Amphiphilic Styrene-Based Pyrene Derivatives: Tunable Aggregation Luminescence and Their Photo-Induced Dimerization Behavior
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis Process and Characterization
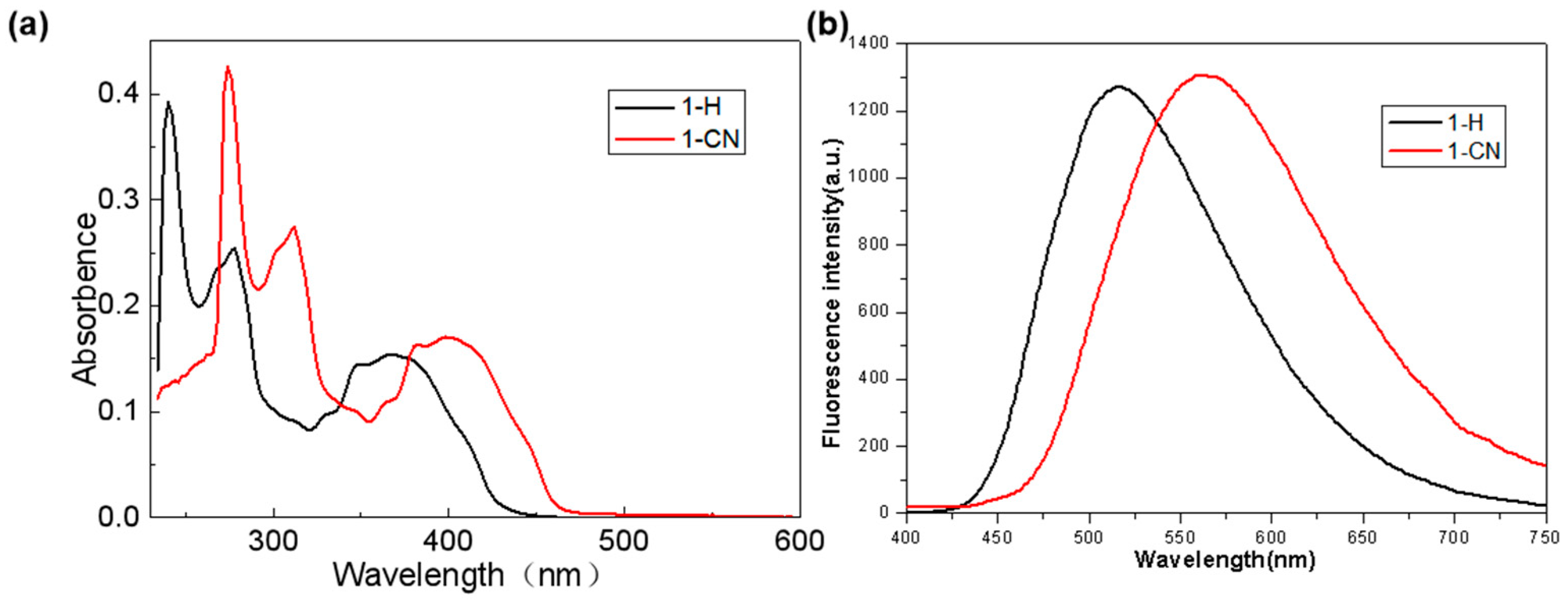
2.2. Photophysical Measurements
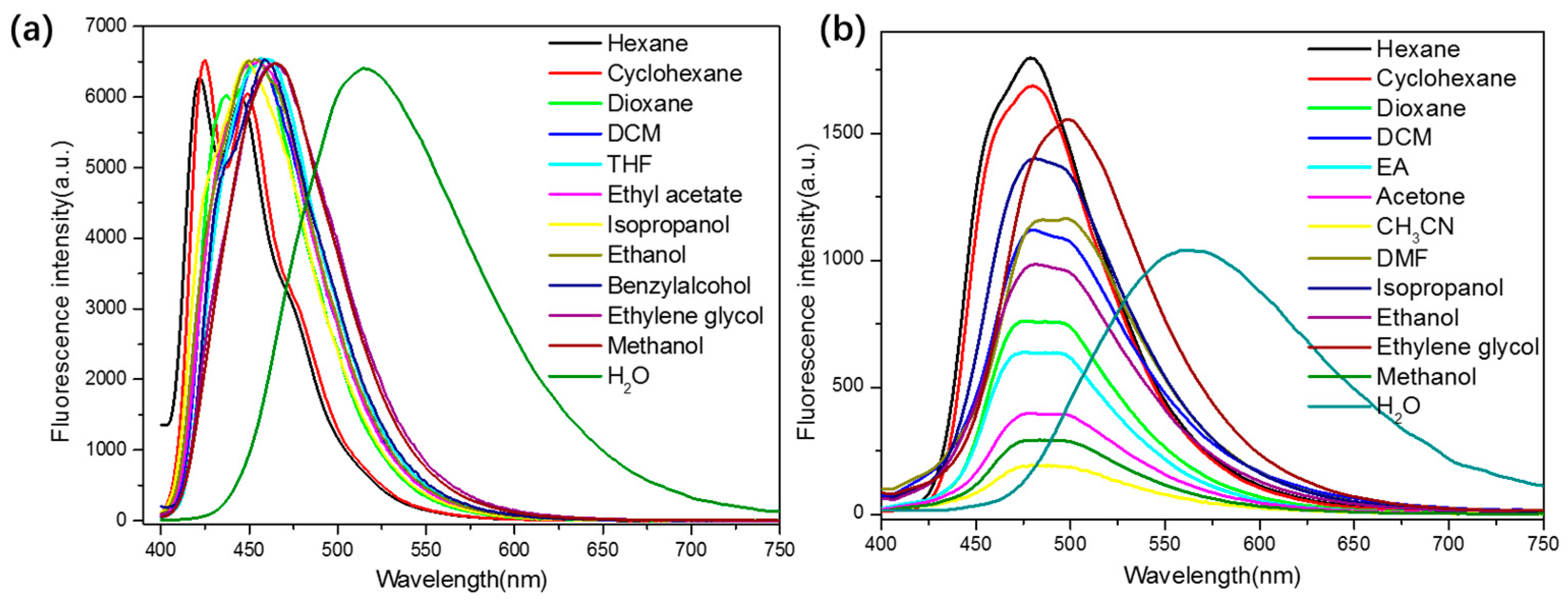
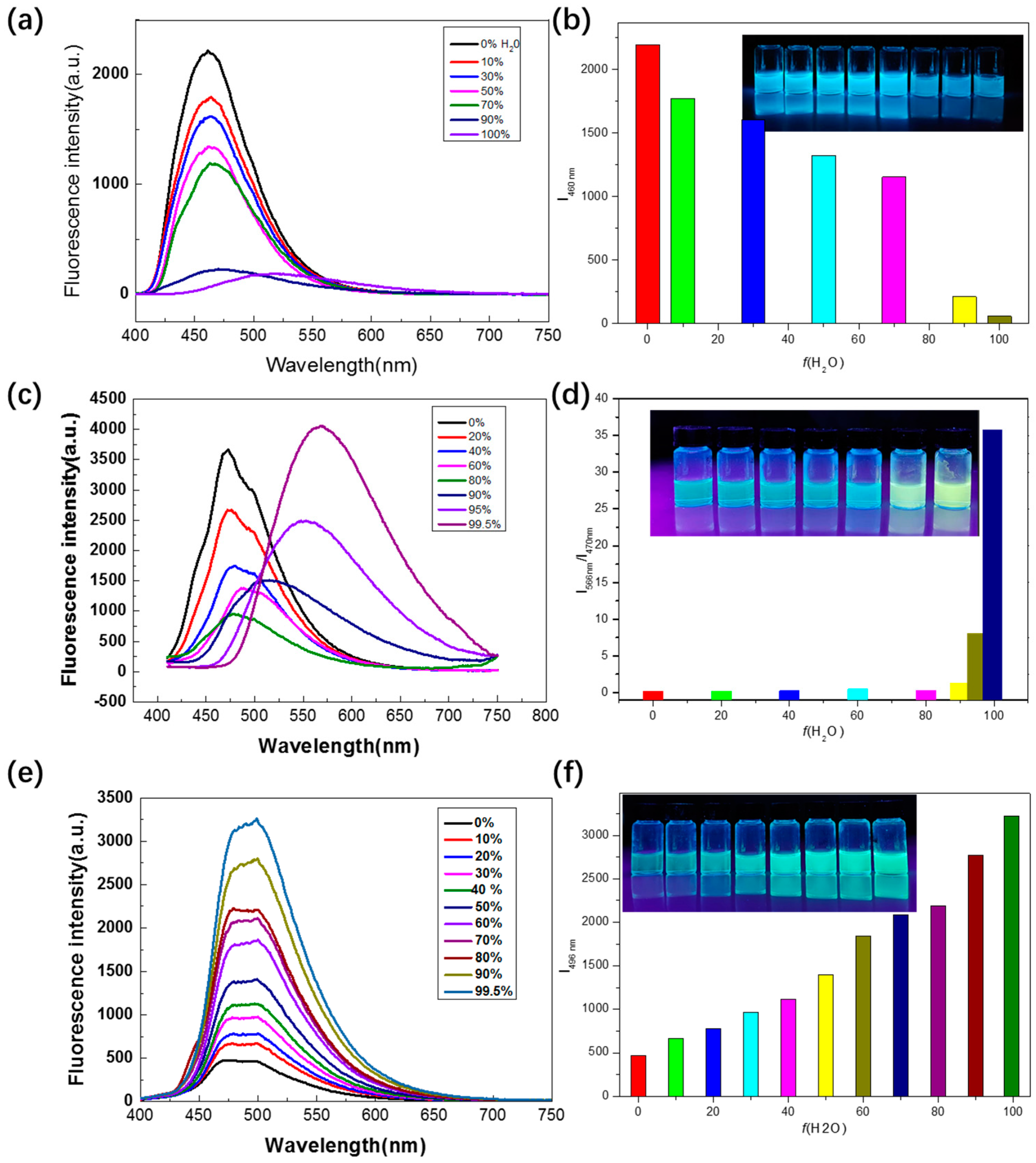
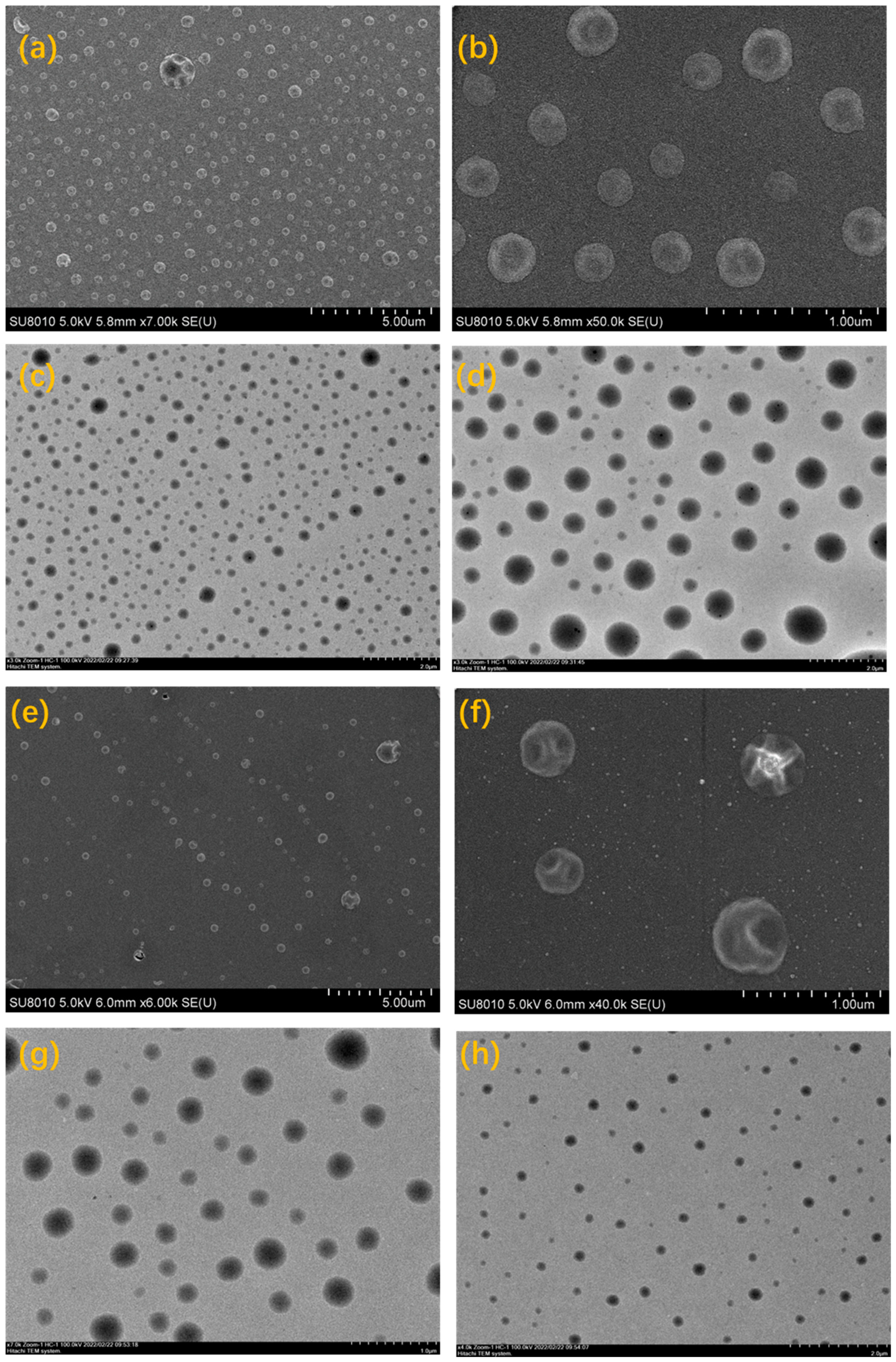
2.3. Aggregation Behavior Study
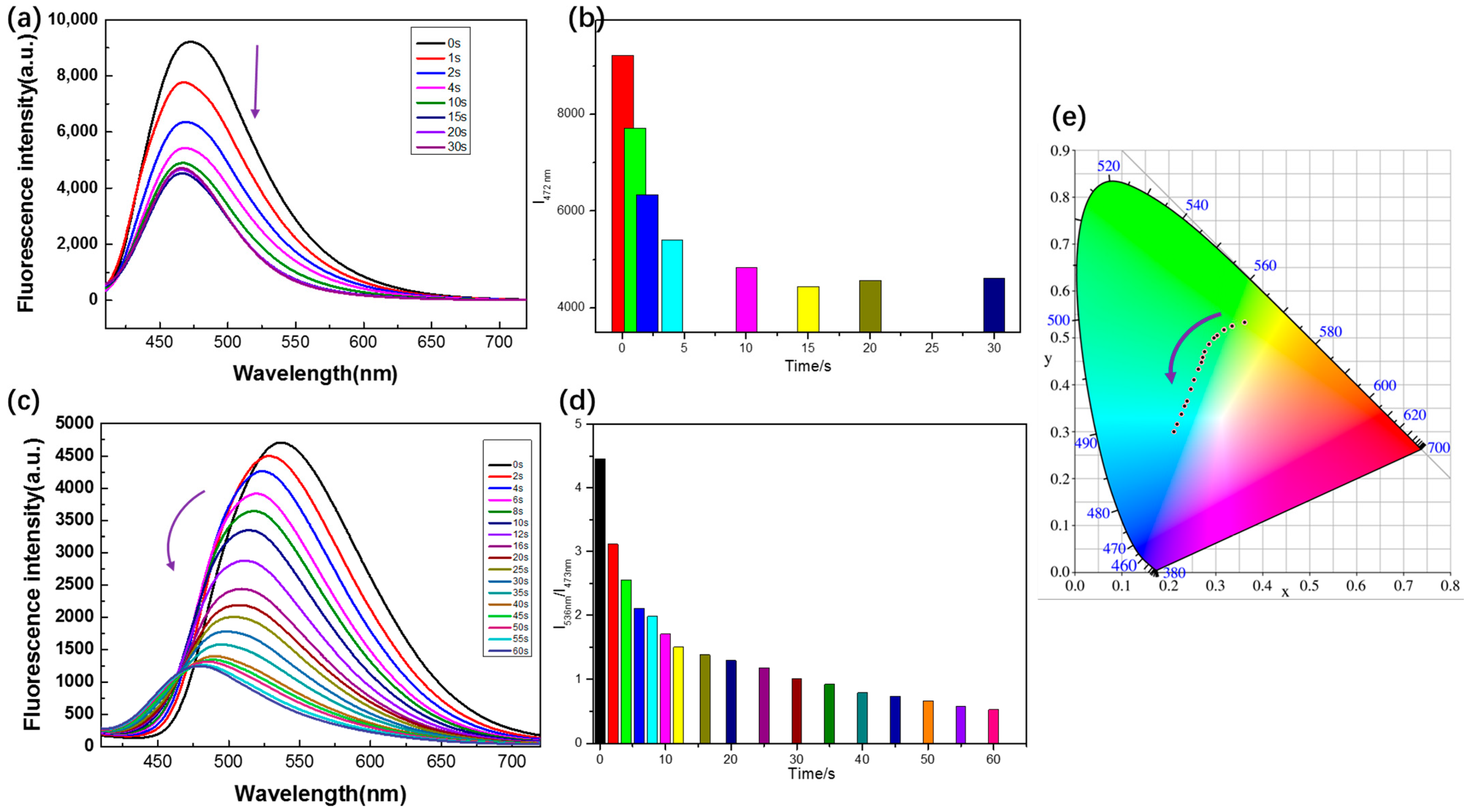
2.4. Study on Photochemical Reaction
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Synthesis
3.2.1. Synthesis of Compound 4
3.2.2. Synthesis of Compound 1-H
3.2.3. Synthesis of Compound 1-CN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Zhang, H.K.; Lam, J.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem.-Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gao, A.; Hou, J.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, Z.; Bei, Y.; Feng, S.; Xu, X. A cationic amphiphilic tetraphenylethylene derivative with hydrochromic sensitive property: Applications in anti-counterfeiting ink and rewritable paper. Chin. Chem. Lett. 2022, 33, 4838–4841. [Google Scholar] [CrossRef]
- Wang, N.; Yang, W.; Feng, L.; Xu, X.; Feng, S. A supramolecular artificial light-harvesting system based on a luminescent platinum(ii) metallacage. Dalton Trans. 2023, 52, 15524–15529. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, S.; Wang, D. Organosilicon fluorescent materials. Polymers 2023, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wang, J.; Kang, H.; Li, H.; Peng, X.; Yoon, J. Photon-driven dye induction pyroptosis: An emerging anti-tumor immunotherapy paradigm. Angew. Chem. Int. Ed. 2025, 64, e202417899. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, W.; Xu, X. Tetraphenylethene-based macrocycles with dual-ring topology: Synthesis, structures, and applications. Org. Chem. Front. 2023, 10, 6225–6239. [Google Scholar] [CrossRef]
- Li, F.; Dong, Y.; Miao, J.; Nie, Y.; Zhang, Y.; Li, T.; Xu, C.; Liu, G.; Jiang, X. Halogen-containing bridged carborane-tetraphenylethene compounds: Efficient and wide-range shifted excitation-dependent emissions. New J. Chem. 2024, 48, 15532–15539. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, C.; Shi, X.; Yang, H. Aie-active metal-organic coordination complexes based on tetraphenylethylene unit and their applications. Chin. J. Polym. Sci. 2019, 37, 372–382. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, X.; Wang, D.; Zhang, Z.; Sun, H.; Xu, X.; Xie, X.; Shi, X.; Peng, H.; Yang, H.; et al. Light-responsive supramolecular liquid-crystalline metallacycle for orthogonal multimode photopatterning. Angew. Chem.-Int. Ed. 2023, 63, e202315061. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Caruso, U.; Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Piotto, S. Aie/acq effects in two dr/nir emitters: A structural and dft comparative analysis. Molecules 2018, 23, 1947. [Google Scholar] [CrossRef]
- Wang, N.; Feng, H.; Hao, X.; Cao, Y.; Xu, X.; Feng, S. Dynamic covalent bond and metal coordination bond-cross-linked silicone elastomers with excellent mechanical and aggregation-induced emission properties. Polym. Chem. 2023, 14, 1396–1403. [Google Scholar] [CrossRef]
- Wang, N.; Feng, L.; Xu, X.D.; Feng, S. Dynamic covalent bond cross-linked luminescent silicone elastomer with self-healing and recyclable properties. Macromol. Rapid Commun. 2022, 43, 2100885. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Biswas, D.; Chowdhury, P.; Bar, N.; Das, G.K. Pyrene-attached new schiff base polymer: Acq to aie conversion and its prospects. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2025, 329, 125551. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, G.; Lai, X.; Su, L.; He, W.; Lai, W.; Deng, S. Polyethyleneimine-induced fluorescence enhancement strategy for aiegen: The mechanism and application. Anal. Bioanal. Chem. 2023, 415, 1347–1355. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, W.; Luo, F.; Jin, Y.; Pan, L.; Deng, Q.; Wang, Q.; Yu, M. Construction of heterogeneous aggregation-induced emission microspheres with enhanced multi-mode information encryption. Molecules 2024, 29, 5852. [Google Scholar] [CrossRef]
- Peng, Q.; Shuai, Z. Molecular mechanism of aggregation-induced emission. Aggregate 2021, 2. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, J.; Ji, H.; Xu, X.; Feng, S. Tetraphenylethene-based macrocycles: Visualized monitoring the hydrolysis of silicon-oxygen bond and their tunable luminescent properties. Chem. Eng. J. 2023, 463, 142241. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Redshaw, C.; Tang, B.Z. Aggregation behaviour of pyrene-based luminescent materials, from molecular design and optical properties to application. Chem. Soc. Rev. 2023, 52, 6715–6753. [Google Scholar] [CrossRef] [PubMed]
- Camerel, F.; Bonardi, L.; Schmutz, M.; Ziessel, R. Highly luminescent gels and mesogens based on elaborated borondipyrromethenes. J. Am. Chem. Soc. 2006, 128, 4548–4549. [Google Scholar] [CrossRef]
- Kodura, D.; Rodrigues, L.L.; Walden, S.L.; Goldmann, A.S.; Frisch, H.; Barner-Kowollik, C. Orange-light-induced photochemistry gated by ph and confined environments. J. Am. Chem. Soc. 2022, 144, 6343–6348. [Google Scholar] [CrossRef]
- Zhang, J.; Han, Y.; Fang, J.; An, J.; Peng, J.; Zhu, X.; Liu, Y. Achieving precise luminescence regulation through manipulating aggregation and protonation in pyrene-based materials. Chem. Eng. J. 2025, 503, 158623. [Google Scholar] [CrossRef]
- Tang, S.; Wang, N.; Xu, X.; Feng, S. A ratiometric fluorescent thermometer based on amphiphilic alkynylpyrene derivatives. New J. Chem. 2019, 43, 6461–6464. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, S.; Fu, R.; Xu, X.; Feng, S. Aggregation-induced photodimerization of an alkynylpyrene derivative as a photoresponsive fluorescent ink. J. Mater. Chem. C 2019, 7, 13786–13793. [Google Scholar] [CrossRef]
- Zhu, F.; Mei, L.; Tian, R.; Li, C.; Wang, Y.; Xiang, S.; Zhu, M.; Tang, B.Z. Recent advances in super-resolution optical imaging based on aggregation-induced emission. Chem. Soc. Rev. 2024, 53, 3350–3383. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Wang, H.; Kang, W.; Xu, X. Photo-induced tunable luminescence from an aggregated amphiphilic ethylene-pyrene derivative in aqueous media. Chin. Chem. Lett. 2024, 35, 109216. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, Z.; Wang, N.; Feng, S.; Xu, X. Tetraphenylethylene based amphiphilic derivatives: The self-assembly, photo-responsiveness and their application for erasable fluorescent ink. Dyes Pigment. 2021, 193, 109479. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, J.; Zhao, Z.; Chen, Y.; He, X.; Chen, M.; Gong, J.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Multiple yet controllable photoswitching in a single aiegen system. J. Am. Chem. Soc. 2018, 140, 1966–1975. [Google Scholar] [PubMed]
- Jana, P.; Kanvah, S. Aggregation-induced emission and organogels with chiral and racemic pyrene-substituted cyanostyrenes. Langmuir 2020, 36, 2720–2728. [Google Scholar] [PubMed]
- Chen, Q.; Cheng, K.; Wang, W.; Yang, L.; Xie, Y.; Feng, L.; Zhang, J.; Zhang, H.; Sun, H. A pyrene-based ratiometric fluorescent probe with a large stokes shift for selective detection of hydrogen peroxide in living cells. J. Pharm. Anal. 2020, 10, 490–497. [Google Scholar] [PubMed]
- Yang, Y.J.; Yang, J.; Fang, M.M.; Li, Z. Recent process of photo-responsive materials with aggregation-induced emission. Chem. Res. Chin. Univ. 2021, 37, 598–614. [Google Scholar] [CrossRef]
- Ping, X.; Pan, J.; Peng, X.; Yao, C.; Li, T.; Feng, H.; Qian, Z. Recent advances in photoresponsive fluorescent materials based on [2+2] photocycloaddition reactions. J. Mater. Chem. C 2023, 11, 7510–7525. [Google Scholar] [CrossRef]
- Hermann, C. The International Commission on Illumination-CIE: What It Is and How It Works. In Symposium-International Astronomical Union; Cambridge University Press: Cambridge, UK, 2001; Volume 196, pp. 60–68. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Luo, X.; Qiu, J. Amphiphilic Styrene-Based Pyrene Derivatives: Tunable Aggregation Luminescence and Their Photo-Induced Dimerization Behavior. Molecules 2025, 30, 1719. https://doi.org/10.3390/molecules30081719
Zhang J, Luo X, Qiu J. Amphiphilic Styrene-Based Pyrene Derivatives: Tunable Aggregation Luminescence and Their Photo-Induced Dimerization Behavior. Molecules. 2025; 30(8):1719. https://doi.org/10.3390/molecules30081719
Chicago/Turabian StyleZhang, Junying, Xingwei Luo, and Juan Qiu. 2025. "Amphiphilic Styrene-Based Pyrene Derivatives: Tunable Aggregation Luminescence and Their Photo-Induced Dimerization Behavior" Molecules 30, no. 8: 1719. https://doi.org/10.3390/molecules30081719
APA StyleZhang, J., Luo, X., & Qiu, J. (2025). Amphiphilic Styrene-Based Pyrene Derivatives: Tunable Aggregation Luminescence and Their Photo-Induced Dimerization Behavior. Molecules, 30(8), 1719. https://doi.org/10.3390/molecules30081719





