Combination of Cu-BTC- and FeCo-MOF-Derived Carbon Enhanced Molecularly Imprinted Electrochemical Sensor for Highly Sensitive and Selective Detection of Benomyl in Fruits and Vegetables
Abstract
:1. Introduction
2. Results and Discussion
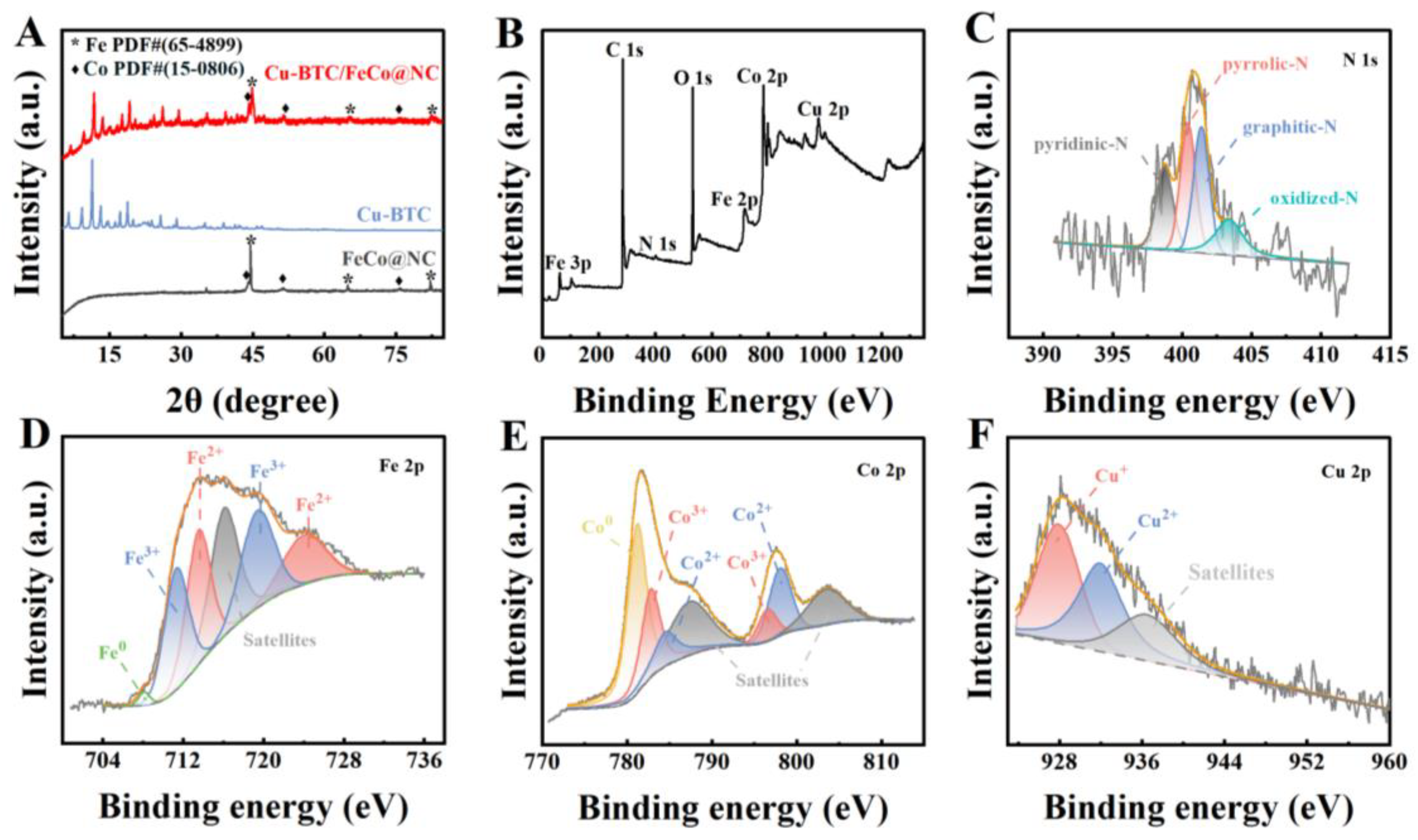
2.1. Surface Morphology and Structure Characterization
2.2. Electrochemical Characterization
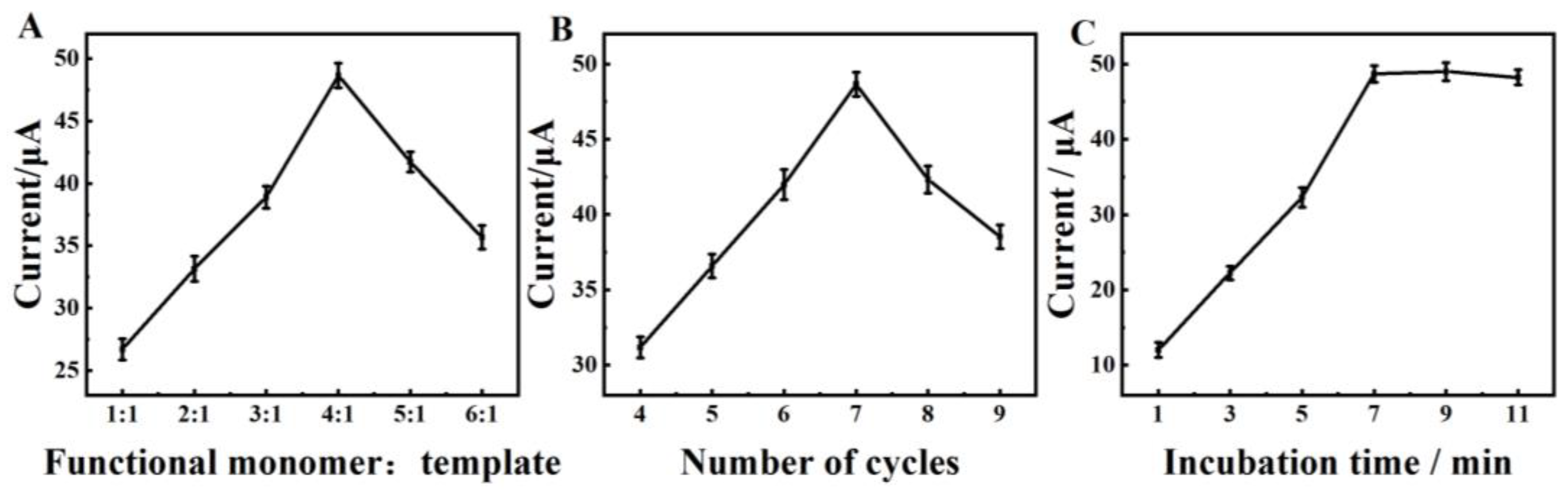
2.3. Optimization of the Experimental Conditions
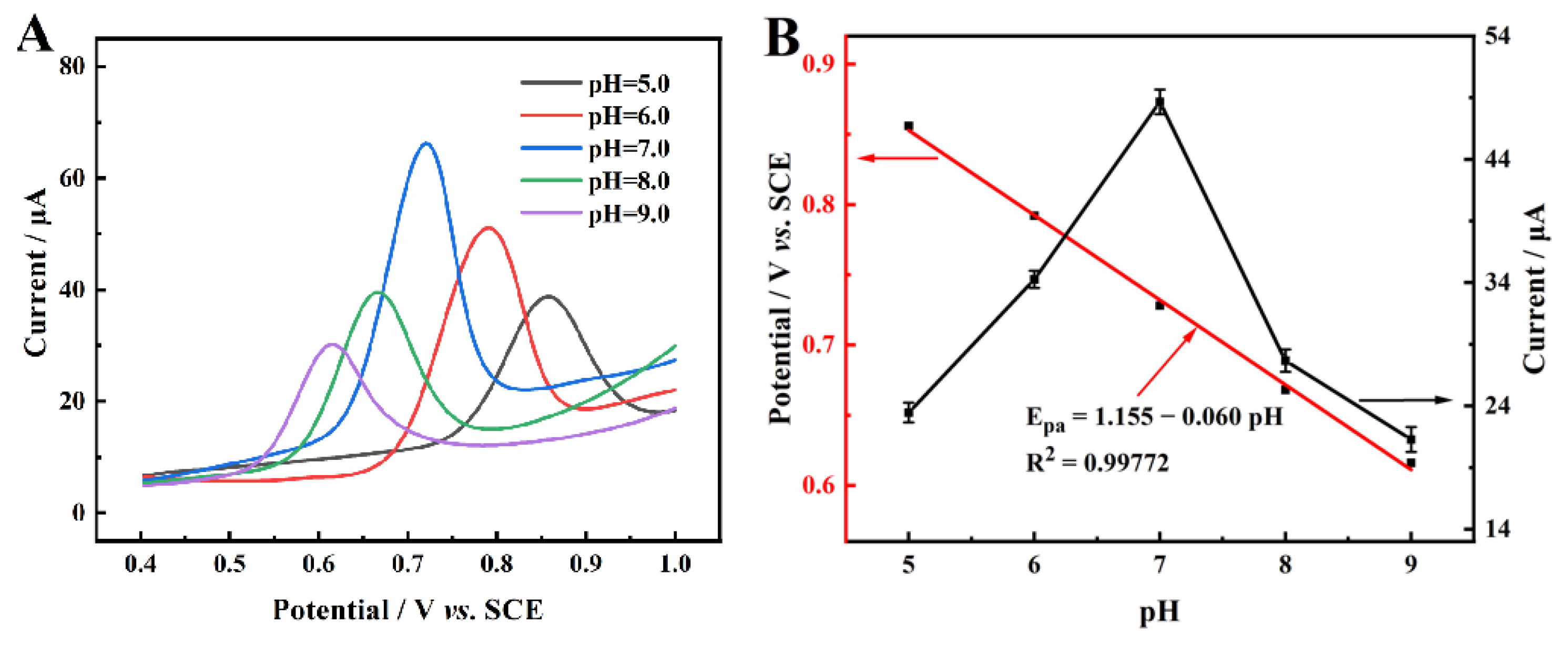
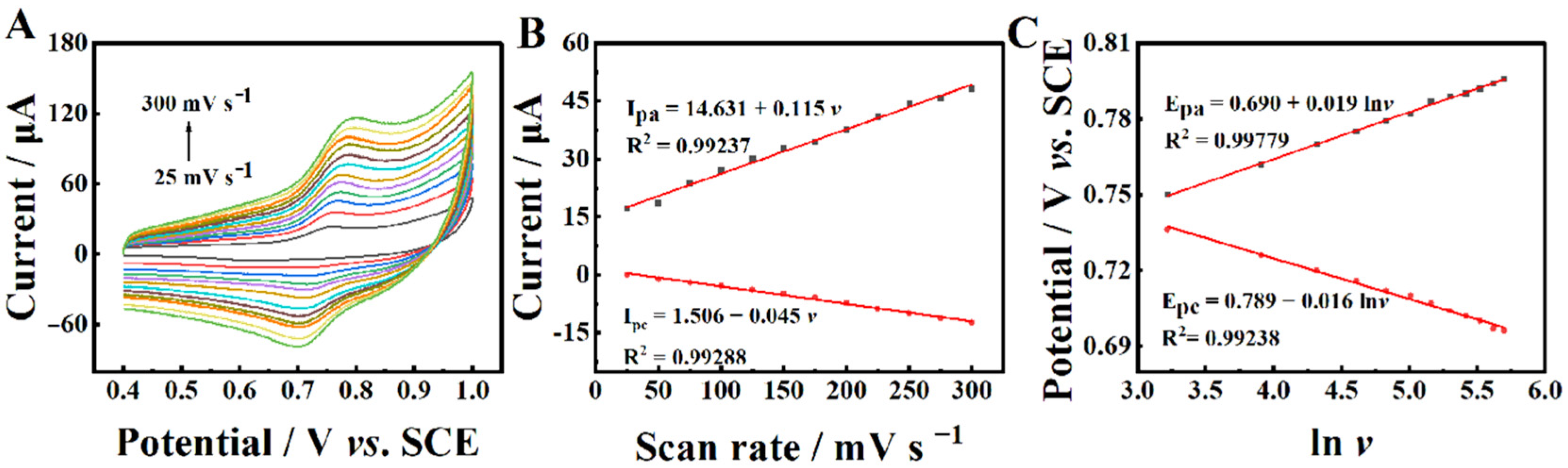
2.4. Electrochemical Reaction Kinetics
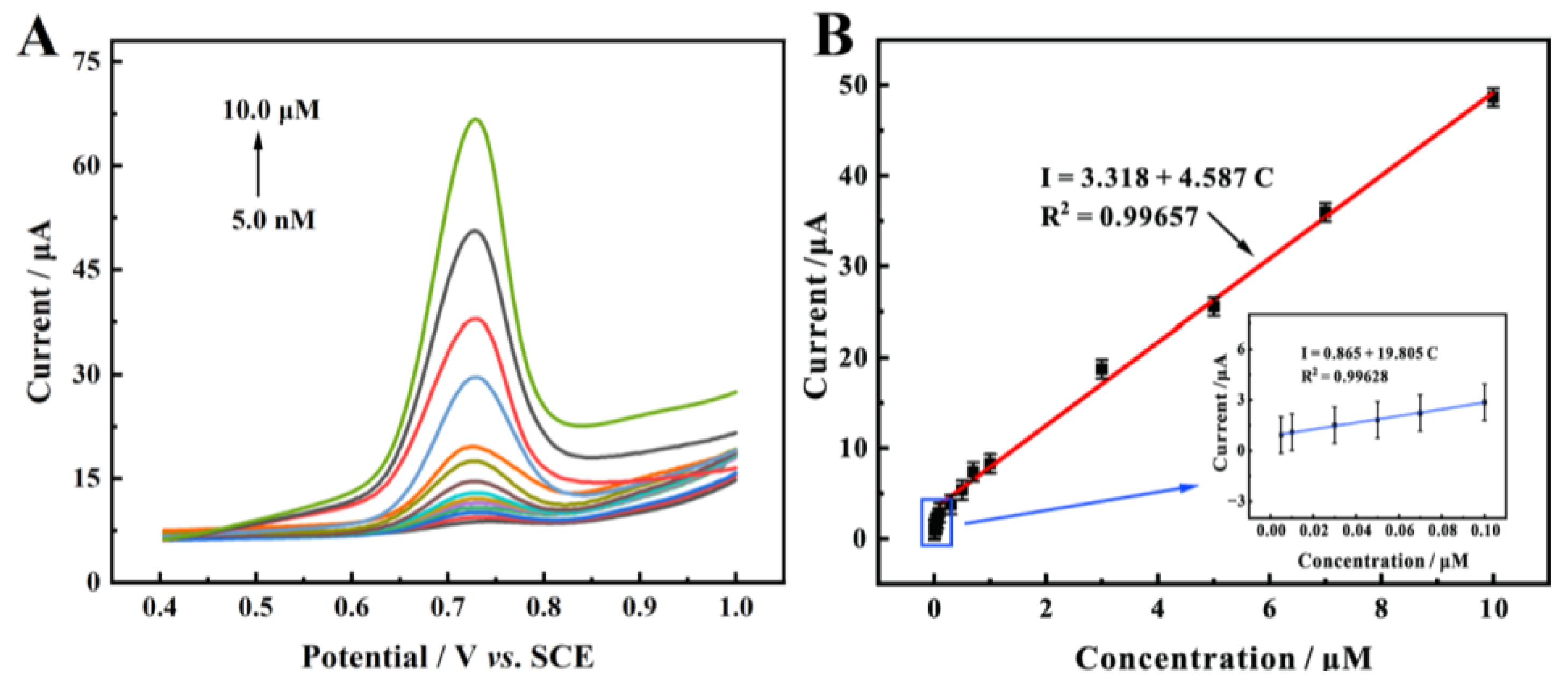
2.5. Electrochemical Detection of BN
2.6. Reproducibility, Repeatability, Stability, and Anti-Interference Ability
2.7. Real Samples Detection of BN
3. Experimental Section
3.1. Materials
3.2. Instruments
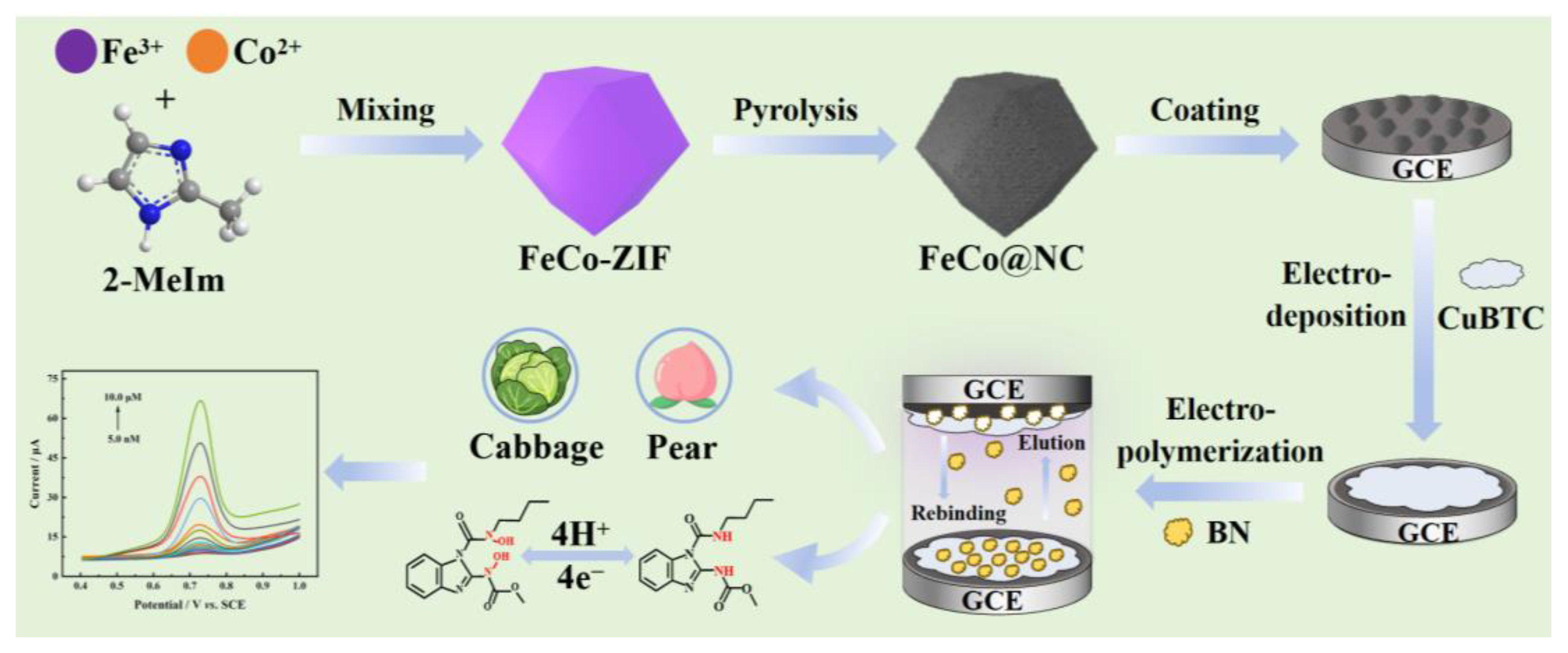
3.3. Synthesis of the FeCo@NC
3.4. Synthesis of Cu-BTC/FeCo@NC/GCE
3.5. Synthesis of MIP/Cu-BTC/FeCo@NC/GCE
3.6. Electrochemical Measurements
3.7. Preparation of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides Classification and its Impact on Environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Fernandes, I.d.A.A.; Maciel, G.M.; Bortolini, D.G.; Pedro, A.C.; Rubio, F.T.V.; de Carvalho, K.Q.; Haminiuk, C.W.I. The bitter side of teas: Pesticide residues and their impact on human health. Food Chem. Toxicol. 2023, 179, 113955. [Google Scholar] [CrossRef] [PubMed]
- Shiny Raj, R.; Anoop Krishnan, K. A comprehensive review on the impact of emerging organophosphorous pesticides and their remedial measures: Special focus on acephate. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100813. [Google Scholar] [CrossRef]
- Bai, J.; Deng, S.; Fu, H.; Yang, Q.; Ren, F.; Zeng, S.; Chen, Z.; Yang, Y.; Wu, Z. Chlorpyrifos induces placental oxidative stress and barrier dysfunction by inducing mitochondrial apoptosis through the ERK/MAPK signaling pathway: In vitro and in vivo studies. Sci. Total Environ. 2023, 903, 166449. [Google Scholar] [CrossRef]
- Levario-Carrillo, M.; Amato, D.; Ostrosky-Wegman, P.; González-Horta, C.; Corona, Y.; Sanin, L.H. Relation between pesticide exposure and intrauterine growth retardation. Chemosphere 2004, 55, 1421–1427. [Google Scholar] [CrossRef]
- Costa, M.B.; Farias, I.R.; da Silva Monte, C.; Filho, L.I.P.F.; de Paula Borges, D.; de Oliveira, R.T.G.; Ribeiro-Junior, H.L.; Magalhães, S.M.M.; Pinheiro, R.F. Chromosomal abnormalities and dysregulated DNA repair gene expression in farmers exposed to pesticides. Environ. Toxicol. Pharmacol. 2021, 82, 103564. [Google Scholar] [CrossRef]
- Kaur, R.; Choudhary, D.; Bali, S.; Bandral, S.S.; Singh, V.; Ahmad, M.A.; Rani, N.; Singh, T.G.; Chandrasekaran, B. Pesticides: An alarming detrimental to health and environment. Sci. Total Environ. 2024, 915, 170113. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Sabzevari, S.; Hofman, J. A worldwide review of currently used pesticides’ monitoring in agricultural soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef]
- Khan, M.; Sun, X.; Attique, F.; Saleh, M.T.; Ahmad, N.; Atiq, K.; Shafi, M.; Ahmed, I.A.; Barsoum, I.; Rafique, M.S.; et al. Unveiling the future: Breakthroughs and innovations in MXene-based electrochemical sensors. Chem. Eng. J. Adv. 2025, 507, 160392. [Google Scholar] [CrossRef]
- Aydoğdu Tığ, G.; Marrazza, G.; Turan, K.; Erdoğan, N.Ö.; Şimşek, N. Recent developments in electrochemical sensors and biosensors for food additives determination: Principle and application. Trends Anal. Chem. 2025, 183, 118127. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-Palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly imprinted polymers (MIPs) combined with nanomaterials as electrochemical sensing applications for environmental pollutants. Trends Environ. Anal. Chem. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Farooq, S.; Xu, L.; Ostovan, A.; Qin, C.; Liu, Y.; Pan, Y.; Ping, J.; Ying, Y. Assessing the greenification potential of cyclodextrin-based molecularly imprinted polymers for pesticides detection. Food Chem. 2023, 429, 136822. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kim, K.-H. Use of molecular imprinted polymers as sensitive/selective luminescent sensing probes for pesticides/herbicides in water and food samples. Environ. Pollut. 2022, 299, 118824. [Google Scholar] [CrossRef]
- Geleta, G.S. Recent advances in electrochemical sensors based on molecularly imprinted polymers and nanomaterials for detection of ascorbic acid, dopamine, and uric acid: A review. Sens. Biosens. Res. 2024, 43, 100610. [Google Scholar] [CrossRef]
- Xue, S.; Zou, J.; Li, J.; Xu, J.; Chen, H.; Wang, L.; Gao, Y.; Duan, X.; Lu, L. Electrochemical detection of carbendazim using molecularly imprinted poly(3,4-ethylenedioxythiophene) on Co,N co-doped hollow carbon nanocage@CNTs-modified electrode. Food Chem. 2024, 456, 140063. [Google Scholar] [CrossRef]
- Ding, L.-H.; Wang, Y.-W.; Li, Q.-W.; Zhang, L.-L.; Wang, A.-Z. A highly selective electrochemical impedimetric sensor for imidacloprid determination based on WO3/MoS2 nanosheets/molecularly imprinted polymer composite. Rare Met. 2023, 43, 1309–1315. [Google Scholar] [CrossRef]
- Mutlu, E.; Şenocak, A.; Demirbaş, E.; Koca, A.; Akyüz, D. Selective and sensitive molecularly imprinted polymer-based electrochemical sensor for detection of deltamethrin. Food Chem. 2025, 463, 141121. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.-B.; Tang, Z.-S.; Qiu, Z.-D.; Zhu, H.-X.; Song, Z.-X.; Jia, A.-L. Synthesis, performance, and application of molecularly imprinted membranes: A review. J. Environ. Chem. Eng. 2021, 9, 106352. [Google Scholar] [CrossRef]
- Li, Y.; Yao, B.; Chen, Y.; Zhou, Y.; Duan, X. Metal-organic frameworks (MOFs) as efficient catalysts for electro-Fenton (EF) reactions: Current progress and prospects. Chem. Eng. J. 2023, 463, 142287. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, Y.; Yuan, Y.; Zhou, X.; Liu, Y.; Meng, X. Recent advances of metal–organic frameworks in corrosion protection: From synthesis to applications. Chem. Eng. J. 2022, 430, 132823. [Google Scholar] [CrossRef]
- Zhao, T.; Xiao, P.; Nie, S.; Luo, M.; Zou, M.; Chen, Y. Recent progress of metal-organic frameworks based high performance batteries separators: A review. Coord. Chem. Rev. 2024, 502, 215592. [Google Scholar] [CrossRef]
- Song, G.; Shi, Y.; Jiang, S.; Pang, H. Recent Progress in MOF-Derived Porous Materials as Electrodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2303121. [Google Scholar] [CrossRef]
- Liu, C.-S.; Li, J.; Pang, H. Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coord. Chem. Rev. 2020, 410, 213222. [Google Scholar] [CrossRef]
- Manoj, D.; Rajendran, S.; Murphy, M.; Jalil, A.A.; Sonne, C. Recent progress and perspectives of metal organic frameworks (MOFs) for the detection of food contaminants. Chemosphere 2023, 340, 139820. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Zhang, J.; Zhao, X.; Feng, H.; Luo, H. Preparation of MOFs derived nitrogen self-doped porous carbon and itselectrochemical performance in mixed electrolytes. Appl. Surf. Sci. 2020, 500, 143936. [Google Scholar] [CrossRef]
- Chen, H.; Guo, P.; Huang, Z.; Sun, J.; Lei, Y.; Xu, J. Enhanced stability and conductivity of montmorillonite and sucrose loaded Fe-MOFs for degradation of chlortetracycline hydrochloride via electrochemically activated persulfate. Appl. Clay Sci. 2024, 249, 107231. [Google Scholar] [CrossRef]
- Ding, M.; Chen, J.; Jiang, M.; Zhang, X.; Wang, G. Ultrathin trimetallic metal–organic framework nanosheets for highly efficient oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 14163–14168. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.; Lu, S.; Hu, J.; Zhu, H.; Duan, F.; Du, M. Conductive metal and covalent organic frameworks for electrocatalysis: Design principles, recent progress and perspective. Nanoscale 2022, 14, 277–288. [Google Scholar] [CrossRef]
- Li, C.; Wu, K. Cu-BTC frameworks based electrochemical sensor for hazardous malachite green in aquaculture. Anal. Chim. Acta 2021, 1162, 338473. [Google Scholar] [CrossRef]
- Cao, A.D.H.; Ha, T.M.; Cao, H.L.N.; Luong, T.H.V.; Nguyen, T.T.; Nguyen, T.Q.C.; Dang, G.H. FeCo-ZIFs–Catalyzed indigo carmine removal: A promising approach for wastewater treatment. Inorg. Chem. Commun. 2025, 173, 113818. [Google Scholar]
- Wu, T.; Ma, Z.; Li, P.; Liu, M.; Liu, X.; Li, H.; Zhang, Y.; Yao, S. Colorimetric detection of ascorbic acid and alkaline phosphatase activity based on the novel oxidase mimetic of Fe–Co bimetallic alloy encapsulated porous carbon nanocages. Talanta 2019, 202, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, Q.; Zhou, M.; Li, C.; Sun, J.; Shi, W.; Ai, S. Degradation of methylene blue by a heterogeneous Fenton reaction catalyzed by FeCo2O4-N-C nanocomposites derived by ZIFs. Powder Technol. 2021, 383, 212–219. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wei, Y.-P.; Chen, J.-S.; Liu, X.-P.; Mao, C.-J.; Jin, B.-K. Self-catalyzed nitrogen-doped carbon nanotubes connected FeCo nanostructures for electrochemical sensitive detection of metol. Talanta 2025, 290, 127761. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Yang, Z.; Xiong, W.; Xu, Z.; Song, P.; Jia, M.; Sun, S.; Zhang, Y.; Li, W. Fabricating iron-cobalt layered double hydroxide derived from metal-organic framework for the activation of peroxymonosulfate towards tetracycline degradation. J. Solid State Chem. 2021, 294, 121857. [Google Scholar] [CrossRef]
- Kamali, K.; Prasad, S.; Sahoo, M.K.; Behera, J.N.; Waghmare, U.V.; Narayana, C. Unusual CO2 Adsorption in ZIF-7: Insight from Raman Spectroscopy and Computational Studies. Inorg. Chem. 2022, 61, 11571–11580. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J. Degradation of Cephalosporin C using MOF-derived Fe-Co bimetal in carbon cages as electro-Fenton catalyst at natural pH. Sep. Purif. Technol. 2023, 323, 124388. [Google Scholar] [CrossRef]
- Zou, L.; Xiao, J.; Liu, Q.; Huang, Y.; Liang, Z. Strong interaction between efficient magnetic tri-metallic PdFeCo nano-alloy towards formic acid dehydrogenation and application of in-situ hydrogenation. Int. J. Hydrogen Energy 2024, 58, 1417. [Google Scholar] [CrossRef]
- Liang, C.; Han, X.; Zhang, T.; Dong, B.; Li, Y.; Zhuang, Z.; Han, A.; Liu, J. Cu Nanoclusters Accelerate the Rate-Determining Step of Oxygen Reduction on Fe─N─C in All pH Range. Adv. Energy Mater. 2024, 14, 2303935. [Google Scholar] [CrossRef]
- Sun, D.; Cui, H.; Sha, J.; Chen, B.; Shi, C.; Kang, J.; Ma, L. FeCo Alloy Nanoparticles on Porous Carbon for Zinc–Air Batteries. ACS Appl. Nano Mater. 2024, 7, 14760–14768. [Google Scholar] [CrossRef]
- Song, Z.; Wu, X.; He, Q.; Xiang, P.; Ma, P.; Miao, Z. Degradation of phenol in high-salt wastewater by three-dimensional FeCu co-doped materials: Interaction of FeCu with g-C3N4–rGO and synergistic catalytic mechanism. J. Environ. Chem. Eng. 2024, 12, 112164. [Google Scholar] [CrossRef]
- Fort, C.I.; Rusu, M.M.; Cotet, L.C.; Vulpoi, A.; Florea, I.; Tuseau-Nenez, S.; Baia, M.; Baibarac, M.; Baia, L. Carbon Xerogel Nanostructures with Integrated Bi and Fe Components for Hydrogen Peroxide and Heavy Metal Detection. Molecules 2020, 26, 117. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Peng, G.; Xue, S.; Xu, J.; Gao, Y.; Liu, S.; Duan, X.; Lu, L. 2D Leaf-Like Structured ZIF-L Embedded Electrochemically Reduced Graphene Oxide Composite as an Electrochemical Sensing Platform for Sensitively Detecting Benomyl. Molecules 2022, 27, 6857. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, S.; Li, L.; Luo, C.; Huang, X.; Chen, H.; Gao, Y.; Wang, L.; Lu, L. Directional construction of multi-doped hollow carbon foam with macropores-walls for ultrasensitive electrochemical detection of carbendazim. Chem. Eng. J. 2024, 499, 156440. [Google Scholar] [CrossRef]
- Sarıgül, T.; İnam, R.; Demir, E.; Aboul-Enein, H.Y. Electro-Oxidation and Determination of Benomyl by Square-Wave Adsorptive Stripping Voltammetry. J. AOAC Int. 2014, 97, 995–1000. [Google Scholar] [CrossRef]
- Hernández, P.; García, S.; Hernández, L. Voltammetric use of a graphite electrode modified with OV-17 silicone for the determination of organic compounds. Anal. Chim. Acta 1992, 259, 325–331. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Dong, Y.; Zhang, L. One-Step Fabrication of a Multifunctional Magnetic Nickel Ferrite/Multi-walled Carbon Nanotubes Nanohybrid-Modified Electrode for the Determination of Benomyl in Food. J. Agric. Food Chem. 2015, 63, 4746–4753. [Google Scholar] [CrossRef]
- Min, X.; Li, L.; Zhong, R.; Wan, C.; Wu, C. MOF-derived porous nitrogen and phosphorus codoped carbon nanosheets: An emerging material for constructing robust electrochemical sensing platform. Sens. Actuators B Chem. 2022, 369, 132263. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Shi, S.; Zhou, X.; Wu, C.; Wu, K. Ti3C2Tx/laser-induced graphene-based micro-droplet electrochemical sensing platform for rapid and sensitive detection of benomyl. Anal. Chim. Acta 2024, 1304, 342526. [Google Scholar] [CrossRef]
- Zeng, Y.; Tang, Y.; Liu, M.; Wu, C. Two-dimensional MXene@ZIF-8 hybrid-derived TiO2/TiN@N-C heterostructure as an emerging material for electrochemical sensing. Carbon Lett. 2024, 34, 1887–1898. [Google Scholar] [CrossRef]
- Zhong, W.; Zou, J.; Xie, Y.; Yang, J.; Li, M.; Liu, S.; Gao, Y.; Wang, X.; Lu, L. Three-dimensional nano-CuxO-MWCNTs-COOH/MXene heterostructure: An efficient electrochemical platform for highly sensitive and selective sensing of benomyl in fruit samples. J. Environ. Chem. Eng. 2022, 920, 116586. [Google Scholar] [CrossRef]
- Tang, S.; Li, C.; Li, L.; Huang, J.; Wang, B.; Zhong, R.; Tang, Y.; Wu, C.; Wu, K. Graphene/metal-organic framework nano-sandwiches derived N, P-codoped porous carbon nanosheets as robust material for electrochemical analysis. Anal. Chim. Acta 2023, 1277, 341675. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Xue, S.-Y.; Peng, G.-W.; Xu, J.-K.; Gao, Y.-S.; Liu, S.-W.; Duan, X.-M.; Lu, L.-M. Electrochemical determination of benomyl using MWCNTs interspersed graphdiyne as enhanced electrocatalyst. Mikrochim. Acta 2023, 190, 98. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Muhammad, T.; Ma, L.; Huang, Z.-F.; Wang, S.; Wang, L.; Zou, J.-J.; Zhang, X. MOF-derived C-doped ZnO prepared via a two-step calcination for efficient photocatalysis. Appl. Catal. B-Environ. 2016, 189, 181–191. [Google Scholar] [CrossRef]


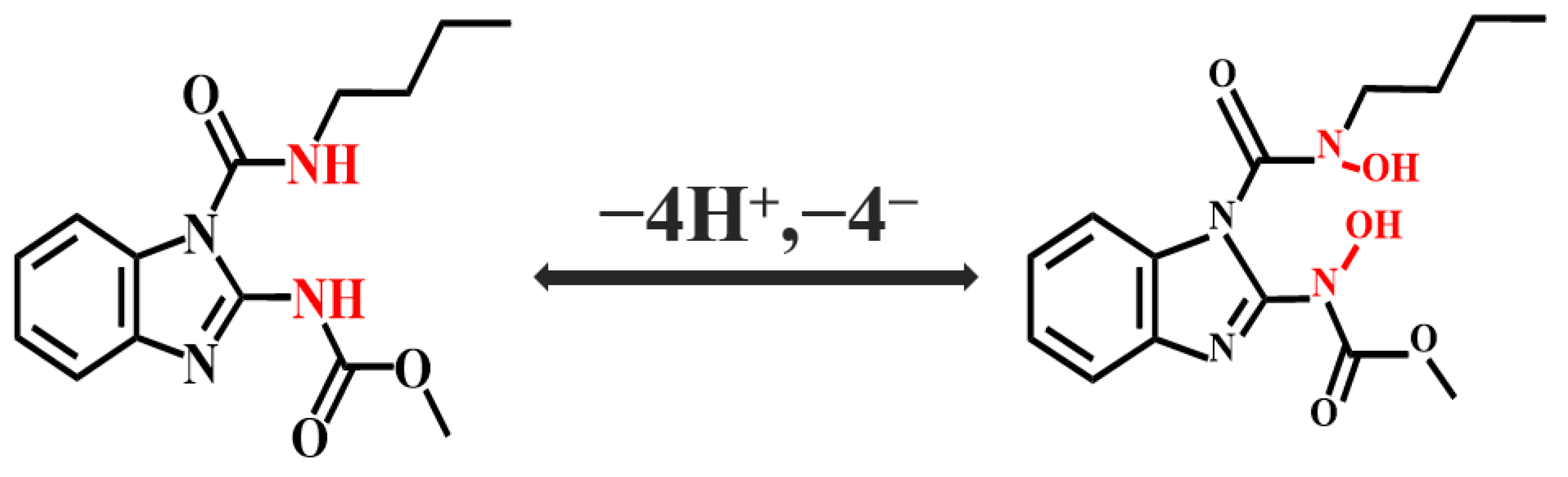

| Sensor | Detection Technology | Sensitivity (μA μM−1 cm−2) | Peak Potential (V) | Linear Range (μM) | Detection Limit (nM) | References |
|---|---|---|---|---|---|---|
| Bare GCE | adsSWSV a | – | 1.09 | 0.27–5.17 | 82.66 | [45] |
| OV-17 silicon/CPE | DPV | – | 0.67 | 0.34–13.77 | 237.66 | [46] |
| NiFe2O4 b/MWCNTs/GCE | DPV | – | 0.78 | 0.09–9.98 | 24.8 | [47] |
| N,P-codoped CNS c | DPV | 381.43 | 0.77 | 0.025–2 | 7.50 | [48] |
| MXene-Ti3C2Tx/LIG d | DPV | 864.3 | 0.77 | 0.01–6 | 5.8 | [49] |
| TiO2/TiN@N-C | DPV | 8.529 | 0.74 | 0.01–1 | 4.5 | [50] |
| Nano e-CuxO-MWCNTs-COOH/MXene | DPV | 70.35 | 0.76 | 0.01–10.00 | 3.0 | [51] |
| ERGO f@ZIF-L g/GCE | DPV | 192.92 | 0.73 | 0.009–10.00 | 3.0 | [43] |
| N,P-codoped PCN h | DPV | 272.86 | 0.73 | 0.005–1.50 | 2.10 | [52] |
| GDY@PDA i/MWCNTs-NH2 | DPV | 0.45 | 0.72 | 0.007–10.00 | 1.8 | [53] |
| MIP/Cu-BTC/FeCo@NC/GCE | DPV | 36.93 | 0.74 | 0.005–10.00 | 1.67 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Cabbage | 1.0 | 0.98 ± 0.03 | 98.0 | 1.96 |
| 3.0 | 2.89 ± 0.15 | 96.3 | 3.55 | |
| 5.0 | 5.06 ± 0.20 | 101.2 | 2.98 | |
| Pear | 1.0 | 1.01 ± 0.03 | 101.0 | 2.03 |
| 3.0 | 2.93 ± 0.13 | 97.7 | 2.59 | |
| 5.0 | 5.12 ± 0.23 | 102.4 | 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Xue, S.; Li, X.; Deng, L.; Li, J.; Zhou, J.; Gao, Y.; Duan, X.; Lu, L. Combination of Cu-BTC- and FeCo-MOF-Derived Carbon Enhanced Molecularly Imprinted Electrochemical Sensor for Highly Sensitive and Selective Detection of Benomyl in Fruits and Vegetables. Molecules 2025, 30, 1869. https://doi.org/10.3390/molecules30091869
Chen L, Xue S, Li X, Deng L, Li J, Zhou J, Gao Y, Duan X, Lu L. Combination of Cu-BTC- and FeCo-MOF-Derived Carbon Enhanced Molecularly Imprinted Electrochemical Sensor for Highly Sensitive and Selective Detection of Benomyl in Fruits and Vegetables. Molecules. 2025; 30(9):1869. https://doi.org/10.3390/molecules30091869
Chicago/Turabian StyleChen, Lili, Shuya Xue, Xin Li, Linbo Deng, Jiapeng Li, Jing Zhou, Yansha Gao, Xuemin Duan, and Limin Lu. 2025. "Combination of Cu-BTC- and FeCo-MOF-Derived Carbon Enhanced Molecularly Imprinted Electrochemical Sensor for Highly Sensitive and Selective Detection of Benomyl in Fruits and Vegetables" Molecules 30, no. 9: 1869. https://doi.org/10.3390/molecules30091869
APA StyleChen, L., Xue, S., Li, X., Deng, L., Li, J., Zhou, J., Gao, Y., Duan, X., & Lu, L. (2025). Combination of Cu-BTC- and FeCo-MOF-Derived Carbon Enhanced Molecularly Imprinted Electrochemical Sensor for Highly Sensitive and Selective Detection of Benomyl in Fruits and Vegetables. Molecules, 30(9), 1869. https://doi.org/10.3390/molecules30091869







