Synthesis, Structural Characterization, and Hydrogen Release of Al-Based Amidoboranes Derived from MAlH4 (Li, Na)-BH3NH2CH2CH2NH2BH3
Abstract
1. Introduction
2. Results
2.1. Synthesis of M[Al(BH3NHCH2CH2NHBH3)2] (M = Li and Na)
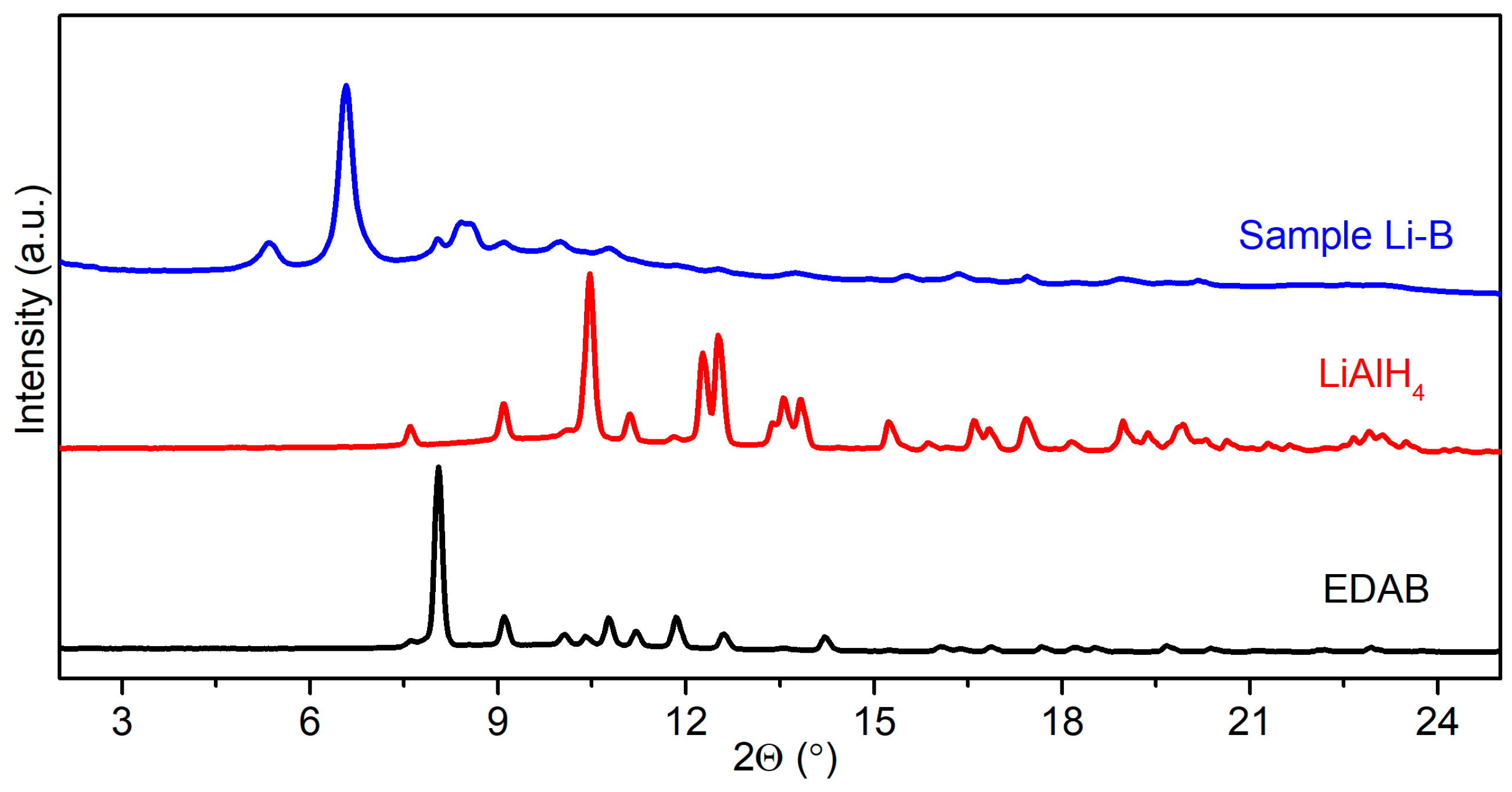
2.1.1. Synthesis of Li[Al(BH3NHCH2CH2NHBH3)2]
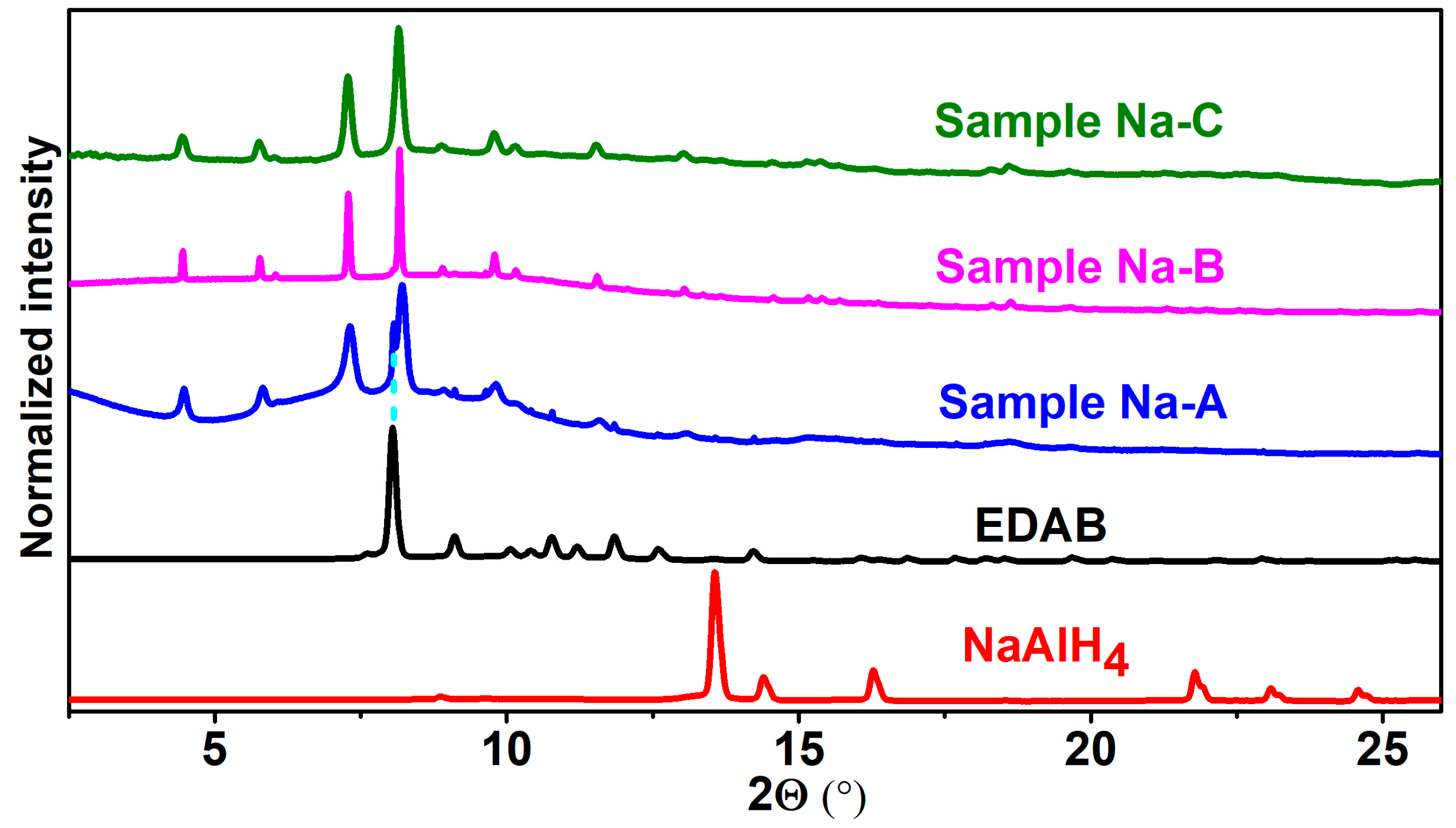
2.1.2. Synthesis of Na(THF)[Al(BH3NHCH2CH2NHBH3)2]
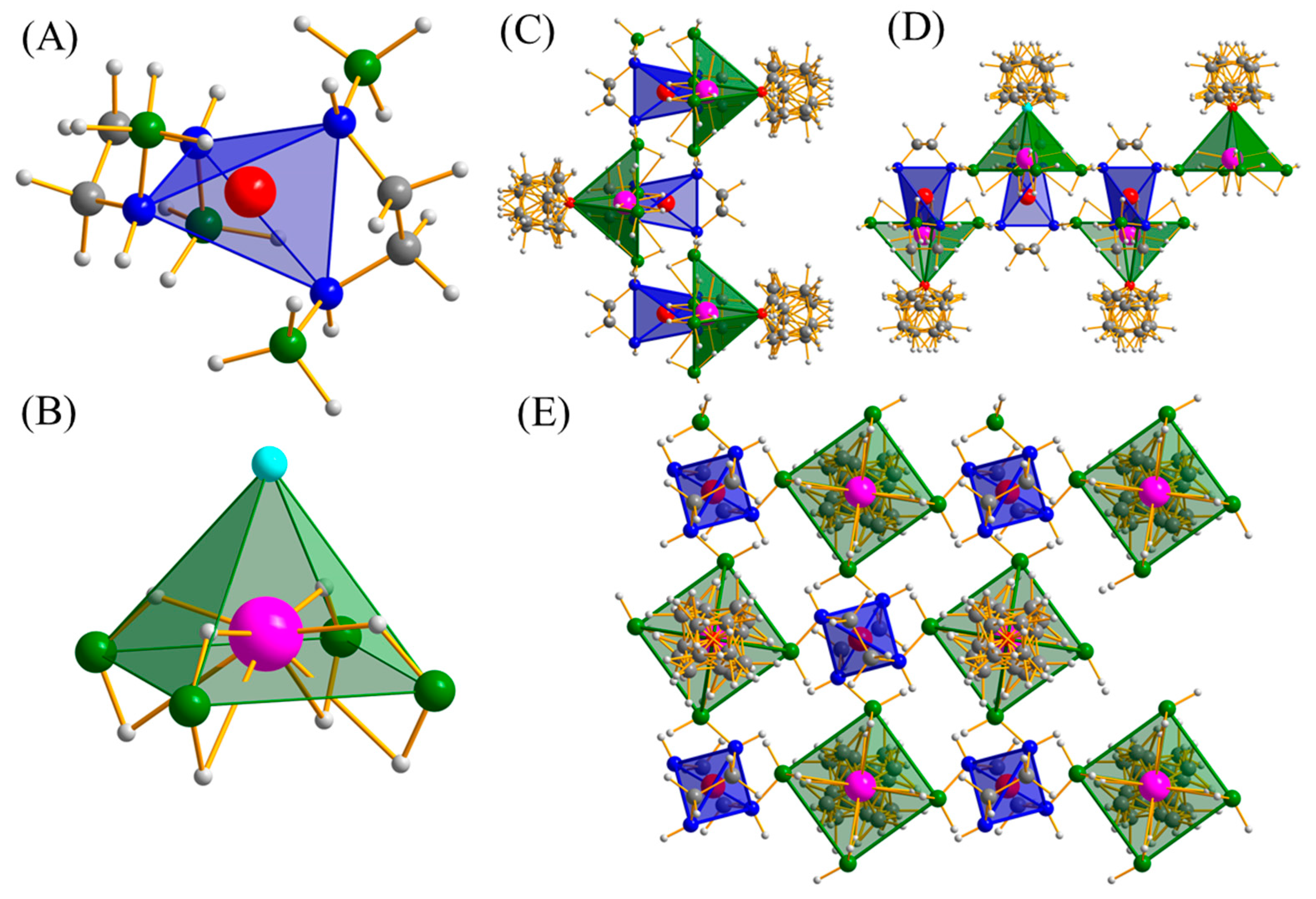
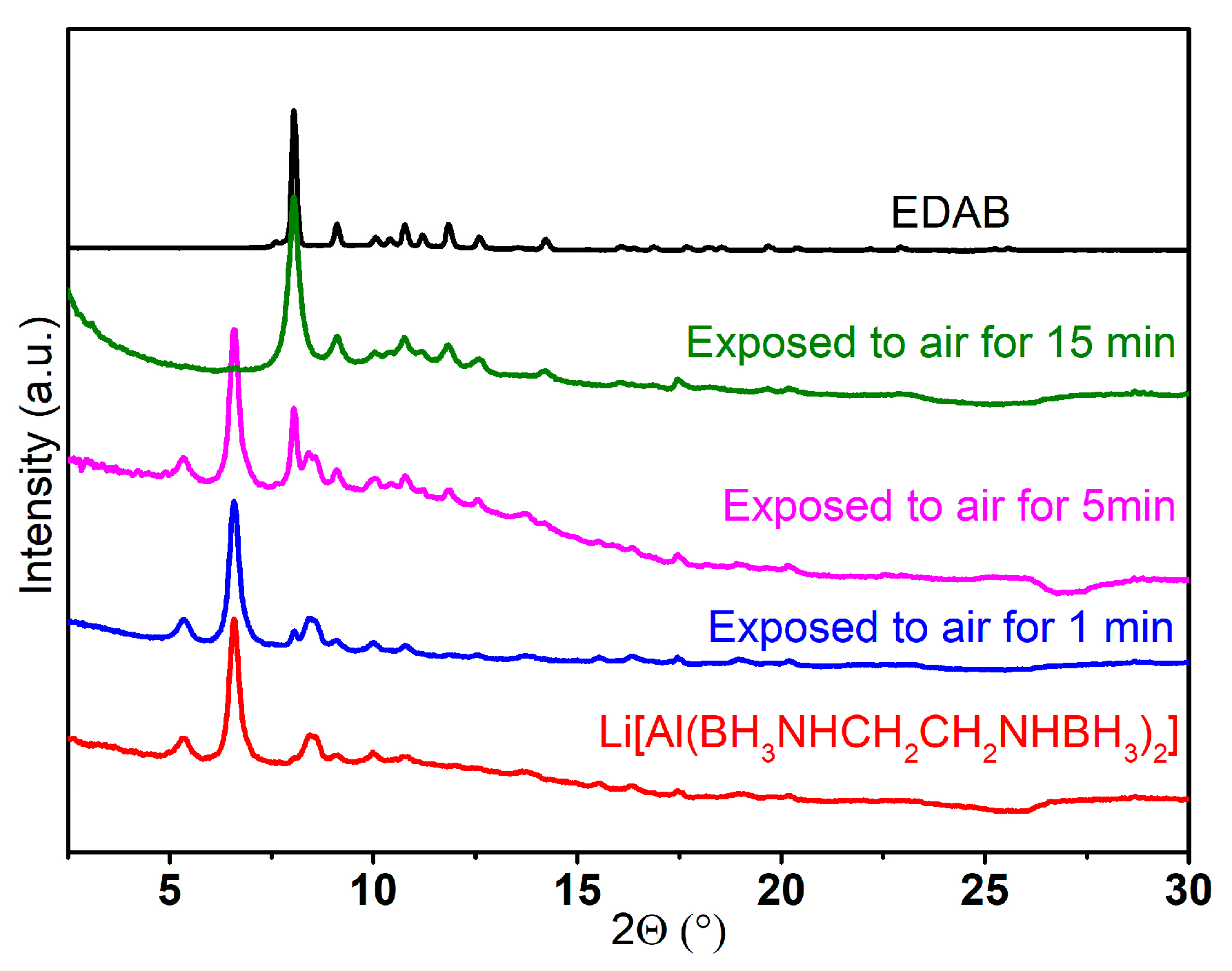
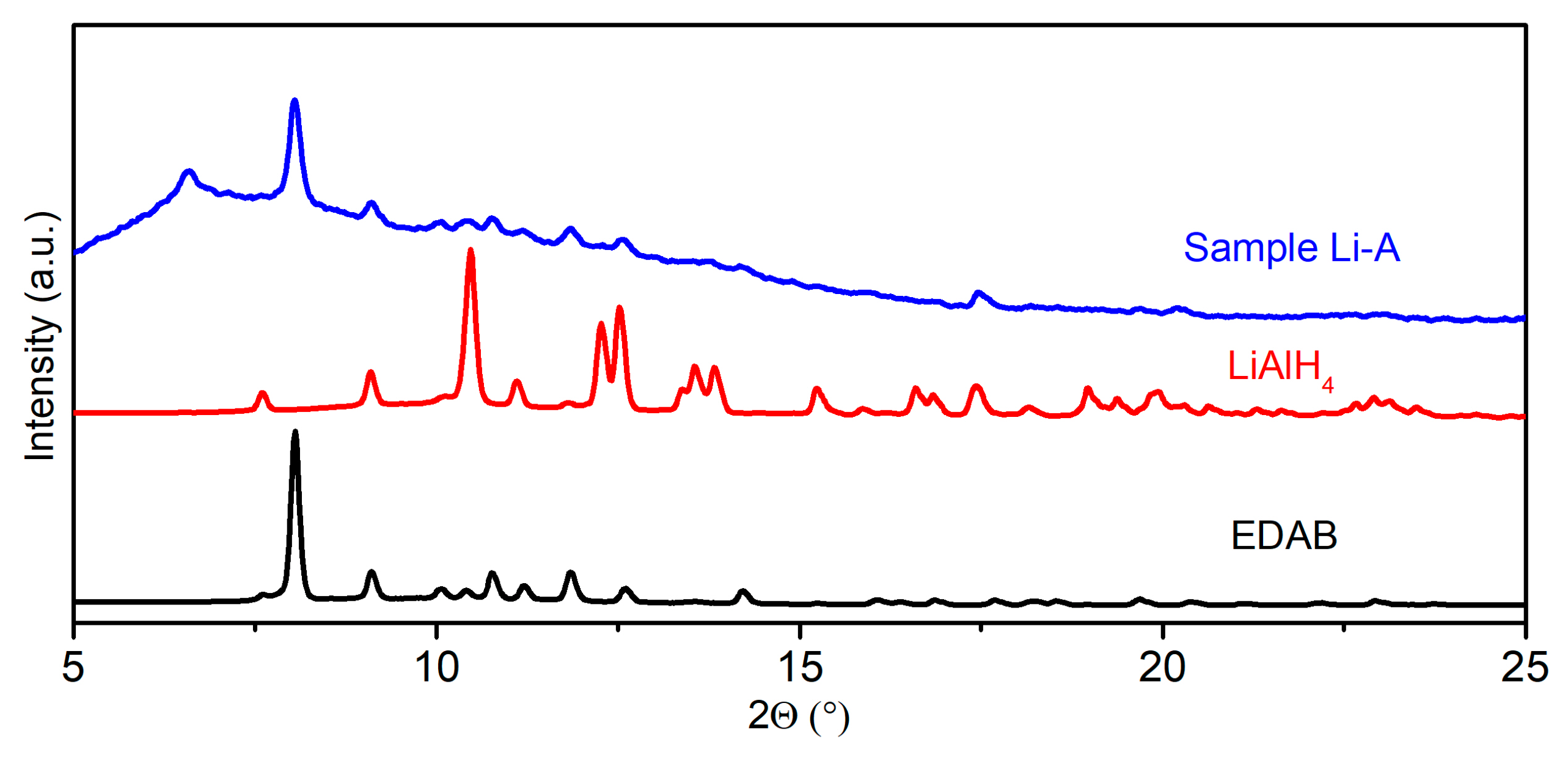
2.2. Characterization of M[Al(BH3NHCH2CH2NHBH3)2] (M = Li, Na)
2.2.1. The Structure of Na(THF)[Al(BH3NHCH2CH2NHBH3)2]
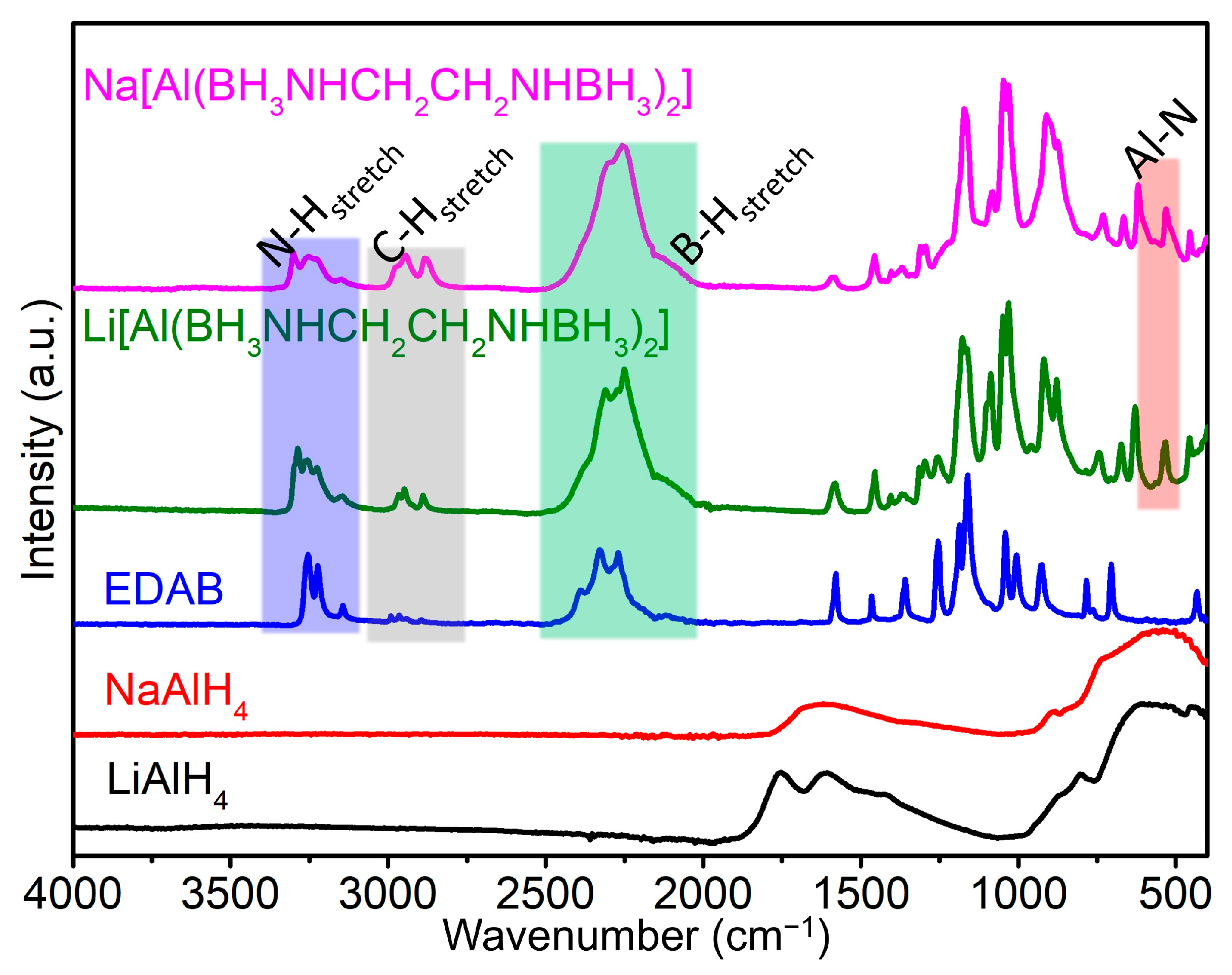
2.2.2. The IR Spectra of M[Al(BH3NHCH2CH2NHBH3)2] (M = Li and Na)
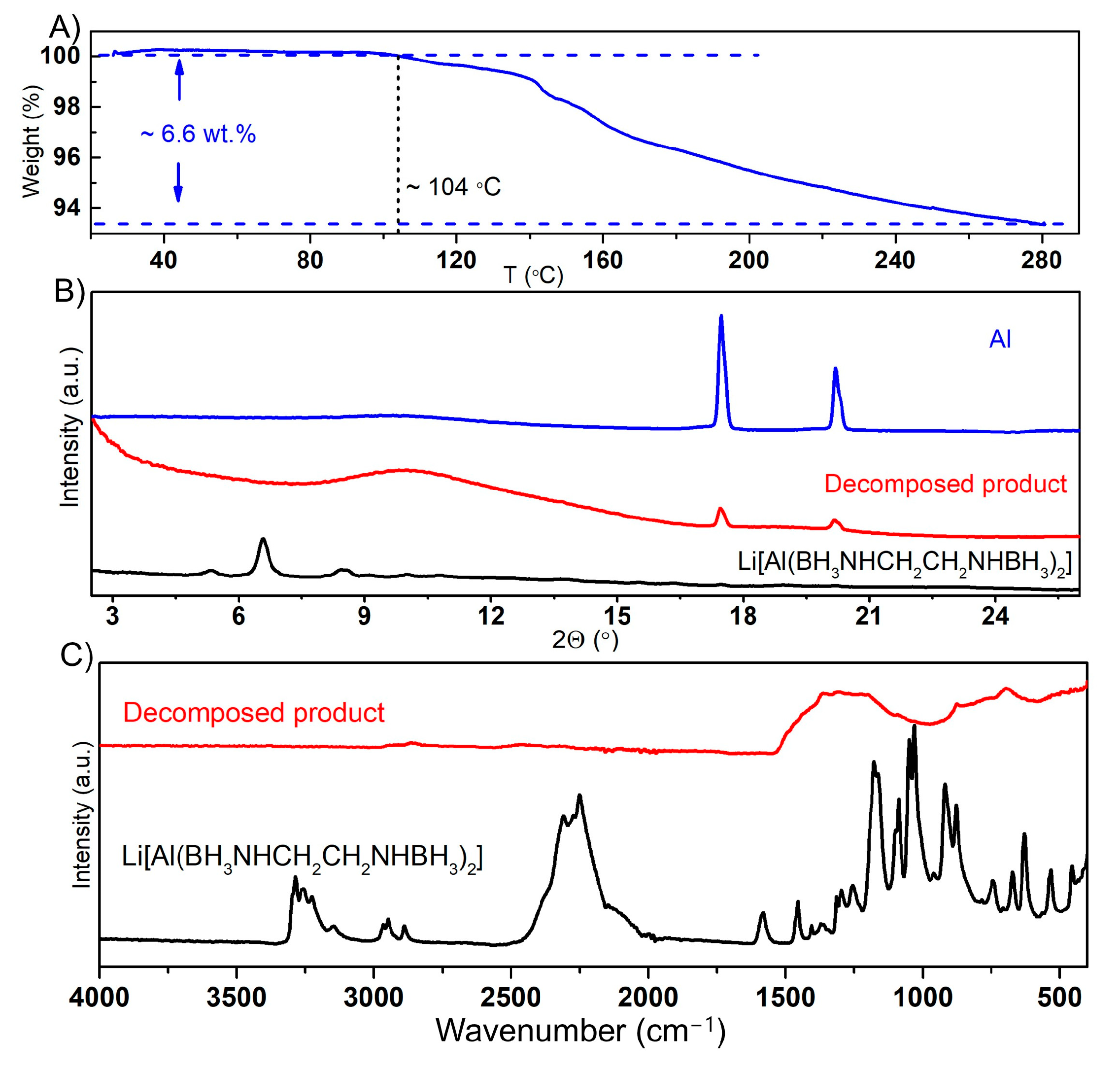
2.3. Thermal Dehydrogenation of M[Al(BH3NHCH2CH2NHBH3)2] (M = Li and Na)
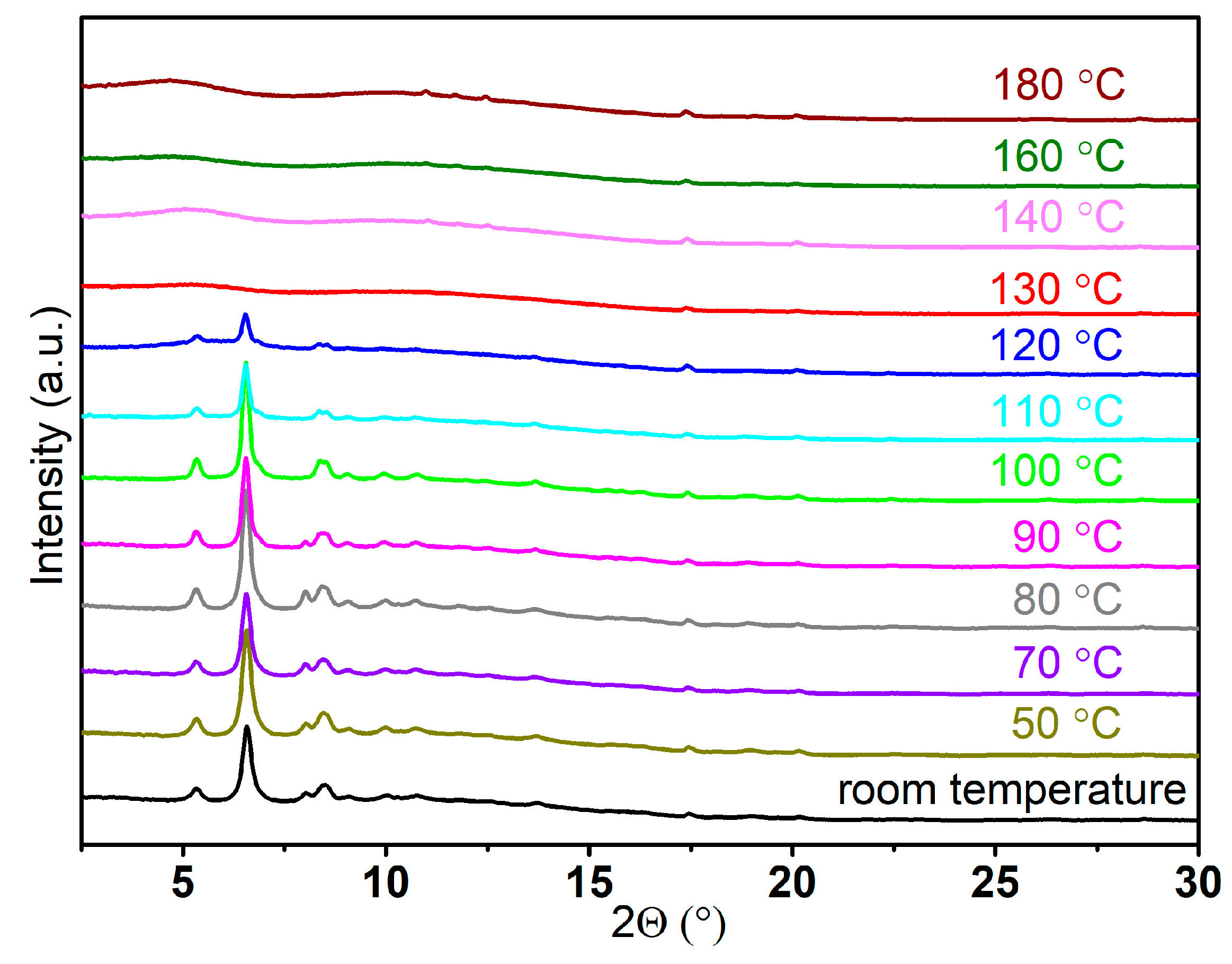
2.3.1. Thermal Dehydrogenation of Li[Al(BH3NHCH2CH2NHBH3)2]
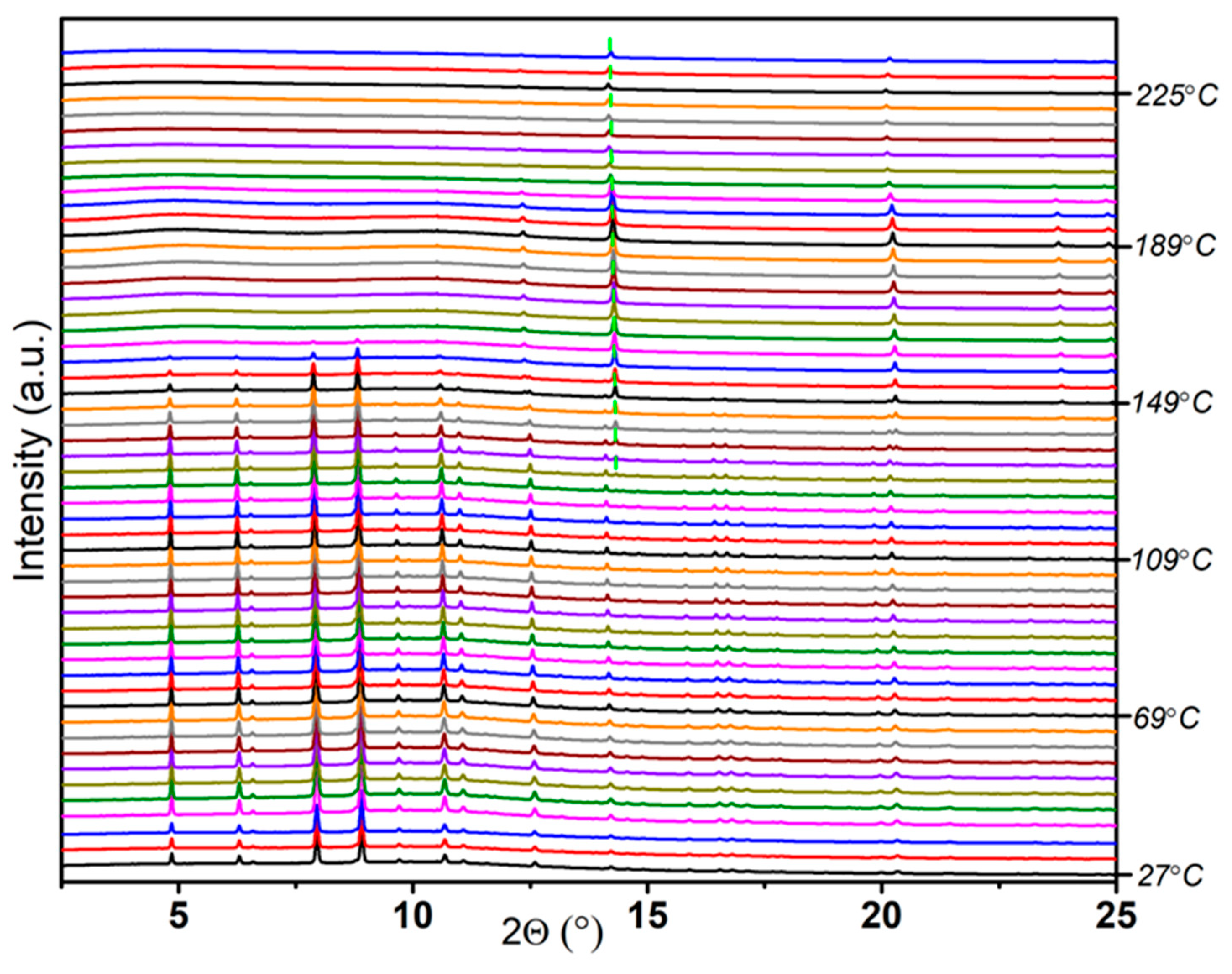
2.3.2. Thermal Dehydrogenation of Na(THF)[Al(BH3NHCH2CH2NHBH3)2]
3. Materials and Methods
3.1. Synthesis of BH3NH2CH2CH2NH2BH3 (EDAB)
3.2. Synthesis Method of Li[Al(BH3NHCH2CH2NHBH3)2]
3.3. Synthesis Method of Na(THF)[Al(BH3NHCH2CH2NHBH3)2]
3.4. NMR Experiments
3.5. Powder X-Ray Diffraction
3.6. Crystal Structure Determination
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
3.8. Thermogravimetric Analysis (TGA)
3.9. Mass Spectrometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabel, T.S.; Bassim, M. Reasons for Shifting and Barriers to Renewable Energy: A Literature Review. Int. J. Energy Econ. Policy 2020, 10, 89–94. [Google Scholar]
- Widera, B. Renewable Hydrogen Implementations for Combined Energy Storage, Transportation and Stationary Applications. Therm. Sci. Eng. Prog. 2020, 16, 100460. [Google Scholar]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of Hydrogen Storage Techniques For on board Vehicle Applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current Research Trends and Perspectives on Materials-Based Hydrogen Storage Solutions: A Critical Review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar]
- Schneemann, A.; White, J.L.; Kang, S.Y.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A Review on the Current Progress of Metal Hydrides Material for Solid-State Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar]
- Kumar, R.; Karkamkar, A.; Bowden, M.; Autrey, T. Solid-State Hydrogen Rich Boron–Nitrogen Compounds for Energy Storage. Chem. Soc. Rev. 2019, 48, 5350–5380. [Google Scholar]
- Dewangan, S.K.; Mohan, M.; Kumar, V.; Sharma, A.; Ahn, B. A Comprehensive Review of the Prospects for Future Hydrogen Storage in Materials-Application and Outstanding Issues. Int. J. Energy Res. 2022, 46, 16150–16177. [Google Scholar] [CrossRef]
- Abdechafik, E.H.; Ait Ousaleh, H.; Mehmood, S.; Filali Baba, Y.; Bürger, I.; Linder, M.; Faik, A. An Analytical Review of Recent Advancements on Solid-State Hydrogen Storage. Int. J. Hydrogen Energy 2024, 52, 1182–1193. [Google Scholar]
- Castilla-Martinez, C.A.; Moury, R.; Demirci, U.B. Amidoboranes and Hydrazinidoboranes: State of the Art, Potential for Hydrogen Storage, and Other Prospects. Int. J. Hydrogen Energy 2020, 45, 30731–30755. [Google Scholar] [CrossRef]
- Wang, K.; Pan, Z.; Yu, X. Metal B-N-H Hydrogen-Storage Compound: Development and Perspectives. J. Alloys Compd. 2019, 794, 303–324. [Google Scholar]
- Demirci, U.B. Mechanistic Insights into the Thermal Decomposition of Ammonia Borane, a Material Studied for Chemical Hydrogen Storage. Inorg. Chem. Front. 2021, 8, 1900–1930. [Google Scholar]
- Baitalow, F.; Baumann, J.; Wolf, G.; Jaenicke-Rößler, K.; Leitner, G. Thermal Decomposition of B–N–H Compounds Investigated by Using Combined Thermoanalytical Methods. Thermochim. Acta 2002, 391, 159–168. [Google Scholar] [CrossRef]
- Astorino, C.; De Nardo, E.; Lettieri, S.; Ferraro, G.; Bartoli, M.; Etzi, M.; Chiodoni, A.M.; Pirri, C.F.; Bocchini, S. Investigation of Solid-State Thermal Decomposition of Ammonia Borane Mix with Sulphonated Poly(Ellagic Acid) for Hydrogen Release. Polymers 2024, 16, 3471. [Google Scholar] [CrossRef]
- Chen, Y.; Lang, Z.; Feng, K.; Wang, K.; Li, Y.; Kang, Z.; Guo, L.; Zhong, J.; Lu, J. Practical H2 Supply from Ammonia Borane Enabled by Amorphous Iron Domain. Nat. Comm. 2024, 15, 9113. [Google Scholar]
- Xiong, Z.; Yong, C.K.; Wu, G.; Chen, P.; Shaw, W.; Karkamkar, A.; Autrey, T.; Jones, M.O.; Johnson, S.R.; Edwards, P.P.; et al. High-Capacity Hydrogen Storage in Lithium and Sodium Amidoboranes. Nat. Mater. 2007, 7, 138. [Google Scholar]
- Diyabalanage, H.V.K.; Nakagawa, T.; Shrestha, R.P.; Semelsberger, T.A.; Davis, B.L.; Scott, B.L.; Burrell, A.K.; David, W.I.F.; Ryan, K.R.; Jones, M.O.; et al. Potassium(I) Amidotrihydroborate: Structure and Hydrogen Release. J. Am. Chem. Soc. 2010, 132, 11836–11837. [Google Scholar]
- Wu, C.; Wu, G.; Xiong, Z.; Han, X.; Chu, H.; He, T.; Chen, P. LiNH2BH3·NH3BH3: Structure and Hydrogen Storage Properties. Chem. Mater. 2010, 22, 3–5. [Google Scholar]
- Owarzany, R.; Jaroń, T.; Leszczyński, P.J.; Fijalkowski, K.J.; Grochala, W. Amidoboranes of Rubidium and Cesium: The Last Missing Members of the Alkali Metal Amidoborane Family. Dalton Trans. 2017, 46, 16315–16320. [Google Scholar]
- Diyabalanage, H.V.K.; Shrestha, R.P.; Semelsberger, T.A.; Scott, B.L.; Bowden, M.E.; Davis, B.L.; Burrell, A.K. Calcium Amidotrihydroborate: A Hydrogen Storage Material. Angew. Chem. Int. Ed. 2007, 46, 8995–8997. [Google Scholar]
- Zhang, Q.; Tang, C.; Fang, C.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. Synthesis, Crystal Structure, and Thermal Decomposition of Strontium Amidoborane. J. Phys. Chem. C 2010, 114, 1709–1714. [Google Scholar] [CrossRef]
- Shcherbina, N.A.; Kazakov, I.V.; Timoshkin, A.Y. Synthesis and Characterization of Barium Amidoborane. Russ. J. Gen. Chem. 2017, 87, 2875–2877. [Google Scholar]
- Chua, Y.S.; Wu, G.; Xiong, Z.; Karkamkar, A.; Guo, J.; Jian, M.; Wong, M.W.; Autrey, T.; Chen, P. Synthesis, Structure and Dehydrogenation of Magnesium Amidoborane Monoammoniate. Chem. Commun. 2010, 46, 5752–5754. [Google Scholar]
- Luo, J.; Kang, X.; Wang, P. Synthesis, Formation Mechanism, and Dehydrogenation Properties of the Long-Sought Mg(NH2BH3)2 Compound. Energy Environ. Sci. 2013, 6, 1018–1025. [Google Scholar]
- Fijalkowski, K.J.; Genova, R.V.; Filinchuk, Y.; Budzianowski, A.; Derzsi, M.; Jaroń, T.; Leszczyński, P.J.; Grochala, W. Na[Li(NH2BH3)2]—The First Mixed-Cation Amidoborane with Unusual Crystal Structure. Dalton Trans. 2011, 40, 4407–4413. [Google Scholar] [CrossRef]
- Kang, X.; Luo, J.; Zhang, Q.; Wang, P. Combined Formation and Decomposition of Dual-Metal Amidoborane NaMg(NH2BH3)3 for High-Performance Hydrogen Storage. Dalton Trans. 2011, 40, 3799–3801. [Google Scholar]
- Wu, H.; Zhou, W.; Pinkerton, F.E.; Meyer, M.S.; Yao, Q.; Gadipelli, S.; Udovic, T.J.; Yildirim, T.; Rush, J.J. Sodium Magnesium Amidoborane: The First Mixed-Metal Amidoborane. Chem. Commun. 2011, 47, 4102–4104. [Google Scholar]
- Chua, Y.S.; Li, W.; Wu, G.; Xiong, Z.; Chen, P. From Exothermic to Endothermic Dehydrogenation—Interaction of Monoammoniate of Magnesium Amidoborane and Metal Hydrides. Chem. Mater. 2012, 24, 3574–3581. [Google Scholar]
- Biliškov, N.; Borgschulte, A.; Užarević, K.; Halasz, I.; Lukin, S.; Milošević, S.; Milanović, I.; Novaković, J.G. In-Situ and Real-Time Monitoring of Mechanochemical Preparation of Li2Mg(NH2BH3)4 and Na2Mg(NH2BH3)4 and Their Thermal Dehydrogenation. Chem.—A Eur. J. 2017, 23, 16274–16282. [Google Scholar]
- Milanović, I.; Biliškov, N.; Užarević, K.; Lukin, S.; Etter, M.; Halasz, I. Mechanochemical Synthesis and Thermal Dehydrogenation of Novel Calcium-Containing Bimetallic Amidoboranes. ACS Sustain. Chem. Eng. 2021, 9, 2089–2099. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Jalisatgi, S.S.; Safronov, A.V.; Lee, H.B.; Wu, J. Chemical Hydrogen Storage Using Polyhedral Borane Anions and Aluminum-Ammonia-Borane Complexes; University of Missouri: Columbia, MI, USA, 2010. [Google Scholar]
- Xia, G.; Tan, Y.; Chen, X.; Guo, Z.; Liu, H.; Yu, X. Mixed-Metal (Li, Al) Amidoborane: Synthesis and Enhanced Hydrogen Storage Properties. J. Mater. Chem. A 2013, 1, 1810–1820. [Google Scholar]
- Dovgaliuk, I.; Jepsen, L.H.; Safin, D.A.; Łodziana, Z.; Dyadkin, V.; Jensen, T.R.; Devillers, M.; Filinchuk, Y. A Composite of Complex and Chemical Hydrides Yields the First Al-Based Amidoborane with Improved Hydrogen Storage Properties. Chem.—A Eur. J. 2015, 21, 14562–14570. [Google Scholar]
- Yang, J.; Beaumont, P.R.; Humphries, T.D.; Jensen, C.M.; Li, X. Efficient Synthesis of an Aluminum Amidoborane Ammoniate. Energies 2015, 8, 9107–9116. [Google Scholar] [CrossRef]
- Møller, K.T.; Jørgensen, M.; Andreasen, J.G.; Skibsted, J.; Łodziana, Z.; Filinchuk, Y.; Jensen, T.R. Synthesis and Thermal Decomposition of Potassium Tetraamidoboranealuminate, K[Al(NH2BH3)4]. Int. J. Hydrogen Energy 2018, 43, 311–321. [Google Scholar]
- Zhang, T.; Steenhaut, T.; Li, X.; Devred, F.; Devillers, M.; Filinchuk, Y. Aluminum Methylamidoborane Complexes: Mechanochemical Synthesis, Structure, Stability, and Reactive Hydride Composites. Sustain. Energy Fuels 2023, 7, 1119–1126. [Google Scholar]
- Bowden, M.E.; Brown, I.W.M.; Gainsford, G.J.; Wong, H. Structure and Thermal Decomposition of Methylamine Borane. Inorganica Chim. Acta 2008, 361, 2147–2153. [Google Scholar]
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-Borane and Related Compounds as Dihydrogen Sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar]
- Leardini, F.; Valero Pedraza, M.J.; Perez Mayoral, E.; Cantelli, R.; Bañares, M.A. Thermolytic Decomposition of Ethane 1,2 Diamineborane Investigated by Thermoanalytical Methods and in Situ Vibrational Spectroscopy. J. Phys. Chem. C 2014, 118, 17221–17230. [Google Scholar]
- Nakagawa, Y.; Shinzato, K.; Nakagawa, T.; Nakajima, K.; Isobe, S.; Goshome, K.; Miyaoka, H.; Ichikawa, T. Synthesis, Structural Characterization, and Hydrogen Desorption Properties of Na[Al(NH2BH3)4]. Int. J. Hydrogen Energy 2017, 42, 6173–6180. [Google Scholar]
- Jacobs, H.; Jänichen, K.; Hadenfeldt, C.; Juza, R. Lithiumaluminiumamid, LiAl(NH2)4, Darstellung, Röntgenographische Untersuchung, Infrarotspektrum Und Thermische Zersetzung. Z. Anorg. Allg. Chem. 1985, 531, 125–139. [Google Scholar] [CrossRef]
- Lutz, H.D.; Lange, N.; Jacobs, H.; Nöcker, B. Gitterschwingungsspektren. Lxxi. Wasserstoffbrückenbindungen Und Synergetischer Effekt in Kristallinen Amiden Am Beispiel Von NaAl(NH2)4. Z. Anorg. Allg. Chem. 1992, 613, 83–87. [Google Scholar]
- Jacobs, H.; Nöcker, B. Neubestimmung Von Struktur Und Eigenschaften Isotyper Natriumtetraamidometallate Des Aluminiums Und Galliums. Z. Anorg. Allg. Chem. 1993, 619, 381–386. [Google Scholar]
- Favre-Nicolin, V.; Cerny, R. Fox, ‘Free Objects for Crystallography’: A Modular Approach to Ab Initio Structure Determination from Powder Diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar]
- Spek, A. Single-Crystal Structure Validation with the Program Platon. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar]
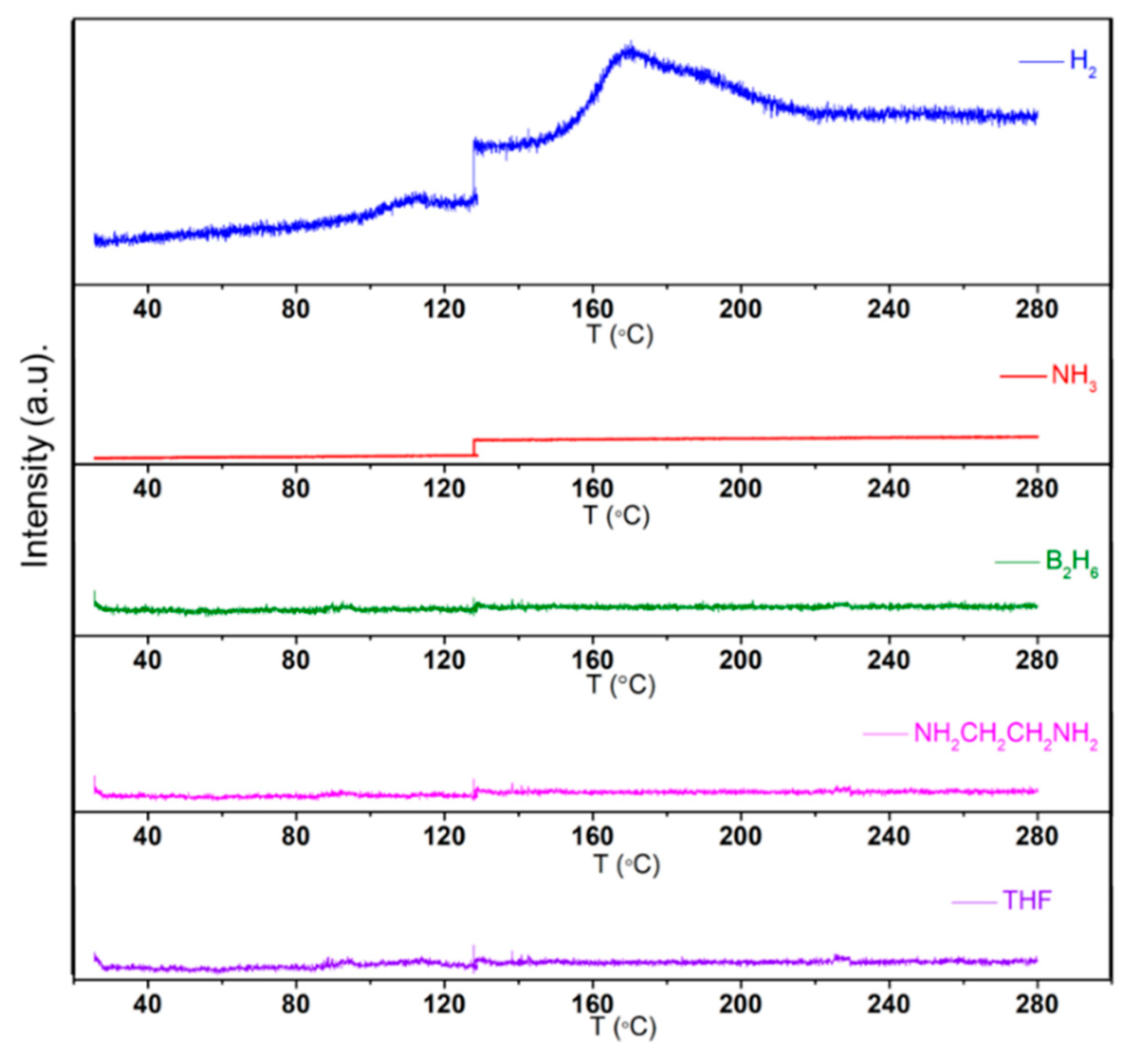


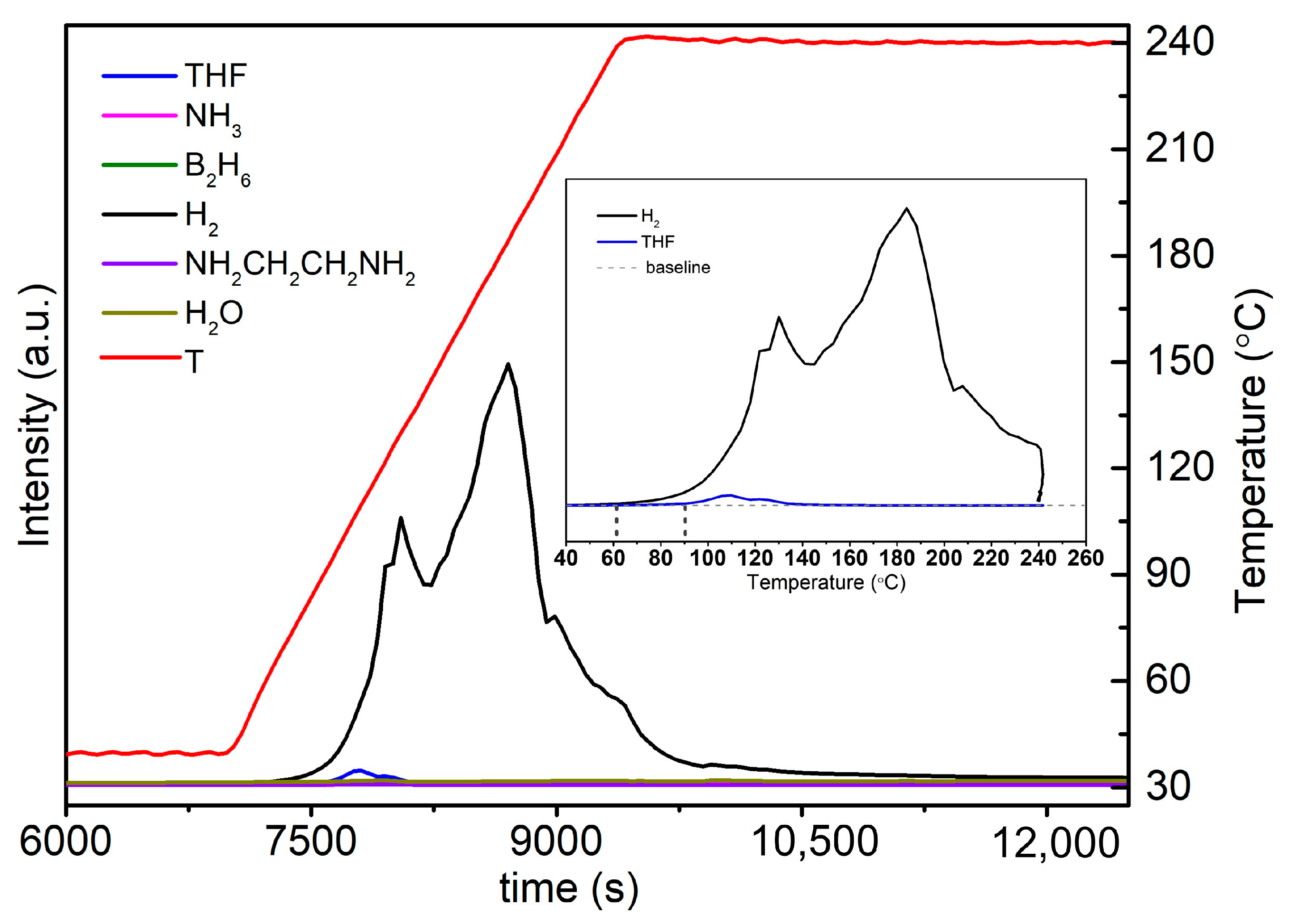

| Pstart (bar) | Tstart (°C) | Pend (bar) | Tend (°C) | Δn Gas (mmol) | Δn Gas/nLiAlH4 |
|---|---|---|---|---|---|
| 1.0 | 23.6 | 1.7 | 23.1 | 2.4 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Li, X.; Li, H.-W.; Devillers, M.; Filinchuk, Y. Synthesis, Structural Characterization, and Hydrogen Release of Al-Based Amidoboranes Derived from MAlH4 (Li, Na)-BH3NH2CH2CH2NH2BH3. Molecules 2025, 30, 1559. https://doi.org/10.3390/molecules30071559
Zhang T, Li X, Li H-W, Devillers M, Filinchuk Y. Synthesis, Structural Characterization, and Hydrogen Release of Al-Based Amidoboranes Derived from MAlH4 (Li, Na)-BH3NH2CH2CH2NH2BH3. Molecules. 2025; 30(7):1559. https://doi.org/10.3390/molecules30071559
Chicago/Turabian StyleZhang, Ting, Xiao Li, Hai-Wen Li, Michel Devillers, and Yaroslav Filinchuk. 2025. "Synthesis, Structural Characterization, and Hydrogen Release of Al-Based Amidoboranes Derived from MAlH4 (Li, Na)-BH3NH2CH2CH2NH2BH3" Molecules 30, no. 7: 1559. https://doi.org/10.3390/molecules30071559
APA StyleZhang, T., Li, X., Li, H.-W., Devillers, M., & Filinchuk, Y. (2025). Synthesis, Structural Characterization, and Hydrogen Release of Al-Based Amidoboranes Derived from MAlH4 (Li, Na)-BH3NH2CH2CH2NH2BH3. Molecules, 30(7), 1559. https://doi.org/10.3390/molecules30071559








