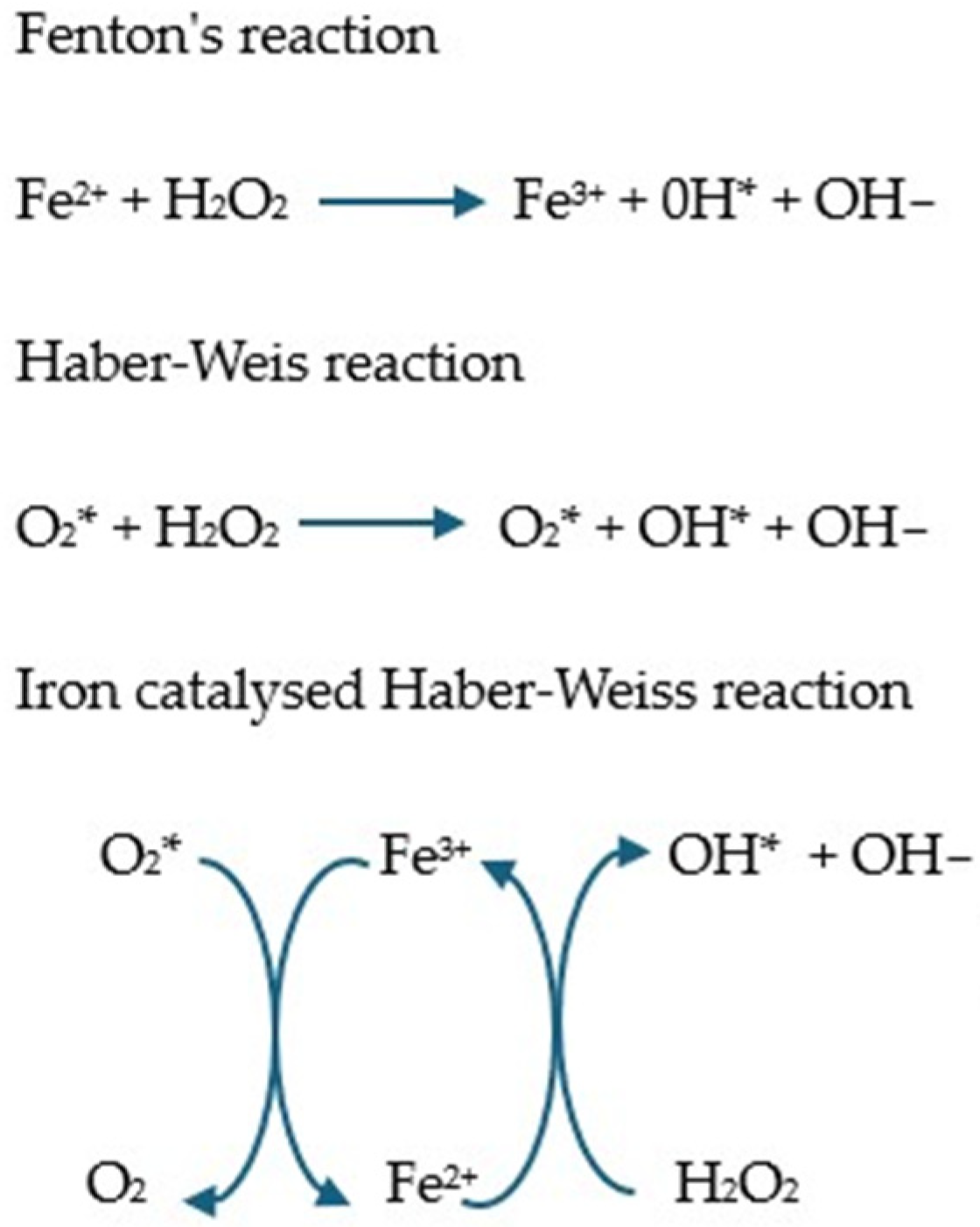
5.6.1. Iron and Its Role in ROS Generation
5.6.2. Antioxidant Properties of Lactoferrin
| Tests | Cell Lines | Lactoferrin Used in the Test | Results |
|---|---|---|---|
| Safaeian et al. [147] | Human umbilical vein endothelial cells (HUVECs) subjected to H2O2 | Sigma-Aldrich human LF (St. Louis, MO, USA) at concentrations of 6.25–100 μg/mL | - LF at concentrations of 6.25–100 μg/mL significantly increased FRAP levels in intracellular fluid and at concentrations of 12.5–100 μg/mL in extracellular fluid. - LF at concentrations of 6.25–100 μg/mL significantly decreased intracellular and extracellular hydroperoxide levels compared to the control group. |
| Hou et al. [144] | SH-SY5Y cells from the SK-N-SH neuroblastoma tumor cell line (cell model for neurodegenerative disorders) | bird’s nest (EBN) and its components, lactoferrin (LF) and ovotransferrin (OVF), induced by H2O2 | - EBN and its components attenuated H2O2-induced cytotoxicity and reduced radical oxygen species (ROS) through increased scavenging activity. - LF, OVF, and EBN induced transcriptional changes in antioxidant-related genes that tended to be neuroprotective compared to the H2O2-treated group |
| Burrow et al. [145] | HT29 colon cancer cells exposed to H2O2 | Apo-bLF and bLF 100% iron-saturated (Fe-bLF) | - Significant reduction in the activity of antioxidant enzymes (catalase, glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GsT), and superoxide dismutase (SOD)) after treatment with Apo-bLF or Fe-bLF, with or without exposure to H2O2. |
| Pan et al. [122] | Human non-neoplastic colorectal fibroblasts CCD-841-CON and CCD-18co, - human colorectal adenocarcinoma cells HT29 | rekombinacyjnie wyrażone fragmenty laktoferryny rtHLF4, rteHLF1 i rpHLF2 | In non-malignant cells, flHLF and lactoferrin variants (rtHLF4, rteHLF1, and rpHLF2) inhibited TNF-α-induced ROS generation. In cancer cells, rteHLF1 and rpHLF2 had no effect on ROS production. rtHLF4 in both TNF-α-treated fibroblast cells resulted in a decrease of more than 40% in ROS produced after treatment with 10 μM protein compared to untreated CCD-841-CON and CCD-18co fibroblast cells, and rteHLF1 and rpHLF2 reduced ROS generation by 10–20%. rtHLF4 also showed the highest suppression of ROS generation in HT29 cells after treatment with 0.1 μM protein. flHLF and rpHLF2 could suppress ROS generation to a lesser extent, while rteHLF1 showed no inhibition of ROS generation. |
| Ianiro et al. [149] | Human stellate glioma cells (U373-MG) and human neuroblastoma cells (SH-SY5Y) constitutively expressing the HIV-1 Tat viral protein (U373-Tat). | BLF native (Nat-bLF) Iron saturation~11%, and iron saturation (Holo-bLF) > 95%. (100 μg/mL) | In human cells (U373-Tat), both Nat-bLF and Holo-bLF increased the host antioxidant response by up-regulating System X c—and the cellular iron exporter Ferroportin via the erythroid nuclear factor 2 (Nrf2) pathway, thereby reducing ROS-dependent lipid peroxidation and DNA damage in astrocytes. |
| Park et al. [143] | Human mesenchymal stem cells (hMSCs) exposed to H2O2 | Lf Sigma (USA) | - Inhibition of intracellular ROS production induced by hydrogen peroxide. - Reducing hydrogen peroxide-induced apoptosis through the inhibition of caspase-3 and Akt activation. |
| Burrow et al. [148] | HT29 human colorectal epithelial cancer cell line exposed to H2O2 | 98% selenium-saturated form of bLF | - Changes in the activity of all antioxidant enzymes (glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-s-transferase (GsT), catalase and superoxide dismutase (SOD)). |
| Zhang et al. [146] | RAW264.7 macrophages stimulated with lipopolysaccharide | LF and Cu-enriched LF products: 1. 0.16 Cu mg/g LF, 2. 0.32 Cu mg/g LF, doses of 10–80 μg/mL | - LF and Cu-enriched products at doses of 10 and 20 μg/mL showed different effects on stimulated cells, by partially reducing or increasing ROS production depending on the Cu enrichment and dose levels used. - Compared to LF, Cu-enriched LF (0.16 mg Cu/g LF) at 10 μg/mL showed increased inhibition of ROS production, and the inhibition of the Cu-enriched LF product (0.32 mg Cu/g LF) at 20 μg/mL on ROS production was reduced. |
| Tests | Type of Study | Dose/Intervention Time | Test Group | Results |
|---|---|---|---|---|
| Mulder et al. 2008 [151] | Dose-response study | 1 placebo capsule for 7 days, 100 mg bovine lactoferrin (bLF) (Glycomax Lactoferrin) for 7 days, followed by 200 mg lactoferrin. Intervention time: 7 days | 8 healthy men aged 30 to 55 years | Statistically significant increases between pre-supplementation and post-200 mg supplementation levels in the hydrophilicity of the antioxidant capacity. |
| Cieślicka et al. 2022 [155] | Clinical trial | Bovine colostrum supplements (total 3.2 g; in divided doses 4 times daily) produced by AGRAPAK, Poland. Intervention time: 6 months | 20 highly trained female athletes (11 in the colostrum supplementation group/9 in the placebo group) | Compared with the placebo group, the colostrum group showed a significant decrease in thiobarbituric acid reactive substance (TBARS) levels at all time points, while a marked increase was observed for superoxide dismutase (SOD) activity. In the colostrum-supplemented group, higher hemopexin levels were observed immediately after exercise, as well as after 3 h of restitution. |
| Derosa et al. 2020 [153] | Randomized trial | Undenatured whey protein isolate (WPI; ≥92.5%) with high native cysteine content (2.7%) and standardized lactoferrin content (≥0.7%) Intervention time: 3 months. | 120 white patients with type 2 diabetes and glycosylated hemoglobin ≥6.5%. Patients received daily supplementation with (1) WPI or (2) placebo. | Markers of oxidation (SOD, glutathione peroxidase, glutathione, and the ratio of reduced glutathione to oxidized glutathione) were significantly lower in the WPI group than in the placebo group |
| Mohamed et al. 2019 [152] | Randomized trial | Group 1: Alzheimer’s patients without LF. Group 2: LF capsules (Jarrow Formulas®®, USA, 250 mg/day, LF). | 50 patients with a clinical diagnosis of probable Alzheimer’s disease (28 men and 22 women) Intervention time: 3 months | Serum antioxidant markers such as MDA, glutathione, total antioxidant capacity (TAC), and nitric oxide (NO) improved significantly after the daily administration of LF. There was a significant decrease in the expression of the PTEN, tau, and MAPK1 genes and serum amyloid A42. |
| Trentini et al. 2020 [154] | Randomized trial | Group 1: (n = 20) vaginal lactoferrin 300 mg 4 h before amniocentesis, Group 2: (n = 20) intravaginal lactoferrin 12 h before amniocentesis, Group 3: no treatment | 60 pregnant patients undergoing amniocentesis at week 16 randomized in a 1:1:1 ratio to 3 groups | Administration of lactoferrin 4 h before running decreased thiobarbituric acid reactive substances (TBARSs, as a measure of ROS) and the oxidative stress index (OSI), and it increased the total antioxidant status (TAS). Administration of lactoferrin 12 h earlier was associated with a decrease in TBARSs, but to a lesser extent. There was no statistically significant difference for OSI or TAS. |
5.7. Lactoferrin and the Microbiota
6. Inhibition of Anemia by Lactoferrin
7. Efficacy of Lactoferrin from Dietary Sources and New Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 10 November 2024).
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron Deficiency Anaemia Revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Babitt, J.L. Liver Iron Sensing and Body Iron Homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.-Z. Cellular Iron Metabolism and Regulation. Adv. Exp. Med. Biol. 2019, 1173, 21–32. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The Role of Iron in Cancer Progression. Front. Oncol. 2021, 11, 778492. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Mina, E.; Roetto, A.; Porporato, P.E. Iron: An Essential Element of Cancer Metabolism. Cells 2020, 9, 2591. [Google Scholar] [CrossRef] [PubMed]
- Forciniti, S.; Greco, L.; Grizzi, F.; Malesci, A.; Laghi, L. Iron Metabolism in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2257. [Google Scholar] [CrossRef]
- Ying, J.-F.; Lu, Z.-B.; Fu, L.-Q.; Tong, Y.; Wang, Z.; Li, W.-F.; Mou, X.-Z. The Role of Iron Homeostasis and Iron-Mediated ROS in Cancer. Am. J. Cancer Res. 2021, 11, 1895–1912. [Google Scholar]
- Basak, T.; Kanwar, R.K. Iron Imbalance in Cancer: Intersection of Deficiency and Overload. Cancer Med. 2022, 11, 3837–3853. [Google Scholar] [CrossRef]
- Henle, E.S.; Linn, S. Formation, Prevention, and Repair of DNA Damage by Iron/Hydrogen Peroxide. J. Biol. Chem. 1997, 272, 19095–19098. [Google Scholar] [CrossRef]
- Hamaï, A.; Gong, C.; Mehrpour, M. Editorial: The Role of Iron in Cancer Progression. Front. Oncol. 2022, 12, 1026420. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species—Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Pfeifhofer-Obermair, C.; Tymoszuk, P.; Petzer, V.; Weiss, G.; Nairz, M. Iron in the Tumor Microenvironment—Connecting the Dots. Front. Oncol. 2018, 8, 549. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin123. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Anaemia in Cancer Patients. Available online: https://www.termedia.pl/onkologia/Niedokrwistosc-u-chorych-na-nowotwory,40664.html (accessed on 10 November 2024).
- Yami, H.A.; Tahmoorespur, M.; Javadmanesh, A.; Tazarghi, A.; Sekhavati, M.H. The Immunomodulatory Effects of Lactoferrin and Its Derived Peptides on NF-κB Signaling Pathway: A Systematic Review and Meta-analysis. Immun. Inflamm. Dis. 2023, 11, e972. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Xu, S.; Fan, F.; Liu, H.; Cheng, S.; Tu, M.; Du, M. Novel Anticoagulant Peptide from Lactoferrin Binding Thrombin at the Active Site and Exosite-I. J. Agric. Food Chem. 2020, 68, 3132–3139. [Google Scholar] [CrossRef]
- Hayashida, K.-I.; Takeuchi, T.; Ozaki, T.; Shimizu, H.; Ando, K.; Miyamoto, A.; Harada, E. Bovine Lactoferrin Has a Nitric Oxide-Dependent Hypotensive Effect in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R359–R365. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Schaalan, M.F. Antidiabetic Efficacy of Lactoferrin in Type 2 Diabetic Pediatrics; Controlling Impact on PPAR-γ, SIRT-1, and TLR4 Downstream Signaling Pathway. Diabetol. Metab. Syndr. 2018, 10, 89. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The Role of Iron in the Pathogenesis of COVID-19 and Possible Treatment with Lactoferrin and Other Iron Chelators. Biomed. Pharmacother. 2021, 136, 111228. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-Mediated Transcytosis of Lactoferrin through the Blood-Brain Barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef]
- Naidu, S.A.G.; Wallace, T.C.; Davies, K.J.A.; Naidu, A.S. Lactoferrin for Mental Health: Neuro-Redox Regulation and Neuroprotective Effects across the Blood-Brain Barrier with Special Reference to Neuro-COVID-19. J. Diet. Suppl. 2023, 20, 218–253. [Google Scholar] [CrossRef]
- Zwirzitz, A.; Reiter, M.; Skrabana, R.; Ohradanova-Repic, A.; Majdic, O.; Gutekova, M.; Cehlar, O.; Petrovčíková, E.; Kutejova, E.; Stanek, G.; et al. Lactoferrin Is a Natural Inhibitor of Plasminogen Activation. J. Biol. Chem. 2018, 293, 8600–8613. [Google Scholar] [CrossRef]
- Wang, A.; Duncan, S.E.; Lesser, G.J.; Ray, W.K.; Dietrich, A.M. Effect of Lactoferrin on Taste and Smell Abnormalities Induced by Chemotherapy: A Proteome Analysis. Food Funct. 2018, 9, 4948–4958. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W.; MacDonald, I.A.; Zeisel, S.H. Present Knowledge in Nutrition, 10th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; ISBN 978-0-470-95917-6. [Google Scholar]
- Gattermann, N.U.; Muckenthaler, M.E.; Kulozik, A.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856. [Google Scholar] [CrossRef]
- Ems, T.; St Lucia, K.; Huecker, M.R. Biochemistry, Iron Absorption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wallace, D.F. The Regulation of Iron Absorption and Homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62. [Google Scholar]
- Patel, M.; Ramavataram, D.V.S.S. Non Transferrin Bound Iron: Nature, Manifestations and Analytical Approaches for Estimation. Indian. J. Clin. Biochem. 2012, 27, 322–332. [Google Scholar] [CrossRef]
- Kakhlon, O.; Cabantchik, Z.I. The Labile Iron Pool: Characterization, Measurement, and Participation in Cellular Processes(1). Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar] [CrossRef]
- Badran, O.; Cohen, I.; Bar-Sela, G. The Impact of Iron on Cancer-Related Immune Functions in Oncology: Molecular Mechanisms and Clinical Evidence. Cancers 2024, 16, 4156. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Senussi, N.H.; Fertrin, K.Y.; Kowdley, K.V. Iron Overload Disorders. Hepatol. Commun. 2022, 6, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Macrophages and Iron Metabolism. Microbiol. Spectr. 2016, 4, 492–504. [Google Scholar] [CrossRef]
- Camaschella, C. Iron Deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements—Iron. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 27 December 2021).
- Moustarah, F.; Mohiuddin, S.S. Dietary Iron. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Iron Absorption—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/iron-absorption (accessed on 23 July 2024).
- Crescenzi, E.; Leonardi, A.; Pacifico, F. Iron Metabolism in Cancer and Senescence: A Cellular Perspective. Biology 2023, 12, 989. [Google Scholar] [CrossRef]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef]
- Fonseca, Ó.; Ramos, A.S.; Gomes, L.T.S.; Gomes, M.S.; Moreira, A.C. New Perspectives on Circulating Ferritin: Its Role in Health and Disease. Molecules 2023, 28, 7707. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Vogt, A.-C.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Jäger, L.; Rachamin, Y.; Senn, O.; Burgstaller, J.M.; Rosemann, T.; Markun, S. Ferritin Cutoffs and Diagnosis of Iron Deficiency in Primary Care. JAMA Netw. Open 2024, 7, e2425692. [Google Scholar] [CrossRef]
- Iron Storage: Ferritin. Available online: https://chem.libretexts.org/Courses/Saint_Marys_College_Notre_Dame_IN/CHEM_342%3A_Bio-inorganic_Chemistry/Readings/Metals_in_Biological_Systems_(Saint_Mary%27s_College)/Iron_Storage%3A_Ferritin (accessed on 3 July 2024).
- Ramey, G.; Deschemin, J.-C.; Durel, B.; Canonne-Hergaux, F.; Nicolas, G.; Vaulont, S. Hepcidin Targets Ferroportin for Degradation in Hepatocytes. Haematologica 2010, 95, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and Anemia: A Tight Relationship. Front. Physiol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin and Iron in Health and Disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Dwilewicz-Trojaczek, J.; Waszczuk-Gajda, A. Chelation therapy in patients with iron overload due to frequent blood transfusions. Hematol. Clin. Pract. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and Transferrin Receptors Update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Ogun, A.S.; Adeyinka, A. Biochemistry, Transferrin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Artym, J. The role of lactoferrin in the iron metabolism. Part I. Effect of lactofferin on intake, transport and iron storage. Postep. Hig. Med. Dosw. 2008, 62, 599–612. [Google Scholar]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Tan, E.-K. Iron Regulatory Protein (IRP)-Iron Responsive Element (IRE) Signaling Pathway in Human Neurodegenerative Diseases. Mol. Neurodegener. 2017, 12, 75. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.-X.; Tang, M.; Zhang, Q.; Deng, L.; Song, C.-H.; Li, W.; Yang, M.; Shi, H.-P.; Cong, M.-H. A Comprehensive Analysis of the Association between Anemia and Systemic Inflammation in Older Patients with Cancer. Support. Care Cancer 2024, 32, 39. [Google Scholar] [CrossRef]
- Aapro, M.; Österborg, A.; Gascón, P.; Ludwig, H.; Beguin, Y. Prevalence and Management of Cancer-Related Anaemia, Iron Deficiency and the Specific Role of i.v. Iron. Ann. Oncol. 2012, 23, 1954–1962. [Google Scholar] [CrossRef]
- Bolkun, L.; Kloczko, J. Anemia in Cancer Patients. Acta Haematol. Pol. 2021, 52, 397–401. [Google Scholar] [CrossRef]
- Madeddu, C.; Gramignano, G.; Astara, G.; Demontis, R.; Sanna, E.; Atzeni, V.; Macciò, A. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front. Physiol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Natalucci, V.; Virgili, E.; Calcagnoli, F.; Valli, G.; Agostini, D.; Zeppa, S.D.; Barbieri, E.; Emili, R. Cancer Related Anemia: An Integrated Multitarget Approach and Lifestyle Interventions. Nutrients 2021, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Tałasiewicz, K.; Kapała, A. Anemia in Cancer Patients: Addressing a Neglected Issue—Diagnostics and Therapeutic Algorithm. Nowotw. J. Oncol. 2023, 73, 309–316. [Google Scholar] [CrossRef]
- De Domenico, I.; McVey Ward, D.; Kaplan, J. Regulation of Iron Acquisition and Storage: Consequences for Iron-Linked Disorders. Nat. Rev. Mol. Cell Biol. 2008, 9, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and Disorders of Iron Metabolism. Annu. Rev. Med. 2011, 62, 347–360. [Google Scholar] [CrossRef]
- Weiss, B.D.; Goodnough, L.T. Anemia of Chronic Disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Naoum, F.A. Iron Deficiency in Cancer Patients. Rev. Bras. Hematol. Hemoter. 2016, 38, 325–330. [Google Scholar] [CrossRef]
- Osborne, N.J.; Gurrin, L.C.; Allen, K.J.; Constantine, C.C.; Delatycki, M.B.; McLaren, C.E.; Gertig, D.M.; Anderson, G.J.; Southey, M.C.; Olynyk, J.K.; et al. HFE C282Y Homozygotes Are at Increased Risk of Breast and Colorectal Cancer. Hepatology 2010, 51, 1311–1318. [Google Scholar] [CrossRef]
- Youkhana, K.; Tutaeva, V.; Williams, H.; Corbali, O.; Krishnamurthy, S.; Metpally, R.; Kip, N. Assessing Cancer Risk in Patients with HFE Gene Variants and Type 1 Hereditary Hemochromatosis. Ann. Oncol. 2017, 28, v242. [Google Scholar] [CrossRef]
- Jayachandran, A.; Shrestha, R.; Bridle, K.R.; Crawford, D.H.G. Association between Hereditary Hemochromatosis and Hepatocellular Carcinoma: A Comprehensive Review. Hepatoma Res. 2020, 6, 8. [Google Scholar] [CrossRef]
- Sorensen: The Proteins in Whey. Available online: https://scholar.google.com/scholar_lookup?title=The+Proteins+in+Whey&author=Sorensen,+M.&author=Sorensen,+S.&publication_year=1939&pages=55%E2%80%9399 (accessed on 5 January 2025).
- Johanson, B.; Virtanen, A.I.; Tweit, R.C.; Dodson, R.M. Isolation of an Iron-Containing Red Protein from Human Milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Iyer, S.; Lönnerdal, B. Lactoferrin, Lactoferrin Receptors and Iron Metabolism. Eur. J. Clin. Nutr. 1993, 47, 232–241. [Google Scholar] [PubMed]
- Bluard-Deconinck, J.M.; Masson, P.L.; Osinski, P.A.; Heremans, J.F. Amino acid sequence of cysteic peptides of lactoferrin and demonstration of similarities between lactoferrin and transferrin. Biochim. Biophys. Acta (BBA) Protein Struct. 1974, 365, 311–317. [Google Scholar] [CrossRef]
- Furmanski, P.; Li, Z.P.; Fortuna, M.B.; Swamy, C.V.; Das, M.R. Multiple Molecular Forms of Human Lactoferrin. Identification of a Class of Lactoferrins That Possess Ribonuclease Activity and Lack Iron-Binding Capacity. J. Exp. Med. 1989, 170, 415–429. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. A Structural Framework for Understanding the Multifunctional Character of Lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef]
- Siebert, P.D.; Huang, B.C.B. Identification of an Alternative Form of Human Lactoferrin mRNA That Is Expressed Differentially in Normal Tissues and Tumor-Derived Cell Lines. Proc. Natl. Acad. Sci. USA 1997, 94, 2198–2203. [Google Scholar]
- Teng, C.T. Lactoferrin Gene Expression and Regulation: An Overview. Biochem. Cell Biol. 2002, 80, 7–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer Effects of Lactoferrin: Underlying Mechanisms and Future Trends in Cancer Therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Capucchio, M.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef] [PubMed]
- Steijns, J.M.; van Hooijdonk, A.C.M. Occurrence, Structure, Biochemical Properties and Technological Characteristics of Lactoferrin. Br. J. Nutr. 2000, 84, 11–17. [Google Scholar] [CrossRef]
- Cooper, C.; Maga, E.; Murray, J. Production of Human Lactoferrin and Lysozyme in the Milk of Transgenic Dairy Animals: Past, Present, and Future. Transgenic Res. 2015, 24, 605–614. [Google Scholar] [CrossRef]
- Lactoferrin in Relation to Biological Functions and Applications: A Review. Available online: https://scialert.net/fulltext/?doi=ijds.2011.79.111 (accessed on 22 May 2022).
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A Natural Antimicrobial Protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional Roles of Lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.; Colavizza, D.; Benaissa, M.; Maes, P.; Tartar, A.; Montreuil, J.; Spik, G. Molecular Cloning and Sequence Analysis of Bovine Lactotransferrin. Eur. J. Biochem. 1991, 196, 177–184. [Google Scholar] [CrossRef]
- Mao, R.; Ma, X.; Hao, Y.; Pen, G.; Zheng, X.; Yang, N.; Teng, D.; Wang, J. Perspective: A Proposal on Solutions of Modern Supply Chain Construction for Lactoferrin. J. Dairy Sci. 2023, 106, 7329–7335. [Google Scholar] [CrossRef]
- Bonaccorsi di Patti, M.C.; Cutone, A.; Polticelli, F.; Rosa, L.; Lepanto, M.S.; Valenti, P.; Musci, G. The Ferroportin-Ceruloplasmin System and the Mammalian Iron Homeostasis Machine: Regulatory Pathways and the Role of Lactoferrin. Biometals 2018, 31, 399–414. [Google Scholar] [CrossRef]
- Baker, E.N. Structure and Reactivity of Transferrins. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1994; Volume 41, pp. 389–463. [Google Scholar]
- Zhao, X.; Kruzel, M.; Aronowski, J. Lactoferrin and Hematoma Detoxification after Intracerebral Hemorrhage. Biochem. Cell Biol. 2021, 99, 97–101. [Google Scholar] [CrossRef]
- Mancinelli, R.; Rosa, L.; Cutone, A.; Lepanto, M.S.; Franchitto, A.; Onori, P.; Gaudio, E.; Valenti, P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules 2020, 25, 1997. [Google Scholar] [CrossRef]
- Narmuratova, Z.; Hentati, F.; Girardet, J.-M.; Narmuratova, M.; Cakir-Kiefer, C. Equine Lactoferrin: Antioxidant Properties Related to Divalent Metal Chelation. LWT 2022, 161, 113426. [Google Scholar] [CrossRef]
- Artym, J. Lactoferrin—A Sensor and Regulator of Iron Absorption. Postep. Biol. Komorki 2015, 42, 283–308. [Google Scholar]
- Du, M.; Liu, M.; Fan, F.; Shi, P.; Tu, M. Structure, Function, and Nutrition of Lactoferrin. In Mineral Containing Proteins; Springer: Berlin/Heidelberg, Germany, 2017; pp. 33–61. [Google Scholar] [CrossRef]
- Hu, F.; Pan, F.; Sawano, Y.; Makino, T.; Kakehi, Y.; Komiyama, M.; Kawakami, H.; Tanokura, M. Studies of the Structure of Multiferric Ion-Bound Lactoferrin: A New Antianemic Edible Material. Int. Dairy J. 2008, 18, 1051–1056. [Google Scholar] [CrossRef]
- Fu, J.; Yang, L.; Tan, D.; Liu, L. Iron Transport Mechanism of Lactoferrin and Its Application in Food Processing. Food Sci. Technol. 2023, 43, e121122. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Mild Thermal Treatment and In-Vitro Digestion of Three Forms of Bovine Lactoferrin: Effects on Functional Properties. Int. Dairy J. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. A Structural Perspective on Lactoferrin Function. Biochem. Cell Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F. Iron Homeostasis and Tumorigenesis: Molecular Mechanisms and Therapeutic Opportunities. Protein Cell 2015, 6, 88–100. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Zou, X.; Wu, W.; Di, Y.; Li, N.; Fu, A. Iron Promotes Ovarian Cancer Malignancy and Advances Platinum Resistance by Enhancing DNA Repair via FTH1/FTL/POLQ/RAD51 Axis. Cell Death Dis. 2024, 15, 329. [Google Scholar] [CrossRef]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and Cancer: Recent Insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Bonaccorsi di Patti, M.C.; Iacovelli, F.; Conte, M.P.; Ianiro, G.; Romeo, A.; Campione, E.; Bianchi, L.; Valenti, P.; et al. Lactoferrin Binding to SARS-CoV-2 Spike Glycoprotein Blocks Pseudoviral Entry and Relieves Iron Protein Dysregulation in Several In Vitro Models. Pharmaceutics 2022, 14, 2111. [Google Scholar] [CrossRef] [PubMed]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.B.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin Differently Modulates the Inflammatory Response in Epithelial Models Mimicking Human Inflammatory and Infectious Diseases. Biometals 2014, 27, 843–856. [Google Scholar] [CrossRef]
- Guillen, C.; McInnes, I.; Kruger, H.; Brock, J. Iron, Lactoferrin and Iron Regulatory Protein Activity in the Synovium; Relative Importance of Iron Loading and the Inflammatory Response. Ann. Rheum. Dis. 1998, 57, 309–314. [Google Scholar] [PubMed]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin Efficiently Counteracts the Inflammation-Induced Changes of the Iron Homeostasis System in Macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef]
- Guschina, T.A.; Soboleva, S.E.; Nevinsky, G.A. Recognition of Specific and Nonspecific DNA by Human Lactoferrin. J. Mol. Recognit. 2013, 26, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar]
- Fujisawa, K.; Takami, T.; Matsumoto, T.; Yamamoto, N.; Yamasaki, T.; Sakaida, I. An Iron Chelation-Based Combinatorial Anticancer Therapy Comprising Deferoxamine and a Lactate Excretion Inhibitor Inhibits the Proliferation of Cancer Cells. Cancer Metab. 2022, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron Homeostasis and Ferroptosis in Human Diseases: Mechanisms and Therapeutic Prospects. Sig Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Simonart, T.; Boelaert, J.R.; Mosselmans, R.; Andrei, G.; Noel, J.-C.; De Clercq, E.; Snoeck, R. Antiproliferative and Apoptotic Effects of Iron Chelators on Human Cervical Carcinoma Cells. Gynecol. Oncol. 2002, 85, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Niro, A.; Rosa, L.; Valenti, P.; Musci, G.; Cutone, A. To Boost or to Reset: The Role of Lactoferrin in Energy Metabolism. Int. J. Mol. Sci. 2023, 24, 15925. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory Effects of Lactoferrin on Antigen Presenting Cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.F.; Zubair, D.; Bashir, M.N.; Alagawany, M.; Ahmed, S.; Shah, Q.A.; Buzdar, J.A.; Arain, M.A. Nutraceutical and Health-Promoting Potential of Lactoferrin, an Iron-Binding Protein in Human and Animal: Current Knowledge. Biol. Trace Elem. Res. 2023, 202, 56–72. [Google Scholar] [CrossRef]
- Legrand, D.; Pierce, A.; Elass, E.; Carpentier, M.; Mariller, C.; Mazurier, J. Lactoferrin Structure and Functions. In Bioactive Components of Milk; Bösze, Z., Ed.; Springer: New York, NY, USA, 2008; pp. 163–194. ISBN 978-0-387-74087-4. [Google Scholar]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Sorimachi, K.; Akimoto, K.; Hattori, Y.; Ieiri, T.; Niwa, A. Activation of Macrophages by Lactoferrin: Secretion of TNF-Alpha, IL-8 and NO. Biochem. Mol. Biol. Int. 1997, 43, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Z.; Wang, Y.; Zhang, L.; Chua, N.; Dai, L.; Chen, J.; Ho, C.L. Evaluation of the Anti-Inflammatory and Anti-Oxidative Effects of Therapeutic Human Lactoferrin Fragments. Front. Bioeng. Biotechnol. 2021, 9, 779018. [Google Scholar] [CrossRef]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S. Bovine Lactoferrin Counteracts Toll-like Receptor Mediated Activation Signals in Antigen Presenting Cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Jugert, C.-S.; Didier, A.; Plötz, M.; Jessberger, N. Strain-specific Antimicrobial Activity of Lactoferrin-based Food Supplements. J. Food Prot. 2023, 86, 100153. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Ertürk, M.; Karav, S. The Potential of Lactoferrin as Antiviral and Immune-Modulating Agent in Viral Infectious Diseases. Front. Immunol. 2024, 15, 1402135. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Virmani, R.; Finn, A.V. New Insights into the Role of Iron in Inflammation and Atherosclerosis. eBioMedicine 2019, 47, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Arora, G.; Dickson, K.; Lehmann, C. Iron Chelation in Local Infection. Molecules 2021, 26, 189. [Google Scholar] [CrossRef]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food. Molecules 2022, 25, 5451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muhoberac, B.B. What Can Cellular Redox, Iron, and Reactive Oxygen Species Suggest About the Mechanisms and Potential Therapy of COVID-19? Front. Cell Infect. Microbiol. 2020, 10, 569709. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. On the Irrelevancy of Hydroxyl Radical to DNA Damage from Oxidative Stress and Implications for Epigenetics. Chem. Soc. Rev. 2020, 49, 6524–6528. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M.A. On the Relevance of Hydroxyl Radical to Purine DNA Damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Barbusiński, K. Fenton reaction—Controversy concerning the chemistry. Ecol. Chem. Eng. 2009, 16, 347–358. [Google Scholar]
- Fibach, E.; Rachmilewitz, E.A. The Role of Antioxidants and Iron Chelators in the Treatment of Oxidative Stress in Thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Cabantchik, Z.I. Labile Iron in Cells and Body Fluids: Physiology, Pathology, and Pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef]
- Zeidan, R.S.; Han, S.M.; Leeuwenburgh, C.; Xiao, R. Iron Homeostasis and Organismal Aging. Ageing Res. Rev. 2021, 72, 101510. [Google Scholar] [CrossRef]
- Ginzburg, Y.Z. Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019, 110, 17. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Rascón-Cruz, Q.; Siqueiros-Cendón, T.S.; Siañez-Estrada, L.I.; Villaseñor-Rivera, C.M.; Ángel-Lerma, L.E.; Olivas-Espino, J.A.; León-Flores, D.B.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview. Int. J. Mol. Sci. 2025, 26, 125. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, A.-J.; Kim, G.-Y.; Jo, A.; Lee, J.E.; Leem, S.-H.; Yoon, J.-H.; Ye, S.K.; Chung, J.W. Lactoferrin Protects Human Mesenchymal Stem Cells from Oxidative Stress-Induced Senescence and Apoptosis. J. Microbiol. Biotechnol. 2017, 27, 1877–1884. [Google Scholar] [CrossRef]
- Hou, Z.; Imam, M.U.; Ismail, M.; Azmi, N.H.; Ismail, N.; Ideris, A.; Mahmud, R. Lactoferrin and Ovotransferrin Contribute toward Antioxidative Effects of Edible Bird’s Nest against Hydrogen Peroxide-Induced Oxidative Stress in Human SH-SY5Y Cells. Biosci. Biotechnol. Biochem. 2015, 79, 1570–1578. [Google Scholar] [CrossRef]
- Burrow, H.; Kanwar, R.K.; Kanwar, J.R. Antioxidant Enzyme Activities of Iron-Saturated Bovine Lactoferrin (Fe-bLf) in Human Gut Epithelial Cells under Oxidative Stress. Med. Chem. 2011, 7, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, H.-J.; Huang, L.-Y.; Song, C.-L.; Li, H.-Q.; Zhao, X.-H. Low-Level Cu-Fortification of Bovine Lactoferrin: Focus on Its Effect on in Vitro Anti-Inflammatory Activity in LPS-Stimulated Macrophages. Curr. Res. Food Sci. 2023, 6, 100520. [Google Scholar] [CrossRef]
- Safaeian, L.; Javanmard, S.H.; Mollanoori, Y.; Dana, N. Cytoprotective and Antioxidant Effects of Human Lactoferrin against H2O2-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Adv. Biomed. Res. 2015, 4, 188. [Google Scholar] [CrossRef]
- Burrow, H.; Kanwar, R.K.; Mahidhara, G.; Kanwar, J.R. Effect of Selenium-Saturated Bovine Lactoferrin (Se-bLF) on Antioxidant Enzyme Activities in Human Gut Epithelial Cells under Oxidative Stress. Anti-Cancer Agents Med. Chem. 2011, 11, 762–771. [Google Scholar] [CrossRef]
- Ianiro, G.; D’Ezio, V.; Carpinelli, L.; Casella, C.; Bonaccorsi di Patti, M.C.; Rosa, L.; Valenti, P.; Colasanti, M.; Musci, G.; Cutone, A.; et al. Iron Saturation Drives Lactoferrin Effects on Oxidative Stress and Neurotoxicity Induced by HIV-1 Tat. Int. J. Mol. Sci. 2023, 24, 7947. [Google Scholar] [CrossRef]
- Ginet, V.; van de Looij, Y.; Petrenko, V.; Toulotte, A.; Kiss, J.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during Lactation Reduces Lipopolysaccharide-Induced Brain Injury. BioFactors 2016, 42, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine Lactoferrin Supplementation Supports Immune and Antioxidant Status in Healthy Human Males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.; Salama, R.M.; Schaalan, M.F. A Pilot Study on the Effect of Lactoferrin on Alzheimer’s Disease Pathological Sequelae: Impact of the p-Akt/PTEN Pathway. Biomed. Pharmacother. 2019, 111, 714–723. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. Change of Some Oxidative Stress Parameters after Supplementation with Whey Protein Isolate in Patients with Type 2 Diabetes. Nutrition 2020, 73, 110700. [Google Scholar] [CrossRef]
- Trentini, A.; Maritati, M.; Rosta, V.; Cervellati, C.; Manfrinato, M.C.; Hanau, S.; Greco, P.; Bonaccorsi, G.; Bellini, T.; Contini, C. Vaginal Lactoferrin Administration Decreases Oxidative Stress in the Amniotic Fluid of Pregnant Women: An Open-Label Randomized Pilot Study. Front. Med. 2020, 7, 555. [Google Scholar] [CrossRef]
- Cieślicka, M.; Ostapiuk-Karolczuk, J.; Buttar, H.S.; Dziewiecka, H.; Kasperska, A.; Skarpańska-Stejnborn, A. Effects of Long-Term Supplementation of Bovine Colostrum on Iron Homeostasis, Oxidative Stress, and Inflammation in Female Athletes: A Placebo-Controlled Clinical Trial. Nutrients 2022, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.O.; Ochoa-Repáraz, J. The Gut Microbiome in Multiple Sclerosis: A Potential Therapeutic Avenue. Med. Sci. 2018, 6, 69. [Google Scholar] [CrossRef]
- Artemev, A.; Naik, S.; Pougno, A.; Honnavar, P.; Shanbhag, N.M. The Association of Microbiome Dysbiosis With Colorectal Cancer. Cureus 2022, 14, e22156. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron Fortification Adversely Affects the Gut Microbiome, Increases Pathogen Abundance and Induces Intestinal Inflammation in Kenyan Infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Simonyté Sjödin, K.; Domellöf, M.; Lagerqvist, C.; Hernell, O.; Lönnerdal, B.; Szymlek-Gay, E.A.; Sjödin, A.; West, C.E.; Lind, T. Administration of Ferrous Sulfate Drops Has Significant Effects on the Gut Microbiota of Iron-Sufficient Infants: A Randomised Controlled Study. Gut 2019, 68, 2095–2097. [Google Scholar] [CrossRef]
- Balamurugan, R.; Mary, R.R.; Chittaranjan, S.; Jancy, H.; Shobana Devi, R.; Ramakrishna, B.S. Low Levels of Faecal Lactobacilli in Women with Iron-Deficiency Anaemia in South India. Br. J. Nutr. 2010, 104, 931–934. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Cleary, T.G. Effect of Lactoferrin on Enteric Pathogens. Biochimie 2009, 91, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-S.; Chen, P.-W. Featured Prebiotic Agent: The Roles and Mechanisms of Direct and Indirect Prebiotic Activities of Lactoferrin and Its Application in Disease Control. Nutrients 2023, 15, 2759. [Google Scholar] [CrossRef]
- Chen, P.-W.; Liu, Z.-S.; Kuo, T.-C.; Hsieh, M.-C.; Li, Z.-W. Prebiotic Effects of Bovine Lactoferrin on Specific Probiotic Bacteria. Biometals 2017, 30, 237–248. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Prebiotic and Modulatory Evidence of Lactoferrin on Gut Health and Function. J. Funct. Food 2023, 108, 105741. [Google Scholar] [CrossRef]
- Berding, K.; Wang, M.; Monaco, M.H.; Alexander, L.S.; Mudd, A.T.; Chichlowski, M.; Waworuntu, R.V.; Berg, B.M.; Miller, M.J.; Dilger, R.N.; et al. Prebiotics and Bioactive Milk Fractions Affect Gut Development, Microbiota, and Neurotransmitter Expression in Piglets. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 688–697. [Google Scholar] [CrossRef]
- Pammi, M.; Preidis, G.A.; Tarnow-Mordi, W.O. Evidence from Systematic Reviews of Randomized Trials on Enteral Lactoferrin Supplementation in Preterm Neonates. Biochem. Cell Biol. 2021, 99, 20–24. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-Pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, G.; Chen, H.; Cao, Y.; Dong, X.; Li, H.; Liu, C. Dose Effect of Bovine Lactoferrin Fortification on Iron Metabolism of Anemic Infants. J. Nutr. Sci. Vitaminol. 2020, 66, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral Administration of Lactoferrin Increases Hemoglobin and Total Serum Iron in Pregnant Women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.M.; Assem, H.; Ahmed, D.; Abd Elmaksoud, M.S. Lactoferrin versus Iron Hydroxide Polymaltose Complex for the Treatment of Iron Deficiency Anemia in Children with Cerebral Palsy: A Randomized Controlled Trial. Eur. J. Pediatr. 2021, 180, 2609–2618. [Google Scholar] [CrossRef]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Siciliano, R.A.; Paesano, R.; Costi, R.; Musci, G.; Valenti, P. Influence of Oral Administration Mode on the Efficacy of Commercial Bovine Lactoferrin against Iron and Inflammatory Homeostasis Disorders. Biometals 2020, 33, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and Efficacy of Lactoferrin versus Ferrous Sulphate in Curing Iron Deficiency and Iron Deficiency Anaemia in Hereditary Thrombophilia Pregnant Women: An Interventional Study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Elsawy, A.; Elsheikh, M.; Donia, A.; Nassar, M. Lactoferrin for Iron-Deficiency Anemia in Children with Inflammatory Bowel Disease: A Clinical Trial. Pediatr. Res. 2022, 92, 762–766. [Google Scholar] [CrossRef]
- Mahmoud, R.M.A.; Mohammed, A. Lactoferrin: A Promising New Player in Treatment of Iron Deficiency Anemia in Patients on Regular Hemodialysis: A Randomized Controlled Trial. Saudi J. Kidney Dis. Transpl. 2023, 34, 235–241. [Google Scholar] [CrossRef]
- Nappi, C.; Tommaselli, G.A.; Morra, I.; Massaro, M.; Formisano, C.; Di Carlo, C. Efficacy and Tolerability of Oral Bovine Lactoferrin Compared to Ferrous Sulfate in Pregnant Women with Iron Deficiency Anemia: A Prospective Controlled Randomized Study. Acta Obs. Gynecol. Scand. 2009, 88, 1031–1035. [Google Scholar] [CrossRef]
- Christofi, M.-D.; Giannakou, K.; Mpouzika, M.; Merkouris, A.; Stylianide, M.V.; Charalambous, A. The Effectiveness of Oral Bovine Lactoferrin Compared to Iron Supplementation in Patients with a Low Hemoglobin Profile: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. BMC Nutr. 2024, 10, 20. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Pantanella, F.; Pacifici, E.; Goolsbee, W.; Valenti, P. Lactoferrin Efficacy versus Ferrous Sulfate in Curing Iron Deficiency and Iron Deficiency Anemia in Pregnant Women. Biometals 2010, 23, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Xu, T.; Luo, J.; Luo, Y.; An, P. Comparative Effects between Oral Lactoferrin and Ferrous Sulfate Supplementation on Iron-Deficiency Anemia: A Comprehensive Review and Meta-Analysis of Clinical Trials. Nutrients 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Bovine Lactoferrin. EFSA J. 2012, 10, 2811. [Google Scholar] [CrossRef]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of Intestinal Polyp Growth by Oral Ingestion of Bovine Lactoferrin and Immune Cells in the Large Intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef]
- Takeuchi, T.; Jyonotsuka, T.; Kamemori, N.; Kawano, G.; Shimizu, H.; Ando, K.; Harada, E. Enteric-Formulated Lactoferrin Was More Effectively Transported into Blood Circulation from Gastrointestinal Tract in Adult Rats. Exp. Physiol. 2006, 91, 1033–1040. [Google Scholar] [CrossRef]
- Kawakami, H.; Park, H.; Park, S.; Kuwata, H.; Shephard, R.J.; Aoyagi, Y. Effects of Enteric-Coated Lactoferrin Supplementation on the Immune Function of Elderly Individuals: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. Dairy J. 2015, 47, 79–85. [Google Scholar] [CrossRef]
- Ong, R.; Cornish, J.; Wen, J. Nanoparticular and Other Carriers to Deliver Lactoferrin for Antimicrobial, Antibiofilm and Bone-Regenerating Effects: A Review. Biometals 2023, 36, 709–727. [Google Scholar] [CrossRef]
- Nangare, S.; Ramraje, G.; Patil, P. Formulation of lactoferrin decorated dextran based chitosan-coated europium metal-organic framework for targeted delivery of curcumin. Int. J. Biol. Macromol. 2024, 259, 129325. [Google Scholar] [CrossRef]
- Guzmán-Mejía, F.; Godínez-Victoria, M.; Molotla-Torres, D.E.; Drago-Serrano, M.E. Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus. Pharmaceuticals 2023, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneem, M.A.; Elwakil, M.M.A.; Khattab, S.N.; Helmy, M.W.; Bekhit, A.A.; Abdulkader, M.A.; Zaky, A.; Teleb, M.; Elkhodairy, K.A.; Albericio, F.; et al. Lactoferrin-dual drug nanoconjugate: Synergistic anti-tumor efficacy of docetaxel and the NF-κB inhibitor celastrol. Mater. Sci. Eng. C 2021, 118, 111422. [Google Scholar] [CrossRef]

| Author | Test/Intervention Time | Dose | Study Group | Results |
|---|---|---|---|---|
| Lepanto et al. 2018 [168] | Study period: 30 days | Women included in Arm A, C, E, and G bLF oral 100 mg 20–30% iron-saturated (70–84 μg elemental iron) 2× daily, women included in Arm B, D, F, and H standard Italian therapy—oral 329.7 mg ferrous sulfate 1× daily (105 mg elemental iron). | - 20 pregnant and 9 not pregnant women, anemia and β-thalassaemia; 70 pregnant and 79 not pregnant with hereditary thrombophilia (HT) affected by anemia of inflammation (AI). - 20 pregnant women with anemia suffering from various pathologies. | In anemic pregnant and nonpregnant women with minor β-thalassemia, bLF decreased IL-6 and increased total serum iron (TSI) concentrations. bLF was more effective than ferrous sulfate in the treatment of AI in pregnant and nonpregnant women with HT, reducing both serum IL-6 and hepcidin, increasing hematological parameters such as red blood cell counts (RBCs), hemoglobin (Hb), TSI and serum ferritin. bLF has been effective in treating anemia in other pathological pregnancies. |
| Mahmoud et al. 2023 [175] | Study period: 6 months | 1. 100 mg 20–30% iron-saturated bLF (70–84 μg iron) 2× daily 2. 576 mg iron glycine sulfate (100 mg iron) 2× daily | 70 bLF/70 iron glycine sulfate patients on regular hemodialysis with iron deficiency anemia. | bLF significantly reduced serum hepcidin levels and significantly increased Hb concentration and transferrin saturation (TSAT), significantly more strongly than iron (II) glycine sulfate. Iron (II) glycine sulfate significantly decreased serum hepcidin levels and significantly increased Hb and TSAT. |
| El Amrousy et al. 2022 [174] | Study period: 3 months | 1. Ferrous sulfate 6 mg/kg/day 2. LF 100 mg/day | 80 children with inflammatory bowel disease (IBD) and iron deficiency anemia (IDA), iron sulfate group (n = 40), lactoferrin group (n = 40). | Hb, mean blood cell volume, serum iron, transferrin saturation, and serum ferritin significantly increased, while total iron binding capacity (TIBC) significantly decreased after ferrous sulfate or LF administration compared with the baseline data. LF significantly increased Hb, serum iron, TS, and serum ferritin compared to ferrous sulphate. LF significantly decreased IL-6 and hepcidin levels. |
| Paesano et al. 2010 [178] | Study period: 30–90 days | 1. bLF 100 mg/day saturated with iron about 30% 2. Ferrous sulfate 520 mg/day 3. Control group | 71 pregnant/189 non-pregnant women with iron deficiency and iron deficiency anemia Arm A: with bLF (30/90), Arm B: with ferrous sulfate (30/90), Arm C: control group (11/9). | In pregnant women, bLF decreased serum IL-6 levels and increased prohepcidin. In non-pregnant women, bLF did not alter low levels of IL-6, while it increased prohepcidin. Ferrous sulfate increased IL-6 levels and decreased prohepcidin. |
| Paesano et al. [173] | Study period: from inclusion to delivery | 1. Orally 100 mg bLF 2× daily 2. 520 mg ferrous sulfate 1× daily | 295 pregnant women with hereditary HT thrombophilia (≥18 years) suffering from anemia (156 women)/iron deficiency anemia (139 women). | Red blood cells, Hb, total serum iron, and serum ferritin were significantly higher in the bLF-treated group than in the iron sulfate group. Serum IL-6 levels decreased significantly in women treated with bLF and increased in women treated with iron sulfate, bLF had no adverse effects. Adverse effects were reported in 16.5% of women treated with ferrous sulfate. |
| Nappi et al. 2009 [176] | Study period: 30 days | 1. 100 mg bLF 2× daily (group A; n = 49) 2. 520 mg ferrous sulfate 1× daily (group B; n = 48) | 97 pregnant women with iron deficiency anemia, bLF (n = 49), ferrous sulfate (n = 48). | In both groups, Hb, serum ferritin, and iron were significantly increased, while TIBC was significantly decreased compared to baseline values. No significant differences were observed between groups. The median scores for abdominal pain and constipation were significantly higher in patients treated with ferrous sulfate compared to patients treated with bLF. |
| Rosa et al. 2020 [172] | Study period: 30 days | bLF (100 mg 2× daily) orally 1. Before or 2. during meals | Pregnant women with hereditary thrombophilia and inflammatory anemia. | A significant increase in RBCs, Hb, TSI, and serum ferritin levels, together with a significant decrease in interleukin-6, was detected in group A, but not in group B. |
| Chen et al. 2020 [169] | Intervention time: 3 months | Milk mixture enriched with bLF: group 1 with bLF concentration of 38 mg/100 g, group 2 with 76 mg/100 g bLF, and group 0 without bLF | 108 infants born at term, aged 6–9 months. | Formula enriched with 76 mg/100 g bLF significantly increased infants’ Hb levels compared to infants in the other two groups |
| Paesano et al. 2006 [170] | Study period: 30 days | 1. Oral1 administration of ferrous sulfate (520 mg once daily) 2. 30% iron-saturated bovine lactoferrin (bLF) (100 mg twice daily) 3. Control group without treatment | 300 women in various trimesters of pregnancy. | In women without treatment, Hb and total serum iron values decreased significantly, especially in women 18–31 weeks of pregnancy. After 30 days of oral administration of bLF, Hb and total serum iron values increased and to a greater extent than observed in women treated with ferrous sulfate, irrespective of the trimester of pregnancy. In contrast to ferrous sulfate, bLF did not cause any side effects. |
| Omar et al. 2021 [171] | Intervention time: 4 weeks | 1. Iron hydroxide-polymaltose complex IPC 6 mg/kg/day of elemental iron in 2 divided doses 2. LF (30% iron-saturated) | 66 children aged 1–10 years with cerebral palsy and iron deficiency anemia, 1. 32 children randomly received IPC, 2. 34 received LF 200 mg per day. | In both the IPC and LF groups, significant improvements in Hb, ferritin and serum iron, total iron binding capacity, mean red cell volume, and mean red cell hemoglobin compared to baseline values were observed. Adjusted mean changes in Hb and SF in the LF group were significantly higher than in the IPC group. Constipation was less frequent in the LF group than in the IPC group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolesławska, I.; Bolesławska-Król, N.; Jakubowski, K.; Przysławski, J.; Drzymała-Czyż, S. Lactoferrin—A Regulator of Iron Homeostasis and Its Implications in Cancer. Molecules 2025, 30, 1507. https://doi.org/10.3390/molecules30071507
Bolesławska I, Bolesławska-Król N, Jakubowski K, Przysławski J, Drzymała-Czyż S. Lactoferrin—A Regulator of Iron Homeostasis and Its Implications in Cancer. Molecules. 2025; 30(7):1507. https://doi.org/10.3390/molecules30071507
Chicago/Turabian StyleBolesławska, Izabela, Natasza Bolesławska-Król, Karol Jakubowski, Juliusz Przysławski, and Sławomira Drzymała-Czyż. 2025. "Lactoferrin—A Regulator of Iron Homeostasis and Its Implications in Cancer" Molecules 30, no. 7: 1507. https://doi.org/10.3390/molecules30071507
APA StyleBolesławska, I., Bolesławska-Król, N., Jakubowski, K., Przysławski, J., & Drzymała-Czyż, S. (2025). Lactoferrin—A Regulator of Iron Homeostasis and Its Implications in Cancer. Molecules, 30(7), 1507. https://doi.org/10.3390/molecules30071507








