Copper(II)-Catalyzed Direct C3 Chalcogenylation of Indoles
Abstract
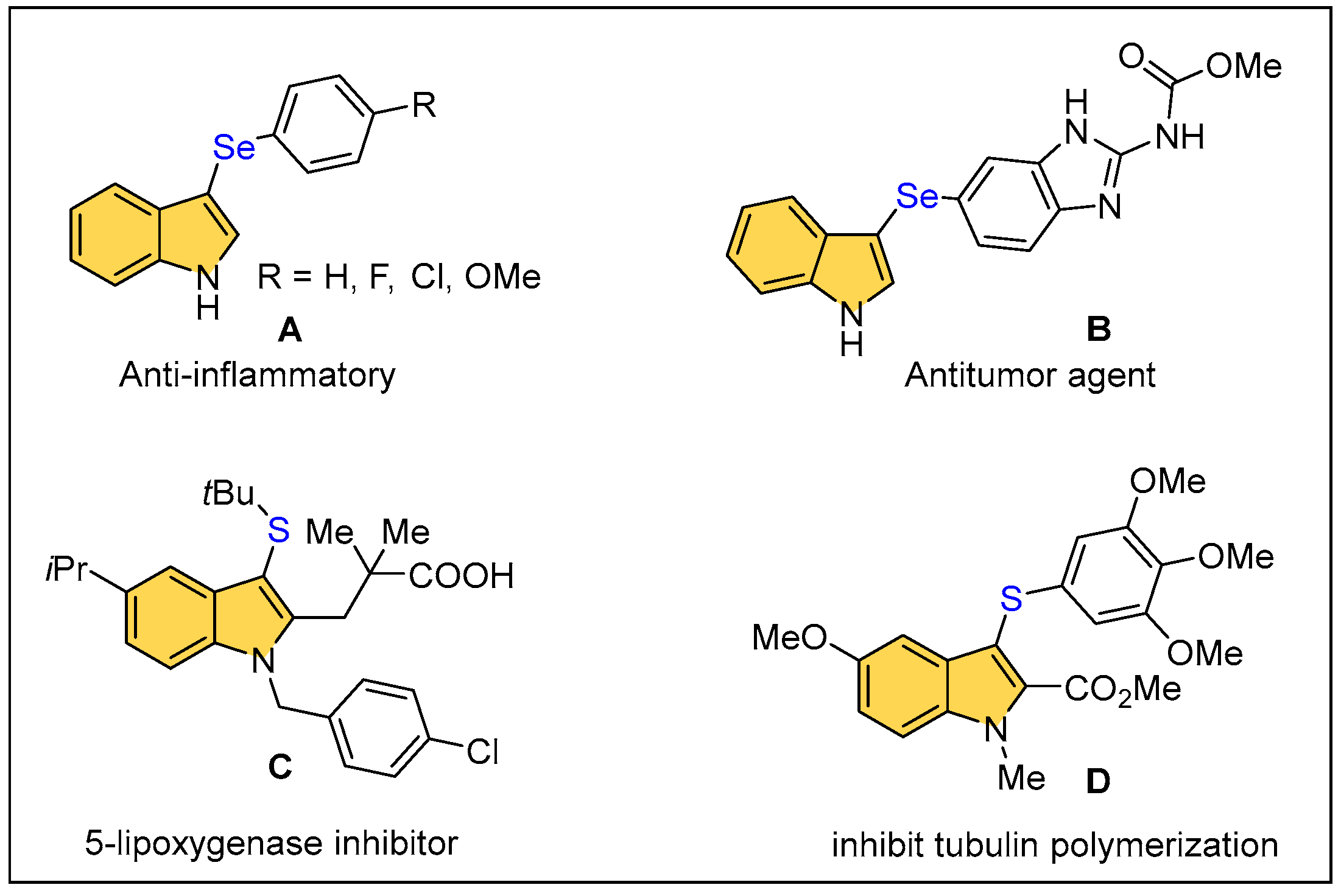
:1. Introduction
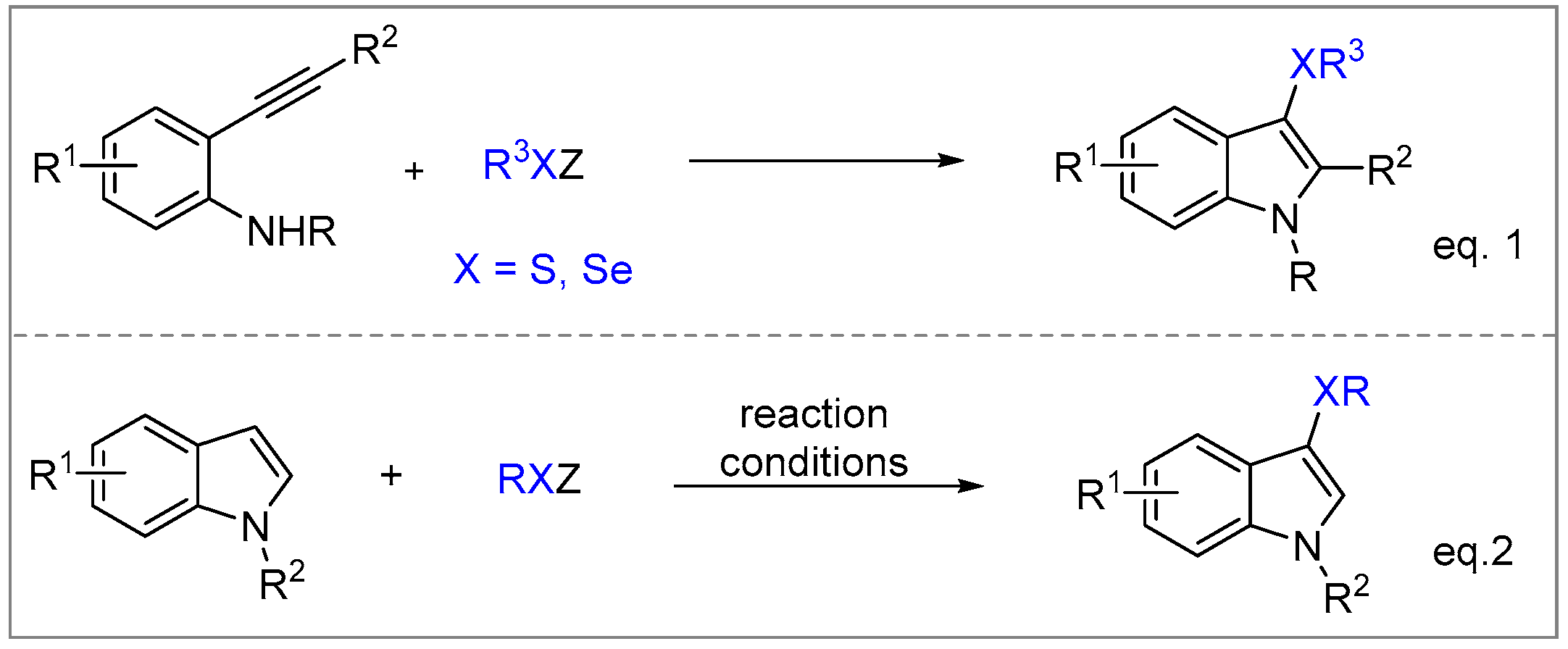
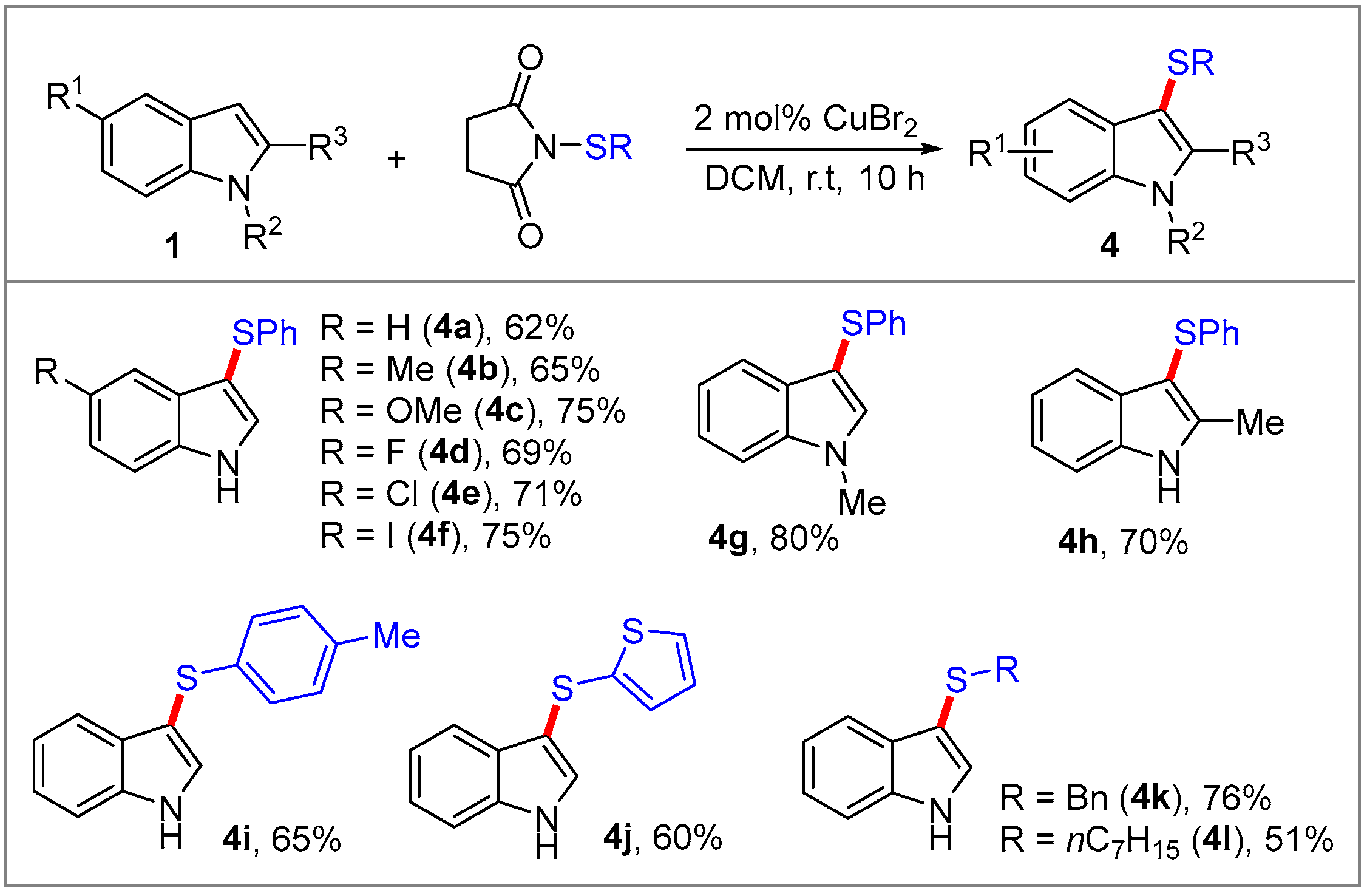
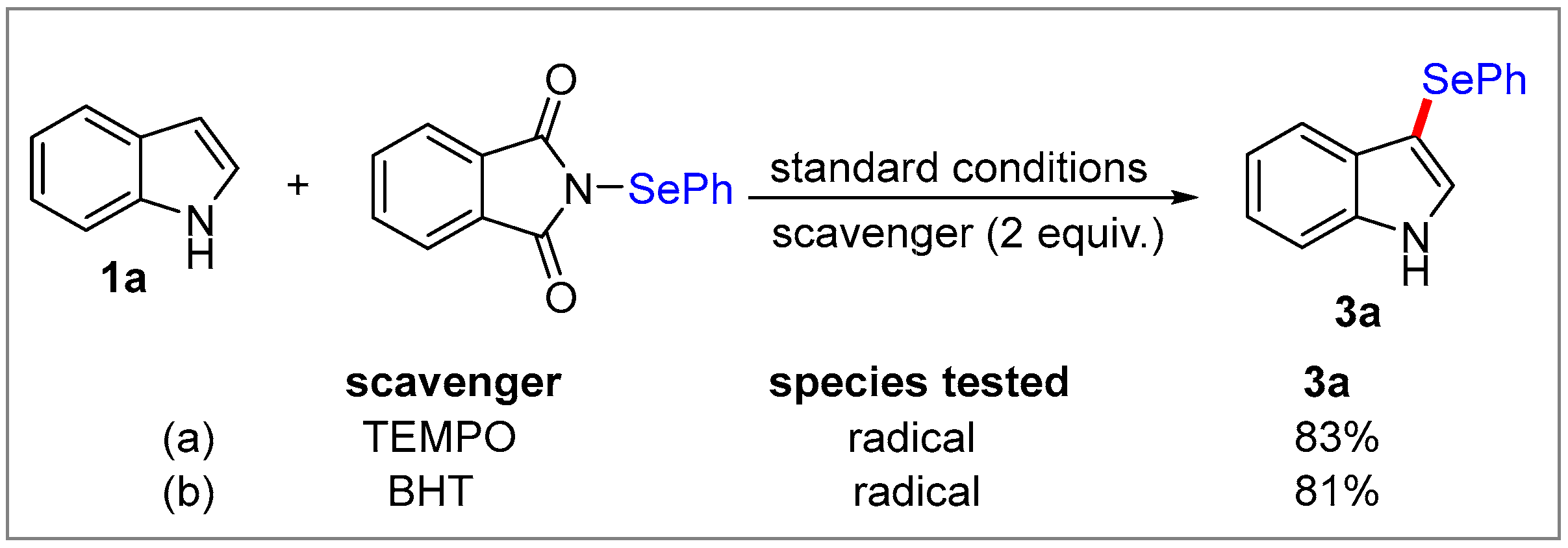
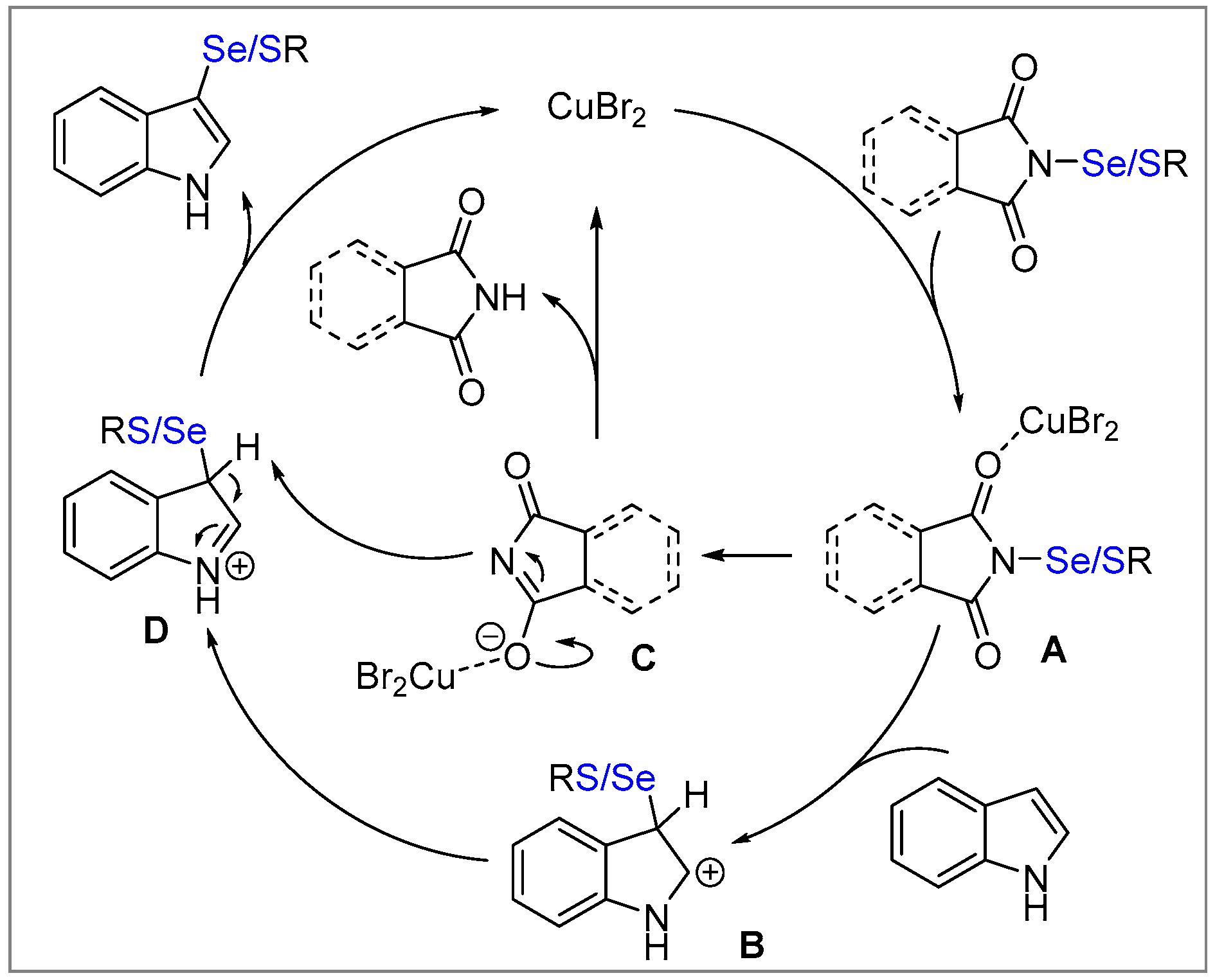
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of 3-Chalcogenylindoles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolognesi, M.L.; Cavalli, A. Multitarget Drug Discovery and Polypharmacology. Chem. Med. Chem. 2016, 11, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uliassi, E.; Bolognesi, M.L. Two diseases, one approach: Multitarget drug discovery in Alzheimer’s and neglected tropical diseases. Med. Chem. Commun. 2014, 5, 853–861. [Google Scholar] [CrossRef]
- Song, K.; Li, M.; Yang, Y.; Zhang, Z.; Zhu, Q.; Liu, J.; Wang, A. Natural flavonolignans as potential therapeutic agents against common diseases. J. Pharm. Pharmacol. 2022, 74, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-N.; Lu, Y.-J.; Chu, C.-J.; Wu, Y.-N.; Huang, H.-L.; Fan, B.-Y.; Chen, G.-T. Biotransformation of betulonic acid by the fungus Rhizopus arrhizus CGMCC 3.868 and antineuroinflammatory activity of the biotransformation products. J. Nat. Prod. 2021, 84, 2664–2674. [Google Scholar] [CrossRef]
- Choi, S.-K. Synthetic Multivalent Molecules; Wiley-VCH: New York, NY, USA, 2004. [Google Scholar]
- Ivasiv, V.; Albertini, C.; Goncalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular Hybridization as a Tool for Designing Multitarget Drug Candidates for Complex Diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef]
- Casaril, A.M.; Ignasiak, M.T.; Chuang, C.Y.; Vieira, B.; Padilha, N.B.; Carroll, L.; Lenardão, E.J.; Savegnago, L.; Davies, M.J. Selenium-containing indolyl compounds: Kinetics of reaction with inflammation-associated oxidants and protective effect against oxidation of extracellular matrix proteins. Free Radic. Biol. Med. 2017, 113, 395–405. [Google Scholar] [CrossRef]
- Guan, Q.; Han, C.; Zuo, D.; Zhai, M.; Li, Z.; Zhang, Q.; Zhai, Y.; Jiang, X.; Bao, K.; Wu, Y.; et al. Synthesis and evaluation of benzimidazole carbamates bearing indole moieties for antiproliferative and antitubulin activities. Eur. J. Med. Chem. 2014, 87, 306–315. [Google Scholar] [CrossRef]
- Cianchi, F.; Cortesini, C.; Magnelli, L.; Fanti, E.; Papucci, L.; Schiavone, N.; Messerini, L.; Vannacci, A.; Capaccioli, S.; Perna, F.; et al. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol. Cancer Ther. 2006, 5, 2716–2726. [Google Scholar] [CrossRef]
- De Martino, G.; Edler, M.C.; La Regina, G.; Coluccia, A.; Barbera, M.C.; Barrow, D.; Nicholson, R.I.; Chiosis, G.; Brancale, A.; Hamel, E.; et al. New Arylthioindoles: Potent Inhibitors of Tubulin Polymerization. 2. Structure−Activity Relationships and Molecular Modeling Studies. J. Med. Chem. 2006, 49, 947–954. [Google Scholar] [CrossRef]
- Chen, Y.; Cho, C.-H.; Larock, R.C. A Novel Synthetic Route to 3-Sulfenyl- and 3-Selenylindoles by n-Bu4NI-Induced Electrophilic Cyclization. Org. Lett. 2009, 11, 173–176. [Google Scholar] [CrossRef]
- Chen, Y.; Cho, C.-H.; Shi, F.; Larock, R.C. Synthesis of 3-Sulfenyl- and 3-Selenylindoles by the Pd/Cu-Catalyzed Coupling of N,N-Dialkyl-2-iodoanilines and Terminal Alkynes, Followed by n-Bu4NI-Induced Electrophilic Cyclization. J. Org. Chem. 2009, 74, 6802–6811. [Google Scholar] [CrossRef] [PubMed]
- Du, H.-A.; Tang, R.-Y.; Deng, C.-L.; Liu, Y.; Li, J.-H.; Zhang, X.-G. Iron-Facilitated Iodine-Mediated Electrophilic Annulation of N,N-Dimethyl-2-alkynylanilines with Disulfides or Diselenides. Adv. Synth. Catal. 2011, 353, 2739–2748. [Google Scholar] [CrossRef]
- Ferreira, N.L.; Azeredo, J.B.; Fiorentin, B.L.; Braga, A.L. Synthesis of 3-Selenylindoles under Eco-friendly Conditions. Eur. J. Org. Chem. 2015, 2015, 5070–5074. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Song, Z.; Liang, G. An efficient t-BuOK promoted C3-chalcogenylation of indoles with dichalcogenides. Org. Biomol. Chem. 2018, 16, 4958–4962. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Das, P.; Jana, R. Atom-economical selenation of electron-rich arenes and phosphonates with molecular oxygen at room temperature. Org. Biomol. Chem. 2018, 16, 9243–9250. [Google Scholar] [CrossRef]
- Prasad, C.D.; Kumar, S.; Sattar, M.; Adhikary, A.; Kumar, S. Metal free sulfenylation and bis-sulfenylation of indoles: Persulfate mediated synthesis. Org. Biomol. Chem. 2013, 11, 8036–8040. [Google Scholar] [CrossRef]
- Rao, H.; Wang, P.; Wang, J.; Li, Z.; Sun, X.; Cao, S. K2S2O8/arenesulfinate: An unprecedented thiolating system enabling selective sulfenylation of indoles under metal-free conditions. RSC Adv. 2014, 4, 49165–49169. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Franco, M.S.; Bettanin, L.; Schneider, A.R.; Silva, L.T.; Braga, A.L. Direct, Metal-free C(sp2)–H Chalcogenation of Indoles and Imidazopyridines with Dichalcogenides Catalysed by KIO3. Chem. Eur. J. 2018, 24, 4173–4180. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Wolf, L.; Martins, G.M.; von Muhlen, L. Efficient synthesis of 3-selanyl- and 3-sulfanylindoles employing trichloroisocyanuric acid and dichalcogenides. Tetrahedron 2012, 68, 10464–10469. [Google Scholar] [CrossRef]
- Benchawan, T.; Saeeng, R. Controllable Synthesis of Mono- and Bis-sulfenylindoles from Indoles and Various Sulfenylation Agents Using KI/SeO2 System. Eur. J. Org. Chem. 2022, 2022, e202200752. [Google Scholar] [CrossRef]
- Pedroso, G.J.; Costa, D.M.S.; Felipe Kokuszi, L.T.; da Silva, E.B.V.; Cavalcante, M.F.O.; Junca, E.; Moraes, C.A.O.; Pich, C.T.; de Lima, V.R.; Saba, S.; et al. Selenylated indoles: Synthesis, effects on lipid membrane properties and DNA cleavage. New J. Chem. 2023, 47, 2719–2726. [Google Scholar] [CrossRef]
- Menezes, J.R.; Gularte, M.M.; dos Santos, F.C.; Roehrs, J.A.; Azeredo, J.B. Synthesis of 3-chalcogenyl-indoles mediated by the safer reagent urea-hydrogen peroxide (UHP). Tetrahedron Lett. 2023, 120, 154446. [Google Scholar] [CrossRef]
- Rai, A.; Prabhakar, N.S.; Kishor, K.; Singh, K.N. Iodine/DMF-Mediated Regioselective Sulfenylation Using Arenediazonium Tosylates and Sodium Metabisulfite: An Easy Access to 3-Arylthioindoles. J. Org. Chem. 2024, 89, 15075–15082. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.B.; Godoi, M.; Martins, G.M.; Silveira, C.C.; Braga, A.L. A Solvent- and Metal-Free Synthesis of 3-Chalcogenyl-indoles Employing DMSO/I2 as an Eco-friendly Catalytic Oxidation System. J. Org. Chem. 2014, 79, 4125–4130. [Google Scholar] [CrossRef]
- Palomba, M.; Angeli, A.; Galdini, R.; Hughineata, A.J.; Perin, G.; Lenardão, E.J.; Marini, F.; Santi, C.; Supuran, C.T.; Bagnoli, L. Iodine/Oxone® oxidative system for the synthesis of selenylindoles bearing a benzenesulfonamide moiety as carbonic anhydrase I, II, IX, and XII inhibitors. Org. Biomol. Chem. 2024, 22, 6532–6542. [Google Scholar] [CrossRef]
- Matsumura, M.; Umeda, A.; Sumi, Y.; Aiba, N.; Murata, Y.; Yasuike, S. Bismuth(III)-Catalyzed Regioselective Selenation of Indoles with Diaryl Diselenides: Synthesis of 3-Selanylindoles. Molecules 2024, 29, 3227. [Google Scholar] [CrossRef]
- Luz, E.Q.; Seckler, D.; Araújo, J.S.; Angst, L.; Lima, D.B.; Maluf Rios, E.A.; Ribeiro, R.R.; Rampon, D.S. Fe(III)-Catalyzed direct C3 chalcogenylation of indole: The effect of iodide ions. Tetrahedron 2019, 75, 1258–1266. [Google Scholar] [CrossRef]
- Benchawan, T.; Maneewong, J.; Saeeng, R. Selective Synthesis of 3-Chalcogenylindoles via Silver-Catalyzed Direct Chalcogenation of Indoles with Dichalcogenides. ChemistrySelect 2023, 8, e202301988. [Google Scholar] [CrossRef]
- He, X.; Song, W.; Liu, X.; Huang, J.; Feng, R.; Zhou, S.; Hong, J.; Ge, X. Micelle-mediated multicomponent cross-coupling in water: General construction of 3-chalcogenylindoles. Green Chem. 2023, 25, 1311–1321. [Google Scholar] [CrossRef]
- Rios, E.A.M.; Gomes, C.M.B.; Silvério, G.L.; Luz, E.Q.; Ali, S.; D’Oca, C.d.R.M.; Albach, B.; Campos, R.B.; Rampon, D.S. Silver-catalyzed direct selanylation of indoles: Synthesis and mechanistic insights. RSC Adv. 2023, 13, 914–925. [Google Scholar] [CrossRef]
- Zhang, Q.-B.; Ban, Y.-L.; Yuan, P.-F.; Peng, S.-J.; Fang, J.-G.; Wu, L.-Z.; Liu, Q. Visible-light-mediated aerobic selenation of (hetero)arenes with diselenides. Green Chem. 2017, 19, 5559–5563. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Ramesh, V.; Gangadhar, M.; Vijaykumar, S. Catalyst and Sensitizer-Free Visible-Light-Induced C(sp2)−H Chalcogenation of Arenes/Heteroarenes with Dichalcogenides. Asian J. Org. Chem. 2018, 7, 1689–1697. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Franco, M.S.; Schneider, A.R.; Espíndola, L.; Silva, D.O.; Braga, A.L. Rose Bengal catalysed photo-induced selenylation of indoles, imidazoles and arenes: A metal free approach. Org. Biomol. Chem. 2018, 16, 880–885. [Google Scholar] [CrossRef]
- Rathore, V.; Kumar, S. Visible-light-induced metal and reagent-free oxidative coupling of sp2 C–H bonds with organo-dichalcogenides: Synthesis of 3-organochalcogenyl indoles. Green Chem. 2019, 21, 2670–2676. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Jiang, H.; Sun, L. Convenient synthesis of selenyl-indoles via iodide ion-catalyzed electrochemical C–H selenation. Chem. Commun. 2018, 54, 8781–8784. [Google Scholar] [CrossRef]
- Chen, C.; Niu, P.; Shen, Z.; Li, M. Electrochemical Sulfenylation of Indoles with Disulfides Mediated by Potassium Iodide. J. Electrochem. Soc. 2018, 165, G67. [Google Scholar] [CrossRef]
- Quadros, G.T.; de Medeiros, S.P.; de Oliveira, C.A.; Rambo, M.W.; Abenante, L.; Lenardão, E.J.; Penteado, F. Benzeneseleninic Acids (BSA) and Photocatalysis: An Alternative Duo for the Synthesis of 3-Selanylindoles. Asian J. Org. Chem. 2023, 12, e202300517. [Google Scholar] [CrossRef]
- Kumar, N.; Venkatesh, R.; Singh, S.; Kandasamy, J. Potassium Persulfate-Glucose Mediated Synthesis of (3)-S-Arylthioindoles from Indole and Thiophenols in Water. Eur. J. Org. Chem. 2023, 26, e202300679. [Google Scholar] [CrossRef]
- Huang, Q.; Peng, X.; Li, H.; He, H.; Liu, L. Visible-Light-Induced, Graphene Oxide-Promoted C3-Chalcogenylation of Indoles Strategy under Transition-Metal-Free Conditions. Molecules 2022, 27, 772. [Google Scholar] [CrossRef]
- Chen, M.; Luo, Y.; Zhang, C.; Guo, L.; Wang, Q.; Wu, Y. Graphene oxide mediated thiolation of indoles in water: A green and sustainable approach to synthesize 3-sulfenylindoles. Org. Chem. Front. 2019, 6, 116–120. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; He, J.; Han, S.; Liu, Y. Iodophor-catalyzed sulfenylation of indoles with sulfonyl hydrazides for the synthesis of 3-sulfenylindoles. RSC Adv. 2024, 14, 29891–29895. [Google Scholar] [CrossRef] [PubMed]
- Priya, V.R.P.; Mercy, A.A.H.; Natarajan, K.; Nandi, G.C. Sulfoximines as S-Aryl Surrogates: A Photocatalytic Rapid, Metal-Free, Mild Protocol to Access 3-Arylsulfenyl Indoles. Synlett 2023, 35, 279–284. [Google Scholar]
- Equbal, D.; Singh, R.; Saima; Lavekar, A.G.; Sinha, A.K. Synergistic Dual Role of [hmim]Br-ArSO2Cl in Cascade Sulfenylation–Halogenation of Indole: Mechanistic Insight into Regioselective C–S and C–S/C–X (X = Cl and Br) Bond Formation in One Pot. J. Org. Chem. 2019, 84, 2660–2675. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-F.; Yi, W.; Ling, Y.; Ming, L.; Liu, G.-Q.; Zhao, Y. Preparation of selenofunctionalized heterocycles via iodosobenzene-mediated intramolecular selenocyclizations of olefins with diselenides. Chin. Chem. Lett. 2021, 32, 2587–2591. [Google Scholar] [CrossRef]
- Liang, Z.-P.; Yi, W.; Wang, P.-F.; Liu, G.-Q.; Ling, Y. Iodosobenzene-mediated three-component selenofunctionalization of olefins. J. Org. Chem. 2021, 86, 5292–5304. [Google Scholar] [CrossRef]
- Zhou, C.-F.; Zhang, Y.-Q.; Ling, Y.; Ming, L.; Xi, X.; Liu, G.-Q.; Zhang, Y. Time-economical synthesis of selenofunctionalized heterocycles via I2O5-mediated selenylative heterocyclization. Org. Biomol. Chem. 2022, 20, 420–426. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, Y.-Q.; Zhou, C.-F.; Jiang, Y.-Q.; Xu, Y.; Zeng, X.; Liu, G.-Q. Iodine pentoxide-mediated oxidative selenation and seleno/thiocyanation of electron-rich arenes. Org. Biomol. Chem. 2022, 20, 5463–5469. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Wang, Y.-H.; Zhou, C.-F.; Zhang, Y.-Q.; Ling, Y.; Zhao, Y.; Liu, G.-Q. N-Fluorobenzenesulfonimide-Mediated Intermolecular Carboselenenylation of Olefins with Aromatics and Diselenides. J. Org. Chem. 2022, 87, 14609–14622. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Jiang, Y.-Q.; Wang, Y.-H.; Qi, C.; Ling, Y.; Zhang, Y.; Liu, G.-Q. Oxidative Three-Component Selenofunctionalization of Alkenes: Convenient Access to Vicinally Functionalized Selenides. J. Org. Chem. 2023, 88, 7431–7447. [Google Scholar] [CrossRef]
- Qu, P.; Jiang, Y.-Q.; Wu, H.; Wang, Y.-H.; Ling, Y.; Zhang, Y.; Liu, G.-Q. Photoinduced, trans-Diastreoselective Oxyselenenylation of Allylic Alcohols to Form Selenylated Cyclic Boronic Esters. Adv. Synth. Catal. 2024, 366, 3160–3165. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Yi, W.; Wang, P.-F.; Liu, J.; Ma, M.; Hao, D.-Y.; Ming, L.; Ling, Y. Visible-light-induced oxidative coupling of vinylarenes with diselenides leading to α-aryl and α-alkyl selenomethyl ketones. Green Chem. 2021, 23, 1840–1846. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Zhou, C.-F.; Zhang, Y.-Q.; Yi, W.; Wang, P.-F.; Liu, J.; Ling, Y. Visible-light-induced intermolecular aminoselenation of alkenes. Green Chem. 2021, 23, 9968–9973. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Claremon, D.A.; Barnette, W.E.; Seitz, S.P. N-Phenylselenophthalimide (N-PSP) and N-phenylselenosuccinimide (N-PSS). Two versatile carriers of the phenylseleno group. Oxyselenation of olefins and a selenium-based macrolide synthesis. J. Am. Chem. Soc. 1979, 101, 3704–3706. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Petasis, N.A.; Claremon, D.A. N-phenylselenophthalimide (NPSP): A valuable selenenylating agent. Tetrahedron 1985, 41, 4835–4841. [Google Scholar] [CrossRef]
- Doraghi, F.; Aledavoud, S.P.; Ghanbarlou, M.; Larijani, B.; Mahdavi, M. N-Sulfenylsuccinimide/phthalimide: An alternative sulfenylating reagent in organic transformations. Beilstein J. Org. Chem. 2023, 19, 1471–1502. [Google Scholar] [CrossRef]
- Qi, C.; Lu, Z.; Gu, Y.; Bao, X.; Xiong, B.; Liu, G.-Q. Zn(OTf)2-catalyzed intra- and intermolecular selenofunctionalization of alkenes under mild conditions. RSC Adv. 2024, 14, 23147–23151. [Google Scholar] [CrossRef]
- Qu, P.; Jiang, Y.-Q.; Wang, Y.-H.; Liu, G.-Q. Recent progress in the electrochemical selenofunctionalization of alkenes and alkynes. Green Chem. 2023, 25, 7485–7507. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Jiang, Y.-Q.; Zhang, Y.-Q.; Ling, Y.; Ming, L.; Liu, G.-Q. Photocatalytic Aerobic Cyclization of N-Propargylamides Enabled by Selenium-π-Acid Catalysis. Chem. Eur. J. 2023, 29, e202300530. [Google Scholar] [CrossRef]
- Tudge, M.; Tamiya, M.; Savarin, C.; Humphrey, G.R. Development of a Novel, Highly Efficient Halide-Catalyzed Sulfenylation of Indoles. Org. Lett. 2006, 8, 565–568. [Google Scholar] [CrossRef]
- Kumar, P.P.; Reddy, Y.D.; Reddy, C.V.R.; Devi, B.R.; Dubey, P.K. Indium chloride: A versatile Lewis acid catalyst for the synthesis of 3-sulfenylindoles. J. Sulfur Chem. 2014, 35, 356–361. [Google Scholar] [CrossRef]
- Nalbandian, C.J.; Miller, E.M.; Toenjes, S.T.; Gustafson, J.L. A conjugate Lewis base-Brønsted acid catalyst for the sulfenylation of nitrogen containing heterocycles under mild conditions. Chem. Commun. 2017, 53, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, J.-Q.; Cui, X.-Y.; Qin, Y.-H.; Ma, S.-J.; An, Z.-A.; Sun, W.-W.; Wu, B. A Method for the Synthesis of Thioindoles through Copper-Catalyzed C–S Bond Coupling Reaction. J. Org. Chem. 2024, 89, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wu, G.; Yang, H.; Liu, M.; Gao, W.; Huang, X.; Chen, J.; Wu, H. Copper-Catalyzed Three-Component Reaction for Regioselective Aryl- and Heteroarylselenation of Indoles using Selenium Powder. J. Org. Chem. 2016, 81, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Equbal, D.; Kumar, S.; Gayathri, P.R.; Kumar, R.; Sinha, A.K. Arylsulfonic Anhydride as Thiol Surrogate for Sulfenylation of sp2 C−H Bond Through De-oxygenative Reduction with Neutral Ionic Liquid: Scope and Mechanistic Studies. Adv. Synth. Catal. 2023, 365, 4163–4169. [Google Scholar] [CrossRef]
- Jing, Z.; Du, J.; Wang, C.; Ablajan, K. Iodine-promoted metal-free synthesis of mono-/di- and tri-substituted aryl sulfides/selenides in aqueous medium. Tetrahedron 2024, 156, 133941. [Google Scholar] [CrossRef]
- Choudhury, P.; Roy, B.; Basu, B. Sustainable and Site-Selective C-H Sulfenylation of Aromatic Compounds with Thiol using Catalytic Graphene Oxide and NaI. Asian J. Org. Chem. 2017, 6, 1569–1574. [Google Scholar] [CrossRef]


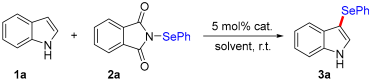 | |||
|---|---|---|---|
| Entry | cat. | Solvent | Isolated Yield (%) |
| 1 | Zn(OTf)2 | DCM | 75 |
| 2 | Cu(OTf)2 | DCM | 70 |
| 3 | AgOTf | DCM | 62 |
| 4 | Yb(OTf)3 | DCM | 71 |
| 5 | In(OTf)3 | DCM | 75 |
| 6 | ZnBr2 | DCM | 77 |
| 7 | FeCl3 | DCM | 82 |
| 8 | CaBr2 | DCM | 79 |
| 9 | CuBr2 | DCM | 87 |
| 10 | MnBr2 | DCM | 80 |
| 11 | CuBr2 | MeOH | 53 |
| 12 | CuBr2 | DMF | 44 |
| 13 | CuBr2 | EtOAc | 71 |
| 14 | CuBr2 | CH3CN | 63 |
| 15 | CuBr2 | hexane | 47 |
| 16 b | CuBr2 | DCM | 86 (82) d |
| 17 c | CuBr2 | DCM | 62 |
| 18 | - | DCM | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Chen, S.; Wu, D.; Shao, J.; Bao, X.; Liu, G.-Q. Copper(II)-Catalyzed Direct C3 Chalcogenylation of Indoles. Molecules 2025, 30, 1870. https://doi.org/10.3390/molecules30091870
Pan L, Chen S, Wu D, Shao J, Bao X, Liu G-Q. Copper(II)-Catalyzed Direct C3 Chalcogenylation of Indoles. Molecules. 2025; 30(9):1870. https://doi.org/10.3390/molecules30091870
Chicago/Turabian StylePan, Liuyan, Shengwei Chen, Dongfang Wu, Jian Shao, Xiaofeng Bao, and Gong-Qing Liu. 2025. "Copper(II)-Catalyzed Direct C3 Chalcogenylation of Indoles" Molecules 30, no. 9: 1870. https://doi.org/10.3390/molecules30091870
APA StylePan, L., Chen, S., Wu, D., Shao, J., Bao, X., & Liu, G.-Q. (2025). Copper(II)-Catalyzed Direct C3 Chalcogenylation of Indoles. Molecules, 30(9), 1870. https://doi.org/10.3390/molecules30091870





