Preparation and Modification of Sucrose-Based Non-Isocyanate Polyurethane Adhesives for Plywood Bonding
Abstract
1. Introduction
2. Results and Discussions
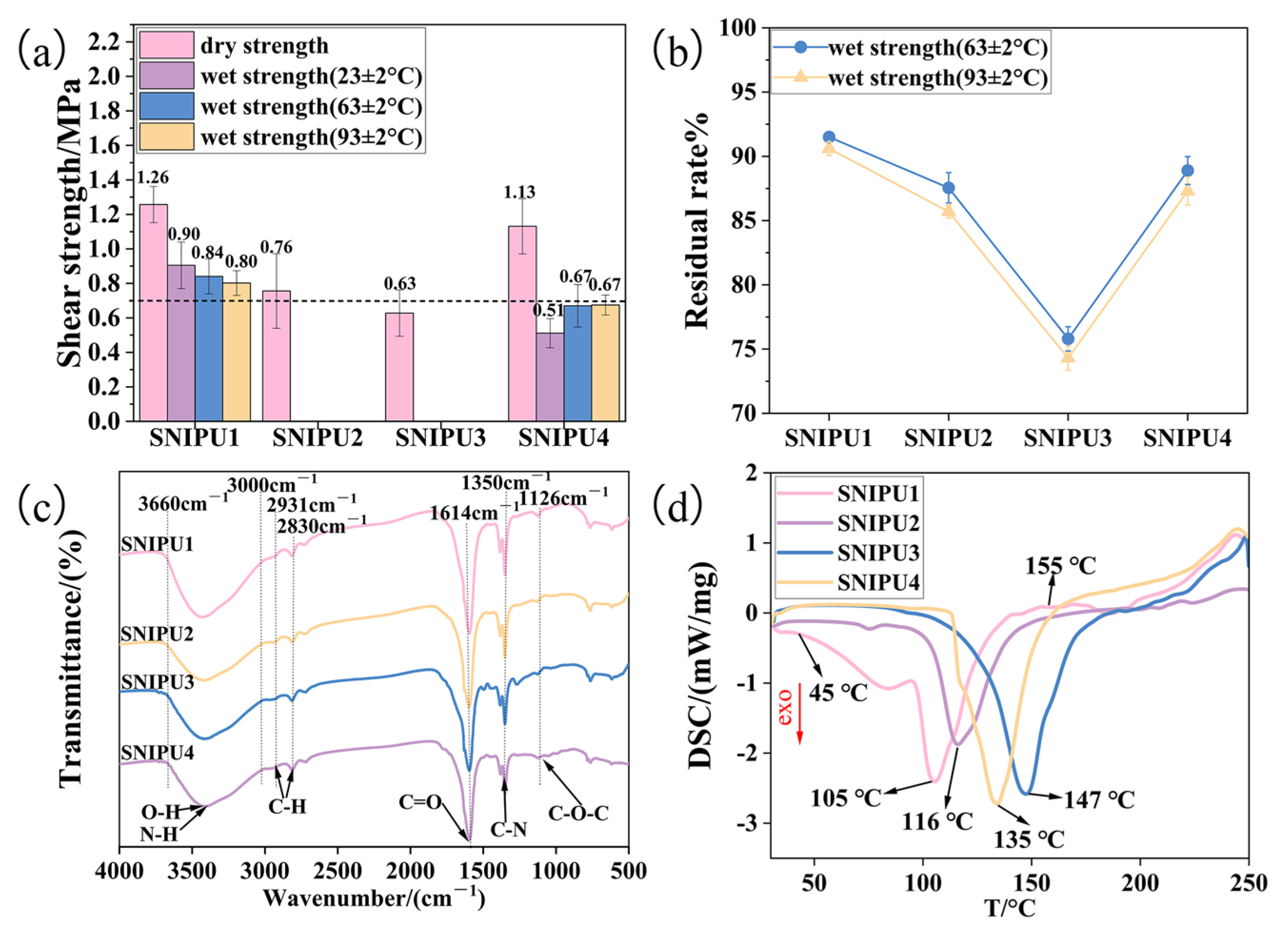
2.1. Effect of Polyamine Type on SNIPU Adhesives
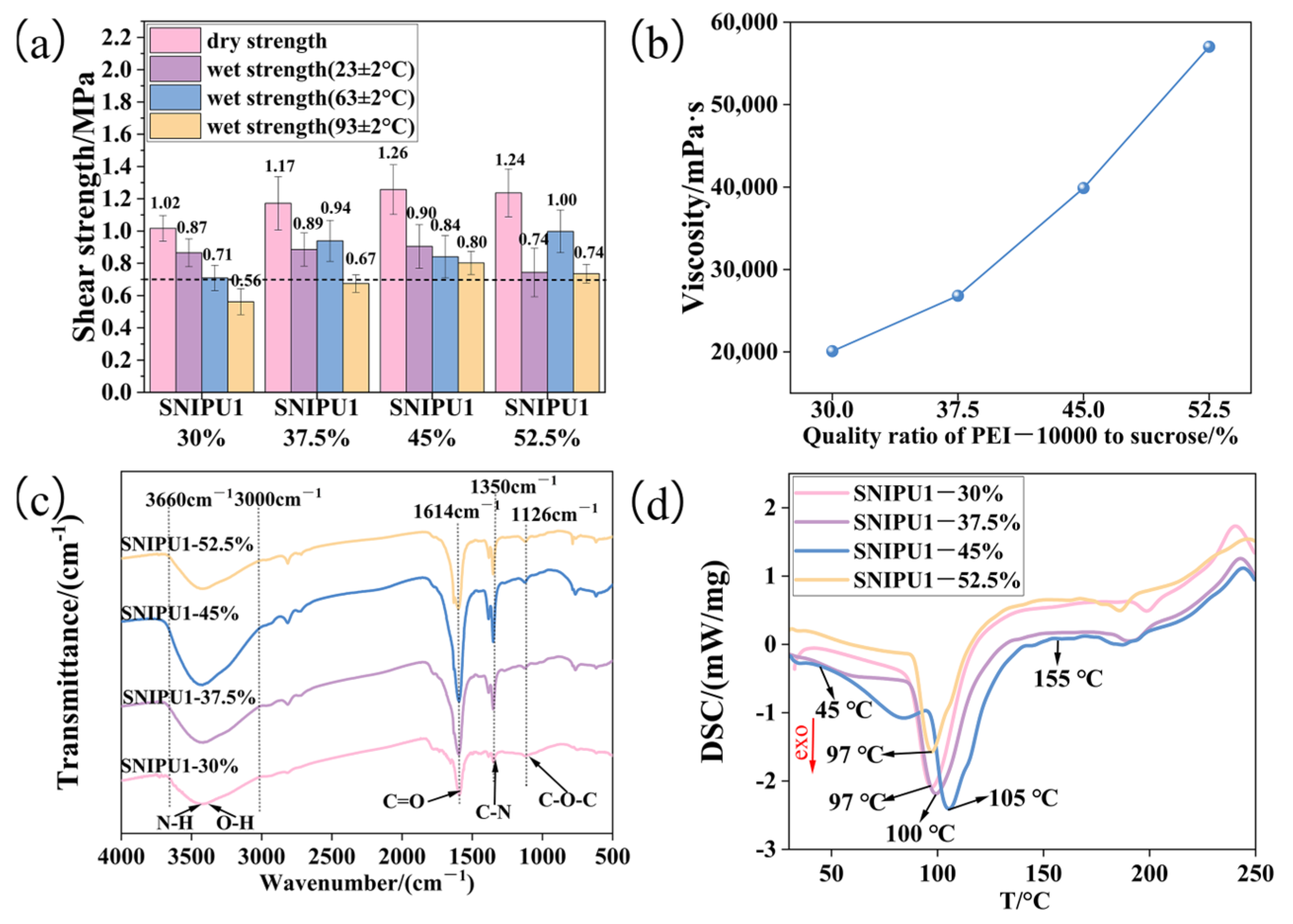
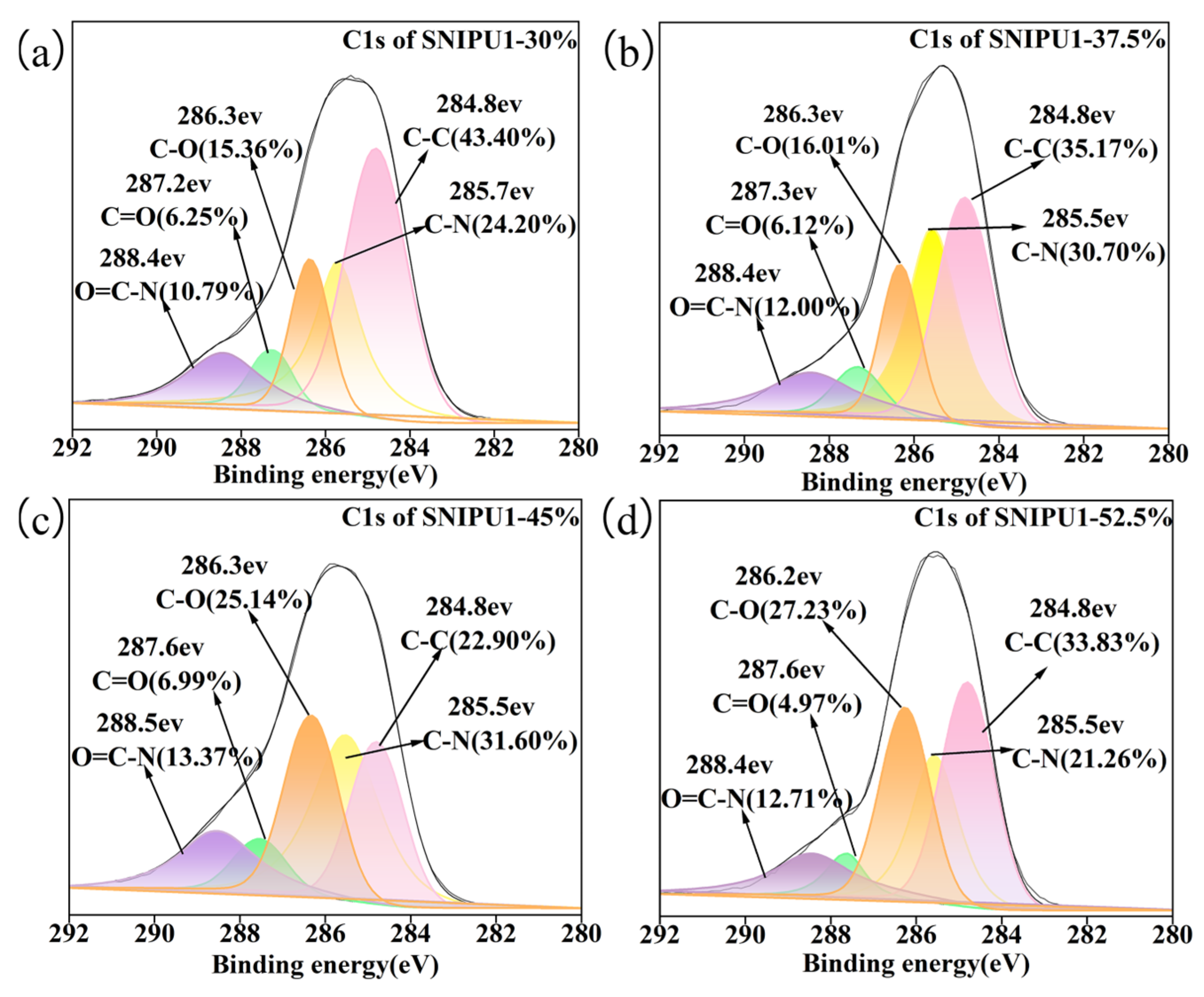
2.2. Effect of PEI-10000 Dosage on Adhesives
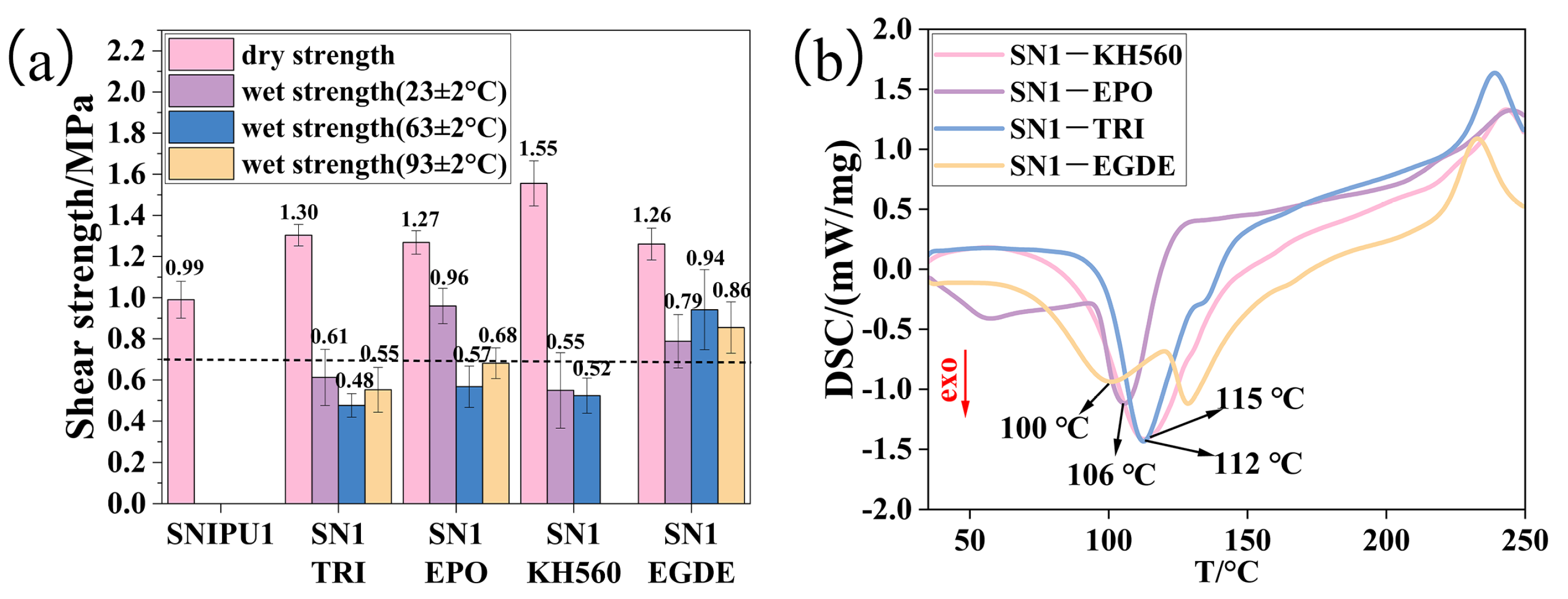
2.3. Effect of Modifier Type on SNIPU Adhesive at the Lower Hot-Pressing Temperature of 180 °C
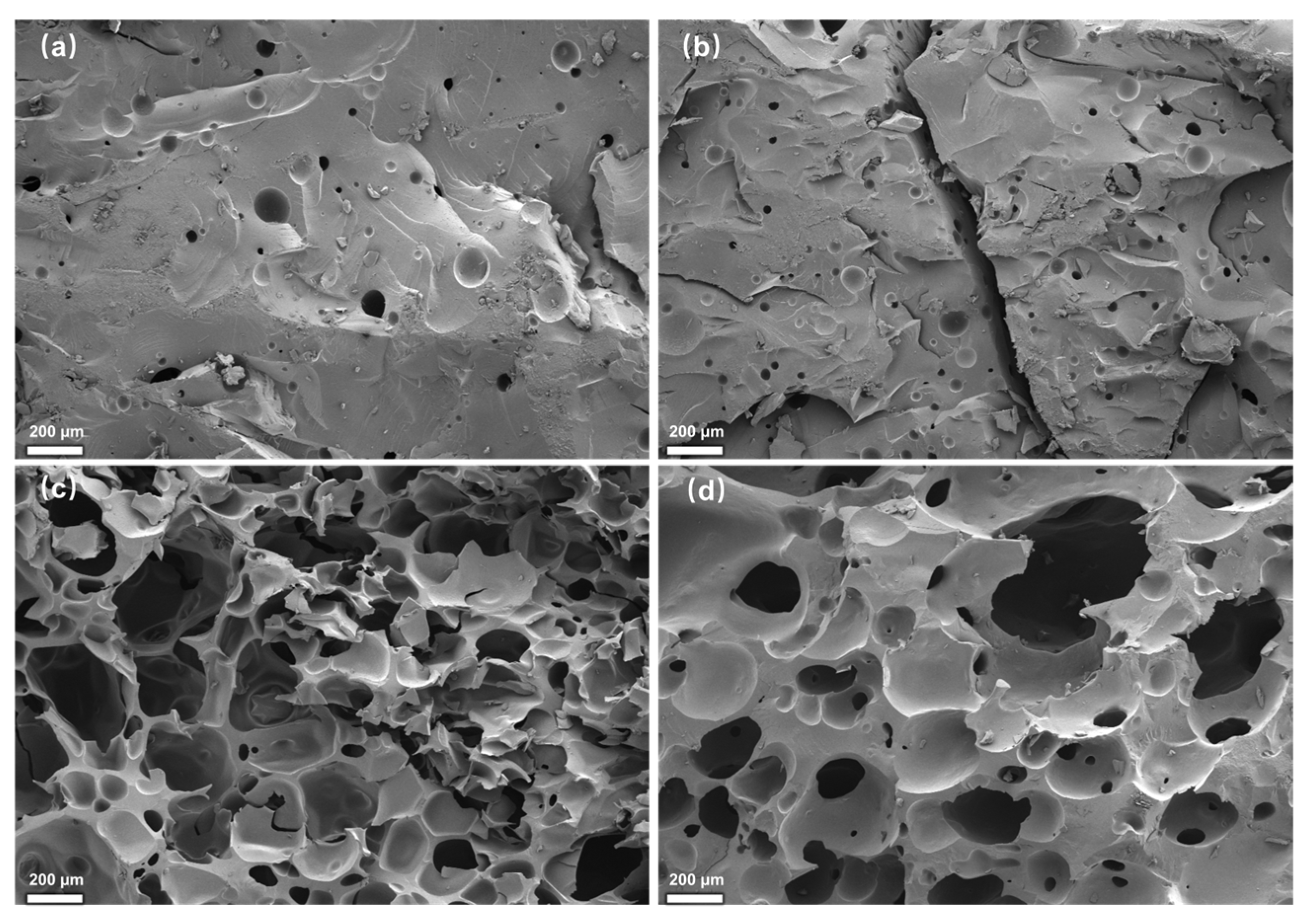
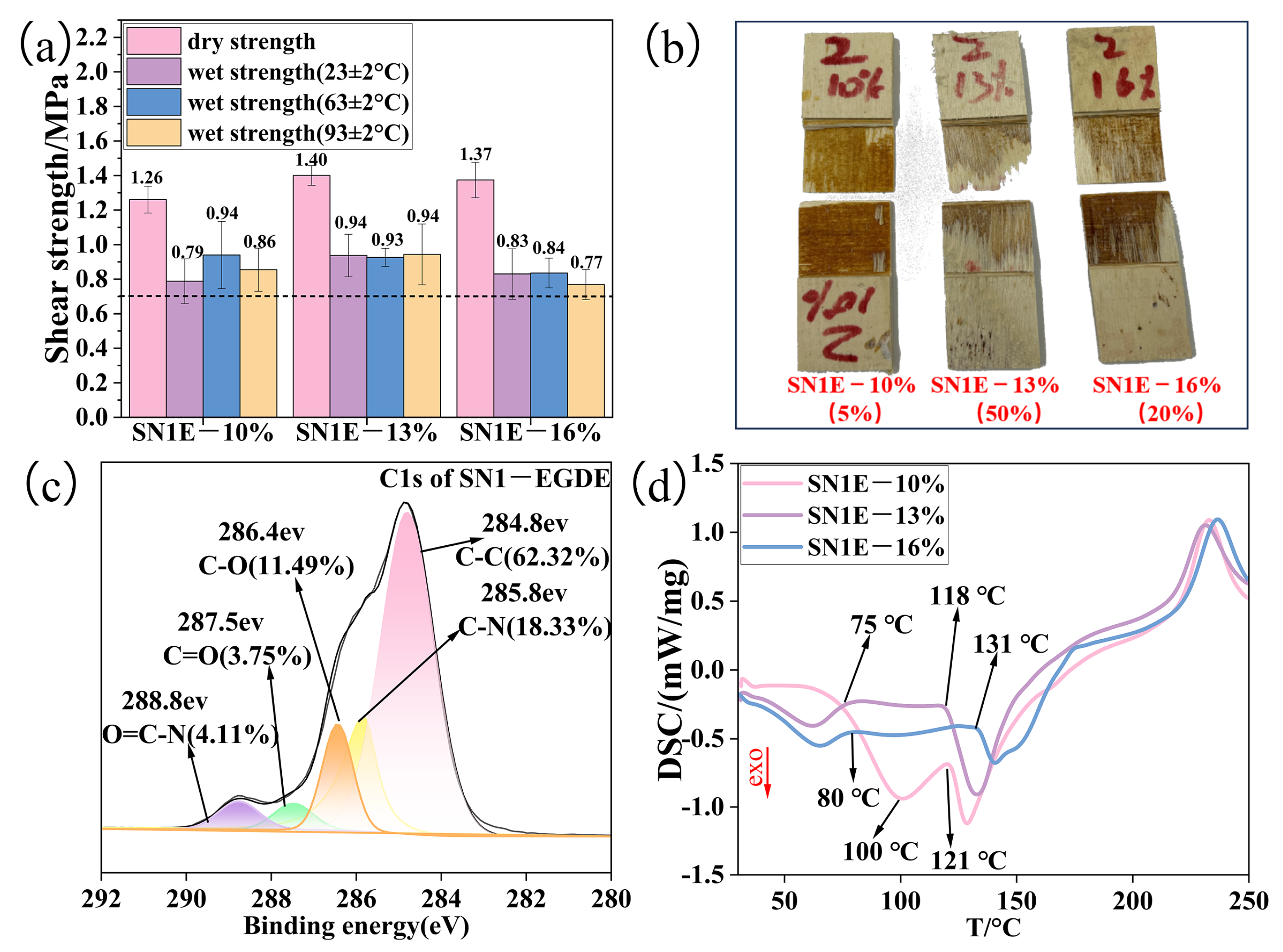
2.4. The Effect of the Amount of the Modifier EGDE on the Adhesive
3. Materials and Methods
3.1. Materials
3.2. Preparation of SNIPU Adhesives
3.3. Preparation of Three-Layer Plywood and Bonding Strength Test
3.4. Viscosity Evaluation
3.5. Water Resistance Test of Cured Adhesives
3.6. Fourier Transforms Infrared (FT-IR) Spectrosco
3.7. X-Ray Photo Spectroscopy (XPS) Analysis
3.8. Differential Scanning Calorimetry (DSC) Analysis
3.9. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suryawanshi, Y.; Sanap, P.; Wani, V. Advances in the synthesis of non-isocyanate polyurethanes. Polym. Bull. 2019, 76, 3233–3246. [Google Scholar] [CrossRef]
- Mouren, A.; Avérous, L. Sustainable cycloaliphatic polyurethanes: From synthesis to applications. Chem. Soc. Rev. 2023, 52, 277–317. [Google Scholar]
- Garipov, R.M.; Sysoev, V.A.; Mikheev, V.V.; Zagidullin, A.I.; Deberdeev, R.Y.; Irzhak, V.I.; Berlin, A.A. Reactivity of Cyclocarbonate Groups in Modified Epoxy–Amine Compositions. Dokl. Phys. Chem. 2003, 393, 289–292. [Google Scholar]
- Zhang, P.; Guo, H.; Qin, C.; Yuan, H.; Cao, Y.; Wang, Z.; Jin, C. Solvent-free synthesis of bio-based non-isocyanate polyurethane (NIPU) with robust adhesive property and resistance to low temperature. Polym. Test 2021, 140, 108616. [Google Scholar]
- Huang, Z.; Guo, H.; Yuan, H.; Cao, Y.; Wang, Z.; Jin, C. Bio-based thermosetting non-isocyanate polyurethane as high-performance adhesives for bonding wood. Eur. Polym. J. 2025, 225, 113733. [Google Scholar]
- Zhang, P.; Yuan, H.; Wang, Y.; Cao, Y.; Wang, Z.; Jin, C. Synthesis of CO2-based biomass derived non-isocyanate polyurethane hybrid adhesives with excellent mechanical properties and water resistance. Green. Chem. 2025, 27, 2102. [Google Scholar] [CrossRef]
- Guan, J.; Song, Y.; Lin, Y.; Yin, X.; Zuo, M.; Zhao, Y.; Tao, X.; Zheng, Q. Progress in Study of Non-Isocyanate Polyurethane. Ind. Eng. Chem. Res. 2011, 50, 6517–6527. [Google Scholar]
- Zhou, Y.; Li, C.; Xu, D.; Wang, Z.; Chen, Z.; Lei, H.; Du, G. A tannin–oxidized glucose wood adhesive with high performance. Ind. Crops Prod. 2024, 222, 119603. [Google Scholar] [CrossRef]
- Bansode, A.; Barde, M.; Asafu-Adjaye, O.; Patil, V.; Hinkle, J.; Via, B.K.; Adhikari, S.; Adamczyk, A.J.; Farag, R.; Elder, T.; et al. Synthesis of Biobased Novolac Phenol–Formaldehyde Wood Adhesives from Biorefinery-Derived Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 10990–11002. [Google Scholar]
- Chang, Z.; Pang, H.; Huang, A.; Li, J.; Zhang, S. Reinforcement of Bonding Strength and Water Resistance of Soybean Meal-Based Adhesive via Construction of an Interactive Network from Biomass Residues. Polymers 2019, 11, 967. [Google Scholar] [CrossRef]
- Iswanto, A.; Lubis, M.; Sutiawan, J.; Al-Edrus, S.; Lee, S.; Antov, P.; Kristak, L.; Reh, R.; Mardawati, E.; Santoso, A. Latest Advancements in the Development of High-Performance Lignin- and Tannin-Based Non-Isocyanate Polyurethane Adhesive for Wood Composites. Polymers 2023, 15, 3864. [Google Scholar] [CrossRef] [PubMed]
- Anttila, A.-K.; Pirttilä, A.M.; Häggman, H.; Harju, A.; Venäläinen, M.; Haapala, A.; Holmbom, B.; Julkunen-Tiitto, R. Condensed conifer tannins as antifungal agents in liquid culture. Holz 2013, 67, 825–832. [Google Scholar] [CrossRef]
- Li, D.; Xue, B.; Zhao, Q.; Wang, W.; Li, X.; Wen, J.; Wang, Z.; Zhao, W. Green synthesis of lignin-based non-isocyanate polyurethanes as reusable, self-healable and removable adhesives. Eur. Polym. J. 2024, 221, 113553. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Xi, X.; Wei, Z.; Wu, Z.; Yan, Z.; Lei, H.; Du, G. Thermosetting Sucrose-Based Wood Adhesive with Enhanced Water Resistance and Bonding Performance by Constructing a Schiff Base Cross-Linking Network. ACS Appl. Polym. Mater. 2024, 6, 13927–13936. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.; Ren, X.; Li, Z.; Yang, H.; Zhang, X.; Huang, T.; Ran, X.; Gao, W.; Ni, K.; et al. Developing sugar-based wood adhesives using Schiff base chemistry derived from carbohydrates. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133485. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.; Zheng, L.; Luo, R.; Essawy, H.; Huang, H.; Wang, Y.; Deng, S.; Zhang, J. Development and Characterization of Bio-Based Formaldehyde Free Sucrose-Based Adhesive for Fabrication of Plywood. Polymers 2024, 16, 640. [Google Scholar] [CrossRef]
- Kusumah, S.S.; Umemura, K.; Guswenrivo, I.; Yoshimura, T.; Kanayama, K. Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard II: Influences of pressing temperature and time on particleboard properties. J. Wood Sci. 2017, 63, 161–172. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green. Chem. 2013, 15, 1740–1763. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Hu, X.-F.; Zou, C.; Shi, J.; Du, Q.-Z.; Teng, B.-T.; Yin, J.-F. Effect of saccharides on sediment formation in green tea concentrate. LWT 2017, 78, 352–360. [Google Scholar] [CrossRef]
- Benítez, E.I.; Genovese, D.B.; Lozano, J.E. Effect of typical sugars on the viscosity and colloidal stability of apple juice. Food Hydrocoll. 2009, 23, 519–525. [Google Scholar] [CrossRef]
- Zhao, Z.; Miao, Y.; Yang, Z.; Wang, H.; Sang, R.; Fu, Y.; Huang, C.; Wu, Z.; Zhang, M.; Sun, S.; et al. Effects of Sulfuric Acid on the Curing Behavior and Bonding Performance of Tannin–Sucrose Adhesive. Polymers 2018, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, R.; Xiong, Y.; Lei, H.; Du, G.; Pizzi, A.; Puangsin, B.; Xi, X. Preparation and characterization of polymeric cellulose wood adhesive with excellent bonding properties and water resistance. Carbohydr. Polym. 2025, 347, 122705. [Google Scholar]
- Keil, T.W.M.; Deiringer, N.; Friess, W.; Merkel, O.M. Evaluation of adsorption of DNA/PEI polyplexes to tubing materials. Eur. J. Pharm. Biopharm. 2022, 179, 58–64. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.; Wang, S.; Hu, J.; Liu, X. Hyperbranched flame retardant for epoxy resin modification: Simultaneously improved flame retardancy, toughness and strength as well as glass transition temperature. Chem. Eng. J. 2022, 428, 131226. [Google Scholar]
- Li, K.; Geng, X.; Simonsen, J.; Karchesy, J. Novel wood adhesives from condensed tannins and polyethylenimine. Int. J. Adhes. Adhes. 2004, 24, 327–333. [Google Scholar]
- Zhou, Y.; Dong, F.; Chen, X.; Huang, X.; Guo, L.; Liu, H.; Xu, X. A novel rosin-based non-isocyanate polyurethane with high-strength, self-healing, and recyclable properties for wood adhesives. Ind. Crops Prod 2024, 211, 118203. [Google Scholar]
- GB/T9846-2015; Plywood for General Use. Standardization Administration of China: Beijing, China, 2015.
- Islam, M.N.; Rahman, F.; Das, A.K.; Hiziroglu, S. An overview of different types and potential of bio-based adhesives used for wood products. Int. J. Adhes. Adhes. 2022, 112, 102992. [Google Scholar]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar]
- Ma, Q.; Mohawk, D.; Jahani, B.; Wang, X.; Chen, Y.; Mahoney, A.; Zhu, J.Y.; Jiang, L. UV-curable cellulose nanofiber-reinforced soy protein resins for 3D printing and conventional molding. ACS Appl. Polym. Mater. 2020, 2, 4666–4676. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 675–905. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Camara, F.; Benyahya, S.; Besse, V.; Boutevin, G.; Auvergne, R.; Boutevin, B.; Caillol, S. Reactivity of secondary amines for the synthesis of non-isocyanate polyurethanes. Eur. Polym. J. 2014, 55, 17–26. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, Z.; Xi, X.; Lei, H.; Li, C.; Chen, Z.; Shi, J.; Du, G. A bio-based soy wood adhesive modified by dual-crosslinking strategy with excellent mechanical strength and water-resistance. Ind. Crops Prod. 2024, 222, 119417. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, P.; Yang, W.; Chu, H.; Wang, W.; Dong, W.; Chen, M.; Bai, H.; Ma, P. Soy protein-based adhesive with superior bonding strength and water resistance by designing densely crosslinking networks. Eur. Polym. J. 2021, 142, 110128. [Google Scholar] [CrossRef]
- Luo, H.; Yin, Y.; Wang, Y.; Li, Q.; Tang, A.; Liu, Y. Enhanced properties of a soybean adhesive by modification with a cycloaliphatic epoxy resin. Int. J. Adhes. Adhes. 2022, 114, 103026. [Google Scholar] [CrossRef]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Gui, C.; Zhang, M.; Umemura, K.; Yong, Q. Investigation of Synthesis Mechanism, Optimal Hot-Pressing Conditions, and Curing Behavior of Sucrose and Ammonium Dihydrogen Phosphate Adhesive. Polymers 2020, 12, 216. [Google Scholar] [CrossRef]
- Ye, R.; Wang, C.; Shi, X.; Zhang, D.; Lai, C.; Chen, X.; Wang, C.; Chu, F. A facile strategy to fabricate low viscosity and high cold tack soy protein-based adhesive for particleboard production. Ind. Crops Prod. 2024, 214, 118612. [Google Scholar] [CrossRef]
- Yao, X.; Liu, H.; Li, C.J.H. Development of Eco-Friendly Soy Meal Adhesives Enhanced by Ethylene Glycol Diglycidyl Ether. Adv. Polym. Technol. 2019, 2019, 8697047. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Zhang, Q.; Lei, H.; Zhou, X.; Zhang, J.; Du, G.; Pizzi, A.; Xi, X. Preparation and Modification of Sucrose-Based Non-Isocyanate Polyurethane Adhesives for Plywood Bonding. Molecules 2025, 30, 1541. https://doi.org/10.3390/molecules30071541
Zhong H, Zhang Q, Lei H, Zhou X, Zhang J, Du G, Pizzi A, Xi X. Preparation and Modification of Sucrose-Based Non-Isocyanate Polyurethane Adhesives for Plywood Bonding. Molecules. 2025; 30(7):1541. https://doi.org/10.3390/molecules30071541
Chicago/Turabian StyleZhong, Hongyi, Qianyu Zhang, Hong Lei, Xiaojian Zhou, Jun Zhang, Guanben Du, Antonio Pizzi, and Xuedong Xi. 2025. "Preparation and Modification of Sucrose-Based Non-Isocyanate Polyurethane Adhesives for Plywood Bonding" Molecules 30, no. 7: 1541. https://doi.org/10.3390/molecules30071541
APA StyleZhong, H., Zhang, Q., Lei, H., Zhou, X., Zhang, J., Du, G., Pizzi, A., & Xi, X. (2025). Preparation and Modification of Sucrose-Based Non-Isocyanate Polyurethane Adhesives for Plywood Bonding. Molecules, 30(7), 1541. https://doi.org/10.3390/molecules30071541







