Colon-Targeted Mucoadhesive PLGA Microspheres Loaded with Ramulus Mori Alkaloids for Enhanced Water-Soluble Drug Delivery in Ulcerative Colitis Treatment
Abstract
1. Introduction
2. Results and Discussion
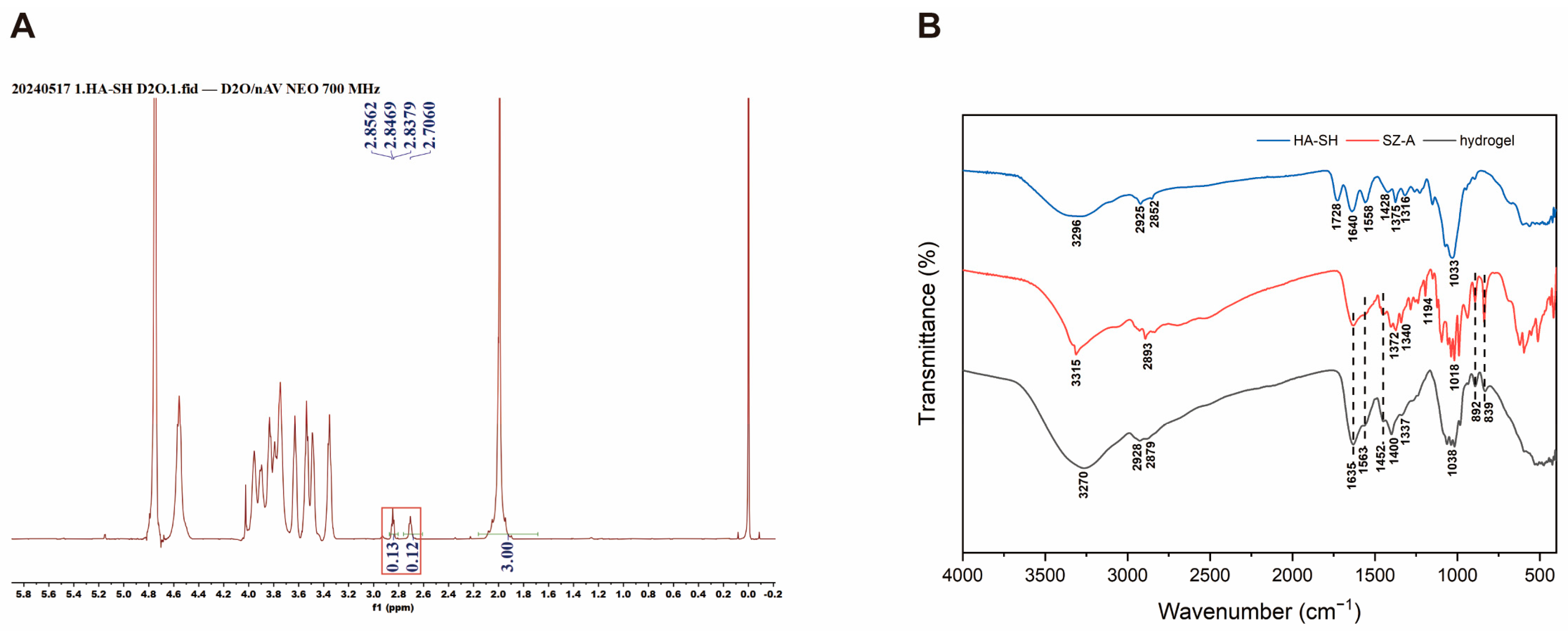
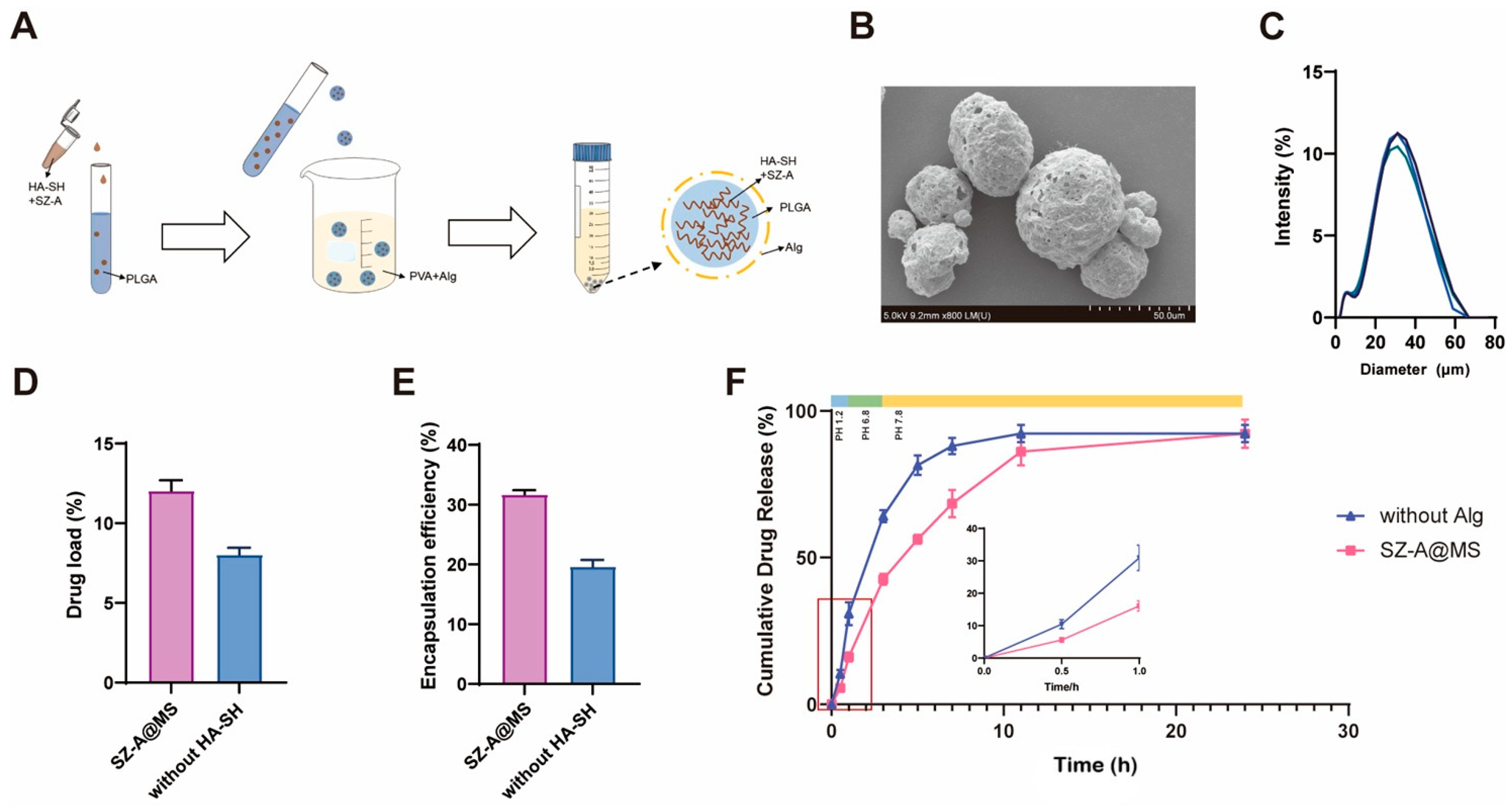
2.1. Preparation and Characterization of SZ-A@MSs
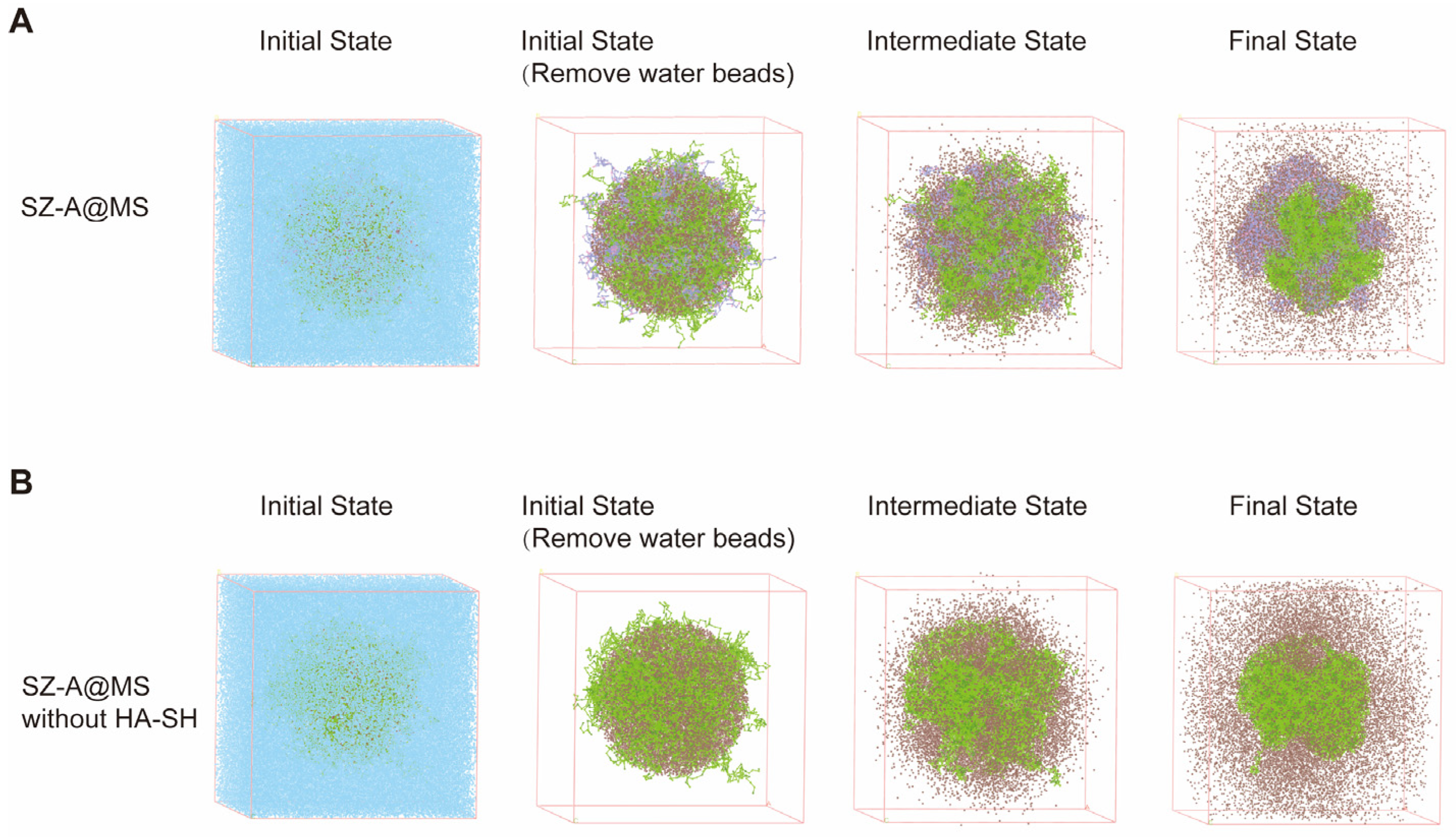
2.2. Mesoscopic Simulations of SZ-A@MSs Based on Dissipative Particle Dynamics (DPD)
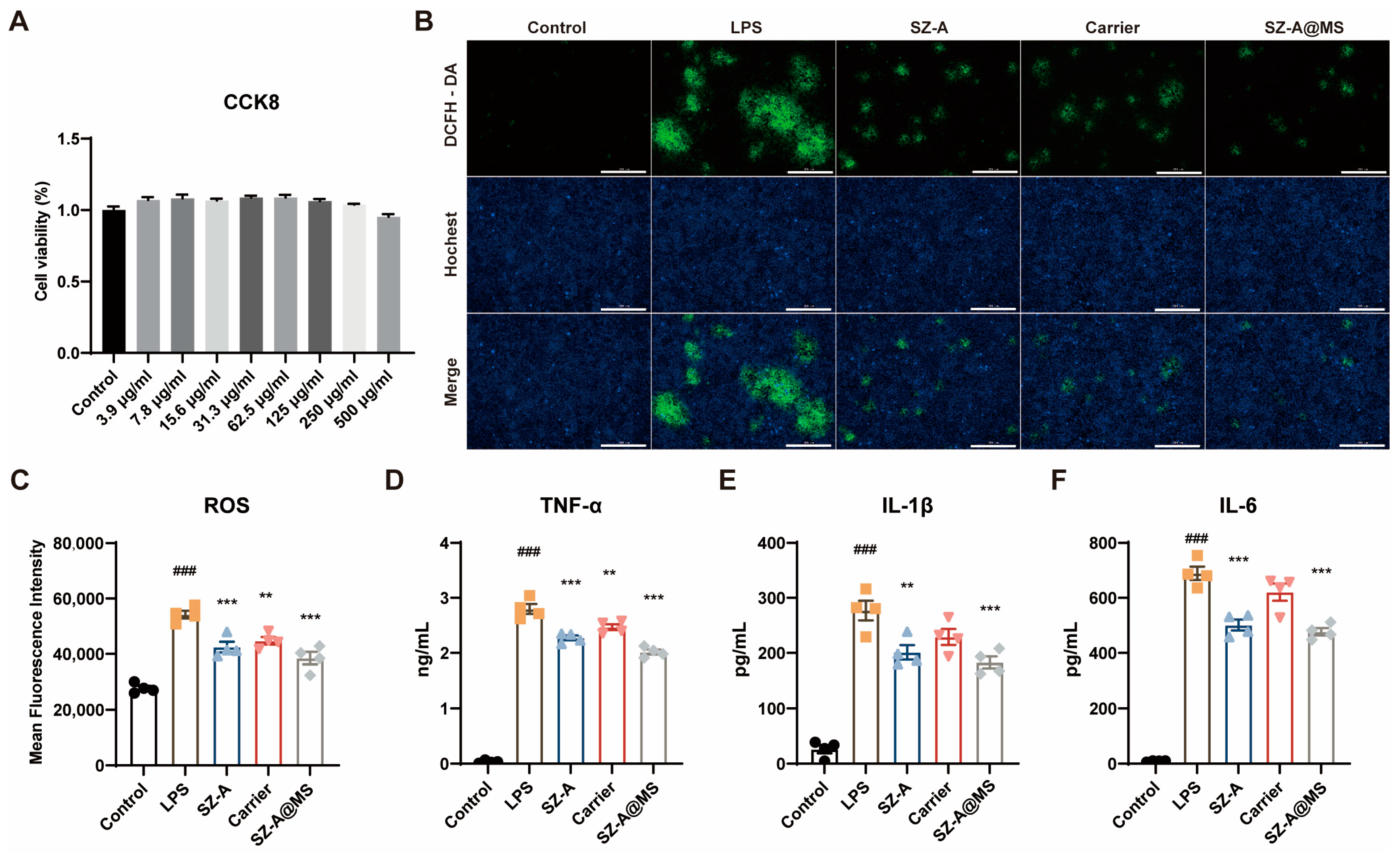
2.3. In Vitro Antioxidant and Anti-Inflammatory Effects of SZ-A@MS
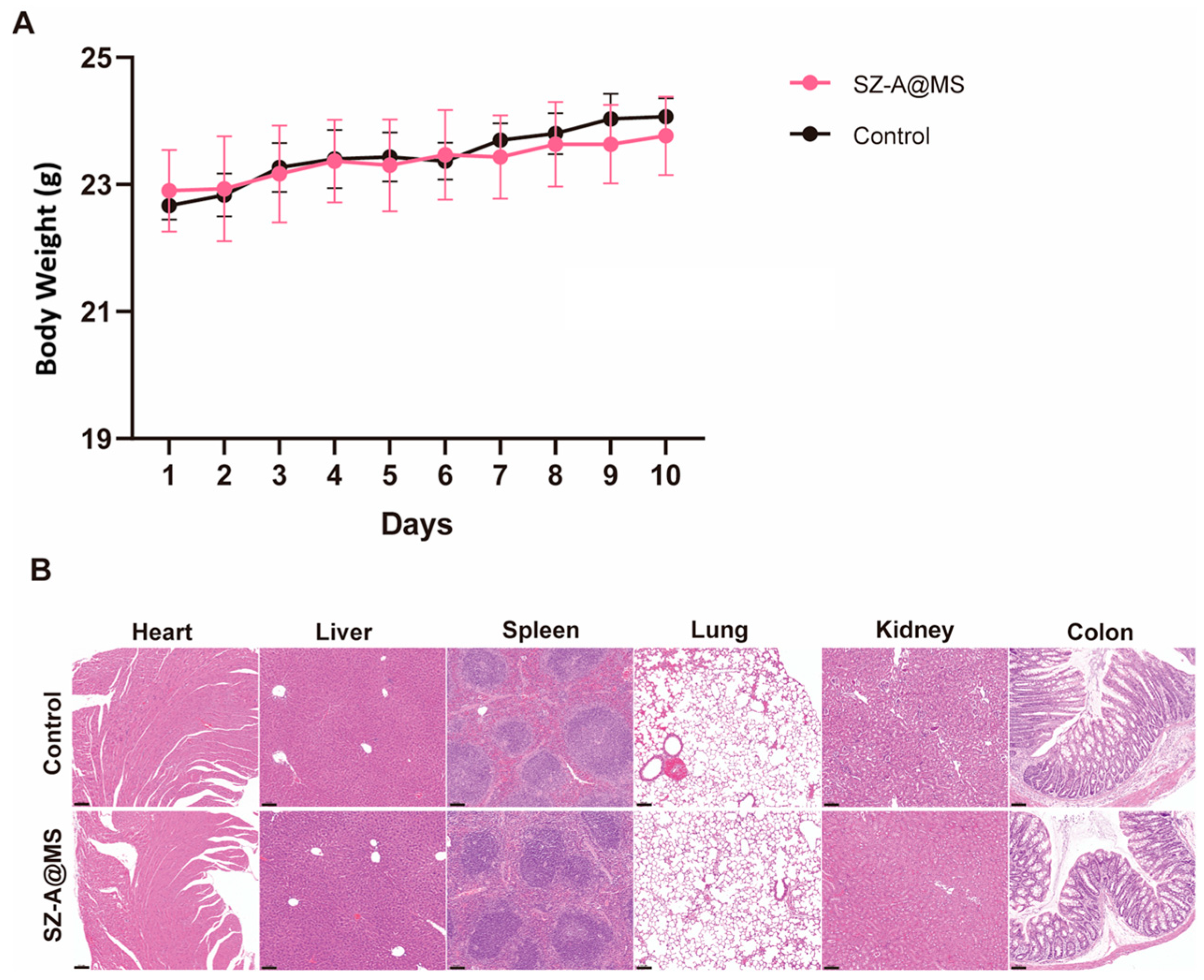
2.4. Bio-Safety Assessment
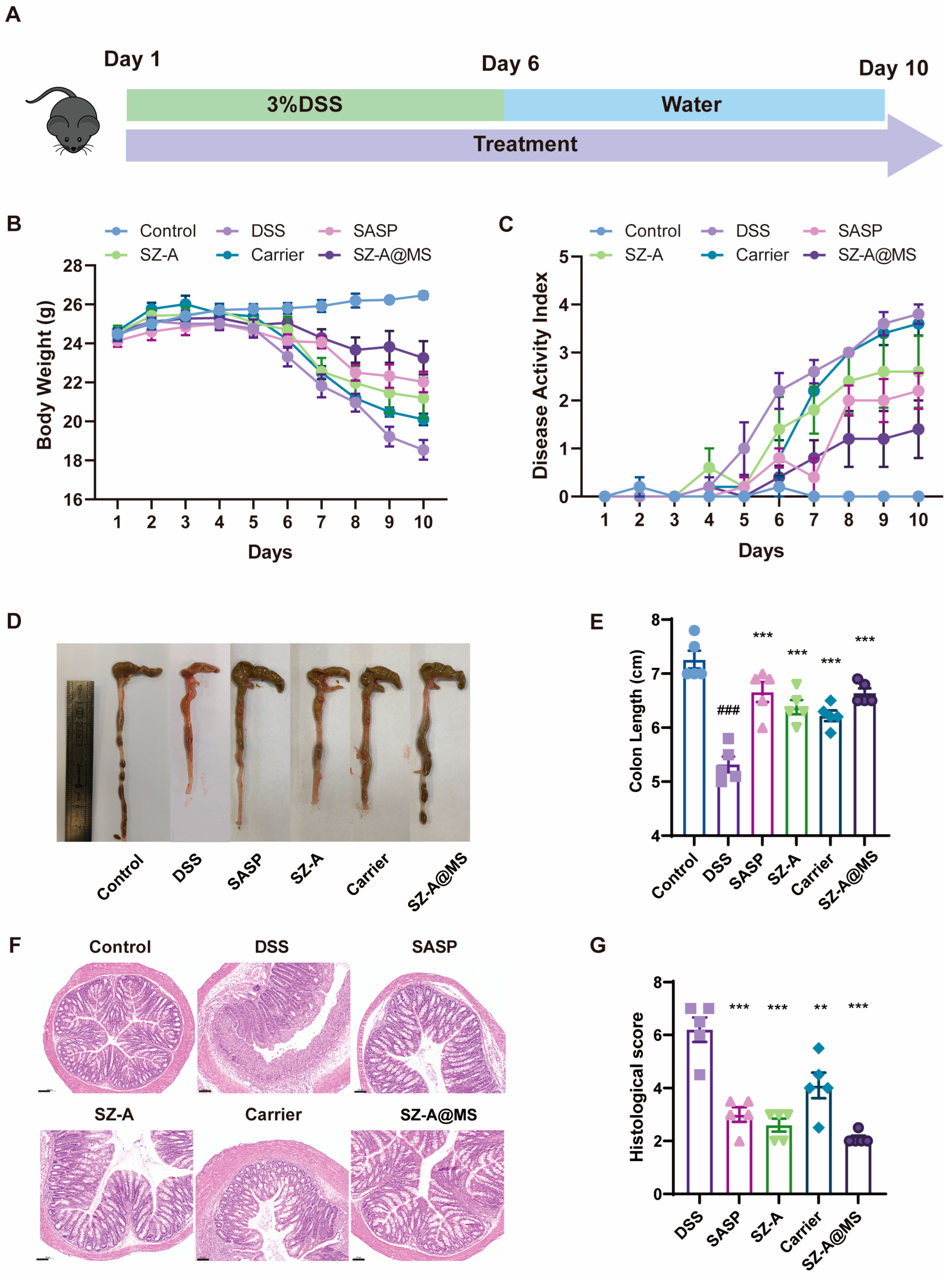
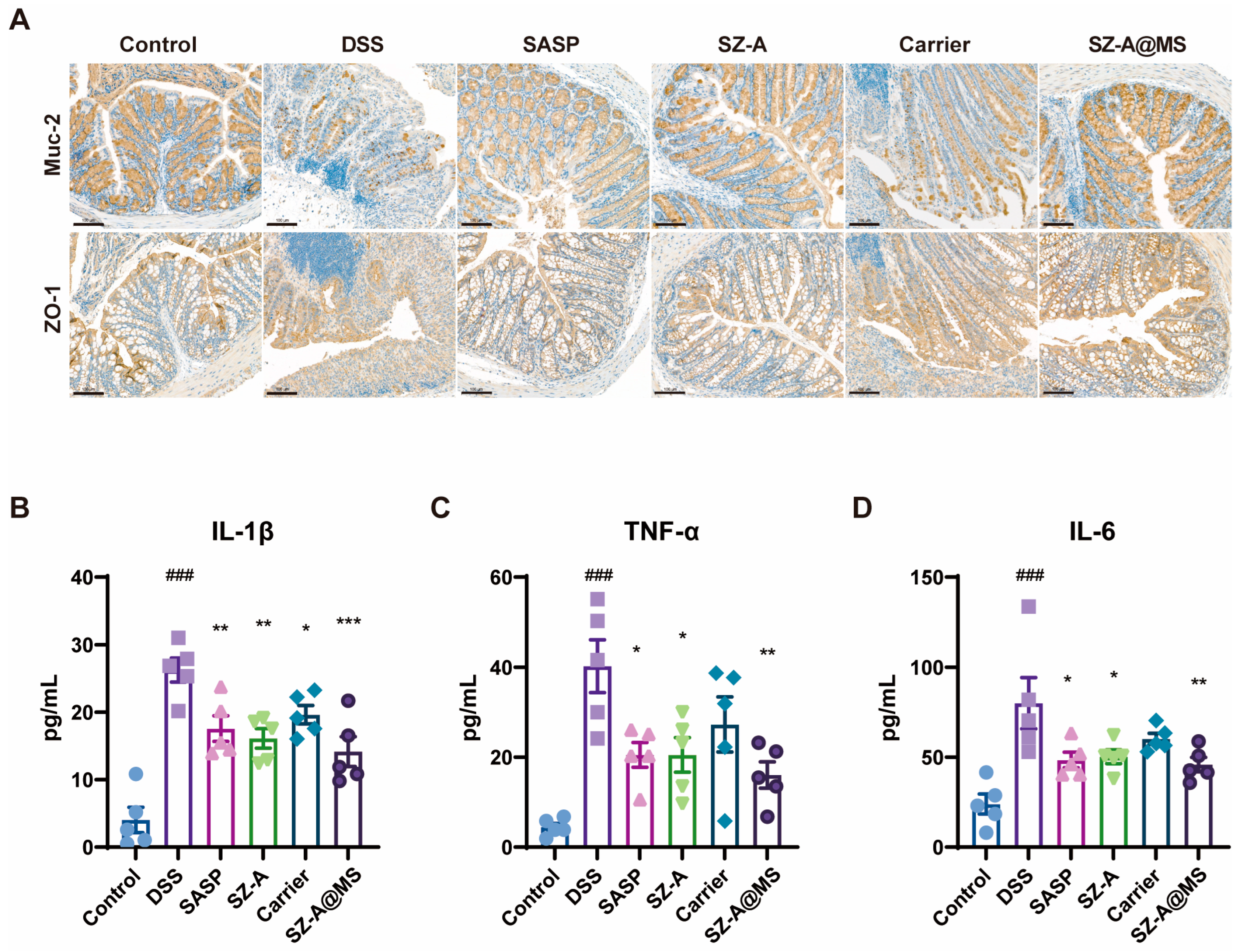
2.5. Protective Effects of SZ-A@MSs on DSS-Induced Colitis
3. Materials and Methods
3.1. Materials
3.2. Preparation of the HA-SH
3.3. Characterization of Materials
3.4. Preparation of the SZ-A@MS
3.5. Characterization of SZ-A@MSs
3.6. In Vitro Release Profile of SZ-A@MSs
3.7. In Vivo IVIS Imaging
3.8. Ex Vivo Adhesion Experiments
3.9. Cell Cytotoxicity Assessment
3.10. In Vitro Antioxidant and Anti-Inflammatory Evaluation
3.11. Mesoscopic Simulations of SZ-A@MSs Based on Dissipative Particle Dynamics (DPD)
3.12. Animals and Experimental Design
3.13. Disease Activity Index (DAI)
3.14. H&E Staining and Immunohistochemistry Staining
3.15. Histopathological Scoring
3.16. Serum and Cell Culture Supernatant ELISA Analysis
3.17. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Cannatelli, R.; Monico, M.C.; Maconi, G.; Ardizzone, S. An Update on Current Pharmacotherapeutic Options for the Treatment of Ulcerative Colitis. J. Clin. Med. 2022, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Sands, B.E. New Therapeutics for Ulcerative Colitis. Annu. Rev. Med. 2021, 72, 199–213. [Google Scholar] [CrossRef]
- Gupta, M.; Mishra, V.; Gulati, M.; Kapoor, B.; Kaur, A.; Gupta, R.; Tambuwala, M.M. Natural compounds as safe therapeutic options for ulcerative colitis. Inflammopharmacology 2022, 30, 397–434. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Cao, H.; Ji, W.; Li, C.; Huan, Y.; Lei, L.; Fu, Y.; Gao, X.; Liu, Y.; et al. Ramulus Mori (Sangzhi) Alkaloids (SZ-A) Ameliorate Glucose Metabolism Accompanied by the Modulation of Gut Microbiota and Ileal Inflammatory Damage in Type 2 Diabetic KKAy Mice. Front. Pharmacol. 2021, 12, 642400. [Google Scholar] [CrossRef]
- Qu, L.; Liang, X.; Tian, G.; Zhang, G.; Wu, Q.; Huang, X.; Cui, Y.; Liu, Y.; Shen, Z.; Xiao, C.; et al. Efficacy and Safety of Mulberry Twig Alkaloids Tablet for the Treatment of Type 2 Diabetes: A Multicenter, Randomized, Double-Blind, Double-Dummy, and Parallel Controlled Clinical Trial. Diabetes Care 2021, 44, 1324–1333. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Shen, Y.; Wang, J.; Zhou, L.; Chen, Z. Preparation of baicalin colon-targeted granules and its intervention effect on ulcerative colitis in rats. Particuology 2023, 85, 13–21. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Liu, J.; Huang, X.; Yan, X. Investigations of the gingerol oil colon targeting pellets for the treatment of ulcerative colitis. Fitoterapia 2023, 169, 105607. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Fernandes, A.I.; Jozala, A.F. Polymers Enhancing Bioavailability in Drug Delivery. Pharmaceutics 2022, 14, 2199. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Ahmad, N.; Alkholifi, F.K.; Ullah, Z.; Khalid, M.S.; Akhtar, S.; Farooqui, S.; Khan, N.; Chaudhary, A.A.; Alawam, A.S.; et al. Preparation of novel S-allyl cysteine chitosan based nanoparticles for use in ischemic brain treatment. RSC Adv. 2024, 14, 160–180. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y.; Sheng, S.; Yang, H.; Li, Z.; Han, Q.; Zhang, Q.; Su, J. DNA-based hydrogels for bone regeneration: A promising tool for bone organoids. Mater. Today Bio 2025, 31, 101502. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liu, Y.; Zhu, J.; Shang, J.; Bai, L.; Jin, Z.; Li, W.; Hu, Y.; Zheng, X.; Qian, J. Mapping the intellectual structure and emerging trends on nanomaterials in colorectal cancer: A bibliometric analysis from 2003 to 2024. Front. Oncol. 2024, 14, 1514581. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Leitner, V.M.; Walker, G.F.; Bernkop-Schnürch, A. Thiolated polymers: Evidence for the formation of disulphide bonds with mucus glycoproteins. Eur. J. Pharm. Biopharm. 2003, 56, 207–214. [Google Scholar] [CrossRef]
- Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. JCR 2007, 122, 338–344. [Google Scholar] [CrossRef]
- Abdelghany, S.; Parumasivam, T.; Pang, A.; Roediger, B.; Tang, P.; Jahn, K.; Britton, W.J.; Chan, H.-K. Alginate modified-PLGA nanoparticles entrapping amikacin and moxifloxacin as a novel host-directed therapy for multidrug-resistant tuberculosis. Int. J. Drug Deliv. Technol. 2019, 52, 642–651. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Z.; Wang, F.; Hong, L.; Deng, L.; Zhong, J.; Wang, Z.; Cui, W. Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora. Adv. Sci. 2021, 8, e2101619. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Akhtar, N.; Manickam, S. Interfacial film stabilized W/O/W nano multiple emulsions loaded with green tea and lotus extracts: Systematic characterization of physicochemical properties and shelf-storage stability. J. Nanobiotechnol. 2014, 12, 20. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J. Surface functionalization of PLGA nanoparticles for potential oral vaccine delivery targeting intestinal immune cells. Colloids Surf. B Biointerfaces 2023, 222, 113121. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Liu, Q.; Li, S.; Cheng, Y.; Cheng, C.; Sun, Z.; Du, Y.; Butch, C.J.; Wei, H. An Orally Administered CeO2@Montmorillonite Nanozyme Targets Inflammation for Inflammatory Bowel Disease Therapy. Adv. Funct. Mater. 2020, 30, 2004692. [Google Scholar] [CrossRef]
- Zhang, S.; Ermann, J.; Succi, M.D.; Zhou, A.; Hamilton, M.J.; Cao, B.; Korzenik, J.R.; Glickman, J.N.; Vemula, P.K.; Glimcher, L.H.; et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 2015, 7, 300ra128. [Google Scholar] [CrossRef]
- Salathia, S.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P.; Censi, R. Hyaluronic Acid-Based Nanosystems for CD44 Mediated Anti-Inflammatory and Antinociceptive Activity. Int. J. Mol. Sci. 2023, 24, 7286. [Google Scholar] [CrossRef]
- Zhang, G.; Baidin, V.; Pahil, K.S.; Moison, E.; Tomasek, D.; Ramadoss, N.S.; Chatterjee, A.K.; McNamara, C.W.; Young, T.S.; Schultz, P.G.; et al. Cell-based screen for discovering lipopolysaccharide biogenesis inhibitors. Proc. Natl. Acad. Sci. USA 2018, 115, 6834–6839. [Google Scholar] [CrossRef]
- Zhao, X.-N.; Bai, Z.-Z.; Li, C.-H.; Sheng, C.-L.; Li, H.-Y. The NK-1R Antagonist Aprepitant Prevents LPS-Induced Oxidative Stress and Inflammation in RAW264.7 Macrophages. Drug Des. Dev. Ther. 2020, 14, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Cross-Talk between NADPH Oxidase and Mitochondria: Role in ROS Signaling and Angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Alnuqaydan, A.M.; Almutary, A.G.; Azam, M.; Manandhar, B.; De Rubis, G.; Madheswaran, T.; Paudel, K.R.; Hansbro, P.M.; Chellappan, D.K.; Dua, K. Phytantriol-Based Berberine-Loaded Liquid Crystalline Nanoparticles Attenuate Inflammation and Oxidative Stress in Lipopolysaccharide-Induced RAW264.7 Macrophages. Nanomaterials 2022, 12, 4312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Z.; Li, C.; Liu, D.; Li, X.; Xu, J.; Chen, N.; Wang, X.; Li, Q.; Li, Y. Multiple Beneficial Effects of Aloesone from Aloe vera on LPS-Induced RAW264.7 Cells, Including the Inhibition of Oxidative Stress, Inflammation, M1 Polarization, and Apoptosis. Molecules 2023, 28, 1617. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. IBD 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Leoncini, G.; Cari, L.; Ronchetti, S.; Donato, F.; Caruso, L.; Calafà, C.; Villanacci, V. Mucin Expression Profiles in Ulcerative Colitis: New Insights on the Histological Mucosal Healing. Int. J. Mol. Sci. 2024, 25, 1858. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Fan, P.; Dong, X.; Zhu, K.; Liu, X.; Zhang, N.; Cao, Y. Dioscin prevents DSS-induced colitis in mice with enhancing intestinal barrier function and reducing colon inflammation. Int. Immunopharmacol. 2021, 99, 108015. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Arumugam, P.; Saha, K.; Nighot, P. Intestinal Epithelial Tight Junction Barrier Regulation by Novel Pathways. IBD 2025, 31, 259–271. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Neurath, M.F. Cytokines in inflammatory bowel diseases—Update 2020. Pharmacol. Res. 2020, 158, 104835. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, K.; Bao, C.; Wang, X.; Sun, X.; Xia, H.; Lin, Q.; Yang, Y.; Zhu, L. Precise Construction of Cell-Instructive 3D Microenvironments by Photopatterning a Biodegradable Hydrogel. Chem. Mater. 2019, 31, 4710–4719. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Jiang, Y.; Chen, Z.; Jiang, D.; Jiang, X.; Ye, J.; Wang, H.; Liu, Y. Colon-Targeted Mucoadhesive PLGA Microspheres Loaded with Ramulus Mori Alkaloids for Enhanced Water-Soluble Drug Delivery in Ulcerative Colitis Treatment. Molecules 2025, 30, 1878. https://doi.org/10.3390/molecules30091878
Wang M, Jiang Y, Chen Z, Jiang D, Jiang X, Ye J, Wang H, Liu Y. Colon-Targeted Mucoadhesive PLGA Microspheres Loaded with Ramulus Mori Alkaloids for Enhanced Water-Soluble Drug Delivery in Ulcerative Colitis Treatment. Molecules. 2025; 30(9):1878. https://doi.org/10.3390/molecules30091878
Chicago/Turabian StyleWang, Mo, Yu Jiang, Zhiyang Chen, Dengbao Jiang, Xuan Jiang, Jun Ye, Hongliang Wang, and Yuling Liu. 2025. "Colon-Targeted Mucoadhesive PLGA Microspheres Loaded with Ramulus Mori Alkaloids for Enhanced Water-Soluble Drug Delivery in Ulcerative Colitis Treatment" Molecules 30, no. 9: 1878. https://doi.org/10.3390/molecules30091878
APA StyleWang, M., Jiang, Y., Chen, Z., Jiang, D., Jiang, X., Ye, J., Wang, H., & Liu, Y. (2025). Colon-Targeted Mucoadhesive PLGA Microspheres Loaded with Ramulus Mori Alkaloids for Enhanced Water-Soluble Drug Delivery in Ulcerative Colitis Treatment. Molecules, 30(9), 1878. https://doi.org/10.3390/molecules30091878






