Optimization of Ultrasonic-Assisted Extraction of Diene Urushiol from Lacquer Tree Leaves Using Response Surface Methodology
Abstract
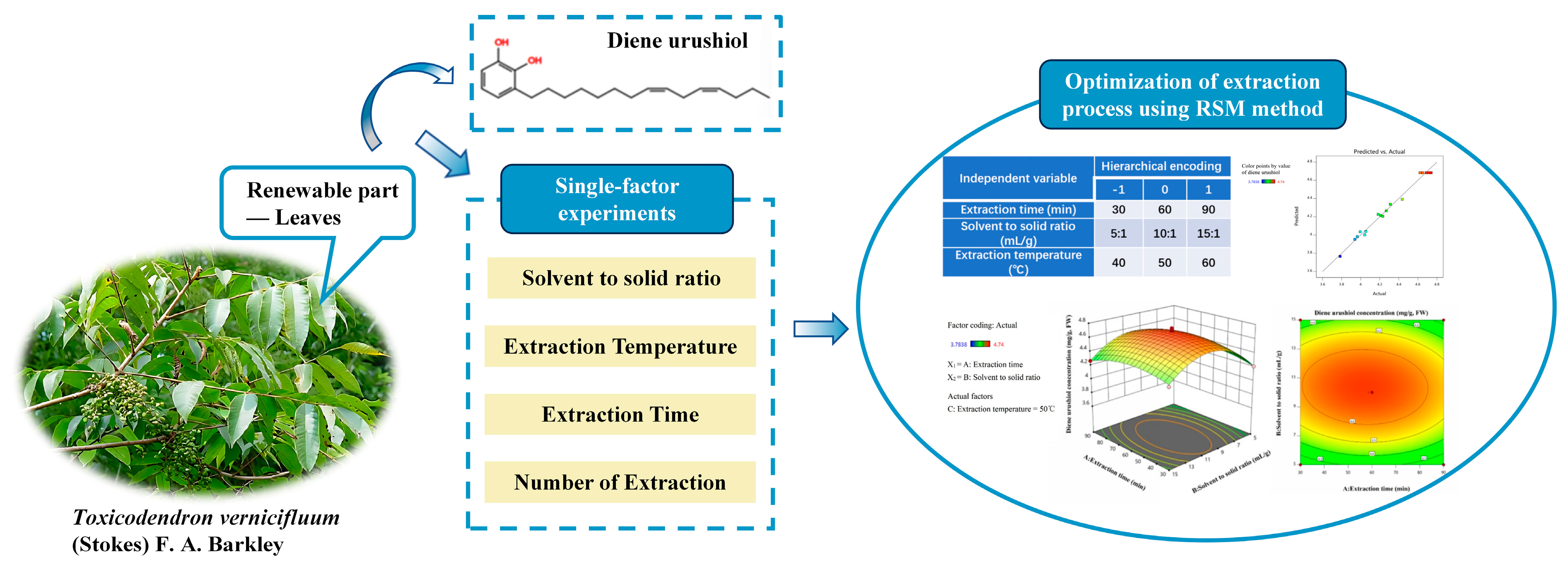
1. Introduction
2. Results and Discussion
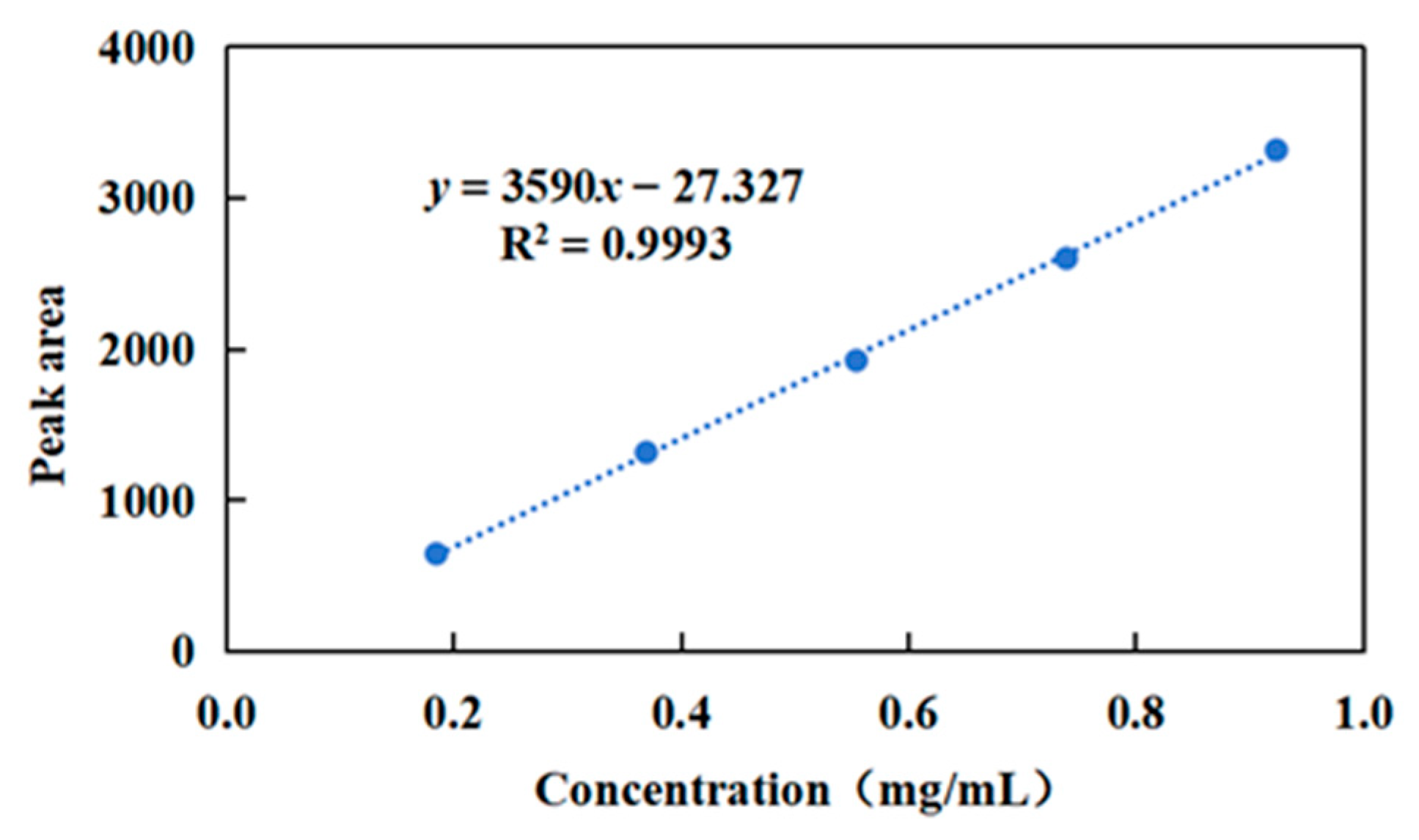
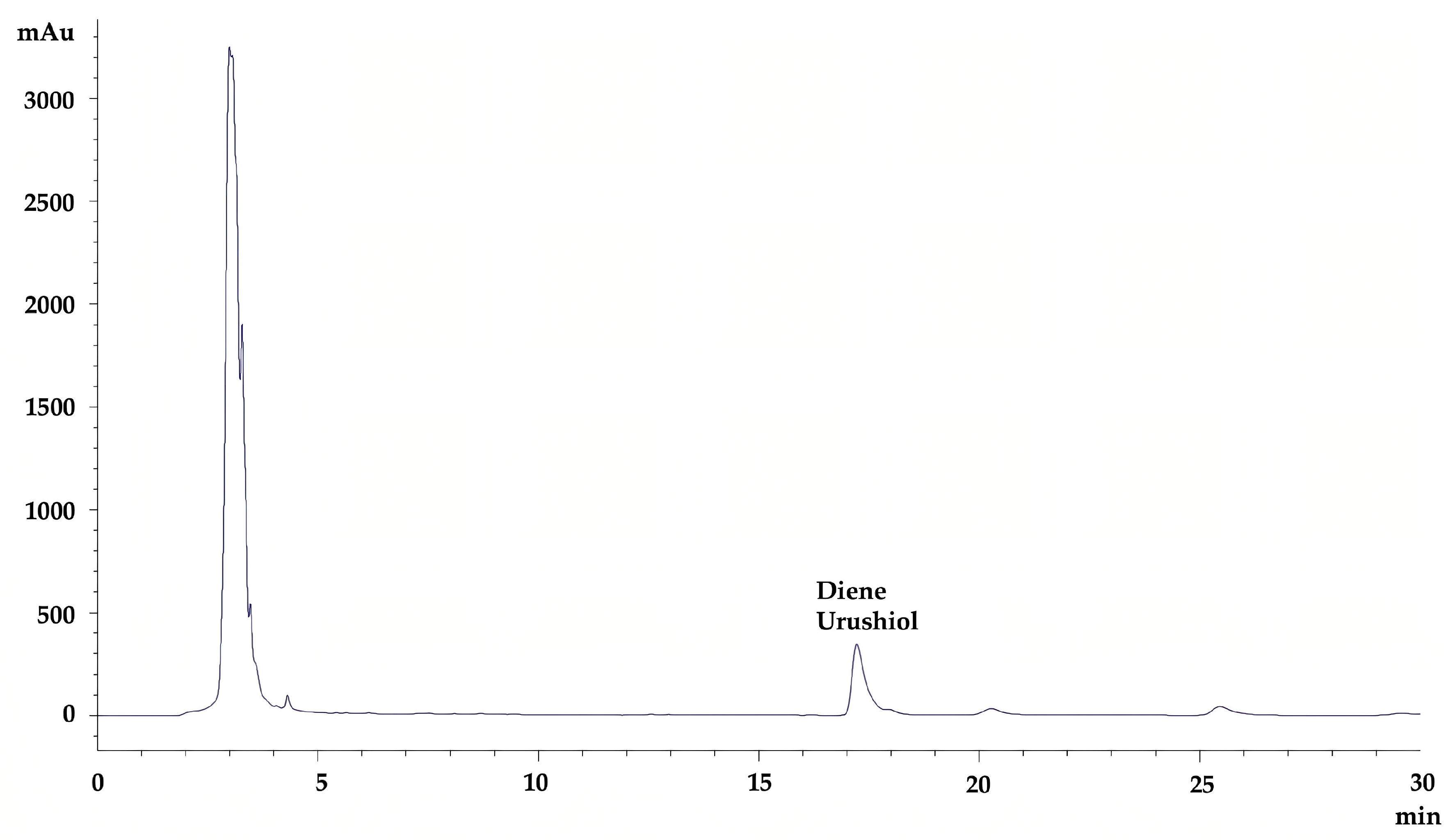
2.1. High-Performance Liquid Chromatography (HPLC) Analysis
2.2. Results of Single-Factor Experiments
2.2.1. Effect of Solvent to Solid Ratio on the Extraction Content of Diene Urushiol
2.2.2. Effect of Extraction Time on the Extraction Content of Diene Urushiol
2.2.3. Effect of Extraction Temperature on the Extraction Content of Diene Urushiol
2.2.4. Effect of the Number of Extractions on the Extraction Content of Diene Urushiol
2.3. Establishment of the Response Surface Box–Behnken Design
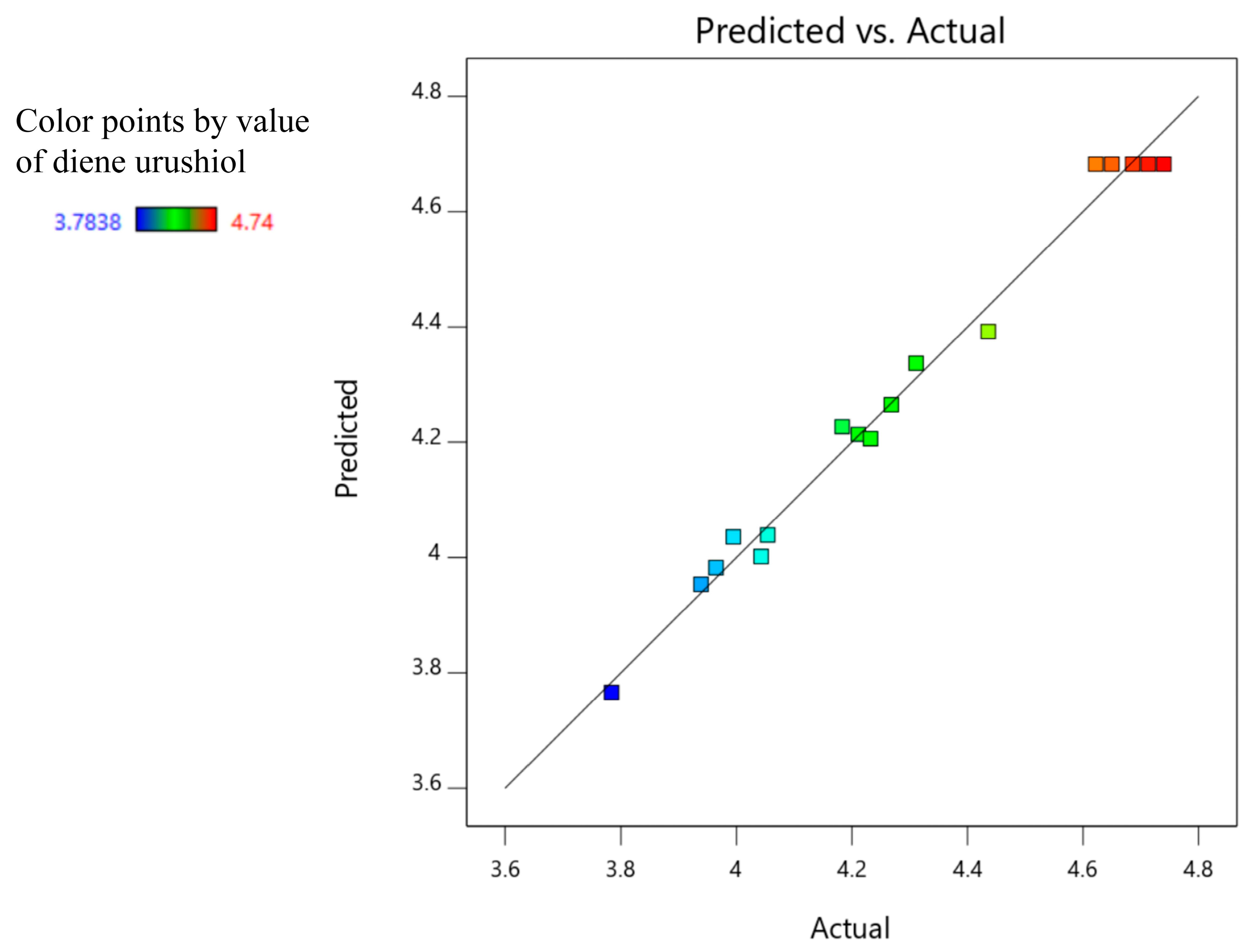
2.4. Model Fitting Statistics and Variance Analysis
0.1101A2 − 0.3166B2 + 0.4192C2 (R2 = 0.9876)
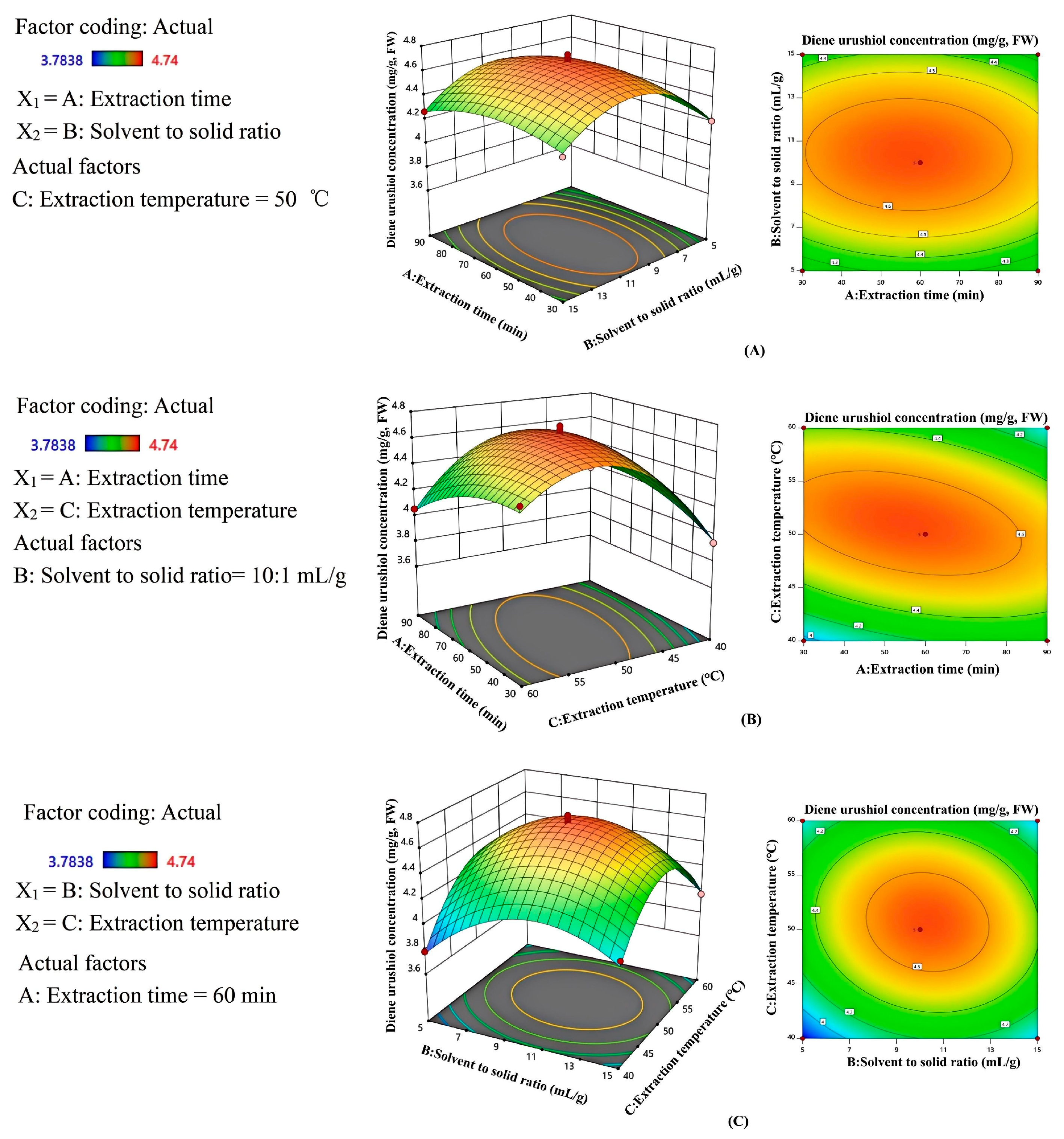
2.5. Analysis of Response Surface Interactions
2.6. Model Validation
3. Materials and Methods
3.1. Materials and Reagents
3.2. Quantitative Determination of Diene Urushiol by HPLC
3.3. Single-Factor Experimental Design
3.4. Response Surface Design
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.C.; Zhang, Y.Q.; Meng, C.W.; Gao, J.G.; Xie, C.J.; Liu, J.Y.; Xu, Y.N. Traditional uses, phytochemistry, and pharmacology of Toxicodendron vernicifluum (Stokes) F.A. Barkley—A review. J. Ethnopharmacol. 2021, 276, 113476. [Google Scholar] [CrossRef]
- Du, Y.; Oshima, R.; Kumanotani, J. Reversed-phase liquid chromatographic separation and identification of constituents of uroshiol in the sap of the lac tree, rhus vernicifera. J. Chromatogr. A 1984, 284, 463–473. [Google Scholar] [CrossRef]
- Lim, K.T.; Hu, C.; Kitts, D.D. Antioxidant activity of a Rhus verniciflua stokes ethanol extract. Food Chem. Toxicol. 2001, 39, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Ryu, J.H. Urushiol V suppresses cell proliferation and enhances antitumor activity of 5-fu in human colon cancer cells by downregulating foxm1. Biomol. Ther. 2022, 30, 257–264. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Chun, W.J.; Kim, T.Y.; Sun, J.; Yu, C.Y.; Kim, M.J. Rhus verniciflua stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010, 120, 539–543. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kang, J.Y.; Oh, M.J. Antiviral activities of flavonoids isolated from the bark of Rhus verniciflua stokes against fish pathogenic viruses in vitro. J. Microbiol. 2012, 50, 293–300. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, W.C.; Barshinikov, A.M.; Kobayashi, A. Inhibitory effects of the extract of Rhus verniciflua stokes on the reverse transcriptase of aids. Korean J. Med. Crop Sci. 2002, 10, 284–287. [Google Scholar]
- Resnick, S.D. Poison-ivy and poison-oak dermatitis. Clin. Dermatol. 1986, 4, 208–212. [Google Scholar] [CrossRef]
- Reda, F.M.; Alagawany, M.; Alsolami, A.M.; Mahmoud, H.K.; Salah, A.S.; Momenah, M.A.; Alshahrani, M.Y.; EL-Tarabily, K.A.; Saleh, R.M. The incorporation of sumac seed powder (Rhus coriaria L.) into the diet of quail breeders as a novel feed additive. Poult. Sci. 2024, 103, 103593. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jung, G.O.; Lee, K.T.; Choi, J.; Choi, M.Y.; Kim, G.T.; Jung, H.J.; Park, H.J. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J. Ethnopharmacol. 2004, 90, 73–79. [Google Scholar] [CrossRef]
- Lee, J.D.; Huh, J.E.; Jeon, G.S.; Yang, H.R.; Woo, H.S.; Choi, D.Y.; Park, D.S. Flavonol-rich rvhxr from Rhus verniciflua stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 2009, 9, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Chamorro, F.; Simal-Gandara, J.; Proetp, M.A.; Cassani, L. Improving phenolic compound extraction from arnica montana flowers through multivariate optimization of heat and ultrasound-assisted methods. Sustain. Chem. Pharm. 2024, 41, 101722. [Google Scholar] [CrossRef]
- Mokaizh, A.A.B.; Nour, A.H.; Ali, G.A.; Ukaegbu, C.I.; Hawege, E.F. Eco-friendly and efficient extraction of phenolic compounds from Commiphora gileadensis bark using microwave-assisted extraction. J. Ind. Eng. Chem. 2009, 142, 321–328. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.L.; Chen, S.J.; Zhang, Y.; Wu, J. Study on Aqueous Two-Phase Extraction of Teapolypheols from in Green Tea. Key Eng. Mater. 2022, 915, 101–106. [Google Scholar] [CrossRef]
- Xiaolong, J.; Qiang, P.; Yuepeng, Y.; Fang, L.; Min, W. Extraction and physicochemical properties of polysaccharides from Ziziphus Jujuba cv. Muzao by ultrasound-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 108, 541–549. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive componentts: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Pandey, V.K.; Tripathi, A.; Singh, R.; Dash, K. Ultrasound-assisted oxalate reduction vs. conventional methods: A comparative study in elephant foot yam (Amorphophallus paeoniifolius) and process optimization using response surface methodology. Food Humanit. 2024, 2, 100217. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Kingwascharapong, P.; Mazumder, M.A.R.; Rawdkuen, S. Optimization of extraction of phenolic compounds and antioxidants from passion fruit and rambutan seeds using response surface methodology. J. Agric. Food Res. 2023, 14, 100888. [Google Scholar] [CrossRef]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of ultraso-und-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wan, Y.; Xu, J.Y.; Wu, G.H.; Li, L.; Yao, X.H. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydr. Polym. 2016, 137, 473–479. [Google Scholar] [CrossRef]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of ultrasonic-assisted extraction of flavonoids, polysaccharides, and eleutherosides from Acanthopanax senticosus using response surface methodology in development of health wine. LWT 2022, 165, 113725. [Google Scholar] [CrossRef]
- Sofia, C.; Constantina, T. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT-Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Tian, J.; Zhou, Z.; Gao, J.; Qin, L.; Qiu, J. Evaluation of six phenolic compounds content and antifungal activity against wood-decay fungi of tobacco waste extracts obtained by ultrasound-assisted extraction. Sustain. Chem. Pharm. 2024, 41, 101718. [Google Scholar] [CrossRef]
- Rosa, A.M.; Basile, T.; Alba, V.; Gasparro, M.; Giannandrea, M.A.; Debiase, G.; Genghi, R.; Antonacci, D. Optimized ultrasonic-assisted extraction of phenolic antioxidants from grape (Vitis vinifera L.) skin using response surface methodology. J. Food Sci. Technol. 2019, 56, 4417–4428. [Google Scholar] [CrossRef]
- Li, Q.; Yu, N.; Wang, Y.; Sun, Y.; Lu, K.; Guan, W. Extraction optimization of Bruguiera gymnorrhiza polysaccharides with radical scavenging activities. Carbohydr. Polym. 2013, 96, 148–155. [Google Scholar] [CrossRef]
- Liao, J.; Xue, H.; Li, J. Extraction of phenolics and anthocyanins from purple eggplant peels by multi-frequency ultrasound: Effects of different extraction factors and optimization using uniform design. Ultrason. Sonochem. 2022, 90, 1350–4177. [Google Scholar] [CrossRef]
- Xie, J.H.; Shen, M.Y.; Xie, M.Y.; Nie, S.P.; Chen, Y.; Li, C.; Huang, D.F.; Wang, Y.X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of cyclocarya paliurus (batal.) iljinskaja polysaccharides. Carbohydr. Polym. 2012, 89, 177–184. [Google Scholar] [CrossRef]
- Han, G.G.D.; Li, H.; Grossman, J.C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Dong, H.; Liu, Z.; Li, S.; Yang, S.; Li, X. Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum Caudatum Ait and evaluation of its biological activities. Ultrason. Sonochem. 2012, 19, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.M.; Tian, Y.T.; Yan, C.L.; Guo, C.Y. Ultrasound-assisted extraction of total phenolic compounds from Inula helenium. Sci. World J. 2013, 2013, 157527. [Google Scholar] [CrossRef]
- He, S.; Wang, X.; Chen, J.; Li, X.; Gu, W.; Zhang, F.; Cao, G.; Yu, J. Optimization of the ultrasonic-assisted extraction Technology of Steroidal Saponins from Polygonatum kingianum Collett & Hemsl and evaluating its quality planted in different areas. Molecules 2022, 27, 1463. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.A.; Sánchez-Chero, M.; Yarlequé, M.V.; Aguilar, G.F.V.; Carrión-Barco, G.; Santa Cruz, A.G.Y. An overview on the use of response surface methodology to model and optimize extraction processes in the food industry. Curr. Res. Nutr. Food Sci. J. 2021, 9, 745–754. [Google Scholar] [CrossRef]
- Lee, L.S.; Lee, N.; Kim, Y.H.; Lee, C.H.; Hong, S.P.; Jeon, Y.W.; Kim, Y.E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef] [PubMed]
- Senrayan, J.; Venkatachalam, S. Optimization of ultrasound-assisted solvent extraction (UASE) based on oil yield, antioxidant activity and evaluation of fattyacid composition and thermal stability of Coriandrum sativum L. Seed oil. Food Sci. Biotechnol. 2019, 28, 377–386. [Google Scholar] [CrossRef]

| Independent Variable | Symbol | Hierarchical Encoding | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Extraction time (min) | A | 30 | 60 | 90 |
| Solvent to solid ratio (mL/g) | B | 5:1 | 10:1 | 15:1 |
| Extraction temperature (°C) | C | 40 | 50 | 60 |
| Serial Number | Extraction Time (min) | Solvent to Solid Ratio (mL/g) | Extraction Temperature (°C) | Diene Urushiol Content 1 (mg/g, FW) |
|---|---|---|---|---|
| 1 | 90 | 10:1 | 60 | 4.05 |
| 2 | 60 | 10:1 | 50 | 4.65 |
| 3 | 60 | 10:1 | 50 | 4.71 |
| 4 | 60 | 10:1 | 50 | 4.74 |
| 5 | 30 | 10:1 | 40 | 3.94 |
| 6 | 90 | 5:1 | 50 | 4.23 |
| 7 | 60 | 15:1 | 60 | 3.96 |
| 8 | 90 | 15:1 | 50 | 4.27 |
| 9 | 30 | 15:1 | 50 | 4.31 |
| 10 | 60 | 15:1 | 40 | 4.04 |
| 11 | 60 | 10:1 | 50 | 4.62 |
| 12 | 90 | 10:1 | 40 | 4.18 |
| 13 | 30 | 5:1 | 50 | 4.21 |
| 14 | 60 | 5:1 | 40 | 3.78 |
| 15 | 30 | 10:1 | 60 | 4.44 |
| 16 | 60 | 10:1 | 50 | 4.69 |
| 17 | 60 | 5:1 | 60 | 3.99 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.4900 | 9 | 0.1658 | 62.1700 | <0.0001 *** |
| A-Extraction time | 0.0032 | 1 | 0.0032 | 1.1900 | 0.3110 |
| B-Solvent to solid ratio | 0.0166 | 1 | 0.0166 | 6.2400 | 0.0411 * |
| C-Extraction temperature | 0.0314 | 1 | 0.0314 | 11.7700 | 0.0110 * |
| AB | 0.0010 | 1 | 0.0010 | 0.3840 | 0.5551 |
| AC | 0.0981 | 1 | 0.0981 | 36.8000 | 0.0005 ** |
| BC | 0.0209 | 1 | 0.0209 | 7.8300 | 0.0266 * |
| A2 | 0.0511 | 1 | 0.0511 | 19.1500 | 0.0033 ** |
| B2 | 0.4220 | 1 | 0.4220 | 158.2700 | <0.0001 *** |
| C2 | 0.7400 | 1 | 0.7400 | 2.7751 | <0.0001 *** |
| Residual | 0.0187 | 7 | 0.0027 | ||
| Lack of fit | 0.0097 | 3 | 0.0032 | 1.4400 | 0.3554 |
| Pure error | 0.0090 | 4 | 0.0022 | ||
| Cor total | 1.5100 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, F.; He, H.; Ma, J.; Jin, Y.; Qiao, Q.; Long, P.; Li, P.; Sun, R. Optimization of Ultrasonic-Assisted Extraction of Diene Urushiol from Lacquer Tree Leaves Using Response Surface Methodology. Molecules 2025, 30, 1663. https://doi.org/10.3390/molecules30081663
Xia F, He H, Ma J, Jin Y, Qiao Q, Long P, Li P, Sun R. Optimization of Ultrasonic-Assisted Extraction of Diene Urushiol from Lacquer Tree Leaves Using Response Surface Methodology. Molecules. 2025; 30(8):1663. https://doi.org/10.3390/molecules30081663
Chicago/Turabian StyleXia, Fengming, Haojiang He, Jize Ma, Yutian Jin, Qing Qiao, Peng Long, Ping Li, and Rui Sun. 2025. "Optimization of Ultrasonic-Assisted Extraction of Diene Urushiol from Lacquer Tree Leaves Using Response Surface Methodology" Molecules 30, no. 8: 1663. https://doi.org/10.3390/molecules30081663
APA StyleXia, F., He, H., Ma, J., Jin, Y., Qiao, Q., Long, P., Li, P., & Sun, R. (2025). Optimization of Ultrasonic-Assisted Extraction of Diene Urushiol from Lacquer Tree Leaves Using Response Surface Methodology. Molecules, 30(8), 1663. https://doi.org/10.3390/molecules30081663







