Quantitative Study of Spodumene by Time-of-Flight Secondary Ion Mass Spectrometry (tof-SIMS)
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantification Theory of tof-SIMS
2.2. Element Determination of Spodumene by tof-SIMS Using the Matrix-Matched Standard
2.3. Comparison of tof-SIMS to LA-ICPMS and EPMA for Spodumene Quantification
2.4. Element Behavior Study of the Non-Matrix-Matched Standard NIST SRM 610
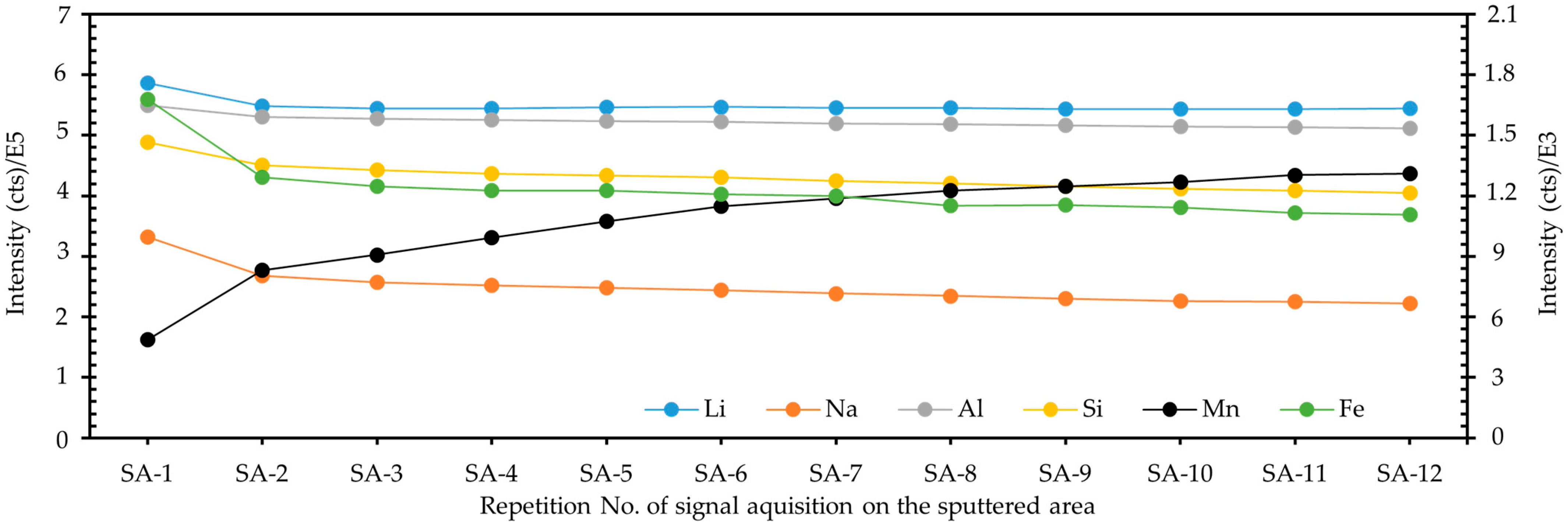
2.5. Effect Study of Surface Sputtering on Spodumene Analysis
2.6. Application of the Proposed tof-SIMS Quantification Method for Spodumene Analysis
3. Materials and Methods
3.1. Spodumene Sample and Silicate Glass Standard Material
3.2. tof-SIMS Apparatus
3.3. Data Acquisition and Processing
3.4. LA-ICPMS Analysis
3.5. EPMA Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macholdt, D.S.; Jochum, K.P.; Pöhlker, C.; Stoll, B.; Weis, U.; Weber, B.; Müller, M.; Kappl, M.; Buhre, S.; Kilcoyne, A.; et al. Microanalytical methods for in-situ high-resolution analysis of rock varnish at the micrometer to nanometer scale. Chem. Geol. 2015, 411, 57–68. [Google Scholar] [CrossRef]
- Li, X.H.; Li, Q.L. Major advances in microbeam analytical techniques and their applications in Earth Science. Sci. Bull. 2016, 61, 1785–1787. [Google Scholar]
- Pownceby, M.I.; MacRae, C.M.; Wilson, N.C. Mineral characterisation by EPMA mapping. Miner. Eng. 2007, 20, 444–451. [Google Scholar] [CrossRef]
- Pisonero, J.; Fernández, B.; Günther, D. Critical revision of GD-MS, LA-ICP-MS and SIMS as inorganic mass spectrometric techniques for direct solid analysis. J. Anal. At. Spectrom. 2009, 24, 1145–1160. [Google Scholar]
- Yang, P.; Wu, G.; Nuriel, P.; Nguyen, A.D.; Chen, Y.; Yang, S.; Feng, Y.; Ren, Z.; Zhao, J. In situ LA-ICPMS Upb dating and geochemical characterization of fault-zone calcite in the central Tarim Basin, northwest China: Implications for fluid circulation and fault reactivation. Chem. Geol. 2021, 568, 120125. [Google Scholar]
- Harris, B.J.R.; De Hoog, J.C.M.; Halama, R. The behaviour of nitrogen during subduction of oceanic crust: Insights from in situ SIMS analyses of high-pressure rocks. Geochim. Cosmochim. Acta 2022, 321, 16–34. [Google Scholar]
- Ma, M.; Wan, Y.; Santosh, M.; Xu, Z.; Xie, H.; Dong, C.; Liu, D.; Guo, C. Decoding multiple tectonothermal events in zircons from single rock samples: SHRIMP zircon U–Pb data from the late Neoarchean rocks of Daqingshan, North China Craton. Gondwana Res. 2012, 22, 810–827. [Google Scholar]
- Massonnet, P.; Heeren, R.M.A. A concise tutorial review of TOF-SIMS based molecular and cellular imaging. J. Anal. At. Spectrom. 2019, 34, 2217–2228. [Google Scholar]
- Stephan, T. TOF-SIMS in cosmochemistry. Planet. Space Sci. 2001, 49, 859–906. [Google Scholar]
- Hagenhoff, B. High resolution surface analysis by TOF-SIMS. Microchim. Acta 2000, 132, 259–271. [Google Scholar]
- Long, T.; Clement, S.W.J.; Xie, H.; Liu, D. Design, construction and performance of a TOF-SIMS for analysis of trace elements in geological materials. Int. J. Mass. Spectrom. 2020, 450, 116289. [Google Scholar]
- Wickramasinghe, R.C.; Pasterski, M.J.; Kenig, F.; Ievlev, A.V.; Lorenz, M.; Gross, J.M.; Hanley, L. Femtosecond laser desorption postionization ms vs tof-sims imaging for uncovering biomarkers buried in geological samples. Anal. Chem. 2021, 93, 15949–15957. [Google Scholar] [PubMed]
- North, R.; Tanner, D.; Nancarrow, M.; Pasic, B.; Mavrogenes, J.A. Resolving sub-micrometer-scale zonation of trace elements in quartz using TOF-SIMS. Am. Mineral. 2022, 107, 955–969. [Google Scholar]
- North, R.; White, L.T.; Nancarrow, M.; Dosseto, A. Sub-micrometre resolution FIB-SEM-based ToF-SIMS used to map geochemical zoning in four zircon reference materials. Geostand. Geoanal. Res. 2023, 47, 125–142. [Google Scholar]
- Saunders, K.; Rinnen, S.; Blundy, J.; Dohmen, R.; Klemme, S.; Arlinghaus, H.F. TOF-SIMS and electron microprobe investigations of zoned magmatic orthopyroxenes: First results of trace and minor element analysis with implications for diffusion modeling. Am. Mineral. 2012, 97, 532–542. [Google Scholar]
- Song, T.; Liu, J.; Zhang, C.; Yang, X.; Chen, T.; Jiang, S.; Xu, F.; Li, N.; Zhu, M.; Li, S.; et al. Characterization of the micro-morphology and compositional distribution of Chang’e-5 lunar soil mineral surfaces using TOF-SIMS. Ad. Sci. 2025, 12, 2416639. [Google Scholar]
- Marques, A.F.A.; Scott, S.D.; Sodhi, R.N.S. Determining major and trace element compositions of exposed melt inclusions in minerals using ToF-SIMS. Surf. Interface Anal. 2011, 43, 436–442. [Google Scholar]
- Wang, M.Q.; Cai, K.D.; Li, Z.P.; Guo, C. Quantitative accuracy assessment of trace elements and halogens in apatite by time-of-flight secondary ion mass spectrometry (TOF-SIMS). J. Anal. At. Spectrom. 2024, 39, 1609–1615. [Google Scholar]
- Graham, J. Some notes on α-spodumene, LiAlSi2O6. Am. Mineral. 1975, 60, 919–923. [Google Scholar]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The lithium market, resources and processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Tan, X.J.; Koch, J.; Günther, D.; Reusser, E.; Hattendorf, B. In situ element analysis of spodumenes by fs-LA-ICPMS with non-matrix-matched calibration: Signal beat and accuracy. Chem. Geol. 2021, 583, 120463. [Google Scholar]
- Liu, C.; Wang, R.C.; Wu, F.Y.; Xie, L.; Liu, X.-C.; Li, X.-K.; Yang, L.; Li, X.-J. Spodumene pegmatites from the Pusila pluton in the higher Himalaya, South Tibet: Lithium mineralization in a highly fractionated leucogranite batholith. Lithos 2020, 358, 105421. [Google Scholar]
- Rinaldi, R.; Llovet, X. Electron probe microanalysis: A review of the past, present, and future. Microsc. Microanal. 2015, 21, 1053–1069. [Google Scholar]
- Stevie, F.A.; Griffis, D.P. Quantification in dynamic SIMS: Current status and future needs. Appl. Surf. Sci. 2008, 255, 1364–1367. [Google Scholar]
- Van der Heide, P. Secondary Ion Mass Spectrometry: An Introduction to Principles and Practices; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Longerich, H.P.; Jackson, S.E.; Gunther, D. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar]
- Werner, H.W. Introduction to Secondary Ion Mass Spectrometry (SIMS). In Electron and Ion Spectroscopy of Solids; Fiermans, L., Vennik, J., Dekeyser, W., Eds.; Springer: Boston, MA, USA, 1978. [Google Scholar]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A new geochemical database for reference materials and isotopic standards. Geostand. Geoanal. Res. 2005, 29, 333–338. [Google Scholar]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar]
- Denny, A.C.; Zimmer, M.M.; Cunningham, H.S.; Sievers, N.E. Sources of Li isotope bias during SIMS analysis of standard glasses. Chem. Geol. 2024, 656, 122015. [Google Scholar]
- Ray, G.; Hart, S.R. Quantitative analysis of silicates by ion microprobe. Int. J. Mass. Spectrom. Ion. Phys. 1982, 44, 231–255. [Google Scholar]
- Andersen, C.A.; Hinthorne, J.R. Ion microprobe mass analyzer. Science 1972, 175, 853–860. [Google Scholar]
- Tan, X.J.; Ren, Y.X.; Liang, T.; Wang, D. Study of ultrasound-assisted low-pressure closed acid digestion method for trace element determination in rock samples by inductively coupled plasma mass spectrometry. Molecules 2025, 30, 342. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Guo, L.; Liu, C.; Wang, L.; Liu, Y.; Tan, X. Analytical effect of stabilizer volume and shape on zircon U–Pb dating by nanosecond LA-ICP-QMS. J. Anal. Sci. Technol. 2022, 13, 13. [Google Scholar]
- Fricker, M.B. Design of Ablation Cells for LA-ICP-MS. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2012. [Google Scholar]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Gunther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar]
- Armstrong, J.T. CITZAF: A package of correction programs for the quantitative electron microbeam X-ray analysis of thick polished materials, thin Films, and particles. Microbeam Anal. 1995, 4, 177–200. [Google Scholar]

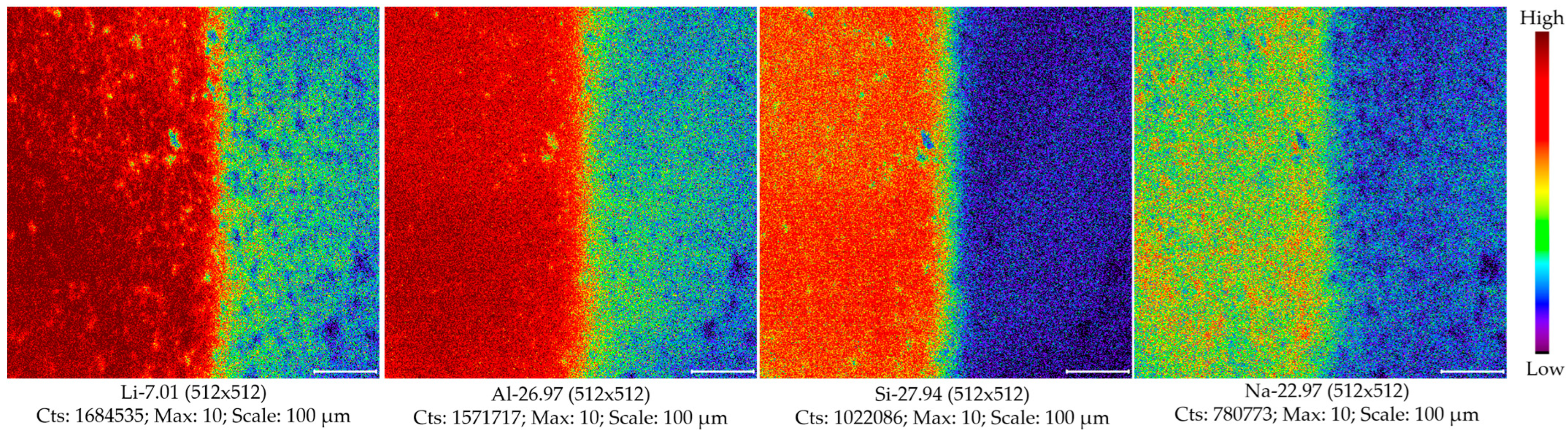
| IS | Element | Raster 1 | Raster 2 | Raster 3 | Raster 4 | Raster 5 | Raster 6 |
|---|---|---|---|---|---|---|---|
| Al | Li | 16,783 | 19,333 | 19,588 | 20,068 | 19,732 | 19,535 |
| Na | 406,209 | 467,922 | 474,098 | 485,705 | 477,575 | 472,809 | |
| Al | 6202 | 7144 | 7238 | 7416 | 7291 | 7219 | |
| Si | 1075 | 1238 | 1255 | 1285 | 1264 | 1251 | |
| Mn | 738,830 | 851,076 | 862,309 | 883,420 | 868,634 | 859,965 | |
| Fe | 62,219 | 71,671 | 72,617 | 74,395 | 73,150 | 72,420 | |
| Si | Li | 16,583 | 19,504 | 19,680 | 20,117 | 19,861 | 19,732 |
| Na | 401,346 | 472,049 | 476,306 | 486,891 | 480,706 | 477,566 | |
| Al | 6128 | 7207 | 7272 | 7434 | 7339 | 7291 | |
| Si | 1062 | 1249 | 1261 | 1289 | 1272 | 1264 | |
| Mn | 729,985 | 858,582 | 866,326 | 885,577 | 874,327 | 868,616 | |
| Fe | 61,474 | 72,303 | 72,955 | 74,577 | 73,629 | 73,148 |
| IS | Element | Raster 1 | Raster 2 | Raster 3 | Raster 4 | Raster 5 | Raster 6 | Average | 2σ | RSD% |
|---|---|---|---|---|---|---|---|---|---|---|
| Al | Li2O | 7.89 | 6.97 | 7.60 | 7.79 | 7.60 | 7.70 | 7.59 | 0.24 | 4.3 |
| Na2O | 0.094 | 0.085 | 0.089 | 0.091 | 0.087 | 0.088 | 0.089 | 0.002 | 3.5 | |
| Al2O3 | 27.59 | 27.59 | 27.59 | 27.59 | 27.59 | 27.59 | 27.59 | – | – | |
| SiO2 | 63.13 | 64.46 | 64.20 | 64.06 | 64.32 | 64.54 | 64.12 | 0.38 | 0.8 | |
| MnO | 0.058 | 0.054 | 0.060 | 0.057 | 0.059 | 0.058 | 0.058 | 0.001 | 3.4 | |
| FeO | 0.24 | 0.22 | 0.24 | 0.23 | 0.24 | 0.24 | 0.24 | 0.01 | 3.3 | |
| Total | 99.02 | 99.40 | 99.78 | 99.82 | 99.89 | 100.2 | 99.69 | 0.31 | 0.4 | |
| Si | Li2O | 7.99 | 6.91 | 7.56 | 7.77 | 7.55 | 7.62 | 7.57 | 0.27 | 4.8 |
| Na2O | 0.095 | 0.084 | 0.088 | 0.091 | 0.086 | 0.087 | 0.089 | 0.003 | 4.3 | |
| Al2O3 | 27.93 | 27.35 | 27.47 | 27.53 | 27.42 | 27.32 | 27.50 | 0.17 | 0.8 | |
| SiO2 | 63.90 | 63.90 | 63.90 | 63.90 | 63.90 | 63.90 | 63.90 | – | – | |
| MnO | 0.059 | 0.054 | 0.060 | 0.057 | 0.058 | 0.057 | 0.058 | 0.002 | 3.6 | |
| FeO | 0.25 | 0.22 | 0.24 | 0.23 | 0.23 | 0.24 | 0.23 | 0.007 | 3.8 | |
| Total | 100.2 | 98.53 | 99.32 | 99.57 | 99.24 | 99.22 | 99.35 | 0.41 | 0.6 |
| Method | No. | Li2O | Al2O3 | SiO2 | Na2O | MnO | FeO |
|---|---|---|---|---|---|---|---|
| tof-SIMS | Raster 1 | 7.97 | 27.87 | 63.76 | 0.095 | 0.058 | 0.25 |
| Raster 2 | 7.02 | 27.76 | 64.86 | 0.085 | 0.055 | 0.23 | |
| Raster 3 | 7.61 | 27.66 | 64.34 | 0.089 | 0.061 | 0.24 | |
| Raster 4 | 7.81 | 27.65 | 64.17 | 0.091 | 0.057 | 0.23 | |
| Raster 5 | 7.61 | 27.62 | 64.39 | 0.087 | 0.059 | 0.24 | |
| Raster 6 | 7.68 | 27.53 | 64.40 | 0.088 | 0.058 | 0.24 | |
| Average | 7.62 | 27.68 | 64.32 | 0.089 | 0.058 | 0.24 | |
| 2σ | 0.27 | 0.10 | 0.29 | 0.003 | 0.002 | 0.007 | |
| LA-ICPMS | Spot 1 | 7.93 | 27.59 | 63.96 | 0.108 | 0.060 | 0.24 |
| Spot 2 | 8.03 | 27.82 | 63.71 | 0.113 | 0.053 | 0.20 | |
| Spot 3 | 8.09 | 27.94 | 63.52 | 0.099 | 0.055 | 0.23 | |
| Spot 4 | 8.04 | 27.64 | 63.70 | 0.101 | 0.066 | 0.25 | |
| Spot 5 | 7.95 | 27.17 | 64.24 | 0.090 | 0.052 | 0.23 | |
| Spot 6 | 8.16 | 27.63 | 63.77 | 0.081 | 0.048 | 0.23 | |
| Spot 7 | 8.03 | 27.68 | 63.85 | 0.086 | 0.055 | 0.24 | |
| Spot 8 | 7.98 | 27.71 | 63.88 | 0.077 | 0.046 | 0.22 | |
| Spot 9 | 7.89 | 27.25 | 64.40 | 0.077 | 0.066 | 0.26 | |
| Spot 10 | 7.96 | 27.53 | 63.93 | 0.074 | 0.064 | 0.24 | |
| Average | 8.00 | 27.59 | 63.90 | 0.091 | 0.057 | 0.23 | |
| 2σ | 0.05 | 0.15 | 0.16 | 0.009 | 0.004 | 0.010 | |
| EPMA | Point 1 | – | 27.30 | 63.45 | 0.100 | 0.015 | 0.08 |
| Point 2 | – | 27.69 | 64.87 | 0.059 | 0.030 | 0.09 | |
| Point 3 | – | 27.40 | 63.52 | 0.093 | 0.060 | 0.02 | |
| Point 4 | – | 27.15 | 63.60 | 0.044 | 0.098 | 0.08 | |
| Point 5 | – | 27.22 | 63.83 | 0.076 | 0.053 | 0.10 | |
| Point 6 | – | 27.33 | 63.78 | 0.057 | 0.023 | 0.14 | |
| Point 7 | – | 27.55 | 64.10 | 0.045 | 0.053 | 0.25 | |
| Point 8 | – | 27.45 | 63.41 | 0.065 | 0.030 | 0.18 | |
| Point 9 | – | 27.62 | 64.60 | 0.122 | 0.059 | 0.14 | |
| Point 10 | – | 27.40 | 63.73 | 0.045 | 0.053 | 0.12 | |
| Average | – | 27.41 | 63.89 | 0.071 | 0.047 | 0.12 | |
| 2σ | – | 0.10 | 0.30 | 0.016 | 0.018 | 0.038 | |
| Rtof-SIMS/LA-ICPMS | 0.95 | 1.00 | 1.01 | 0.98 | 1.02 | 1.01 | |
| Rtof-SIMS/EPMA | – | 1.01 | 1.01 | 1.26 | 1.22 | 1.99 | |
| Method | Li2O | Al2O3 | SiO2 | Na2O | MnO | FeO | |
|---|---|---|---|---|---|---|---|
| tof-SIMS | Raster 1 | 7.93 | 26.92 | 64.87 | 0.115 | 0.145 | 0.0188 |
| Raster 2 | 7.94 | 27.41 | 64.36 | 0.117 | 0.149 | 0.0197 | |
| Raster 3 | 8.00 | 27.38 | 64.33 | 0.118 | 0.148 | 0.0197 | |
| Raster 4 | 7.50 | 26.53 | 65.69 | 0.108 | 0.153 | 0.0183 | |
| Raster 5 | 7.81 | 27.42 | 64.48 | 0.115 | 0.149 | 0.0205 | |
| Raster 6 | 7.85 | 27.67 | 64.19 | 0.117 | 0.150 | 0.0204 | |
| Average | 7.84 | 27.22 | 64.65 | 0.115 | 0.149 | 0.0196 | |
| 2σ | 0.15 | 0.003 | 0.34 | 0.457 | 0.002 | 0.001 | |
| LA-ICPMS 2 | 7.92 ± 0.12 | 27.44 ± 0.18 | 64.33 ± 0.23 | 0.12 ± 0.004 | 0.15 ± 0.019 | 0.02 ± 0.007 | |
| EPMA 2 | – | 27.45 ± 0.12 | 64.5 ± 0.12 | 0.11 ± 0.014 | 0.12 ± 0.021 | 0.02 ± 0.012 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X. Quantitative Study of Spodumene by Time-of-Flight Secondary Ion Mass Spectrometry (tof-SIMS). Molecules 2025, 30, 1552. https://doi.org/10.3390/molecules30071552
Tan X. Quantitative Study of Spodumene by Time-of-Flight Secondary Ion Mass Spectrometry (tof-SIMS). Molecules. 2025; 30(7):1552. https://doi.org/10.3390/molecules30071552
Chicago/Turabian StyleTan, Xijuan. 2025. "Quantitative Study of Spodumene by Time-of-Flight Secondary Ion Mass Spectrometry (tof-SIMS)" Molecules 30, no. 7: 1552. https://doi.org/10.3390/molecules30071552
APA StyleTan, X. (2025). Quantitative Study of Spodumene by Time-of-Flight Secondary Ion Mass Spectrometry (tof-SIMS). Molecules, 30(7), 1552. https://doi.org/10.3390/molecules30071552





