Bioresorbable High-Strength HA/PLLA Composites for Internal Fracture Fixation
Abstract
1. Introduction
2. Results and Discussion
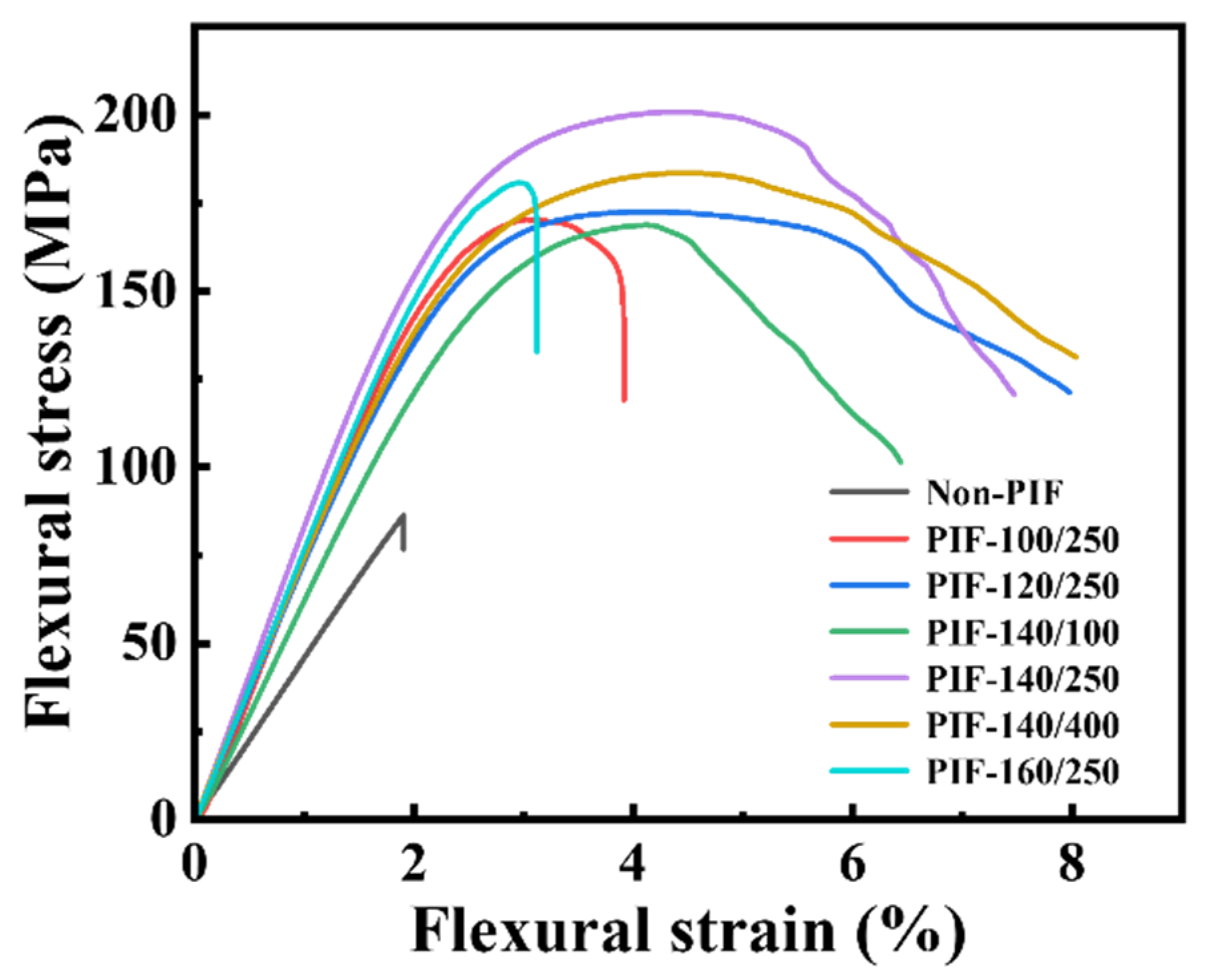
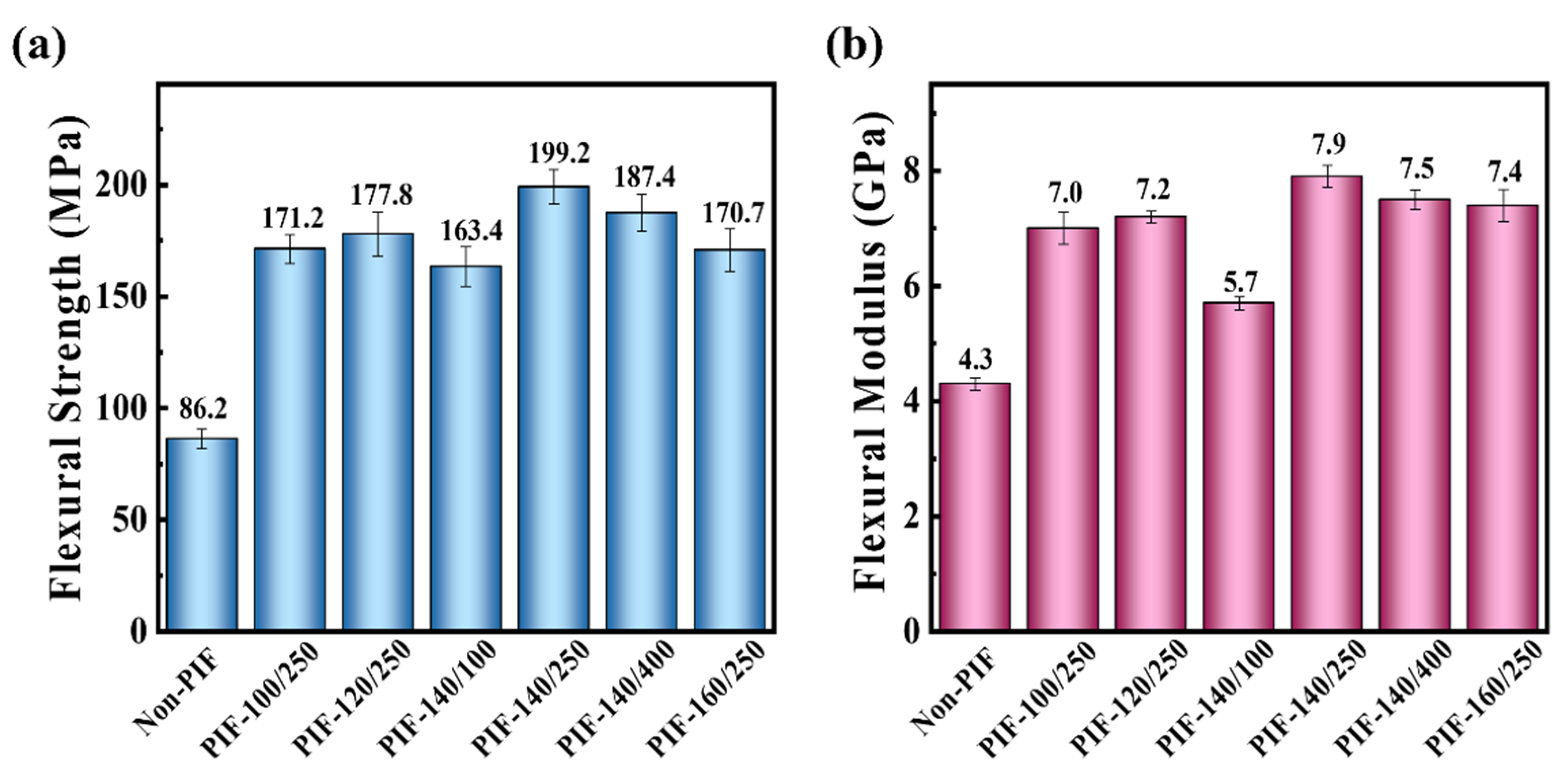
2.1. Mechanical Properties of the HA/PLLA Composites
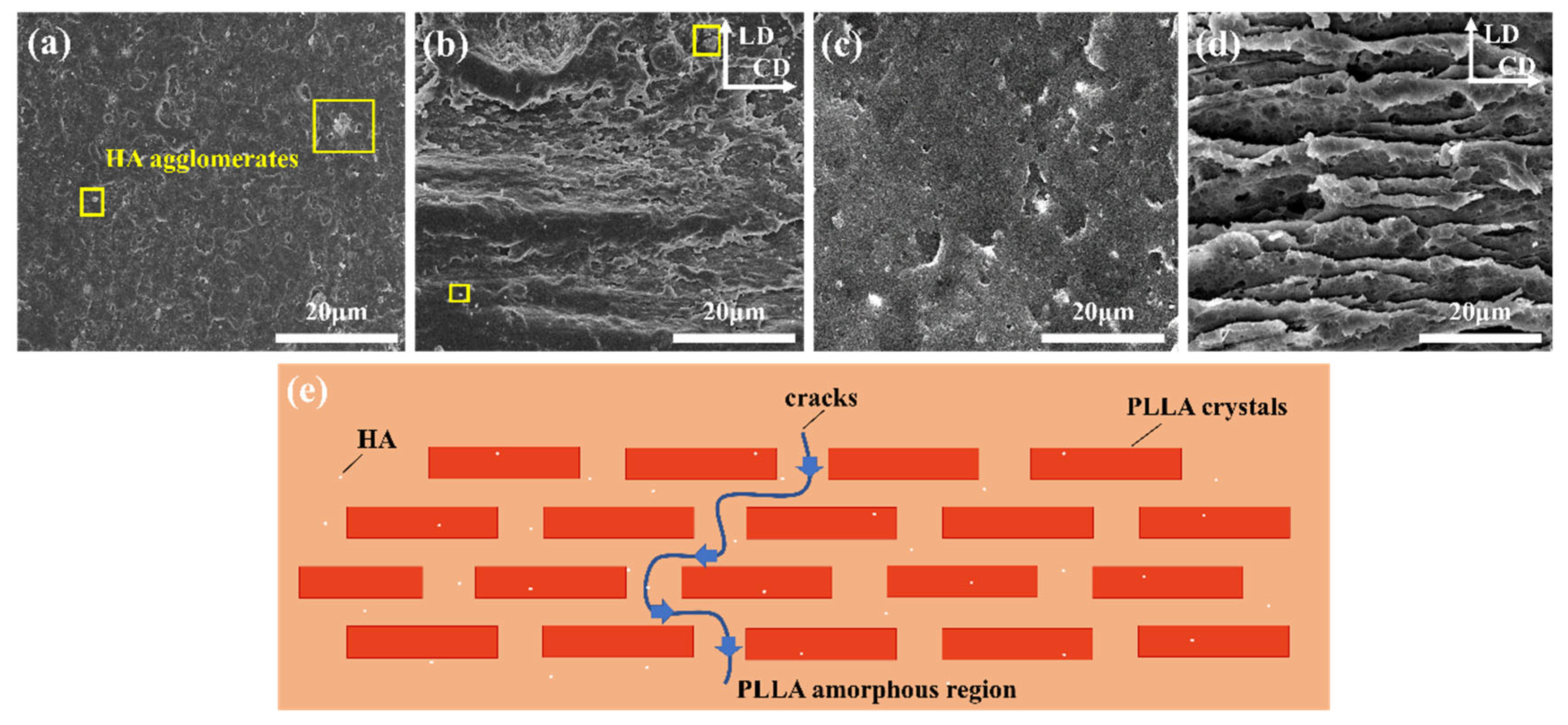
2.2. Microstructure of the HA/PLLA Composites
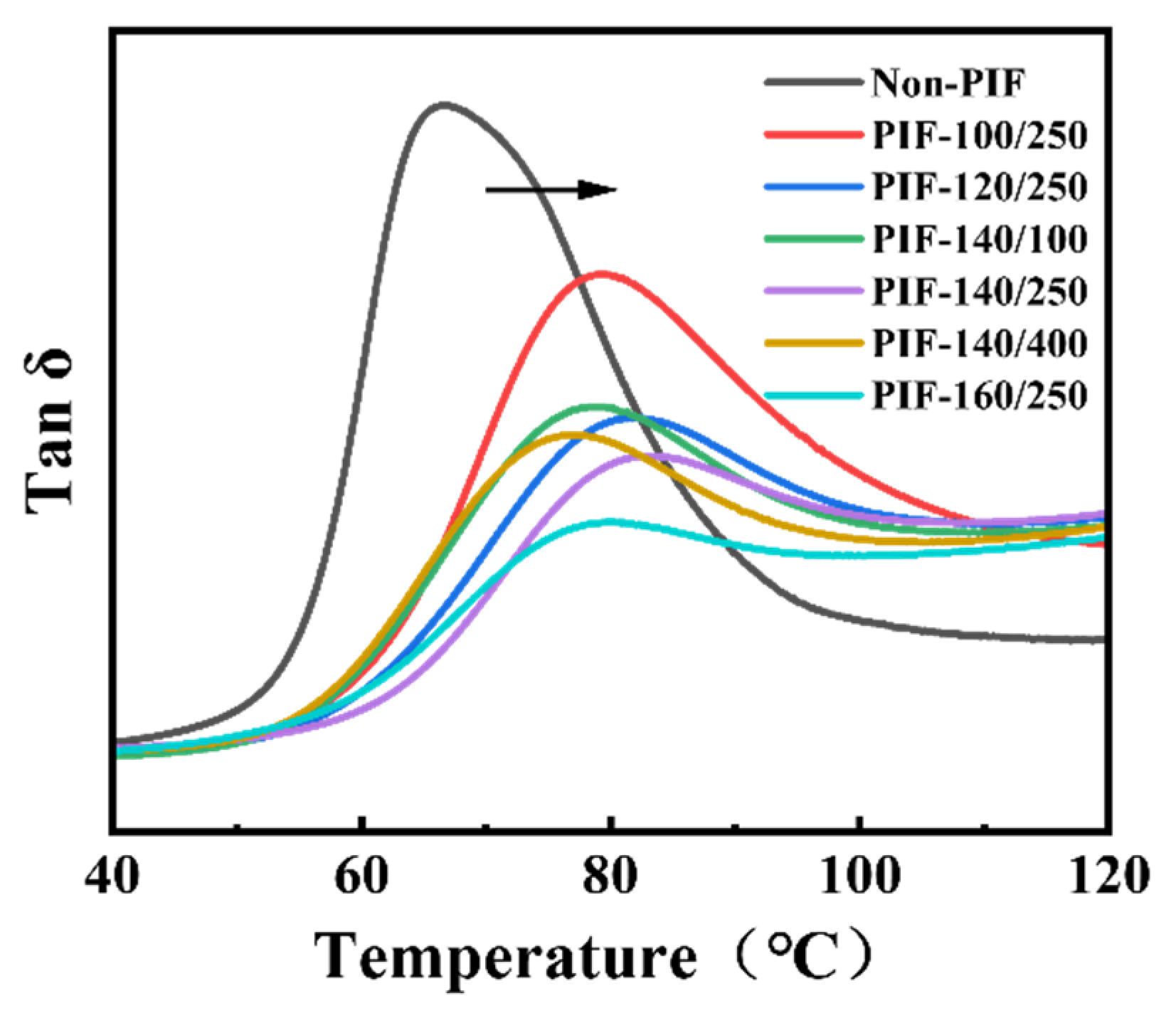
2.3. Formation of Multi-Level Stacked Crystal Layers
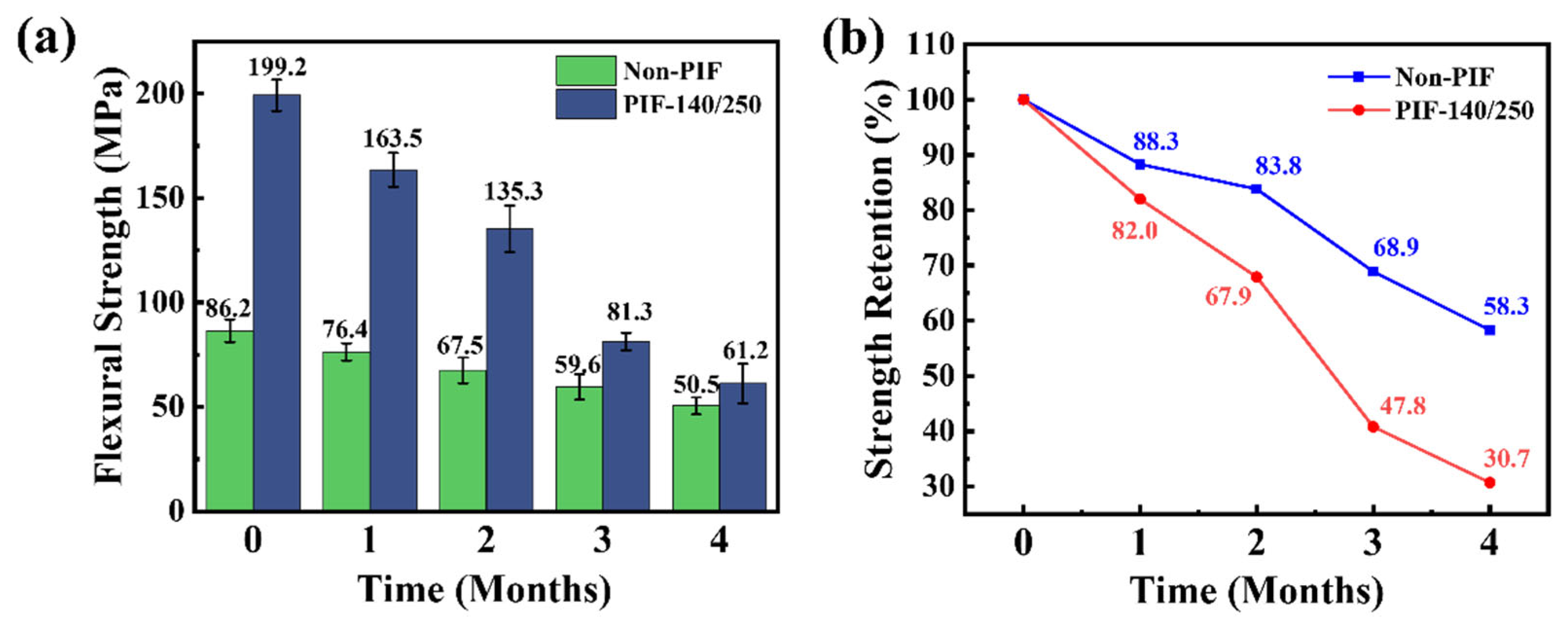
2.4. In Vitro Degradation Property of the HA/PLLA Composites
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Characterization
3.3.1. Mechanical Properties Tests
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. Differential Scanning Calorimetry (DSC)
3.3.4. Wide-Angle X-Ray Diffraction (WAXD)
3.3.5. Dynamic Mechanical Analysis (DMA)
3.3.6. In Vitro Degradation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Migliorini, F.; Giorgino, R.; Hildebrand, F.; Spiezia, F.; Peretti, G.M.; Alessandri-Bonetti, M.; Eschweiler, J.; Maffulli, N. Fragility fractures: Risk factors and management in the elderly. Medicina 2021, 57, 1119. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.P.; Njoku-Austin, C.; Charles, S.; Como, M.; Singh-Varma, A.; Okundaye, O.; Fogg, D.; Karimi, A.; Lin, A. Dual mini-fragment plate fixation of midshaft clavicle fractures demonstrates fewer union complications but similar patient-reported outcomes compared to nonoperative management: A cohort study with mean 3.4-year follow-up. J. Shoulder Elb. Surg. 2024, in press. [Google Scholar] [CrossRef]
- Tamburini, L.M.; Mayo, B.C.; Edgar, C. Dual- versus single-plate fixation of clavicle fractures: Understanding the rationale behind both approaches. Clin. Sports Med. 2023, 42, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, C.; Rodriguez, K.; Zeng, S.; Klifto, K.M.; Klifto, C.S.; Ruch, D.S. Complications following volar locking plate fixation of distal radius fractures in adults: A systematic review of randomized control trials. J. Hand Surg. 2023, 48, 861–874. [Google Scholar] [CrossRef]
- Dantas, F.L.; Rodriguez Llanos, J.H.; Pereira Rodrigues, I.C.; Pereira, K.D.; Luchessi, A.D.; Sawazaki, R.; Najar Lopes, É.S.; Gabriel, L.P. PLLA scaffolds functionalized with ketoprofen via rotary jet spinning for biomedical applications. J. Mater. Res. Technol. 2024, 30, 9020–9027. [Google Scholar] [CrossRef]
- Qu, D.; Xiang, J.; Tian, J.; Zhang, S.; Li, L.; Zhou, C. Enhancing bone repair efficiency through synergistic modification of recombinant human collagen onto PLLA membranes. Int. J. Biol. Macromol. 2024, 283, 137631. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H.; Qiu, Z.; Liu, W.; Hu, Y.; Wang, C.; Ding, J. Enhancement of poly-L-lactic acid stents through polydopamine coating: Boosting endothelialization and suppressing inflammation. Alex. Eng. J. 2023, 81, 525–531. [Google Scholar] [CrossRef]
- Slepička, P.; Slepičková Kasálková, N.; Musílková, J.; Bačáková, L.; Frýdlová, B.; Sajdl, P.; Kolská, Z.; Rebollar, E.; Švorčík, V. PLLA honeycombs activated by plasma and high-energy excimer laser for stem cell support. Appl. Surf. Sci. Adv. 2025, 25, 100662. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Skwarek, E. Composites of hydroxyapatite and their application in adsorption, medicine and as catalysts. Adv. Colloid Interface Sci. 2024, 334, 103308. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, S.; Rehman Khan, A.; Liu, J.; Fu, Q. Development of nano-hydroxyapatite composite photo-crosslinked bone decellularized matrix based microsphere for large bone defect regeneration. J. Ind. Eng. Chem. 2024, 146, 656–667. [Google Scholar] [CrossRef]
- Pańtak, P.; Czechowska, J.P.; Kashimbetova, A.; Čelko, L.; Montufar, E.B.; Wójcik, Ł.; Zima, A. Improving the processability and mechanical strength of self-hardening robocasted hydroxyapatite scaffolds with silane coupling agents. J. Mech. Behav. Biomed. Mater. 2025, 161, 106792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, S.; Ding, X.; Xiong, M.; Duan, P. Design of internal fixation implants for fracture: A review. J. Orthop. Transl. 2025, 50, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, H.; Lee, J.; Kim, G. Mechanically and biologically enhanced 3D-printed HA/PLLA/dECM biocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2022, 218, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.B.; Mohan, S.; Genasan, K.; Murali, M.R.; Naveen, S.V.; Talebian, S.; McKean, R.; Kamarul, T. Synergistic interaction of platelet derived growth factor (PDGF) with the surface of PLLA/Col/HA and PLLA/HA scaffolds produces rapid osteogenic differentiation. Colloids Surf. B Biointerfaces 2016, 139, 68–78. [Google Scholar] [CrossRef]
- Nishida, Y.; Domura, R.; Sakai, R.; Okamoto, M.; Arakawa, S.; Ishiki, R.; Salick, M.R.; Turng, L.-S. Fabrication of PLLA/HA composite scaffolds modified by DNA. Polymer 2015, 56, 73–81. [Google Scholar] [CrossRef]
- Ikawa, H.; Moroi, A.; Yoshizawa, K.; Saida, Y.; Hotta, A.; Tsutsui, T.; Fukaya, K.; Hiraide, R.; Takayama, A.; Tsunoda, T.; et al. Bone regeneration enhancement by ultra-violet (UV) treatment for uHA/PLLA absorbable mesh. J. Cranio-Maxillofac. Surg. 2017, 45, 634–641. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Wang, H.; Liu, C.; Jiang, L.; Liu, W.; Wu, Y.; Wang, Y.; Yan, H.; Lin, J.; et al. A Sr@Ag-based spatiotemporal and step-release scaffold against chronic osteomyelitis, fabricated by coaxial 3D-printing. Biomaterials 2025, 314, 122899. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, J.; Fang, S.; Li, T.; Chen, Y.; Li, L.; Chen, N. A biodegradable, osteo-regenerative and biomechanically robust polylactide bone screw for clinical orthopedic surgery. Int. J. Biol. Macromol. 2024, 283, 137477. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Gazińska, M.; Kryszak, B.; Grzymajło, M.; Pigłowski, J.; Wiglusz, R.J.; Okamoto, M. The influence of hydroxyapatite content on properties of poly(L-lactide)/hydroxyapatite porous scaffolds obtained using thermal induced phase separation technique. Eur. Polym. J. 2019, 113, 313–320. [Google Scholar] [CrossRef]
- Todo, M.; Park, S.D.; Arakawa, K.; Takenoshita, Y. Relationship between microstructure and fracture behavior of bioabsorbable HA/PLLA composites. Compos. Part A. Sci. Manuf. 2006, 37, 2221–2225. [Google Scholar] [CrossRef]
- Oktay, B.; Ahlatcıoğlu Özerol, E.; Sahin, A.; Gunduz, O.; Ustundag, C.B. Production and characterization of PLA/HA/GO nanocomposite scaffold. ChemistrySelect 2022, 7, e202200697. [Google Scholar] [CrossRef]
- Wang, T.; Chow, L.C.; Frukhtbeyn, S.A.; Ting, A.H.; Dong, Q.; Yang, M.; Mitchell, J.W. Improve the strength of PLA/HA composite through the use of surface initiated polymerization and phosphonic acid coupling agent. J. Res. Natl. Inst. Stand. Technol. 2011, 116, 785–796. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2502-17; Standard Specification and Test Methods for Absorbable Plates and Screws for Internal Fixation Implants. ASTM: West Conshohocken, PA, USA, 2024.
- Qin, L.; Qiu, J.; Liu, M.; Ding, S.; Shao, L.; Lü, S.; Zhang, G.; Zhao, Y.; Fu, X. Mechanical and thermal properties of Poly(Lactic Acid) composites with rice straw fiber modified by Poly(butyl acrylate). Chem. Eng. J. 2011, 166, 772–778. [Google Scholar] [CrossRef]
- Fernändez, J.; Etxeberria, A.; Sarasu, J. Synthesis, structure andproperties of poly(L-lactide-co-ε-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef]
- Huan, Q.; Zhu, S.; Ma, Y.; Zhang, J.; Zhang, S.; Feng, X.; Han, K.; Yu, M. Markedly improving mechanical properties for isotactic polypropylene with large-size spherulites by pressure-induced flow processing. Polymer 2013, 54, 1177–1183. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.-P.; Feldmann, M.; Mariatti, M. Synergized high-load bearing bone replacement compositefrom poly(lactic acid) reinforced with hydroxyapatite/glassfiber hybrid filler—Mechanical and dynamic mechanicalproperties. Polym. Compos. 2021, 42, 57–69. [Google Scholar] [CrossRef]
- Chen, C.; Tian, Y.; Li, F.; Hu, H.; Wang, K.; Kong, Z.; Ying, W.B.; Zhang, R.; Zhu, J. Toughening polylactic acid by a biobased poly(butylene 2,5-furandicarboxylate)-b-poly(ethylene glycol) copolymer: Balanced mechanical properties and potential biodegradability. Biomacromolecules 2021, 22, 374–385. [Google Scholar] [CrossRef]
- Zhu, S.; Yan, T.; Huang, X.; Hassan, E.A.M.; Zhou, J.; Zhang, S.; Xiong, M.; Yu, M.; Li, Z. Bioinspired nacre-like PEEK material with superior tensile strength and impact toughness. RSC Adv. 2022, 12, 15584–15592. [Google Scholar] [CrossRef]
- Lu, Y.; Li, W.; Zhou, J.; Ren, Y.; Wang, X.; Li, J.; Zhu, S. Strengthening and toughening behaviours and mechanisms of carbon fiber reinforced polyetheretherketone composites (CF/PEEK). Compos. Commun. 2023, 37, 101397. [Google Scholar] [CrossRef]
- Hyon, S.-H.; Jin, F.; Jamshidi, K.; Tsutsumi, S.; Kanamoto, T. Biodegradable ultra high strength poly(L-lactide) rods for bone fixation. Macromol. Symp. 2003, 197, 355–368. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Yan, C.; Jiang, Y.-P.; Hou, D.-F.; Yang, W.; Yang, M.-B. High-efficient crystallization promotion and melt reinforcement effect of diblock PDLA-b-PLLA copolymer on PLLA. Polymer 2020, 186, 122021. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Li, M.; Cao, W.; Liu, C.; Shen, C. Orientation and structural development of semicrystalline poly(lactic acid) under uniaxial drawing assessed by infrared spectroscopy and X-ray diffraction. Polym. Test. 2015, 41, 163–171. [Google Scholar] [CrossRef]
- Hara, S.; Watanabe, S.; Takahashi, K.; Shimizu, S.; Ikake, H. Preparation of crystallites for oriented poly(lactic acid) films using a casting method under a magnetic field. Polymers 2018, 10, 1083. [Google Scholar] [CrossRef]
- Mao, Y.; Su, Y.; Hsiao, B.S. Probing structure and orientation in polymers using synchrotron small- and wide-angle X-ray scattering techniques. Eur. Polym. J. 2016, 81, 433–446. [Google Scholar] [CrossRef]
- Ghimire, S.; Miramini, S.; Richardson, M.; Mendis, P.; Zhang, L. Effects of dynamic loading on fracture healing under different locking compression plate configurations: A finite element study. J. Mech. Behav. Biomed. Mater. 2019, 94, 74–85. [Google Scholar] [CrossRef]
- Perren, S.M.; Regazzoni, P.; Fernandez, A.A. Biomechanical and biological aspects of defect treatment in fractures using helical plates. Acta Chir. Orthop. Traumatol. Cech. 2014, 81, 267–271. [Google Scholar] [CrossRef]
- Bethell, M.A.; Doyle, T.R.; Hurley, E.T.; Allen, H.; Pidgeon, T.S.; Péan, C.A.; Anakwenze, O.; Klifto, C.S. Tension band wiring and plate fixation for olecranon fractures—A systematic review and meta-analysis. JSES Rev. Rep. Tech. 2025, in press. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; He, Y.; Yan, W.; Yu, M.; Han, K. Synergistic toughening of polylactide by layer structure and network structure. Polymer 2025, 317, 127969. [Google Scholar] [CrossRef]
- ISO 178:2019; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.
- ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. ISO: Geneva, Switzerland, 2019.
- ISO 180:2023; Plastics—Determination of Izod Impact Strength. ISO: Geneva, Switzerland, 2019.
- Zhang, H.; Li, S.; Yan, Y. Dissolution behavior of hydroxyapatite powder in hydrothermal solution. Ceram. Int. 2001, 27, 451–454. [Google Scholar] [CrossRef]
- Sun, M.; Liu, J.; Yu, M.; Yan, W.; Han, K. The impact of template effect at different temperatures on homocrystallization-to-stereocomplexation transformation in poly(L-lactide)/poly(D-lactide) racemic blends. Polymer 2024, 309, 127436. [Google Scholar] [CrossRef]



| Classification | Name | Peculiarity |
|---|---|---|
| bio-polyester | poly(D,L-lactic acid) | amorphous, degraded quickly |
| bio-polyester | poly(lactic-co-glycolic acid) | low strength, degraded quickly |
| bio-ceramics | tricalcium phosphate (Ca3(PO4)2) | osteoinductivity |
| Sample | Tensile Strength (MPa) | Notched Izod Impact Strength (kJ/m2) |
|---|---|---|
| Non-PIF | 35.3 (±4.3) | 3.4 (±0.1) |
| PIF-140/250 | 84.2 (±6.7) | 16.7 (±1.6) |
| Samples | Tg (℃) | ΔHc (J/g) | ΔHm (J/g) | Tm (℃) | Xc (%) |
|---|---|---|---|---|---|
| Non-PIF | 57.2 | 21.7 | 41.1 | 173.4 | 23.1 |
| PIF-100/250 | 77.2 | 3.4 | 43.7 | 173.4 | 47.8 |
| PIF-120/250 | 77.5 | 1.5 | 43.8 | 173.8 | 50.2 |
| PIF-140/100 | 64.5 | 0 | 42.4 | 174.4 | 50.3 |
| PIF-140/250 | 78.8 | 0 | 44.7 | 174.3 | 53.1 |
| PIF-140/400 | 63.8 | 0 | 44.1 | 173.7 | 52.4 |
| PIF-160/250 | 77.3 | 0 | 50.9 | 175.1 | 60.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Sun, M.; He, Y.; Yan, W.; Yu, M.; Han, K. Bioresorbable High-Strength HA/PLLA Composites for Internal Fracture Fixation. Molecules 2025, 30, 1889. https://doi.org/10.3390/molecules30091889
Liu J, Sun M, He Y, Yan W, Yu M, Han K. Bioresorbable High-Strength HA/PLLA Composites for Internal Fracture Fixation. Molecules. 2025; 30(9):1889. https://doi.org/10.3390/molecules30091889
Chicago/Turabian StyleLiu, Jie, Mingtao Sun, Yipeng He, Weixia Yan, Muhuo Yu, and Keqing Han. 2025. "Bioresorbable High-Strength HA/PLLA Composites for Internal Fracture Fixation" Molecules 30, no. 9: 1889. https://doi.org/10.3390/molecules30091889
APA StyleLiu, J., Sun, M., He, Y., Yan, W., Yu, M., & Han, K. (2025). Bioresorbable High-Strength HA/PLLA Composites for Internal Fracture Fixation. Molecules, 30(9), 1889. https://doi.org/10.3390/molecules30091889





