Opuntia stricta (Haw.) Fruit Pulp and Seeds as Source of Bioactive Phytochemicals with Promising Functional Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenol and Flavonoid Content
2.2. Chemical Profile of Opuntia Stricta Freeze-Dried Fruit Pulp (OSF) and Its Hydroalcoholic Extract (OSC)
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) of Opuntia Stricta Oil
2.4. Antioxidant Activity
2.5. Carbohydrate-Hydrolizing Enzymes and Lipase Inhibitory Activities
3. Materials and Methods
3.1. Plant Materials and Extraction Procedure
3.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.3. Total Phenolic, Flavonoid, and Carotenoid Compounds
3.4. Phytochemical Analysis of Opuntia Stricta Fruit Pulp Samples
3.5. Evaluation of Opuntia Stricta Samples’ Antioxidant Activities
3.6. Evaluation of α-Amylase, α-Glucosidase, and Lipase Inhibitory Activities
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilà, M.; Burriel, J.A.; Pino, J.; Chamizo, J.; Llach, E.; Porterias, M.; Vives, M. Association between Opuntia species invasion and changes in land-cover in the Mediterranean region. Glob. Change Biol. 2003, 9, 1234–1239. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; Merra, R.; Badalamenti, N.; Lazzara, G.; Bruno, M.; Sottile, F. Opuntia ficus-indica (L.) Mill. and Opuntia stricta (Haw.) Haw. Mucilage-Based Painting Binders for Conservation of Cultural Heritage. Sustainability 2023, 15, 14487. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Izquierdo-Vega, J.; Delgado-Olivares, L.; Vargas-Mendoza, N.; Álvarez-González, I.; Morales-González, Á.; et al. Opuntia spp. in Human Health: A Comprehensive Summary on Its Pharmacological, Therapeutic and Preventive Properties. Part 2. Plants 2022, 11, 2333. [Google Scholar] [CrossRef]
- Hultine, K.R.; Hernández-Hernández, T.; Williams, D.G.; Albeke, S.E.; Tran, N.; Puente, R.; Larios, E. Global change impacts on cacti (Cactaceae): Current threats, challenges and conservation solutions. Ann. Bot. 2023, 132, 671–683. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- ISTAT. Aspetti Della Vita Quotidiana. Fattori di Rischio per la Salute: Fumo, Obesità, Alcol e Sedentarietà; Italian National Institute of Health. ISS, Pass.: Rome, Italy, 2021; Available online: https://www.epicentro.iss.it/passi/dati/diabete (accessed on 10 February 2025).
- Liu, X.; Zeng, X.; Liu, W.; Lu, Y.; Cheng, J.; Chen, Y. An overview of dietary supplements on obesity and type 2 diabetes: Efficacy and mechanisms. Curr. Drug. Metab. 2021, 22, 415–440. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative stress in type 2 diabetes: Impacts from pathogenesis to lifestyle modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Loukili, E.H.; Abrigach, F.; Bouhrim, M.; Bnouham, M.; Fauconnier, M.L.; Ramdani, M. Chemical composition and physicochemical analysis of Opuntia dilleniii extracts grown in Morocco. J. Chem. 2021, 2021, 8858929. [Google Scholar] [CrossRef]
- Loukili, E.H.; Merzouki, M.; Taibi, M.; Elbouzidi, A.; Hammouti, B.; Kumar Yadav, K.; Khalid, M.; Addi, M.; Ramdani, M.; Kumar, P.; et al. Phytochemical, biological, and nutritional properties of the prickly pear, Opuntia dilleniii: A review. Saudi Pharm. J. 2024, 32, 102167. [Google Scholar] [CrossRef]
- Bouhrim, M.; Ouassou, H.; Boutahiri, S.; Daoudi, N.E.; Mechchate, H.; Gressier, B.; Eto, B.; Imtara, H.; Alotaibi, A.A.; Al-Zharani, M.; et al. Opuntia dilleniiid (Ker Gawl.) Haw., seeds oil antidiabetic potential using in vivo, in vitro, in situ, and ex vivo approaches to reveal its underlying mechanism of action. Molecules 2021, 26, 1677. [Google Scholar] [CrossRef]
- Montalbano, G.; La Rosa, M. Flora della Sicilia; Palumbo Editore: Palermo, Italy, 2010; pp. 1–900. [Google Scholar]
- Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760. [Google Scholar] [CrossRef] [PubMed]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Chahdoura, H.; Mzoughi, Z.; Ellouze, I.; Generalić Mekinić, I.; Čmiková, N.; El Bok, S.; Majdoub, H.; Ben Hsouna, A.; Ben Saad, R.; Mnif, W.; et al. Opuntia species: A comprehensive review of chemical composition and bio-pharmacological potential with contemporary applications. S. Afr. J. Bot. 2024, 174, 645–677. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef]
- Shirazinia, R.; Golabchifar, A.A.; Rahimi, V.B.; Jamshidian, A.; Samzadeh-Kermani, A.; Hasanein, P.; Hajinezhad, M.; Askari, V.R. Protective Effect of Opuntia dilleniii Haw Fruit Against Lead Acetate-Induced Hepatotoxicity: In Vitro and In Vivo Studies. Evid. Based Complement. Alternat. Med. 2021, 2021, 6698345. [Google Scholar] [CrossRef]
- Marhri, A.; Rbah, Y.; Allay, A.; Boumediene, M.; Tikent, A.; Benmoumen, A.; Melhaoui, R.; Elamrani, A.; Abid, M.; Addi, M. Comparative Analysis of Antioxidant Potency and Phenolic Compounds in Fruit Peel of Opuntia robusta, Opuntia dilleniii, and Opuntia ficus-indica Using HPLC-DAD Profiling. J. Food Qual. 2024, 2024, 2742606. [Google Scholar] [CrossRef]
- Reis, C.M.G.; Gouveia, C.; Vitorino, M.C.; Gazarini, L.C.; Ribeiro, M.M.; Peres, F. Bioactive compounds and morphology In Opuntia spp. fruits from Portuguese ecotypes. Bulg. J. Agric. Sci. 2017, 23, 929–938. [Google Scholar]
- Díaz-Medina, E.M.; Rodríguez-Rodríguez, E.M.; Díaz-Romero, C. Chemical characterization of Opuntia dilleniii and Opuntia ficus-indica fruits. Food Chem. 2007, 103, 38–45. [Google Scholar]
- Moussa-Ayoub, T.E.; El-Samahy, S.K.; Rohn, S.; Kroh, L.W. Flavonols, betacyanins content and antioxidant activity of Cactus Opuntia macrorhiza fruits. Food Res. Int. 2011, 44, 2169–2174. [Google Scholar]
- Kunyanga, C.N.; Imungi, J.K.; Vadivel, V. Nutritional quality, phytochemical composition and health protective effects of an under-utilized prickly cactus fruit (Opuntia stricta Haw.) collected from Kenya. Afr. J. Food Agric. Develop. 2014, 7, 9561–9577. [Google Scholar] [CrossRef]
- Florian, C.S.; Jürgen, C.; Iris, K.; Uwe, B.; Reinhold, C. Structural investigations on betacyanin pigments by LC NMR and 2D NMR spectroscopy. Phytochemistry 2004, 65, 415–422. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC-DAD-ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef]
- Otalora, C.M.; Bonifazi, E.; Fissore, E.N.; Basanta, F.; Gerschenson, L.N. Thermal Stability of Betalains in By-Products of the Blanching and Cutting of Beta vulgaris L. var conditiva. Pol. J. Food Nutr. Sci. 2020, 70, 15–24. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. HPLC Characterization of Betalains from Plants in the Amaranthaceae. J. Chromatogr. Sci. 2005, 43, 454–460. [Google Scholar] [CrossRef]
- Gómez-López, I.; Mendiola, J.A.; Portillo, M.P.; Cano, M.P. Pressurized green liquid extraction of betalains and phenolic compounds from Opuntia stricta var. dilleniii whole fruit: Process optimization and biological activities of green extracts. Innov. Food Sci. Emerg. Technol. 2022, 80, 103066. [Google Scholar] [CrossRef]
- Abdnim, R.; Lafdil, F.Z.; Elrherabi, A.; El Fadili, M.; Kandsi, F.; Benayad, O.; Legssyer, A.; Ziyyat, A.; Mekhfi, H.; Bnouham, M. Fatty acids characterisation by GC-MS, antiglycation effect at multiple stages and protection of erythrocytes cells from oxidative damage induced by glycation of albumin of Opuntia ficus-indica (L.) Mill seed oil cultivated in Eastern Morocco: Experimental and computational approaches. J. Ethnopharmacol. 2024, 329, 118106. [Google Scholar] [CrossRef]
- Ghazi, Z.; Ramdani, M.; Fauconnier, M.L.; El Mahi, B.; Cheikh, R. Fatty acids Sterols and Vitamin E composition of seed oil of Opuntia ficus-indica and Opuntia dilleniii from Morocco. J. Mater. Environ. Sci. 2013, 4, 967. [Google Scholar]
- El Mannoubi, I. Tunisian Opuntia stricta seed oil: Extraction, characterization, and prediction of fatty acid methyl ester properties as biodiesel fuel. Chem. Nat. Compd. 2021, 57, 614–619. [Google Scholar] [CrossRef]
- Alsaad, A.J.A.; Altemimi, A.B.; Aziz, S.N.; Lakhssassi, N. Extraction and Identification of Cactus Opuntia dilleniii Seed Oil and Its added Value for Human Health Benefits. Pharmacogn. J. 2019, 11, 579–587. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef]
- Katanić, J.; Yousfi, F.; Caruso, M.C.; Matić, S.; Monti, D.M.; Loukili, E.H.; Boroja, T.; Mihailović, V.; Galgano, F.; Imbimbo, P.; et al. Characterization of bioactivity and phytochemical composition with toxicity studies of different Opuntia dilleniii extracts from Morocco. Food Biosci. 2019, 30, 100410. [Google Scholar] [CrossRef]
- Ghazi, Z.; Ramdani, M.; Tahri, M.; Rmili, R.; Elmsellem, H.; El Mahi, B.; Fauconnier, M.L. Chemical composition and antioxidant activity of seeds oils and fruit juice of Opuntia ficus indica and Opuntia dilleniii from Morocco. J. Mater. 2015, 6, 2338–2345. [Google Scholar]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef]
- Sakihama, Y.; Kato, T.; Sawatdee, S.; Yakushi, Y.; Asano, J.; Hayashi, H.; Goto, Y.; Hashimoto, M.; Hashidoko, Y. Isolation of High-Purity Betanin from Red Beet and Elucidation of Its Antioxidant Activity Against Peroxynitrite: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 15411. [Google Scholar] [CrossRef]
- Muramatsu, D.; Uchiyama, H.; Higashi, H.; Kida, H.; Iwai, A. Effects of heat degradation of betanin in red beetroot (Beta vulgaris L.) on biological activity and antioxidant capacity. PLoS ONE 2023, 18, e0286255. [Google Scholar] [CrossRef]
- Gliszczyńska-Swigło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef]
- Tsai, P.J.; Sheu, C.H.; Wu, P.H.; Sun, Y.F. Thermal and pH stability of betacyanin pigment of Djulis (Chenopodium formosanum) in Taiwan and their relation to antioxidant activity. J. Agric. Food Chem. 2010, 58, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Vieira Teixeira da Silva, D.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [PubMed]
- Airen, Z.; Yu, Y.; Jing, L.; Xu, B.; Yu, X.; Qiu, Y.; Cao, S. Study on the relation of structure and antioxidant activity of isorhamnetin, quercetin, phloretin, silybin and phloretin isonicotinyl hydrazone. Free Rad. Antiox. 2011, 1, 39–47. [Google Scholar] [CrossRef]
- Mhiri, R.; Soltani, S.; Baccouche, N.; Allouche, N.; Hichem, B.S. Chemical composition and biological activities of essential oils from two Opuntia species growing in Tunisia: An in vitro and in silico studies. J. Essent. Oil Bear. Plants 2024, 27, 1460–1470. [Google Scholar] [CrossRef]
- Dubey, K.; Dubey, R.; Gupta, R.; Gupta, A. α-Amylase, α-glucosidase and aldose reductase inhibitory potential of betanin for the management of diabetes and its complications. J. Adv. Sci. Res. 2020, 11, 92–95. [Google Scholar]
- Lee, H.S.; Choi, S.M.; Lim, S.H.; Choi, C.-I. Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes. Pharmaceuticals 2023, 16, 1727. [Google Scholar] [CrossRef]
- Serrano-Sandoval, S.N.; Parralejo-Sanz, S.; Lobo, M.G.; Cano, M.P.; Antunes-Ricardo, M. A bio-guided search of anti-steatotic compounds in Opuntia stricta var. dilleniii by fast centrifugal partition chromatography. Food Chem. 2025, 464 Pt 2, 141682. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Inhibitory effects on the digestive enzyme alpha-amylase of three Salsola species (Chenopodiaceae) in vitro. Pharmazie 2007, 62, 473–475. [Google Scholar]
- Metibemu, D.S.; Saliu, J.A.; Metibemu, A.O.; Oluwadahunsi, O.J.; Oboh, G.; Omotuyi, I.O.; Akinloye, O.A. Molecular Docking Studies of Isorhamnetin from Corchorus olitorius with Target Alpha-Amylase Related to Type 2 Diabetes. J. Chem. Pharm. Res. 2016, 8, 1262–1266. [Google Scholar]
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In Vitro Studies to Assess the α-Glucosidase Inhibitory Activity and Insulin Secretion Effect of Isorhamnetin 3-O-Glucoside and Quercetin 3-O-Glucoside Isolated from Salicornia herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Y.; Ma, Y.; Cheng, G.; Cai, S. Phenolic Composition, Antioxidant Properties, and Inhibition toward Digestive Enzymes with Molecular Docking Analysis of Different Fractions from Prinsepia utilis Royle Fruits. Molecules 2018, 23, 3373. [Google Scholar] [CrossRef]
- González-Arceo, M.; Gomez-Lopez, I.; Carr-Ugarte, H.; Eseberri, I.; González, M.; Cano, M.P.; Portillo, M.P.; Gómez-Zorita, S. Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. Int. J. Mol. Sci. 2023, 24, 299. [Google Scholar] [CrossRef] [PubMed]
- Türkyilmaz, M.; Tagı, S.; Dereli, U.; Ozkan, M. Effects of various pressing programs and yields on the antioxidant activity, antimicrobial activity, phenolic content and colour of pomegranate juices. Food Chem. 2013, 138, 1810–1818. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Leporini, M.; Sicari, V.; Falco, T.; Pellicanò, M.T.; Tundis, R. Investigating the in vitro hypoglycaemic and antioxidant properties of Citrus × clementina Hort. Juice. Eur. Food Res. Technol. 2017, 244, 523–534. [Google Scholar] [CrossRef]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan Prickly pear fruits (Opuntia ficus indica). Arab. J. Chem. 2016, 9 (Suppl. S1), S278–S281. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Leporini, M.; D’Urso, G.; Gagliano Candela, R.; Falco, T.; Piacente, S.; Bruno, M.; Sottile, F. Almond (Prunus dulcis cv. Casteltermini) Skin Confectionery By-Products: New Opportunity for the Development of a Functional Blackberry (Rubus ulmifolius Schott) Jam. Antioxidants 2021, 10, 1218. [Google Scholar] [CrossRef]

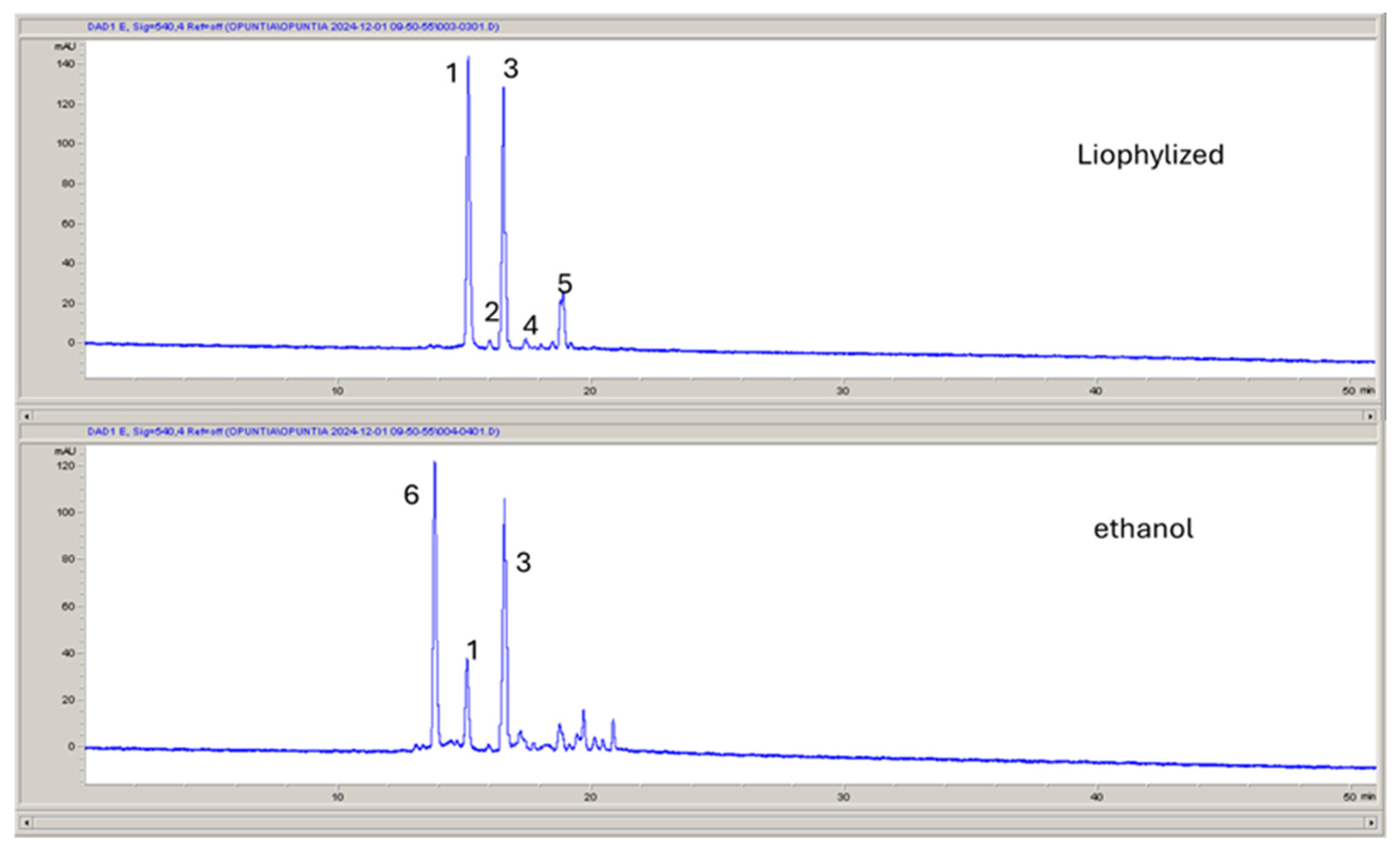
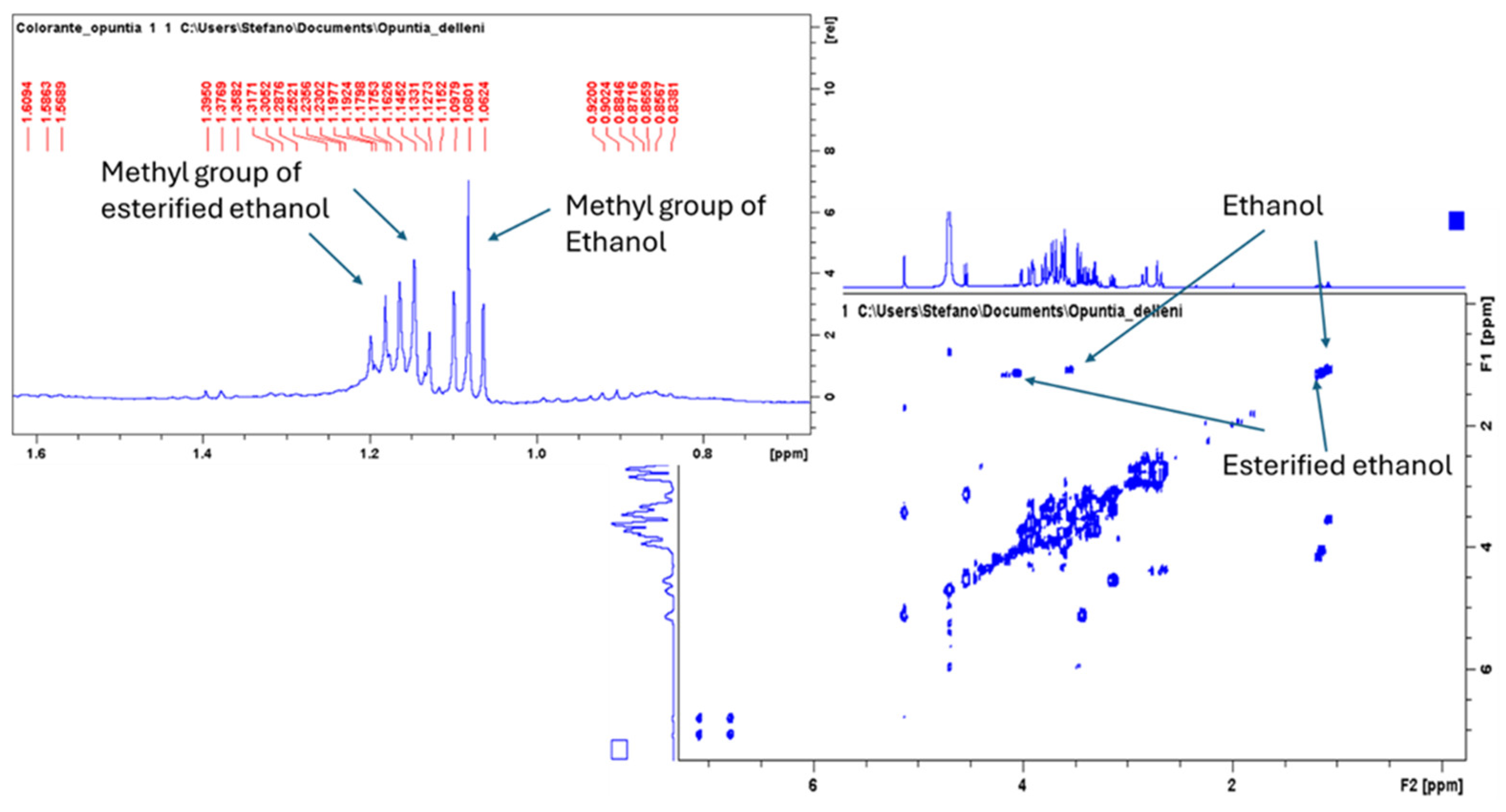
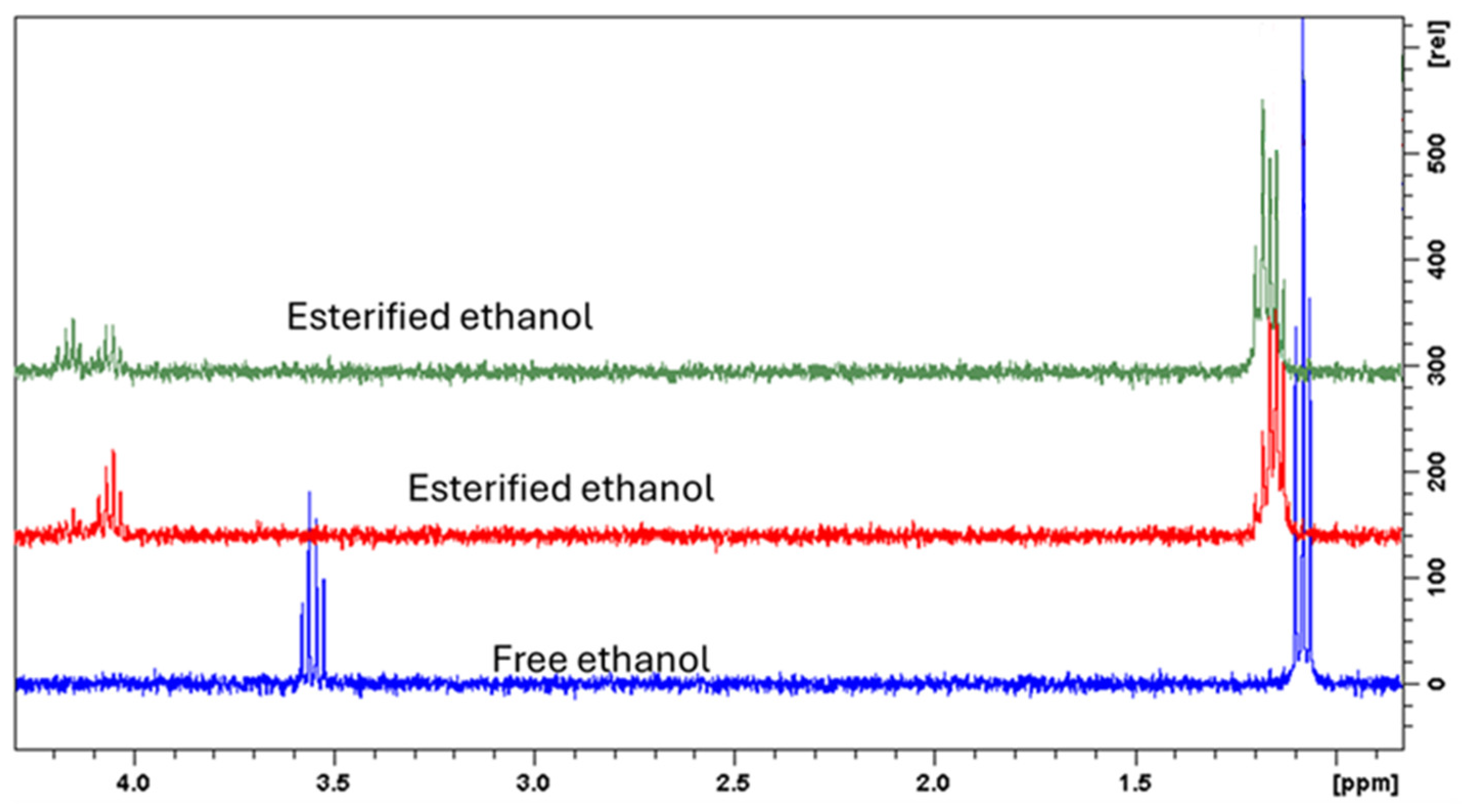

| Samples | Total Phenol Content (TPC) (mg of Chlorogenic Acid Equivalent (CAE)/g Fresh Weight (FW)) | Total Flavonoid Content (TFC) (mg of Quercetin Equivalent (QE)/g Fresh Weight (FW)) |
|---|---|---|
| OSF | 546.09 ± 11.34 a | 276.02 ± 6.31 a |
| OSC | 458.12 ± 10.65 b | 125.23 ± 5.20 b |
| Sign. | *** | *** |
| Retention Time | [M + H]+ | Fragments | Identification | Freeze-Dried Fruit Pulp Sample | Freeze-Dried Fruit Pulp Hydroalcoholic Extract |
|---|---|---|---|---|---|
| 15.3 | 551 | MS2: 389 MS3(389):345 343 301 246 194 150 | Betanin | √ | √ |
| 16.7 | 551 | MS2: 389 MS3(389):345 343 301 246 194 150 | Isobetanin | √ | √ |
| 11.9 | 507 | MS2: 345 MS3(345) 301 314 150 | Decarboxy betanin | √ | |
| 12.9 | 507 | MS2: 345 MS3(345) 301 314 150 | Decarboxy betanin | √ | |
| 13.9 | 507 | MS2: 345 MS3(345) 301 314 150 | Decarboxy betanin | √ | |
| Retention time | [M + 2H]2+ | Fragments | Identification | Freeze-dried sample | Hydroalcoholic extract |
| 12.3 | 318 | 157 | Triethanol-betanin | √ | |
| Retention time | [M − H]− | Fragments | Identification | Freeze-dried sample | Hydroalcoholic extract |
| 12.0 | 255 | Piscidic acid | √ | √ | |
| 26.7 | 623 | 315 | Isorhamnetin-3-O-rutinoside | √ | √ |
| 26.4 | 477 | 315 | Isorhamnetin-3-O-glucoside | √ | √ |
| Fatty Acids | % |
|---|---|
| Myristic acid (C14:0) | 0.25 ± 0.01 |
| Palmitoleic acid (C16:1) | 1.55 ± 0.65 |
| Palmitic acid (C16:0) | 19.32 ± 1.28 |
| Linoleic acid (C18:2) | 41.95 ± 2.66 |
| Oleic acid (C18:1) | 8.03 ± 1.33 |
| Stearic acid (C18:0) | 10.84 ± 1.85 |
| Gondoic acid (C20:1) | 2.01 ± 0.07 |
| Arachidic acid (C20:0) | 1.55 ± 0.65 |
| Erucid acid (C22:1) | 0.65 ± 0.03 |
| Behenic acid (C22:0) | 1.08 ± 0.08 |
| Lignoceric acid (C24:0) | 0.58 ± 0.02 |
| Total identified | 87.81 |
| SFA | 33.62 |
| UFA | 54.19 |
| UFA/SFA | 1.61 |
| Oleic/linoleic | 0.20 |
| Sample | DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) | β-Carotene Bleaching Test IC50 (µg/mL) | FRAP ^ μMFe (II)/g |
|---|---|---|---|---|
| OSF | 23.77 ± 1.26 a | 14.82 ± 1.33 b | 25.78 ± 1.23 a | 89.91 ± 3.26 a |
| OSC | 44.94 ± 2.65 b | 13.24 ± 1.56 a | 23.15 ± 1.44 b | 76.89 ± 3.12 b |
| OSS | 57.91 ± 2.48 c | 32.40 ± 2.12 b | 75.99 ± 3.45 c | 23.15 ± 1.23 c |
| Sign. | *** | *** | *** | *** |
| Ascorbic acid | 5.10 ± 0.98 | 1.71 ± 0.86 | ||
| Propyl gallate | 0.09 ± 0.00 | |||
| BHT | 63.02 ± 11.45 |
| Sample | α-Amylase | α-Glucosidase | Pancreatic lipase |
|---|---|---|---|
| OSF | 105.43 ± 3.77 a | 130.37 ± 3.85 a | 33.54 ± 3.92 a |
| OSC | 415.50 ± 3.56 b | 158.51 ± 3.77 b | 81.77 ± 3.44 b |
| OSS | 459.44 ± 3.56 b | 169.94 ± 3.81 c | 100.45 ± 3.44 c |
| Sign. | *** | *** | *** |
| Acarbose | 50.18 ± 1.32 | 35.57 ± 1.99 | |
| Orlistat | 37.44 ± 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, R.; Badalamenti, N.; Dall’Acqua, S.; Tundis, R.; Bruno, M.; Sottile, F.; Sut, S.; Loizzo, M.R. Opuntia stricta (Haw.) Fruit Pulp and Seeds as Source of Bioactive Phytochemicals with Promising Functional Properties. Molecules 2025, 30, 1580. https://doi.org/10.3390/molecules30071580
Pino R, Badalamenti N, Dall’Acqua S, Tundis R, Bruno M, Sottile F, Sut S, Loizzo MR. Opuntia stricta (Haw.) Fruit Pulp and Seeds as Source of Bioactive Phytochemicals with Promising Functional Properties. Molecules. 2025; 30(7):1580. https://doi.org/10.3390/molecules30071580
Chicago/Turabian StylePino, Roberta, Natale Badalamenti, Stefano Dall’Acqua, Rosa Tundis, Maurizio Bruno, Francesco Sottile, Stefania Sut, and Monica Rosa Loizzo. 2025. "Opuntia stricta (Haw.) Fruit Pulp and Seeds as Source of Bioactive Phytochemicals with Promising Functional Properties" Molecules 30, no. 7: 1580. https://doi.org/10.3390/molecules30071580
APA StylePino, R., Badalamenti, N., Dall’Acqua, S., Tundis, R., Bruno, M., Sottile, F., Sut, S., & Loizzo, M. R. (2025). Opuntia stricta (Haw.) Fruit Pulp and Seeds as Source of Bioactive Phytochemicals with Promising Functional Properties. Molecules, 30(7), 1580. https://doi.org/10.3390/molecules30071580















