Abstract
A superhydrophilic silica coating was prepared using a sequential dipping process involving acid-catalyzed silica, base-catalyzed silica, and 3-(trihydroxysilyl)propanesulfonic acid. Acid-catalyzed and base-catalyzed silica particles with varying diameters were synthesized by hydrolyzing tetraethyl orthosilicate using HCl and NH3·H2O as catalysts, respectively. 3-(Trihydroxysilyl)propanesulfonic acid was obtained by oxidizing mercaptopropyl trimethoxysilane with hydrogen peroxide under acidic conditions. The resulting silica coating exhibited exceptional superhydrophilicity, with a water static contact angle of 5.0°, and demonstrated underwater superoleophobicity, with a hexadecane underwater contact angle exceeding 140°. Surfaces coated with the superhydrophilic silica coatings showed excellent performances in oil–water separation, anti-protein adsorption, and anti-fogging applications.
1. Introduction
Superhydrophilic surfaces are characterized by water contact angles (WCAs) lower than 5° within 0.5 s and the near-instantaneous spreading of condensed water droplets [1]. These surfaces exhibit exceptional wetting properties, which are governed by the chemical composition and topographic structure of the surface [2]. Superhydrophilic coatings have huge potential for applications in self-cleaning, water/oil separation, anti-fogging, and anti-fouling [3,4,5,6,7,8]. Typically, superhydrophilic surfaces are achieved by combining hydrophilic materials with carefully engineered rough topographies [9].
Various methods can be used to fabricate superhydrophilic surfaces, including vapor deposition [10], ultrasonic spray pyrolysis [11], layer-by-layer (LbL) assembly [12], the sol–gel method [13], etching [14], electrospinning [15], interfacial polymerization [16], and bottom-up assembly [17]. Surface coating offers a simple and effective way to modify surfaces for biocompatibility and hydrophilicity without chemically altering the substrate [18,19]. Dip coating [20,21,22,23], spray coating [24,25,26], doctoral blading [27], and spin coating [28] are common surface coating techniques used to create hydrophilic surfaces [29]. Dip coating, also known as immersion precipitation, involves immersing a substrate in a liquid coating dispersion, withdrawing it at a controlled rate, and then drying or curing the coating [30].
Hydrophilic surfaces are primarily achieved through the use of nanoparticles [31], nanoparticle/polyelectrolyte composites [32], and polymer/polyelectrolyte composites [33]. Titanium dioxide (TiO2) nanoparticles [34] and silica (SiO2) nanoparticles [35] are commonly used hydrophilic components due to their diverse morphology and hydrophilic properties. TiO2-based coatings, however, require activation via UV exposure to maintain their super-wetting properties, limiting their practical applications in environments with light [36]. In contrast, silica nanoparticles possess an inherent hydrophilicity due to the abundance of surface hydroxyl groups [37]. Additionally, their network structure offers excellent hydrolytic stability. Unlike polymer-based coatings, which are susceptible to swelling and cracking after prolonged exposure to aqueous environments, silica nanoparticles are widely employed in the fabrication of superhydrophilic surfaces. The formation of superhydrophilic properties also relies on microstructural roughness, which can be tailored using techniques such as LbL assembly [38], chemical vapor deposition (CVD) [39,40], and the sol–gel method [41]. Among these, the sol–gel method is usually favored due to its simpler process, lower costs, and eco-friendliness [42]. However, the superhydrophilic surfaces solely based on silica nanoparticles can be prone to fouling and a loss of oil repellency over time, limiting their long-term applicability [43].
In this work, acid- and base-catalyzed silica nanoparticles were synthesized by hydrolyzing tetraethyl orthosilicate (TEOS) using HCl and NH3·H2O as catalysts, respectively. Additionally, 3-(trihydroxysilyl)propanesulfonic acid was prepared by oxidizing mercaptopropyl trimethoxysilane with hydrogen peroxide under acidic conditions. A superhydrophilic coating, incorporating the above components, was fabricated via facile dip coating. The resulting coating exhibited a surface hardness of 2H, adhesion grade of 0, high abrasion resistance, superhydrophilicity, and underwater superoleophobicity. This coating shows great potential for applications including oil–water separation, anti-protein adsorption, and anti-fogging.
2. Results and Discussion
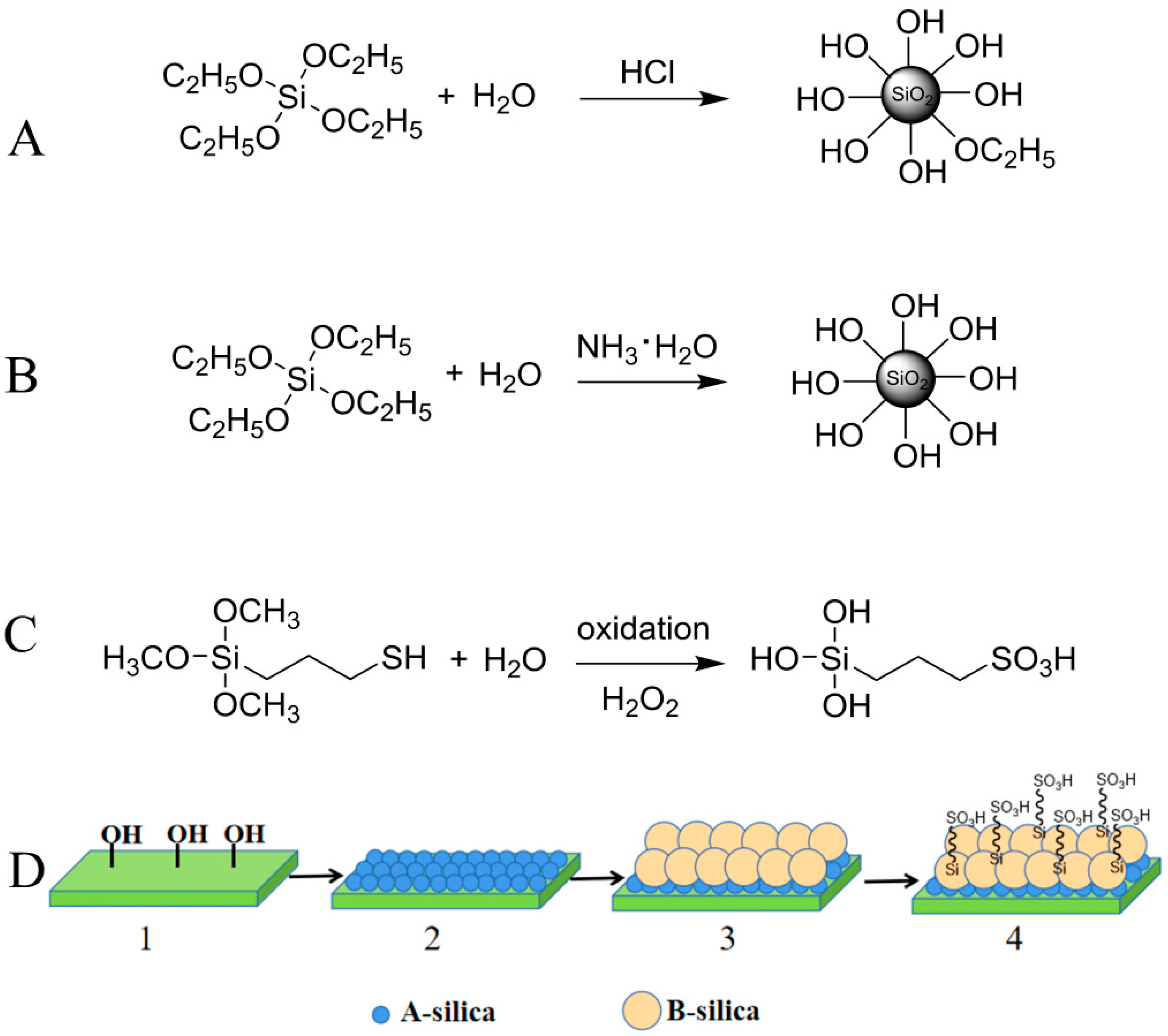
As shown in Figure 1A,B, A-silica and B-silica were prepared with HCl and NH3·H2O as catalysts, respectively, following the Stöber method, [44,45]. During the oxidation of MPTMS using hydrogen peroxide in an acidic aqueous solution, the sulfhydryl group in MPTMS was oxidized to a sulfonic acid group and the methoxy groups were hydrolyzed to hydroxyl groups, forming TPS (Figure 1C).
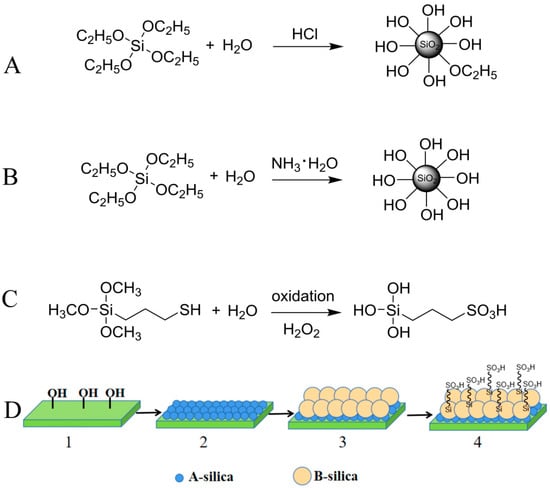
Figure 1.
Schematic of synthesis process: (A) A-silica, (B) B-silica, and (C) TPS, and (D) preparation process and structure of formed ABS-silica coating.
The cleaned glass slides, PET films, nylon fabrics, and medical cotton were immersed sequentially in the A-silica, B (or B′, B″)-silica, and TPS dispersions. The schematic of the sequential coating process and structure of the formed ABS-silica coating are shown in Figure 1D.
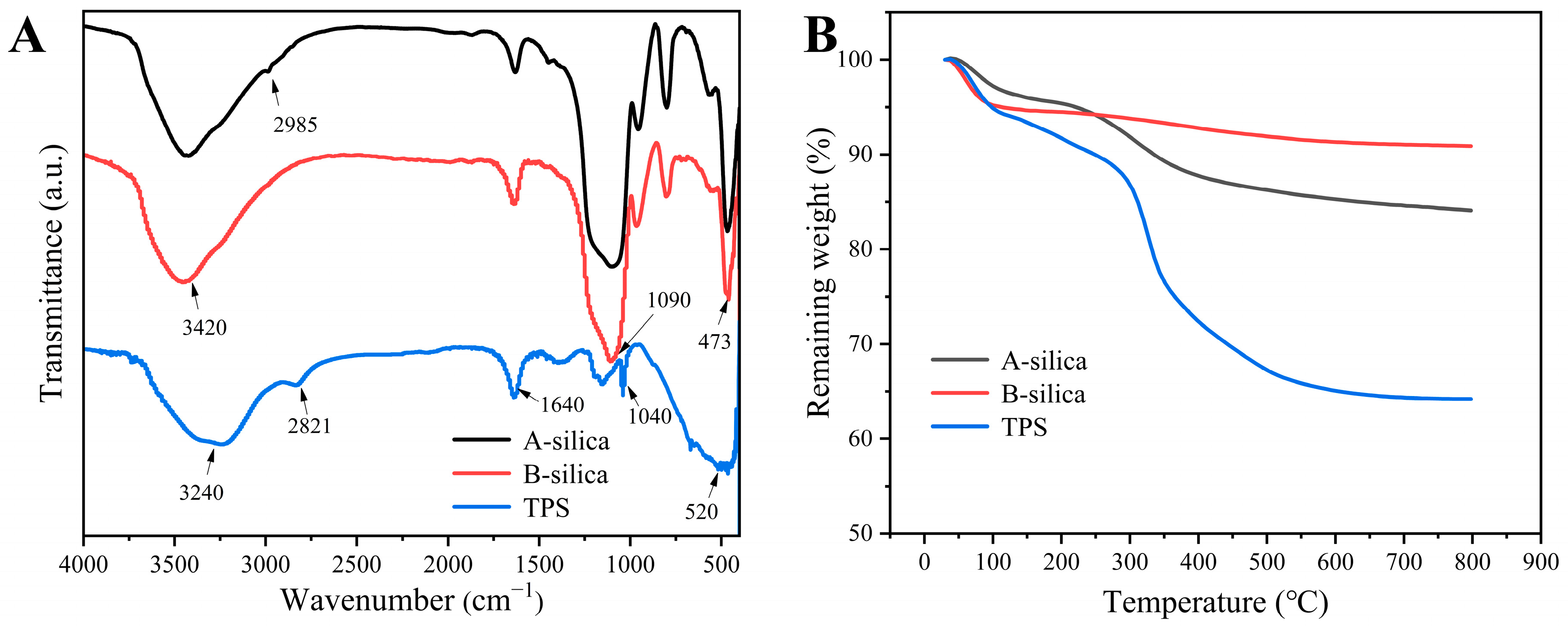
The FTIR spectra of the samples are presented in Figure 2A. The absorption bands at 1090 and 473 cm−1 in the A-silica and B-silica correspond to the stretching and bending vibrations of the Si-O-Si bonds, respectively [46,47]. The broad absorption band at ~3420 cm−1 is attributed to the stretching vibrations of Si-OH groups [48]. Under acidic conditions, the TEOS was not fully hydrolyzed, leaving a small amount of ethoxy groups, which results in a characteristic C-H absorption peak at 2985 cm−1 [49]. In contrast, the TEOS underwent complete hydrolysis under basic conditions. The absorption band at 1640 cm−1 in the A-silica, B-silica, and TPS is assigned to the bending vibration of O-H groups from the adsorbed water. For TPS, the absorption bands at 1040 and 520 cm−1 are characteristic of the -SO3H group [50]. Additionally, the absorption peak at 2821 cm−1 in TPS arises from the stretching vibration of the C-H bonds in the –CH2CH2SO3H group [51].
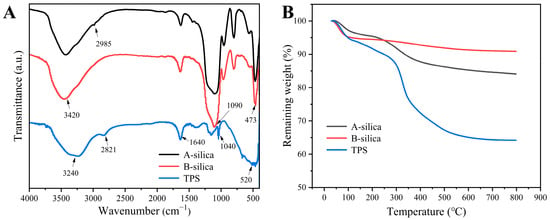
Figure 2.
(A) FTIR spectra and (B) TGA thermograms of the A-silica, B-silica, and TPS.
Figure 2B presents the TGA thermograms of the A-silica, B-silica, and TPS. The thermograms of the A-silica and B-silica reveal two distinct mass loss stages. In the range of 30–180 °C, the weight loss is primarily attributed to the evaporation of adsorbed moisture, with the A-silica losing 4.4% of its mass and the B-silica losing 5.5%. The slightly higher moisture content of the B-silica is likely due to its more complete hydrolysis and higher surface hydroxyl group density compared to the A-silica. The second stage, from 200 to 650 °C, is characterized by weight loss due to dehydration condensation and the detachment of ethoxy groups on the silica surface. The A-silica exhibits a weight loss of approximately 11.3%, compared to 3.7% for the B-silica. This notable difference is primarily attributed to the following mechanisms: First, the ethoxy groups in A-silica are more susceptible to chemical bond cleavage. Additionally, they have a higher molecular weight than hydroxyl groups. Second, the smaller particle size of A-silica particles provides a larger specific surface area, increasing the density of surface hydroxyl groups (–OH). Third, the high content of surface hydroxyl groups releases more H2O molecules during the high-temperature dehydration stage, resulting in a more substantial mass loss [52].
In contrast, the TGA thermogram of the TPS demonstrates notable differences. Between 30 and 180 °C, TPS shows a weight loss of approximately 7.6%, attributed to the hydrophilic nature of the sulfonic acid groups, which promote greater moisture adsorption. From 200 to 650 °C, the weight loss is approximately 26.8%, primarily due to the decomposition of the sulfonic acid group, hydrocarbon segments, and the dehydration condensation [53,54].
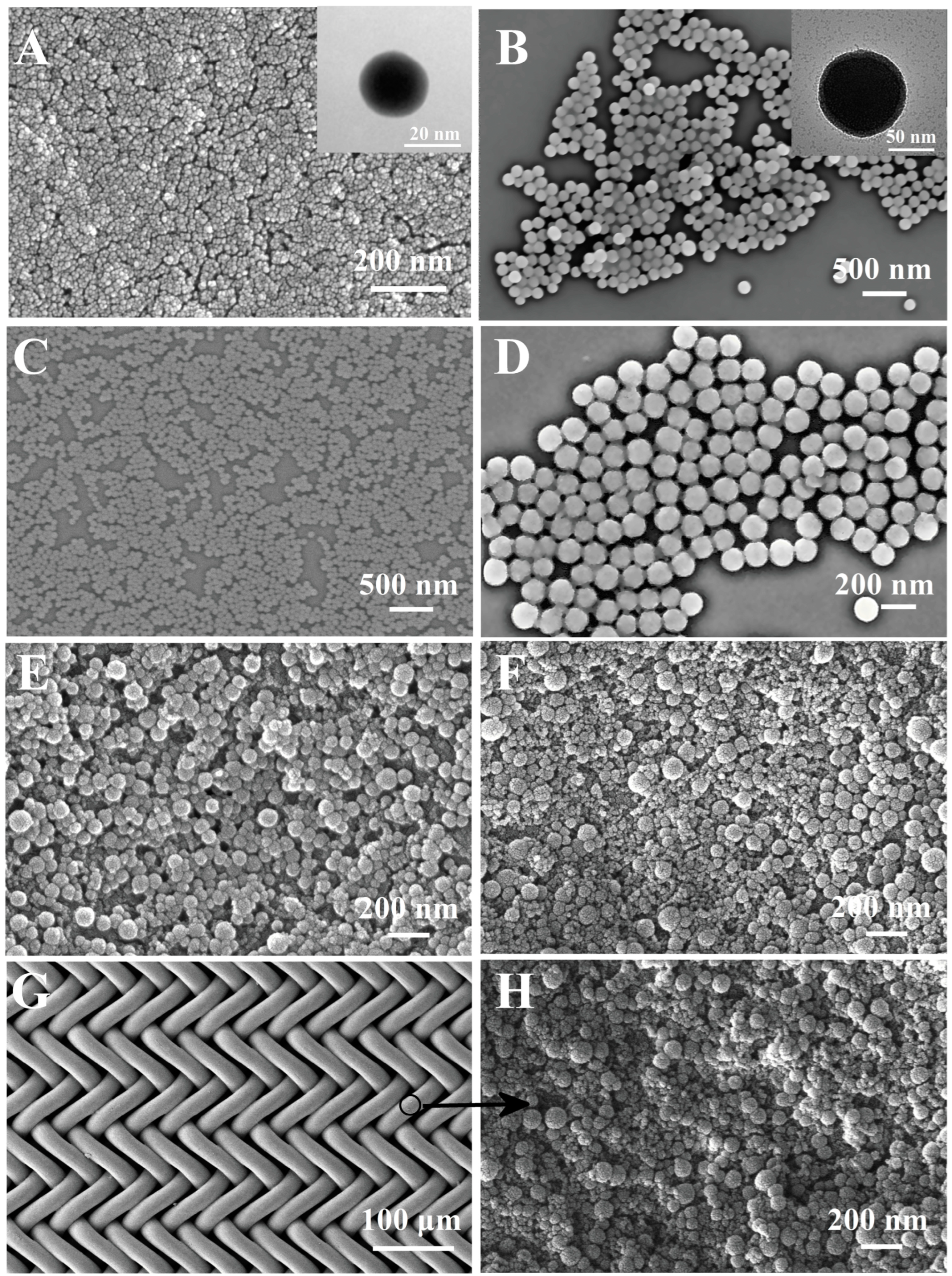
The SEM images of the A-silica and B-silica are shown in Figure 3A and Figure 3B, respectively. The A-silica coating exhibits a typical nanoscale porous structure, composed of spherical silica nanoparticles with diameters of ca. 20 nm, while the B-silica particles are much larger, having a diameter of ca. 100 nm. Due to its smaller particle size and high hydroxyl group density, the A-silica was applied as the bottom layer to enhance the adhesion between the substrate and the B-silica, which will be discussed in more detail later. The SEM images of the B′-silica and B″-silica are shown in Figure 3C and Figure 3D, respectively. The diameter of the B′-silica particles is ca. 65 nm, while the diameter of the B″-silica particles is ca. 150 nm. This observation suggests that the NH3·H2O catalyst concentration significantly influences the particle size of the silica formed via base catalysis; a lower catalyst content results in a corresponding decrease in silica particle size, which is consistent with earlier reports [55,56,57]. After coating B-silica on top of the A-silica layer, the surface roughness of the formed AB-silica coating was enhanced due to the larger particle size of B-silica, as shown in Figure 3E. TPS, known for its strong hydrophilicity, was coated as the top layer to endow the coating with superhydrophilic properties. Figure 3F demonstrates that after applying TPS onto the B-silica layer, the formed ABS-silica coating retains its high surface roughness.
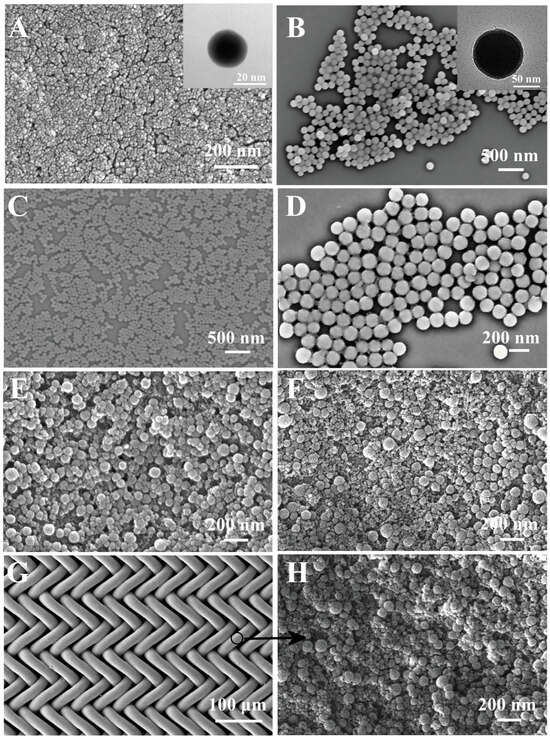
Figure 3.
(A,B) SEM images of A-silica (A) and B-silica (B); insets in (A,B) show corresponding TEM images. (C,D) SEM images of B′-silica (C) and B″-silica (D); (E) SEM images of AB-silica coating on glass slide. (F) SEM image of ABS-silica coating on glass slide. (G) SEM image of coated nylon fabric. (H) SEM image of nylon fabric coated with ABS-silica coating.
Figure 3G,H reveal that the nylon fabric, after being coated with the ABS-silica coating using the same sequential dip coating method, exhibits a surface morphology similar to that of the ABS-silica coating on cleaned glass slides. This result highlights the adaptability of the coating method to various substrates.
Using this sequential dip coating method, the first layer of the small A-silica particles enhances the adhesion to the substrate, the second layer of larger basic silica particles improves the surface roughness, and the third layer of TPS introduces polar hydrophilic groups, further increasing the substrate’s hydrophilicity [58]. Condensation reactions between each layer can improve the layer adhesion [59,60].
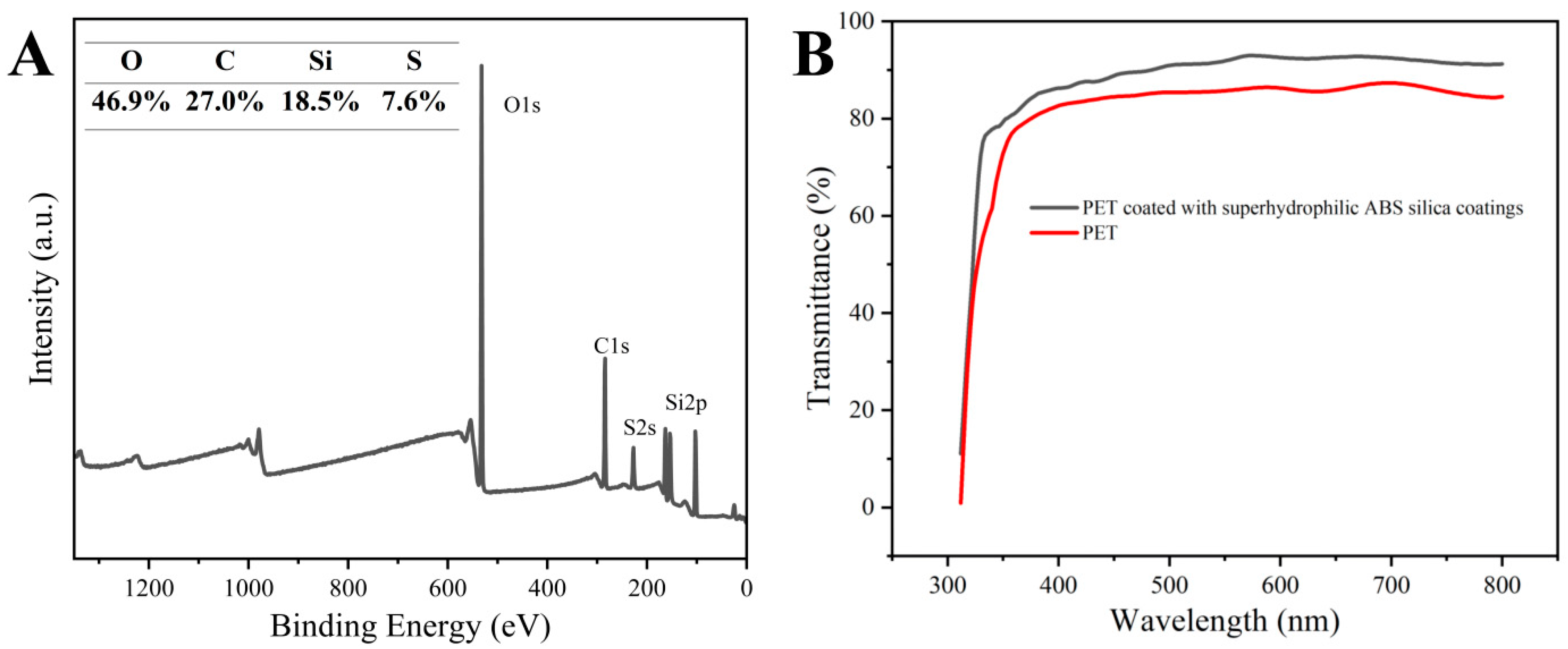
The presence of sulfur in the outer layer of the ABS-silica coatings was confirmed by the XPS analysis, as shown in Figure 4A. The XPS peaks at 533.1, 291.9, 229.0, and 103.8 eV correspond to O 1s, C 1s, S 2s, and Si 2p, respectively [61]. These results confirm the successful grafting of TPS onto the coating surface. The presence of sulfonic acid groups in the TPS on the coating surface enhances the surface polarity, a critical factor for achieving superhydrophilicity [62].
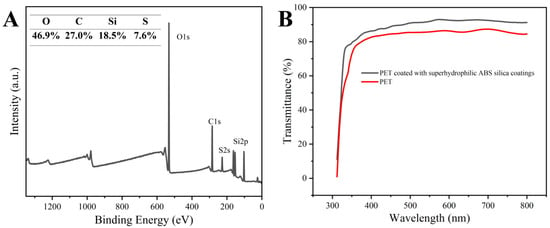
Figure 4.
(A) XPS survey spectrum of ABS-silica coatings and (B) transmittance of uncoated PET and PET coated with ABS-silica coating.
The UV-Vis transmittance spectra of the uncoated PET and the PET coated with the ABS-silica coating are presented in Figure 4B. The coated PET film exhibited a slightly higher transmittance compared to the uncoated PET, which can be attributed to the antireflection effect [63]. This result indicates that the ABS-silica coating enhances the optical clarity of the substrate, making it suitable for applications where high optical transparency is essential.
The ABS-silica coating demonstrated a stronger adhesion than the BS-silica coating, as evaluated according to ISO standard 2409 [64] (Table 1), which rates coating adhesion on a scale from 0 to 5, with 0 indicating the highest adhesion. The ABS-silica and BS-silica coatings received adhesion grades of 0 and 3, respectively, confirming the firm attachment of the ABS-silica coating to the glass substrate. Similarly, the wear damage analysis based on ASTM standard D4060 demonstrated that the ABS-silica coating exhibited a greater wear durability than the BS-silica coating [65]. After 300 friction cycles, the ABS-silica coating retained its excellent superhydrophilic properties, further confirming its strong wear resistance, whereas the BS-silica coating sustained only 50 friction cycles before significant degradation. This enhanced adhesion and durability are attributed to the smaller particle size and higher hydroxyl group density of A-silica, which promote stronger bonding with the glass substrate. Additionally, the effective interlayer bonding further improves the overall mechanical performance of the ABS-silica coating [66].

Table 1.
Properties of BS and ABS-silica coatings on glass slides.
A pencil hardness test was adopted to assess the hardness of the ABS-silica and BS-silica coatings, revealing a hardness level of 2H for both, as anticipated. The high hardness, along with the superior adhesion and durability of the ABS-silica coating, as discussed above, makes it highly advantageous for various surface-related applications.
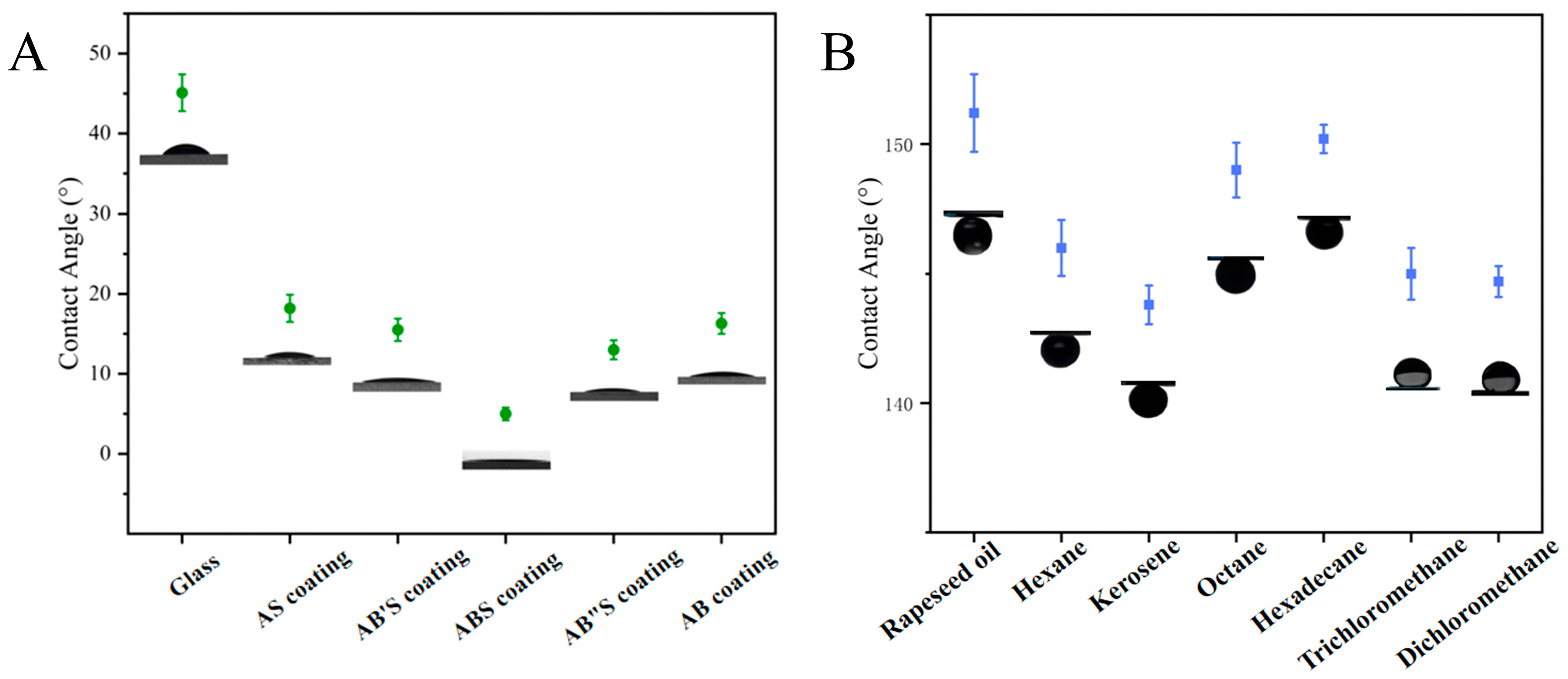
The wettability of the silica coating was systematically evaluated by measuring the static water contact angle and underwater oil contact angle. As shown in Figure 5A, the water contact angle of an uncoated glass slide was measured at 45.1° ± 2.3°, indicating its moderate wettability. In contrast, the static water contact angles of the AS-, AB′S-, ABS-, and AB″S-silica coatings were 18.2° ± 1.7°, 15.5° ± 1.4°, 5.0° ± 0.8°, and 13.0° ± 1.2°, respectively. This observed trend can be attributed to variations in the particle size of the silica layers, which were controlled by adjusting the concentration of the base catalyst, ammonium hydroxide (NH3·H2O). Specifically, for AS-, AB′S-, ABS-, and AB″S-silica coatings, the top silica layer consisted of particles with diameters of approximately 20 (A-silica), 65, 100, and 150 nm, respectively.
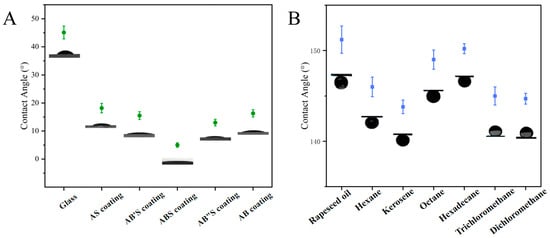
Figure 5.
(A) Water contact angles of the uncoated and coated glass slides and (B) underwater oil contact angles of the ABS-silica coating.
The AS-silica coating was formed by depositing A-silica particles with a diameter of 20 nm, resulting in a relatively smooth surface (Figure 3A). Such a smooth surface is suboptimal for achieving superhydrophilicity because it lacks the sufficient surface roughness to enhance the capillary action and promote rapid water spreading [53], leading to a relatively high contact angle of 18.2°. As the deposited silica particle size increased, the surface roughness also increased, which, combined with the inherently hydrophilic nature of silica, enhanced wettability and lowered the contact angle. The ABS-silica coating, which incorporated B-silica particles of ca. 100 nm, exhibited the lowest water contact angle (5.0°), indicating superhydrophilicity. However, when the particle size increased further to 150 nm in the AB″S-silica coating, the contact angle increased to 13.0°. This trend reversal can be explained by the Cassie–Baxter wetting state, where an excessively large surface roughness can lead to air entrapment, reducing the effective water spreading and thus increasing the contact angle [67].
To further confirm the role of the top TPS layer, a control AB-silica coating (without the top TPS layer) was fabricated and tested. This sample exhibited a water contact angle of 16.3° ± 1.3° (Figure 5A), demonstrating that the introduction of the TPS top layer is essential for achieving ultra-low contact angles. The TPS layer enhances hydrophilicity by providing additional polar functional groups that facilitate water spreading, further reducing the contact angle.
Figure 5B illustrates the underwater oil contact angles of the ABS-silica coating. Various oils, including n-hexane, n-octane, hexadecane, kerosene, rapeseed oil, dichloromethane, and trichloromethane, were tested, all yielding underwater oil contact angles exceeding 140.0°. These findings demonstrate that the ABS-silica coating possesses excellent hydrophilic and underwater oleophobic properties, achieving the desired superhydrophilic and underwater oleophobic performance.
Given that the ABS-silica coating demonstrates an optimal superhydrophilic and underwater oleophobic performance, along with superior durability, adhesion, and hardness, the following application demonstrations, including oil–water separation, anti-protein adhesion, and anti-fouling, were conducted exclusively on the ABS-silica coating.
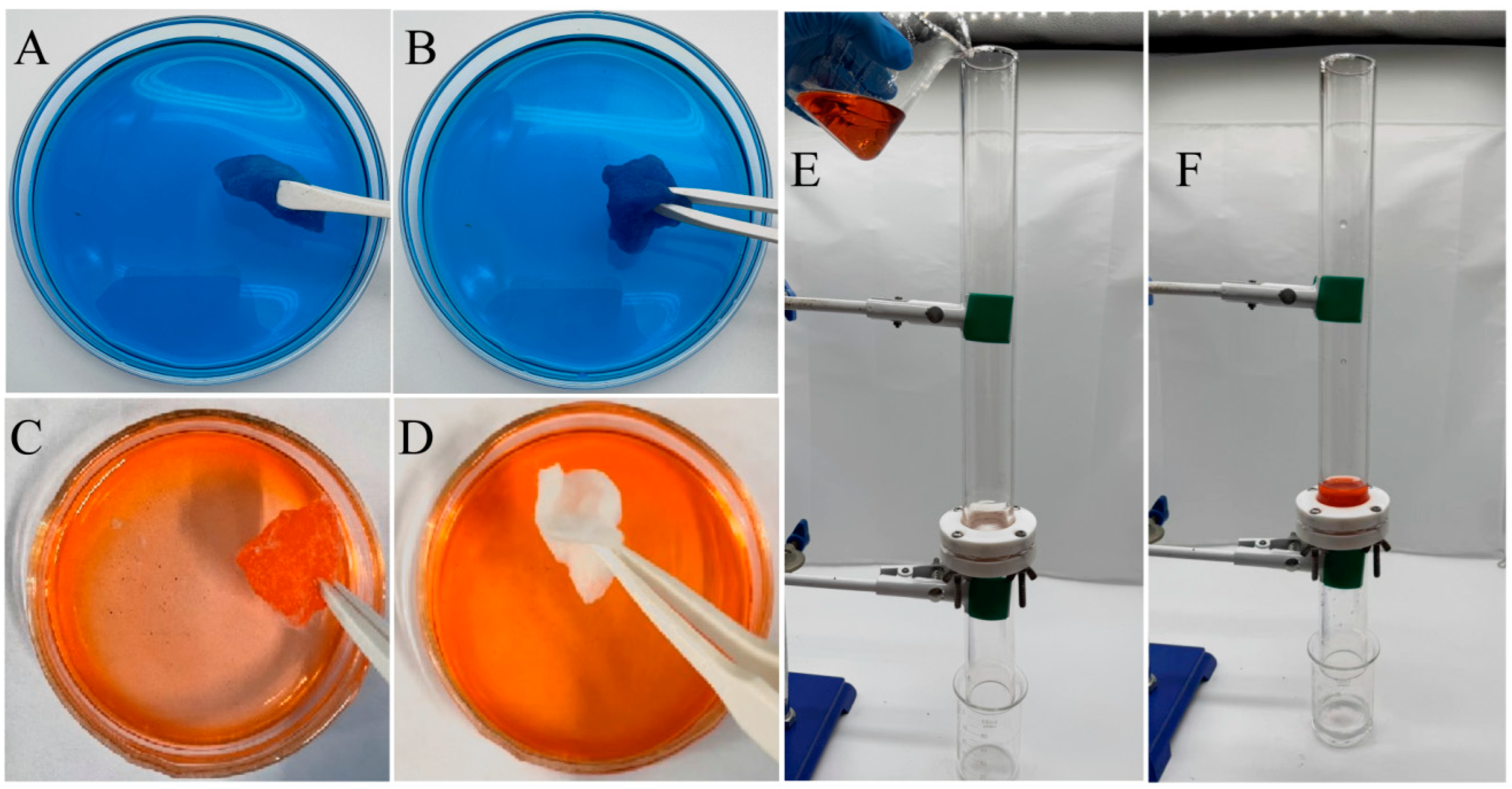
Figure 6A,B illustrate the soaking behavior of the untreated and ABS-silica-coated medical cotton in water, while Figure 6C,D depict their soaking behavior in an oil (n-hexadecane). These images highlight the water absorption and oil-repelling properties of the medical cotton following the ABS-silica coating treatment. Notably, Figure 6B shows the coated cotton immersed in a methylene blue-stained aqueous solution. The cotton absorbs water readily, becoming wet and stained with the blue dye, suggesting its strong water absorption capability. In Figure 6C,D, the untreated and ABS-silica-coated medical cotton were immersed in n-hexadecane dyed with Sudan III for 10 s, respectively. The untreated cotton became visibly wet with oil and stained by the dye, whereas the coated cotton remained clean and free from contamination after its removal from the solution. These tests clearly demonstrate that the superhydrophilic ABS-silica coating imparts cotton with remarkable superhydrophilic and superoleophobic properties, effectively repelling oil.
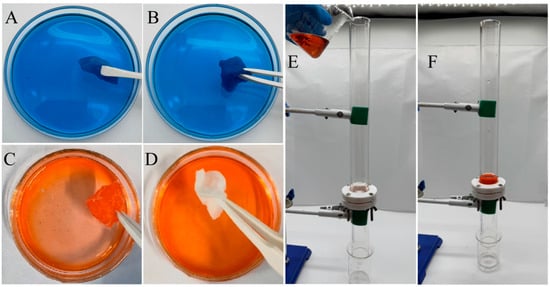
Figure 6.
(A,B) Untreated (A) and ABS-silica-coated (B) medical cotton soaked in aqueous solution; (C,D) untreated (C) and ABS-silica-coated (D) medical cotton soaked in oil solution; and (E,F) oil/water separation demonstration using nylon fabric treated with ABS-silica coating.
The oil/water separation performance testing and demonstration are presented in Figure 6E,F, using the nylon fabric treated with the superhydrophilic ABS-silica coating. As shown in Figure 6E, the oil/water mixture consisted of 20 mL of deionized water and 20 mL of n-hexane dyed red with Sudan III (Macklin Biochemical Co., Ltd., Shanghai, China). To achieve uniform mixing, the oil and water were mixed using ultrasonic vibration. During the test, the treated nylon fabric was securely clamped between two glass tubes. The oil/water mixture (1:1 by volume) was charged into the upper glass tube. Due to the superhydrophilic properties of the filter cloth, nearly all the water (20 mL) passed rapidly through the coated filter solely under gravity and was collected in the lower beaker. Simultaneously, almost all the n-hexane (20 mL) was retained above the coated filter. The water collected in the lower beaker was clear and colorless, with no trace of red n-hexane, indicating effective separation.
This remarkable performance is attributed to the superhydrophilicity and underwater superoleophobicity of the treated nylon filter. These properties create a hydration layer on the substrate surface, preventing n-hexane droplets from wetting or penetrating the filter. The results visually demonstrate the excellent oil/water separation capabilities of the nylon fabric treated with the superhydrophilic ABS-silica coating.
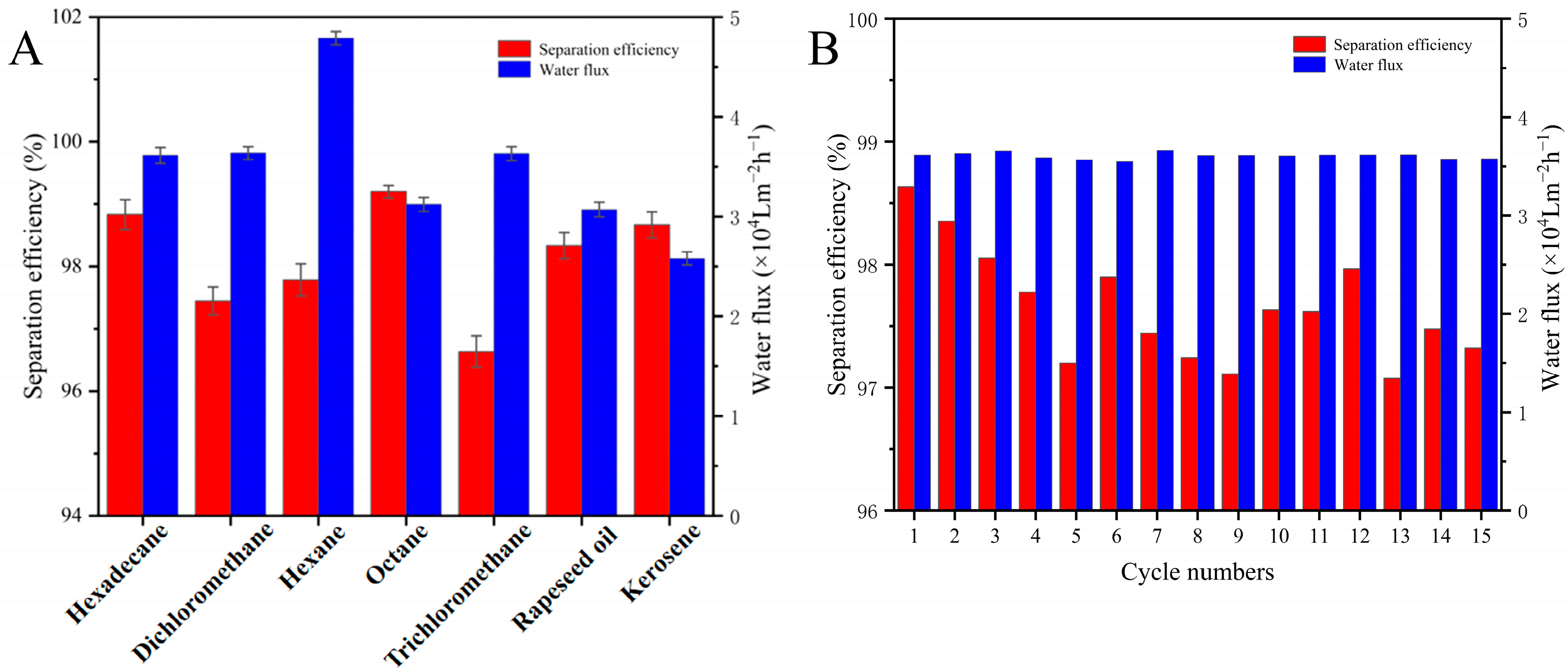
The separation efficiency, water flux, recyclability, and durability of the coated nylon fabric were systematically evaluated, with the results shown in Figure 7. As seen in Figure 7A, the nylon fabric treated with the superhydrophilic ABS-coating exhibited a separation efficiency exceeding 96.0% for various oil/water mixtures (vol/vol = 1:1), including hexadecane, dichloromethane, n-hexane, n-octane, trichloromethane, rapeseed oil, and kerosene, demonstrating an excellent separation performance and indicating its broad applicability in oil/water separation. The water flux for n-hexane reached 48,000 L·m−2·h−1.
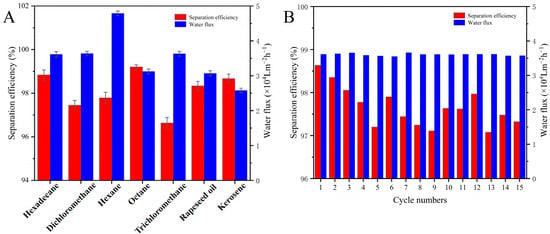
Figure 7.
(A) Separation efficiency of nylon fabric coated with superhydrophilic ABS-silica for various oil–water mixtures. (B) Separation efficiency of nylon fabric coated with superhydrophilic ABS-silica for hexadecane–water mixture during cycling experiments.
To test the recyclability and durability of the coated nylon fabric, multiple cycles of separation experiments were conducted, as shown in Figure 7B, using a hexadecane/water mixture as the sample. During the first separation of the hexadecane/water mixture, the treated nylon fabric achieved a separation efficiency of approximately 98.8%. After 15 cycles, the oil/water separation efficiency remained above 96.0%. These results demonstrate that the superhydrophilic ABS-silica coated nylon fabric not only provides an excellent oil/water separation efficiency, exceeding 96.0%, but is also recyclable, significantly enhancing its practicality.
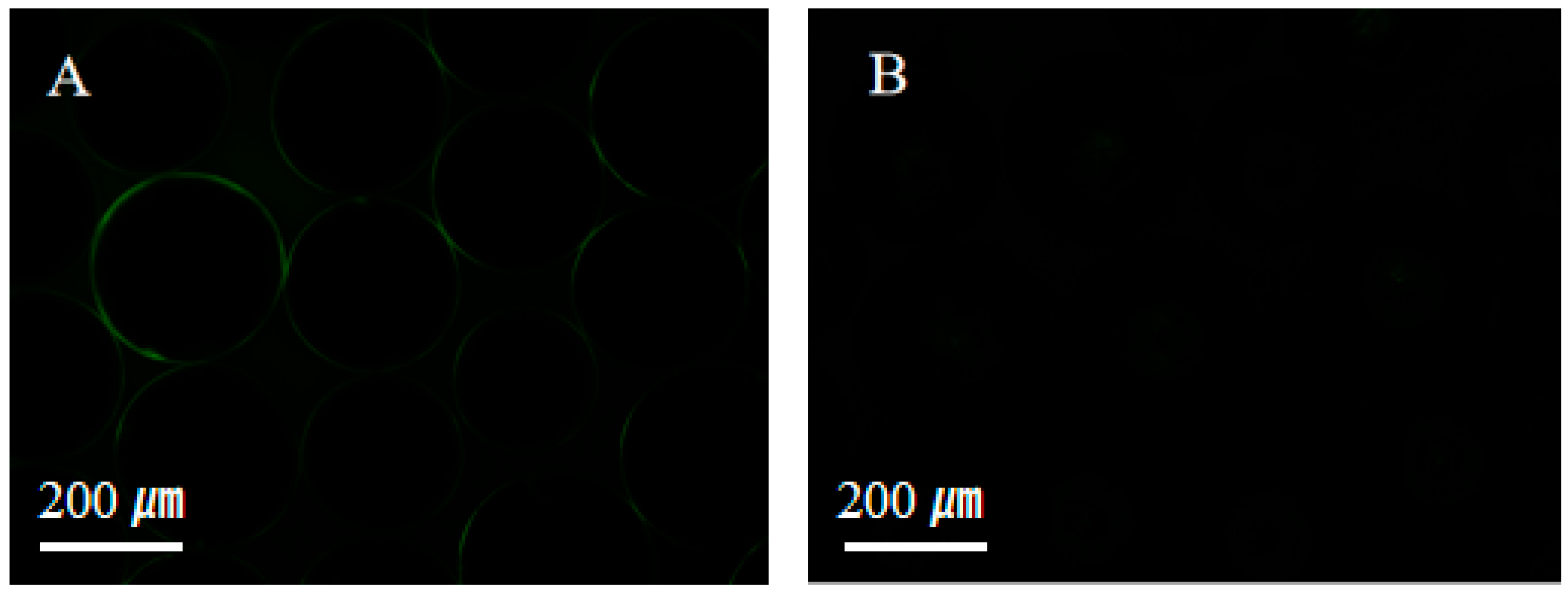
The resistance to protein adsorption was evaluated to assess the anti-fouling properties of the superhydrophilic ABS-silica coating, as shown in Figure 8. Bovine serum albumin labeled with fluorescein isothiocyanate (BSA-FITC) was used as a model biofoulant [68]. Uncoated glass beads readily adsorbed BSA-FITC, exhibiting strong fluorescence signals (Figure 8A). In contrast, glass beads coated with the superhydrophilic ABS-silica coatings showed a significantly reduced protein adsorption, with minimal fluorescence observed. This result demonstrates that the superhydrophilic ABS-silica coating effectively resists protein adsorption, highlighting its anti-fouling capability.
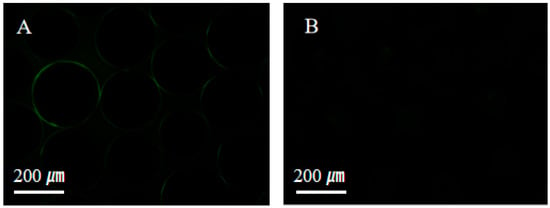
Figure 8.
Fluorescence microscopy images of (A) uncoated glass beads and (B) glass beads coated with the superhydrophilic ABS-silica coating.
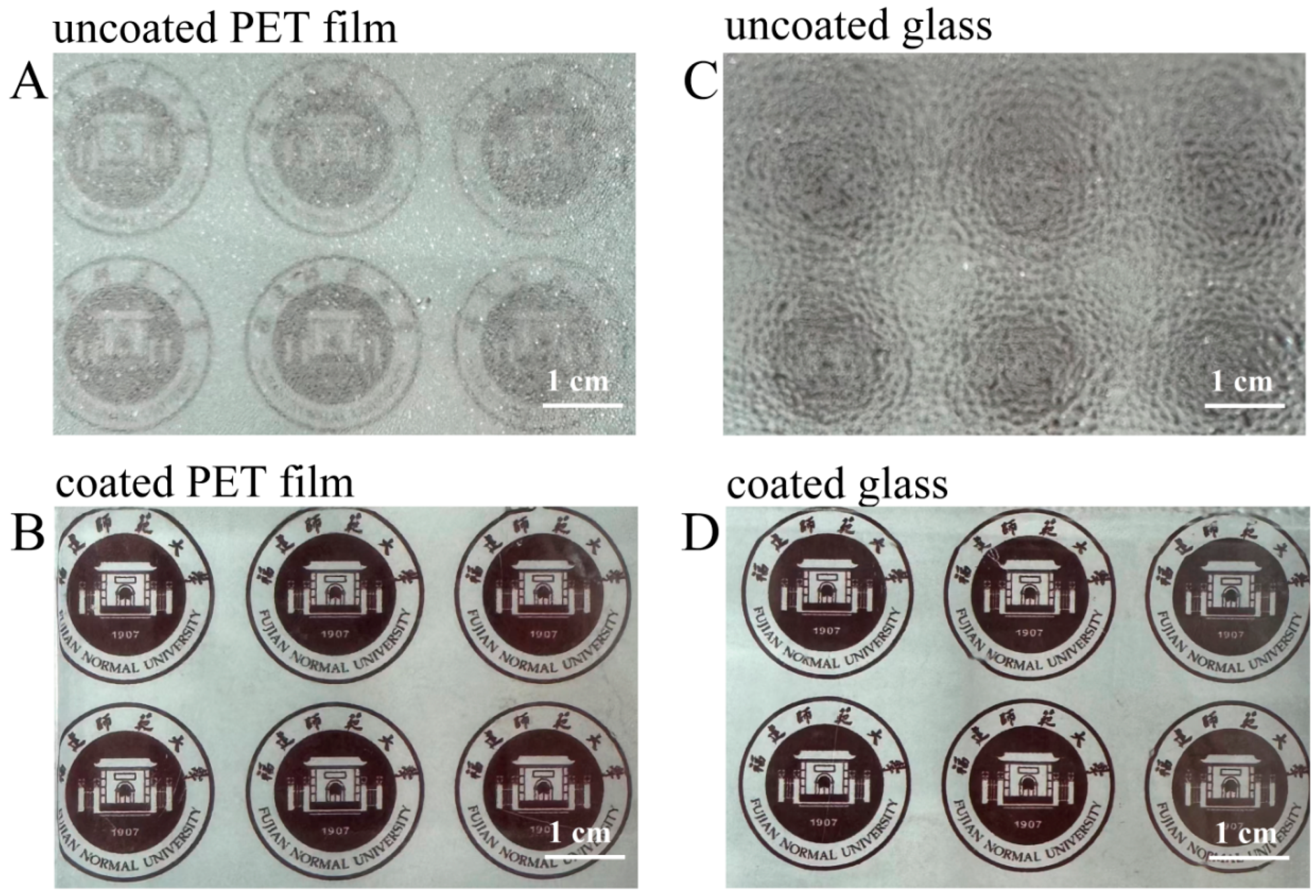
The anti-fogging properties of the superhydrophilic ABS-silica coatings were evaluated using the PET film and glass slide as substrates. The uncoated and coated substrates were placed above hot water, and their light transparency was assessed after an exposure to water vapor for 10 s (Figure 9). On the uncoated PET film and glass surfaces, a fog layer formed, rendering the surfaces opaque. In contrast, the coated PET film and glass slides maintained high transparency under the same conditions. This outcome can be attributed to the superior hydrophilic nature of the superhydrophilic ABS-silica coating, which prevents fog formation by promoting a uniform water film dispersion [69].
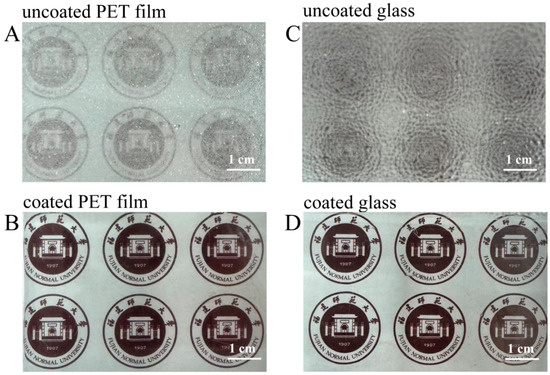
Figure 9.
Photos from anti-fogging tests: (A,B) PET film substrate and (C,D) glass slide substrate.
3. Materials and Methods
3.1. Materials
Tetraethyl orthosilicate (TEOS, 99%) and 3-mercaptopropyl trimethoxy silane (MPTMS, 99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrogen peroxide (H2O2, 30%), nitric acid (HNO3), ethanol (99.5%), ammonium hydroxide (NH3·H2O, 25 wt. % in water), and dichloromethane (CH2Cl2) were sourced from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The nylon fabric, supplied by Hengyi Petrochemical Co., Ltd. (Hangzhou, China), has a thickness of 0.4 mm. Medical cotton was sourced from Johnson & Johnson (New Brunswick, NJ, USA). Microscope slides (75 mm × 25 mm) were supplied by VWR International, LLC (Radnor, PA, USA). Polyethylene terephthalate (PET) films with a thickness of 22 μm were obtained from Toyobo Plastics Co., Ltd. (Osaka, Japan). Fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (BSA) (BSA-FITC) was supplied by Arbio Technology Co., Ltd. (Beijing, China).
3.2. Preparation of Superhydrophilic Silica Coating via Sequential Dipping
Acid-catalyzed silica (A-silica) was synthesized using HCl as the catalyst through a sol–gel process following the Stöber method [70]. Specifically, 7.20 g of deionized water, 20.00 g of TEOS, 200.00 g of absolute ethanol, and 10.00 g of HCl (1.00 wt. %) were mixed and stirred for 10 min. The solution was then kept stirring at 65 °C for 4 h to prepare A-silica. Base-catalyzed silica was synthesized using NH3·H2O as the catalyst through a sol–gel process following the Stöber method [47,71,72]. Specifically, to prepare base-catalyzed silica with different particle sizes, 1.00 g of deionized water, 1.00 g of TEOS, and 10.00 g of anhydrous ethanol were mixed with 0.50, 1.00, and 2.00 g of ammonium hydroxide solution, respectively, and stirred at 45 °C for 4 h to obtain B′-silica, B-silica, and B″-silica, respectively. 3-(Trihydroxysilyl)propanesulfonic acid (TPS) was prepared by oxidizing MPTMS with hydrogen peroxide under acidic conditions. In this process, 1.00 g of MPTMS was mixed with 3.00 g of deionized water and ultrasonicated for 20 min. Next, 6.00 g of hydrogen peroxide solution (H2O2, 30%) was slowly added to the MPTMS/water mixture at room temperature under mechanical stirring [73]. Hydrochloric acid was then used to adjust the pH of the solution to 6.0, and the mixture was stirred continuously for 24 h to complete the oxidation, resulting in a transparent TPS solution. The resulting A-silica, B-silica (and B′-silica and B″-silica), and TPS were diluted to 0.5 wt. % in ethanol for coating.
Prior to coating, the glass slides were treated with piranha solution (concentrated sulfuric acid/hydrogen peroxide = 7:3) for 30 s, followed by thorough rinsing with deionized water (2–3 times) and natural drying [74]. This treatment introduces hydroxyl groups on the surface, ensuring effective modification. The cleaned glass slides, PET films, nylon fabrics, and medical cotton were sequentially immersed in A-silica, B-silica (or B′-silica, B″-silica), and TPS dispersions for 5 s each. After immersion, the substrates were withdrawn at a rate of ca. 80 mm/min and dried at 80 °C for 2 min after each coating cycle. The first layer of coating, consisting of A-silica, was designated as the A-silica coating. After drying the A-silica coating, a second layer of B-silica was applied, resulting in the AB silica coating (i.e., A-silica + B-silica). Finally, after the AB-silica coating was dried, a third layer of TPS was applied, yielding the ABS-silica coating (i.e., A-silica + B-silica + TPS).
Moreover, a series of control samples were prepared for comparison purposes, including AB-silica coating (i.e., A-silica + B-silica), AS-silica (i.e., A-silica + TPS), BS-silica coating (i.e., B-silica + TPS), AB′S silica coating (i.e., A-silica + B′-silica + TPS), and AB″S silica coating (i.e., A-silica + B″-silica + TPS).
3.3. Characterization
Contact angles (CAs) were measured using a drop shape analyzer (DSA-25, Kruss Scientific Instrument Co., Hamburg, Germany) at room temperature, with 5 μL droplets of deionized water or hexadecane placed on the coating surface [74,75]. For water contact angle measurements in air, the water droplet was directly applied to the substrate. For underwater oil wetting performance characterization, the substrates were immersed in a transparent quartz container filled with water [76]. The CAs were determined by averaging ten measurements from ten different locations on the coating surface.
Fourier-transform infrared (FTIR) spectroscopy was performed using a Nicolet 577 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with the KBr pellet method. The A-silica, B-silica, and TPS were ground separately, mixed with KBr, and pressed into transparent pellets.
The surface morphologies of the coatings were characterized by field emission scanning electron microscopy (FE-SEM, JEOL 7800F, Tokyo, Japan). Transmission electron microscopy (TEM, JEOL JEM-2100F, Tokyo, Japan) was used to observe the morphology of individual silica nanoparticles.
The A-silica and B-silica samples were freeze-dried and then placed in a vacuum oven at 60 °C to dry. The samples were uniformly spread as a thin layer at the bottom of a crucible. The thermogravimetric analysis (TGA) was conducted at a heating rate of 10 °C/min under a nitrogen atmosphere with a flow rate of 50 mL/min using a Q600 thermal analyzer (TA Instruments, New Castle, DE, USA).
Elemental analysis was performed using X-ray photoelectron spectroscopy (XPS) to analyze the chemical compositions of the ABS-silica coating.
The light transmittance of the PET film coated with the ABS-silica coating was measured using a UV-1750 ultraviolet-visible spectrophotometer (Shimadzu Corporation, Kyoto, Japan), with the wavelength range set from 300 to 800 nm.
Film hardness was measured according to ASTM D3363 standard using pencil hardness testing to assess coating durability [77]. Adhesion was evaluated using the ISO 2409 standard with the tape adhesion test, where a defined grid was cut into the coating, and a 3M 810 tape was applied and peeled off at a 60° angle [64]. Wear resistance was determined using ASTM standard D4060-10, employing a CS-10 abrader at a friction speed of 60 rpm and a 100 g weight load to assess the abrasion resistance of the ABS-silica coating [65].
3.4. Oil–Water Separation Test
The oil–water separation experiment was conducted using an oil–water separation device composed of two transparent glass tubes (diameter: 3.15 cm), with a nylon fabric coated with the ABS-silica coating clamped between them. First, the coated nylon fabric was immersed in deionized water to ensure it was thoroughly wetted. The wetted nylon fabric was then secured between the two glass tubes. Finally, the oil–water separation glass tubes were fixed onto a ring stand to conduct the oil–water separation experiment. A mixture of oil and water, with Sudan III dye added to the oil for visual tracking, was placed at the top of the test tube. The separation of oil and water occurred under the influence of gravity, allowing the process to be visually observed.
The efficiency of oil–water separation was calculated using the following formula:
where m0 is the mass of the initial water, and m1 is the mass of the water collected after oil/water separation.
3.5. Anti-Protein Adsorption Test
The anti-protein adsorption performance of the ABS-silica coating was evaluated using a fluorescence inverted microscope (ECLIPSE Ts2R-FL, Nikon Corporation, Tokyo, Japan). Fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (BSA-FITC) was employed as the detection substance to assess the protein anti-adsorption characteristics. The coated glass beads were immersed in a 0.10 mg/mL solution of BSA-FITC and incubated in the dark at 25 °C for 30 min. After incubation, the beads were washed three times with PBS solution to remove any unbound excess protein. The fluorescence intensity of the adsorbed fluorescent protein on the glass bead surfaces was then observed using the inverted fluorescence microscope, with a 385 nm light as the excitation source, to assess the anti-protein performance of the coating [78,79].
3.6. Anti-Fogging Test
The glass slides and PET films, with one half coated with the ABS-silica coating and the other half uncoated, were placed above boiling water (100 °C) to observe the anti-fogging effect on both the coated and uncoated sides. By comparing the fogging intensity under the same conditions, the performance of the ABS-silica coating in preventing fog was evaluated.
4. Conclusions
Silica particles of varying sizes were synthesized using hydrochloric acid and ammonium hydroxide as hydrolysis catalysts. A superhydrophilic ABS-silica coating with a rough nanostructured surface was then fabricated by sequentially depositing A-silica, B-silica, and TPS onto the substrate. This coating exhibited an excellent adhesion, wear durability, and hardness. The ABS-silica coating achieved a remarkably low static water contact angle of just 5°, while its underwater oil contact angle exceeded 140.0° for all tested oils, confirming its superior hydrophilicity and underwater oleophobicity.
When applied to the nylon fabric, the superhydrophilic ABS-silica coating demonstrated an exceptional oil–water separation efficiency, exceeding 96%. Furthermore, it maintained this high efficiency even after 15 separation cycles, highlighting its durability and recyclability. In addition to the oil–water separation, the ABS-silica coating exhibited a strong resistance to protein adsorption and excellent anti-fogging properties. Overall, the ABS-silica coating holds great promise for applications in oil–water separation, anti-fouling, and anti-fogging technologies.
Author Contributions
J.X.: Writing—original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. A.L.: Writing—original draft, Formal analysis, Methodology, Conceptualization. Q.L.: Data curation. N.C.: Formal analysis. A.A.: Visualization, Methodology, Data curation. X.L.: Writing—review and editing, Supervision, Resources, Methodology, Formal analysis, Conceptualization. R.J.: Writing—review and editing, Supervision, Resources, Methodology, Formal analysis, Conceptualization. L.S.: Writing—review and editing, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. F.D.: Writing—review and editing, Visualization, Supervision, Resources, Formal analysis, Data curation, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
The Fujian Provincial Industry-University Corporation Research Project (Grant no. 2022H6023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
F.D. acknowledges the financial support by the Fujian Provincial Industry-University Corporation Research Project (Grant no. 2022H6023).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Superhydrophilic (superwetting) surfaces: A review on fabrication and application. J. Ind. Eng. Chem. 2017, 47, 19–40. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, C.; Zuhlke, C.; Alexander, D.; Francisco, J.S.; Zeng, X.C. Turning a Superhydrophilic Surface Weakly Hydrophilic: Topological Wetting States. J. Am. Chem. Soc. 2020, 142, 18491–18502. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Bioinspired, roughness-induced, water and oil super-philic and super-phobic coatings prepared by adaptable layer-by-layer technique. Sci. Rep. 2015, 5, 14030. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Wen, L.; Tian, Y.; Jiang, L. Bioinspired Super-Wettability from Fundamental Research to Practical Applications. Angew. Chem. Int. Ed. 2015, 54, 3387–3399. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.N.; Wu, S.Z.; Chen, Q.D.; Zhao, S.; Zhang, H.; Sun, H.B.; Jiang, L. Three-Level Biomimetic Rice-Leaf Surfaces with Controllable Anisotropic Sliding. Adv. Funct. Mater. 2011, 21, 2927–2932. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired Multifunctional Foam with Self-Cleaning and Oil/Water Separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Xiong, L.; Guo, W.; Alameda, B.M.; Sloan, R.K.; Walker, W.D.; Patton, D.L. Rational Design of Superhydrophilic/Superoleophobic Surfaces for Oil–Water Separation via Thiol–Acrylate Photopolymerization. ACS Omega 2018, 3, 10278–10285. [Google Scholar]
- Kuo, C.-S.; Tseng, Y.-H.; Li, Y.-Y. Wettability and Superhydrophilic TiO2 Film Formed by Chemical Vapor Deposition. Chem. Lett. 2006, 35, 356–357. [Google Scholar] [CrossRef]
- Simić, L.; Stopic, S.; Friedrich, B.; Zadravec, M.; Jelen, Ž.; Bobovnik, R.; Anžel, I.; Rudolf, R. Synthesis of Complex Concentrated Nanoparticles by Ultrasonic Spray Pyrolysis and Lyophilisation. Metals 2022, 12, 1802. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, D.; Li, T.; Zhou, Q.; Shen, L.; Li, R.; Xu, Y.; Lin, H. Facile fabrication of superhydrophilic nanofiltration membranes via tannic acid and irons layer-by-layer self-assembly for dye separation. Appl. Surf. Sci. 2020, 515, 146063. [Google Scholar] [CrossRef]
- Liu, X.; He, J. Hierarchically structured superhydrophilic coatings fabricated by self-assembling raspberry-like silica nanospheres. J. Colloid Interface Sci. 2007, 314, 341–345. [Google Scholar] [CrossRef]
- Yu, E.; Kim, S.-C.; Lee, H.J.; Oh, K.H.; Moon, M.-W. Extreme wettability of nanostructured glass fabricated by non-lithographic, anisotropic etching. Sci. Rep. 2015, 5, 9362. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.V.M.; Silva, A.S.; Melo, G.F.S.; Vasconscellos, L.M.R.; Marciano, F.R.; Lobo, A.O. Influence of low contents of superhydrophilic MWCNT on the properties and cell viability of electrospun poly (butylene adipate-co-terephthalate) fibers. Mater. Sci. Eng. C 2016, 59, 782–791. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Ghorbani, F.; Amini, M.; Naslhajian, H. Polyoxometalate based thin film nanocomposite forward osmosis membrane: Superhydrophilic, anti-fouling, and high water permeable. J. Colloid Interface Sci. 2019, 536, 328–338. [Google Scholar] [CrossRef]
- Polakiewicz, A.; Dodiuk, H.; Kenig, S. Super-hydrophilic coatings based on silica nanoparticles. J. Adhes. Sci. Technol. 2014, 28, 466–478. [Google Scholar] [CrossRef]
- Bu, X.; Li, J.; Yang, S.; Sun, J.; Deng, Y.; Yang, Y.; Wang, G.; Peng, Z.; He, P.; Wang, X.; et al. Surface Modification of C3N4 through Oxygen-Plasma Treatment: A Simple Way toward Excellent Hydrophilicity. ACS Appl. Mater. Interfaces 2016, 8, 31419–31425. [Google Scholar] [CrossRef]
- Joo, H.; Park, J.; Sutthiwanjampa, C.; Kim, H.; Bae, T.; Kim, W.; Choi, J.; Kim, M.; Kang, S.; Park, H. Surface Coating with Hyaluronic Acid-Gelatin-Crosslinked Hydrogel on Gelatin-Conjugated Poly(dimethylsiloxane) for Implantable Medical Device-Induced Fibrosis. Pharmaceutics 2021, 13, 269. [Google Scholar] [CrossRef]
- Ding, F.; Liu, J.; Zeng, S.; Xia, Y.; Wells, K.M.; Nieh, M.-P.; Sun, L. Biomimetic nanocoatings with exceptional mechanical, barrier, and flame-retardant properties from large-scale one-step coassembly. Sci. Adv. 2017, 3, e1701212. [Google Scholar] [CrossRef]
- Liu, J.; Chavez, S.E.; Ding, H.; Farooqui, M.M.; Hou, Z.; Lin, S.; D’Auria, T.D.; Kennedy, J.M.; LaChance, A.M.; Sun, L. Ultra-transparent nanostructured coatings via flow-induced one-step coassembly. Nano Mater. Sci. 2022, 4, 97–103. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Williams, B.L.; Hou, Z.; Bodin, J.N.; Lofink, B.J.; Santos, V.H.; Becher, E.M.; Shrestha, S.B.; Nasir, Z.; et al. Enhancing flame retardancy of flexible polyurethane foams through one-step coassembled nanocoatings. Adv. Compos. Hybrid Mater. 2024, 7, 230. [Google Scholar] [CrossRef]
- Xue, Y.; LaChance, A.M.; Liu, J.; Farooqui, M.; Dabaghian, M.D.; Ding, F.; Sun, L. Polyvinyl alcohol/α-zirconium phosphate nanocomposite coatings via facile one-step coassembly. Polymer 2023, 265, 125580. [Google Scholar] [CrossRef]
- Williams, B.L.; Ding, H.; Hou, Z.; Paul, P.O.; Lewis, F.A.; Smith, A.T.; Sun, L. Highly efficient polyvinyl alcohol/montmorillonite flame retardant nanocoating for corrugated cardboard. Adv. Compos. Hybrid Mater. 2021, 4, 662–669. [Google Scholar] [CrossRef]
- Chavez, S.E.; Ding, H.; Williams, B.L.; Nam, S.; Hou, Z.; Zhang, D.; Sun, L. One-step Coassembled Nanocoatings on Paper for Potential Packaging Applications. ES Mater. Manuf. 2022, 15, 72–77. [Google Scholar] [CrossRef]
- Smith, A.T.; Shen, K.; Hou, Z.; Zeng, S.; Jin, J.; Ning, C.; Zhao, Y.; Sun, L. Dual Photo and Mechanochromisms of Graphitic Carbon Nitride/Polyvinyl Alcohol Film. Adv. Funct. Mater. 2021, 32, 2110285. [Google Scholar] [CrossRef]
- LaChance, A.M.; Hou, Z.; Farooqui, M.M.; Carr, S.A.; Serrano, J.M.; Odendahl, C.E.; Hurley, M.E.; Morrison, T.E.; Kubachka, J.L.; Samuels, N.T.; et al. Doctor-Blade-Assisted Casting for Forming Thin Composite Coatings of Montmorillonite and Poly(vinyl alcohol). Ind. Eng. Chem. Res. 2022, 61, 3766–3774. [Google Scholar] [CrossRef]
- LaChance, A.M.; Hou, Z.; Farooqui, M.M.; Samuels, N.T.; Carr, S.A.; Serrano, J.M.; Odendahl, C.E.; Hurley, M.E.; Morrison, T.E.; Kubachka, J.L.; et al. Spin Coating for Forming Thin Composite Coatings of Montmorillonite and Poly(vinyl alcohol). Ind. Eng. Chem. Res. 2022, 61, 4168–4177. [Google Scholar] [CrossRef]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Afshar, A.; Ghorbani, M.; Saeri, M.R. Adsorption/Desorption Phenomena of Plasma Sprayed Hydroxyapatite Coating Surface during Immersion in the Ca-Free SBF. J. Ceram. Soc. Jpn. 2004, 112, 77–81. [Google Scholar] [CrossRef]
- Hill, D.; Barron, A.R.; Alexander, S. Controlling the wettability of plastic by thermally embedding coated aluminium oxide nanoparticles into the surface. J. Colloid Interface Sci. 2020, 567, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Soike, T.; Streff, A.K.; Guan, C.; Ortega, R.; Tantawy, M.; Pino, C.; Shastri, V.P. Engineering a Material Surface for Drug Delivery and Imaging using Layer-by-Layer Assembly of Functionalized Nanoparticles. Adv. Mater. 2010, 22, 1392–1397. [Google Scholar] [CrossRef]
- Budy, S.M.; Hamilton, D.J.; Cai, Y.; Knowles, M.K.; Reed, S.M. Polymer mediated layer-by-layer assembly of different shaped gold nanoparticles. J. Colloid Interface Sci. 2017, 487, 336–347. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES–TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef]
- Xu, L.; He, J. Antifogging and Antireflection Coatings Fabricated by Integrating Solid and Mesoporous Silica Nanoparticles without Any Post-Treatments. ACS Appl. Mater. Interfaces 2012, 4, 3293–3299. [Google Scholar] [CrossRef] [PubMed]
- Arturi, K.R.; Jepsen, H.; Callsen, J.N.; Søgaard, E.G.; Simonsen, M.E. Superhydrophilicity and durability of fluoropolymer-TiO2 coatings. Prog. Org. Coat. 2016, 90, 132–138. [Google Scholar] [CrossRef]
- Du, X.; Li, X.; He, J. Facile Fabrication of Hierarchically Structured Silica Coatings from Hierarchically Mesoporous Silica Nanoparticles and Their Excellent Superhydrophilicity and Superhydrophobicity. ACS Appl. Mater. Interfaces 2010, 2, 2365–2372. [Google Scholar] [CrossRef]
- Aghdam, A.S.; Ghorbani, M.; Deprem, G.; Cebeci, F.Ç.; Koşar, A. A New Method for Intense Cavitation Bubble Generation on Layer-by-Layer Assembled SLIPS. Sci. Rep. 2019, 9, 11600. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Merlano, A.S.; Hoyos Palacio, L.M.; Cacua, K.; Rudas, J.S.; Meneses Munera, S.; Vázquez-Fletes, R.C.; Cornelio, J.A.C. Facile chemical vapor deposition (CVD) method for synthesis of high purity carbon nano-onions. Fuller. Nanotub. Carbon Nanostructures 2025, 33, 171–177. [Google Scholar] [CrossRef]
- Kim, T.H.; Song, K.C. Low-temperature preparation of superhydrophilic coatings using tetraethoxysilane and colloidal silica by sol-gel method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129105. [Google Scholar] [CrossRef]
- Okkay, H.; Satı, S.; Cengiz, U. Mechanically stable superhydrophilic antifog surface by microwave assisted sol-gel method. J. Taiwan Inst. Chem. Eng. 2021, 120, 360–367. [Google Scholar] [CrossRef]
- Liao, Z.; Wu, G.; Lee, D.; Yang, S. Ultrastable Underwater Anti-Oil Fouling Coatings from Spray Assemblies of Polyelectrolyte Grafted Silica Nanochains. ACS Appl. Mater. Interfaces 2019, 11, 13642–13651. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, H.; Fei, T.; Zhang, J. Highly transparent superhydrophobic organic–inorganic nanocoating from the aggregation of silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 51–62. [Google Scholar] [CrossRef]
- Nakamura, M.; Ozaki, S.; Abe, M.; Doi, H.; Matsumoto, T.; Ishimura, K. Size-controlled synthesis, surface functionalization, and biological applications of thiol-organosilica particles. Colloids Surf. B Biointerfaces 2010, 79, 19–26. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Kavale, M.S.; Mukherjee, S.K.; Rao, A.V. Transparent Superhydrophobic silica coatings on glass by sol–gel method. Appl. Surf. Sci. 2010, 257, 333–339. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Y.; Song, C.; Li, Y.; Jiang, B. A simple sol-gel method to prepare superhydrophilic silica coatings. Mater. Lett. 2017, 188, 316–318. [Google Scholar] [CrossRef]
- Guo, C.; Ding, H.; Xie, M.; Zhang, H.; Hong, X.; Sun, L.; Ding, F. Multifunctional superamphiphobic fluorinated silica with a core-shell structure for anti-fouling and anti-corrosion applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126155. [Google Scholar] [CrossRef]
- Kohns, R.; Meyer, R.; Wenzel, M.; Matysik, J.; Enke, D.; Tallarek, U. In situ synthesis and characterization of sulfonic acid functionalized hierarchical silica monoliths. J. Sol-Gel Sci. Technol. 2020, 96, 67–82. [Google Scholar] [CrossRef]
- Marschall, R.; Rathouský, J.; Wark, M. Ordered Functionalized Silica Materials with High Proton Conductivity. Chem. Mater. 2007, 19, 6401–6407. [Google Scholar] [CrossRef]
- Cheng, Z.; Shan, H.; Sun, Y.; Zhang, L.; Jiang, H.; Li, C. Evolution mechanism of surface hydroxyl groups of silica during heat treatment. Appl. Surf. Sci. 2020, 513, 145766. [Google Scholar] [CrossRef]
- Wang, C.; Feng, Z.; Zhao, Y.; Li, X.; Li, W.; Xie, X.; Wang, S.; Hou, H. Preparation and properties of ion exchange membranes for PEMFC with sulfonic and carboxylic acid groups based on polynorbornenes. Int. J. Hydrogen Energy 2017, 42, 29988–29994. [Google Scholar] [CrossRef]
- Alekseev, S.A.; Zaitsev, V.N.; Fraissard, J. Organosilicas with Covalently Bonded Groups under Thermochemical Treatment. Chem. Mater. 2006, 18, 1981–1987. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Z.; Teng, Z.; Liang, J.; Guo, Z.; Wang, D.; Han, M.-Y.; Yang, W. Unraveling the Growth Mechanism of Silica Particles in the Stöber Method: In Situ Seeded Growth Model. Langmuir 2017, 33, 5879–5890. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Saeki, S.; Yoshida, M.; Nagao, D.; Konno, M. Synthesis of spherical submicron-sized magnetite/silica nanocomposite particles. J. Sol-Gel Sci. Technol. 2008, 45, 35–41. [Google Scholar] [CrossRef]
- Norazmi, F.; Chaudhary, K.; Mazalan, E.; Hader, Z.; Ali, J. Effect of various amount of ammonium hydroxide on morphology of silica nanoparticles grown by sol-gel. Malays. J. Fundam. Appl. Sci. 2018, 14, 482–484. [Google Scholar] [CrossRef]
- Liu, H.; Fu, S.; Li, H.; Zhan, H. Layer-by-layer assembly of lignosulfonates for hydrophilic surface modification. Ind. Crops Prod. 2009, 30, 287–291. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, X. Theoretical study on the interactions between silica and the products of 3-mercaptopropyltriethoxysilane (MPTS) with different hydrolysis degrees. Appl. Surf. Sci. 2020, 502, 143853. [Google Scholar] [CrossRef]
- Peng, S.; Zeng, Z.; Zhao, W.; Chen, J.; Han, J.; Wu, X. Performance evaluation of mercapto functional hybrid silica sol–gel coating on copper surface. Surf. Coat. Technol. 2014, 251, 135–142. [Google Scholar] [CrossRef]
- Yuan, R.; Wu, S.; Yu, P.; Wang, B.; Mu, L.; Zhang, X.; Zhu, Y.; Wang, B.; Wang, H.; Zhu, J. Superamphiphobic and Electroactive Nanocomposite toward Self-Cleaning, Antiwear, and Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2016, 8, 12481–12493. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, L.; Luo, Y.; Wu, R.; Jiang, H.; Chen, Y.; Zeng, G.; Liu, Y. Facile transition from hydrophilicity to superhydrophilicity and superhydrophobicity on aluminum alloy surface by simple acid etching and polymer coating. Appl. Surf. Sci. 2013, 280, 193–200. [Google Scholar] [CrossRef]
- Geng, Z.; He, J.; Xu, L. Fabrication of superhydrophilic and antireflective silica coatings on poly(methyl methacrylate) substrates. Mater. Res. Bull. 2012, 47, 1562–1567. [Google Scholar] [CrossRef]
- ISO 2409:2020; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- ASTM D4060-10; Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser. ASTM International: West Conshohocken, PA, USA, 2010.
- Lee, J.-g.; Cho, W.; Kim, Y.; Cho, H.; Lee, H.; Kim, J.H. Formation of a conductive overcoating layer based on hybrid composites to improve the stability of flexible transparent conductive films. RSC Adv. 2019, 9, 4428–4434. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Qi, H.; Zheng, W.; Zhou, X.; Zhang, C.; Zhang, L. A mussel-inspired chimeric protein as a novel facile antifouling coating. Chem. Commun. 2018, 54, 11328–11331. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Chu, Z.; Fan, X.; Jia, Z.; Teng, M. Study on surface wetting property regulation of greenhouse film and its antifogging performance. J. Coat. Technol. Res. 2022, 19, 1199–1209. [Google Scholar] [CrossRef]
- Qi, D.; Lin, C.; Zhao, H.; Liu, H.; Lü, T. Size regulation and prediction of the SiO2 nanoparticles prepared via Stöber process. J. Dispers. Sci. Technol. 2017, 38, 70–74. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, J.; Hao, T.; Wang, Z.; Zhang, Y. A Scalable Method toward Superhydrophilic and Underwater Superoleophobic PVDF Membranes for Effective Oil/Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 14896–14904. [Google Scholar] [CrossRef]
- Li, Y.; Lan, J.Y.; Liu, J.; Yu, J.; Luo, Z.; Wang, W.; Sun, L. Synthesis of Gold Nanoparticles on Rice Husk Silica for Catalysis Applications. Ind. Eng. Chem. Res. 2015, 54, 5656–5663. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, R.; Ding, F.; Brittain, A.D.; Liu, J.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Sulfonic Acid-Functionalized α-Zirconium Phosphate Single-Layer Nanosheets as a Strong Solid Acid for Heterogeneous Catalysis Applications. ACS Appl. Mater. Interfaces 2014, 6, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Noshadi, I.; Ding, H.; Liu, J.; Parnas, R.S.; Clearfield, A.; Xiao, M.; Meng, Y.; Sun, L. Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification. Catalysts 2018, 8, 17. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Song, J.; Yang, J.; Pan, C.; Xu, T.; Zhang, L. Novel Balanced Charged Alginate/PEI Polyelectrolyte Hydrogel that Resists Foreign-Body Reaction. ACS Appl. Mater. Interfaces 2018, 10, 6879–6886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Cheng, J.; Hou, K.; Zhao, A.; Pi, P.; Wen, X.; Xu, S. Superhydrophilic and underwater superoleophobic titania nanowires surface for oil repellency and oil/water separation. Chem. Eng. J. 2016, 301, 249–256. [Google Scholar] [CrossRef]
- ASTM D3363-22; Standard Test Method for Film Hardness by Pencil Test. ASTM International: West Conshohocken, PA, USA, 2022.
- Zhang, C.; Qi, Y.; Guo, Y.; Zhang, S.; Xiong, G.; Wang, K.; Zhang, Z. Anti-marine biofouling adhesion performance and mechanism of PDMS fouling-release coating containing PS-PEG hydrogel. Mar. Pollut. Bull. 2023, 194, 115345. [Google Scholar] [CrossRef]
- Han, M.; Dong, Z.; Li, J.; Luo, J.; Yin, D.; Sun, L.; Tao, S.; Zhen, L.; Yang, J.; Li, J. Mussel-inspired self-assembly engineered implant coatings for synergistic anti-infection and osteogenesis acceleration. J. Mater. Chem. B 2021, 9, 8501–8511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).