Bioactive Polysaccharides from Hericium erinaceus: Extraction, Structure, Bioactivities, and Applications
Abstract
:1. Introduction
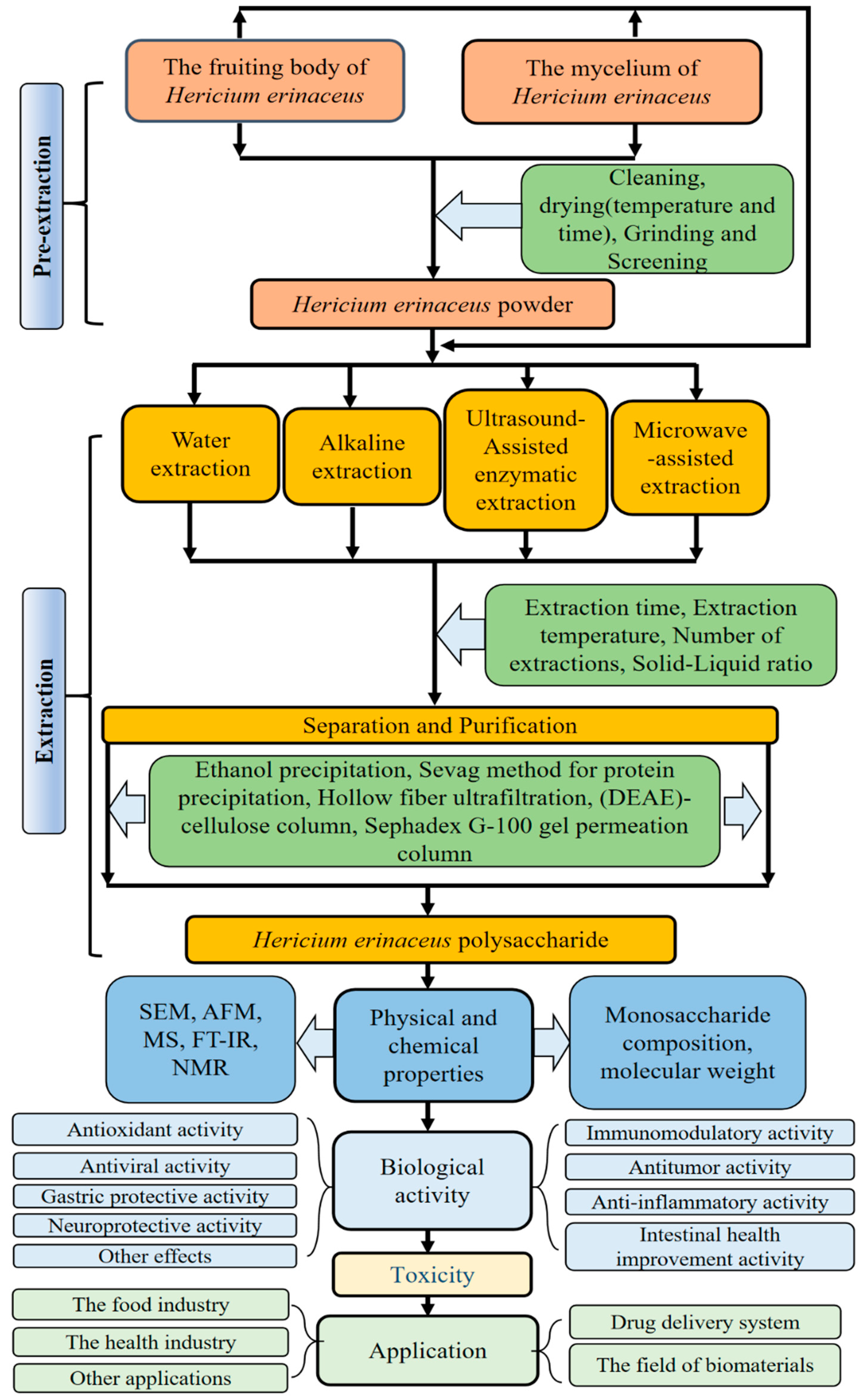
2. Extraction, Separation, and Purification
2.1. Extraction
2.2. Separation and Purification
| Type | Pretreatment | Polysaccharide Names | Extraction Method | Separation and Purification Methods | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drying Condition | Granularity (Item) | Solid/Liquid Ratio (w:v) | Extraction Frequency | Total Extraction Time | Extraction Temperature (°C) | Extraction Rate | ||||
| Dried Hericium erinaceus powder | - | - | HEP | 1:10 | 1 | 6 h | 100 | - | 95% ethanol precipitation Sevag method for protein precipitation | [25] |
| HEFB | 40 °C, 6 h | 24 | HEP | - | 1 | 4 h | 80 | - | Ethanol precipitation Sevag method for protein precipitation DEAE-52 column Sephadex G-100 gel permeation column | [17] |
| Dried HEFB | - | 80 | HEFPs | 1:40 | 1 | 4 h | 80 | - | Precipitate proteins with 10.6% potassium ferrocyanide and 21.9% zinc acetate solution at a 9:9:100 ratio (potassium ferrocyanide, zinc acetate, and sample, respectively), then dialyze with distilled water Ethanol precipitation | [26] |
| HEM | 40 °C | - | PFHE | 1:10 | 1 | 24 h | 70 | - | Lipid removal with 96% ethanol | [29] |
| HEFB | - | - | Water-soluble polysaccharides | 1:15 | 1 | 2 h | 100 | - | Ethanol precipitation | [30] |
| HEFB powder after semi-solid enzymolysis | 80 °C | - | - | - | ||||||
| Dried and ground Hericium erinaceus | - | - | HEP-W | 1:15 | 1 | 2 h | 100 | 0.3% | Sevage method for protein precipitation Ethanol precipitation | [21] |
| - | - | HEP-A | 1:10 | 2 | 6 h | 25 | 3.1% | Acid precipitation Sevage method for protein precipitation Ethanol precipitation | [21] | |
| Hericium erinaceus | - | 100 | Hericium erinaceus polysaccharide | 3:100 | 1 | 3.5 h | 50 | - | - | [31,32] |
| HEFB | 50 °C, 10 h | 50 | HEP | 1:15 | 2 | 6 h | 95 | - | Ethanol precipitation Sevag method for protein precipitation | [33] |
| HEFB | 50 °C, 10 h | 50 | HEP50 | 1:15 | 2 | 6 h | 95 | 1.87 ± 0.11% | [34] | |
| HEFB | Freeze-dried | - | Hericium erinaceus polysaccharide | 1:1 | 1 | 10 min | 100 | - | - | [14] |
| Dried HEFB | - | - | Hericium erinaceus polysaccharide | 1:30 | 4 | 4 h | 100 | - | - | [35] |
| HEFB | - | - | HEP-1, HEP-2, HEP-3, HEP-4, HEP-5 | 1:10 | 1 | 8 h | 100 | 2.735% | Ethanol precipitation DEAE Cellulose-52 column Sephadex G-100 column | [36] |
| Fermented HEM | - | - | PHEB | - | 2 | 6 h | 80 | - | Ethanol precipitation Sevag method for protein precipitation DEAE Sepharose Fast Flow HiLoad 16/600 Superdex 200 prep grade Column | [32,37] |
| Dried HEFB | - | - | HEPs | 1:10 | 1 | 8 h | 100 | 2.735% | - | [38] |
| HEM | - | - | wHEP-1, wHEP-2, wHEP-3 | 1:5 | 3 | 12 h | 80 | - | Ethanol precipitation 3-K hollow fiber ultrafiltration cartridge DEAE-Sephadex A-50 column 0.2-K hollow fiber ultrafiltration column P30 column | [39] |
| Hericium erinaceus residue | - | - | HRPs | 3:50 | 1 | 4 h | 90 | - | Ethanol precipitation Sevage method for protein precipitation | [40] |
| HEFB | - | - | HEP 1, HEP 2, HEP 3, HEP 4 | 1:20 | 2 | 4 h | 100 | 5% | 95% ethanol precipitation Sevag method for protein precipitation DEAE Sepharose Fast Flow column | [28] |
| HEFB | 50 °C, 72 h | 100 | HEFP-2b | 1:1 | 1 | 3 h | 80 | - | Ethanol precipitation DEAE-cellulose-52ion exchange column A gel permeation chromatography column of Sephacryl S-400 | [41] |
| HEFB | 50 °C, 72 h | 100 | HEFPs | 1:40 | 1 | 3 h | 80 | - | 10.6% potassium ferricyanide Solution, 21.9% zinc acetate solution, and the concentrated supernatant were mixed at a 1:1:10 ratio, precipitation Ethanol precipitation | [42] |
| Dried Hericium erinaceus | - | - | HEP | 1:20 | 2 | 4 h | 110 | - | Ethanol precipitation Sevage method for protein precipitation anion-exchange chromatography Sephadex G-100 column | [43] |
| Cultured HEM | - | - | wHEP-1 | 1:5 | 3 | 36 h | 80 | - | Ethanol precipitation A hollow fiber ultra-filtration cartridge 0.2 K hollow fiber ultrafiltration column DEAE Sephadex A-50 | [44] |
| The mature HEFB | - | - | H6PC20 | 1:2 | 2 | 4 h | 100 | 0.25% | Ethanol precipitation | [45] |
| Cultured HEM | - | - | Hep-1, Hep-2, Hep-3 | - | 2 | 12 | 70 | - | Ethanol precipitation Hollow-fiber ultrafiltration cartridge DEAE-Sephadex A-50 column Bio-Gel P-30 | [46] |
| HEM | - | - | EP-1 | 1:5 | 3 | 12 | 70 | - | Ethanol precipitation hollow fiber ultrafiltration cartridges (3 K) hollow fiber ultrafiltration column (0.2 K) DEAE-Sephadex column | [47] |
| Cultured HEM | - | - | EP-1 | 1:5 | 3 | 12 | 70 | - | Ethanol precipitation Hollow fiber ultrafiltration cartridges (3 K) Hollow fiber ultra-filtration column of 0.2 K DEAE-Sephadex column | [48] |
3. Physicochemical Properties and Structural Characteristics
3.1. Monosaccharide Composition
3.2. Molecular Weight
3.3. Conformational Characteristics
3.4. Chemical Structure Analysis
| Polysaccharide Name | Monosaccharide Composition | Mw (kDa) | Conformational Characteristics | Structural Analysis Techniques and Results | Reference | |||
|---|---|---|---|---|---|---|---|---|
| SEM | AFM | FT-IR | MS | NMR | ||||
| HEP | Rha, Fru, Gal, Glc | 23.5 | - | - | O-H stretching vibration, C-H asymmetric stretching vibration, bound water, O-H bending vibration of carboxyl group, C-O glycosidic bond, stretching vibration of pyran ring, α-glycosidic bond and β-glycosidic bond, C-C stretching vibration. | - | - | [16] |
| AHEP-A-b | Glc, Gal, Man, GlcA, Fuc | 20 | - | - | O-H stretching vibration, C-H stretching vibration, bound water and C = O groups, variable angle vibration of C-H groups, pyranose ring, β-linked glycosyl residues. | The polymerization degree of the [→3)-β-D-Glcp-(1→)] side chain is 2~8. | The main chain is composed of [→6)-β-D-Glcp-(1→], and the side chain is composed of [→3)-β-D-Glcp-(1→] and β-D-Glcp-(1→] linked to the C-3 main chain of Glcp. | [20] |
| HEP | Glc, Man, Gal, GlcN | 27.5 | The dense and smooth surface has a layered structure, which is characterized by small and rough fragments. | - | O-H stretching vibration, C-H stretching vibration, C-O-C stretching vibration of pyranose, C-O-H or C-O-C bending vibration of C-O bond. | - | - | [25] |
| HEP | Man, Rha, Glc, Gal, Fuc | - | - | - | O-H antisymmetric stretching vibration, C-H symmetric stretching vibration, carbonyl (C = O) antisymmetric stretching vibration, C-H antisymmetric bending vibration, pyranose ring. | - | - | [17] |
| HEFPs | Fuc, GlcN·HCl, Gal, Glc, Xyl, Man | 17.21 | - | - | O-H stretching vibration, C-H stretching vibration, COO-asymmetric stretching vibration, pyranose ring. | - | - | [26] |
| HEP10 | Fuc, Ara, Gal, Glc, Man, Xyl | 9.9 | - | - | O-H stretching vibration, C-H stretching vibration, asymmetric and symmetric stretching vibration of carboxyl group, pyranose ring, furanose ring, β configuration, α-glycosidic bond. | - | The glycosidic linkage of HEP10 might be (1→2) and (1→6). | [55] |
| Gal-HE | Fuc, Gal, Glc, Man | 22.1 | - | - | - | - | Mannan galactomannan is composed of a 1,6-linked α-D-Gal backbone, which contains a small amount of α-1,6-D-Me-Galp, and is branched by a single-unit β-Manp residue at O-2. Fucoidan contains α-1,6-linked DGal, and the t-α-L-Fucp side chain is replaced at O-2. | [21] |
| HMP | - | - | Granular structure, with rough surface and irregular crystal morphology. | - | O-H stretching vibration, C-H stretching vibration, β-pyran bond, pyranose ring appeared. | - | - | [56] |
| HFP | - | - | Block structure, smooth surface, regular crystal morphology. | - | - | - | ||
| HEP-30 | Fru, Man, Glc, Gal | 15.019 ± 1.59 | The surface morphology of the three polysaccharides was mainly flat, layered, smooth, and wrinkled. The polysaccharide structure of HEP-30 was slightly larger and sparser than those of the other two polysaccharides. | - | O-H stretching vibration, C-H stretching vibration, C = O group or C = C group, C-H variable angle vibration, pyranose ring appeared. | - | - | [18] |
| HEP-50 | Fru, Man, Glc, Gal | 16.723 ± 2.11 | - | - | - | |||
| HEP-70 | Fru, Man, Glc, Gal | 4.771 ± 0.21 | - | - | - | |||
| PHEB | Gal, Glc, Man, GlcA | 36.1 | - | - | O-H stretching vibration, C-H stretching vibration, crystal water, C-O symmetric stretching vibration, O-H variable angle vibration, pyranose ring. | - | There exists →6)-α-D-Galp-(1→6)-α-D-Galp-(1→). | [37] |
| HEP | Man, Gal, Glc, Fuc, GlcA | 19.7 | Layered, with a small amount of debris, loose shape, and a smooth surface. | - | O-H stretching vibration, C-H stretching vibration, − C = O stretching vibration, pyranose ring, β-pyranoside bond, α-glycosidic bond. | - | Gal, Fuc. | [15] |
| HEP | Xyl, Ara, Fuc, GalA, Man, Glc, Gal | - | - | - | - | - | - | [22] |
| wHEP-1 | Man, Glc, Gal | 5.01 | - | - | O-H stretching vibration, C-H stretching vibration, β-glycosidic bond, α-glycosidic bond. | - | α-glycosidic bond, β-glycosidic bond. | [39] |
| wHEP-2 | Glc, Gal | 1.812 | - | - | - | |||
| wHEP-3 | Glc, Gal | 1.11 | - | - | - | |||
| SHRPs | Xyl, Ara, Rha, Fuc, Man, Gal, Glc, GlcA | - | - | - | O-H stretching vibration, C-H stretching vibration, C = O bond in carboxyl group, stretching vibration of uronic acid, furan sugar ring, β-glycosidic bond. | - | - | [40] |
| HEP-1 | Fuc, Gal, Glc | - | - | - | - | - | - | [28] |
| HEP-2 | Fuc, Gal, Glc | - | - | - | - | - | - | |
| HEP-3 | Glc | 13.3 | - | - | - | - | The β-glucan could be tentatively deduced as a (1→6)-linked glucan main chain with (1→3)-linked glucopyranosyl branching unit(s). | |
| HEFP-2b | Fuc, Gal, Glc, Man | 32.52 | Flat and smooth shape, and the particles are layered. | - | O-H stretching vibration, C-H stretching vibration, C-H bond deformation vibration, C = O stretching vibration, pyranose ring, β and α configurations. | - | The backbone of HEFP-2b might mainly consist of (1→6)-linked-α-D-glucose residues, (1→4)-linked-β-D-galactose residues, (1→3,6)-linked-α-D-mannose residues, and terminal glucose and fucose. Two branches consisted of (1→6)-linked-β-D-galactose and (1→3)-linked-α-D-mannose residues. | [41] |
| HER | - | - | Aggregate and irregular shape, rough surface. | - | - | - | - | [57] |
| HEFPs | Ara, Gal, Glc, Man | - | Rough and blocky surface with dense honeycomb structure. | - | O-H stretching vibration, C-H stretching vibration, C = O stretching vibration, C-H bond deformation vibration, pyranose ring. | - | - | [42] |
| HEPN | Man, Glc, Gal | 12.713 | Bright luster, showing irregular, uneven distribution of particles, concave, wrinkled, and relatively fluffy. | - | O-H stretching vibration, C-H stretching vibration, bound water stretching vibration, C = O stretching vibration, in-plane bending vibration of C-H, glycosidic bond and α configuration. | - | β-D-Glc and α-D-Gal. | [53] |
| H6PC20 | Glc | - | - | - | - | - | H6PC20 has a backbone of (1→3)- linked-β-D-glucopyranosyl units, with one single-unit β-D-glucopyranosyl branch substituted at O-6 on every third backbone unit. | [45] |
| EP-1 | - | 3.1 | - | - | - | - | - | [47] |
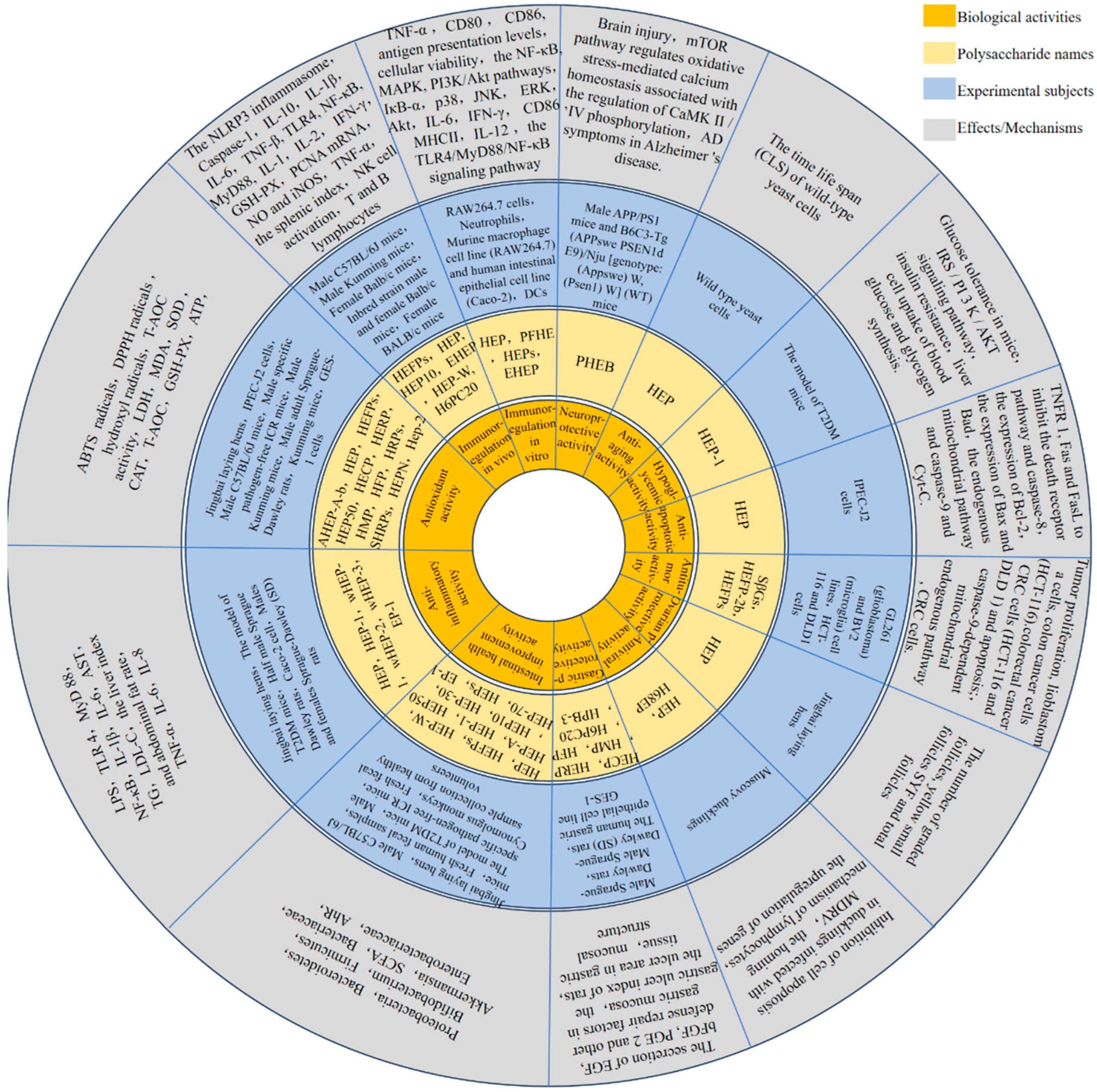
4. Biological Activity
4.1. Immunomodulatory Activity
4.1.1. Immunoregulation In Vitro
4.1.2. Immunoregulation In Vivo
4.2. Antitumor Activity
4.3. Antioxidant Activity
4.4. Anti-Inflammatory Activity
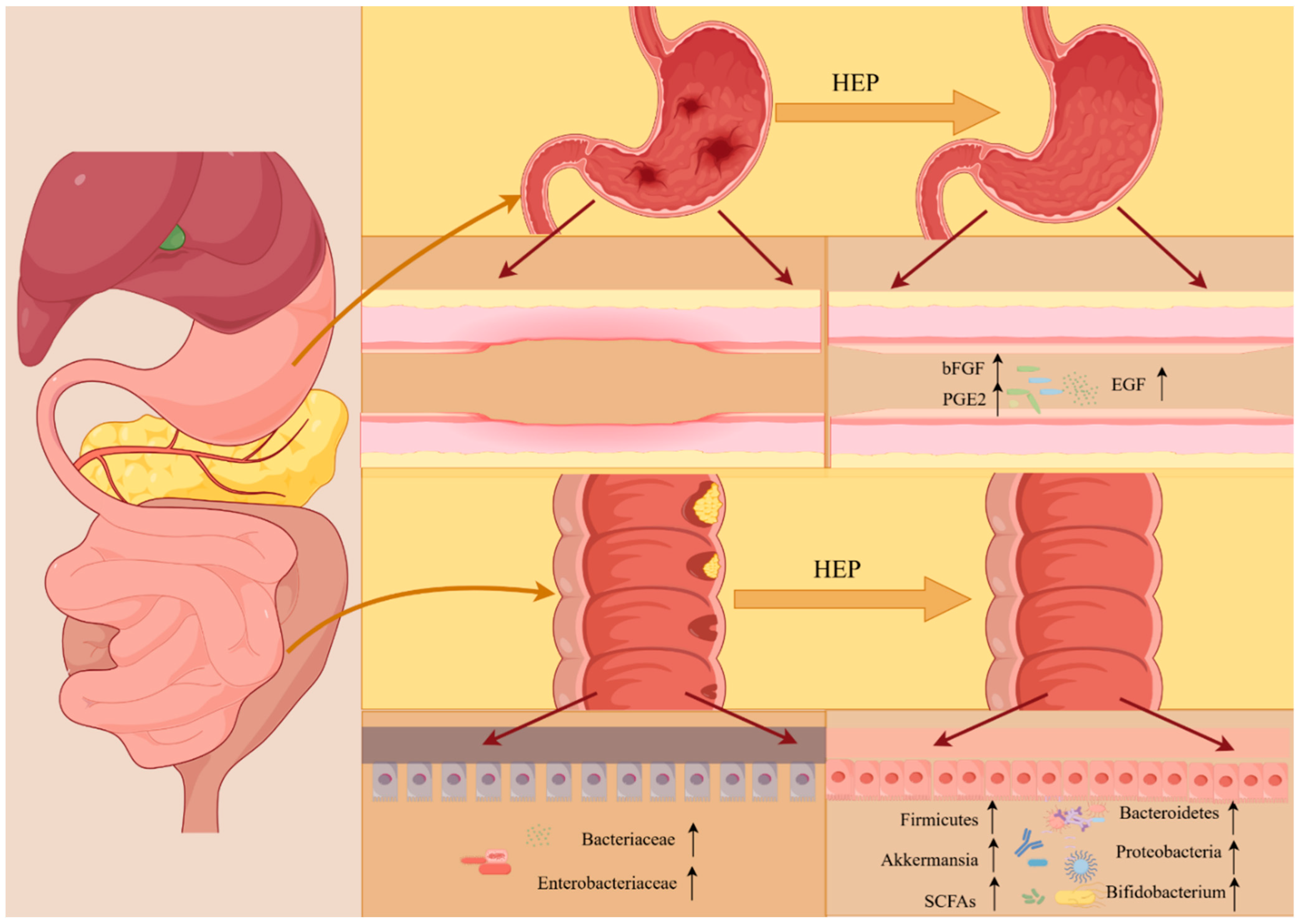
4.5. Intestinal Health Improvement Activity
4.6. Gastric Protective Activity
4.7. Neuroprotective Activity
4.8. Antiviral Activity
4.9. Other Effects
5. Toxicity
6. Applications
6.1. Application in the Food Industry
6.2. Application in the Health Industry
6.3. Application in Drug Delivery Systems
6.4. Application in the Field of Biomaterials
6.5. Other Applications
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Gao, Y.; Xu, D.; Konishi, T.; Gao, Q. Hericium erinaceus (yamabushitake): A unique resource for developing functional foods and medicines. Food Funct. 2014, 5, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J.; Yamaguchi, R.; Nagai, K.; Liu, C.; Choi, J.; Hirai, H.; Xie, X.; Kobayashi, S.; Kawagishi, H. Uncovering hericenones from the fruiting bodies of Hericium erinaceus through interdisciplinary collaboration. J. Nat. Prod. 2025, 88, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, S.; Sun, Y.; Zhang, Q. Medicinal properties of Hericium erinaceus and its potential to formulate novel mushroom-based pharmaceuticals. Appl. Microbiol. Biotechnol. 2014, 98, 7661–7670. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complement. Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef]
- Kala, K.; Cicha-Jelen, M.; Hnatyk, K.; Krakowska, A.; Sulkowska-Ziaja, K.; Szewczyk, A.; Lazur, J.; Muszynska, B. Coffee with cordyceps militaris and Hericium erinaceus fruiting bodies as a source of essential bioactive substances. Pharmaceuticals 2024, 17, 955. [Google Scholar] [CrossRef]
- Tzeng, T.T.; Chen, C.C.; Chen, C.C.; Tsay, H.J.; Lee, L.Y.; Chen, W.P.; Shen, C.C.; Shiao, Y.J. The cyanthin diterpenoid and sesterterpene constituents of Hericium erinaceus mycelium ameliorate alzheimer’s disease-related pathologies in APP/PS1 transgenic mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Bao, L.; Yang, Y.; Ma, K.; Liu, H. Three new cyathane diterpenes with neurotrophic activity from the liquid cultures of Hericium erinaceus. J. Antibiot. 2018, 71, 818–821. [Google Scholar] [CrossRef]
- He, J.; Fan, P.; Feng, S.; Shao, P.; Sun, P. Isolation and purification of two isoflavones from Hericium erinaceum mycelium by high-speed counter-current chromatography. Molecules 2018, 23, 560. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (lion’s mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, T.; Qiu, F.; Song, Y.; Lin, D.; Ma, Y.; Li, J.; Huang, Y. Immunomodulatory effects of hydroxyethylated Hericium erinaceus polysaccharide on macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 105, 879–885. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Li, Y.H.; Wang, X.; Guo, Y.Q.; Song, X.X.; Nie, S.P.; Yin, J.Y. Evaluation of the “relative ordered structure of Hericium erinaceus polysaccharide” from different origins: Based on similarity and dissimilarity. J. Agric. Food Chem. 2023, 71, 17886–17898. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawahara, K.; Osawa, H.; Song, L.T.; Numazaki, K.; Kawai, J.; Onoue, S.; Nishioka, T.; Nemoto, E.; Matsushita, K.; et al. Hericium erinaceus ethanol extract and ergosterol exert anti-inflammatory activities by neutralizing lipopolysaccharide-induced pro-inflammatory cytokine production in human monocytes. Biochem. Biophys. Res. Commun. 2022, 636, 1–9. [Google Scholar] [CrossRef]
- Tu, J.Q.; Liu, H.P.; Wen, Y.H.; Chen, P.; Liu, Z.T. A novel polysaccharide from hericium erinaceus: Preparation, structural characteristics, thermal stabilities, and antioxidant activities In Vitro. J. Food Biochem. 2021, 45, e13871. [Google Scholar] [CrossRef]
- Shi, X.Z.; Zhang, X.Y.; Wang, Y.Y.; Zhao, Y.M.; Wang, J. Polysaccharides from Hericium erinaceus and its immunomodulatory effects on RAW 264.7 macrophages. Int. J. Biol. Macromol. 2024, 278, 134947. [Google Scholar] [CrossRef]
- Li, J.; Mo, J.R.; Hu, S.Y.; Dong, X.; Li, J.W.; Yang, L.Y.; Wu, Y.J. Effects of Hericium erinaceus polysaccharide in porcine IPEC-j2 intestinal epithelial cells against apoptosis induced by oxidative stress. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2024, 280, 109902. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Xu, T.; Zou, X.; Mao, R.; Pi, X.; Wu, W.; Huang, L.; Yang, K.; Zeng, X.; et al. Digestive characteristics of Hericium erinaceus polysaccharides and their positive effects on fecal microbiota of male and female volunteers during In Vitro fermentation. Front. Nutr. 2022, 9, 858585. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, J.; Li, S. Quantitative estimation of enzymatic released specific oligosaccharides from Hericium erinaceus polysaccharides using CE-LIF. J. Pharm. Anal. 2023, 13, 201–208. [Google Scholar] [CrossRef]
- Qiao, Z.; Jia, X.; Wang, Y.; Wang, Y.; Zhou, Y.; Li, F.; Qu, Y.; Cheng, H. Structural analysis and antioxidant activity of alkaline-extracted glucans from Hericium erinaceus. Foods 2024, 13, 2742. [Google Scholar] [CrossRef]
- Zhuang, H.; Dong, H.; Zhang, X.; Feng, T. Antioxidant activities and prebiotic activities of water-soluble, alkali-soluble polysaccharides extracted from the fruiting bodies of the fungus Hericium erinaceus. Polymers 2023, 15, 4165. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.S.; Liang, J.; Zhang, Y.; Kuang, H.X.; Xia, Y.G. Enzymatic-fingerprinting workflow of polysaccharides in Hericium erinaceus fruiting bodies: From HILIC-ESI(-)-MS screening to targeted MIM profiling. Int. J. Biol. Macromol. 2021, 173, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Pan, X.; Chen, M.; Ma, J.; Xu, B.; Zhao, Y. Ultrasound-assisted enzymatic extraction of soluble dietary fiber from Hericium erinaceus and its in vitro lipid-lowering effect. Food Chem. X 2024, 23, 101657. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Hutmacher, A.; Lu, B.; Steiger, N.; Nystrom, L.; Narciso, J.O. Vegan shrimp alternative made with pink oyster and lion’s mane mushrooms: Nutritional profiles, presence of conjugated phenolic acids, and prototyping. Curr. Res. Food Sci. 2023, 7, 100572. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Z.; Lv, Y.; Ge, C.; Luo, X.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Hericium erinaceus polysaccharides ameliorate nonalcoholic fatty liver disease via gut microbiota and tryptophan metabolism regulation in an aged laying hen model. Int. J. Biol. Macromol. 2024, 273, 132735. [Google Scholar] [CrossRef]
- Li, H.; Feng, J.; Liu, C.; Hou, S.; Meng, J.; Liu, J.Y.; Zilong, S.; Chang, M.C. Polysaccharides from an edible mushroom, Hericium erinaceus, alleviate ulcerative colitis in mice by inhibiting the NLRP3 inflammasomes and reestablish intestinal homeostasis. Int. J. Biol. Macromol. 2024, 267, 131251. [Google Scholar] [CrossRef]
- Qu, Y.; Yan, J.; Zhang, X.; Song, C.; Zhang, M.; Mayo, K.H.; Sun, L.; Cheng, H.; Zhou, Y. Structure and antioxidant activity of six mushroom-derived heterogalactans. Int. J. Biol. Macromol. 2022, 209, 1439–1449. [Google Scholar] [CrossRef]
- Xie, B.; Yi, L.; Zhu, Y.; Chang, X.; Hao, J.; Pang, L.; Ouyang, Y.; Yuan, S.; Zhang, Z. Structural elucidation of a branch-on-branch beta-glucan from Hericium erinaceus with a HPAEC-PAD-MS system. Carbohydr. Polym. 2021, 251, 117080. [Google Scholar] [CrossRef]
- Zaitseva, O.; Sergushkina, M.; Popyvanov, D.; Nazarova, Y.; Polezhaeva, T.; Solomina, O.; Khudyakov, A. The influence of polysaccharide fractions of the lion’s mane medicinal mushroom Hericium erinaceus (agaricomycetes) on the immunomodulatory and antioxidant activity of neutrophils exposed to stress of different durations. Int. J. Med. Mushrooms 2024, 26, 11–25. [Google Scholar] [CrossRef]
- Cui, M.; Ma, Q.; Zhang, Z.; Li, W.; Chen, W.; Liu, P.; Wu, D.; Yang, Y. Semi-solid enzymolysis enhanced the protective effects of fruiting body powders and polysaccharides of Herinaceus erinaceus on gastric mucosal injury. Int. J. Biol. Macromol. 2023, 251, 126388. [Google Scholar] [CrossRef]
- Su, Y.; Cheng, S.; Ding, Y.; Wang, L.; Sun, M.; Man, C.; Zhang, Y.; Jiang, Y. A comparison of study on intestinal barrier protection of polysaccharides from Hericium erinaceus before and after fermentation. Int. J. Biol. Macromol. 2023, 233, 123558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, S.; Hu, W.; Teng, M.; Wang, X.; Qu, Y.; Liu, Y.; Yuan, Y.; Wang, D. The neuroprotective properties of Hericium erinaceus in glutamate-damaged differentiated PC12 cells and an alzheimer’s disease mouse model. Int. J. Mol. Sci. 2016, 17, 1810. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, R.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Yang, K.; Zeng, X.; Peilong, S. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J. Sci. Food. Agric. 2023, 103, 3050–3064. [Google Scholar] [CrossRef]
- Tian, B.; Wang, P.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Sun, P.; Yang, K. Ameliorating effects of Hericium erinaceus polysaccharides on intestinal barrier injury in immunocompromised mice induced by cyclophosphamide. Food Funct. 2023, 14, 2921–2932. [Google Scholar] [CrossRef]
- Tripodi, F.; Falletta, E.; Leri, M.; Angeloni, C.; Beghelli, D.; Giusti, L.; Milanesi, R.; Sampaio-Marques, B.; Ludovico, P.; Goppa, L.; et al. Anti-aging and neuroprotective properties of grifola frondosa and Hericium erinaceus extracts. Nutrients 2022, 14, 4368. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Hong, Q.; Zhang, X.; Liu, Z.; Zhang, T. Polysaccharides from Hericium erinaceus fruiting bodies: Structural characterization, immunomodulatory activity and mechanism. Nutrients 2022, 14, 3721. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, M.; Wang, C.; Guo, Z.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Hericium erinaceus fermented mycelium and its pharmacological basis for application in alzheimer’s disease: Oxidative stress related calcium homeostasis. Int. J. Biol. Macromol. 2021, 193, 358–369. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.; Zhao, C.; Ren, L.; Wang, C.; Georgiev, M.I.; Xiao, J.; Zhang, T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef]
- Wang, D.; Xu, D.; Zhao, D.; Wang, M. Screening and comparison of anti-intestinal inflammatory activities of three polysaccharides from the mycelium of lion’s mane culinary-medicinal mushroom, Hericium erinaceus (agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 63–71. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Liu, W.; Feng, Y.; Zhang, Y.; Yuan, F.; Jia, L. Liver and brain protective effect of sulfated polysaccharides from residue of lion’s mane medicinal mushroom, Hericium erinaceus (agaricomycetes), on d-galactose-induced aging mice. Int. J. Med. Mushrooms 2021, 23, 55–65. [Google Scholar] [CrossRef]
- Liu, J.; Hou, X.; Li, Z.; Shan, S.; Chang, M.; Feng, C.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at s-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, J.; Li, Z.; Chang, M.; Guo, M.; Feng, C.; Shi, J. Fruiting body polysaccharides of Hericium erinaceus induce apoptosis in human colorectal cancer cells via ROS generation mediating caspase-9-dependent signaling pathways. Food Funct. 2020, 11, 6128–6138. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Liu, X.; Luo, Y.; Yu, R.; Chen, S.; Zhang, J.; Xu, Y.; Meng, Z.; Huang, Y.; Ren, Z. Characterization of polysaccharides isolated from Hericium erinaceus and their protective effects on the DON-induced oxidative stress. Int. J. Biol. Macromol. 2020, 152, 1265–1273. [Google Scholar] [CrossRef]
- Wang, D.; Xu, D.; Zhang, Y.; Zhao, D.; Wang, M. A novel oligosaccharide isolated from Hericium erinaceus and its protection against LPS-induced caco-2 cells via the TLR4/NF-kappab pathway. J. Food Biochem. 2020, 44, e13135. [Google Scholar] [CrossRef]
- Wu, D.; Tang, C.; Liu, Y.; Li, Q.; Wang, W.; Zhou, S.; Zhang, Z.; Cui, F.; Yang, Y. Structural elucidation and immunomodulatory activity of a beta-d-glucan prepared by freeze-thawing from Hericium erinaceus. Carbohydr. Polym. 2019, 222, 114996. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, W.; Wang, M.; Xiao, X.; Gao, M.; Gao, Q.; Xu, D. Molecular properties, structure, and antioxidant activities of the oligosaccharide hep-2 isolated from cultured mycelium of Hericium erinaceus. J. Food Biochem. 2019, 43, e12985. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wang, D.; Zheng, W.; Li, X.; Zhang, H.; Zhao, D.; Wang, M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int. Immunopharmacol. 2019, 71, 411–422. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Yang, S.; Zhao, D.; Wang, M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int. J. Biol. Macromol. 2019, 125, 572–579. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Z.; Yu, R.; Chen, S.; Zhang, J.; Xu, Y.; Meng, Z.; Luo, Y.; Zhang, W.; Huang, Y.; et al. Structural characterization of enzymatic modification of Hericium erinaceus polysaccharide and its immune-enhancement activity. Int. J. Biol. Macromol. 2021, 166, 1396–1408. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, M.; Yin, J.Y.; Song, Y.H.; Wang, Y.X.; Nie, S.P.; Xie, M.Y. Gastroprotective activity of polysaccharide from the fruiting body of Hericium erinaceus against acetic acid-induced gastric ulcer in rats and structure of one bioactive fraction. Int. J. Biol. Macromol. 2022, 210, 455–464. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Song, X.; Li, X.; Jia, L.; Zhang, C. Structural characterization of Hericium erinaceus polysaccharides and the mechanism of anti-t2DM by modulating the gut microbiota and metabolites. Int. J. Biol. Macromol. 2023, 242, 125165. [Google Scholar] [CrossRef]
- Liao, B.; Zhou, C.; Liu, T.; Dai, Y.; Huang, H. A novel Hericium erinaceus polysaccharide: Structural characterization and prevention of h(2)o(2)-induced oxidative damage in GES-1 cells. Int. J. Biol. Macromol. 2020, 154, 1460–1470. [Google Scholar] [CrossRef]
- Wu, F.; Huang, H. Surface morphology and protective effect of Hericium erinaceus polysaccharide on cyclophosphamide-induced immunosuppression in mice. Carbohydr. Polym. 2021, 251, 116930. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, Q.; Gao, R.; Sheng, Y.; Guan, T.; Li, W.; Zhou, L.; Liu, C.; Li, H.; Lu, Z.; et al. Low weight polysaccharide of Hericium erinaceus ameliorates colitis via inhibiting the NLRP3 inflammasome activation in association with gut microbiota modulation. Nutrients 2023, 15, 739. [Google Scholar] [CrossRef]
- Hou, C.; Liu, L.; Ren, J.; Huang, M.; Yuan, E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2022, 375, 131896. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. Extraction of a novel fungal chitin from Hericium erinaceus residue using multistep mild procedures. Int. J. Biol. Macromol. 2020, 156, 1279–1286. [Google Scholar] [CrossRef]
- Wu, L.; Lv, Y.; Ge, C.; Luo, X.; Hu, Z.; Huang, W.; Zhan, S.; Shen, X.; Yu, D.; Liu, B. Polysaccharide from Hericium erinaceus improved laying performance of aged hens by promoting yolk precursor synthesis and follicle development via liver-blood-ovary axis. Poult. Sci. 2024, 103, 103810. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Cuende, E.; Martinez, C. Compartmentalization of the peripheral immune system. Adv. Immunol. 1993, 53, 157–216. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, Y.; Bi, J.; Huang, Y.; Cheng, Y.; Li, Y.; Wu, Y.; Cao, G.; Tian, Z. The use of supercytokines, immunocytokines, engager cytokines, and other synthetic cytokines in immunotherapy. Cell. Mol. Immunol. 2022, 19, 192–209. [Google Scholar] [CrossRef]
- Yu, R.; Sun, M.; Meng, Z.; Zhao, J.; Qin, T.; Ren, Z. Immunomodulatory effects of polysaccharides enzymatic hydrolysis from Hericium erinaceus on the MODE-k/DCs co-culture model. Int. J. Biol. Macromol. 2021, 187, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, J.; Zhao, C.; Zhang, F.; Gu, Y.; Wang, C.; Jin, E. Hericium erinaceus polysaccharide improves the microstructure, immune function, proliferation and reduces apoptosis of thymus and spleen tissue cells of immunosuppressed mice. Biosci. Biotechnol. Biochem. 2023, 87, 279–289. [Google Scholar] [CrossRef]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, X.; Tang, X.; Li, H.; Yizhen, X.; Chen, D. Auxiliary antitumor effects of fungal proteins from Hericium erinaceus by target on the gut microbiota. J. Food Sci. 2020, 85, 1872–1890. [Google Scholar] [CrossRef] [PubMed]
- Niesel, K.; Schulz, M.; Anthes, J.; Alekseeva, T.; Macas, J.; Salamero-Boix, A.; Mockl, A.; Oberwahrenbrock, T.; Lolies, M.; Stein, S.; et al. The immune suppressive microenvironment affects efficacy of radio-immunotherapy in brain metastasis. EMBO Mol. Med. 2021, 13, e13412. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, C.C.; Li, M. Macrophages/microglia in the glioblastoma tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 5775. [Google Scholar] [CrossRef]
- Folgado-Dorado, C.; Caracena-De, L.C.J.; Aguilera-Velazquez, J.R.; Santana-Villalona, R.; Rivera-Ramos, A.; Carbonero-Aguilar, M.P.; Talaveron, R.; Bautista, J.; Sarmiento, S.M. Isolation and purification of fungal beta-glucan as an immunotherapy strategy for glioblastoma. J. Vis. Exp. 2023, 196, e64924. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kaldunska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Wang, F.; Wang, J.; Liang, X.; Wu, Z.; Xue, J.; Yin, L.; Wei, L.; Zhang, X. Ghrelin inhibits myocardial pyroptosis in diabetic cardiomyopathy by regulating ERS and NLRP3 inflammasome crosstalk through the PI3k/AKT pathway. J. Drug Target. 2024, 32, 148–158. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of rich in beta-glucans edible mushrooms on aging gut microbiota characteristics: An in vitro study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of intestinal barrier function by microbial metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, Z.; Amakye, W.K.; Liu, W.; Zhao, Z.; Gao, L.; Wang, M.; Ren, J. Hericium erinaceus mycelium-derived polysaccharide alleviates ulcerative colitis and modulates gut microbiota in cynomolgus monkeys. Mol. Nutr. Food Res. 2023, 67, e2200450. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Choi, S.; Seo, K.; Kim, K.H.; Jeon, J.H.; Kim, C.H.; Lim, S.; Jeong, S.; Chun, J.L. Gut microbiota profiling in aged dogs after feeding pet food contained Hericium erinaceus. J. Anim. Sci. Technol. 2022, 64, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Jin, X.; Li, J.; Li, R.; Gao, Y.; Zhang, J.; Wang, X.; Wang, G. The global burden of peptic ulcer disease in 204 countries and territories from 1990 to 2019: A systematic analysis for the global burden of disease study 2019. Int. J. Epidemiol. 2022, 51, 1666–1676. [Google Scholar] [CrossRef]
- Chen, W.; Wu, D.; Jin, Y.; Li, Q.; Liu, Y.; Qiao, X.; Zhang, J.; Dong, G.; Li, Z.; Li, T.; et al. Pre-protective effect of polysaccharides purified from Hericium erinaceus against ethanol-induced gastric mucosal injury in rats. Int. J. Biol. Macromol. 2020, 159, 948–956. [Google Scholar] [CrossRef]
- Yuan, E.; Nie, S.; Liu, L.; Ren, J. Study on the interaction of Hericium erinaceus mycelium polysaccharides and its degradation products with food additive silica nanoparticles. Food Chem. X 2021, 12, 100172. [Google Scholar] [CrossRef]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of In Silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef]
- Szucko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and neuroprotective effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef]
- Wang, T.; Toriumi, K.; Suzuki, K.; Miyashita, M.; Ozawa, A.; Masada, M.; Itokawa, M.; Arai, M. Amyloban, extracted from Hericium erinaceus, ameliorates social deficits and suppresses the enhanced dopaminergic system in social defeat stress mice. Neuropsychopharmacol. Rep. 2024, 44, 728–736. [Google Scholar] [CrossRef]
- Lee, S.L.; Hsu, J.Y.; Chen, T.C.; Huang, C.C.; Wu, T.Y.; Chin, T.Y. Erinacine a prevents lipopolysaccharide-mediated glial cell activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo. Int. J. Mol. Sci. 2022, 23, 810. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lin, Y.C.; Huang, C.C.; Villaflores, O.B.; Wu, T.Y.; Huang, S.M.; Chin, T.Y. Hericium erinaceus mycelium and its isolated compound, erinacine a, ameliorate high-fat high-sucrose diet-induced metabolic dysfunction and spatial learning deficits in aging mice. J. Med. Food 2019, 22, 469–478. [Google Scholar] [CrossRef]
- Arunachalam, K.; Sasidharan, S.P.; Yang, X. A concise review of mushrooms antiviral and immunomodulatory properties that may combat against COVID-19. Food Chem. Adv. 2022, 1, 100023. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yan, P.; Zhu, Z.; Liao, L.; Chen, Q.; Luo, Y.; Li, H.; Li, J.; Wang, Q.; et al. Transcriptome analysis of the effects of Hericium erinaceus polysaccharide on the lymphocyte homing in muscovy duck reovirus-infected ducklings. Int. J. Biol. Macromol. 2019, 140, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liao, L.; Chen, Q.; Lin, S.; Luo, Y.; Qin, T.; Li, J.; Wang, Q.; Wu, B.; Huang, Y.; et al. Effects of Hericium erinaceus polysaccharide on immunity and apoptosis of the main immune organs in muscovy duck reovirus-infected ducklings. Int. J. Biol. Macromol. 2021, 171, 448–456. [Google Scholar] [CrossRef]
- Poniedzialek, B.; Siwulski, M.; Wiater, A.; Komaniecka, I.; Komosa, A.; Gasecka, M.; Magdziak, Z.; Mleczek, M.; Niedzielski, P.; Proch, J.; et al. The effect of mushroom extracts on human platelet and blood coagulation: In vitro screening of eight edible species. Nutrients 2019, 11, 3040. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Biochemical characterization and cytotoxicity of polylactosamine-extended n-glycans binding isolectins from the mushroom Hericium erinaceus. Int. J. Biol. Macromol. 2023, 226, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Ulziijargal, E.; Yang, J.H.; Lin, L.Y.; Chen, C.P.; Mau, J.L. Quality of bread supplemented with mushroom mycelia. Food Chem. 2013, 138, 70–76. [Google Scholar] [CrossRef]
- Crawford, L.M.; Kahlon, T.S.; Wang, S.C.; Friedman, M. Acrylamide content of experimental flatbreads prepared from potato, quinoa, and wheat flours with added fruit and vegetable peels and mushroom powders. Foods 2019, 8, 228. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, N.; Banerjee, A.; Chatterjee, T.; Acharya, K. Mycochemical profiling and antioxidant activity of two different tea preparations from lion’s mane medicinal mushroom, Hericium erinaceus (agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 59–70. [Google Scholar] [CrossRef]
- Tian, B.; Pan, Y.; Wang, J.; Cai, M.; Ye, B.; Yang, K.; Sun, P. Insoluble dietary fibers from by-products of edible fungi industry: Basic structure, physicochemical properties, and their effects on energy intake. Front. Nutr. 2022, 9, 851228. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Shevchuk, Y.; Kuypers, K.; Janssens, G.E. Fungi as a source of bioactive molecules for the development of longevity medicines. Ageing Res. Rev. 2023, 87, 101929. [Google Scholar] [CrossRef] [PubMed]
- Docherty, S.; Doughty, F.L.; Smith, E.F. The acute and chronic effects of lion’s mane mushroom supplementation on cognitive function, stress and mood in young adults: A double-blind, parallel groups, pilot study. Nutrients 2023, 15, 4842. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Luo, Y.; Meng, Z.; Zhang, J.; Yu, R.; Sun, M.; Xu, T.; Li, J.; Ma, Y.; Huang, Y.; et al. Multi-walled carbon nanotube polysaccharide modified Hericium erinaceus polysaccharide as an adjuvant to extend immune responses. Int. J. Biol. Macromol. 2021, 182, 574–582. [Google Scholar] [CrossRef]
- Matalqah, S.M.; Aiedeh, K.; Mhaidat, N.M.; Alzoubi, K.H.; Bustanji, Y.; Hamad, I. Chitosan nanoparticles as a novel drug delivery system: A review article. Curr. Drug Targets 2020, 21, 1613–1624. [Google Scholar] [CrossRef]
- Ren, Z.; Luo, Y.; Liu, X.; Zhang, J.; Chen, S.; Yu, R.; Xu, Y.; Meng, Z.; Li, J.; Ma, Y.; et al. Preparation, characterization and controlled-release property of CS crosslinked MWCNT based on Hericium erinaceus polysaccharides. Int. J. Biol. Macromol. 2020, 153, 1310–1318. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, Z.; Bo, R.; Liu, X.; Zhang, J.; Yu, R.; Chen, S.; Meng, Z.; Xu, Y.; Ma, Y.; et al. Designing selenium polysaccharides-based nanoparticles to improve immune activity of Hericium erinaceus. Int. J. Biol. Macromol. 2020, 143, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ge, M.; Wu, D.; Li, W.; Chen, W.; Liu, P.; Zhang, H.; Yang, Y. Resveratrol-loaded sulfated Hericium erinaceus beta-glucan-chitosan nanoparticles: Preparation, characterization and synergistic anti-inflammatory effects. Carbohydr. Polym. 2024, 332, 121916. [Google Scholar] [CrossRef]
- Ma, G.; Tao, Q.; Li, X.; Han, Y.; Du, H.; Hu, Q.; Xiao, H. Metabolomics study of dietary pleurotus eryngii beta-type glycosidic polysaccharide on colitis induced by dextran sodium sulfate in mice—Exploration for the potential metabolic indicators in urine and serum. Food Chem. 2024, 458, 140195. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, J.; Huang, K.; Liang, Z. Heat-killed saccharomyces boulardii alleviates dextran sulfate sodium-induced ulcerative colitis by restoring the intestinal barrier, reducing inflammation, and modulating the gut microbiota. Nutrients 2024, 16, 702. [Google Scholar] [CrossRef]
- Naiel, S.; Dowdall, N.; Zhou, Q.; Ali, P.; Hayat, A.; Vierhout, M.; Wong, E.Y.; Couto, R.; Yepez, B.; Seifried, B.; et al. Modulating pro-fibrotic macrophages using yeast beta-glucan microparticles prepared by pressurized gas expanded liquid (PGX) technology(r). Biomaterials 2025, 313, 122816. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Liu, J.J.; Wang, X.; Luo, H.; He, W.W.; Song, X.X.; Nie, S.P.; Yin, J.Y. Hericium erinaceus beta-glucan/tannic acid hydrogels based on physical cross-linking and hydrogen bonding strategies for accelerating wound healing. Int. J. Biol. Macromol. 2024, 279, 135381. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Huang, Q.; Huang, H. A self-healing hydrogel based on oxidized microcrystalline cellulose and carboxymethyl chitosan as wound dressing material. Int. J. Biol. Macromol. 2022, 221, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. A fungal chitin derived from Hericium erinaceus residue: Dissolution, gelation and characterization. Int. J. Biol. Macromol. 2020, 152, 456–464. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Xiao, M.; Tang, L.; Huang, H. Smart ph-sensitive hydrogel based on the pineapple peel-oxidized hydroxyethyl cellulose and the Hericium erinaceus residue carboxymethyl chitosan for use in drug delivery. Biomacromolecules 2022, 23, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Song, P.; Zhou, C.; Huang, H. Electric-field-sensitive hydrogel based on pineapple peel oxidized hydroxyethyl cellulose/gelatin/Hericium erinaceus residues chitosan and its study in curcumin delivery. Int. J. Biol. Macromol. 2024, 271, 132591. [Google Scholar] [CrossRef]
- Kim, N.; Yoo, H.S.; Ju, Y.J.; Oh, M.S.; Lee, K.T.; Inn, K.S.; Kim, N.J.; Lee, J.K. Synthetic 3’,4’-dihydroxyflavone exerts anti-neuroinflammatory effects in BV2 microglia and a mouse model. Biomol. Ther. 2018, 26, 210–217. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Magnetic sensitive Hericium erinaceus residue chitin/cu hydrogel nanocomposites for h(2) generation by catalyzing NaBH(4) hydrolysis. Carbohydr. Polym. 2020, 229, 115426. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Green magnetic hydrogels synthesis, characterization and flavourzyme immobilization based on chitin from Hericium erinaceus residue and polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 138, 462–472. [Google Scholar] [CrossRef]
- Liao, J.; Dai, H.; Huang, H. Construction of hydrogels based on the homogeneous carboxymethylated chitin from Hericium erinaceus residue: Role of carboxymethylation degree. Carbohydr. Polym. 2021, 262, 117953. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar] [CrossRef]
- Hou, C.; Yin, M.; Lan, P.; Wang, H.; Nie, H.; Ji, X. Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: Extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agric. 2021, 8, 13. [Google Scholar] [CrossRef]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125–131. [Google Scholar] [CrossRef] [PubMed]

| Polysaccharide Name | Biological Activity | Experimental Subject | Effects and Mechanisms | Reference |
|---|---|---|---|---|
| HECP, HERP | Antioxidant activity | Male adult Sprague Dawley rats | Enhancing the activities of SOD and GSH-PX in gastric tissues of gastric ulcer rats; reducing the content of MDA. | [50] |
| HEP | Antioxidant activity | In vitro | The scavenging ability of Hericium erinaceus polysaccharide on hydroxyl radical increased with an increase in the polysaccharide concentration. | [15] |
| Hep-2 | Antioxidant activity | GES-1 cell | Producing T-SOD and GSH-px in a concentration-responsive manner to protect cells from oxidative damage. | [46] |
| HEP | Anti-inflammatory activity | Jingbai laying hens | Reduced inflammatory cell infiltration; alleviated inflammation; exhibited fewer or smaller cytoplasmic lipid vacuoles. | [58] |
| wHEP-1, wHEP-2, wHEP-3 | Anti-inflammatory activity | Half male Sprague Dawley rats | Improvements in mucosal degeneration and necrosis, cryptitis and crypt dilation, and mucosal and submucosal neutrophilic and monocytic infiltration. | [39] |
| wHEP-1 | Anti-inflammatory activity | Caco-2 cell | The protective effect on epithelial cells induced by LPS through the TLR4/NF-κB pathway is significant. | [44] |
| HEFPs | Intestinal health improvement activity | Male C57BL/6J mice | Enhancing the richness of the gut microbiota, which is composed of Firmicutes, Bacteroidetes, Verrucomicrobia, Proteobacteria, Actinobacteria, and a few other microorganisms. | [26] |
| HEP | Intestinal health improvement activity | Male specific pathogen-free ICR mice | Enhancing the species richness and diversity of the gut microbiota; significantly increasing the expression levels of SCFA receptors; regulating and restoring imbalance in the gut microbiota. | [33] |
| HEPs | Intestinal health improvement activity | Fresh feces and intestinal contents (small intestine, cecum, and colon) | Significantly increasing the relative abundance of Ruminococcaceae and Akkermansiales in the gut microbiota. | [38] |
| HECP, HERP | Gastric protective activity | Male Sprague Dawley rats | Reducing the ulcer area in gastric tissue; improving mucosal structure; gastric gland structure tending to be complete; alleviating the inflammatory response of gastric mucosa in ulcerative rats; increasing the release of gastric defensive factors; promoting the regeneration of gastric mucosa. | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, F.; Chen, Y.; Wang, B.; Zhou, W.; Du, B.; Hou, L. Bioactive Polysaccharides from Hericium erinaceus: Extraction, Structure, Bioactivities, and Applications. Molecules 2025, 30, 1850. https://doi.org/10.3390/molecules30081850
Ge F, Chen Y, Wang B, Zhou W, Du B, Hou L. Bioactive Polysaccharides from Hericium erinaceus: Extraction, Structure, Bioactivities, and Applications. Molecules. 2025; 30(8):1850. https://doi.org/10.3390/molecules30081850
Chicago/Turabian StyleGe, Fangzhi, Yan Chen, Binshuo Wang, Wenxin Zhou, Baoxiang Du, and Lin Hou. 2025. "Bioactive Polysaccharides from Hericium erinaceus: Extraction, Structure, Bioactivities, and Applications" Molecules 30, no. 8: 1850. https://doi.org/10.3390/molecules30081850
APA StyleGe, F., Chen, Y., Wang, B., Zhou, W., Du, B., & Hou, L. (2025). Bioactive Polysaccharides from Hericium erinaceus: Extraction, Structure, Bioactivities, and Applications. Molecules, 30(8), 1850. https://doi.org/10.3390/molecules30081850






