The Influence of Integrated and Intensive Grain Production on the Content and Properties of Chemical Components in Rye Grain
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Grain Milling and Analysis
3.2. Extraction of Rye Starch and Its Analysis
3.3. Extraction of Rye Arabinoxylan and Its Analysis
3.4. Statistical Evaluation of Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horoszkiewicz-Janka, J.; Mrówczyński, M. Metodyka integrowanej ochrony i produkcji żyta dla doradców. In Instytut Ochrony Roślin—Państwowy Instytut Badawczy; Mrówczyński, M., Ed.; Zakład Transferu i Innowacji: Poznań, Poland, 2016; p. 5. [Google Scholar]
- Muhie, S.H. Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Korstad, J. Ecological and Practical Applications for Sustainable Agriculture; Springer: Singapore, 2020. [Google Scholar]
- Smith, G.; Nandwani, D.; Kankarla, V. Facilitating resilient rural-to-urban sustainable agriculture and rural communities. Intern. J. Sustain. Dev. World Ecol. 2017, 24, 485–501. [Google Scholar] [CrossRef]
- Rydhmer, L.; Gourdine, J.L.; de Greef, K.; Bonneau, M. Evaluation of the sustainability of contrasted pig farming systems: Breeding programmes. Animals 2014, 8, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Grabiński, J.; Sułek, A.; Wyzińska, M.; Stuper-Szablewska, K.; Cacak-Pietrzak, G.; Nieróbca, A.; Dziki, D. Impact of Genotype, Weather Conditions and Production Technology on the Quantitative Profile of Anti-Nutritive Compounds in Rye Grains. Agronomy 2021, 11, 151. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Budzyński, W.S.; Dubis, B. Cultivar-related and agronomic conditions of rye yielding on good rye complex soil. Electron. J. Polish Agric. Univ. 2003, 6, 1. Available online: http://www.ejpau.media.pl/volume6/issue1/agronomy/abs-05.html (accessed on 12 February 2025).
- Moitzi, G.; Neugschwandtner, R.W.; Kaul, H.-P.; Wagentristl, H. Energy Efficiency of Continuous Rye, Rotational Rye and Barley in Different Fertilization Systems in a Long-Term Field Experiment. Agronomy 2021, 11, 229. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Mahli, N.S. Interactions of nitrogen with other nutrients and water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration and environmental pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar] [CrossRef]
- Studnicki, M.; Macholdt, J.; Macdonald, A.; Stępień, W. Effects of Fertilizers and Manures on Temporal Yield Variability of Winter Rye. Agronomy 2021, 11, 519. [Google Scholar] [CrossRef]
- Vaughn, K.; Adeyemi, O.; Zandvakili, O.; Hunter, D.; Nair, J.; Still, S.; Sadeghpour, A. Winter rye cover crop biomass, nutrient uptake, and quality in response to fall and spring N fertilization. Cogent Food Agric. 2022, 8, 2132843. [Google Scholar] [CrossRef]
- Laszlo, M. Precipitation and Fertilization Level Impacts on Winter Rye (Secale cereale L.) yield. Cereal Res. Commun. 2013, 35, 1509–1517. [Google Scholar] [CrossRef]
- Chukhina, O.; Karbasnikova, E.; Obriaeva, O. Influence of fertilizers on harvest yield, uptake of nutrients, and “raw” protein content in witner rye grains of Vologda region. In Proceedings of the Web of Conferences, Prague, Czech Republic, 27–28 February 2020. [Google Scholar] [CrossRef]
- D’yachenko, E.N.; Shevelev, A.T. Meteorological conditions, mineral fertilizers and lime aftereffect influence on the barley grain yield and quality in the Irkustk region. IOP Conf. Ser. Earth Environ. Sci. 2020, 54, 052013. [Google Scholar] [CrossRef]
- Schachtman, D.P. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Theodorou, M.E.; Plaxton, W.C. Metabolic Adaptations of Plant Respiration to Nutritional Phosphate Deprivation. Plant Physiol. 1993, 101, 339–344. [Google Scholar] [CrossRef]
- Rufty, T.W.; Mackown, C.T.; Israel, D.W. Phosphorus stress effects on assimilation of nitrate. Plant Physiol. 1990, 94, 328–333. [Google Scholar] [CrossRef] [PubMed]
- De Magalhaes, J.V.; Alves, V.M.C.; de Novais, R.F.; Mosquim, P.R.; Magalhaes, J.R.; Bahia, A.F.C.; Huber, D.M. Nitrate uptake by corn under increasing periods of phosphorus starvation. J. Plant Nutr. 1998, 21, 1753–1998. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Tripler, C.E.; Kaushal, S.S.; Linkens, G.E.; Walter, M.T. Patterns in potassium dynamics in forest ecosystems. Ecol. Lett. 2006, 9, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Linina, A.; Augspole, I.; Romanova, I.; Kuzel, S. Winter rye (Secale cereale L.) antioxidant capacity, total phenolic content and quality indices. In Proceedings of the Biosystems Engineering, Tartu, Estonia, 6–8 May 2020. [Google Scholar] [CrossRef]
- Rodehutscor, M.; Rücker, C.; Maurer, H.P.; Schenkel, H.; Schipprack, W.; Knudsen, K.E.B.; Schollenberger, M.; Laux, M.; Eklund, M.; Siegert, W.; et al. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 2016, 70, 1477–2817. [Google Scholar] [CrossRef]
- Shewry, P.R.; Piironen, V.; Lampi, A.M.; Edelmann, M.; Kariluoto, S.; Nurmi, T.; Fernandez-Orozco, R.; Andersson, A.A.M.; Åman, P.; Fraś, A.; et al. Effects of Genotype and Environment on the Content and Composition of Phytochemicals and Dietary Fiber Components in Rye in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2010, 58, 9372–9383. [Google Scholar] [CrossRef]
- Hansen, H.B.; Rasmussen, C.V.; Knudsen, K.E.B.; Hansen, A. Effects of genotype and harvest year on content and composition of dietary fibre in rye (Secale cereale L.) grain. J. Sci. Food Agric. 2003, 83, 76–85. [Google Scholar] [CrossRef]
- Hansen, H.B.; Moller, B.; Andersen, S.B.; Jorgensen, J.R.; Hansen, A. Grain Characteristics, Chemical Composition, and Functional Properties of Rye (Secale cereale L.) As Influenced by Genotype and Harvest Year. J. Agric. Food Chem. 2004, 52, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Lasztity, R.; Laszitiy, B.; Hidvegi, M.; Simon-Sarkadi, L. Effect of fertilizers on the yield, protein content and amino acid composition of winter cereals. Period. Polytech. Ser. Chem. Eng. 1992, 36, 25–41. [Google Scholar]
- Klikocka, H.; Skwaryło-Bednarz, B.; Podleśna, A.; Narolski, B. The Response of Spring Rye (Secale cereale L.) to NPK and S Fertilizers. The content and uptake of macroelements and the value of ionic ratios. J. Elem. 2022, 27, 249–263. [Google Scholar] [CrossRef]
- Łysoń, E.; Biel, W. Ocena Składu Chemicznego Ziarna Wybranych Odmian Żyta (Secale cereale L.) z Uprawy Ekologicznej I Konwencjonalnej. Żywność Nauka Technol. Jakość 2016, 3, 91–101. [Google Scholar] [CrossRef]
- Menkovska, M.; Levkov, V.; Damjanovski, D.; Gjorgovska, N.; Knezevic, D.; Nikolova, N.; Andreevska, D. Content of TDF, SDF, IDF in Cereals Grown by Organic and Conventional Farming—A Short Report. Polish J. Food Nutr. Sci. 2017, 67, 241–244. [Google Scholar] [CrossRef]
- Kargin, V.; Zaharkina, R.A.; Danilin, S.I.; Geraskin, M.M.; Erofeev, A.A. Economic evaluation of winter rye cultivation technology. Rev. Espac. 2019, 40, 22. [Google Scholar] [CrossRef]
- Slukova, M.; Jurkaninova, L.; Sves, I.; Skrivan, P. Rye—The nutritional and technological evaluation in Czech cereal technology—A review: Grain and flours. Czech J. Food Sci. 2021, 39, 03–08. [Google Scholar] [CrossRef]
- Stępień, A.; Wojtkowiak, K.; Pietrusewicz, M.; Skłodowski, M.; Pietrzak-Fiećko, R. The yield and grain quality of winter rye (Secale cereale L.) under the conditions of foliar fertilization with micronutrients (Cu, Zn, and Mn). Polish J. Nat. Sci. 2016, 31, 33–46. [Google Scholar]
- Järvan, M.; Lukme, L.; Tupits, I.; Akk, A. The productivity, quality and bread-making properties of organically and conven-tionally grown winter rye. Zemdirb.-Agric. 2018, 105, 323–330. [Google Scholar] [CrossRef]
- Kindred, D.R.; Verhoeven, T.M.O.; Weightman, R.M.; Swanston, J.S.; Agu, R.C.; Brosnan, J.M.; Sylvester-Bradley, R. Effects of ariety and fertilizer nitrogen on alcohol yield, grain yield, starch and protein content and protein composition of winter wheat. J. Cereal Sci. 2008, 48, 46–57. [Google Scholar] [CrossRef]
- Safar -Noori, M.; Dong, Q.; Saneoka, H. Improvement of Grain Yield, Nutritional and Antinutritional Quality and Seed Phys-iological Performance of Wheat by NPK. J. Agric. Sci. Technol. 2018, 20, 1467–1477. Available online: https://jast.modares.ac.ir/browse.php?a_id=17204&sid=23&slc_lang=fa (accessed on 12 February 2025).
- Studnicki, M.; Ghafoor, A.Z.; Wijata, M.; Rozbicki, J.; Krysztofik, R.; Banaszak, K.; Karim, H.; Derejko, A. Influence of crop man-agement on stability rye yield and some grain quality traits. Agron. J. 2024, 116, 2263–2274. [Google Scholar] [CrossRef]
- Jarecki, W.; Czernicka, M. Reaction of Winter Wheat (Triticum aestivum L.) Depending on the Multi-Component Foliar Ferti-lization. Chem. Proc. 2022, 10, 68. [Google Scholar] [CrossRef]
- Jarecki, W.; Buczek, J.; Bobrecka-Jamro, D. Response of facultative cultivars of spring wheat to autumn sowing and foliar fertilization. J. Elem. 2019, 24, 817–828. [Google Scholar] [CrossRef]
- Bazitov, R.; Mihaylova, M.; Ganchev, G. Influence of nitrogen fertilization on the energy value of maize grain in non-ruminants. Agric. Sci. Technol. 2023, 15, 46–50. [Google Scholar] [CrossRef]
- Shahbazi, F.; Nematollahi, A. Influences of phosphorus and foliar fertilization rate on quality parameters of whole wheat grain. Food Sci. Nutr. 2019, 7, 442–448. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Haliniarz, M.; Tomczyńska-Mleko, M.; Mleko, S.; Kawecka-Radomska, M. The content of dietary fiber, amino acids, dihydroxyphenols and some macro- and micronutrients in grain of conventionally and organically grown common wheat, spelt wheat and proso millet. Agric. Food Sci. 2015, 24, 195–205. [Google Scholar] [CrossRef]
- Wilczewski, E. Content of Macroelements and Crude Fiber in Grain of Spring Barley Cultivated in Different Agronomic Conditions. Acta Sci. Pol. Agric. 2014, 13, 73–83. [Google Scholar]
- Tingtin, Z.; Wang, S.; Wang, M.; Mao, J.; Xu, Y.; Ren, J.; Liu, Y.; Liu, S.; Qiao, Z.; Cao, X. Effect of Different Fertilizer Types on Quality of Foxtail Millet Under Low Nitrogen Conditions. Plants 2024, 13, 1830. [Google Scholar] [CrossRef]
- Güler, M. Barley grain beta-glucan content as affected by nitrogen and irrigation. Field Crops Res. 2003, 84, 335–340. [Google Scholar] [CrossRef]
- Jaśkiewicz, B.; Szczepanek, M. Crop management and variety have influence on alkyloresorcinols content in triticale grain. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2016, 66, 570–574. [Google Scholar] [CrossRef]
- Buerkert, A.; Haake, C.; Ruckwied, M.; Marschner, H. Phosphorus application affects the nutritional quality of millet grain in the Sahel. Fields Crops Res. 1998, 57, 223–235. [Google Scholar] [CrossRef]
- Michniewicz, P. Żyto Hybrydowe i Populacyjne Różnice w Uprawie, 1st ed.; WARMIŃSKO-Mazurski Ośrodek Doradztwa Rolniczego z siedzibą w Olsztynie: Olsztyn, Poland, 2021; pp. 3–7. [Google Scholar]
- Miedaner, T.; Lauenstein, S.; Lieberherr, B. Comparison of Hybrid Rye and Wheat for Grain Yield and Other Agronomic Traits Under Less Favourable Environmental Conditions and Two Input Levels. Agriculture 2025, 15, 163. [Google Scholar] [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G.; Studnicki, M.; Grabiński, J.; Nieróbca, A.; Wyzińska, M.; Różewicz, M. Influence of Nitrogen Ferti-lisation Level and Weather Conditions on Yield and Quantitative Profile of Anti-Nutritional Compounds in Grain of Selected Rye Cultivars. Agriculture 2024, 14, 418. [Google Scholar] [CrossRef]
- Wrigley, C.; Batey, I.; Miskelly, D. Cereal Grains: Assessing and Managing Quality, 2nd ed.; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Bushuk, W. Rye: Production, Chemistry and Technology, 2nd ed.; American Association of Cereal Chemistry: St. Paul, MN, USA, 2001. [Google Scholar]
- Buksa, K.; Nowotna, A.; Prazner, W.; Gambuś, H.; Ziobro, R.; Krawontka, J. The role of pentosans and starch in baking of wholemeal rye bread. Food Res. Intern. 2010, 43, 2045–2051. [Google Scholar] [CrossRef]
- Poutanen, K.; Katina, K.; Heinio, R.-L. Rye. Bakery Products Science and Technology, 2nd ed.; Zhou, W., Hui, Y.H., De Leyn, I., Pagani, M.A., Rosell, C.M., Selman, J.D., Therdthai, N., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ikram, A.; Saeed, F.; Noor, R.A.; Imran, A.; Afzaal, M.; Rasheed, A.; Islam, F.; Iqbal, A.; Zahoor, T.; Naz, S.; et al. A comprehensive review on biochemical and technological properties of rye (Secale cereale L.). Intern. J. Food Prop. 2023, 26, 2212–2228. [Google Scholar] [CrossRef]
- Glitsot, L.V.; Bach Knudsen, K.E. Milling of whole grain rye to obtain fractions with different dietary fibre characteristics. J. Cereal Sci. 1999, 29, 89–97. [Google Scholar] [CrossRef]
- Błażewicz, J.; Liszewski, M. Ziarno jęczmienia nagiego odmiany Rastik jako surowiec do produkcji słodów typu pilzneńskiego. Acta Sci. Pol. Technol. Aliment. 2003, 2, 63–74. [Google Scholar]
- Litwinek, D.; Buksa, K.; Gambuś, H.; Kowalczyk, M.; Boreczek, J. Ocena Jakości Handlowych Mąk Całoziarnowych—Pszennej Orkiszowej, Pszennej Zwyczajnej I Żytniej Oraz Uzyskanych z Nich Zakwasów Spontanicznych. Żywność Nauka Technol. Jakość 2017, 24, 76–89. [Google Scholar] [CrossRef]
- Buksa, K. Extraction and characterization of rye grain starch and its susceptibility to resistant starch formation. Carbohydr. Pol. 2018, 194, 184–192. [Google Scholar] [CrossRef]
- Buresova, I.; Sedlackova, I.; Famera, O.; Lipavsky, J. Effect of growing conditions on starch and protein content in triticale grain and amylose content in starch. Plant Soil Environ. 2010, 56, 99–104. [Google Scholar] [CrossRef]
- Buksa, K. Application of model bread baking in the examination ofarabinoxylan—Protein complexes in rye bread. Carbohydr. Pol. 2016, 148, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Buksa, K.; Ziobro, R.; Nowotna, A.; Praznik, W.; Gambuś, H. Isolation, modification and characterization of soluble arabinoxylan fractions from rye grain. Eur. Food Res. Technol. 2012, 235, 385–395. [Google Scholar] [CrossRef]
- Warechowska, M.; Warechowski, J.; Tyburski, J.; Siemianowska, E.; Nawrocka, A.; Miś, A.; Skrajda-Brdak, M. Evaluation of physicochemical properties, antioxidant potential and baking quality of grain and flour of primitive rye (Secale cereale var. Multicaule). J. Food Sci. Technol. 2019, 56, 3422–3430. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists International: Gaithesburg, MD, USA, 2006.
- Hashimoto, S.; Shogren, M.D.; Pomeranz, Y. Cereal Pentosans: Their Estimation and Significance. I. Pentosans in Wheat and Milled Wheat Products. Cereal Chem. 1987, 64, 30–34. [Google Scholar]
- Sandberg, A.S.; Adherinne, R.J. HPLC Method for Determination of lnositol Tri-, Tetra-, Penta-, and Hexaphosphates in Foods and Intestinal Contents. J. Food Sci. 1986, 51, 547–550. [Google Scholar] [CrossRef]
- Sandberg, A.S.; Andersson, H.; Carlsson, N.-G.; Sabdström, B. Degradation Products of Bran Phytate Formed during Digestion in the Human Small Intestine: Effects of Extrusion Cooking on Digestibility. J. Nutr. 1987, 117, 2061–2065. [Google Scholar] [CrossRef]
- Morrison, W.R.; Laignelet, B. An Improved Procedure for Determining Apparent and Total Amylose in Cereal and Other Starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Shewry, P.R.; Ward, J.L. Analysis of Bioactive Components in Small Cereal Grain; AACC Int., Inc.: St. Paul, MN, USA, 2009. [Google Scholar]
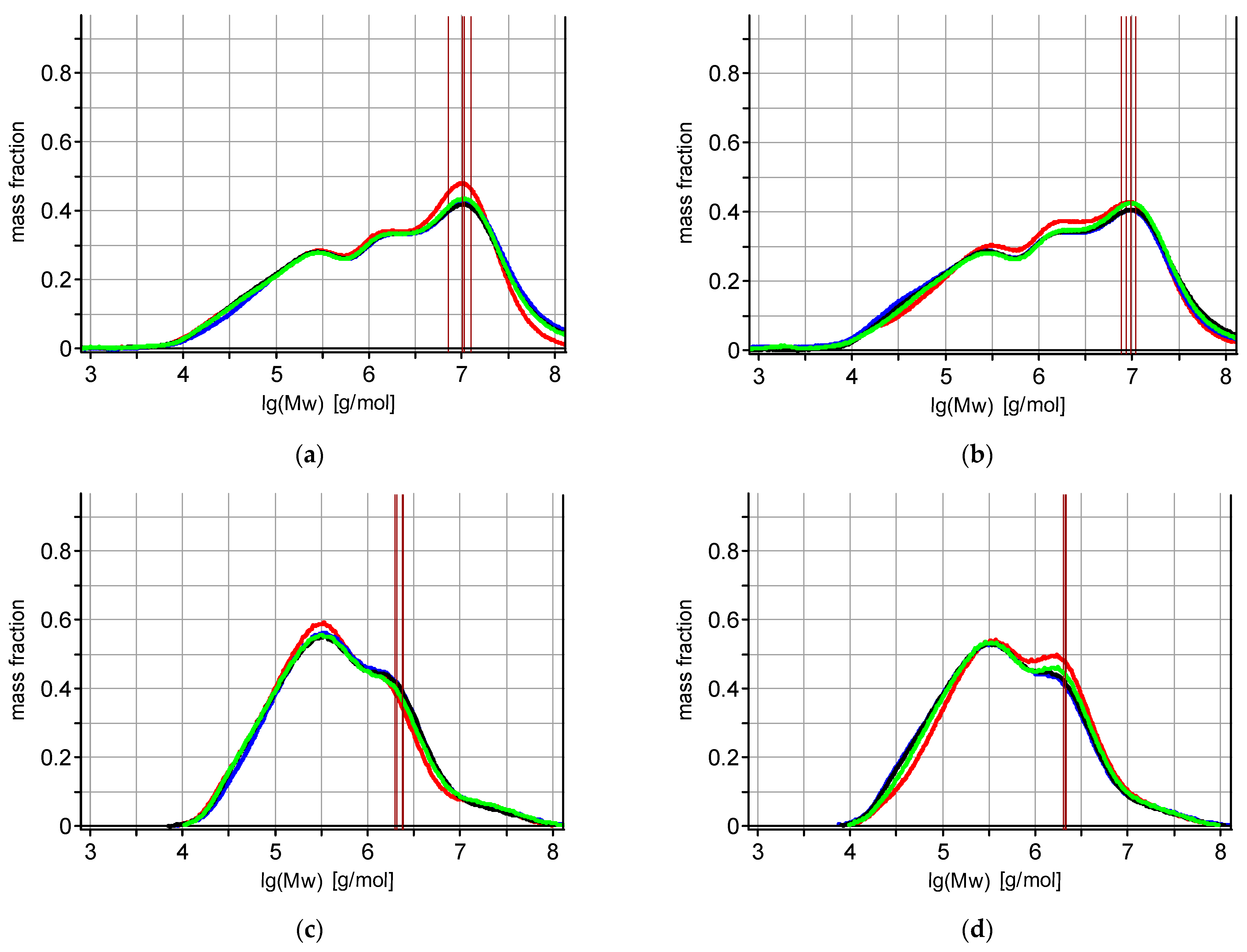
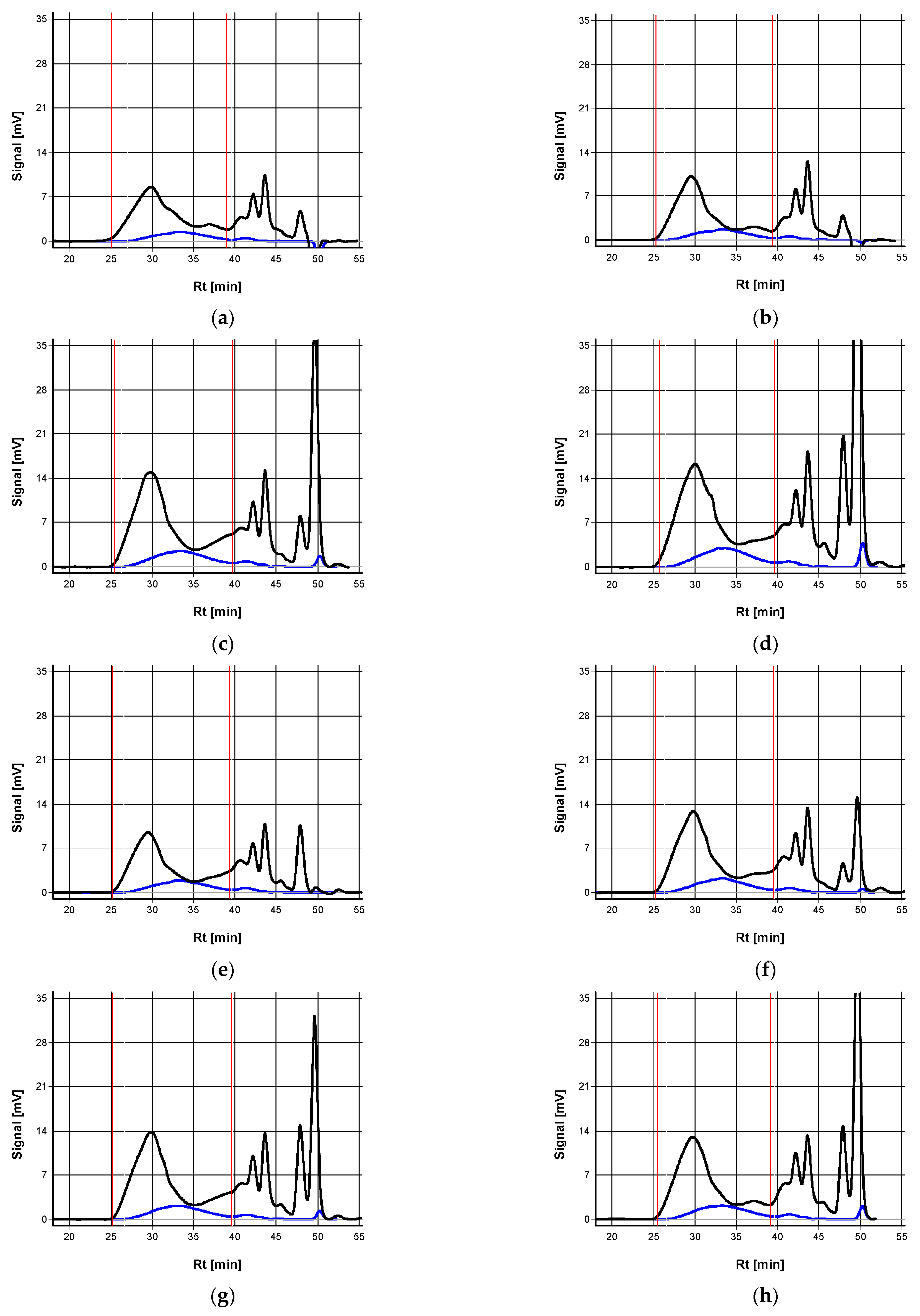
| Tech * | Yield [t/ha] | Starch [%] | Protein [%] | Fat [%] | Ash [%] | TDF ** [%] | SDF ** [%] | IDF ** [%] | WEAX [%] | WUAX [%] | TAX [%] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variety | KWS Vinetto | 6.9 ± 0.2 d | 63.3 ± 6.0 b | 10.7 ± 0.3 a | 2.2 ± 0.1 a | 1.57 ± 0.05 b | 14.4 ± 0.3 a | 3.4 ± 0.1 a | 11.0 ± 0.2 b | 3.1 ± 0.4 a | 5.4 ± 0.7 a | 8.5 ± 0.9 a | |
| Variety | KWS Bono | 6.7 ± 0.6 bc | 64.5 ± 0.8 c | 10.8 ± 0.2 b | 2.3 ± 0.1 a | 1.54 ± 0.02 a | 14.9 ± 0.8 c | 3.4 ± 0.1 a | 11.5 ± 0.8 c | 3.1 ± 0.3 a | 5.3 ± 0.5 a | 8.4 ± 0.3 a | |
| Variety | D. Granat | 6.6 ± 0.2 b | 65.1 ± 0.2 c | 11.9 ± 0.0 d | 2.4 ± 0.1 a | 1.61 ± 0.02 c | 15.9 ± 0.3 d | 3.9 ± 0.1 b | 12.0 ± 0.2 d | 3.7 ± 0.1 b | 6.6 ± 0.6 b | 10.4 ± 0.5 b | |
| Variety | Horyzo | 6.0 ± 0.2 a | 61.2 ± 1.9 a | 11.8 ± 0.4 c | 2.3 ± 0.2 a | 1.57 ± 0.23 b | 14.6 ± 0.1 b | 3.9 ± 0.6 b | 10.7 ± 0.5 a | 3.9 ± 0.6 b | 5.3 ± 0.6 a | 9.2 ± 0.4 a | |
| Tech | ID | 6.3 ± 0.4 a | 61.7 ± 3.1 a | 11.1 ± 0.7 a | 2.3 ± 0.1 a | 1.61 ± 0.11 b | 14.7 ± 0.8 a | 3.5 ± 0.3 a | 11.3 ± 0.6 a | 3.1 ± 0.4 a | 5.8 ± 0.9 a | 9.0 ± 1.2 a | |
| Tech | IE | 6.8 ± 0.5 b | 65.4 ± 2.1 b | 11.5 ± 0.6 b | 2.3 ± 0.1 a | 1.54 ± 0.10 a | 15.1 ± 0.5 b | 3.8 ± 0.4 b | 11.3 ± 0.8 a | 3.7 ± 0.4 b | 5.5 ± 0.6 a | 9.2 ± 0.6 a | |
| Var × Tech *** | KWS Vinetto | ID | 6.9 ± 0.2 cd | 58.2 ± 0.2 a | 10.4 ± 0.1 a | 2.2 ± 0.1 a | 1.53 ± 0.01 b | 14.2 ± 0.1 a | 3.3 ± 0.0 a | 10.9 ± 0.1 bc | 2.8 ± 0.0 a | 4.9 ± 0.4 a | 7.7 ± 0.4 a |
| Var × Tech | KWS Vinetto | IE | 7.0 ± 0.1 cd | 68.5 ± 0.2 e | 10.9 ± 0.0 b | 2.3 ± 0.1 a | 1.62 ± 0.01 c | 14.6 ± 0.0 b | 3.4 ± 0.0 a | 11.1 ± 0.0 bc | 3.4 ± 0.1 bc | 5.8 ± 0.7 ab | 9.2 ± 0.6 abc |
| Var × Tech | KWS Bono | ID | 6.2 ± 0.1 ab | 63.8 ± 0.3 c | 10.7 ± 0.0 a | 2.2 ± 0.1 a | 1.53 ± 0.00 b | 14.2 ± 0.1 a | 3.4 ± 0.1 a | 10.8 ± 0.1 b | 2.8 ± 0.0 a | 5.5 ± 0.4 ab | 8.4 ± 0.4 ab |
| Var × Tech | KWS Bono | IE | 7.3 ± 0.0 d | 65.2 ± 0.3 d | 11.0 ± 0.0 b | 2.3 ± 0.0 a | 1.56 ± 0.01 b | 15.5 ± 0.0 c | 3.4 ± 0.1 a | 12.2 ± 0.1 e | 3.3 ± 0.1 b | 5.1 ± 0.5 a | 8.4 ± 0.4 ab |
| Var × Tech | D. Granat | ID | 6.5 ± 0.3 bc | 65.2 ± 0.3 d | 12.0 ± 0.0 d | 2.4 ± 0.1 a | 1.63 ± 0.01 c | 16.1 ± 0.1 d | 3.9 ± 0.1 b | 12.2 ± 0.0 e | 3.7 ± 0.1 cd | 7.1 ± 0.1 b | 10.8 ± 0.1 c |
| Var × Tech | D. Granat | IE | 6.7 ± 0.1 bc | 65.0 ± 0.1 d | 11.9 ± 0.0 d | 2.4 ± 0.0 a | 1.60 ± 0.01 c | 15.6 ± 0.0 c | 3.9 ± 0.1 b | 11.8 ± 0.0 d | 3.8 ± 0.0 d | 6.0 ± 0.2 ab | 9.9 ± 0.2 bc |
| Var × Tech | Horyzo | ID | 5.8 ± 0.1 a | 59.6 ± 0.2 b | 11.5 ± 0.1 c | 2.2 ± 0.1 a | 1.77 ± 0.01 d | 14.5 ± 0.0 b | 3.3 ± 0.1 a | 11.2 ± 0.1 c | 3.3 ± 0.1 bc | 5.8 ± 0.4 ab | 9.2 ± 0.4 abc |
| Var × Tech | Horyzo | IE | 6.2 ± 0.1 ab | 62.9 ± 0.2 c | 12.1 ± 0.0 d | 2.4 ± 0.2 a | 1.38 ± 0.01 a | 14.7 ± 0.1 b | 4.4 ± 0.0 c | 10.3 ± 0.1 a | 4.3 ± 0.1 e | 4.9 ± 0.4 a | 9.2 ± 0.4 abc |
| Tech * | IP3 ** [%] | IP4 ** [%] | IP5 ** [%] | IP6 ** [%] | Total Phytate [%] | ||
|---|---|---|---|---|---|---|---|
| Variety | KWS Vinetto | 0.004 ± 0.002 a | 0.007 ± 0.002 a | 0.066 ± 0.006 a | 1.720 ± 0.051 a | 1.797 ± 0.057 a | |
| Variety | KWS Bono | 0.005 ± 0.003 a | 0.006 ± 0.002 a | 0.059 ± 0.011 a | 1.686 ± 0.137 a | 1.755 ± 0.145 a | |
| Variety | D. Granat | 0.004 ± 0.003 a | 0.006 ± 0.002 a | 0.069 ± 0.009 a | 1.826 ± 0.273 ab | 1.905 ± 0.280 ab | |
| Variety | Horyzo | 0.005 ± 0.004 a | 0.006 ± 0.001 a | 0.066 ± 0.010 a | 1.946 ± 0.114 b | 2.022 ± 0.119 b | |
| Tech | ID | 0.003 ± 0.002 a | 0.007 ± 0.002 a | 0.067 ± 0.005 a | 1.792 ± 0.175 a | 1.869 ± 0.178 a | |
| Tech | IE | 0.005 ± 0.004 a | 0.006 ± 0.001 a | 0.063 ± 0.012 a | 1.796 ± 0.199 a | 1.870 ± 0.207 a | |
| Var × Tech *** | KWS Vinetto | ID | 0.002 ± 0.001 a | 0.008 ± 0.001 a | 0.069 ± 0.003 a | 1.748 ± 0.015 a | 1.828 ± 0.017 a |
| Var × Tech | KWS Vinetto | IE | 0.005 ± 0.000 a | 0.005 ± 0.001 a | 0.063 ± 0.008 a | 1.692 ± 0.067 a | 1.766 ± 0.075 a |
| Var × Tech | KWS Bono | ID | 0.002 ± 0.001 a | 0.008 ± 0.001 a | 0.066 ± 0.003 a | 1.787 ± 0.077 ab | 1.862 ± 0.077 ab |
| Var × Tech | KWS Bono | IE | 0.007 ± 0.003 a | 0.005 ± 0.001 a | 0.051 ± 0.010 a | 1.585 ± 0.097 a | 1.649 ± 0.105 a |
| Var × Tech | D. Granat | ID | 0.006 ± 0.001 a | 0.006 ± 0.001 a | 0.062 ± 0.007 a | 1.597 ± 0.051 a | 1.670 ± 0.057 a |
| Var × Tech | D. Granat | IE | 0.002 ± 0.002 a | 0.007 ± 0.003 a | 0.076 ± 0.001 a | 2.055 ± 0.104 b | 2.140 ± 0.106 b |
| Var × Tech | Horyzo | ID | 0.002 ± 0.000 a | 0.006 ± 0.002 a | 0.071 ± 0.001 a | 2.038 ± 0.067 b | 2.118 ± 0.068 b |
| Var × Tech | Horyzo | IE | 0.007 ± 0.006 a | 0.005 ± 0.001 a | 0.060 ± 0.014 a | 1.853 ± 0.018 ab | 1.926 ± 0.027 ab |
| Tech * | Mw × 106 [g/mol] | Mn × 106 [g/mol] | Ð | Amylose Mw × 106 [g/mol] | Amylose Mn × 106 [g/mol] | Amylose Ð | Apparent Amylose Content [%] | ||
|---|---|---|---|---|---|---|---|---|---|
| Variety | KWS Vin. | 10.76 ± 2.27 bc | 0.12 ± 0.09 a | 124.3 ± 68.6 d | 2.366 ± 0.151 b | 0.180 ± 0.017 ab | 13.2 ± 0.4 d | 22.9 ± 0.47 a | |
| Variety | KWS Bono | 11.17 ± 0.37 c | 0.16 ± 0.02 c | 70.9 ± 6.0 b | 2.072 ± 0.048 a | 0.176 ± 0.008 a | 11.8 ± 0.3 b | 23.3 ± 0.75 a | |
| Variety | D. Granat | 10.15 ± 0.39 b | 0.13 ± 0.03 a | 84.0 ± 17.3 c | 2.306 ± 0.193 b | 0.183 ± 0.006 ab | 12.6 ± 1.3 c | 23.4 ± 0.56 a | |
| Variety | Horyzo | 7.55 ± 0.25 a | 0.15 ± 0.01 b | 51.6 ± 4.1 a | 2.113 ± 0.086 a | 0.194 ± 0.025 b | 11.0 ± 1.0 a | 23.0 ± 0.44 a | |
| Tech | ID | 10.41 ± 2.06 b | 0.16 ± 0.02 b | 64.5 ± 11.0 a | 2.272 ± 0.222 b | 0.181 ± 0.010 a | 12.5 ± 0.9 b | 22.7 ± 0.24 a | |
| Tech | IE | 9.41 ± 1.45 a | 0.11 ± 0.05 a | 100.9 ± 54.0 b | 2.156 ± 0.092 a | 0.185 ± 0.021 a | 11.8 ± 1.3 a | 23.6 ± 0.37 b | |
| Var × Tech *** | KWS Vin. | ID | 12.71 ± 0.35 d | 0.20 ± 0.00 e | 64.8 ± 0.4 c | 2.480 ± 0.086 c | 0.194 ± 0.005 bc | 12.8 ± 0.1 c | 22.5 ± 0.32 a |
| Var × Tech | KWS Vin. | IE | 8.80 ± 0.36 ab | 0.05 ± 0.00 a | 183.7 ± 0.0 g | 2.252 ± 0.093 abc | 0.166 ± 0.007 a | 13.6 ± 0.0 d | 23.3 ± 0.16 abc |
| Var × Tech | KWS Bono | ID | 11.07 ± 0.46 c | 0.15 ± 0.01 c | 76.1 ± 0.0 e | 2.092 ± 0.058 a | 0.181 ± 0.007 ab | 11.6 ± 0.2 b | 22.7 ± 0.16 a |
| Var × Tech | KWS Bono | IE | 11.27 ± 0.39 c | 0.17 ± 0.00 d | 65.8 ± 0.9 cd | 2.051 ± 0.043 a | 0.171 ± 0.007 ab | 12.0 ± 0.2 b | 24.0 ± 0.34 c |
| Var × Tech | D. Granat | ID | 10.40 ± 0.43 c | 0.15 ± 0.00 c | 69.1 ± 0.9 d | 2.461 ± 0.101 bc | 0.178 ± 0.005 ab | 13.8 ± 0.2 d | 22.9 ± 0.32 ab |
| Var × Tech | D. Granat | IE | 9.91 ± 0.21 bc | 0.10 ± 0.00 b | 98.9 ± 2.0 f | 2.151 ± 0.074 a | 0.187 ± 0.004 ab | 11.5 ± 0.2 b | 23.8 ± 0.17 bc |
| Var × Tech | Horyzo | ID | 7.44 ± 0.31 a | 0.15 ± 0.01 cd | 48.1 ± 0.3 a | 2.056 ± 0.085 a | 0.173 ± 0.007 ab | 11.9 ± 0.0 b | 22.7 ± 0.16 a |
| Var × Tech | Horyzo | IE | 7.67 ± 0.21 a | 0.14 ± 0.01 c | 55.1 ± 0.7 b | 2.170 ± 0.045 ab | 0.214 ± 0.009 c | 10.1 ± 0.2 a | 23.4 ± 0.33 abc |
| Tech * | AX Mw × 106 [g/mol] | AX Mn × 106 [g/mol] | AX Ð | FA ** Content [mg/100g AX] | ||
|---|---|---|---|---|---|---|
| Variety | KWS Vin. | 2.066 ± 0.031 c | 0.149 ± 0.023 b | 14.1 ± 2.4 c | 2.6 ± 0.7 a | |
| Variety | KWS Bono | 2.065 ± 0.112 c | 0.159 ± 0.029 d | 13.2 ± 1.7 b | 2.9 ± 0.7 b | |
| Variety | D. Granat | 1.943 ± 0.053 b | 0.113 ± 0.009 a | 17.2 ± 0.9 d | 4.3 ± 0.2 c | |
| Variety | Horyzo | 1.871 ± 0.159 a | 0.152 ± 0.040 c | 12.8 ± 2.3 a | 4.7 ± 0.3 d | |
| Tech | ID | 1.958 ± 0.171 a | 0.144 ± 0.036 a | 14.1 ± 2.7 a | 3.3 ± 1.2 a | |
| Tech | IE | 2.015 ± 0.051 b | 0.143 ± 0.028 a | 14.5 ± 2.4 b | 4.0 ± 0.7 b | |
| Var × Tech *** | KWS Vin. | ID | 2.039 ± 0.001 e | 0.169 ± 0.001 f | 12.0 ± 0.0 b | 2.0 ± 0.0 a |
| Var × Tech | KWS Vin. | IE | 2.093 ± 0.001 f | 0.129 ± 0.001 d | 16.2 ± 0.2 d | 3.2 ± 0.1 b |
| Var × Tech | KWS Bono | ID | 2.162 ± 0.016 g | 0.184 ± 0.001 g | 11.7 ± 0.0 b | 2.4 ± 0.0 a |
| Var × Tech | KWS Bono | IE | 1.969 ± 0.008 c | 0.135 ± 0.002 e | 14.6 ± 0.1 c | 3.5 ± 0.1 b |
| Var × Tech | D. Granat | ID | 1.897 ± 0.001 b | 0.105 ± 0.001 a | 18.0 ± 0.2 e | 4.4 ± 0.0 c |
| Var × Tech | D. Granat | IE | 1.989 ± 0.001 cd | 0.121 ± 0.001 c | 16.5 ± 0.1 d | 4.2 ± 0.2 c |
| Var × Tech | Horyzo | ID | 1.733 ± 0.004 a | 0.117 ± 0.002 b | 14.8 ± 0.2 c | 4.5 ± 0.0 cd |
| Var × Tech | Horyzo | IE | 2.009 ± 0.007 d | 0.187 ± 0.000 g | 10.7 ± 0.0 a | 5.0 ± 0.3 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buksa, K.; Sułek, A.; Szczypek, M. The Influence of Integrated and Intensive Grain Production on the Content and Properties of Chemical Components in Rye Grain. Molecules 2025, 30, 1880. https://doi.org/10.3390/molecules30091880
Buksa K, Sułek A, Szczypek M. The Influence of Integrated and Intensive Grain Production on the Content and Properties of Chemical Components in Rye Grain. Molecules. 2025; 30(9):1880. https://doi.org/10.3390/molecules30091880
Chicago/Turabian StyleBuksa, Krzysztof, Alicja Sułek, and Michał Szczypek. 2025. "The Influence of Integrated and Intensive Grain Production on the Content and Properties of Chemical Components in Rye Grain" Molecules 30, no. 9: 1880. https://doi.org/10.3390/molecules30091880
APA StyleBuksa, K., Sułek, A., & Szczypek, M. (2025). The Influence of Integrated and Intensive Grain Production on the Content and Properties of Chemical Components in Rye Grain. Molecules, 30(9), 1880. https://doi.org/10.3390/molecules30091880







