Cr(VI) Adsorption by Mg/Al Layered Double Hydroxide-Modified Sphagnum Moss Cellulose Gel: Performance and Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analyses of Morphologies
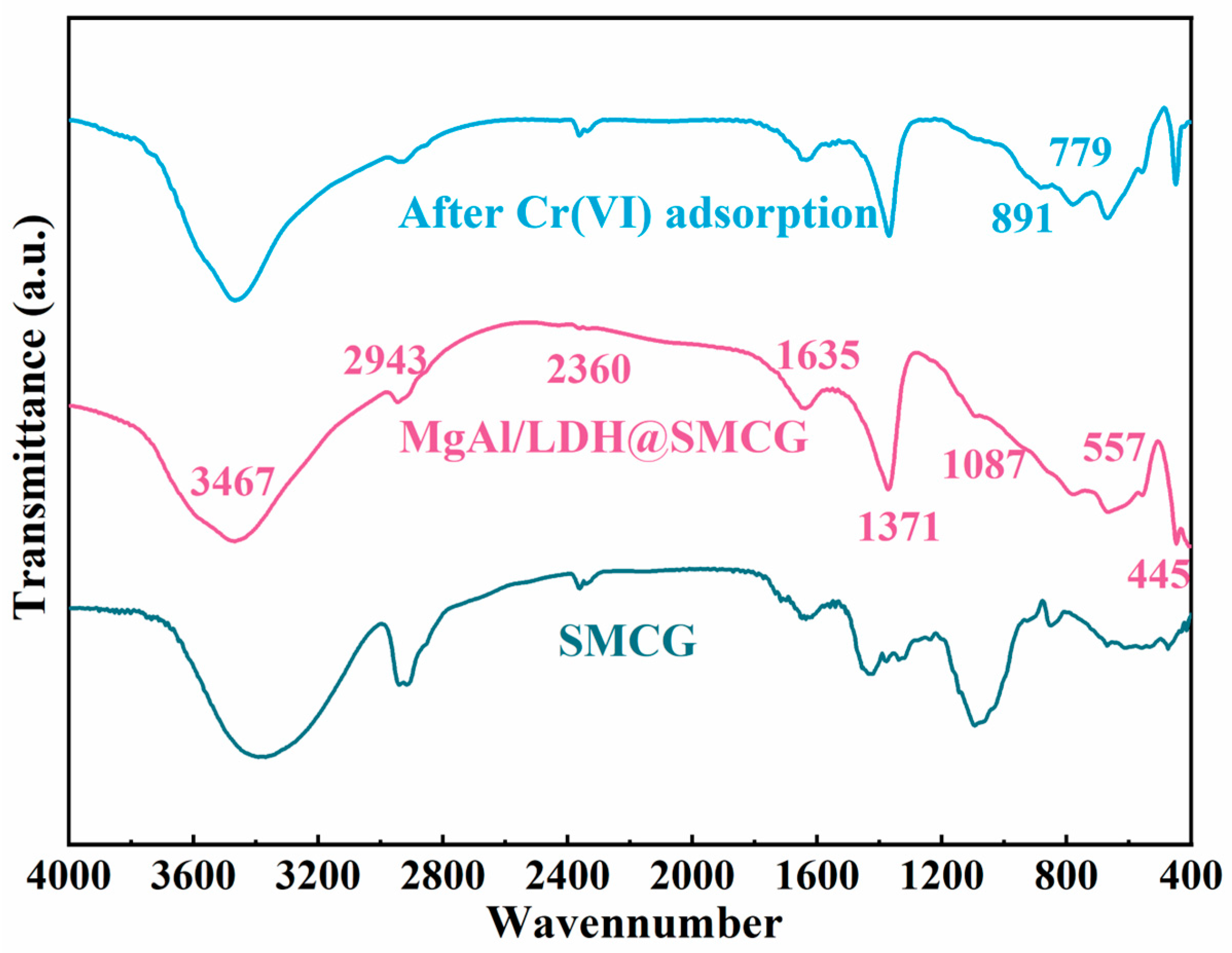
2.2. FT-IR Analysis
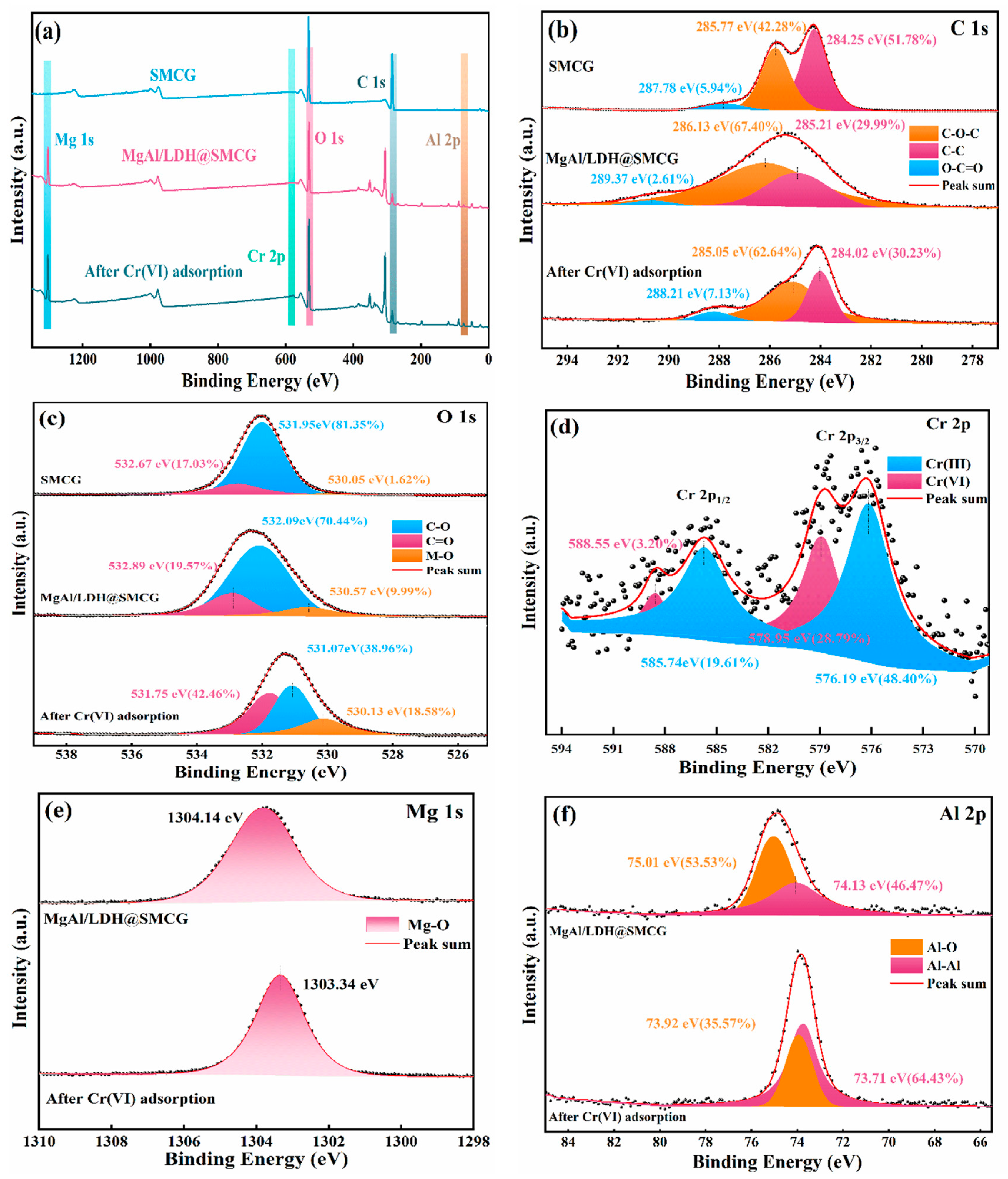
2.3. XPS Analysis
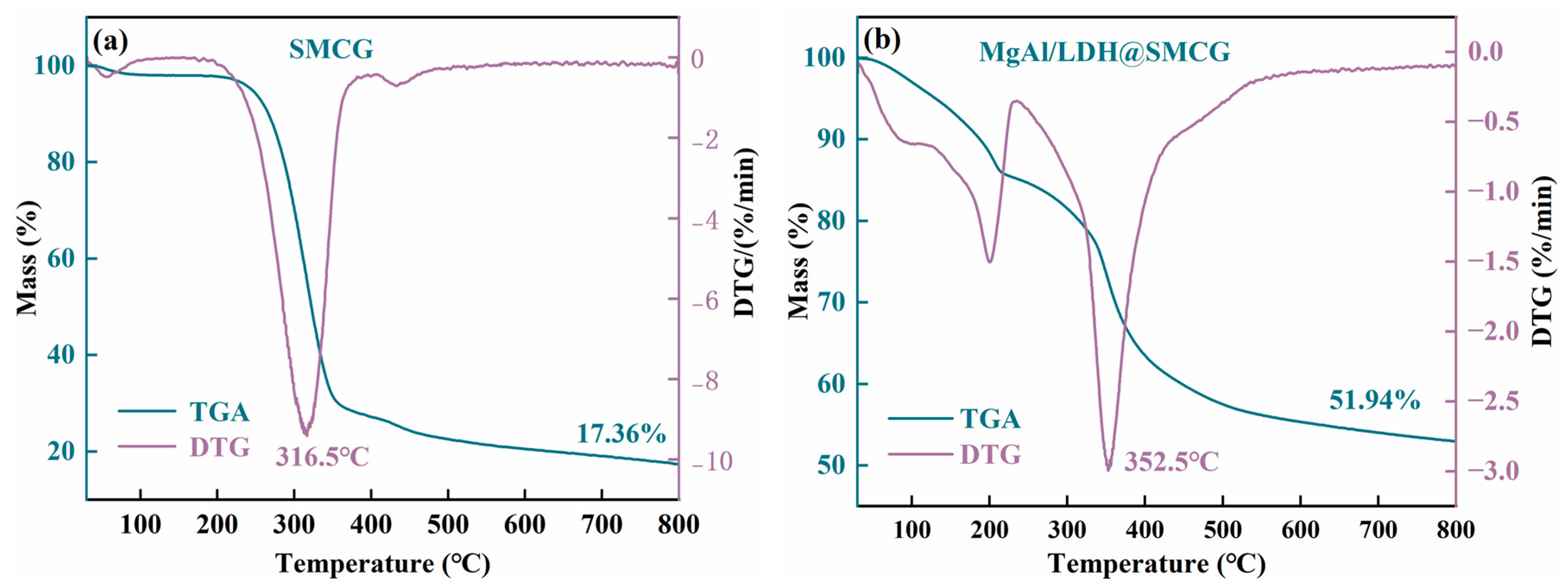
2.4. TG Analysis
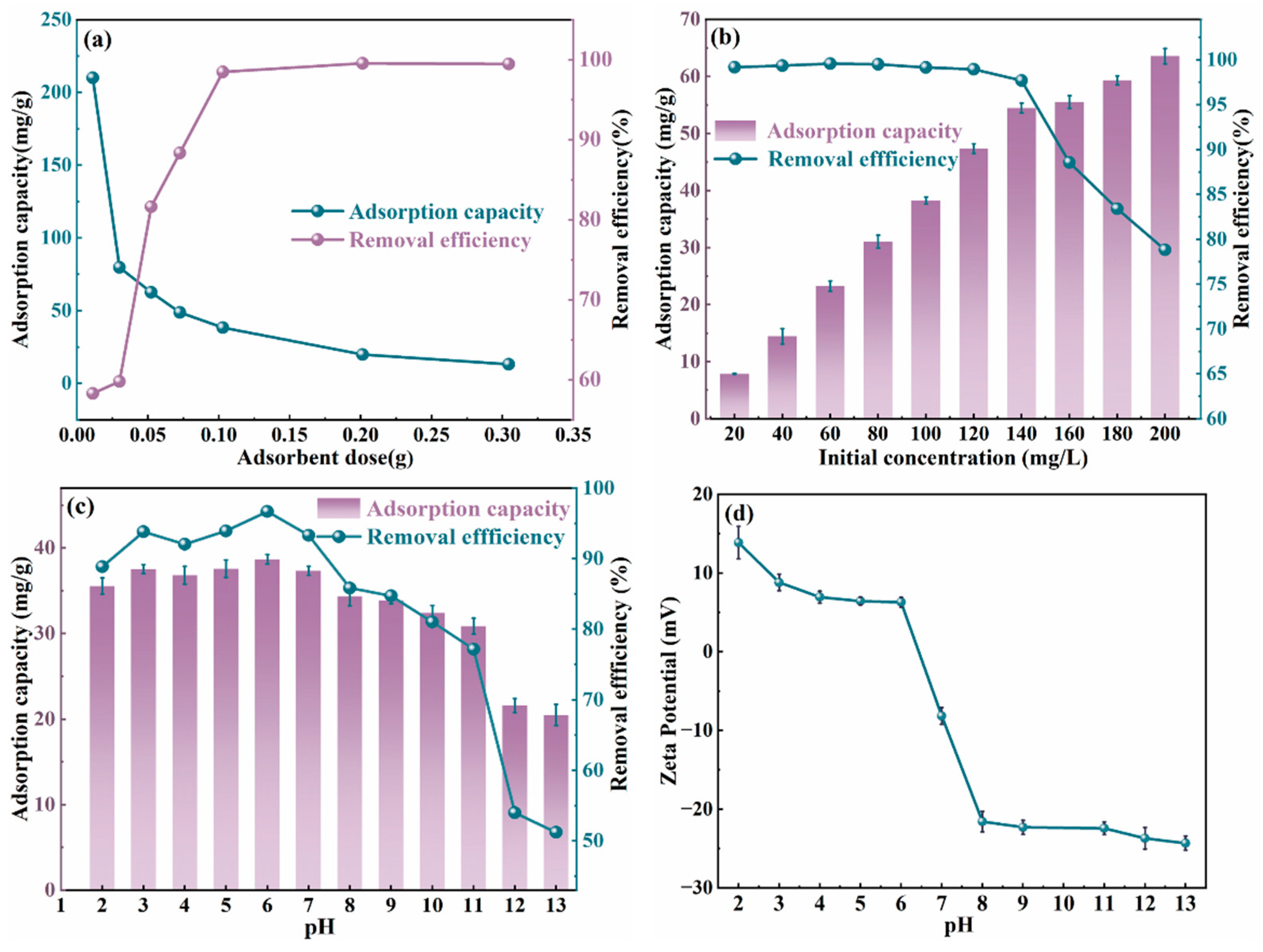
2.5. Adsorption Performance Analysis
2.6. Adsorption Kinetics
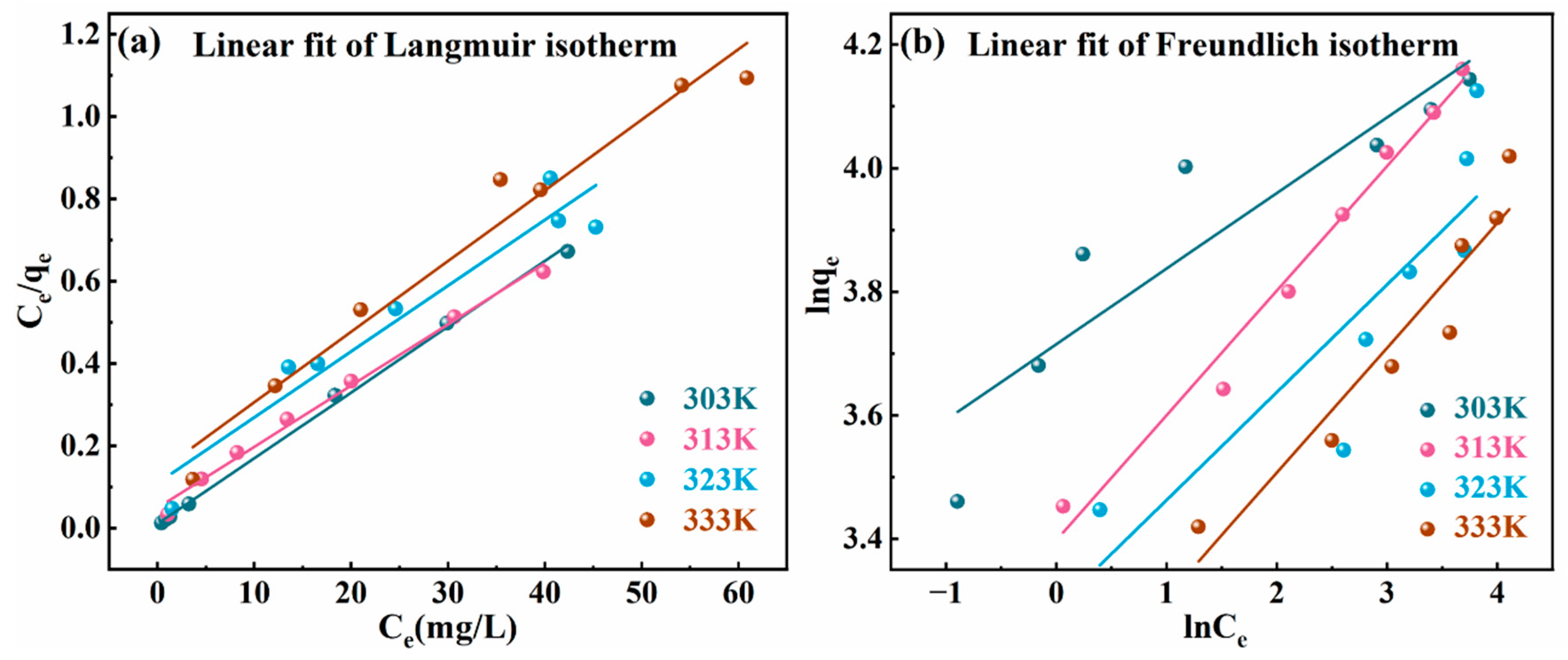
2.7. Adsorption Isotherms
2.8. Thermodynamics Investigation
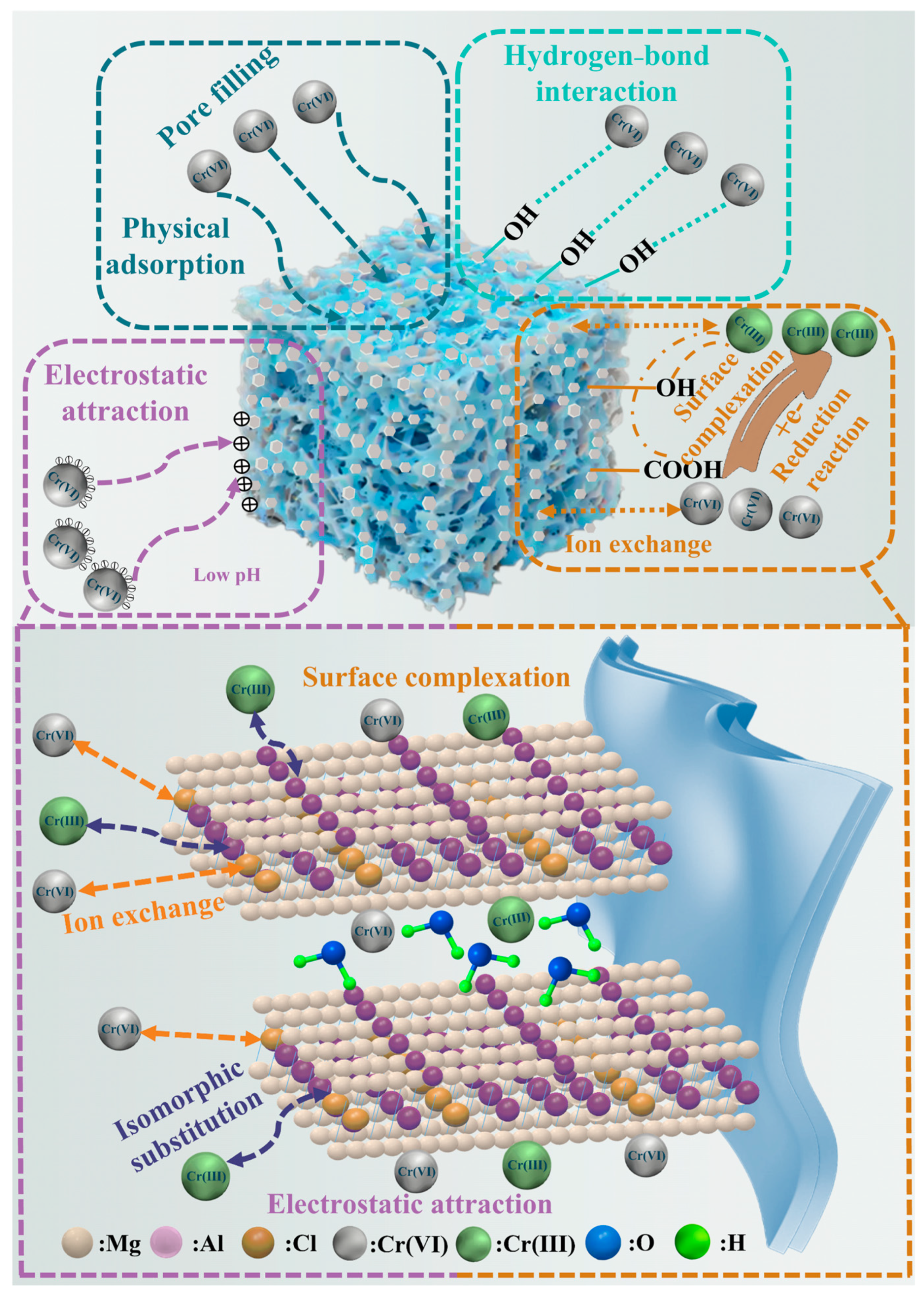
2.9. Adsorption Mechanism
3. Experimental Methods
3.1. Materials
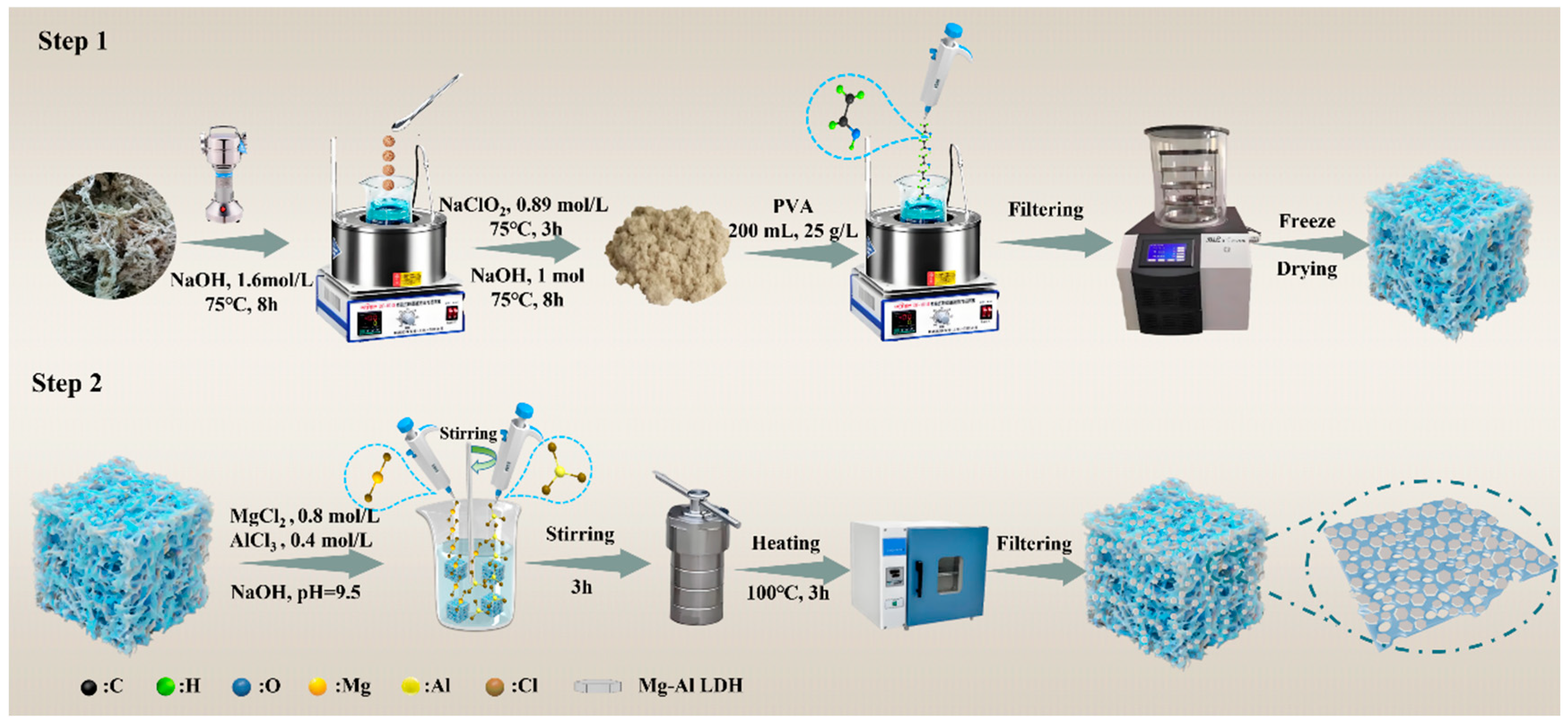
3.2. Preparation of Gel Adsorption Materials
3.3. Characterization
3.4. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Li, W.; Wang, X.; Su, M.; Lin, Q. A novel 3D hierarchical NiFe-LDH/graphitic porous carbon composite as multifunctional adsorbent for efficient removal of cationic/anionic dyes and heavy metal ions. J. Mol. Liq. 2024, 411, 125753. [Google Scholar] [CrossRef]
- Mustapha, L.S.; Kolade, S.O.; Durosinmi, S.O.; Tan, I.S.; Lau, S.Y.; Obayomi, K.S. Anthill clay activated ocimum gratissimum extract for effective adsorption of methylene blue and chromium (VI) ion from wastewater: Insights into the adsorption isotherms, kinetics, thermodynamics, and mechanisms. J. Water Process Eng. 2024, 67, 106286. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, J.; Li, X.; Hao, B.; Xu, F.; Liu, K. Self-assembled morphology-controlled hierarchical Fe3O4 @LDH for Cr(VI) removal. J. Environ. Chem. Eng. 2023, 11, 110129. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Abd-Elhamid, A.I.; Aly, A.A.; Bräse, S.; Nayl, A.A. Synthesis of Ni-Fe-Co3 layered double hydroxide as effective adsorbent to remove Cr(VI) and ARS-dye from aqueous media. Environ. Technol. Innov. 2023, 31, 103214. [Google Scholar] [CrossRef]
- Li, X.; Shi, Z.; Zhang, J.; Gan, T.; Xiao, Z. Aqueous Cr(VI) removal performance of an invasive plant-derived biochar modified by Mg/Al-layered double hydroxides. Colloid Interface Sci. Commun. 2023, 53, 100700. [Google Scholar] [CrossRef]
- Usmani, W.; Inam, M.A.; Iftikhar, R.; Irfan, I.; Adnan, R.; Niazi, M.B.K.; Khan, R.; Hassan, M. Efficient removal of hexavalent chromium Cr(VI) using magnesium-iron layered double hydroxide supported on orange peel (Mg-Fe LDH@OPP): A synthetic experimental and mechanism studies. J. Water Process Eng. 2023, 55, 104233. [Google Scholar] [CrossRef]
- Nemati, S.S.; Dehghan, G.; Khataee, A.; Alidokht, L.; Kudaibergenov, N. Layered double hydroxides as versatile materials for detoxification of hexavalent chromium: Mechanism, kinetics, and environmental factors. J. Environ. Chem. Eng. 2024, 12, 114742. [Google Scholar] [CrossRef]
- Miao, J.; Zhao, Q.; Qian, L.; Fan, K.; Wen, J.; Mo, L.; Qin, Z. Preparation and characterization of functionally antibacterial multi-layer lignin core-shell structure material with adsorption and high efficiency of photocatalysis for Cr(VI). Ind. Crops Prod. 2024, 222, 119574. [Google Scholar] [CrossRef]
- Xiong, J.; Xiao, Y.; Tan, Z.; Xu, X.; Wang, Z.; Zhang, L.; Shi, Y.; Pi, K.; Qiu, G.; Yang, X. Influence of coexisting anions on the one-step electrochemical reduction and precipitation removal of Cr(VI): Implications for advanced wastewater treatment. J. Environ. Manag. 2024, 371, 123167. [Google Scholar] [CrossRef]
- Thiripelu, P.; Manjunathan, J.; Revathi, M.; Ramasamy, P. Removal of hexavalent chromium from electroplating wastewater by ion-exchange in presence of Ni(Ⅱ) and Zn(Ⅱ) ions. J. Water Process Eng. 2024, 58, 104815. [Google Scholar] [CrossRef]
- Verma, R.; Maji, P.K.; Sarkar, S. Removal of hexavalent chromium from impaired water: Polyethylenimine-based sorbents—A review. J. Environ. Chem. Eng. 2023, 11, 109598. [Google Scholar] [CrossRef]
- Yu, K.; Yang, L.; Zhang, S.; Song, H.; Wang, S.; Liu, H. A novel chitosan-based hydrogel microspheres for efficient heavy metal-ion adsorption. Mater. Today Commun. 2024, 41, 110488. [Google Scholar] [CrossRef]
- Cheng, W.; Wen, J.; Yang, W. Removal of hexavalent chromium using one-step synthesized metal-polyphenol composites epigallocatechin gallate/titanium: Performance, interference, and mechanisms. J. Environ. Chem. Eng. 2024, 12, 111866. [Google Scholar] [CrossRef]
- Mabalane, K.; Shooto, N.D.; Thabede, P.M. A novel permanganate and peroxide carbon-based avocado seed waste for the adsorption of manganese and chromium ions from water. Case Stud. Chem. Environ. Eng. 2024, 10, 100782. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Mao, W.; Guan, Y. Fast and efficient removal of Cr(VI) to ppb level together with Cr(Ⅲ) sequestration in water using layered double hydroxide interclated with diethyldithiocarbamate. Sci. Total Environ. 2020, 727, 138701. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Luo, L.; Zhong, Z.; Xie, X. Advances in sorptive removal of hexavalent chromium (Cr(VI)) in aqueous solutions using polymeric materials. Polymers 2023, 15, 388. [Google Scholar] [CrossRef]
- Varamesh, A.; Abraham, B.D.; Wang, H.; Berton, P.; Zhao, H.; Gourlay, K.; Minhas, G.; Lu, Q.; Bryant, S.L.; Hu, J. Multifunctional fully biobased aerogels for water remediation: Applications for dye and heavy metal adsorption and oil/water separation. J. Hazard. Mater. 2023, 457, 131824. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Li, B. Adsorption behaviour of congo red by cellulose/chitosan hydrogel beads regenerated from ionic liquid. Desalination Water Treat. 2016, 57, 16970–16980. [Google Scholar] [CrossRef]
- Riaz, S.; Rehman, A.U.; Akhter, Z.; Najam, T.; Hossain, I.; Karim, M.R.; Assiri, M.A.; Shah, S.S.A.; Nazir, M.A. Recent advancement in synthesis and applications of layered double hydroxides (LDHs) composites. Mater. Today Sustain. 2024, 27, 100897. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; He, J.; Liu, S.; Tang, Y.; Wen, X. Porous NiCo-LDH microspheres obtained by freeze-drying for efficient dye and Cr(VI) adsorption. J. Alloys Compd. 2024, 976, 173107. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, X.; Liu, S.; Ren, S.; Luo, W. Synergistic effect of W(VI) and Ni(Ⅱ) uptakes on an MgAl-layered double hydroxide. Environ. Res. 2025, 266, 120591. [Google Scholar] [CrossRef]
- Mo, W.; He, C.; Yang, Y.; Cheng, B.; Yang, J.; Huang, Y. Adsorption behavior of Mg-Al layered double hydroxide on Pb(Ⅱ), Zn(Ⅱ), Cd(Ⅱ), and As(Ⅴ) coexisting in aqueous solution. Mater. Today Sustain. 2024, 27, 100861. [Google Scholar] [CrossRef]
- Shen, Z.; Li, Q.; Wei, X.; Lu, M.; Chang, Z.; Chong, R.; Li, D. Highly efficient removal of Pb2+ and Cd2+ on MgAl-LDH modified with carbon dots, citric acid and amino silane: Kinetic, isothermal and mechanistic studies. J. Environ. Chem. Eng. 2024, 12, 113601. [Google Scholar] [CrossRef]
- Sarbisheh, F.; Norouzbeigi, R.; Hemmati, F.; Shayesteh, H. Application of response surface methodology for modeling and optimization of malachite green adsorption by modified sphagnum peat moss as a low cost biosorbent. Desalination Water Treat. 2017, 59, 230–242. [Google Scholar] [CrossRef]
- Coupal, B.; Lalancette, J. The treatment of waters with peat moss. Water Res. 1976, 10, 1071–1076. [Google Scholar] [CrossRef]
- Zhang, R.; Leiviskä, T.; Taskila, S.; Tanskanen, J. Iron-loaded sphagnum moss extract residue for phosphate removal. J. Environ. Manag. 2018, 218, 271–279. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, F.; Zhang, F.; He, Y.; Song, P.; Wang, R. Fabrication of 3D flower-shaped MgAl-LDHs with inducing cumof for improving its adsorption and antibacterial performance. J. Mol. Struct. 2025, 1322, 140583. [Google Scholar] [CrossRef]
- Cui, B.; Cui, J.; Liu, C.; Fang, J.; Wang, S.; Du, X.; Li, Z. Synthesis of MgAl-LDH from three alkali sources for boosting flame retardancy of EP with APP. Constr. Build. Mater. 2024, 447, 137997. [Google Scholar]
- Ci, E.; Zhao, F.; Liu, T.; Yang, C.; Liu, F.; Zhao, T. Enhancing adsorption of Cr(VI) from aqueous solutions: Core-shell engineered polyaniline@MOF composites overcome thermodynamic-kinetic trade-off effect. Sep. Purif. Technol. 2025, 362, 131839. [Google Scholar] [CrossRef]
- Peng, L.; Hu, Z.; Cui, J.; Yang, N.; Bao, R.; Dai, Y.; Wang, Q.; Jiang, Y.; Cui, P. Tailoring of a chitosan-based adsorbent with target-specific sites for efficient adsorption-reduction of chromium ion pollutant. Sep. Purif. Technol. 2025, 360, 131055. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S. A new approach to modification of natural adsorbent for heavy metal adsorption. Bioresour. Technol. 2008, 99, 2516–2527. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Chen, J.; Zhu, J.; Han, P. Effects of amino alcohols-intercalated MgAl-LDH nanocomposites on the properties of sulfoaluminate cement-based materials. Constr. Build. Mater. 2024, 452, 138982. [Google Scholar] [CrossRef]
- Mo, W.; Yang, Y.; He, C.; Huang, Y.; Feng, J. Excellent adsorption properties of Mg/Al/Fe LDOs for Cr(VI) in water. J. Environ. Chem. Eng. 2023, 11, 111069. [Google Scholar] [CrossRef]
- Lin, X.; He, X.; Lei, L.; Zhao, Y.; Cui, L.; Wu, G. Development of ionic liquid filled chitosan capsules to remove Cr(VI) from acidic solution: Adsorption properties and mechanism. J. Environ. Chem. Eng. 2022, 10, 108081. [Google Scholar] [CrossRef]
- Lu, J.; Xu, K.; Yang, J.; Hao, Y.; Cheng, F. Nano iron oxide impregnated in chitosan bead as a highly efficient sorbent for Cr(VI) removal from water. Carbohydr. Polym. 2017, 173, 28–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, J.; Zhou, Y.; Wang, J.; Cheng, W. Novel efficient capture of hexavalent chromium by polyethyleneimine/amyloid fibrils/polyvinyl alcohol aerogel beads: Functional design, applicability, and mechanisms. J. Hazard. Mater. 2023, 458, 132017. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Wang, H.; Huang, H.; Xia, C.; Liang, D.; Yang, J.; Zhang, Q.; Meng, Z. Effective removal and expedient recovery of As(Ⅴ) and Cr(VI) from soil by layered double hydroxides coated waste textile. Sep. Purif. Technol. 2021, 263, 118419. [Google Scholar] [CrossRef]
- Shi, Y.; Shan, R.; Lu, L.; Yuan, H.; Jiang, H.; Zhang, Y.; Chen, Y. High-efficiency removal of Cr(VI) by modified biochar derived from glue residue. J. Clean. Prod. 2020, 254, 119935. [Google Scholar] [CrossRef]
- Hong, X.; Ding, C.; Shi, M.; Ding, Z.; Du, P.; Xia, M.; Wang, F. Easy separation dual-function Cu2O@LDH@Fe3O4 adsorbent for the removal of Cr(VI) under dark conditions: Experimental and mechanistic study. Sep. Purif. Technol. 2024, 332, 125734. [Google Scholar] [CrossRef]
- Li, Z.; Fang, X.; Yuan, W.; Zhang, X.; Yu, J.; Chen, J.; Qiu, X. Preparing of layered double hydroxide- alginate microspheres for Cr(VI)-contaminated soil remediation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130655. [Google Scholar] [CrossRef]
- Long, F.; Niu, C.; Tang, N.; Guo, H.; Li, Z.; Yang, Y.; Lin, L. Highly efficient removal of hexavalent chromium from aqueous solution by calcined Mg/Al-layered double hydroxides/polyaniline composites. Chem. Eng. J. 2021, 404, 127084. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Q.; Feng, X.; Rui, Z.; Guo, W.; Zhang, Y.; Li, W.; Li, Z. Chloride ion-responsive active protection epoxy composite coatings realized by 2-mercaptobenzimidazole and 2-mercaptobenzothiazole intercalated Mg-Al layered double hydroxides. Corros. Sci. 2024, 234, 112144. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Ghorbani, M.; Mohammadi, M.; Pezeshki, A. Improvement of the physico-mechanical properties of antibacterial electrospun poly lactic acid nanofibers by incorporation of guar gum and thyme essential oil. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126659. [Google Scholar] [CrossRef]
- Chen, T.; Tang, P.; Feng, Y.; Li, D. Facile color tuning, characterization, and application of acid green 25 and acid yellow 25 co-intercalated layered double hydroxides. Ind. Eng. Chem. Res. 2017, 56, 5495–5504. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Hu, Y.; Yan, S.; Yang, J. Adsorption removal of cationic dyes from aqueous solutions using ceramic adsorbents prepared from industrial waste coal gangue. J. Environ. Manag. 2019, 234, 245–252. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, M.; Chen, B.; Zhang, Y.; Sun, Y.; Chen, K.; Du, Q.; Pi, X.; Wang, Y.; et al. Efficient adsorption of methylene blue in water by nitro-functionalized metal-organic skeleton-calcium alginate composite aerogel. Int. J. Biol. Macromol. 2023, 253, 126458. [Google Scholar] [CrossRef]
- Saha, B.; Orvig, C. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord. Chem. Rev. 2010, 254, 2959–2972. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Bahari, M.B.; Izzuddin, N.M.; Fauzi, N.A.F.M.; Jusoh, N.W.C.; Kamaroddin, M.F.A.; Saravanan, R.; Tehubijuluw, H. A critical review of mxene-based composites in the adsorptive and photocatalysis of hexavalent chromium removal from industrial wastewater. Environ. Res. 2024, 259, 119584. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, Z.; Wei, S. A new hydrotalcite-like absorbent FeMnMg-LDH and its adsorption capacity for Pb2+ ions in water. Appl. Clay Sci. 2018, 153, 29–37. [Google Scholar] [CrossRef]
- Benhiti, R.; Ait Ichou, A.; Aboussabek, A.; Carja, G.; Zerbet, M.; Sinan, F.; Chiban, M. Efficient removal of Cr(VI) from aqueous solution using memory effect property of layered double hydroxide material. Chemosphere 2023, 341, 140127. [Google Scholar] [CrossRef]
- Song, X.; Xiao, W.; Chen, X.; Chen, W.; Mou, H.; Ao, T. Nanohybrid layered clay composite decorated with polyaniline for intensified Cr(VI) removal through enhanced Cr(VI) reduction. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134474. [Google Scholar] [CrossRef]
- Elhamdy, W.A. Sustainable phosphorous porous biochar generated from sugarcane bagasse for effective adsorption coupled reduction of hexavalent chromium: Evaluating the method’s greenness. Inorg. Chem. Commun. 2024, 169, 112931. [Google Scholar] [CrossRef]
- Huang, S.; Ouyang, T.; Chen, J.; Wang, Z.; Liao, S.; Li, X.; Liu, Z. Synthesis of nickel–iron layered double hydroxide via topochemical approach: Enhanced surface charge density for rapid hexavalent chromium removal. J. Colloid Interface Sci. 2022, 605, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Alnasrawi, F.A. Cadmium sequestration with MgCuAl-layered double hydroxide@montmorillonite nanocomposite in batch and circulated fluidized bed column experiments. Desalination Water Treat. 2024, 320, 100744. [Google Scholar] [CrossRef]
- Tian, H.; Yang, H.; Mo, Z.; Guo, W.; Xu, L.; Liu, B. Removal of Cr(VI) from electroplating wastewater by CoNi-LDH@NHCS electrode and its electrocatalytic ammonia production. J. Water Process Eng. 2024, 68, 106471. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Dou, Y.; Xue, Y.; Ji, Y.; Tang, Y.; Hu, M. Removal difference of Cr(VI) by modified zeolites coated with MgAl and ZnAl-layered double hydroxides: Efficiency, factors and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126583. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Chen, Z.; Zhou, X.; Wang, J.; Chen, Z. Engineered biochar with anisotropic layered double hydroxide nanosheets to simultaneously and efficiently capture Pb2+ and CrO42− from electroplating wastewater. Bioresour. Technol. 2020, 306, 123118. [Google Scholar] [CrossRef]
- Li, M.; Guo, X.; Wei, Y.; Liu, A.; Lu, J.; Niu, X.; Ma, Y.; Li, S.; Shang, Z.; Liu, X. Adsorption mechanism and structure-performance relationship of chromium ions by biochar. Water Air Soil Pollut. 2020, 231, 517. [Google Scholar] [CrossRef]



| Parameters | Initial Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|
| 60 | 80 | 100 | 120 | 140 | ||
| , exp(mg·g−1) | 23.6445 | 31.1871 | 39.6445 | 47.4609 | 52.8479 | |
| Pseudo-first- order model | K1 (min−1) | 0.1211 | 0.1067 | 0.1080 | 0.1091 | 0.0472 |
| R2 | 0.9398 | 0.9101 | 0.8931 | 0.8704 | 0.7531 | |
| (mg·g−1) | 9.6194 | 22.8902 | 9.6549 | 16.1710 | 6.8210 | |
| Pseudo-second-order model | K2 (g·mg−1·min−1) | 0.0282 | 0.0050 | 0.0329 | 0.0163 | 0.0523 |
| R2 | 0.9995 | 0.9868 | 0.9998 | 0.9994 | 0.9999 | |
| (mg·g−1) | 24.4559 | 35.3107 | 40.2576 | 48.7567 | 52.6367 | |
| Adsorption Isotherm Models | Parameters | 303 K | 313 K | 323 K | 333 K |
|---|---|---|---|---|---|
| Langmuir | qm | 62.6566 | 67.2495 | 62.5391 | 58.3090 |
| KL | 1.4888 | 0.3035 | 0.1459 | 0.1275 | |
| RL | 0.0083 | 0.0396 | 0.0789 | 0.0893 | |
| R2 | 0.9978 | 0.9913 | 0.9149 | 0.9626 | |
| Freundlich | KF | 41.0767 | 29.9129 | 26.8056 | 22.2771 |
| 1/n | 0.1222 | 0.2019 | 0.1744 | 0.2020 | |
| R2 | 0.8103 | 0.9788 | 0.6903 | 0.8951 |
| ΔH0 (kJ/mol) | ΔS0 (J/(mol·K) | ΔG0 (kJ/mol) | |||
|---|---|---|---|---|---|
| −58.916 | −158.615 | 303 K | 313 K | 323 K | 333 K |
| −10.980 | −8.828 | −8.198 | −5.899 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Zhang, S.; Wang, Y.; Shi, H.; Zhen, C. Cr(VI) Adsorption by Mg/Al Layered Double Hydroxide-Modified Sphagnum Moss Cellulose Gel: Performance and Mechanism. Molecules 2025, 30, 1796. https://doi.org/10.3390/molecules30081796
Ren J, Zhang S, Wang Y, Shi H, Zhen C. Cr(VI) Adsorption by Mg/Al Layered Double Hydroxide-Modified Sphagnum Moss Cellulose Gel: Performance and Mechanism. Molecules. 2025; 30(8):1796. https://doi.org/10.3390/molecules30081796
Chicago/Turabian StyleRen, Junpeng, Shijiang Zhang, Yu Wang, Huixian Shi, and Cheng Zhen. 2025. "Cr(VI) Adsorption by Mg/Al Layered Double Hydroxide-Modified Sphagnum Moss Cellulose Gel: Performance and Mechanism" Molecules 30, no. 8: 1796. https://doi.org/10.3390/molecules30081796
APA StyleRen, J., Zhang, S., Wang, Y., Shi, H., & Zhen, C. (2025). Cr(VI) Adsorption by Mg/Al Layered Double Hydroxide-Modified Sphagnum Moss Cellulose Gel: Performance and Mechanism. Molecules, 30(8), 1796. https://doi.org/10.3390/molecules30081796






