Deep Eutectic Solvents (DESs) as Alternative Sustainable Media for the Extraction and Characterization of Bioactive Compounds from Winemaking Industry Wastes
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Sampling and Instrumentation
3.2. Preparation of Extraction Media
3.3. Chemical Analyses
3.4. Extraction
3.5. Quali-Quantitative Determination of Bioactive Compounds
3.6. Detection of Antioxidant Activity
3.7. Determination of Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muchhadiya, R.M.; Gohil, B.; Yadahalli, V.; HM, A.U.R.; Siddiqua, A.; Khayum, A.; Behera, H.S.; Kumar, S. Feeding the world: Agronomic innovations to meet the challenges of a growing population. Int. J. Res. Agron. 2024, 7, 790–802. [Google Scholar] [CrossRef]
- Roselli, V.; Pugliese, G.; Leuci, R.; Brunetti, L.; Gambacorta, L.; Tufarelli, V.; Piemontese, L. Green Methods to Recover Bioactive Compounds from Food Industry Waste: A Sustainable Practice from the Perspective of the Circular Economy. Molecules 2024, 29, 2682. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A review on the use of agro-industrial CO-products in animals’ diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Brunetti, L.; Leuci, R.; Colonna, M.A.; Carrieri, R.; Celentano, F.E.; Bozzo, G.; Loiodice, F.; Selvaggi, M.; Tufarelli, V.; Piemontese, L. Food Industry Byproducts as Starting Material for Innovative, Green Feed Formulation: A Sustainable Alternative for Poultry Feeding. Molecules 2022, 27, 4735. [Google Scholar] [CrossRef]
- Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases. Foods 2021, 10, 29. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Siller-Sánchez, A.; Luna-Sánchez, K.A.; Bautista-Hernández, I.; Chávez-González, M.L. Use of Grape Pomace from the Wine Industry for the Extraction of Valuable Compounds with potential use in the Food Industry. Curr. Food Sci. Technol. Rep. 2024, 2, 7–16. [Google Scholar] [CrossRef]
- Sanchez-Garcia, E.; Martinez-Falco, J.; Marco-Lajara, B.; Georgantzis, N. Value creation in the wine industry—A bibliometric analysis. Eur. Food Res. Technol. 2024, 250, 1135–1148. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, L.; Poe, N.; Huang, H. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Wani, T.A.; Majid, D.; Dar, B.N.; Makroo, H.A.; Allai, F.M. Utilization of novel techniques in extraction of polyphenols from grape pomace and their therapeutic potential: A review. Food Meas. 2023, 17, 5412–5425. [Google Scholar] [CrossRef]
- Oliveira, J.; Alhinho da Silva, M.; Teixeira, N.; De Freitas, V.; Salas, E. Screening of anthocyanins and anthocyanin-derived pigments in red wine grape pomace using LC-DAD/MS and MALDI-TOF techniques. J. Agric. Food Chem. 2015, 63, 7636–7644. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, 1585. [Google Scholar] [CrossRef]
- Dheyab, A.S.; Abu Bakar, M.F.; AlOmar, M.; Sabran, S.F.; Muhamad Hanafi, A.F.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8, 176. [Google Scholar] [CrossRef]
- de los Ángeles Fernández, M.; Boiteux, J.; Espino, M.; Gomez, F.J.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.F.E.; Ghafoor, K.; Banjanin, T.; Osman, M.A.; Gassem, M.A.; Alqaf, H.A.S. Chemical composition bioactive compounds mineral contents and fatty acid composition of pomace powder of different grape varieties. J. Food Process. Preserv. 2020, 44, e14539. [Google Scholar] [CrossRef]
- Alibade, A.; Lakka, A.; Bozinou, E.; Lalas, S.I.; Chatzilazarou, A.; Makris, D.P. Development of a Green Methodology for Simultaneous Extraction of Polyphenols and Pigments from Red Winemaking Solid Wastes (Pomace) Using a Novel Glycerol- Sodium Benzoate Deep Eutectic Solvent and Ultrasonication Pretreatment. Environments 2021, 8, 90. [Google Scholar] [CrossRef]
- Samorì, C.; Mazzei, L.; Ciurli, S.; Cravotto, G.; Grillo, G.; Guidi, E.; Pasteris, A.; Tabasso, S.; Galletti, P. Urease inhibitory potential and soil ecotoxicity of novel “polyphenols–deep eutectic solvents” formulations. ACS Sustain. Chem. Eng. 2019, 7, 15558–15567. [Google Scholar] [CrossRef]
- Vorobyova, V.; Vasyliev, G.; Skiba, M.; Frolenkova, S.; Zaporozhets, J.; Gnatko, O.; Linyucheva, O. Green extraction of phenolic compounds from grape pomace by deep eutectic solvent extraction: Physicochemical properties, antioxidant capacity. Chem. Pap. 2023, 77, 2447–2458. [Google Scholar] [CrossRef]
- Li, Z.; Cui, R.; Liu, W.; Wang, M.; Li, L.; Liu, F.; Du, B.; Song, L. Application of green deep eutectic solvents for anthocyanins extraction from grape pomace: Optimization, stability, antioxidant activity, and molecular dynamic simulation. LWT-Food Sci. Technol. 2024, 211, 116878. [Google Scholar] [CrossRef]
- Piemontese, L. Plant Food Supplements with Antioxidant Properties for the Treatment of Chronic and Neurodegenerative Diseases: Benefits or Risks? J. Diet. Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Vine-Winery Byproducts as Precious Resource of Natural Antimicrobials: In Vitro Antibacterial and Antibiofilm Activity of Grape Pomace Extracts against Foodborne Pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The evaluation of phenolic content, in vitro antioxidant and antibacterial activity of Mentha piperita extracts obtained by natural deep eutectic solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef]
- Ivanović, M.; Grujić, D.; Cerar, J.; Islamčević Razboršek, M.; Topalić-Trivunović, L.; Savić, A.; Kočar, D.; Kolar, M. Extraction of Bioactive Metabolites from Achillea millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 724. [Google Scholar] [CrossRef]
- Sabo, J.; Čmiková, N.; Kačániová, M. Antimicrobial Activity of Grape Pomace Extracts Against Different Species of Microorganisms. Acta Hortic. Regiotect. 2024, 27, 117–123. [Google Scholar] [CrossRef]
- Mouffok, A.; Bellouche, D.; Debbous, I.; Anane, A.; Khoualdia, Y.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Benguerba, Y. Synergy of Garlic Extract and Deep Eutectic Solvents as Promising Natural Antibiotics: Experimental and COSMO-RS. J. Mol. Liq. 2023, 375, 121321. [Google Scholar] [CrossRef]
- Piemontese, L.; Perna, F.M.; Logrieco, A.; Capriati, V.; Solfrizzo, M. Deep eutectic solvents as novel and effective extraction media for quantitative determination of ochratoxin A in wheat and derived products. Molecules 2017, 22, 121. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Chen, J.; Yu, J. Ascorbic acid and choline chloride: A new natural deep eutectic solvent for extracting tert-butylhydroquinone antioxidant. J. Mol. Liq. 2018, 260, 173–179. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yu, H.; Guo, S.; Chen, D. Deep eutectic solvent as a green solvent for enhanced extraction of narirutin, naringin, hesperidin and neohesperidin from Aurantii Fructus. Phytochem. Anal. 2019, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, Y.R.; Row, K.H. Synthesis of mesoporous siliceous materials in choline chloride deep eutectic solvents and the application of these materials to high-performance size exclusion chromatography. Chromatographia 2016, 79, 375–382. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.; Ferreira, I.C.; Ferreira, O. Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crops Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Sousa, A.M.; Gando-Ferreira, L.M.; Quina, M.J. Grape pomace as a natural source of phenolic compounds: Solvent screening and extraction optimization. Molecules 2023, 28, 2715. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S.; Heredia, F.J.; Hernanz, D.; Romano, A. Extraction of antioxidants from winemaking byproducts: Effect of the solvent on phenolic composition, antioxidant and anti-cholinesterase activities, and electrochemical behaviour. Antioxidants 2020, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Dimcheva, V.; Karsheva, M.; Diankov, S.; Hinkov, I. Optimization of extraction of antioxidants from Bulgarian Mavrud By-Products. J. Chem. Technol. Metall. 2018, 53, 631–639. [Google Scholar]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Massari, F.; Zambonin, C. Determination of polyphenols and vitamins in wine-making by-products by supercritical fluid extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- Garcia-Montalvo, J.; Garcia-Martín, A.; Ibañez Bujan, J.; Santos Mazorra, V.E.; Yustos Cuesta, P.; Bolivar, J.M.; Ladero, M. Extraction of antioxidants from grape and apple pomace: Solvent selection and process kinetics. Appl. Sci. 2022, 12, 4901. [Google Scholar] [CrossRef]
- Gurgenidze, L.; Kanchaveli, T.; Kvartskhava, G. Selecting optimal parameters for obtaining the extract of red grape pomace. Rev. Fac. Nac. Agron. Medellín 2022, 75, 9831–9837. [Google Scholar] [CrossRef]
- AOAC. Official Method 945.15. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- AOAC. Official Method 990.03. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Method 967.05. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Zou, W.; Lusk, C.; Messer, D.; Lane, R. Fat Contents of Cereal Foods: Comparison of Classical with Recently Developed Extraction Techniques. J. AOAC Int. 1999, 82, 141–150. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Method 922.06. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC: Arlington, VA, USA, 2002. [Google Scholar]
- Ageyeva, N.M.; Tikhonova, A.N.; Burtsev, B.V.; Biryukova, S.A.; Globa, E.V. Grape pomace treatment methods and their effects on storage. Foods Raw Mater. 2021, 9, 215–223. [Google Scholar] [CrossRef]
- Chamorro, S.; Goñi, I.; Viveros, A.; Hervert-Hernández, D.; Brenes, A. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res. Technol. 2012, 234, 147–155. [Google Scholar] [CrossRef]
- Sergio, L.; Boari, F.; Pieralice, M.; Linsalata, V.; Cantore, V.; Di Venere, D. Bioactive phenolics and antioxidant capacity of some wild edible greens as affected by different cooking treatments. Foods 2020, 9, 1320. [Google Scholar] [CrossRef]
- Carocci, A.; Barbarossa, A.; Leuci, R.; Carrieri, A.; Brunetti, L.; Laghezza, A.; Catto, M.; Limongelli, F.; Chaves, S.; Tortorella, P.; et al. Novel phenothiazine/donepezil-like hybrids endowed with antioxidant activity for a multi-target approach to the therapy of Alzheimer’s disease. Antioxidants 2022, 11, 1631. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- CLSI M07TM; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2012.
- Barbarossa, A.; Ceramella, J.; Carocci, A.; Iacopetta, D.; Rosato, A.; Limongelli, F.; Carrieri, A.; Bonofiglio, D.; Sinicropi, M.S. Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents. Antibiotics 2023, 12, 1651. [Google Scholar] [CrossRef]

| Grape Pomace | Moisture % | Crude Protein % DM * | Crude Fiber % DM * | Crude Lipids % DM * | Total Ash % DM * |
|---|---|---|---|---|---|
| Cabernet Sauvignon | 48.85 ± 0.47 | 6.32 ± 0.15 | 35.09 ± 0.29 | 5.23 ± 0.09 | 7.82 ± 0.18 |
| Petit Verdot | 50.33 ± 0.39 | 5.49 ± 0.13 | 34.61 ± 0.31 | 4.57 ± 0.11 | 8.80 ± 0.16 |
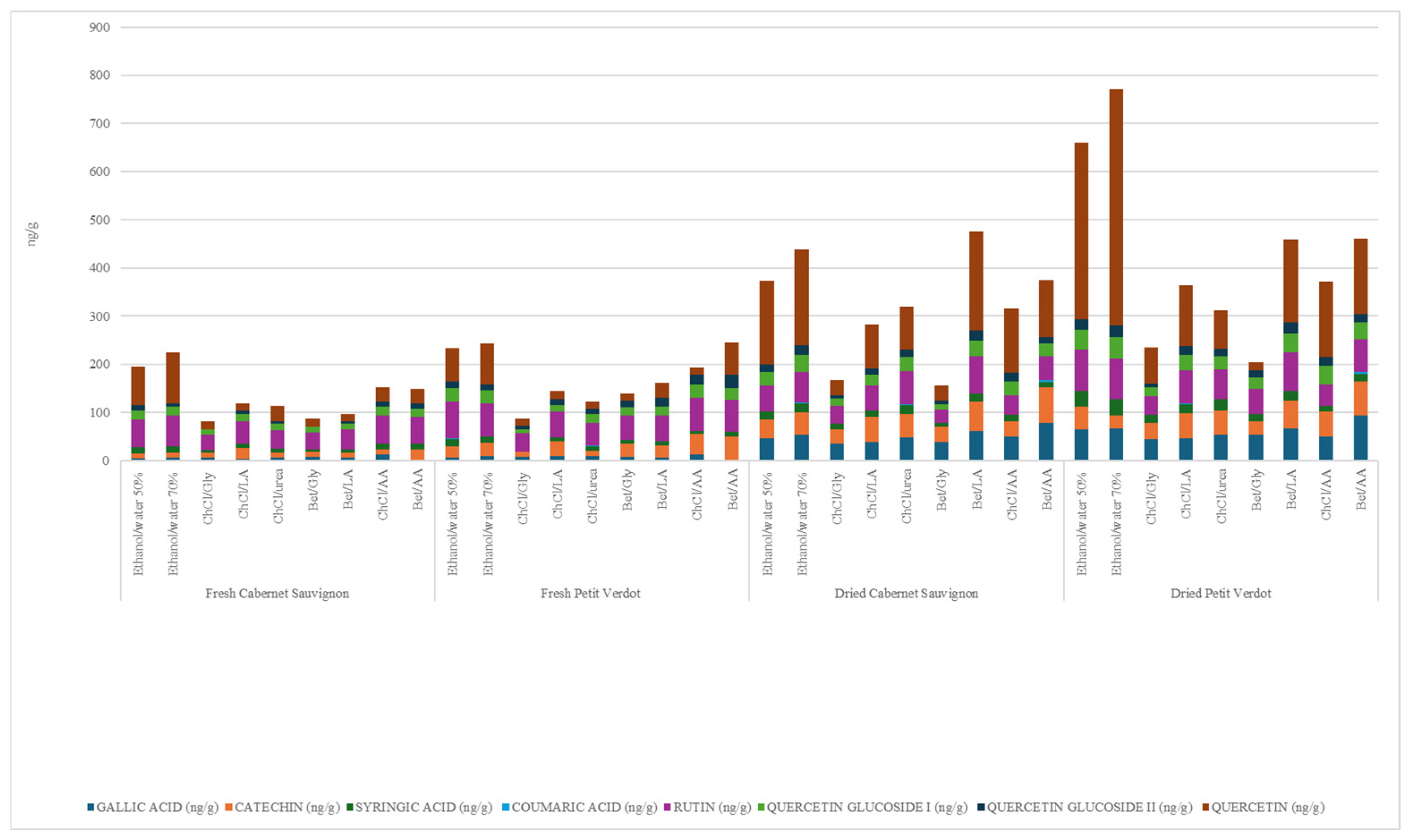
| GRAPE POMACE | SOLVENT | GALLIC ACID (ng/g) | CATECHIN (ng/g) | SYRINGIC ACID (ng/g) | COUMARIC ACID (ng/g) | RUTIN (ng/g) | QUERCETIN GLUCOSIDE I (ng/g) | QUERCETIN GLUCOSIDE II (ng/g) | QUERCETIN (ng/g) |
|---|---|---|---|---|---|---|---|---|---|
| Fresh Cabernet Sauvignon | Ethanol/water 50% | 4.9 | <LOQ | 12.8 | ND | 57.3 | 17.6 | 12.0 | 78.7 |
| Ethanol/water 70% | 6.1 | <LOQ | 14.1 | ND | 63.2 | 18.2 | 6.8 | 106.8 | |
| ChCl/Gly | 5.6 | <LOQ | 6.2 | ND | 32.2 | 10.3 | 1.3 | ND | |
| ChCl/LA | 3.7 | 22.8 | 7.7 | ND | 47.5 | 14.5 | 7.8 | ND | |
| ChCl/urea | 6.5 | <LOQ | 7.7 | ND | 39.5 | 12.5 | 6.0 | <LOQ | |
| Bet/Gly | 7.6 | <LOQ | 6.0 | ND | 34.9 | 10.9 | 1.3 | ND | |
| Bet/LA | 5.7 | <LOQ | 7.4 | ND | 41.7 | 11.2 | 5.7 | ND | |
| ChCl/AA | 12.3 | <LOQ | 12.5 | ND | 58.3 | 18.2 | 10.0 | <LOQ | |
| Bet/AA | 0.1 | 23.4 | 11.3 | ND | 56.0 | 15.8 | 12.2 | <LOQ | |
| Fresh Petit Verdot | Ethanol/water 50% | 6.3 | 23.1 | 16.3 | ND | 77.2 | 27.8 | 14.2 | 68.3 |
| Ethanol/water 70% | 8.9 | 28.1 | 13.6 | ND | 68.9 | 25.5 | 13.1 | 85.7 | |
| ChCl/Gly | 7.4 | <LOQ | <LOQ | ND | 37.3 | 9.9 | 5.5 | ND | |
| ChCl/LA | 9.4 | 30.9 | 7.7 | ND | 54.2 | 14.1 | 11.5 | ND | |
| ChCl/urea | 10.3 | <LOQ | 10.2 | ND | 48.5 | 17.4 | 10.1 | ND | |
| Bet/Gly | 7.5 | 27.0 | 8.7 | ND | 50.1 | 16.9 | 13.4 | ND | |
| Bet/LA | 6.3 | 24.6 | 9.7 | ND | 53.5 | 18.3 | 17.5 | <LOQ | |
| ChCl/AA | 13.8 | 41.4 | 6.8 | ND | 68.3 | 26.5 | 20.4 | 15.5 | |
| Bet/AA | 0.1 | 49.5 | 10.5 | ND | 64.6 | 26.5 | 26.1 | 66.9 | |
| Dried Cabernet Sauvignon | Ethanol/water 50% | 47.0 | 38.8 | 15.5 | ND | 54.9 | 28.8 | 14.3 | 174.2 |
| Ethanol/water 70% | 53.4 | 46.2 | 20.0 | ND | 64.2 | 36.0 | 20.2 | 198.6 | |
| ChCl/Gly | 34.6 | 30.4 | 12.6 | ND | 35.7 | 16.2 | 6.8 | <LOQ | |
| ChCl/LA | 38.2 | 52.8 | 13.0 | ND | 51.8 | 22.2 | 12.7 | 91.9 | |
| ChCl/urea | 48.8 | 48.7 | 18.7 | ND | 70.4 | 28.1 | 14.8 | 88.9 | |
| Bet/Gly | 38.0 | 31.9 | 8.1 | ND | 27.5 | 11.0 | 7.9 | <LOQ | |
| Bet/LA | 61.1 | 61.1 | 16.7 | ND | 78.0 | 32.2 | 21.9 | 204.5 | |
| ChCl/AA | 50.2 | 32.3 | 12.3 | ND | 40.4 | 29.8 | 18.3 | 131.5 | |
| Bet/AA | 79.1 | 73.2 | 10.6 | 5.2 | 48.3 | 26.8 | 12.9 | 118.9 | |
| Dried Petit Verdot | Ethanol/water 50% | 65.9 | 46.4 | 32.1 | ND | 84.6 | 42.9 | 21.7 | 265.8 |
| Ethanol/water 70% | 67.4 | 27.0 | 32.6 | ND | 84.0 | 44.9 | 23.5 | 492.5 | |
| ChCl/Gly | 44.6 | 34.2 | 15.9 | ND | 40.0 | 17.1 | 8.1 | 74.6 | |
| ChCl/LA | 46.7 | 52.4 | 18.9 | ND | 69.8 | 31.8 | 19.0 | 126.5 | |
| ChCl/urea | 52.9 | 51.8 | 22.4 | ND | 62.6 | 27.3 | 14.8 | 80.1 | |
| Bet/Gly | 54.0 | 27.6 | 15.8 | ND | 50.8 | 24.1 | 15.9 | ND | |
| Bet/LA | 67.3 | 57.2 | 20.1 | ND | 80.2 | 37.7 | 24.3 | 170.8 | |
| ChCl/AA | 49.3 | 52.2 | 11.5 | ND | 44.3 | 39.5 | 18.4 | 156.5 | |
| Bet/AA | 93.0 | 70.7 | 15.0 | 6.4 | 66.6 | 35.3 | 17.1 | 155.4 |
| GRAPE POMACE | SOLVENT | μM GAE | GRAPE POMACE | SOLVENT | μM GAE |
|---|---|---|---|---|---|
| Fresh Cabernet Sauvignon | Ethanol/water 50% | ND | Dry Cabernet Sauvignon | Ethanol/water 50% | 5080 ± 1200 |
| Ethanol/water 70% | ND | Ethanol/water 70% | 5980 ± 840 | ||
| ChCl/Gly | 166 ± 23 | ChCl/Gly | 6960 ± 1450 | ||
| ChCl/LA | 1710 ± 150 | ChCl/LA | 8170 ± 1270 | ||
| ChCl/urea | 1910 ± 290 | ChCl/urea | 12,550 ± 2360 | ||
| Bet/Gly | 3530 ± 370 | Bet/Gly | 8960 ± 1350 | ||
| Bet/LA | 5060 ± 440 | Bet/LA | 9800 ± 1160 | ||
| ChCl/AA | ND | ChCl/AA | ND | ||
| Bet/AA | ND | Bet/AA | ND | ||
| Fresh Petit Verdot | Ethanol/water 50% | 1530 ± 80 | Dry Petit Verdot | Ethanol/water 50% | 5880 ± 280 |
| Ethanol/water 70% | 990 ± 70 | Ethanol/water 70% | 4740 ± 210 | ||
| ChCl/Gly | 5340 ± 270 | ChCl/Gly | 3920 ± 180 | ||
| ChCl/LA | 6630 ± 230 | ChCl/LA | 4020 ± 160 | ||
| ChCl/urea | 6970 ± 180 | ChCl/urea | 15,000 ± 1030 | ||
| Bet/Gly | 1850 ± 150 | Bet/Gly | 2350 ± 93 | ||
| Bet/LA | 720 ± 85 | Bet/LA | 3390 ± 280 | ||
| ChCl/AA | ND | ChCl/AA | ND | ||
| Bet/AA | ND | Bet/AA | ND |
| E. faecalis 29212 | S. aureus 29213 | S. aureus 43300 | E. coli 25922 | K. pneumoniae 13883 | P. aeruginosa 27853 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Solvent | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| 70% EtOH | 12.5 | 25.0 | 12.5 | 25 | 25 | 50 | 25 | 50 | 25 | 50 | 25 | 50 |
| ChCl/AA | 1.6 | 3.1 | 1.6 | 3.1 | 3.1 | 6.3 | 3.1 | 6.3 | 3.1 | 6.3 | 6.3 | 12.5 |
| Bet/LA | 0.4 | 0.8 | 0.4 | 0.8 | 0.4 | 0.8 | 0.4 | 0.8 | 0.4 | 0.8 | 0.8 | 1.6 |
| ChCl/Urea | 6.3 | 12.5 | 6.3 | 12.5 | 12.5 | 25 | 12.5 | 25 | 12.5 | 25 | 12.5 | 25 |
| E. faecalis 29212 | S. aureus 29213 | S. aureus 43300 | E. coli 25922 | K. pneumoniae 13883 | P. aeruginosa 27853 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract (a Solvent) | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| 119 (70% EtOH) | 0.8 | 1.6 | 0.4 | 0.8 | 0.4 | 0.8 | 1.6 | 3.1 | 3.1 | 6.3 | 3.1 | 6.3 |
| 122 (ChCl/AA) | 0.8 | 1.6 | 0.4 | 0.8 | 0.4 | 0.8 | 0.8 | 1.6 | 1.6 | 3.1 | 1.6 | 3.1 |
| 140 (Bet/LA) | 0.2 | 0.4 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.4 | 0.2 | 0.4 | 0.4 | 0.8 |
| 152 (ChCl/Urea) | 0.8 | 1.6 | 0.4 | 0.8 | 0.4 | 0.8 | 1.6 | 3.1 | 3.1 | 6.3 | 3.1 | 6.3 |
| levofloxacin | 2.0 | - | 0.5 | - | 1.0 | - | 0.1 | - | 8.0 | - | 4.0 | - |
| HBA | HBD | Molar Ratio | Water Content (%) | Reference |
|---|---|---|---|---|
| ChCl | AA | 2:1 | 40 | [31] |
| Bet | AA | 2:1 | 40 | [31] |
| Bet | Gly | 1:4 | 40 | [32] |
| Bet | LA | 1:4 | 40 | [32] |
| ChCl | Gly | 1:2 | 40 | [33] |
| ChCl | LA | 1:2 | 40 | [34] |
| ChCl | Urea | 1:2 | 40 | [21] |
| Phenolic Compounds | LOD (ng/g) | LOQ (ng/g) |
|---|---|---|
| Gallic acid | 0.18 | 0.37 |
| Catechin | 10.1 | 20.3 |
| Syringic acid | 0.89 | 1.78 |
| Rutin and quercetin glucosides | 2.58 | 5.16 |
| Quercetin | 30.9 | 61.8 |
| Kaempferol 3-galactoside | 19.3 | 38.6 |
| Apigenin | 7.56 | 15.1 |
| Apigenin 3-glucuronide | 1.03 | 2.07 |
| Resveratrol | 0.06 | 0.12 |
| Ferulic acid | 0.19 | 0.39 |
| Chlorogenic acid | 1.39 | 2.78 |
| Caffeic acid | 0.67 | 1.33 |
| Coumaric acid | 0.32 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roselli, V.; Leuci, R.; Pugliese, G.; Barbarossa, A.; Laghezza, A.; Paparella, M.; Carocci, A.; Tufarelli, V.; Gambacorta, L.; Piemontese, L. Deep Eutectic Solvents (DESs) as Alternative Sustainable Media for the Extraction and Characterization of Bioactive Compounds from Winemaking Industry Wastes. Molecules 2025, 30, 1855. https://doi.org/10.3390/molecules30081855
Roselli V, Leuci R, Pugliese G, Barbarossa A, Laghezza A, Paparella M, Carocci A, Tufarelli V, Gambacorta L, Piemontese L. Deep Eutectic Solvents (DESs) as Alternative Sustainable Media for the Extraction and Characterization of Bioactive Compounds from Winemaking Industry Wastes. Molecules. 2025; 30(8):1855. https://doi.org/10.3390/molecules30081855
Chicago/Turabian StyleRoselli, Vincenzo, Rosalba Leuci, Gianluca Pugliese, Alexia Barbarossa, Antonio Laghezza, Marco Paparella, Alessia Carocci, Vincenzo Tufarelli, Lucia Gambacorta, and Luca Piemontese. 2025. "Deep Eutectic Solvents (DESs) as Alternative Sustainable Media for the Extraction and Characterization of Bioactive Compounds from Winemaking Industry Wastes" Molecules 30, no. 8: 1855. https://doi.org/10.3390/molecules30081855
APA StyleRoselli, V., Leuci, R., Pugliese, G., Barbarossa, A., Laghezza, A., Paparella, M., Carocci, A., Tufarelli, V., Gambacorta, L., & Piemontese, L. (2025). Deep Eutectic Solvents (DESs) as Alternative Sustainable Media for the Extraction and Characterization of Bioactive Compounds from Winemaking Industry Wastes. Molecules, 30(8), 1855. https://doi.org/10.3390/molecules30081855







