One-Step Molten Salt Constructing Double S-Scheme K0.2WO3/NiO/NiWO4 Heterojunction for Photocatalytic CO2 Reduction
Abstract
1. Introduction
2. Results and Discussion
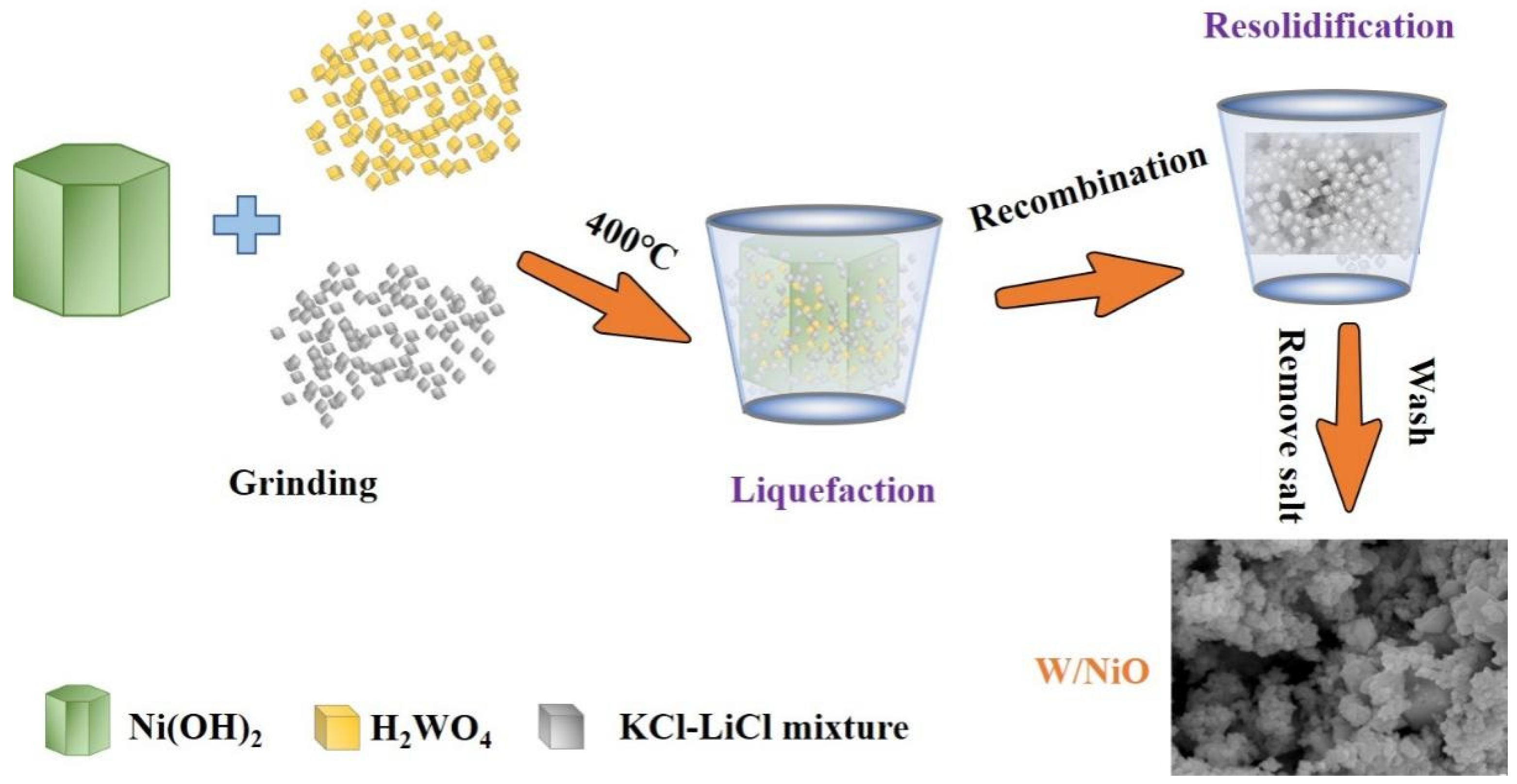
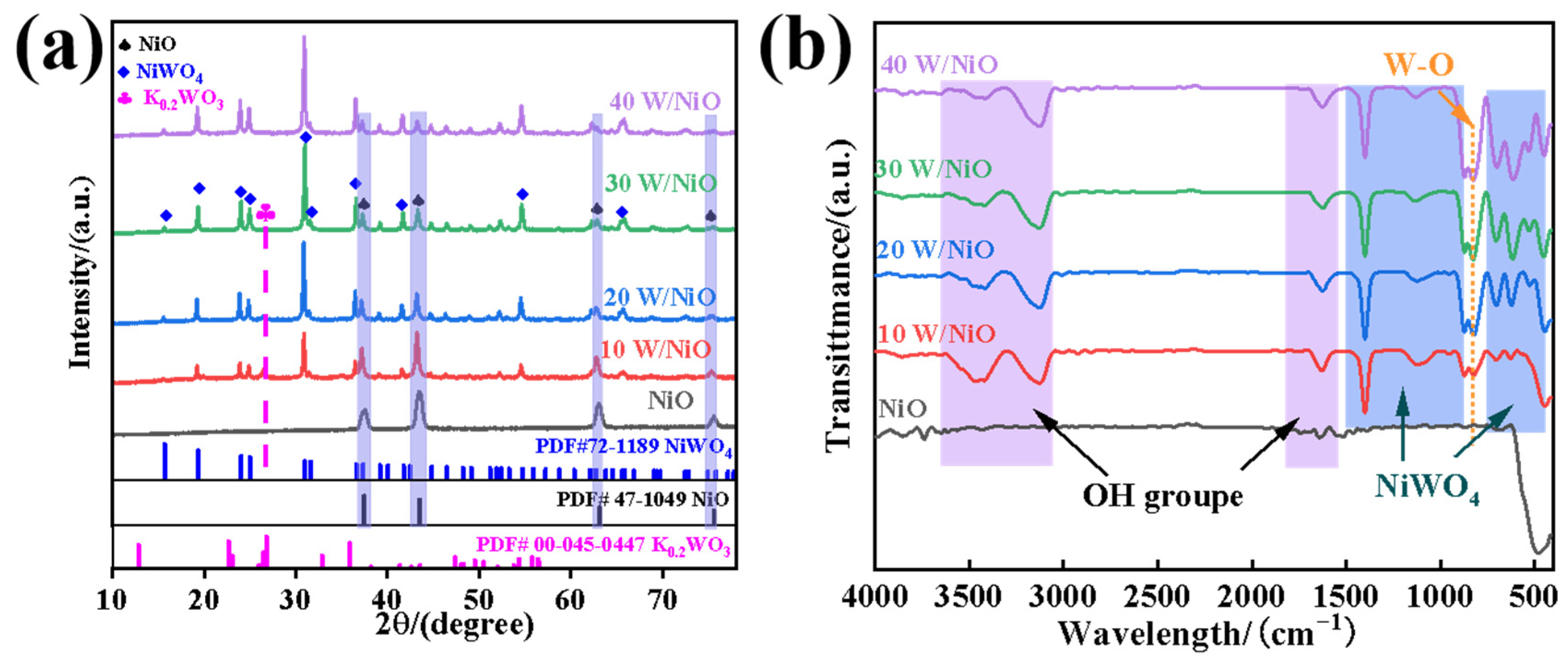
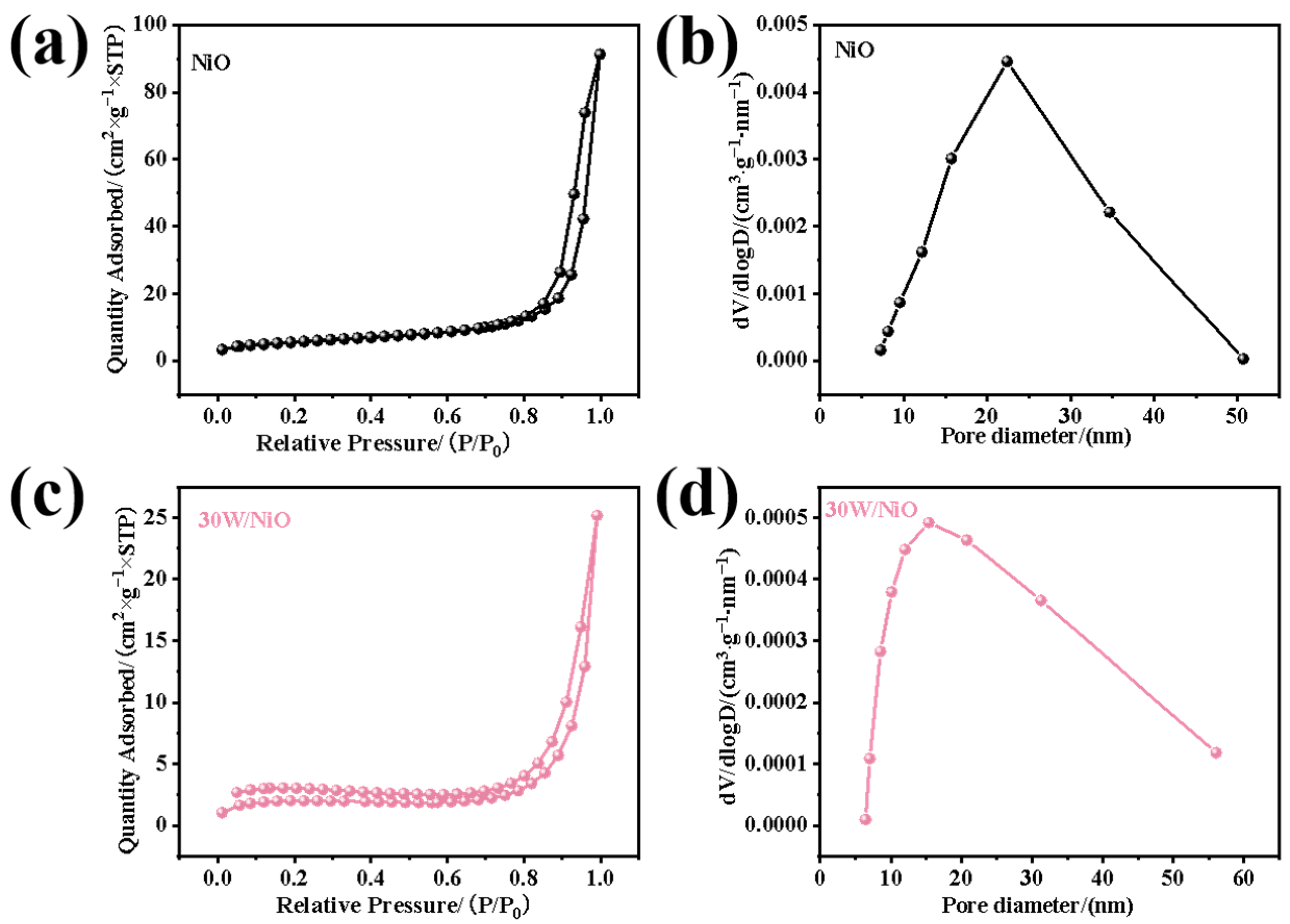
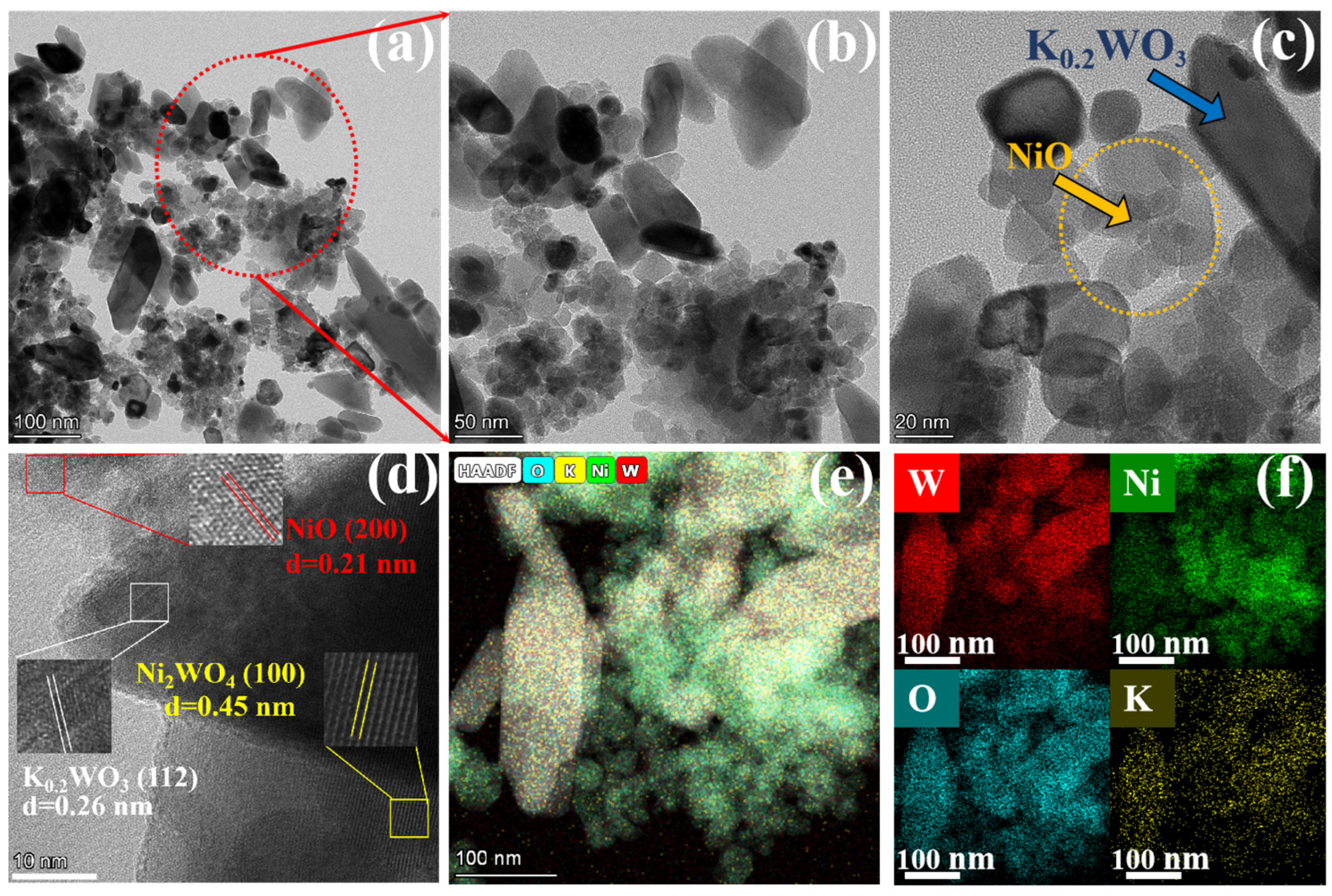
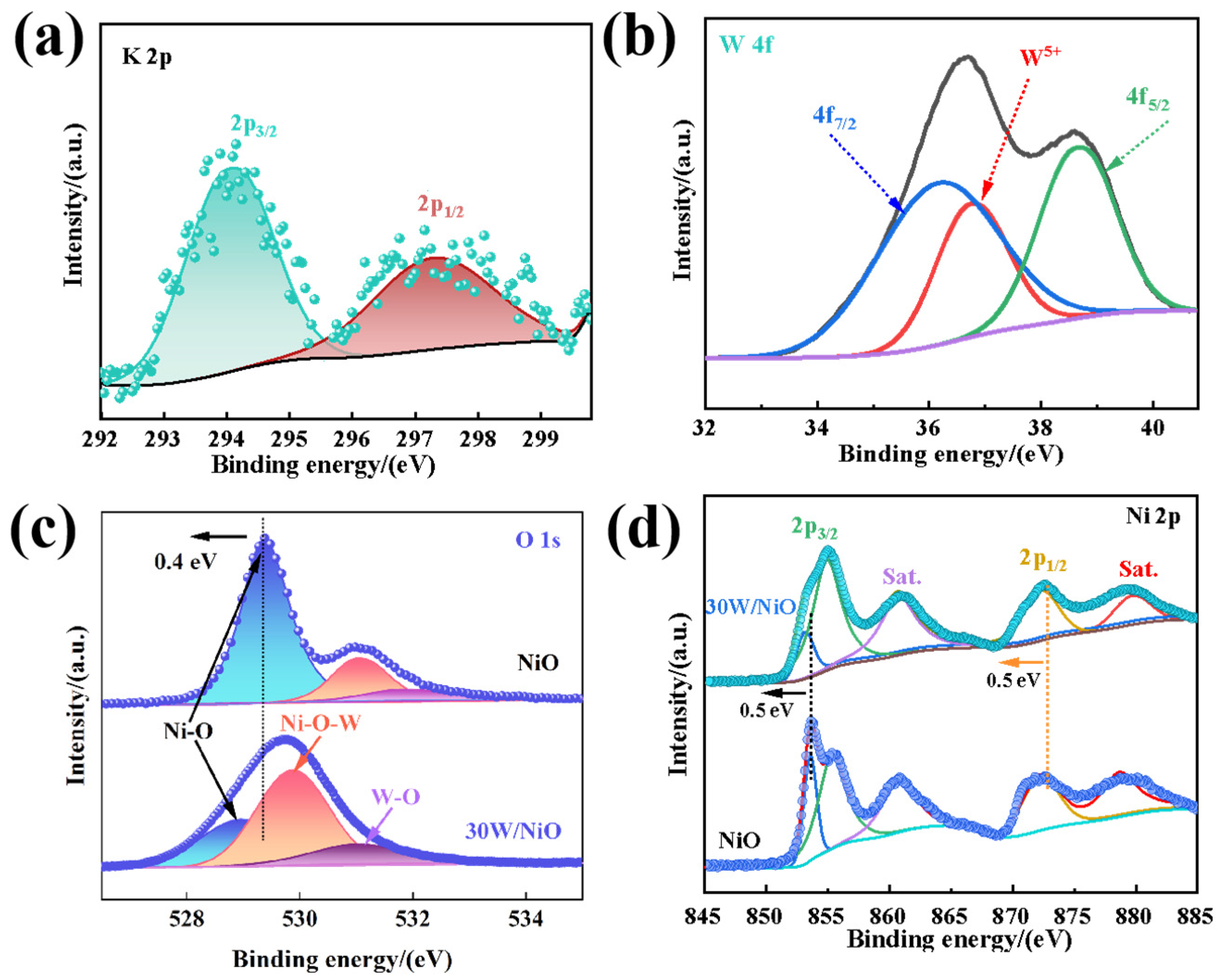
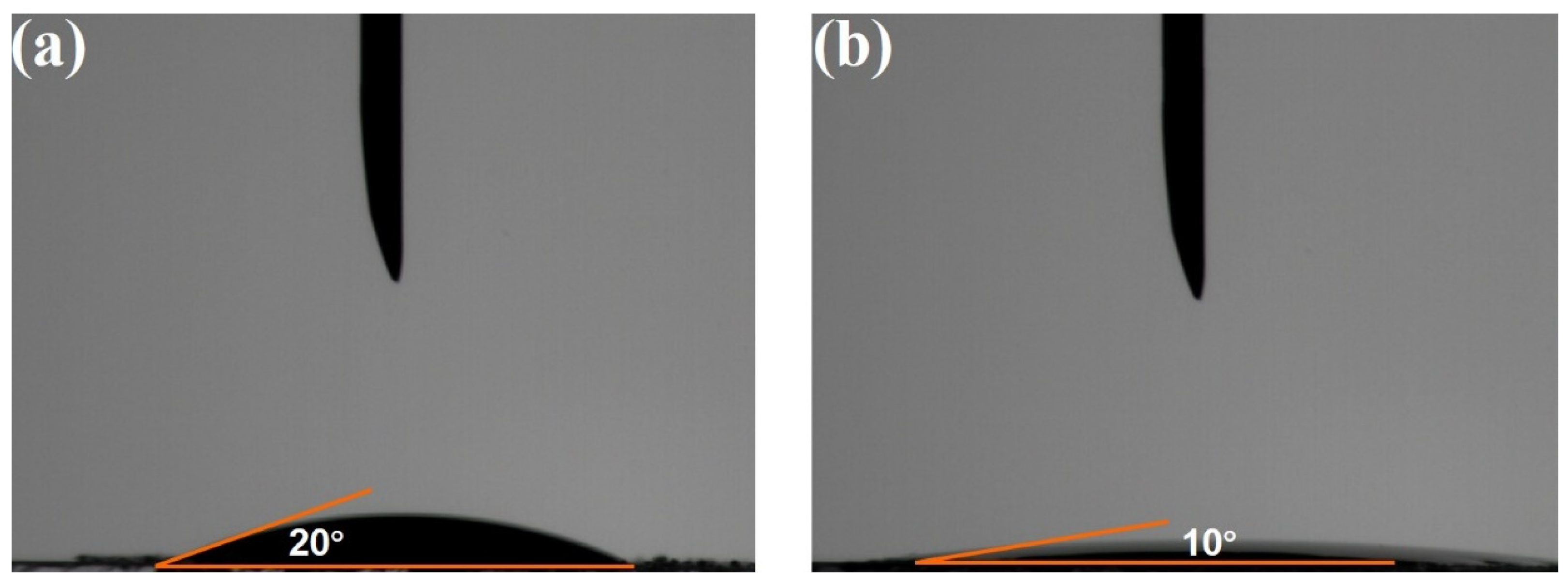
2.1. Structures and Properties of Photocatalysts
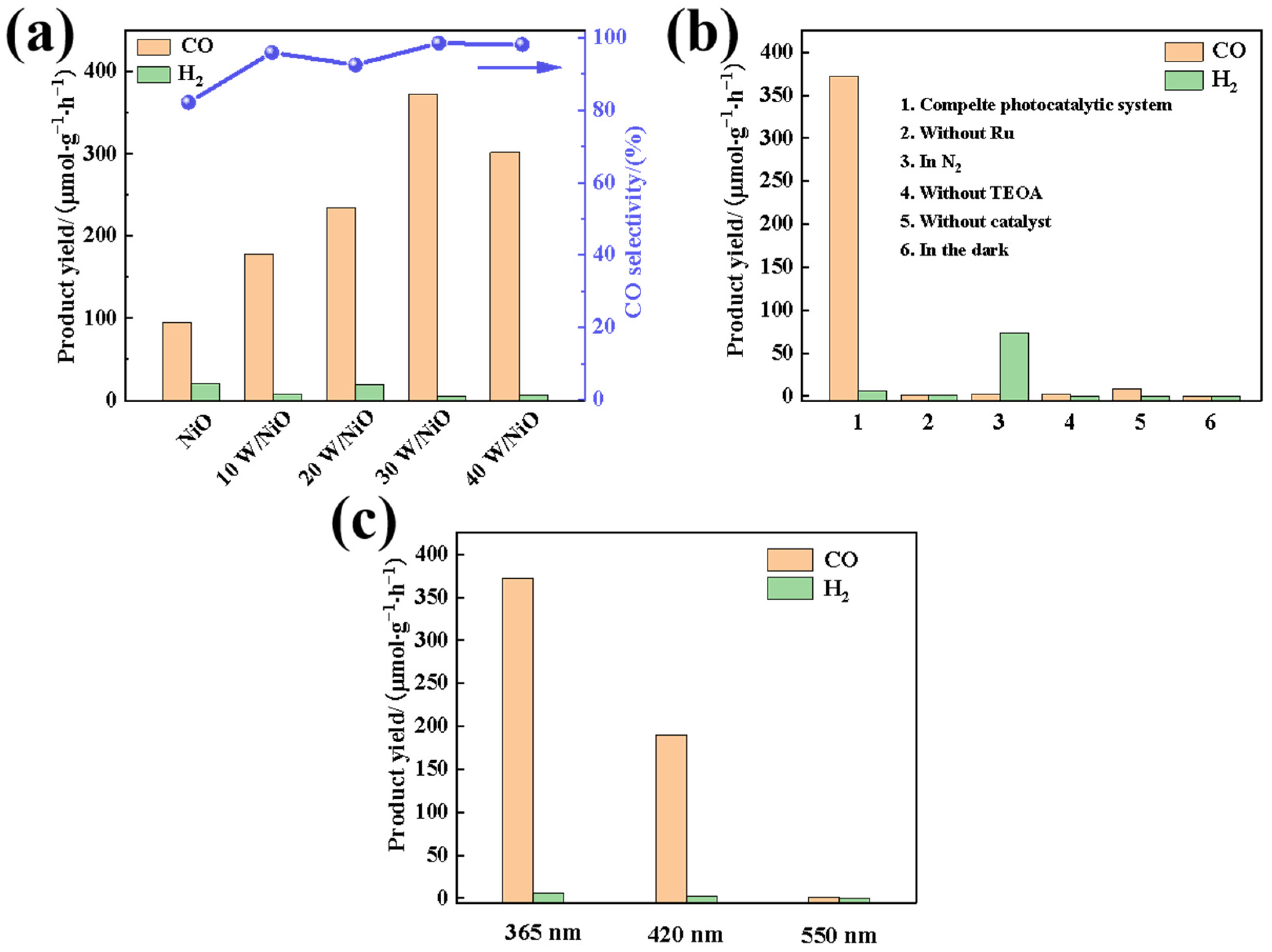
2.2. Photocatalytic CO2 Reduction Performance
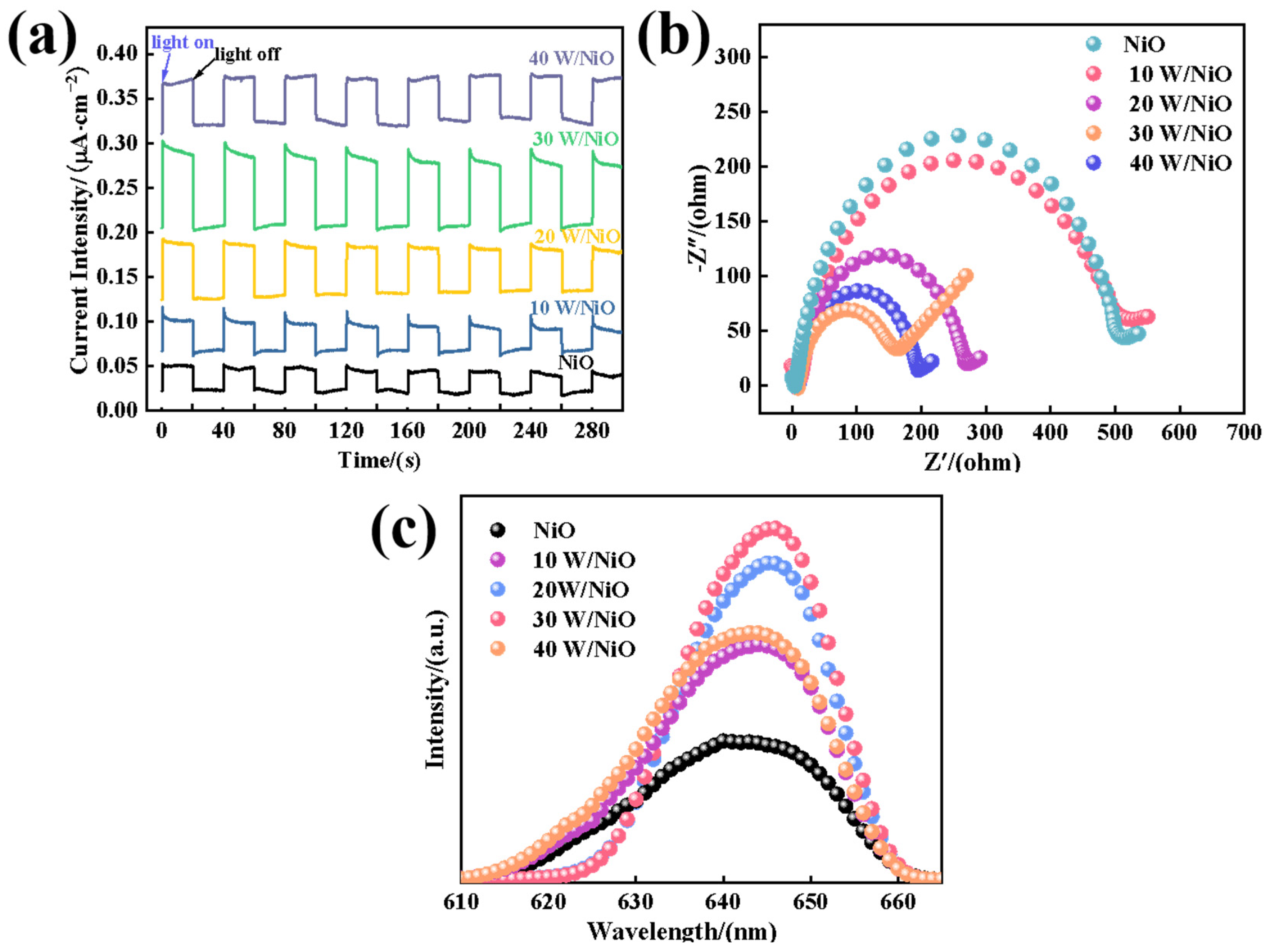
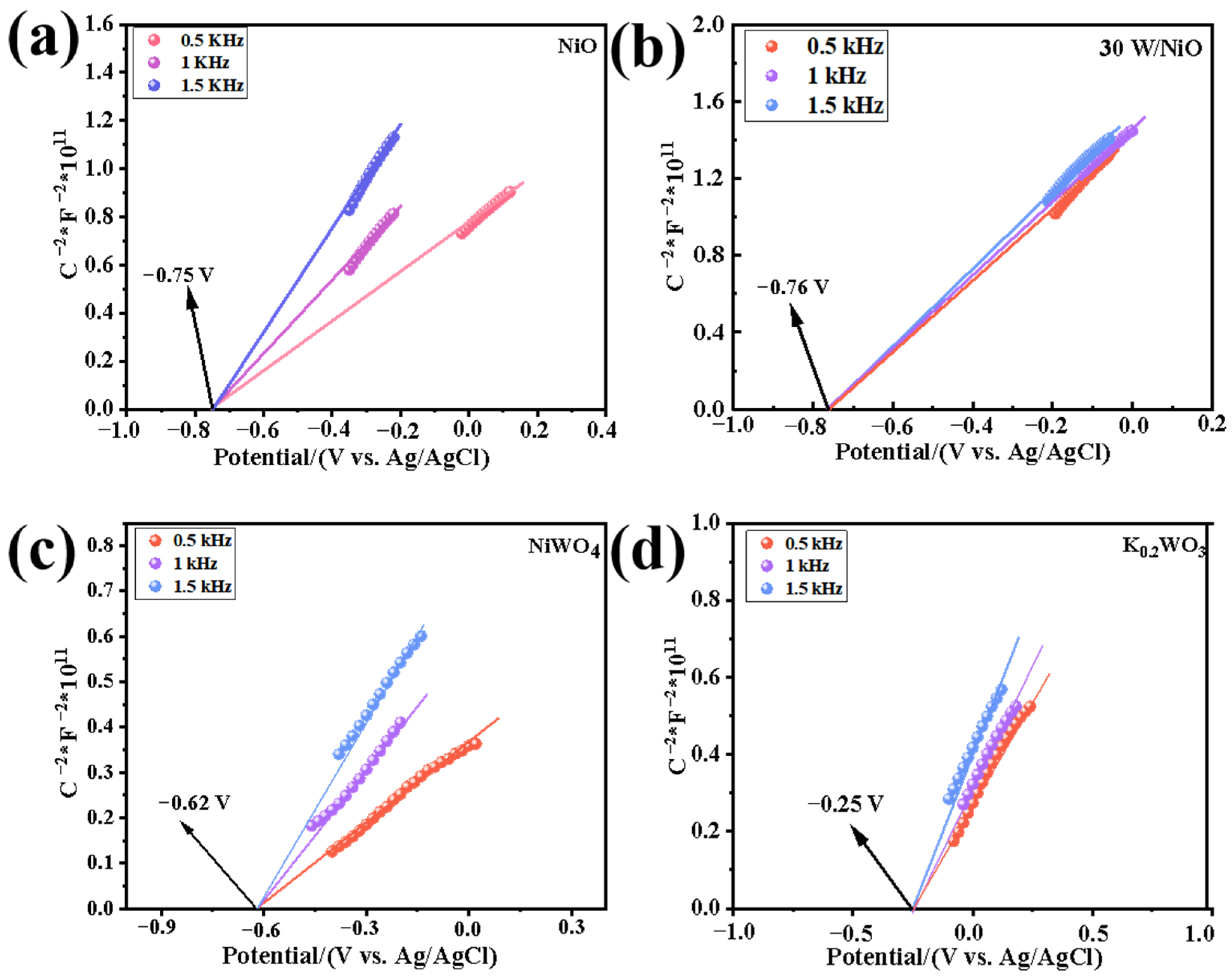
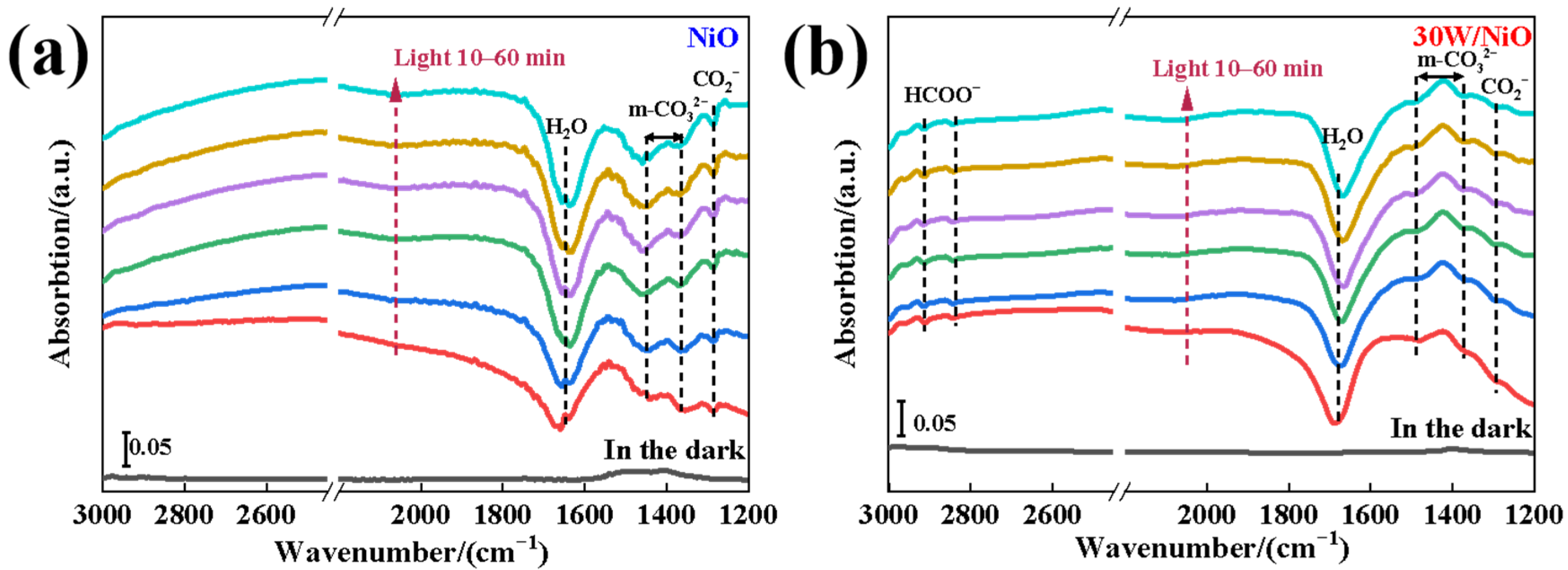
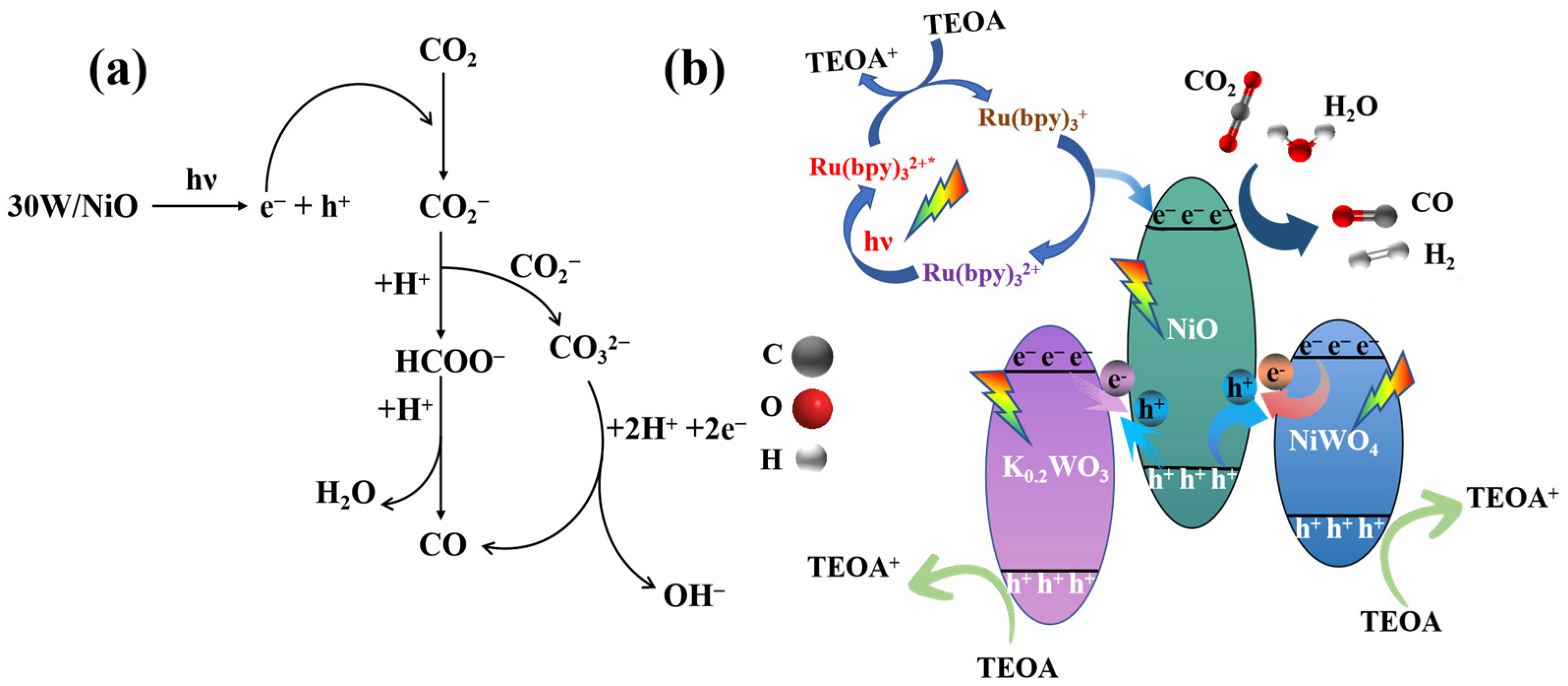
2.3. Discussion of Mechanism of Improving Photocatalytic Performance
3. Experimental Section
3.1. Materials
3.2. Synthesis of Precursor Ni(OH)2
3.3. Synthesis of NiO
3.4. Synthesis of K0.2WO3/NiO/NiWO4 Heterojunction
3.5. Synthesis of K0.2WO3
3.6. Photocatalytic CO2 Reduction
- N: the number of incident photons;
- E: the accumulated light energy in the given area (J);
- λ: the wavelength of the light;
- h: the Plank constant (6.626 × 10−34 J·s);
- c: the velocity of light (3 × 108 m·s−1).
3.7. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Tournet, J.; Dastafkan, K.; Liu, Y.; Ng, Y.H.; Karuturi, S.K.; Zhao, C.; Yin, Z. Noble-Metal-Free Multicomponent Nanointegration for Sustainable Energy Conversion. Chem. Rev. 2021, 121, 10271–10366. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhang, Y.; Yu, J.C.; Guo, Z. Asymmetric Atomic Dual-Sites for Photocatalytic CO2 Reduction. J. Adv. Mater. 2024, 36, 2403153. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Nanostructured Metal Sulfides: Classification, Modification Strategy, and Solar-Driven CO2 Reduction Application. Adv. Funct. Mater. 2020, 31, 2008008. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xiang, M.; He, M.; Zhou, W.; Chen, J.; Lu, J.; Wu, Z.; Su, Y. Transformation of CO2 with Glycerol to Glycerol Carbonate over ETS-10 Zeolite-Based Catalyst. Molecules 2023, 28, 2272. [Google Scholar] [CrossRef]
- He, H.; Wang, Z.; Dai, K.; Li, S.; Zhang, J. LSPR-enhanced carbon-coated In2O3/W18O49 S-scheme heterojunction for efficient CO2 photoreduction. J. Chin. J. Catal. 2023, 48, 267–278. [Google Scholar] [CrossRef]
- Jiao, Z.-F.; Tian, Y.-M.; Zhang, B.; Hao, C.-H.; Qiao, Y.; Wang, Y.-X.; Qin, Y.; Radius, U.; Braunschweig, H.; Marder, T.B.; et al. High photocatalytic activity of a NiO nanodot-decorated Pd/SiC catalyst for the Suzuki-Miyaura cross-coupling of aryl bromides and chlorides in air under visible light. J. Catal. 2020, 389, 517–524. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, H.; Liang, S.; Zhong, X.; Deng, H. Enhancing photocatalytic CO2 reduction reaction on amorphous Ni@NiO aerogel via oxygen incorporated tuning. J. Appl. Catal. B Environ. 2023, 330, 122603. [Google Scholar] [CrossRef]
- Park, H.R.; Pawar, A.U.; Pal, U.; Zhang, T.; Kang, Y.S. Enhanced solar photoreduction of CO2 to liquid fuel over rGO grafted NiO-CeO2 heterostructure nanocomposite. Nano Energy 2021, 79, 105483. [Google Scholar] [CrossRef]
- Qahtan, T.F.; Owolabi, T.O.; Olubi, O.E.; Hezam, A. Emerging tandem S-scheme heterojunction photocatalysts. J. Coord. Chem. Rev. 2024, 514, 215839. [Google Scholar] [CrossRef]
- Zhao, D.; Guan, X.; Shen, S. Design of polymeric carbon nitride-based heterojunctions for photocatalytic water splitting: A review. Environ. Chem. Lett. 2022, 20, 3505–3523. [Google Scholar] [CrossRef]
- Kumar, A.; Khosla, A.; Kumar Sharma, S.; Dhiman, P.; Sharma, G.; Gnanasekaran, L.; Naushad, M.; Stadler, F.J. A review on S-scheme and dual S-scheme heterojunctions for photocatalytic hydrogen evolution, water detoxification and CO2 reduction. Fuel 2023, 333, 126267. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Bilal, M.; Hou, J.; Butt, F.K.; Ahmad, J.; Ali, S.; Hussain, A. Photocatalytic CO2 Reduction Using TiO2-Based Photocatalysts and TiO2 Z-Scheme Heterojunction Composites: A Review. Molecules 2022, 27, 2069. [Google Scholar] [CrossRef]
- Li, J.; Huang, B.; Guo, Q.; Guo, S.; Peng, Z.; Liu, J.; Tian, Q.; Yang, Y.; Xu, Q.; Liu, Z.; et al. Van der Waals heterojunction for selective visible-light-driven photocatalytic CO2 reduction. Appl. Catal. B 2021, 284, 119733. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J. Heterostructured Catalysts for Electrocatalytic and Photocatalytic Carbon Dioxide Reduction. Adv. Funct. Mater. 2020, 30, 1910768. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, H.; Li, J.; Liao, J.; Zhang, H.; Wang, X.; Kuang, D. Z-Scheme 2D/2D Heterojunction of CsPbBr3/Bi2WO6 for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2020, 30, 2004293. [Google Scholar] [CrossRef]
- Li, T.; Tsubaki, N.; Jin, Z. S-scheme heterojunction in photocatalytic hydrogen production. J. Mater. Sci. Technol. 2024, 169, 82–104. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Q.; Ma, Y.; Zhu, R.; Fan, Y.; Huang, J.; Niu, H.; Dong, Y.; Li, D.; Zhang, Y.; et al. Highly efficient photocatalytic hydrogen evolution and simultaneous formaldehyde degradation over S-scheme ZnIn2S4-NiO/BiVO4 hierarchical heterojunction under visible light irradiation. Chem. Eng. J. 2021, 423, 130164. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, L.; Xiang, S.; Tian, S. Enhanced photocatalytic degradation of organic dyes by dual heterojunction of ZnO/NiWO4/V2C MXene. J. Appl. Surf. Sci. 2025, 679, 161260. [Google Scholar] [CrossRef]
- Cai, Y.; Jin, M.; Bai, J.; Niu, S.; Guo, C. Constructing NiWO4/In2S3 hetero-junction to promote photo-generated carriers separation for enhancing photo-catalytic performance. J. Mater. Res. Bull. 2023, 164, 112243. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, K.; Yu, C.; Lu, K.; Huang, W.; Xu, L.; Zou, L.; Wang, S.; Chen, Z.; Hu, J.; et al. Steering Unit Cell Dipole and Internal Electric Field by Highly Dispersed Er atoms Embedded into NiO for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2022, 32, 2111999. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, Y.; Shad, N.A.; Sajid, M.M.; Munawar, A.; Hussain, T.; Imran, M.; Hussain, D. Facile hydrothermal synthesis of nickel tungstate (NiWO4) nanostructures with pronounced supercapacitor and electrochemical sensing activities. J. Alloys Compd. 2021, 878, 160314. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X. Operando Studies Redirect Spatiotemporal Restructuration of Model Coordinated Oxides in Electrochemical Oxidation. J. Adv. Mater. 2024, 37, 2413073. [Google Scholar] [CrossRef]
- Wu, P.; Wu, Y.; Shi, Z.; Chen, B.; Wen, J.; Na, S.; Xia, W.; Yoriya, S.; He, P.; Huang, K.; et al. Z-Scheme Heterojunction CdIn2S4/BiVO4 with a Spherical Structure for Photocatalytic CO2 Reduction. J. ACS Appl. Nano Mater. 2025, 8, 5979–5991. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, J.; Xu, S.; Liu, Z.; Liu, X.; Li, Z.; Shang, Y.; Li, Q. Construction of 3D/3D heterojunction between new noble metal free ZnIn2S4 and non-inert metal NiMoO4 for enhanced hydrogen evolution performance under visible light. Int. J. Hydrogen Energy 2023, 48, 26707–26717. [Google Scholar] [CrossRef]
- Gu, H.-C.; Tang, Y.-B.; Chen, F.-Y.; Li, M.-Y.; Shi, W.-L. Preparation of a novel composite g-C3N4/TiO2/NiWO4 with enhanced photocatalytic activity toward the degradation of rhodamine B. J. Mater. Sci. Mater. Electron. 2022, 33, 14581–14592. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Tahini, H.A.; Lin, Q.; Chen, Y.; Guan, D.; Zhou, C.; Hu, Z.; Lin, H.-J.; Chan, T.-S.; et al. Single-phase perovskite oxide with super-exchange induced atomic-scale synergistic active centers enables ultrafast hydrogen evolution. J. Nat. Commun. 2020, 11, 5657. [Google Scholar] [CrossRef] [PubMed]
- Angineni, R.; Venkataswamy, P.; Veldurthi, N.K.; Ramaswamy, K.; Sudheera, M.; Vithal, M. Photocatalytic degradation studies of carbon and sulfur-doped K2Ta2O6. J. Mater. Sci. Mater. Electron. 2023, 34, 633. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Gao, Q.; Fan, Y. Effect of morphology on the near infrared shielding property and thermal performance of K0.3WO3 blue pigments for smart window applications. Dye. Pigment. 2018, 156, 33–38. [Google Scholar] [CrossRef]
- Shin, J.; Heo, J.N.; Do, J.Y.; Kim, Y.-I.; Yoon, S.J.; Kim, Y.S.; Kang, M. Effective charge separation in rGO/NiWO4@Au photocatalyst for efficient CO2 reduction under visible light. J. Ind. Eng. Chem. 2020, 81, 427–439. [Google Scholar] [CrossRef]
- Wang, D.; Gu, K.; Zhang, J.; Xing, D.; Zhang, M. MOF-derived NiWO4@NiO p-p heterostructure for distinguish detection of TEA and xylene by temperature regulation. J. Alloys Compd. 2021, 875, 160015. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Z.; Shi, K.; Li, X.; Lu, K.; Huang, W.; Yu, C.; Yang, K. One-Pot Synthesis of N-Doped NiO for Enhanced Photocatalytic CO2 Reduction with Efficient Charge Transfer. J. Mol. 2023, 28, 2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fang, T.; Li, X.; Xu, L.; Song, J. Controllable Transient Photocurrent in Photodetectors Based on Perovskite Nanocrystals via Doping and Interfacial Engineering. J. Phys. Chem. C 2021, 125, 5475–5484. [Google Scholar] [CrossRef]
- Mu, P.; Zhou, M.; Yang, K.; Chen, X.; Yu, Z.; Lu, K.; Huang, W.; Yu, C.; Dai, W. Cd0.5Zn0.5S/CoWO4 Nanohybrids with a Twinning Homojunction and an Interfacial S-Scheme Heterojunction for Efficient Visible-Light-Induced Photocatalytic CO2 Reduction. Inorg. Chem. 2021, 60, 14854–14865. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Li, L.; Ye, X.; Zheng, X.; Pan, L.; Ren, W.; Meng, S.; Zhang, S.; Chen, S. Tailoring defects in In2S3/Zn0.3Cd0.7S heterojunctions for efficient photocatalytic CO2 conversion. Appl. Surf. Sci. 2023, 631, 157524. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Y.; Qu, B.; Bai, L.; Liu, Y.; Yang, Z.; Zhang, W.; Jing, L.; Fu, H. Construction of Six-Oxygen-Coordinated Single Ni Sites on g-C3N4 with Boron-Oxo Species for Photocatalytic Water-Activation-Induced CO2 Reduction. Adv. Mater. 2021, 33, 2105482. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Yu, Q.; He, M.; Zhang, W.; Mo, Z.; Yuan, J.; She, Y.; Xu, H.; Li, H. Multidimensional In2O3/In2S3 heterojunction with lattice distortion for CO2 photoconversion. Chin. J. Catal. 2022, 43, 1286–1294. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, K.; Wu, X.; Zhang, G. Highly Enhanced Full Solar Spectru-Driven Photocatalytic CO2 Reduction Performance in Cu2−xS/g-C3N4 Composite: Efficient Charge Transfer and Mechanism Insight. Sol. RRL 2020, 5, 2000326. [Google Scholar] [CrossRef]
- Tan, C.; Ai, L.; Wang, L.; Xu, M.; Guo, N.; Jia, D. Selective CO production via a dual-defective CdS/BiOCl photocatalyst for CO2 photoreduction. J. Mater. Chem. A 2024, 12, 25371–25380. [Google Scholar] [CrossRef]
- Bai, S.; Jing, W.; He, G.; Liao, C.; Wang, F.; Liu, Y.; Guo, L. Near-Infrared-Responsive Photocatalytic CO2 Conversion via In Situ Generated Co3O4/Cu2O. ACS Nano 2023, 17, 10976–10986. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, B.; Zhang, L.; Yu, J.; Tan, H. BiOBr/NiO S-Scheme Heterojunction Photocatalyst for CO2 Photoreduction. Sol. RRL 2022, 6, 2100587. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Fan, J.; Sun, T.; Liu, E. Fabrication of NiWO4/g-C3N4 S-scheme heterojunction photocatalyst for enhanced H2 evolution. J. Surf. Interfaces 2024, 44, 103811. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Lv, X.; Huang, X.; Zhao, Y.; Deng, T.; Zhang, J.; Wan, J.; Zhen, Y.; Wang, T. Insight into photocatalytic chlortetracycline degradation by WO3/AgI S-scheme heterojunction: DFT calculation, degradation pathway and electron transfer mechanism. J. Clean. Prod. 2024, 472, 143521. [Google Scholar] [CrossRef]
- Li, B.; Ma, X.; Lei, M.; Jin, Z. Graphdiyne based Zn0.5Cd0.5S and NiO dual S-scheme heterojunction boosting photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2025, 683, 1064–1077. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Li, Y.-H.; Zhang, F.; Qi, M.-Y.; Chen, X.; Tang, Z.-R.; Yamada, Y.M.A.; Anpo, M.; Conte, M.; Xu, Y.-J. Rationally designed transition metal hydroxide nanosheet arrays on graphene for artificial CO2 reduction. Nat. Commun. 2020, 11, 5181. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, W.; Yu, Z.; Gao, R.; Yi, Z.; Gong, K.; Lu, K.; Huang, W.; Yu, C.; Zhang, Z.; Zhou, M.; et al. One-Step Molten Salt Constructing Double S-Scheme K0.2WO3/NiO/NiWO4 Heterojunction for Photocatalytic CO2 Reduction. Molecules 2025, 30, 1804. https://doi.org/10.3390/molecules30081804
Xiang W, Yu Z, Gao R, Yi Z, Gong K, Lu K, Huang W, Yu C, Zhang Z, Zhou M, et al. One-Step Molten Salt Constructing Double S-Scheme K0.2WO3/NiO/NiWO4 Heterojunction for Photocatalytic CO2 Reduction. Molecules. 2025; 30(8):1804. https://doi.org/10.3390/molecules30081804
Chicago/Turabian StyleXiang, Wentao, Zhenzhen Yu, Renwu Gao, Zhichao Yi, Kun Gong, Kangqiang Lu, Weiya Huang, Changlin Yu, Zeshu Zhang, Man Zhou, and et al. 2025. "One-Step Molten Salt Constructing Double S-Scheme K0.2WO3/NiO/NiWO4 Heterojunction for Photocatalytic CO2 Reduction" Molecules 30, no. 8: 1804. https://doi.org/10.3390/molecules30081804
APA StyleXiang, W., Yu, Z., Gao, R., Yi, Z., Gong, K., Lu, K., Huang, W., Yu, C., Zhang, Z., Zhou, M., & Yang, K. (2025). One-Step Molten Salt Constructing Double S-Scheme K0.2WO3/NiO/NiWO4 Heterojunction for Photocatalytic CO2 Reduction. Molecules, 30(8), 1804. https://doi.org/10.3390/molecules30081804







