Natural (Clinoptilolite) and Synthetic (NaP1) Zeolites in the Adsorption Process for the Removal of Acid Black 1 Dye from Aqueous Solutions
Abstract
1. Introduction
2. Results
2.1. Characteristic of Adsorbents
2.2. Adsorption of ABk 1 onto CLIN and NaP1
2.3. Adsorption Isotherms
2.4. Adsorption Kinetics
3. Materials and Methods
3.1. Adsorbents—Zeolites
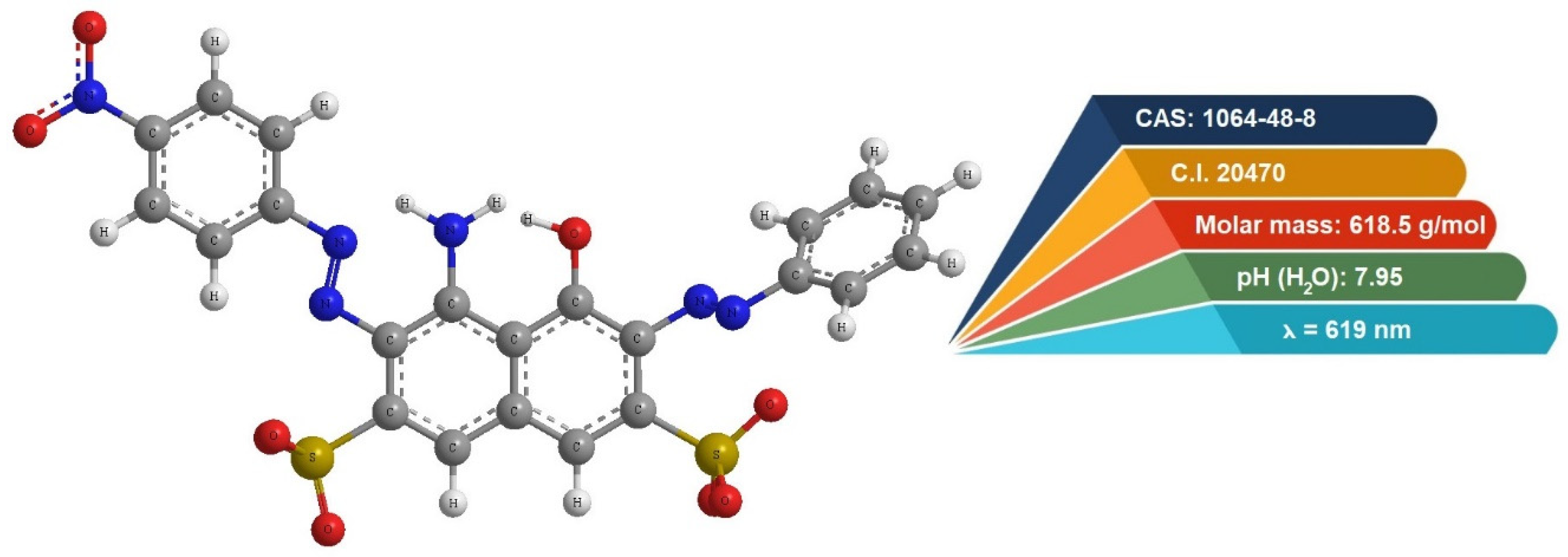
3.2. Adsorbate—Dye
3.3. Adsorption Experiments
3.4. Isotherms
3.5. Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiwaan, H.A.; Mohamed, F.S.; El-Ghamaz, N.A.; Beshry, N.M.; El-Bindary, A.A. Experimental and electrical studies of Na-X zeolite for the adsorption of different dyes. J. Mol. Liq. 2021, 332, 115877. [Google Scholar] [CrossRef]
- Pajak, M. Adsorption Capacity of Smectite Clay and Its Thermal and Chemical Modification for Two Anionic Dyes: Comparative Study. Water Air Soil Pollut. 2021, 232, 83. [Google Scholar] [CrossRef]
- Shankar, S.N.; Dinakaran, D.R.; Chandran, D.K.; Mantha, G.; Srinivasan, B.; Kannaian, U.P.N. Adsorption kinetics, equilibrium and thermodynamics of a textile dye V5BN by a natural nanocomplex material: Clinoptilolite. Energy Nexus 2023, 10, 100197. [Google Scholar] [CrossRef]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Ghanem, A.F.; Sheir, D.H.; Badawy, A.A. Effective single and contest carcinogenic dyes adsorption onto A-zeolite/bacterial cellulose composite membrane: Adsorption isotherms, kinetics, and thermodynamics. J. Environ. Chem. Eng. 2022, 10, 108588. [Google Scholar] [CrossRef]
- Humelnicu, I.; Băiceanu, A.; Ignat, M.-E.; Dulman, V. The removal of Basic Blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Armaǧan, B.; Özdemir, O.; Turan, M.; Çelik, M.S. The removal of reactive azo dyes by natural and modified zeolites. J. Chem. Technol. Biotechnol. 2003, 78, 725–732. [Google Scholar] [CrossRef]
- Pająk, M.; Dzieniszewska, A.; Kyzioł-Komosińska, J. Sorption of Acid Black 1 dye onto bentonite—Equilibrium and kinetic studies. J. Environ. Sci. Health A 2019, 54, 1099–1108. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Babu, J.; Murthy, Z.V.P. Treatment of textile dyes containing wastewaters with PES/PVA thin film composite nanofiltration membranes. Sep. Purif. Technol. 2017, 183, 66–72. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Khan, A.; Chen, Y.; Khan, A.U.; Shah, N.S.; Muhammad, N.; Murtaza, B.; Tahir, K.; Khan, F.U.; Wan, P. Photo catalytic applications of gold nanoparticles synthesized by green route and electrochemical degradation of phenolic Azo dyes using AuNPs/GC as modified paste electrode. J. Alloys Compd. 2017, 725, 869–876. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Zhao, J.; Feng, N. Removal of reactive dyes from wastewater assisted with kaolin clay by magnesium hydroxide coagulation process. Colloid Surface A 2016, 494, 222–227. [Google Scholar] [CrossRef]
- Beyene, H.D. The potential of dyes removal from textile wastewater by using different treatment technology. A review. Int. J. Environ. Monit. Anal. 2014, 2, 347–353. [Google Scholar] [CrossRef]
- Fortunato, L.; Elcik, H.; Blankert, B.; Ghaffour, N.; Vrouwenvelder, J. Textile dye wastewater treatment by direct contact membrane distillation: Membrane performance and detailed fouling analysis. J. Membr. Sci. 2021, 636, 119552. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Jamal, A.; Ilyas, M.; Zubair, M.; Khan, G.; Atieh, M.A. Bioremediation of dyes: Current status and prospects. J. Water Process Eng. 2020, 38, 101680. [Google Scholar] [CrossRef]
- Avila, M.C.; Lick, I.D.; Comelli, N.A.; Ruiz, M.M. Adsorption of an anionic dye from aqueous solution on a treated clay. Groundw. Sustain. Dev. 2021, 15, 100688. [Google Scholar] [CrossRef]
- Dzieniszewska, A.; Pająk, M.; Kyzioł-Komosińska, J. The effect of auxiliary substances on the adsorption of anionic dyes on low-moor peat. Desalin. Water Treat. 2020, 177, 209–226. [Google Scholar] [CrossRef]
- Fabryanty, R.; Valencia, C.; Edi-Soetaredjo, F.; Nyoo Putro, J.; Santoso, S.P.; Kurniawan, A.; Ju, Y.H.; Suryadi, I. Removal of crystal violet dye by adsorption using bentonite—Alginate composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking water treatment residuals: A review of recent uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.-H.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Kyziol-Komosinska, J.; Rosik-Dulewska, C.; Dzieniszewska, A.; Pajak, M. Low-moor peats as biosorbents for removal of anionic dyes from water. Fresen. Environ. Bull. 2018, 27, 6–20. [Google Scholar]
- Pająk, M.; Dzieniszewska, A. Evaluation of the metallurgical dust sorbent efficacy in Reactive Blue 19 dye removal from aqueous solutions and textile wastewater. Environ. Eng. Sci. 2020, 37, 509–518. [Google Scholar] [CrossRef]
- Chaari, I.; Fakhfakh, E.; Medhioub, M.; Jamoussi, F. Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J. Mol. Struct. 2019, 1179, 672–677. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. An update on synthetic dyes adsorption onto clay based minerals: A state-of-art review. J. Environ. Manag. 2017, 191, 35–57. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from the Brazilian pine-fruit-shell for the removal of Procion Red MX 3B from aqueous solution–Kinetic, equilibrium, and thermodynamic studies. Chem. Eng. J. 2009, 155, 627–636. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liang, D.; Xiao, Z.; Xie, Y.; Li, J. Sulfhydryl-modified chitosan aerogel for the adsorption of heavy metal ions and organic dyes. Ind. Eng. Chem. Res. 2020, 59, 14531–14536. [Google Scholar] [CrossRef]
- Lipatova, I.M.; Makarova, L.I.; Yusova, A.A. Adsorption removal of anionic dyes from aqueous solutions by chitosan nanoparticles deposited on the fibrous carrier. Chemosphere 2018, 212, 1155–1162. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Z.J.; Guo, X.; Jiang, X.H.; Liu, R. Process optimization, kinetics and equilibrium of orange G and acid orange 7 adsorptions onto chitosan/surfactant. J. Mol. Liq. 2014, 197, 353–367. [Google Scholar] [CrossRef]
- Georgin, J.; Drumm, F.C.; Grassi, P.; Franco, D.; Allasia, D.; Dotto, G.L. Potential of Araucaria angustifolia bark as adsorbent to remove Gentian Violet dye from aqueous effluents. Water Sci. Technol. 2018, 78, 1693–1703. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, R.; Baskar, C.; Chung, W.J. Removal of methylene blue from aqueous waste using rice husk and rice husk ash. Desalination 2010, 259, 249–257. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Gu, X.; Lu, J. Competitive adsorption of methylene blue and Cu2+ onto citric acid modified pine sawdust. Clean-Soil Air Water 2015, 43, 96–103. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bandyopadhyay, A. Characterizing acidic fly ash with and without biomass combustion residue for adsorptive removal of crystal violet with optimization of mixed adsorbent by response surface modeling. Environ. Earth Sci. 2016, 75, 820. [Google Scholar] [CrossRef]
- Gunes, E.; Kaygusuz, T. Adsorption of Reactive Blue 222 onto an industrial solid waste included Al(III) hydroxide: pH, ionic strength, isotherms, and kinetics studies. Desalin. Water Treat. 2015, 53, 2510–2517. [Google Scholar] [CrossRef]
- Alexander, J.A.; Zaini, M.A.A.; Surajudeen, A.; Aliyu, E.-N.U.; Omeiza, A.U. Surface modification of low-cost bentonite adsorbents–A review. Particul. Sci. Technol. 2018, 37, 538–549. [Google Scholar] [CrossRef]
- Charki, A.; Ahari, M.; Hadoudi, N.; Benyoub, B.; Zaki, N.; Fraiha, O.; El Hammoudani, Y.; Elyoussfi, A.; Salhi, A.; El Ouarghi, H. Natural Moroccan bentonite clay as an adsorbent for nutrient removal from synthetic leachate: Performance and evaluation. Desalin. Water Treat. 2024, 318, 100381. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Munagapati, V.S.; Liao, S.-W.; Shu, C.-M.; Shadangi, K.P.; Sarangi, P.K.; Wen, J.C. Ionic liquid [bmim] [TFSI] templated Na-X zeolite for the adsorption of (Cd2+, Zn2+), and dyes (AR, R6). Environ. Res. 2023, 216, 114525. [Google Scholar] [CrossRef]
- Mortazavi, N.; Bahadori, M.; Marandi, A.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I. Enhancement of CO2 adsorption on natural zeolite, modified clinoptilolite with cations, amines and ionic liquids. Sustain. Chem. Pharm. 2021, 22, 100495. [Google Scholar] [CrossRef]
- Rouhani, M.; Ashrafi, S.D.; Taghavi, K.; Joubani, M.N.; Jaafari, J. Evaluation of tetracycline removal by adsorption method using magnetic iron oxide nanoparticles (Fe3O4) and clinoptilolite from aqueous solutions. J. Mol. Liq. 2022, 356, 119040. [Google Scholar] [CrossRef]
- Bień, T.; Kołodyńska, D.; Franus, W. Functionalization of Zeolite NaP1 for Simultaneous Acid Red 18 and Cu(II) Removal. Materials 2021, 14, 7817. [Google Scholar] [CrossRef]
- Patel, M.G.; Marakana, P.G.; Dey, A.; Saini, B.; Chokshi, H. Coal fly ash derived adsorbent for enhancing waste water treatment. Mater. Today-Proc. 2023, 77, 163–167. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, L.; Sun, X.; Huang, J. Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies. Chin. J. Chem. Eng. 2009, 17, 513–521. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, L.; Chenglun, L. NaP1 zeolite synthesized via effective extraction of Si and Al from red mud for methylene blue adsorption. Adv. Powder Technol. 2021, 32, 3904–3914. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Yongming, J.; Franus, M.; Franus, W. Zeolite NaP1 Functionalization for the Sorption of Metal Complexes with Biodegradable N-(1,2-dicarboxyethyl)-D,L-aspartic Acid. Materials 2021, 14, 2518. [Google Scholar] [CrossRef]
- Franus, W. Characterization of X-type zeolite prepared from coal fly ash. Pol. J. Environ. Stud. 2012, 21, 337–343. [Google Scholar]
- Wdowin, M.; Franus, M.; Panek, R.; Bandura, L.; Franus, W. The conversion technology of fly ash into zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef]
- Rida, K.; Bouraoui, S.; Hadnine, S. Adsorption of methylene blue from aqueous solution by kaolin and zeolite. Appl. Clay. Sci. 2013, 83–84, 99–105. [Google Scholar] [CrossRef]
- Hor, K.Y.; Chee, J.M.C.; Chong, M.N.; Jin, B.; Saint, C.; Poh, P.E.; Aryal, R. Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater. J. Clean. Prod. 2016, 118, 197–209. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- Supelano, G.I.; Gómez Cuaspud, J.A.; Moreno-Aldana, L.C.; Ortiz, C.; Trujillo, C.A.; Palacio, C.A.; Parra Vargas, C.A.; Gómez, M. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel 2020, 263, 116800. [Google Scholar] [CrossRef]
- Google Maps. Available online: www.google.com/maps (accessed on 15 November 2024).
- Drozd, J.; Licznar, M.; Licznar, S.E.; Weber, J. Soil Science with Elements of Mineralogy and Petrography, 1st ed.; Wydawnictwo Akademii Rolniczej we Wrocławiu: Wrocław, Poland, 1998. (In Polish) [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
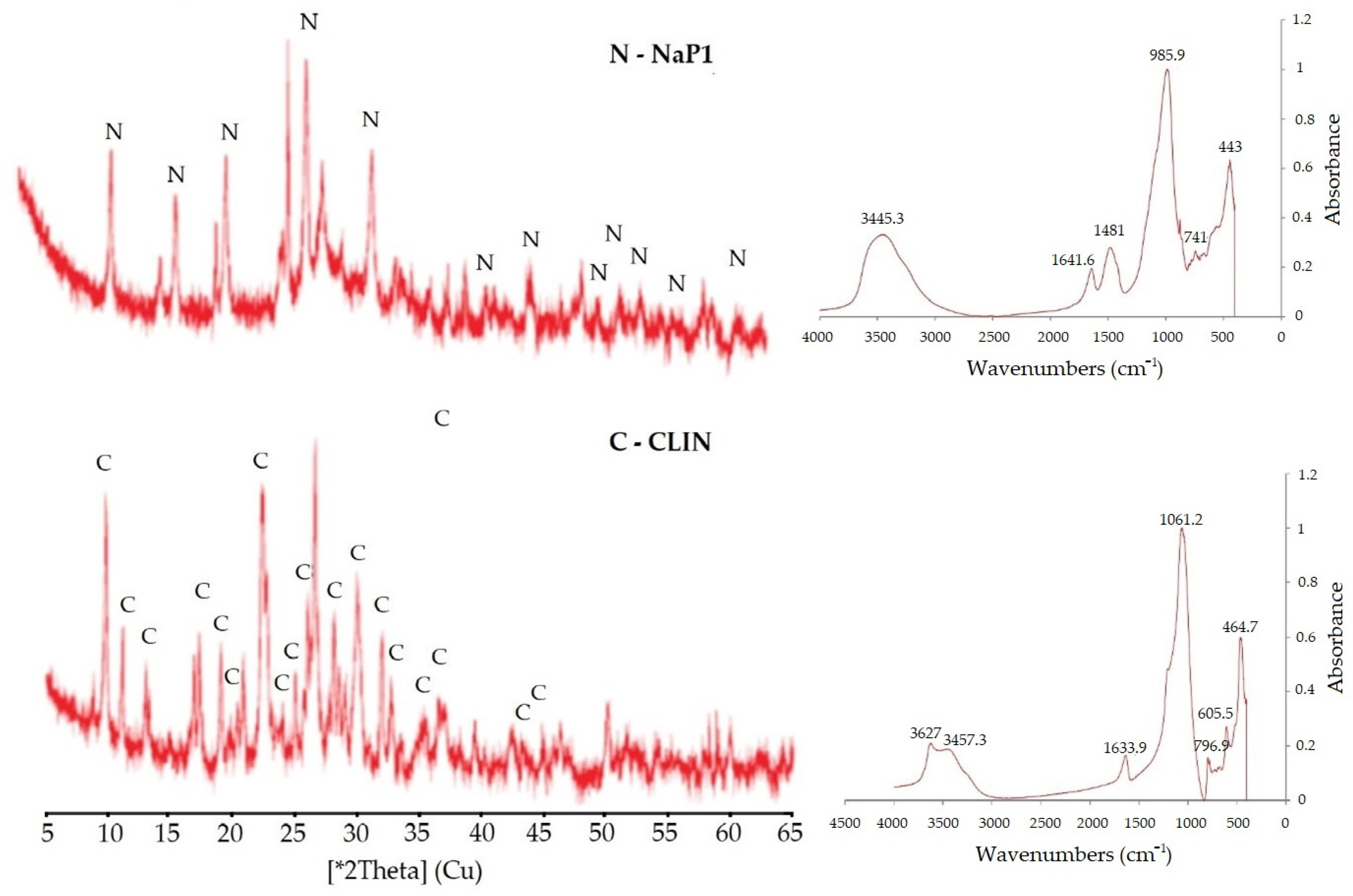
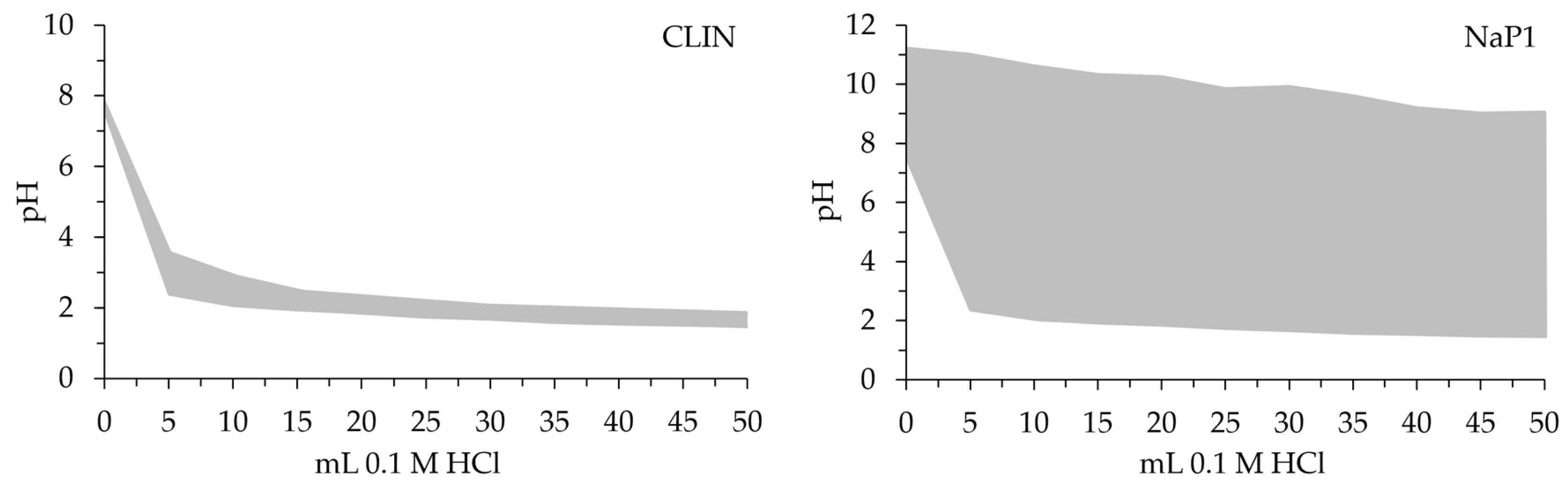
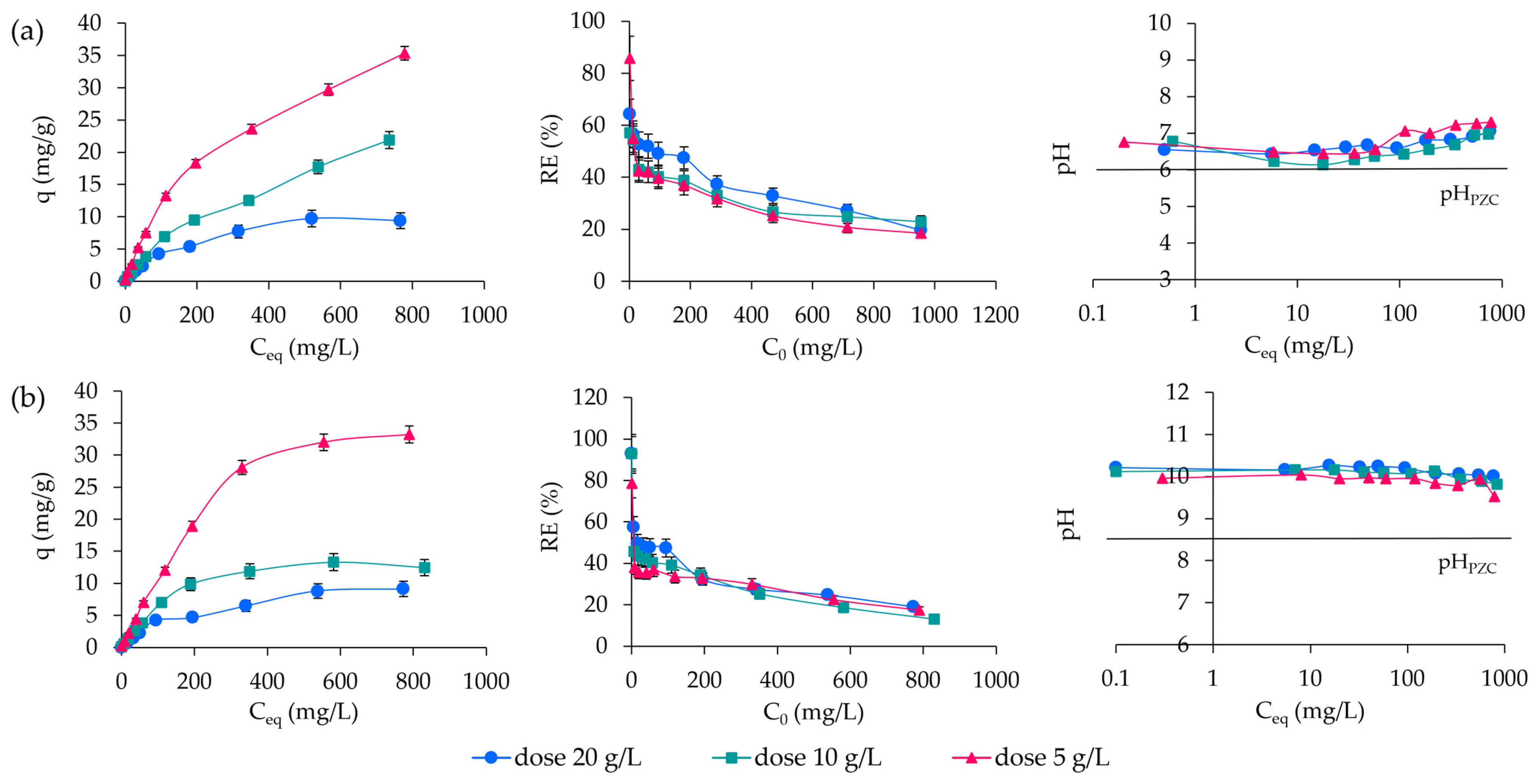


| Zeolites | CLIN | NaP1 |
|---|---|---|
| Specific surface area (m2/g) | 18.33 | 86.85 |
| Total pore area (m3/g) | 11.15 | 80.236 |
| Micropore surface (m2/g) | 10.65 | 32.84 |
| Average pore diameter (µm) | 0.210 | 0.070 |
| Porosity (%) | 54.76 | 75.33 |
| CEC (cmol(+)/kg) | 68.98 | 138.7 |
| pH | 7.84 | 11.22 |
| pHPZC | 6.0 | 8.5 |
| Chemical composition (%) | ||
| SiO2 | 73.9 | 44.3 |
| Al2O3 | 13.6 | 29.0 |
| Fe2O3 | 2.79 | 7.81 |
| MnO | 0.108 | 0.125 |
| Na2O | 0.63 | 3.97 |
| K2O | 4.02 | 1.19 |
| MgO | 0.297 | 0.513 |
| CaO | 3.75 | 10.7 |
| P2O5 | 0.222 | 0.447 |
| TiO2 | 0.296 | 1.26 |
| Cl | 0.0186 | 0.0186 |
| SO3 | 0.076 | 0.183 |
| Si/Al, mol·mol−1 | 4.79 | 1.34 |
| Adsorbent | Dye | Parameters | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|
| Na-X | Reactive Black 5 | pH = 2, dosage = 0.05/25 mL, T = 25 °C, t = 90 min | qmax = 25.3 | [1] |
| Brilliant Green | pH = 10, dosage = 0.05/25 mL, T = 25 °C, t = 90 min | qmax = 24.13 | ||
| Clinoptilolite | Violet 5BN | pH = 5, dosage = 1.5 g/L, T = 30 °C, t = 90 min | qmax = 7.96 | [3] |
| Clinoptilolite | Reactive Black 5 | pH = 6.5, dosage = 0.5 g/100 mL, T = 22.5 °C, t = 10 min | qmax = 60.61 | [7] |
| Reactive Red 239 | qmax = 111.11 | |||
| Reactive Yellow 176 | qmax = 88.50 | |||
| Na-X | Rhodamine 6G | pH = 2–5, dosage = 0.1–0.5 g/30 mL, C0 = 50 mg/L, T = 45 °C | qmax = 65.85 | [37] |
| Alizarin Red S | qmax = 76.33 | |||
| NaP1 | Acid Red 18 | pH = 6, dosage = 0.1 g/L, C0 = 50 mg/L, T = 23 °C, t = 120 min | qmax = 5.39 | [40] |
| NaP1 | Methylene Blue | pH = 7, dosage = 1 g/L, C0 = 50 mg/L, T = 25 °C, t = 120 min | qeq = 48.7 | [43] |
| Zeolite | Methylene Blue | pH = 6.65, dosage = 1 g/L, C0 = 18 mg/L, T = 20 °C, t = 60 min | qeq = 11.21 | [47] |
| Natural zeolite | Methylene Blue | dosage = 0.6 g/L, C0 = 20 mg/L, T = 25 °C, t = 120 min | qeq = 1.50 | [48] |
| Synthetic zeolite | Methylene Blue | pH = 6, dosage = 6 g/L, C0 = 200 mg/L, T = 40 °C, t = 200 min | qeq = 33.57 | [49] |
| Synthetic zeolites from fly ash | Methylene Blue | dosage = 6.25 g/L, T = 20 °C, t = 60 min | qeq = 5.99 | [50] |
| Clinoptilolite | Acid Black 1 | dosage = 5 g/L, C0 = 1000 mg/L, T = 23 °C, t = 24 h | qeq = 35.32 | This study |
| NaP1 | qeq = 33.20 |
| CLIN | NaP1 | |||||
|---|---|---|---|---|---|---|
| Adsorbent Dosage | 20 g/L | 10 g/L | 5 g/L | 20 g/L | 10 g/L | 5 g/L |
| Freundlich isotherm | ||||||
| 1/n | 0.7490 | 0.7749 | 0.6304 | 0.5899 | 0.5675 | 0.6930 |
| KF | 0.1023 | 0.1492 | 0.5630 | 0.2011 | 0.3578 | 0.3749 |
| R2 | 0.9802 | 0.9925 | 0.9912 | 0.9799 | 0.9596 | 0.9710 |
| Langmuir isotherm | ||||||
| qexp | 9.39 | 21.90 | 35.32 | 9.11 | 12.46 | 33.20 |
| Q | 11.99 | 28.41 | 42.19 | 10.83 | 16.08 | 40.65 |
| KL | 0.005534 | 0.003050 | 0.004637 | 0.005964 | 0.007357 | 0.004001 |
| RL | 0.1591 | 0.2556 | 0.1842 | 0.1493 | 0.1246 | 0.2074 |
| R2 | 0.9845 | 0.9161 | 0.9258 | 0.9390 | 0.9631 | 0.8792 |
| CLIN | NaP1 | |||||
|---|---|---|---|---|---|---|
| Adsorbent Dosage | 20 g/L | 10 g/L | 5 g/L | 20 g/L | 10 g/L | 5 g/L |
| Freundlich isotherm | ||||||
| 1/n | 0.4947 | 0.6632 | 0.5638 | 0.5136 | 0.4284 | 0.5644 |
| KF | 0.3977 | 0.2739 | 0.8432 | 0.3206 | 0.8241 | 0.8607 |
| R2 | 0.9621 | 0.9967 | 0.9924 | 0.9763 | 0.9210 | 0.9549 |
| SSE | 4.732 | 1.715 | 10.68 | 2.458 | 19.43 | 70.25 |
| χ2 | 1.463 | 0.4452 | 1.581 | 0.9163 | 3.628 | 5.870 |
| RMSE | 0.6879 | 0.4142 | 1.034 | 0.4958 | 1.394 | 2.650 |
| Langmuir isotherm | ||||||
| qexp | 9.39 | 21.90 | 35.32 | 9.11 | 12.46 | 33.20 |
| Q | 12.51 | 38.18 | 48.17 | 11.82 | 15.90 | 48.92 |
| KL | 0.004943 | 0.001680 | 0.003078 | 0.004305 | 0.007024 | 0.003202 |
| RL | 0.1748 | 0.3840 | 0.2538 | 0.1956 | 0.1297 | 0.2465 |
| R2 | 0.9908 | 0.9913 | 0.9957 | 0.9817 | 0.9858 | 0.9888 |
| SSE | 1.145 | 4.490 | 6.007 | 1.903 | 3.491 | 17.52 |
| χ2 | 0.1635 | 0.9005 | 2.157 | 1.139 | 1.813 | 1.824 |
| RMSE | 0.3384 | 0.6701 | 0.7751 | 0.4362 | 0.5908 | 1.324 |
| CLIN | NaP1 | |||
|---|---|---|---|---|
| 25 mg/L | 250 mg/L | 25 mg/L | 250 mg/L | |
| qe | 2.51 | 6.3 | 1.46 | 5.12 |
| Pseudo-first-order model | ||||
| qe1 | 1.2159 | 2.6797 | 0.9408 | 3.0826 |
| k1 | 0.0044 | 0.0042 | 0.0017 | 0.0042 |
| R2 | 0.8820 | 0.8270 | 0.8461 | 0.9113 |
| Pseudo-second-order model | ||||
| qe2 | 2.5484 | 6.3573 | 1.4384 | 5.2356 |
| k2 | 0.0457 | 0.0568 | 0.0157 | 0.0277 |
| R2 | 0.9997 | 0.9999 | 0.9855 | 0.9999 |
| Isotherm Models | ||||
|---|---|---|---|---|
| Isotherm | Nonlinear Form | Linear Form | Parameters | Ref. |
| Freundlich | KF—Freundlich constant associated with the system’s adsorption capacity and intensity, expressed in units of mg/g(L/mg)1/nF. 1/n—Represents the adsorption favorability and is a dimensionless parameter. | [54] | ||
| Langmuir | qmax—The maximum capacity of adsorption, measured in mg/g. KL—The Langmuir constant, reflecting the binding site affinity and the adsorption energy, with units of L/mg. | [55] | ||
| Error functions | ||||
| Abbreviation | Definition/expression | Parameters | Ref. | |
| SSE | qe,cal—The adsorption capacity value obtained from calculations. qe,exp—The adsorption capacity value measured experimentally. n—The total number of data points in the experimental observations. | [53] | ||
| RMSE | ||||
| χ2 | ||||
| Kinetic Model | Equation | Nonlinear Form | Description of Kinetic Parameters | Ref. |
|---|---|---|---|---|
| Pseudo-first-order | qt (mg/g)—the amount of ABk 1 adsorbed at any specific time (min). qe (mg/g)—the amount of ABk 1 adsorbed at equilibrium. k1 (1/min)—the adsorption rate constant, potentially influenced by the initial concentration of the solute. k2 (g/(mg·min))—the adsorption rate constant for the pseudo-second-order model. h (1/min)—the initial adsorption rate. | [3] | ||
| Pseudo-second-order |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, M.; Dzieniszewska, A.; Kyzioł-Komosińska, J. Natural (Clinoptilolite) and Synthetic (NaP1) Zeolites in the Adsorption Process for the Removal of Acid Black 1 Dye from Aqueous Solutions. Molecules 2025, 30, 1677. https://doi.org/10.3390/molecules30081677
Pająk M, Dzieniszewska A, Kyzioł-Komosińska J. Natural (Clinoptilolite) and Synthetic (NaP1) Zeolites in the Adsorption Process for the Removal of Acid Black 1 Dye from Aqueous Solutions. Molecules. 2025; 30(8):1677. https://doi.org/10.3390/molecules30081677
Chicago/Turabian StylePająk, Magdalena, Agnieszka Dzieniszewska, and Joanna Kyzioł-Komosińska. 2025. "Natural (Clinoptilolite) and Synthetic (NaP1) Zeolites in the Adsorption Process for the Removal of Acid Black 1 Dye from Aqueous Solutions" Molecules 30, no. 8: 1677. https://doi.org/10.3390/molecules30081677
APA StylePająk, M., Dzieniszewska, A., & Kyzioł-Komosińska, J. (2025). Natural (Clinoptilolite) and Synthetic (NaP1) Zeolites in the Adsorption Process for the Removal of Acid Black 1 Dye from Aqueous Solutions. Molecules, 30(8), 1677. https://doi.org/10.3390/molecules30081677







