Research Progress on the Therapeutic Mechanisms of Stigmasterol for Multiple Diseases
Abstract
:1. Introduction
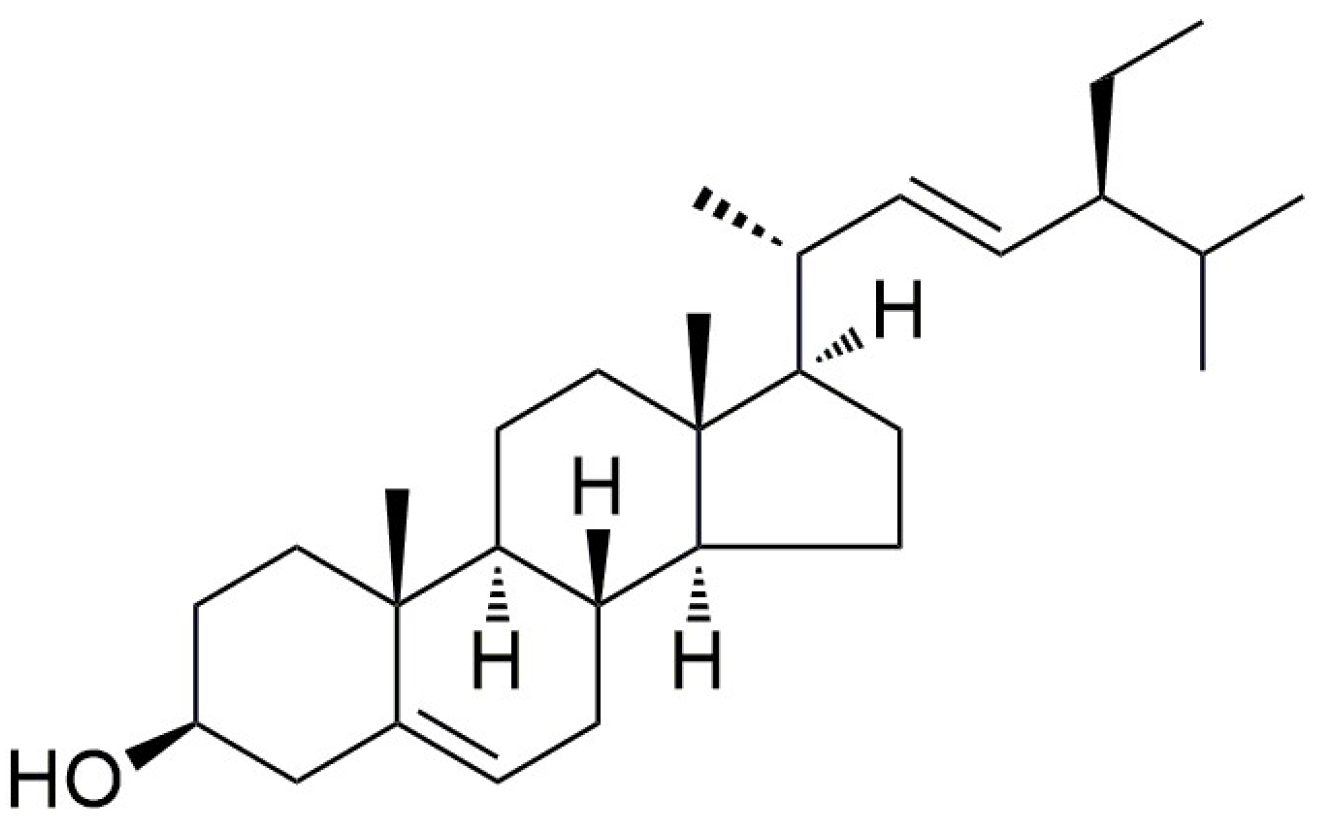
2. Structural Characteristics, Biosynthesis, and Potential Applications of Stigmasterol
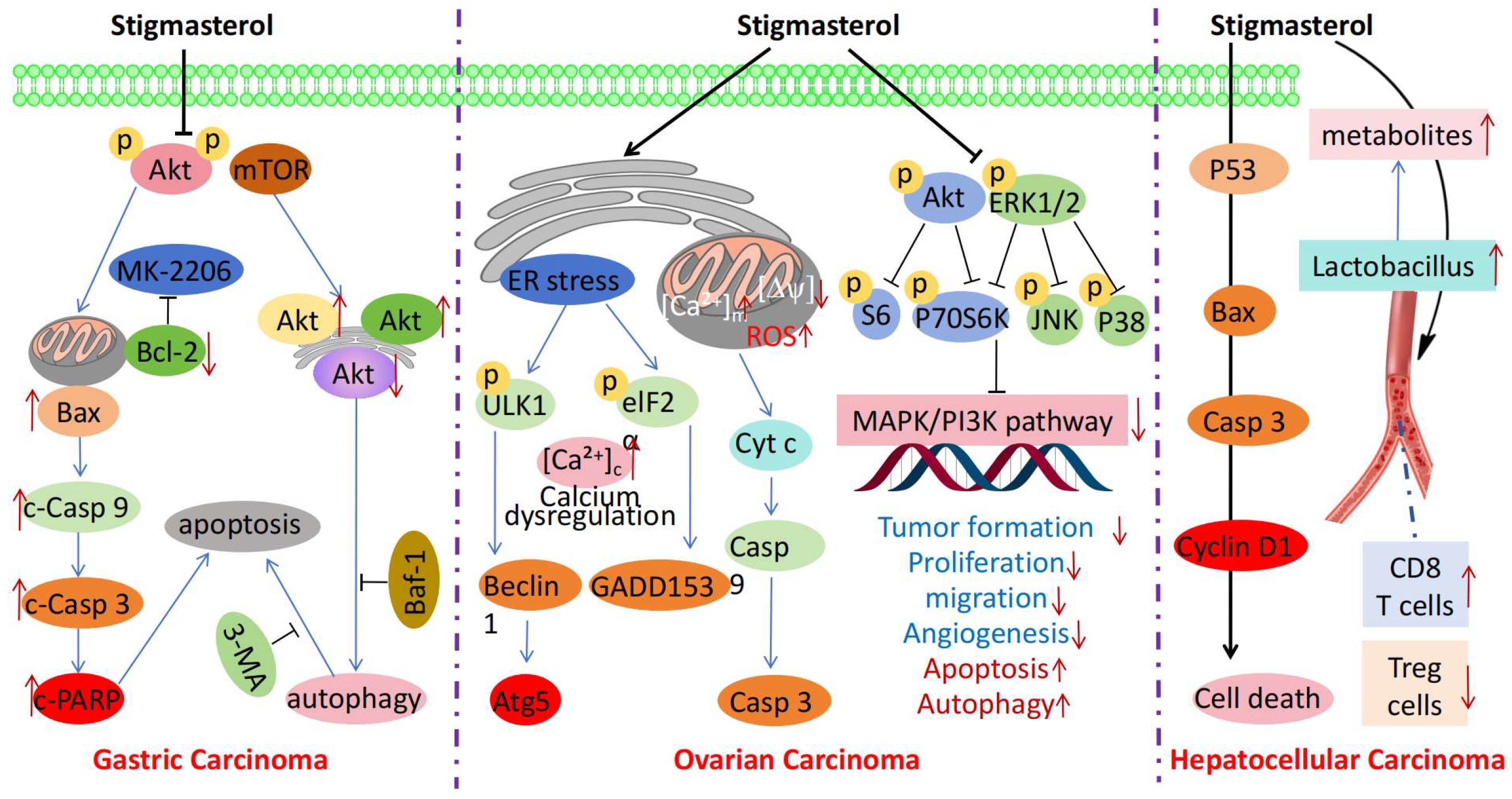
3. Anticancer Mechanisms of Stigmasterol
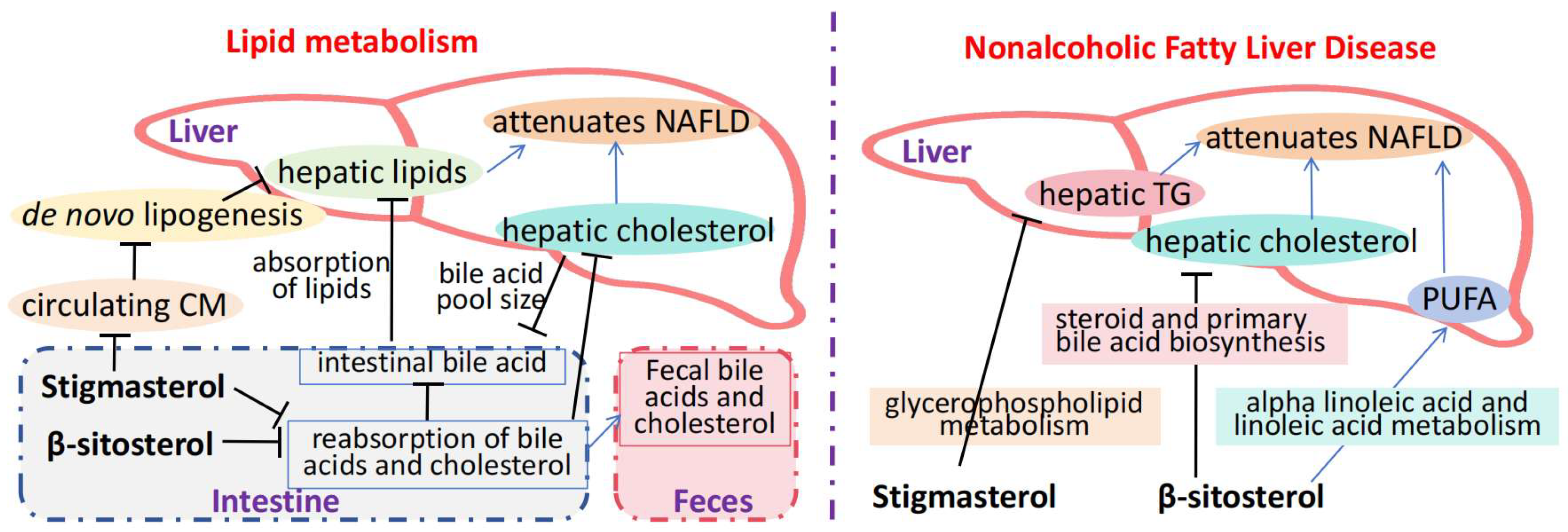
4. Mechanisms Through Which Stigmasterol Alleviates Metabolic Diseases
5. Cardiovascular Protective Mechanisms of Stigmasterol
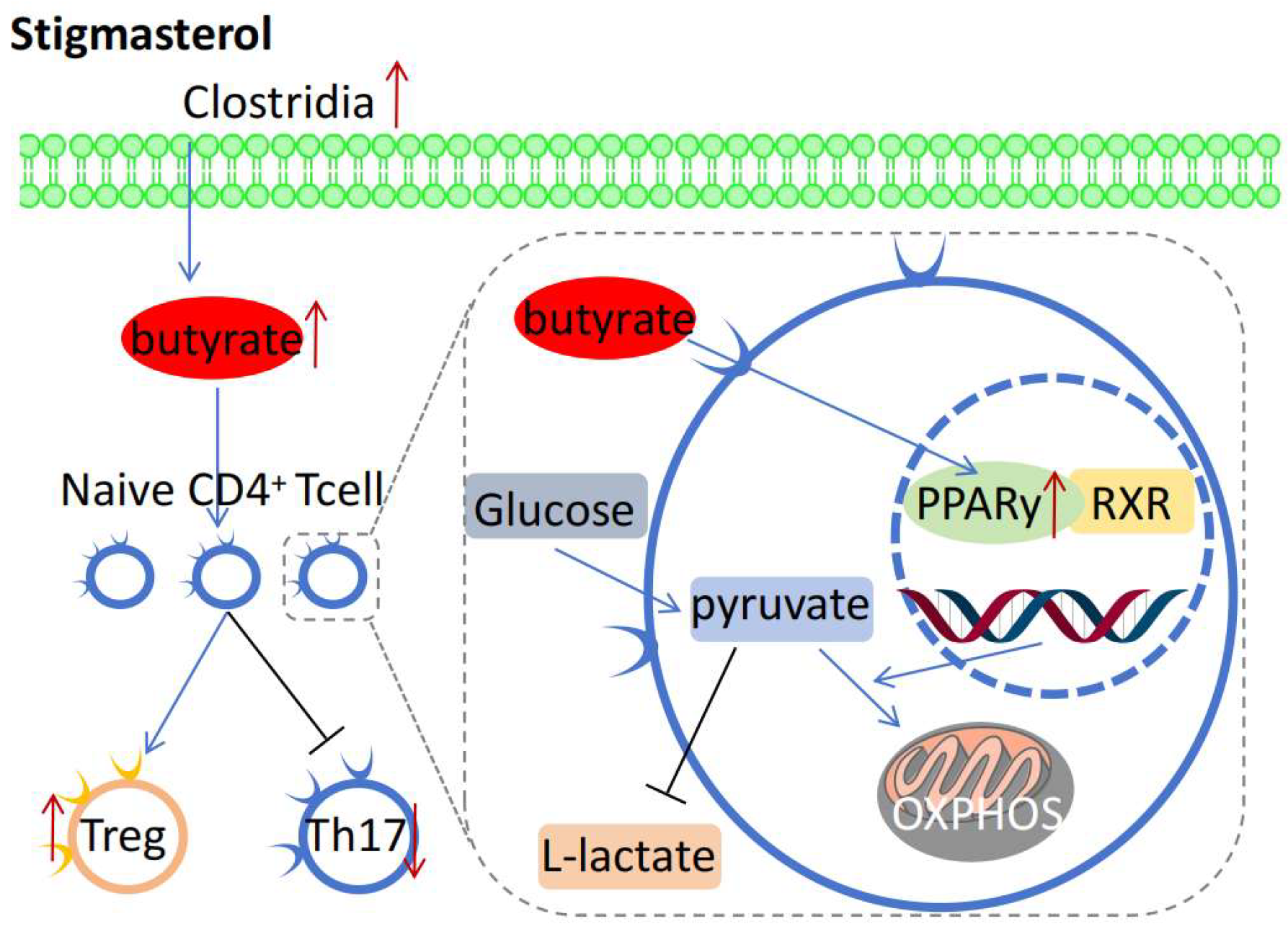
6. Anti-Inflammatory and Immune Regulatory Mechanisms of Stigmasterol
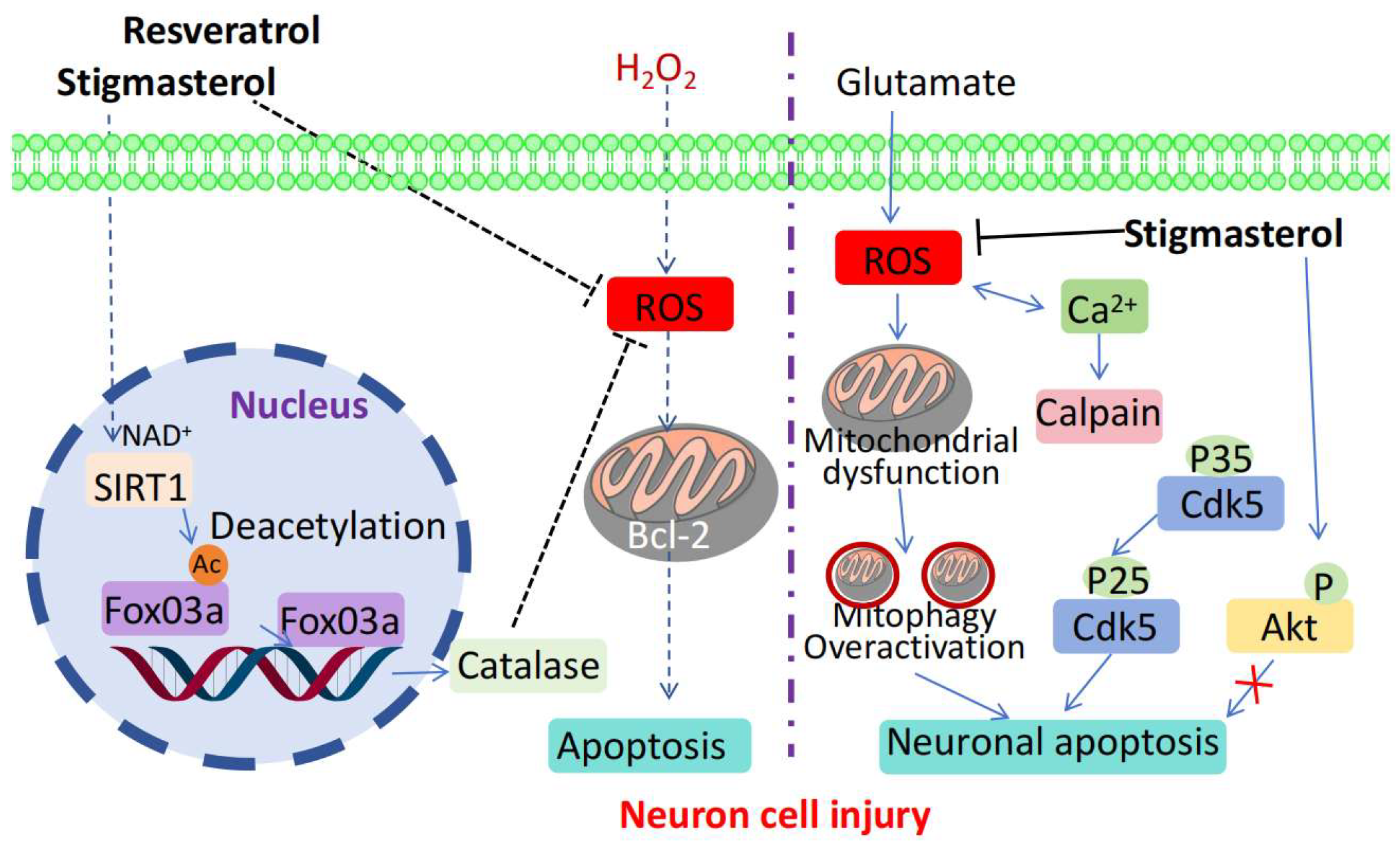
7. Neuroprotective Mechanisms of Stigmasterol
8. Limitations, Challenges, and Future Prospects of Stigmasterol
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Huang, W.; Huang, J.; Luo, Y.; Huang, N. Natural bioactive compounds form herbal medicine in Alzheimer’s disease: From the perspective of GSK-3beta. Front. Pharmacol. 2025, 16, 1497861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shen, S.; Zhang, M.; Luo, H.; Zhang, Y.; Wu, C.; Zeng, L.; Ruan, H. Mechanisms of action and synergetic formulas of plant-based natural compounds from traditional Chinese medicine for managing osteoporosis: A literature review. Front. Med. 2023, 10, 1235081. [Google Scholar] [CrossRef]
- Li, X.H.; Yin, F.T.; Zhou, X.H.; Zhang, A.H.; Sun, H.; Yan, G.L.; Wang, X.J. The Signaling Pathways and Targets of Natural Compounds from Traditional Chinese Medicine in Treating Ischemic Stroke. Molecules 2022, 27, 3099. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xu, D.; Liu, D.; Ran, X.; Li, F.; Wang, J.; Ge, Y.; Liu, Y.; Guo, W.; Liu, J.; et al. Stigmasterol from Prunella vulgaris L. Alleviates LPS-induced mammary gland injury by inhibiting inflammation and ferroptosis. Phytomedicine 2025, 137, 156362. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Duan, S.; Baik, M.Y.; Eom, S.H. Thermal stability of stigmasterol and beta-sitosterol glucosides in fresh-cut bitter melon fruit. Food Chem. 2025, 468, 142414. [Google Scholar] [CrossRef]
- Ding, L.; Lin, H.; Ma, Z.; He, Y.; Ding, S.; Zhang, K.; Zhang, J.; Li, W.; Xiao, L. Stigmasterol mitigates rheumatoid arthritis progression by decreasing Nrf2/NLRP3-mediated pyroptosis in chondrocyte. Mol. Immunol. 2025, 179, 9–17. [Google Scholar] [CrossRef]
- Zhan, T.; Jacoby, C.; Jede, M.; Knapp, B.; Ferlaino, S.; Gunter, A.; Drepper, F.; Muller, M.; Weber, S.; Boll, M. Bacterial stigmasterol degradation involving radical flavin delta-24 desaturase and molybdenum-dependent C26 hydroxylase. J. Biol. Chem. 2024, 300, 107243. [Google Scholar] [CrossRef]
- Angsusing, J.; Singh, S.; Samee, W.; Tadtong, S.; Stokes, L.; O’Connell, M.; Bielecka, H.; Toolmal, N.; Mangmool, S.; Chittasupho, C. Anti-Inflammatory Activities of Yataprasen Thai Traditional Formulary and Its Active Compounds, Beta-Amyrin and Stigmasterol, in RAW264.7 and THP-1 Cells. Pharmaceuticals 2024, 17, 1018. [Google Scholar] [CrossRef]
- Xin, Y.; Li, X.; Zhu, X.; Lin, X.; Luo, M.; Xiao, Y.; Ruan, Y.; Guo, H. Stigmasterol Protects Against Steatohepatitis Induced by High-Fat and High-Cholesterol Diet in Mice by Enhancing the Alternative Bile Acid Synthesis Pathway. J. Nutr. 2023, 153, 1903–1914. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, C.; Chen, C.; Zhang, C.; Zhang, L.; Zhang, Y.; Li, Y.; Dong, Z. Stigmasterol inhibits the progression of lung cancer by regulating retinoic acid-related orphan receptor C. Histol. Histopathol. 2021, 36, 1285–1299. [Google Scholar]
- Zhou, R.; Zhang, Y.; Xu, L.; Sun, Y. Stigmasterol Attenuates Triple-negative Breast Cancer Stem Cell Properties by Inhibiting JAK3. J. Cancer 2025, 16, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Baothman, O.; EM, M.A.; Hosawi, S.; EH, E.K.; Abu Zeid, I.M.; Ahmad, A.; Altayb, H.N. Multi-targeted therapeutic potential of stigmasterol from the Euphorbia ammak plant in treating lung and breast cancer. Comput. Biol. Chem. 2024, 110, 108037. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, D.; Bai, M.; Chen, H.; Yan, S.; Yu, J.; Zhu, H.; Zheng, W.; Fan, G. Stigmasterol sensitizes endometrial cancer cells to chemotherapy by repressing Nrf2 signal pathway. Cancer Cell Int. 2020, 20, 480. [Google Scholar] [CrossRef]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J. BUON 2018, 23, 1420–1425. [Google Scholar] [PubMed]
- AmeliMojarad, M.; AmeliMojarad, M.; Pourmahdian, A. The inhibitory role of stigmasterol on tumor growth by inducing apoptosis in Balb/c mouse with spontaneous breast tumor (SMMT). BMC Pharmacol. Toxicol. 2022, 23, 42. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Wang, M.; Lin, Y.; Zhou, S. Stigmasterol Simultaneously Induces Apoptosis and Protective Autophagy by Inhibiting Akt/mTOR Pathway in Gastric Cancer Cells. Front. Oncol. 2021, 11, 629008. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lim, W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Deol, P.; Kozlova, E.; Valdez, M.; Ho, C.; Yang, E.W.; Richardson, H.; Gonzalez, G.; Truong, E.; Reid, J.; Valdez, J.; et al. Dysregulation of Hypothalamic Gene Expression and the Oxytocinergic System by Soybean Oil Diets in Male Mice. Endocrinology 2020, 161, bqz044. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.; Zhao, P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef]
- Feng, S.; Gan, L.; Yang, C.S.; Liu, A.B.; Lu, W.; Shao, P.; Dai, Z.; Sun, P.; Luo, Z. Effects of Stigmasterol and beta-Sitosterol on Nonalcoholic Fatty Liver Disease in a Mouse Model: A Lipidomic Analysis. J. Agric. Food. Chem. 2018, 66, 3417–3425. [Google Scholar] [CrossRef]
- Rudzinska, M.; Cieslik-Boczula, K.; Grygier, A.; Kmiecik, D.; Dwiecki, K.; Jarzebski, M. Stigmasterol and its esters encapsulated in liposomes: Characterization, stability, and derivative formation. Food Chem. 2025, 465 Pt 2, 142039. [Google Scholar] [CrossRef]
- Wang, H.; Mao, Z.; Xiang, H.; Huang, H.; Yang, X.; Yang, C. Stigmasterol, a Major Component of Cornus Officinalis, Ameliorates Osteoporosis in Diabetes Mellitus Effects by Increasing Bone Mineral Density. J. Musculoskelet. Neuronal Interact. 2025, 25, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Min, J.E.; Long, N.P.; Hong, J.Y.; Kim, S.J.; Anh, N.H.; Wang, D.; Wang, X.; Park, J.H.; Kwon, S.W.; Lee, S.J. The dehiscence process in Panax ginseng seeds and the stigmasterol biosynthesis pathway in terms of metabolomics. J. Ginseng Res. 2022, 46, 225–234. [Google Scholar] [CrossRef]
- Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. Int. J. Mol. Sci. 2024, 25, 8122. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Wang, J.; Lai, Z.; Liang, S.; Zou, W.; Wang, J.; Zheng, D.; Li, Y.; He, Y.; Cheng, J.; et al. Arisaema heterophyllum Blume Monomer Stigmasterol Targets PPARgamma and Inhibits the Viability and Tumorigenicity of Lung Adenocarcinoma Cells NCI-H1975. Evid.-Based Complement. Altern. Med. 2022, 2022, 5377690. [Google Scholar] [CrossRef]
- Wen, S.; He, L.; Zhong, Z.; Zhao, R.; Weng, S.; Mi, H.; Liu, F. Stigmasterol Restores the Balance of Treg/Th17 Cells by Activating the Butyrate-PPARgamma Axis in Colitis. Front. Immunol. 2021, 12, 741934. [Google Scholar] [CrossRef]
- Si, W.; Chen, Z.; Bei, J.; Chang, S.; Zheng, Y.; Gao, L.; Zhao, G.; Li, X.; Zhang, D. Stigmasterol alleviates neuropathic pain by reducing Schwann cell-macrophage cascade in DRG by modulating IL-34/CSF1R. CNS Neurosci. Ther. 2024, 30, e14657. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- Mustafa, M.; Abbas, K.; Ahmad, W.; Ahmad, R.; Islam, S.; Khan, H.; Moinuddin; Hassan, M.I.; Parveen, S.; Habib, S. A Promising Druggable Target for Translational Therapy of Ovarian Cancer: A Molecular Profiling of Therapeutic Innovations, Extracellular Vesicle Acquired Resistance, and Signaling Pathways. Curr. Med. Chem. 2025. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Afshinpour, M.; Fakhr, S.S.; Kalkhoran, P.G.; Shadman-Manesh, V.; Adelian, S.; Beiranvand, S.; Rezaei-Tazangi, F.; Khorram, R.; Hamblin, M.R.; et al. Cancer stem cells in colorectal cancer: Signaling pathways involved in stemness and therapy resistance. Crit. Rev. Oncol. Hematol. 2023, 182, 103920. [Google Scholar] [CrossRef]
- Hargadon, K.M. Genetic dysregulation of immunologic and oncogenic signaling pathways associated with tumor-intrinsic immune resistance: A molecular basis for combination targeted therapy-immunotherapy for cancer. Cell. Mol. Life Sci. 2023, 80, 40. [Google Scholar] [CrossRef]
- Morimoto, Y.; Takada, K.; Takeuchi, O.; Watanabe, K.; Hirohara, M.; Hamamoto, T.; Masuda, Y. Bcl-2/Bcl-xL inhibitor navitoclax increases the antitumor effect of Chk1 inhibitor prexasertib by inducing apoptosis in pancreatic cancer cells via inhibition of Bcl-xL but not Bcl-2. Mol. Cell. Biochem. 2020, 472, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Kohn, E.C.; Brill, E.; Lee, J.M. Apoptosis is augmented in high-grade serous ovarian cancer by the combined inhibition of Bcl-2/Bcl-xL and PARP. Int. J. Oncol. 2017, 50, 1064–1074. [Google Scholar] [CrossRef]
- Khan, H.; Singh, A.; Singh, Y.; Sharma, D.; Dua, K.; Grewal, A.K.; Singh, T.G. Pharmacological modulation of PI3K/PTEN/Akt/mTOR/ERK signaling pathways in ischemic injury: A mechanistic perspective. Metab. Brain Dis. 2025, 40, 131. [Google Scholar] [CrossRef]
- Roopashree, P.G.; Shetty, S.S.; Shetty, V.V.; Suhasini, P.C.; Suchetha, K.N. Inhibitory effects of medium-chain fatty acids on the proliferation of human breast cancer cells via suppression of Akt/mTOR pathway and modulating the Bcl-2 family protein. J. Cell. Biochem. 2024, 125, e30571. [Google Scholar] [CrossRef]
- Tungsukruthai, S.; Reamtong, O.; Roytrakul, S.; Sukrong, S.; Vinayanwattikun, C.; Chanvorachote, P. Targeting AKT/mTOR and Bcl-2 for Autophagic and Apoptosis Cell Death in Lung Cancer: Novel Activity of a Polyphenol Compound. Antioxidants 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Siddiqui, S.; Ahmad, I.; Singh, R.; Mishra, D.P.; Srivastava, A.N.; Ahmad, R. Phytochemicals from Ajwa dates pulp extract induce apoptosis in human triple-negative breast cancer by inhibiting AKT/mTOR pathway and modulating Bcl-2 family proteins. Sci. Rep. 2021, 11, 10322. [Google Scholar] [CrossRef]
- Huo, R.; Yang, W.J.; Liu, Y.; Liu, T.; Li, T.; Wang, C.Y.; Pan, B.S.; Wang, B.L.; Guo, W. Stigmasterol: Remodeling gut microbiota and suppressing tumor growth through Treg and CD8+ T cells in hepatocellular carcinoma. Phytomedicine 2024, 129, 155225. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhou, Y.; He, K.; Lu, Y.; Tao, F.; Huang, H. Glucose Metabolic Reprogramming in Microglia: Implications for Neurodegenerative Diseases and Targeted Therapy. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef]
- Angiulli, S.; Merolla, A.; Borgonovo, E.; De Lorenzo, R.; Spadoni, S.; Fontana, B.; Manganaro, G.; Rela, E.; Bongiovanni, A.; Peracino, R.; et al. Universal capillary screening for chronic autoimmune, metabolic and cardiovascular diseases: Feasibility and acceptability of the UNISCREEN study. Front. Public Health 2025, 13, 1506240. [Google Scholar] [CrossRef]
- Varillas-Delgado, D. Role of the PPARGC1A Gene and Its rs8192678 Polymorphism on Sport Performance, Aerobic Capacity, Muscle Adaptation and Metabolic Diseases: A Narrative Review. Genes 2024, 15, 1631. [Google Scholar] [CrossRef]
- Fan, L.; Li, L.; Zhao, Y.; Zhao, Y.; Wang, F.; Wang, Q.; Ma, Z.; He, S.; Qiu, J.; Zhang, J.; et al. Antagonizing Effects of Chromium Against Iron-Decreased Glucose Uptake by Regulating ROS-Mediated PI3K/Akt/GLUT4 Signaling Pathway in C2C12. Biol. Trace Elem. Res. 2024, 202, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, S.; Gong, C.; Chen, L. Neuregulin-1beta increases glucose uptake and promotes GLUT4 translocation in palmitate-treated C2C12 myotubes by activating PI3K/AKT signaling pathway. Front. Pharmacol. 2022, 13, 1066279. [Google Scholar]
- Feng, S.Y.; Wu, S.J.; Chang, Y.C.; Ng, L.T.; Chang, S.J. Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells. Metabolites 2022, 12, 856. [Google Scholar] [CrossRef]
- Ghosh, C.; Kundu, T.; Pathak, T.; Saini, S.; Das, N.; Saini, S.; Sircar, D.; Kumar, P.; Roy, P. Indian lychee honey ameliorates hepatic glucose uptake by regulating the ChREBP/Glut4 axis under insulin-resistant conditions. Food Funct. 2025, 16, 2031–2056. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.M.; Lanfranconi, M.P.; Hernandez, M.A. Metabolism-lipid droplet-nucleic acid crosstalk to regulate lipid storage and other cellular processes in oleaginous Rhodococcus bacteria. Biol. Cell 2025, 117, e2400094. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, T.; Li, Y.; Wang, Q.; Du, Y.; Li, R.; Lin, J.; Fu, J.; Chen, X.; Luo, S. Adipose stem cells regulate lipid metabolism by upregulating mitochondrial fatty acid beta-oxidation in macrophages to improve the retention rate of transplanted fat. Stem Cell Res. Ther. 2024, 15, 328. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, H.; Ma, B.; Zhang, Z.; Wang, J.; Wang, J.; Chen, O. Lipid metabolism-related genes are involved in the occurrence of asthma and regulate the immune microenvironment. BMC Genom. 2024, 25, 129. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and beta-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar]
- Zhao, Y.; Zhang, H.; Lian, L.; Wang, X.; Gao, W.; Zhu, B.; Lou, D. A molecular beacon-like Ag nanocluster fluorescence probe for nucleic acid detection. Anal. Sci. 2022, 38, 131–136. [Google Scholar] [CrossRef]
- Behr, C.M.; IJzerman, M.J.; Kip, M.M.A.; Groen, H.J.M.; Heuvelmans, M.A.; van den Berge, M.; van der Harst, P.; Vonder, M.; Vliegenthart, R.; Koffijberg, H. Model-Based Cost-Utility Analysis of Combined Low-Dose Computed Tomography Screening for Lung Cancer, Chronic Obstructive Pulmonary Disease, and Cardiovascular Disease. JTO Clin. Res. Rep. 2025, 6, 100813. [Google Scholar] [CrossRef]
- Umishio, W.; Ikaga, T.; Kario, K.; Fujino, Y.; Kagi, N.; Suzuki, M.; Ando, S.; Saeki, K.; Murakami, S. Effect of living in well-insulated warm houses on hypertension and cardiovascular diseases based on a nationwide epidemiological survey in Japan: A modelling and cost-effectiveness analysis. BMJ Public Health 2024, 2, e001143. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, N.; Huang, L.; Chen, C.; Dong, X.; Gao, W. Exploring Potential Drug Targets in Multiple Cardiovascular Diseases: A Study Based on Proteome-Wide Mendelian Randomization and Colocalization Analysis. Cardiovasc. Ther. 2025, 2025, 5711316. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Shapiro, M.D. Immune-Mediated Inflammatory Diseases, Dyslipidemia, and Cardiovascular Risk: A Complex Interplay. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2396–2406. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Satoh, M.; Yoshino, K.; Ishimori, N. Recent advances regarding the potential roles of invariant natural killer T cells in cardiovascular diseases with immunological and inflammatory backgrounds. Int. Immunol. 2024, 36, 377–392. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, A. Causal effects of inflammatory cytokines on cardiovascular diseases: Insights from genetic evidence. Heliyon 2024, 10, e35447. [Google Scholar] [CrossRef]
- Lifsey, H.C.; Kaur, R.; Thompson, B.H.; Bennett, L.; Temel, R.E.; Graf, G.A. Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine. J. Nutr. Biochem. 2020, 76, 108263. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Xie, Z.; Lu, Q.; Luo, S. Stigmasterol protects against Ang II-induced proliferation of the A7r5 aortic smooth muscle cell-line. Food Funct. 2015, 6, 2266–2272. [Google Scholar] [CrossRef]
- Tsutsuki, H.; Zhang, T.; Akaike, T.; Sawa, T. Regulation of innate immune and inflammatory responses by supersulfides. Int. Immunol. 2024, 36, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, H.; Shan, M.; Dong, Y.; Zhang, L.; Chen, L.; Wang, Y. Comprehensive Transcriptome Analysis of Patients with Keratoconus Highlights the Regulation of Immune Responses and Inflammatory Processes. Front. Genet. 2022, 13, 782709. [Google Scholar] [CrossRef]
- Chen, Q.; Li, H.; Liu, Y.; Zhao, M. Epigenetic Regulation of Immune and Inflammatory Responses in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 881191. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Sais, D.; Chowdhury, S.; Dalton, J.P.; Tran, N.; Donnelly, S. Both host and parasite non-coding RNAs co-ordinate the regulation of macrophage gene expression to reduce pro-inflammatory immune responses and promote tissue repair pathways during infection with fasciola hepatica. RNA Biol. 2024, 21, 62–77. [Google Scholar] [CrossRef]
- Makita, S.; Takatori, H.; Nakajima, H. Post-Transcriptional Regulation of Immune Responses and Inflammatory Diseases by RNA-Binding ZFP36 Family Proteins. Front. Immunol. 2021, 12, 711633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Duan, Z.; Yang, B.; Xiao, C. The Effective Regulation of Pro- and Anti-inflammatory Cytokines Induced by Combination of PA-MSHA and BPIFB1 in Initiation of Innate Immune Responses. Open Med. 2017, 12, 299–307. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Zou, Y.; Chen, C.; Wang, S. Stigmasterol and gastrodin, two major components of banxia-baizhu-tianma decoction, alleviated the excessive phlegm-dampness hypertension by reducing lipid accumulation. J. Ethnopharmacol. 2024, 319 Pt 2, 117193. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yang, H.; Guo, C.; Xie, R.; Yu, G.; Li, Y. Integrated Network Pharmacology and Experimental Validation Approach to Investigate the Mechanisms of Stigmasterol in the Treatment of Rheumatoid Arthritis. Drug Des. Devel. Ther. 2023, 17, 691–706. [Google Scholar] [CrossRef]
- Ahmad Khan, M.; Sarwar, A.; Rahat, R.; Ahmed, R.S.; Umar, S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int. Immunopharmacol. 2020, 85, 106642. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Miao, L.; Meng, Z.; Gu, N.; Song, G. Stigmasterol alleviates allergic airway inflammation and airway hyperresponsiveness in asthma mice through inhibiting substance-P receptor. Pharm. Biol. 2023, 61, 449–458. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Xie, W.; Guo, Y.; Cai, J.; Huang, T.; Li, P. Advances in clinical translation of stem cell-based therapy in neurological diseases. J. Cereb. Blood Flow Metab. 2025, 45, 600–616. [Google Scholar] [CrossRef]
- Jiang, S.; Bao, X.; Zhong, C.; Wang, R. Mapping the global clinical landscape of stem cell therapies for neurological diseases from 1998 to 2023: An analysis based on the Trialtrove database. Stem Cell Res. Ther. 2025, 16, 41. [Google Scholar] [CrossRef]
- Ikeuchi, Y.; Kohta, M.; Hosoda, K.; Yamashita, S.; Shose, H.; Yamanishi, S.; Tanaka, K.; Sasayama, T. Systematic review of neurological diseases and carbenoxolone: A double-edged sword? Eur. J. Pharmacol. 2025, 994, 177387. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, F.; Wang, T.; Zhang, X.; Chen, D.; Wang, Y.; Chen, C.; Pan, G. Research progress in the mechanisms and functions of specialized pro-resolving mediators in neurological diseases. Prostaglandins Other Lipid Mediat. 2024, 175, 106905. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, K.; Qin, X.; Du, G.; Gao, L. The mechanisms of perineuronal net abnormalities in contributing aging and neurological diseases. Ageing Res. Rev. 2023, 92, 102092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Tao, J.; Sun, T. Treatments and regulatory mechanisms of acoustic stimuli on mood disorders and neurological diseases. Front. Neurosci. 2023, 17, 1322486. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nantasenamat, C.; Ruankham, W.; Suwanjang, W.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Mechanisms and Neuroprotective Activities of Stigmasterol Against Oxidative Stress-Induced Neuronal Cell Death via Sirtuin Family. Front. Nutr. 2021, 8, 648995. [Google Scholar] [CrossRef]
- Haque, M.N.; Moon, I.S. Stigmasterol promotes neuronal migration via reelin signaling in neurosphere migration assays. Nutr Neurosci. 2020, 23, 679–687. [Google Scholar] [CrossRef]
- Haque, M.N.; Moon, I.S. Stigmasterol upregulates immediate early genes and promotes neuronal cytoarchitecture in primary hippocampal neurons as revealed by transcriptome analysis. Phytomedicine 2018, 46, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Bhuiyan, M.M.H.; Moon, I.S. Stigmasterol activates Cdc42-Arp2 and Erk1/2-Creb pathways to enrich glutamatergic synapses in cultures of brain neurons. Nutr. Res. 2018, 56, 71–78. [Google Scholar] [CrossRef]
- Liang, Q.; Yang, J.; He, J.; Chen, X.; Zhang, H.; Jia, M.; Liu, K.; Jia, C.; Pan, Y.; Wei, J. Stigmasterol alleviates cerebral ischemia/reperfusion injury by attenuating inflammation and improving antioxidant defenses in rats. Biosci. Rep. 2020, 40, BSR20192133. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Liu, J.; Pan, X.; Zhao, Q. Stigmasterol Exerts Neuro-Protective Effect Against Ischemic/Reperfusion Injury Through Reduction of Oxidative Stress and Inactivation of Autophagy. Neuropsychiatr. Dis. Treat. 2019, 15, 2991–3001. [Google Scholar] [CrossRef]
- Si, W.; Li, X.; Jing, B.; Chang, S.; Zheng, Y.; Chen, Z.; Zhao, G.; Zhang, D. Stigmasterol regulates microglial M1/M2 polarization via the TLR4/NF-kappaB pathway to alleviate neuropathic pain. Phytother. Res. 2024, 38, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Mongkolpobsin, K.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Baek, S.J. Stigmasterol isolated from Azadirachta indica flowers attenuated glutamate-induced neurotoxicity via downregulation of the Cdk5/p35/p25 signaling pathway in the HT-22 cells. Phytomedicine 2023, 113, 154728. [Google Scholar] [CrossRef] [PubMed]
| Disease | Study Model | Observed Therapeutic Effects | Mechanism of Action | References |
|---|---|---|---|---|
| Breast Cancer | MCF-7 cell line, Balb/c mouse model of spontaneous breast tumor (SMMT) | Downregulates related genes, induces apoptosis, and inhibits cell proliferation and tumor growth | Downregulates the Bcl-2 and BCL-XL genes, thereby inducing apoptosis | [15] |
| Gastric Cancer | SGC-7901 and MGC-803 cells, xenograft model of gastric cancer in nude mice | Inhibits cell proliferation, induces apoptosis and autophagy, and suppresses tumor growth | Inhibits Akt/mTOR signaling pathway, thereby inducing apoptosis and protective autophagy | [16] |
| Endometrial Cancer | Ishikawa cell line, related animal model (not specified in detail) | Enhances the sensitivity of cancer cells to chemotherapy | Inhibits the Nrf2 signaling pathway, thereby enhancing the sensitivity of cancer cells to chemotherapy | [13] |
| Ovarian Cancer | ES2 and OV90 cells | Inhibits cell growth, induces apoptosis, ROS production, and calcium overload, and suppresses related genes | Induces endoplasmic reticulum and mitochondrial dysfunction, thereby promoting cancer cell apoptosis | [17] |
| Lung Cancer | Unspecified lung cancer cell lines | Inhibits cell proliferation and promotes apoptosis | Modulates retinoic acid-related orphan receptor C, thereby inhibiting cancer cell proliferation and promoting apoptosis | [10] |
| Liver Cancer | Subcutaneous tumor model of Balb/c mice inoculated with liver cancer cells | Suppresses tumor growth and regulates the gut microbiota and immune cell ratios | Modulates gut microbiota, thus inhibiting tumor growth | [38] |
| Disease | Study Model | Observed Therapeutic Effects | Mechanism of Action | References |
|---|---|---|---|---|
| Diabetes | In vitro experiments (L6 cells), KK-Ay mice | Promotes glucose uptake and improves insulin resistance and blood glucose indices | Targets the GLUT4 transporter and regulates its expression and translocation | [19] |
| Nonalcoholic fatty liver disease (NAFLD) | Mouse model of NAFLD induced by a high-fat diet | Reduces hepatic steatosis and improves lipid metabolism indices | Inhibition of NF-κB pathway alleviates hepatitis and steatosis | [45,46,47,48] |
| HFD—induced dyslipidemia, obesity, hepatic steatosis | Rats on HFD, some given ST, fecal microbiota transplant tests, combined—treatment tests | Relieve HFD lipid disorder and improve treatment with combined medication | Reverse the imbalance of flora, change BA metabolism, associated with enterohepatic circulation | [50] |
| Disease | Study Model | Observed Therapeutic Effects | Mechanism of Action | References |
|---|---|---|---|---|
| Alzheimer’s disease | Human neuronal cells (SH-SY5Y cells) | Upregulated FoxO 3a, catalase, Bcl-2, increased SIRT1 expression, decreased acetylated lysine levels, and stimulated SIRT1 activity. | Controlling ROS, promoting anti-oxidative and anti-apoptotic factors, and activating SIRT1 through lysine de-acetylation. | [76,77,78,79] |
| Cerebral Ischemia/Reperfusion Injury | Rat model of cerebral ischemia/reperfusion injury | Reduces damage, improves pathological changes, and inhibits apoptosis | Reduces oxidative stress and inflammation and inhibits autophagy | [80,81] |
| Neuropathic Pain | Rats with CCI and cell cultures (microglia, Schwann cells, macrophages) | Reduced pain hypersensitivity in rats. Altered cytokine levels. Regulated M1/M2 polarization markers. Lowered IL-34, CSF1R, NLRP3 levels in relevant cells and tissues. | Regulates microglial M1/M2 polarization through the TLR4/NF-κB pathway. Reduces IL-34-CSF1R-mediated activation and NLRP3 inflammasome activation. | [82] |
| Glutamate-Induced Neurotoxicity | HT-22 cells | Inhibits cell death, regulates related indicators, and downregulates protein expression | Downregulates the Cdk5/p35/p25 signaling pathway | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zheng, X.; Qi, J. Research Progress on the Therapeutic Mechanisms of Stigmasterol for Multiple Diseases. Molecules 2025, 30, 1874. https://doi.org/10.3390/molecules30091874
Li J, Zheng X, Qi J. Research Progress on the Therapeutic Mechanisms of Stigmasterol for Multiple Diseases. Molecules. 2025; 30(9):1874. https://doi.org/10.3390/molecules30091874
Chicago/Turabian StyleLi, Juan, Xinhua Zheng, and Jinxu Qi. 2025. "Research Progress on the Therapeutic Mechanisms of Stigmasterol for Multiple Diseases" Molecules 30, no. 9: 1874. https://doi.org/10.3390/molecules30091874
APA StyleLi, J., Zheng, X., & Qi, J. (2025). Research Progress on the Therapeutic Mechanisms of Stigmasterol for Multiple Diseases. Molecules, 30(9), 1874. https://doi.org/10.3390/molecules30091874







