Construction of MXene-Based Heterostructured Hybrid Separators for Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Overview of MXenes and MXene-Based Heterostructures
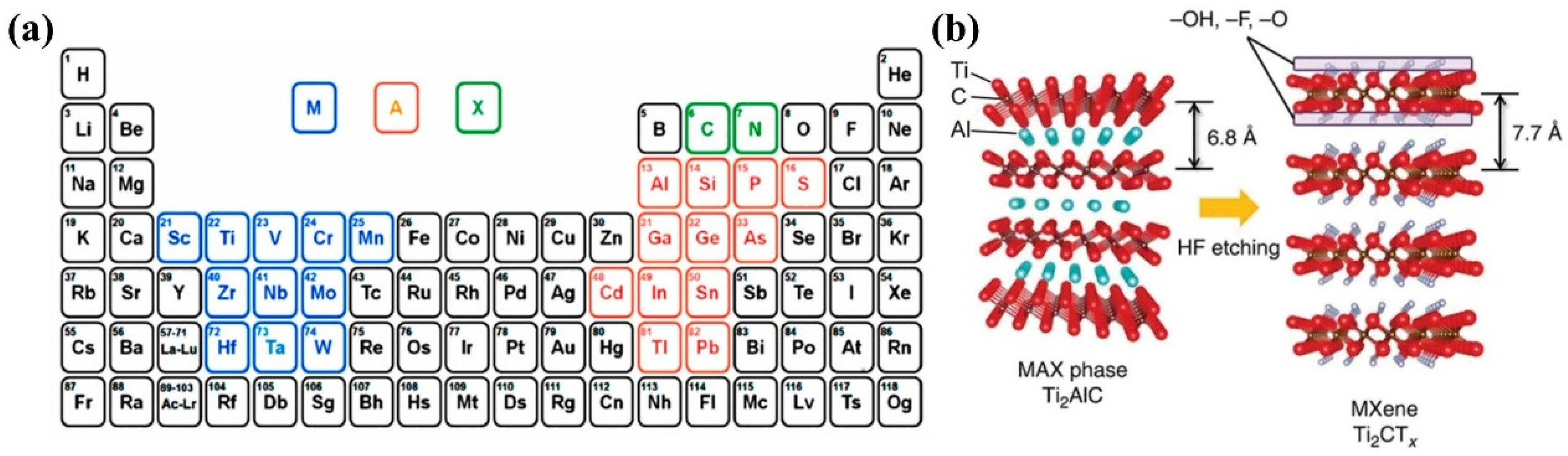
2.1. Overview of MXenes
2.2. Properties of MXene-Based Heterostructures
3. Synthesis of MXenes and MXene-Based Heterostructures
3.1. Synthesis of MXenes
3.1.1. HF Etching
3.1.2. In Situ Generation of HF for Etching
3.1.3. Molten-Salt Etching
3.1.4. CVD Method
3.2. Synthesis of MXene-Based Heterostructures
3.2.1. Hydrothermal/Solvothermal Reaction
3.2.2. Electrostatic Self-Assembly
3.2.3. Lewis Acid Etching and Subsequent Chemical Conversion
3.2.4. Partial Transformation of MXenes
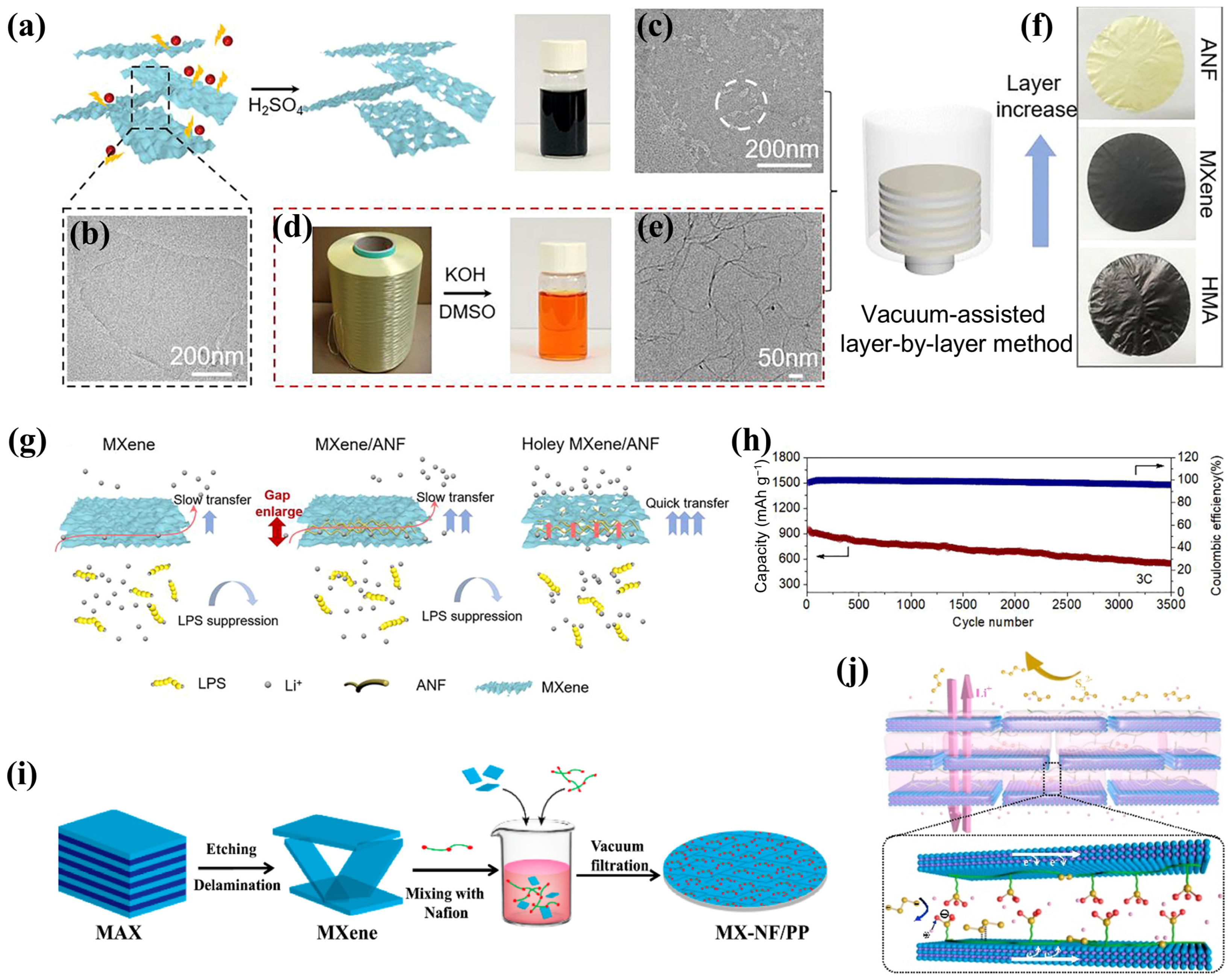
3.2.5. Vacuum Filtration Self-Assembly
4. Construction of MXene-Based Heterostructured Hybrid Separators
4.1. MXene/Inorganic Metal Heterostructures
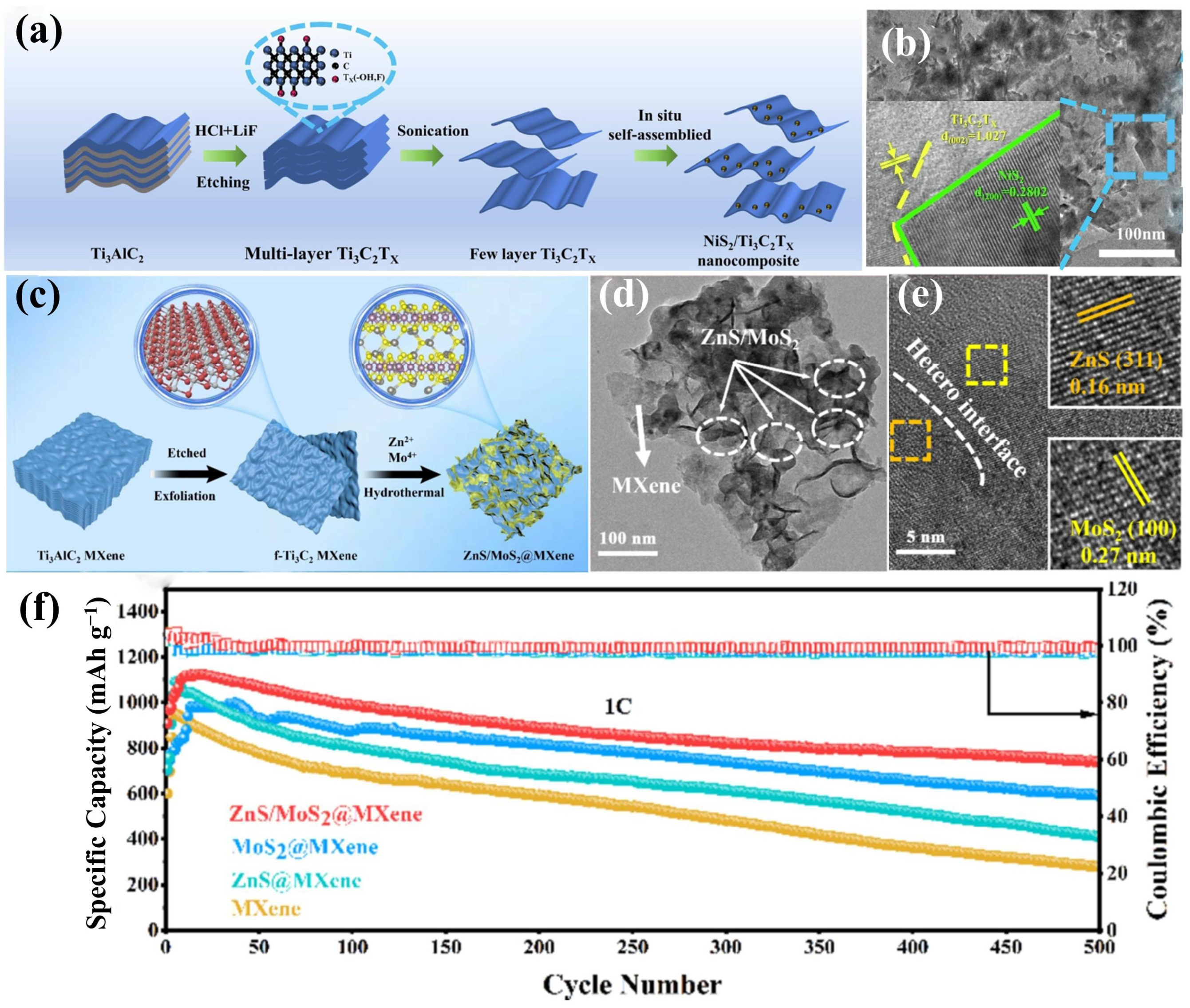
4.1.1. MXene/Metal Sulfide Heterostructures
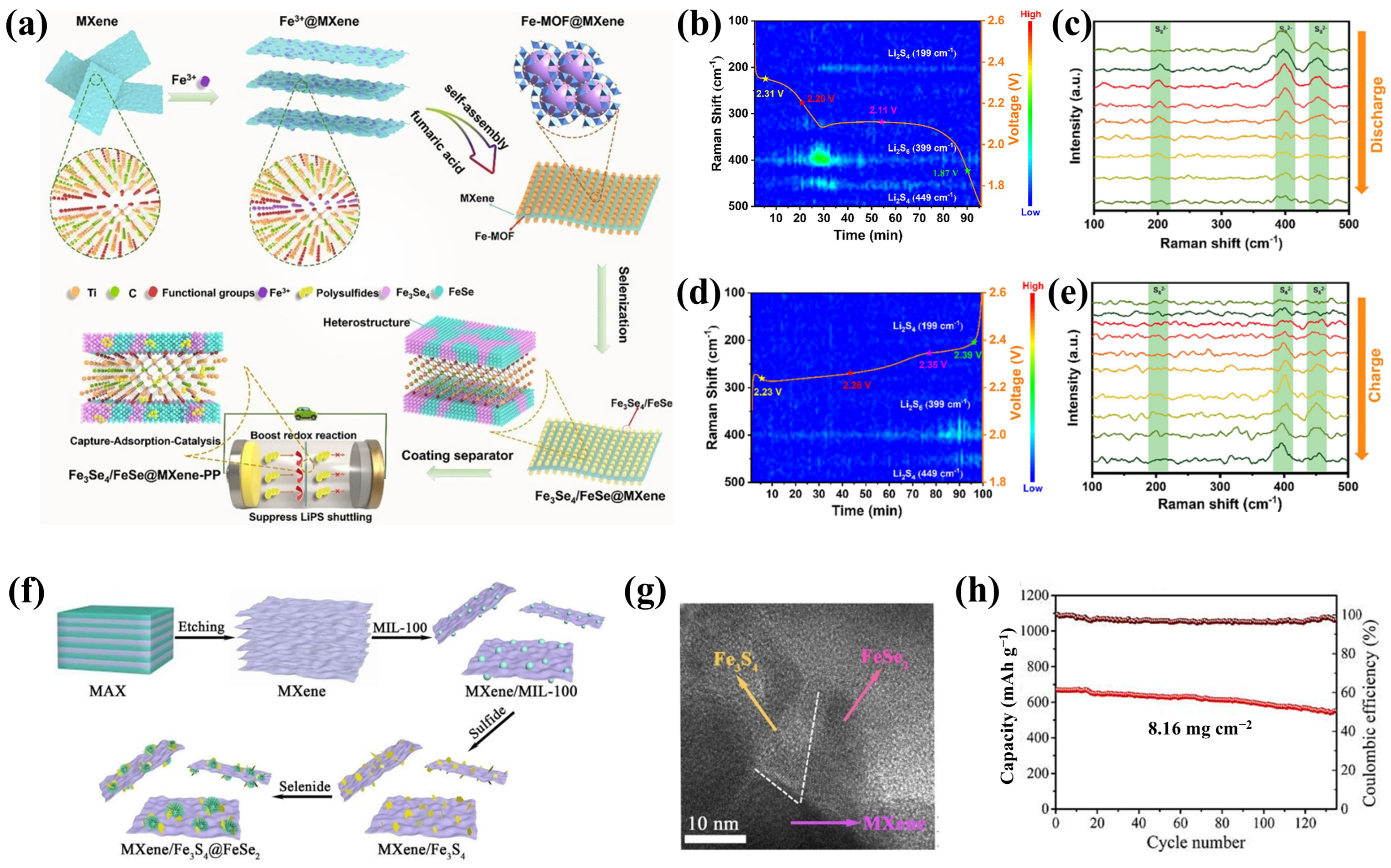
4.1.2. MXene/Metal Selenide Heterostructures
4.1.3. MXene/Other Inorganic Metal Heterostructures
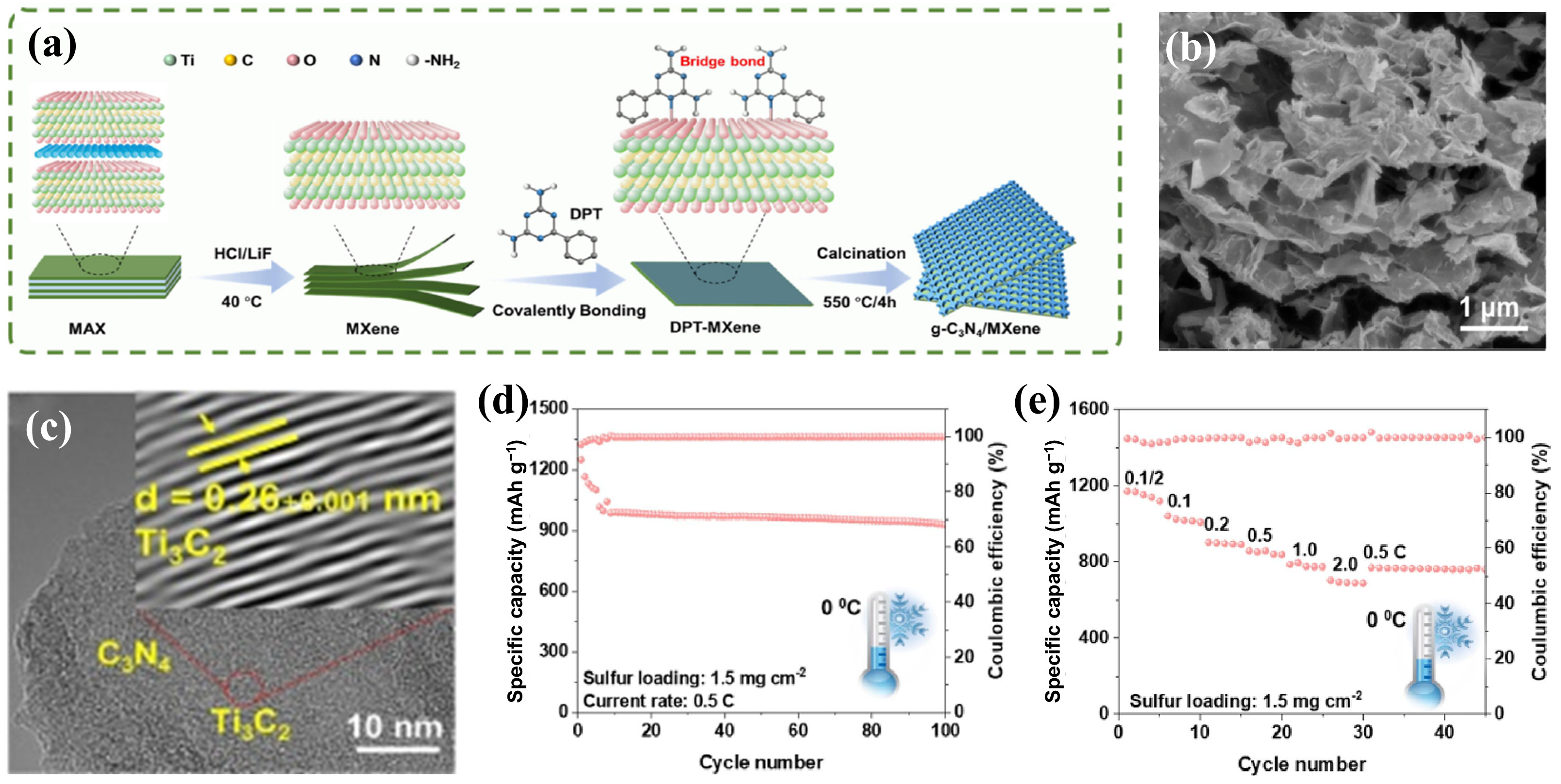
4.2. MXene/Inorganic Non-Metal Heterostructures
4.3. MXene/Organic Framework and Polymer Heterostructures
4.4. MXene/Carbon Heterostructures
5. Conclusions and Prospects
5.1. Conclusions
5.2. Prospects
- (1)
- An atomic-scale understanding of MXene termination effects is needed. Although the terminal groups are known to influence MXene’s adsorption and catalytic properties, their site-specific interactions with polysulfides (e.g., their preferential adsorption on –O vs. –F sites) remain ambiguous due to the lack of dynamic operando characterization tools. By integrating operando testing techniques with DFT simulations, it is possible to correlate the real-time distribution of S species with termination-dependent adsorption energies.
- (2)
- A rational design of the heterointerface charge distribution should be developed. The interfacial electric field in MXene-based heterostructures mainly relies on empirical regulation, lacking precise control over the direction and intensity of charge transfer. By designing the Janus-type heterostructure with asymmetric surface terminations, the internal spontaneous electric field can be created to directionally confine LiPSs. In addition, microscopic probe techniques, such as in situ Kelvin probe force microscopy (KPFM), can be used to directly measure the change in the potential distribution at the heterogeneous interface during charging and discharging, which may establish the quantitative relationship between the charge gradient and polysulfide conversion efficiency.
- (3)
- The expansion of MXene chemical diversity should be undertaken. MXenes, as a large family of 2D materials, have over 100 stoichiometric phases. Yet, only about 30 kinds of MXenes have been successfully synthesized to date, with most research focusing on Ti-based MXenes. Given the vast number of MXene family members and their tunable termination properties, this presents vast and limitless opportunities for realizing high-performance Li-S batteries. Therefore, exploring new MXene species with enhanced stability and electrochemical properties is a promising and intriguing research direction.
- (4)
- Scalable manufacturing and economic feasibility should be addressed. Laboratory-scale MXene synthesis (e.g., HF etching) and coating processes (e.g., vacuum filtration) face severe challenges in cost control and industrial compatibility, particularly in achieving ultra-thin but defect-free MXene coatings required for high-energy-density batteries. For the synthesis of low-cost MXenes, developing Lewis acid salts or molten salts as substitutes for HF not only aligns with the principles of green chemistry but also helps reduce production costs. It is also possible to recycle MXene-based materials from waste separators, improving material recycling. For the low-cost industrialization of MXene-based hybrid separators with ultra-thin coatings, one could attempt to develop a 2D MXene self-assembled monolayer deposition process, which would greatly reduce the loading while still effectively blocking polysulfides. Meanwhile, in combination with the roll-to-roll manufacturing technique, it is possible to develop a coating process suitable for the large-scale production of MXene-based separators with ultra-thin coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Li, Y.; Xu, X.; Zhou, L.; Mai, L. Rechargeable metal (Li, Na, Mg, Al)-sulfur batteries: Materials and advances. J. Energy Chem. 2021, 61, 104–134. [Google Scholar] [CrossRef]
- Ji, X.; Nazar, L.F. Advances in Li-S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, Z.X.; Li, X.Y.; Hou, L.P.; Bi, C.X.; Zhang, X.Q.; Huang, J.Q.; Li, B.Q. Constructing a 700 Wh kg−1 -level rechargeable lithium-sulfur pouch cell. J. Energy Chem. 2023, 76, 181–186. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.W.; Cheng, H.M.; Li, F. More reliable lithiumsulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zou, K.; Qian, Y.; Deng, Y.; Zhang, L.; Chen, G. Insight to the synergistic effect of N-doping level and pore structure on improving the electrochemical performance of sulfur/N-doped porous carbon cathode for Li-S batteries. Carbon 2019, 144, 745–755. [Google Scholar] [CrossRef]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on high-loading and highenergy lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Fang, M.; Han, J.; He, S.; Ren, J.C.; Li, S.; Liu, W. Effective screening descriptor for MXenes to enhance sulfur reduction in lithium–sulfur batteries. J. Am. Chem. Soc. 2023, 145, 12601–12608. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, T.; Zhao, L.; Zhang, B.; Li, W.; Xu, J.; Li, T.; Zhang, L.; Hou, Y. Multifunctional V3S4-nanowire/graphene composites for high performance Li-S batteries. Sci. China Mater. 2020, 63, 1910–1919. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Sun, M.; He, R.; Zhong, W.; Yu, C.; Cheng, S.; Xie, J. Fluorobenzene diluted low-density electrolyte for high-energy density and high-performance lithium-sulfur batteries. J. Energy Chem. 2022, 68, 752–761. [Google Scholar] [CrossRef]
- Sul, H.; Bhargav, A.; Manthiram, A. Lithium Trithiocarbonate as a Dual-Function Electrode Material for High-Performance Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2200680. [Google Scholar] [CrossRef]
- Peng, H.J.; Wang, D.W.; Huang, J.Q.; Cheng, X.B.; Yuan, Z.; Wei, F.; Zhang, Q. Janus Separator of polypropylene-supported cellular graphene framework for sulfur cathodes with high utilization in Lithium-sulfur batteries. Adv. Sci. 2016, 3, 1500268. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Y.; Pasta, M.; Zhou, G.; Li, Y.; Liu, W.; Xiong, F.; Cui, Y. Entrapment of Polysulfides by a black-phosphorus-modified separator for Lithium-sulfur batteries. Adv. Mater. 2016, 28, 9797–9803. [Google Scholar] [CrossRef]
- Lee, H.; Alcoutlabi, M.; Toprakci, O.; Xu, G.; Watson, J.V.; Zhang, X. Preparation and characterization of electrospun nanofiber-coated membrane separators for lithium-ion batteries. J. Solid State Electrochem. 2014, 18, 2451–2458. [Google Scholar] [CrossRef]
- Wang, B.; Guo, W.; Fu, Y. Anodized aluminum oxide separators with aligned channels for high-performance LiS batteries. ACS Appl. Mater. Inter. 2020, 12, 5831–5837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Shao, A.; Xiong, D.; Liu, J.; Lao, C.; Xi, K.; Lu, S.; Jiang, Q.; Yu, J.; et al. Recyclable cobalt-molybdenum bimetallic carbide modified separator boosts the polysulfide adsorption-catalysis of lithium sulfur battery. Sci. China Mater. 2020, 63, 2443–2455. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, L.; Mamoor, M.; Wang, B.; Qu, G.; Jing, Z.; Pang, Y.; Wang, F.; Yang, X.; Wang, D.; et al. Co/MoN invigorated bilateral kinetics modulation for advanced lithium-sulfur batteries. Adv. Mater. 2024, 36, 2310143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Gu, H.; Jia, H.; Long, Z.; Yan, X. Niobium boride/graphene directing high-performance lithium-sulfur batteries derived from favorable surfacepassivation. ACS Nano 2024, 18, 8863–8875. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; He, X.; Khattak, A.M.; Khan, A.; Liang, B.; Iqbal, A.; Wang, J.; Sin, H.; Li, L.; Tang, Z. MoS2/Celgard separator as efficient polysulfide barrier for long-life lithium-sulfur batteries. Adv. Mater. 2017, 29, 1606817. [Google Scholar] [CrossRef]
- Qin, J.; Li, B.; Huang, J.; Kong, L.; Chen, X.; Peng, H.; Xie, J.; Liu, R.; Zhang, Q. Graphene-based Fe-coordinated framework porphyrin as an interlayer for lithium-sulfur batteries. Mater. Chem. Front. 2019, 3, 615–619. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten years of progress in the synthesis and development of MXenes. Adv. Mater. 2021, 33, 2103393–2210402. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Guo, X.; Cao, X.; Wang, S.; Liu, H.; Wang, G. Engineering strategies and active site identiffcation of MXene-based catalysts for electrochemical conversion reactions. Chem. Soc. Rev. 2023, 52, 3215–3264. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic properties and applications of MXenes: A theoretical review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B.; et al. Synthesis of Mo4ValC4 MAX Phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals. ACS Nano 2020, 14, 204–217. [Google Scholar] [CrossRef]
- Eklund, P.; Rosen, J. Persson POÅ Layered ternary Mn+1AXn phases and their 2Dderivative MXene: An overview from a thin-fflm perspective. J. Phys. D Appl. Phys. 2017, 50, 113001. [Google Scholar] [CrossRef]
- Gao, X.T.; Xie, Y.; Zhu, X.D.; Sun, K.N.; Xie, X.M.; Liu, Y.T.; Yu, J.Y.; Ding, B. Ultrathin MXene nanosheets decorated with TiO2 quantum dots as an efficient sulfur host toward fast and stable Li–S batteries. Small 2018, 14, 1802443. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wei, C.; Tian, K.; Zhang, X.; Xiong, S.; Xi, B.; Feng, J. Boosting polysulffdes conversion kinetics through heterostructure optimization and electrons redistribution for robust lithium-sulfur batteries. Chem. Engin. J. 2024, 497, 154658. [Google Scholar] [CrossRef]
- Li, T.; Liu, W.; Liu, Y.; Wang, J.; Hao, H.; Yu, Z.; Liu, H. Covalent organic frameworks integrated MXene as selective “ion-sieving” heterostructure catalyst for kinetics-reinforced Li–S batteries. Chem. Engin. J. 2024, 498, 155817. [Google Scholar] [CrossRef]
- Rehman, Z.; Khan, K.; Yao, S.; Nawaz, M.; Miotello, A.; Assiri, M.A.; Bashir, T.; Tamang, T.; Javed, M. Recent progress in MXene-based materials, synthesis, design, and application in lithium-sulfur batteries. Mater. Today Chem. 2024, 40, 102200. [Google Scholar] [CrossRef]
- Wang, X.; Kajiyama, S.; Iinuma, H.; Hosono, E.; Oro, S.; Moriguchi, I.; Okubo, M.; Yamada, A. Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors. Nat. Commun. 2015, 6, 6544. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Shuck, C.E.; Maleski, K.; Alshareef, H.N.; Zhou, J.; Gogotsi, Y.; Huang, L. An aqueous 2.1 V pseudocapacitor with MXene and V-MnO2 electrodes. Nano Res. 2022, 15, 535–541. [Google Scholar] [CrossRef]
- Zeraati, A.S.; Mirkhani, S.A.; Sun, P.; Naguib, M.; Braun, P.V.; Sundararaj, U. Improved synthesis of Ti3C2Tx MXenes resulting in exceptional electrical conductivity, high synthesis yield, and enhanced capacitance. Nanoscale 2021, 13, 3572–3580. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Gu, J.; Chen, H.; Shi, Y.; Cao, Z.; Du, Z.; Li, B.; Yang, S. Eliminating lightning-rod effect of lithium anodes via sine-wave analogous MXene layers. Adv. Energy Mater. 2022, 12, 2201181. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Li, B.; Gong, Y.; Yang, S. Horizontal growth of lithium on parallelly aligned MXene layers towards dendrite-free metallic lithium anodes. Adv. Mater. 2019, 31, 1901820. [Google Scholar] [CrossRef]
- Chen, L.i.; Yue, L.; Wang, X.; Wu, S.; Wang, W.; Lu, D.; Liu, X.; Zhou, W.; Li, Y. Synergistically accelerating adsorption-electrocataysis of sulfur species via interfacial built-in electric field of SnS2-MXene Mott-Schottky heterojunction in Li-S batteries. Small 2023, 19, 2206462. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Lim, V.Y.; Wang, Y.; Li, Y.; Zhang, D.; Yang, H.Y. Recent advances in heterostructure engineering for lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Pang, J.; Chang, B.; Liu, H.; Zhou, W. Potential of MXenebased heterostructures for energy conversion and storage. ACS Energy Lett. 2022, 7, 78–96. [Google Scholar] [CrossRef]
- Alferov, Z. The history and future of semiconductor heterostructures. Semiconductors 1998, 32, 1–14. [Google Scholar] [CrossRef]
- Huang, J.; Zhuang, Z.; Zhao, Y.; Chen, J.; Zhuo, Z.; Liu, Y.; Lu, N.; Li, H.; Zhai, T. Back-gated van der Waals heterojunction manipulates local charges toward fine-tuning hydrogen evolution. Angew. Chem. Int. Ed. 2022, 61, e202203522. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Xia, L.; Huang, J.; Zhu, P.; Li, Y.; Ye, C.; Xia, M.; Yu, R.; Lang, Z.; Zhu, J.; et al. Continuous modulation of electrocatalytic oxygen reduction activities of singleatom catalysts through p-n junction rectification. Angew. Chem. Int. Ed. 2022, 62, e202212335. [Google Scholar] [CrossRef]
- Li, N.; Peng, J.; Ong, W.; Ma, T.; Arramel; Zhang, P.; Jiang, J.; Yuan, X.; Zhang, C. MXenes: An emerging platform for wearable electronics and looking beyond. Matter 2021, 4, 377–407. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Hou, Y. MXenes: Synthesis strategies and lithium-sulfur battery applications. eScience 2022, 2, 164–182. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Pan, L.; Tu, K.; Du, F.; Qiu, T.; Yang, J.; Cullen, C.; McEvoy, N.; Zhang, C. A robust, freestanding MXene-sulfur conductive paper for long-lifetime Li-S batteries. Adv. Funct. Mater. 2019, 29, 1901907. [Google Scholar] [CrossRef]
- Liu, L.; Orbay, M.; Luo, S.; Duluard, S.; Shao, H.; Harmel, J.; Rozier, P.; Taberna, P.; Simon, P. Exfoliation and delamination of Ti3C2Tx MXene prepared via molten salt etching route. ACS Nano 2022, 16, 111–118. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, C.; Filatov, A.; Cho, W.; Lagunas, F.; Wang, M.; Vaikuntanathan, S.; Liu, C.; Klie, R.; Talapin, D. Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science 2023, 379, 1242–1247. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Xu, K.; Li, Z.; Liu, S.; Sun, L.; Zhao, J.; Liu, H.; Liu, W. In situ self-assembled NiS2 nanoparticles on MXene nanosheets as multifunctional separators: Regulating shuttling effect and boosting redox reaction kinetics of lithium polysulfides. Appl. Surf. Sci. 2024, 645, 158859. [Google Scholar] [CrossRef]
- Zhen, M.; Meng, X.; Wang, X.; Zhang, Z.; Guo, S.; Hu, Z.; Shen, B. Layered double Hydroxides-MXene heterointerfaces with abundant anion vacancies expediting sulfur redox kinetics for High-Performance Lithium–Sulfur batteries. Chem. Eng. J. 2024, 489, 151285. [Google Scholar] [CrossRef]
- Cai, L.; Ying, H.; Huang, P.; Zhang, Z.; Tan, H.; Han, Q.; Han, W. In-situ grown Ti3C2Tx@CoSe2 heterostructure as trapping-electrocatalyst for accelerating polysulffdes conversion in lithium-sulfur battery. Chem. Engin. J. 2023, 474, 145862. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, S.; Jia, X.; Yang, J.; Li, Y.; Shao, D.; Feng, L.; Liao, J.; Song, H. MXene derivative Ta4C3-Ta2O5 heterostructure as bi-functional barrier for Li-S batteries. J. Mater. Sci. Technol. 2023, 151, 89–98. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Zhu, Y.C.; Song, S.; Li, S.N.; Aarons, J.; Tang, L.C.; Bae, J. Polysulffde-inhibiting, thermotolerant and nonffammable separators enabled by DNA co-assembled CNT/MXene networks for stable high-safety Li-S batteries. Compos. Part B 2023, 251, 110465. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Peng, W.; Zhang, F.; Fan, X. In situ N-doped CoS2 anchored on MXene toward an efficient bifunctional catalyst for enhanced lithium-sulfur batteries. Chem. Eng. J. 2022, 427, 131792. [Google Scholar] [CrossRef]
- Zhu, F.; Tao, J.; Yan, M.; Huang, S.; Irshad, M.S.; Mei, T.; Lin, L.; Chen, Y.; Qian, J.; Wang, X. NiS2-MoS2@MXene heterostructures for enhancing polysulfide adsorption and conversion of Li-S battery. Sustain. Mater. Technol. 2024, 39, e00868. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, A.; Qiao, S.; Zhang, Q.; Huang, C.; Lei, D.; Shi, X.; He, G.; Zhang, F. Mott-Schottky MXene@WS2 Heterostructure: Structural and Thermodynamic Insights and Application in Ultra Stable Lithium–Sulfur Batteries. ChemSusChem 2023, 16, e202300507. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Zhang, Y.; Ma, C.; Wang, J.; Qiao, W.; Ling, L. In situ construction of 3D 1T-VS2/V2C heterostructures for enhanced polysulfide trapping and catalytic conversion in lithium-sulfur batteries. J. Colloid Inter. Sci. 2025, 681, 106–118. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, T.; Shen, S.; Cao, Y.; Mao, Y.; Wang, W.; Bao, E.; Jiang, H. In situ synthesis of VS4/Ti3C2Tx MXene composites as modified separators for lithium-sulfur battery. J. Colloid Inter. Sci. 2023, 650, 480–489. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, J.; Liu, S.; Wang, X.; Qi, M.; Dai, B.; Wang, Y.; Ma, L.; Li, J.; Yang, J.; et al. In Situ Built ZnS/MXene Heterostructure by a Mild Method for Inhibiting Polysulfide Shuttle in Li-S Batteries. Chem. Eur. J. 2024, 30, e202403185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, D.; Mao, Y.; Cao, Y.; Wang, W.; Li, Y.; Jiang, H.; Shen, S.; Liao, Q. Hollow Structure Co1-xS/3D-Ti3C2Tx MXene Composite for Separator Modification of Lithium-Sulfur Batteries. ACS Appl. Mater. Inter. 2023, 15, 57088–57098. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Li, D.; Zhang, Y.; Liu, B.; Huo, P. ZnS/MoS2 heterostructures on MXene: A strategy for improved polysulfide adsorption and conversion in Li-S batteries. Chem. Eng. J. 2024, 502, 158151. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, X.; Huang, X.; Xu, L.; Tang, S. Binary Transition-Metal Sulfides/MXene Synergistically Promote Polysulfide Adsorption and Conversion in Lithium-Sulfur Batteries. ACS Appl. Mater. Inter. 2023, 15, 49223–49232. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, S.; Huang, C.; Wang, X.; Cai, C.; He, G.; Zhang, F. Multi-heterostructured MXene/NiS2/Co3S4 with S—Vacancies to Promote Polysulfide Conversion in Lithium-Sulfur Batteries. ACS Appl. Mater. Inter. 2024, 16, 24502–24513. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. A high-efficiency CoSe electrocatalyst with hierarchical porous polyhedron nanoarchitecture for accelerating polysulfides conversion in Li–S batteries. Adv. Mater. 2020, 32, 2002168. [Google Scholar] [CrossRef]
- Wang, M.; Fan, L.; Sun, X.; Guan, B.; Jiang, B.; Wu, X.; Tian, D.; Sun, K.; Qiu, Y.; Yin, X.; et al. Nitrogen-doped CoSe2 as a bifunctional catalyst for high areal capacity and lean electrolyte of Li–S battery. ACS Energy Lett. 2020, 5, 3041–3050. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Zhang, Z.; Sun, B.; Jin, Y.; Jin, Q.; Liu, H.; Wang, G. Heterostructure ZnSe-CoSe2 embedded with yolk-shell conductive dodecahedral as Two-in-one hosts for cathode and anode protection of Lithium-Sulfur full batteries. Energy Storage Mater. 2022, 47, 223–234. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, Y.; Huang, X.; Zhang, Q.; Wang, B.; Tang, S. Interface engineering of Fe3Se4/FeSe heterostructures encapsulated in MXene for boosting LiPS conversion and inhibiting shuttle effect. Chem. Eng. J. 2023, 457, 141139. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, S.; Zhang, Q.; Huang, C.; He, G.; Zhang, F. Vacancy-rich, multi-heterostructured MXene/Fe3S4@FeSe2 catalyst for high performance lithium-sulfur batteries. Chem. Eng. J. 2023, 477, 147100. [Google Scholar] [CrossRef]
- Ng, S.F.; Lau, M.Y.L.; Ong, W.J. Lithium-sulfur battery cathode design: Tailoring metal-based nanostructures for robust polysulfide adsorption and catalytic conversion. Adv. Mater. 2021, 33, 2008654. [Google Scholar] [CrossRef]
- Lee, D.; Choi, C.; Park, J.B.; Jung, S.W.; Kim, D.W. Ingenious Separator Architecture: Revealing the Versatile 3D Heterostructured MXene-Hydrogen Titanate Electrocatalysts for Advanced Lithium-Sulfur Battery. Energy Storage Mater. 2024, 70, 103529. [Google Scholar] [CrossRef]
- Wang, Q.; Deng, B.; Zhang, X.; Cao, L.; Wang, K.; Yao, W.; Chen, C.; Zhao, H.; Xu, J. Partially oxidized Ti3-yNbyC2Tx MXene nanosheets as efficient sulfur hosts and separator modification for Li-S batteries. J. Mater. 2025, 11, 100920. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Wang, J.; Hao, H.; Yu, Z.; Liu, H. Electronic redistribution and fast delivery enabled by Co0.5Ni0.5Te2 grafting 3D MXene heterostructure for accelerating High-Durability sulfur redox kinetics. Chem. Eng. J. 2024, 487, 150502. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, B.; Liu, H.; Wu, H.; Bian, H.; Ma, Y.; Lu, H.; Tang, S.; Meng, X. CoP nanocages intercalated MXene nanosheets as a bifunctional mediator for suppressing polysulffde shuttling and dendritic growth in lithium-sulfur batteries. Chem. Eng. J. 2022, 450, 138046. [Google Scholar] [CrossRef]
- Yang, K.; Huang, Y.; Li, H.; Wang, P.; Zou, J.; Jiang, W.; Zhu, X.; Xu, Y.; Jiang, Q.; Pan, L.; et al. Hierarchical pore CoxP@Ti3C2Tx foams prepared by Co2+ induced Self-assembly and in-situ “micron-reactor” phosphating for novel separator modification of lithium-sulfur batteries. Electrochim. Acta 2025, 512, 145456. [Google Scholar] [CrossRef]
- Guan, B.; Sun, X.; Zhang, Y.; Wu, X.; Qiu, Y.; Wang, M.; Fan, L.; Zhang, N. The discovery of interfacial electronic interaction within cobalt boride@MXene for high performance lithium-sulfur batteries. Chin. Chem. Lett. 2021, 32, 2249–2253. [Google Scholar] [CrossRef]
- Tian, S.; Liu, G.; Xu, S.; Han, C.; Tao, K.; Huang, J.; Peng, S. Deposition Mode Design of Li2S: Transmitted Orbital Overlap Strategy in Highly Stable Lithium-Sulfur Battery. Adv. Funct. Mater. 2023, 34, 2309437. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, W.; Feng, J.; Lin, Z.; Shi, C.; Wang, T.; Lei, Y.; Zhao, X.; Song, J.; Wang, G. Cobalt/MXene-derived TiO2 Heterostructure as a Functional Separator Coating to Trap Polysulfide and Accelerate Redox Kinetics for Reliable Lithium-sulfur Battery. Batter. Supercaps 2024, 7, e202300516. [Google Scholar] [CrossRef]
- Shi, C.; Huang, J.; Tang, Y.; Cen, Z.; Wang, Z.; Liu, S.; Fu, R. A hierarchical porous carbon aerogel embedded with small-sized TiO2 nanoparticles for high-performance Li-S batteries. Carbon 2023, 202, 59–65. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, K.; Ali, A.; Chen, X.; Shen, P.K. 2D TiN@C sheets derived from MXene as highly efficient polysulfides traps and catalysts for lithium-sulfur batteries. Electrochim. Acta 2021, 384, 138187. [Google Scholar] [CrossRef]
- Wan, P.; Guo, X.; Jing, S.; Li, S.; Lu, S.; Zhang, Y.; Fan, H. Multicomponent synergy and simultaneous improvement of catalytic conversion and chemisorption of polysulfides via CoFe/FeN0.0324/TiN modified Li-S battery separator. Chem. Eng. J. 2025, 504, 159014. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, Y.; Zhang, Y.; Ma, C.; Chen, Z.; Wang, J.; Qiao, W.; Ling, L. Defect-rich porous graphitic phase carbon nitride layer grafted MXene as desolvation promoter for efficient sulfur conversion in extremely harsh conditions. J. Colloid Inter. Sci. 2024, 671, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, P.; Wang, Y.; Bi, L.; Liang, Q.; Yao, Y.; Qiu, Y.; He, L.; Xie, Q.; Dong, P.; et al. Insight into Accelerating Polysulfides Redox Kinetics by BN@MXene Heterostructure for Li-S Batteries. Small 2023, 19, 2302386. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Sa, R.; Li, P.; Yuan, D.; Wang, X.; Wang, R. Metalloporphyrin-based covalent organic frameworks composed of the electron donor-acceptor dyads for visible-light-driven selective CO2 reduction. Sci. China Chem. 2020, 63, 1289–1294. [Google Scholar] [CrossRef]
- Sun, T.; Xie, J.; Guo, W.; Li, D.; Zhang, Q. Covalent–Organic Frameworks: Advanced Organic Electrode Materials for Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 1904199. [Google Scholar] [CrossRef]
- Lv, S.; Ma, X.; Ke, S.; Wang, Y.; Ma, T.; Yuan, S.; Jin, Z.; Zuo, J.L. Metal-coordinated covalent organic frameworks as advanced bifunctional hosts for both sulfur cathodes and lithium anodes in lithium-sulfur batteries. J. Am. Chem. Soc. 2024, 146, 9385–9394. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Zhai, L.; Moon, H.; Song, C.; Oh, K.S.; Kong, X.; Han, D.; Zhu, Z.; Wu, Y.; et al. Electrostatic polarity-regulated, vinylene-linked cationic covalent organic frameworks as an ionic sieve membrane for long-cyclable lithium-sulfur batteries. Energy Storage Mater. 2024, 66, 103222. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, F.; Li, J.; Yang, H.; Wang, Y.; He, Y. Designing Organic–Inorganic Hybrid Materials to Construct a Complementary Interface with Versatility for Li-S Batteries. Adv. Funct. Mater. 2024, 16, 2410236. [Google Scholar] [CrossRef]
- Li, P.; Lv, H.; Li, Z.; Meng, X.; Lin, Z.; Wang, R.; Li, X. The Electrostatic Attraction and Catalytic Effect Enabled by Ionic-Covalent Organic Nanosheets on MXene for Separator Modiffcation of Lithium-Sulfur Batteries. Adv. Mater. 2021, 33, 2007803. [Google Scholar] [CrossRef]
- Phung, J.; Zhang, X.; Deng, W.; Li, G. An overview of MOF-based separators for lithium-sulfur batteries. Sustain. Mater. Technol. 2022, 31, e00374. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, H.; Xue, H.; Braunstein, P.; Pang, H. Pillared-layer Ni-MOF nanosheets anchored on Ti3C2 MXene for enhanced electrochemical energy storage. J. Colloid Interface Sci. 2022, 614, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kiai, M.S.; Ponnada, S.; Eroglu, O.; Mansoor, M.; Aslfattahi, N.; Nguyen, V.; Gadkari, S.; Sharma, R.K. Ti3C2Tx nanosheet@Cu/Fe-MOF separators for high-performance lithium–sulfur batteries: An experimental and density functional theory study. Dalton Trans. 2024, 53, 82–92. [Google Scholar] [CrossRef]
- Wang, Y.; Cecen, V.; Wang, S.; Zhao, L.; Liu, L.; Zhu, X.; Huang, Y.; Wang, M. Biomimetic nacre-like aramid nanofiber-holey MXene composites for lithium-sulfur batteries. Cell Rep. Phys. Sci. 2023, 4, 101592. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, P.; Zhao, T.; Li, M.; Yang, Z.; Zhang, H.; Huang, J. Laminar MXene-Nafion-modified separator with highly inhibited shuttle effect for long-life lithiumesulfur batteries. Electrochim. Acta 2019, 320, 134558. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Liu, X.; Fu, C.; Gu, W.; Lu, J.; Wang, C.; Wang, T. Supramolecular macrocycle grafted Nb-MXene nanosheets constructing self-supporting framework for high-performance lithium-sulphur batteries. Appl. Surf. Sci. 2025, 685, 161985. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Meng, X.; Wang, R. MXene-engineered lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 22730–22743. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Li, X.; Xiao, W.; Chen, Z.; Lu, J. Interlayer material selection for lithium-sulfur batteries. Joule 2019, 3, 361–386. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, L.; Lin, H.; Wang, J. Advances and prospects of 2D graphene-based materials/hybrids for lithium metal-sulfur full battery: From intrinsic property to catalysis modification. Adv. Energy Sustain. Res. 2022, 3, 2100187. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Kang, Q.; Zhan, L.; Tang, W.; Yu, Y.; Shen, K.; Wang, H.; Chu, X.; Wang, J.; et al. Synergistic electrocatalysis of polysulfides by a nanostructured VS4-carbon nanofiber functional separator for high-performance lithiumsulfur batteries. J. Mater. Chem. A 2019, 7, 16812–16820. [Google Scholar] [CrossRef]
- Gueon, D.; Hwang, J.T.; Yang, S.B.; Cho, E.; Sohn, K.; Yang, D.K.; Moon, J.H. Spherical macroporous carbon nanotube particles with ultrahigh sulfur loading for lithium-sulfur battery cathodes. ACS Nano 2018, 12, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zheng, N.; Chen, X.; Ding, G.; Li, Y.; Sun, F.; Li, Y. In-situ decoration of MOF-derived carbon on nitrogen-doped ultrathin MXene nanosheets to multifunctionalize separators for stable Li-S batteries. Chem. Eng. J. 2019, 373, 1309–1318. [Google Scholar] [CrossRef]
- Zheng, M.; Luo, Z.; Song, Y.; Zhou, M.; Guo, C.; Shi, Y.; Li, L.; Sun, Q.; Shi, B.; Yi, Z.; et al. Carbon-coated nitrogen, vanadium co-doped MXene interlayer for enhanced polysulffde shuttling inhibition in lithium-sulfur batteries. J. Power Sources 2023, 580, 233445. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, Z.; Bai, X.; Shen, H.; Wang, Z.; Wei, C.; Tian, K.; Xi, B.; Xiong, S.; Feng, J. Hierarchical Porous N-doped Carbon Encapsulated Fluorine-free MXene with Tunable Coordination Chemistry by One-pot Etching Strategy for Lithium-Sulfur Batteries. Adv. Energy Mater. 2023, 13, 2301349. [Google Scholar] [CrossRef]
- Li, N.; Cao, W.; Liu, Y.; Ye, H.; Han, K. Impeding polysulffde shuttling with a three-dimensional conductive carbon nanotubes/MXene framework modiffed separator for highly efficient lithium-sulfur batteries. Colloids Surf. A 2019, 573, 128–136. [Google Scholar] [CrossRef]
- Li, N.; Xie, Y.; Peng, S.; Xiong, X.; Han, K. Ultra-lightweight Ti3C2Tx MXene modified separator for Li-S batteries: Thickness regulation enabled polysulfide inhibition and lithium ion transportation. J. Energy Chem. 2020, 42, 116–125. [Google Scholar] [CrossRef]



| Materials | Core Advantages | Main Disadvantages |
| Carbon |
|
|
| Metal compound |
|
|
| Polymer |
|
|
| MXene |
|
|
| MXene-based heterostructure |
| |
| Heterostructures | Coating/Loading (mg cm−2) | Sulfur Loading/mg cm−2 | Rate Capacity/Rate | Initial Capacity (mAh g−1)/Rate | Decay Rate/Cycles | Ref. | |
|---|---|---|---|---|---|---|---|
| MXene/metal sulfide | NiS2/Ti3C2Tx | Blade/0.8 | 1.07 | 921/2 C | 1129/2C | 0.038%/1000 | [51] |
| N-MX-CoS2 | Filtration/0.2 | 1.2 | 775/4C | 1031/1C | 0.052%/700 | [56] | |
| IL-MoS2/MX | Filtration/- | 1.2–1.5 | 745.4/1C | 764.4/1C | 0.059%/700 | [57] | |
| MXene@WS2 | Filtration/0.6 | 1 | 622.2/3C | 889.1/2C | 0.0286%/2000 | [58] | |
| 1T-VS2/V2C | 818.2/5 C | 1131/1C | 0.056%/1000 | [59] | |||
| VS4/Ti3C2Tx | Filtration/0.15 | 1.2–1.5 | 673/4C | 932/1C | 0.059%/500 | [60] | |
| ZnS/MXene | Blade/- | 1 | 551/5C | 810/0.5C | 0.082%/500 | [61] | |
| Co1−xS/3D-Ti3C2Tx | Filtration/0.15 | 1.2–1.5 | 1056/1C | 0.05%/500 | [62] | ||
| ZnS/MoS2@MXene | -/0.5 | 1 | 802.6/2C | 900/1C | 0.067%/500 | [63] | |
| Bi2S3/MoS2@MX | 0.8–1.2 | 592/5C | 847/2C | 0.069%/500 | [31] | ||
| MXene/MoS2/SnS@C | -/0.5 | 1 | 693.4/4C | ~800/2C | 0.051%/1000 | [64] | |
| MXene/NiS2/Co3S4 | Filtration/0.8 | 1 | 549.0/6C | 956.3/2C | 0.026%/2000 | [65] | |
| MXene/metal selenide | Ti3C2Tx@CoSe2 | Filtration/0.5 | 1.2 | 694/3C | 1032.7/0.5C | 0.059%/800 | [53] |
| Fe3Se4/FeSe@MXene | Blade/0.27 | 1.2 | 768.5/4C | ~975/2C | 0.07%/600 | [69] | |
| MXene/Fe3S4@FeSe2 | Filtration/0.8 | 1 | 557.1/5C | ~825/2C | 0.049%/1000 | [70] | |
| MXene/other inorganic metal | M-HTO-0.5 | Blade/0.3–0.4 | 1.8–2.0 | 822.7/2C | ~920/5C | 0.073%/500 | [72] |
| Ta4C3-Ta2O5 | -/0.9 | 1.0–1.5 | 738.5/1C | 801.9/1C | 0.086%/500 | [54] | |
| Co0.5Ni0.5Te2/MXene | Blade/0.27–0.30 | 1.5 | 773/2C | ~950/1C | 0.0227%/500 | [74] | |
| Ti3C2/CPNC | Blade/0.5 | 1.5 | 525.4/5C | 928.4/1C | 0.039%/1150 | [75] | |
| CoxP@Ti3C2Tx | Blade/- | 751.61/3C | 813.33/2C | 0.037%/400 | [76] | ||
| Co2B@MXene | Filtration/- | 1.2–1.5 | 597/5C | 786/2C | 0.0088%/2000 | [77] | |
| MX@WSSe | Filtration/- | 1.2–1.5 | 504.4/5C | 600.2/2C | 0.016%/1000 | [78] | |
| Vo-LDHs-MXenes | Blade/- | 2 | 342.9/3C | 938.9/1C | 0.084%/300 | [52] | |
| MXene/inorganic non-metal | g-C3N4/MXene | Coating machine/0.18 | 1.5 | 945/4C | 858/1C | 0.035%/1000 | [83] |
| BN@Mxene | Filtration/0.06 | 1.5–2 | 748/1C | 686.9/1C | 0.058%/700 | [84] |
| Heterostructure | Coating/Loading (mg cm−2) | Sulfur Loading/mg cm−2 | Rate Capacity/Rate | Initial Capacity (mAh g−1)/Rate | Decay Rate/Cycles | Ref. |
|---|---|---|---|---|---|---|
| MXene@COF | 1.2–1.5 | 563/3C | ~1050/0.5C | 0.085%/200 | [89] | |
| MXene@TBCOF | -/0.29–0.31 | 1.65 | 493/5C | 952.2/1C | 0.0191%/1500 | [32] |
| Ti3C2@iCON | Filtration/0.1 | 1.2 | 687/5C | 810/2C | 0.006%/2000 | [90] |
| Ti3C2Tx@Cu/Fe-MOF | Filtration/- | 4.1 | 728/983/1C | 1300/1400/1C | 0.051%/0.064%/300 | [93] |
| MXene/ANF | Filtration/- | 1.0–1.5 | 818/5C | ~975/3C | 0.013%/3500 | [94] |
| MXene-Nafion | Filtration/0.2 | 2 | 794/3C | 920/1C | 0.030%/1000 | [95] |
| PA5-COOH/Nb2C | Filtration/- | 1 | 303/10C | 754/1C | 0.047%/500 | [96] |
| Heterostructure | Coating/Loading (mg cm−2) | Sulfur Loading/mg cm−2 | Rate Capacity/Rate | Initial Capacity (mAh g−1)/Rate | Decay Rate/Cycles | Ref. |
|---|---|---|---|---|---|---|
| N-Ti3C2/C | Blade/0.6 | 3.4 | 675/2C | 1101.54/0.5C | 0.07%/500 | [102] |
| CNVM | Blade/0.38 | 1.2 | 723/3C | 867/1C | 0.075%/660 | [103] |
| Ti-N-Ti3C2Cl-C | -/0.12 | 1.0–1.5 | 518.6/4C | 761/2C | 0.053%/500 | [104] |
| DNA-CNT/MXene | Filtration/0.1 | 1 | 588/2C | 798/1C | 0.13%/200 | [55] |
| CNTs/MXene | Filtration/0.16 | 0.8 | 728/2C | 987/1C | 0.06%/600 | [105] |
| Ti3C2Tx/CNTs 10% | Filtration/0.016 | 1.2 | 640/2C | 760/1C | 0.086%/200 | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Jin, G.; Mao, M.; Wang, Z.; Xu, T.; Wan, T.; Zhao, J. Construction of MXene-Based Heterostructured Hybrid Separators for Lithium–Sulfur Batteries. Molecules 2025, 30, 1833. https://doi.org/10.3390/molecules30081833
Zhang X, Jin G, Mao M, Wang Z, Xu T, Wan T, Zhao J. Construction of MXene-Based Heterostructured Hybrid Separators for Lithium–Sulfur Batteries. Molecules. 2025; 30(8):1833. https://doi.org/10.3390/molecules30081833
Chicago/Turabian StyleZhang, Xiao, Guijie Jin, Min Mao, Zirui Wang, Tianyu Xu, Tongtao Wan, and Jinsheng Zhao. 2025. "Construction of MXene-Based Heterostructured Hybrid Separators for Lithium–Sulfur Batteries" Molecules 30, no. 8: 1833. https://doi.org/10.3390/molecules30081833
APA StyleZhang, X., Jin, G., Mao, M., Wang, Z., Xu, T., Wan, T., & Zhao, J. (2025). Construction of MXene-Based Heterostructured Hybrid Separators for Lithium–Sulfur Batteries. Molecules, 30(8), 1833. https://doi.org/10.3390/molecules30081833






