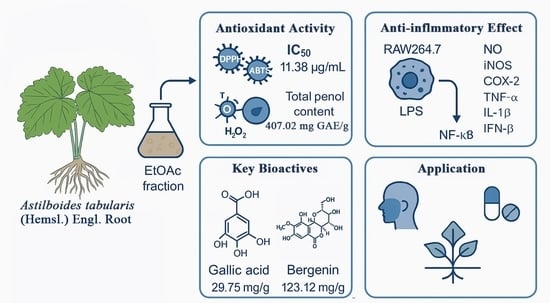
Antioxidant and Anti-Inflammatory Activities of Astilboides tabularis (Hemsl.) Engl. Root Extract
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity of the EtOAc Fraction from A. tabularis Root Extract
2.1.1. DPPH and ABTS Radical Scavenging Activity
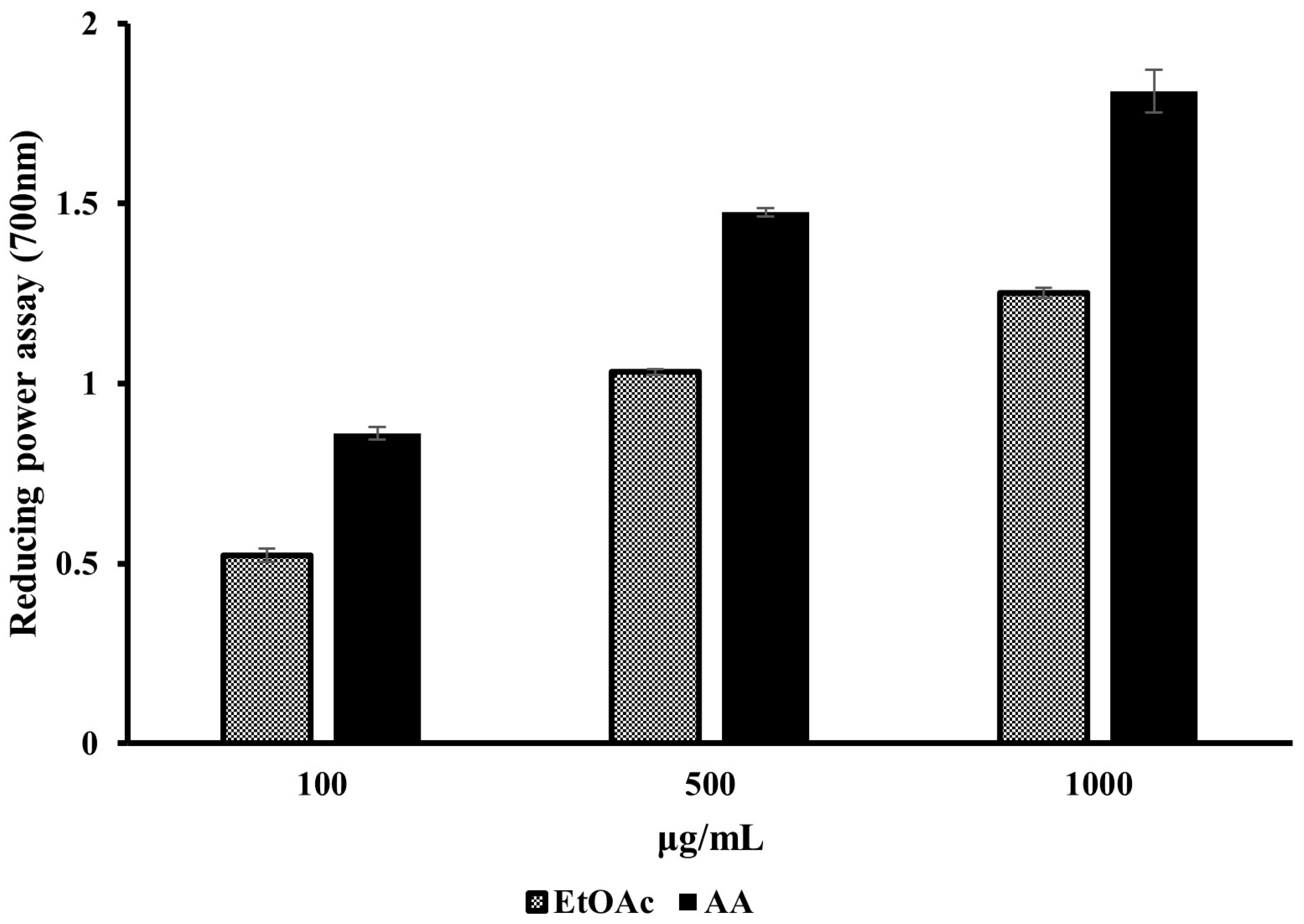
2.1.2. Reducing Power
2.1.3. Total Phenolic Content
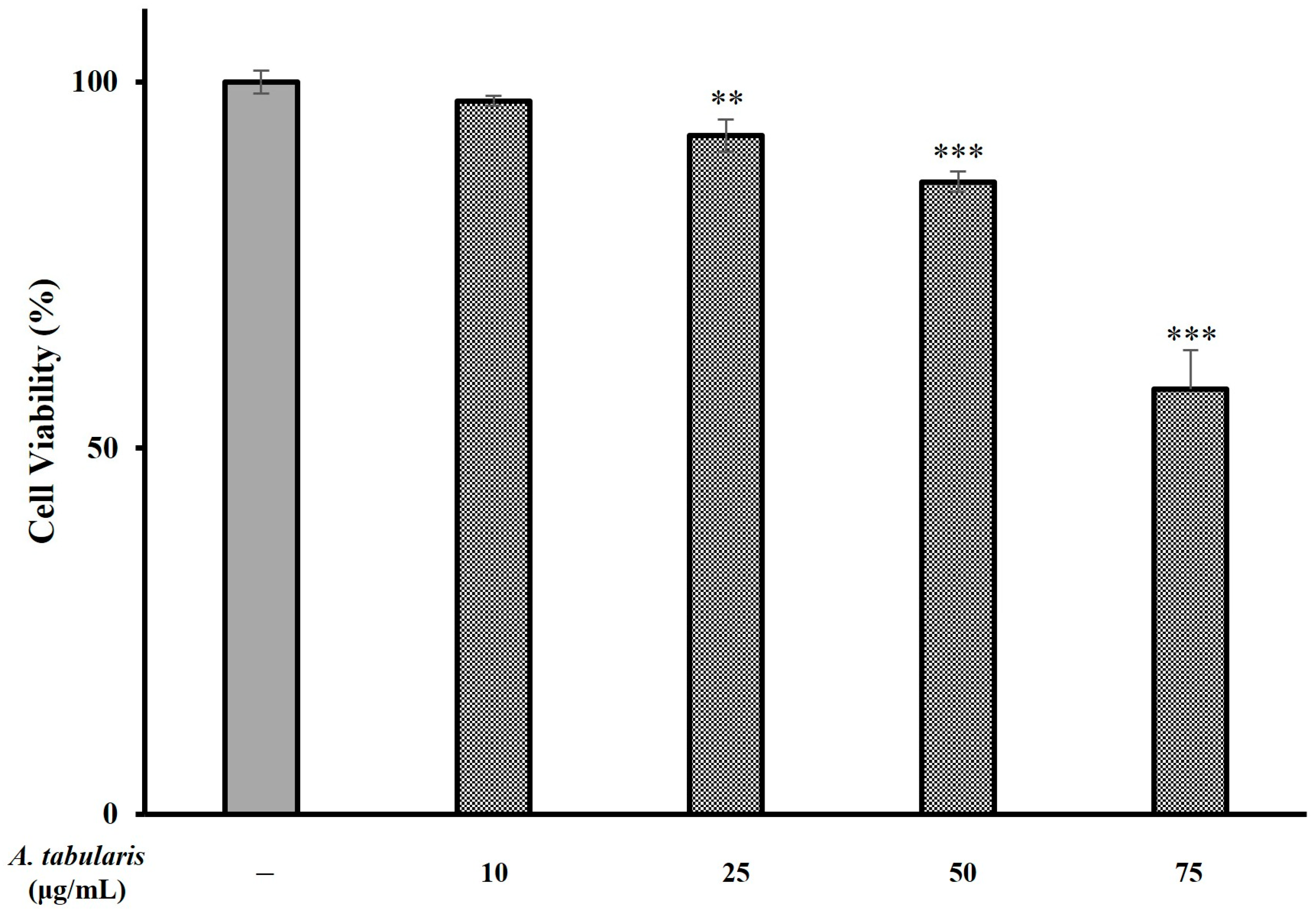
2.2. Cellular Protective Effects Against Oxidative Stress
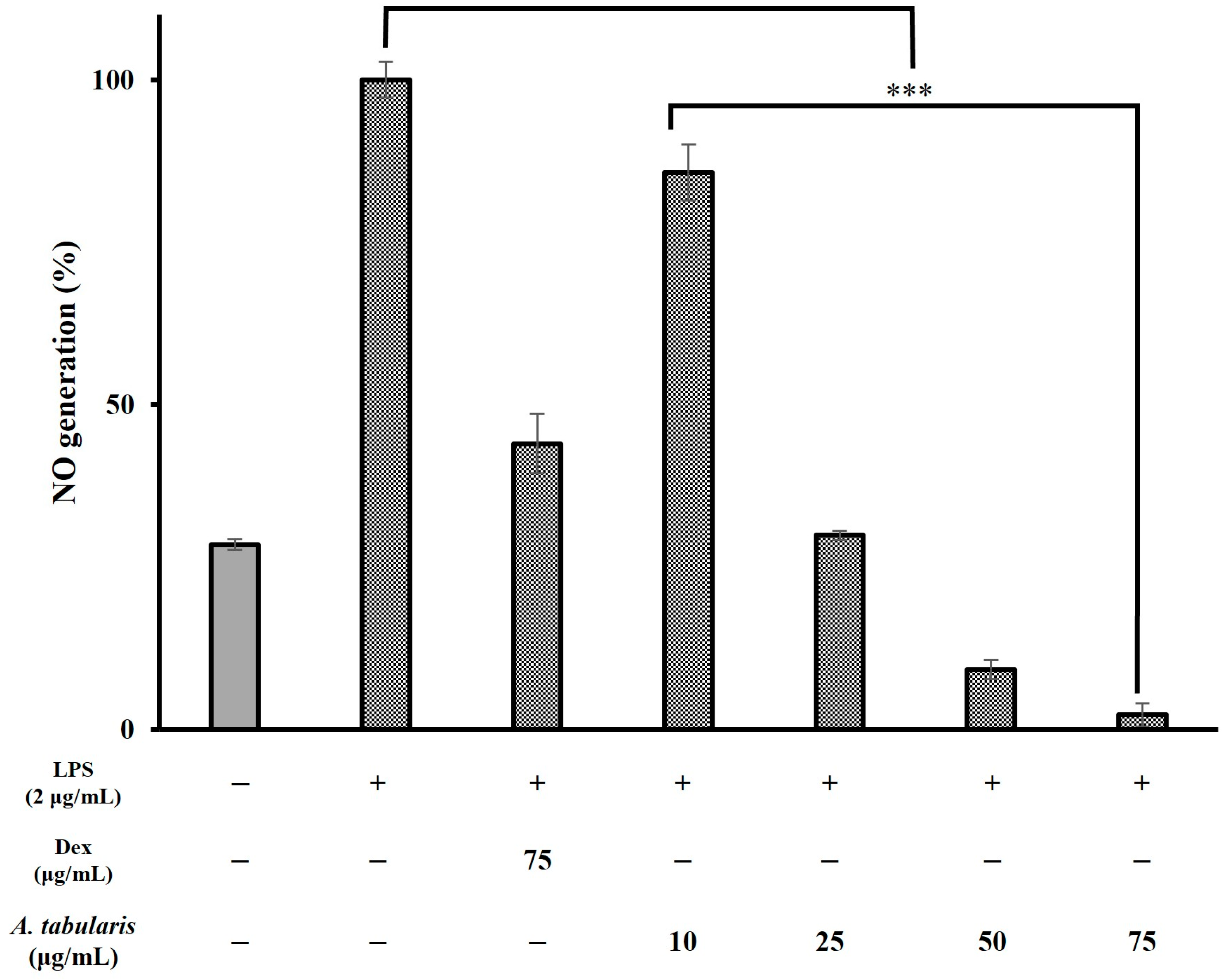
2.3. Anti-Inflammatory Activity of the EtOAc Fraction of A. tabularis Root Extract
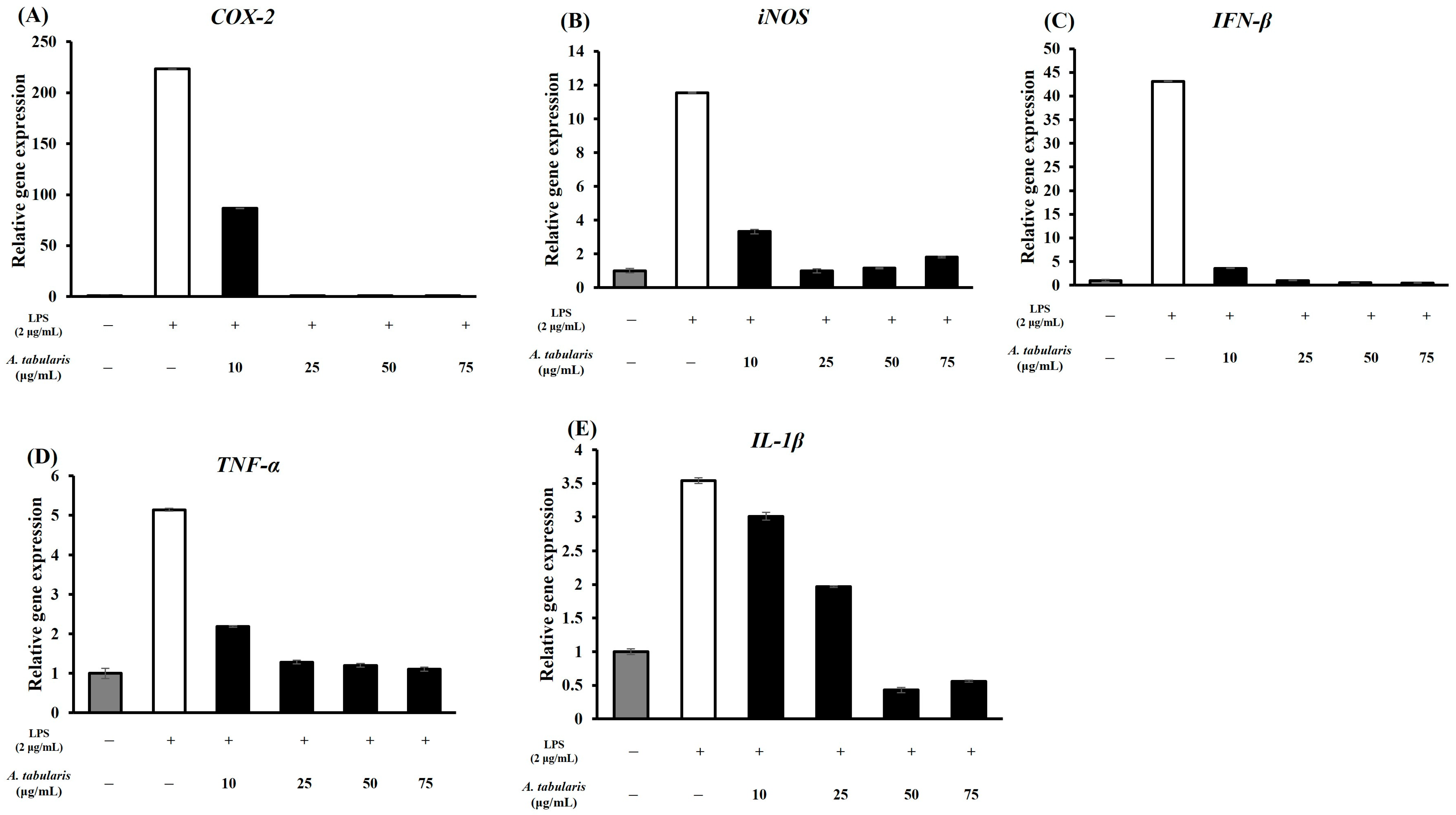
2.4. Anti-Inflammatory Effects and Mechanistic Analysis
2.4.1. T-Helper 1 (TH1) Inflammation Suppression
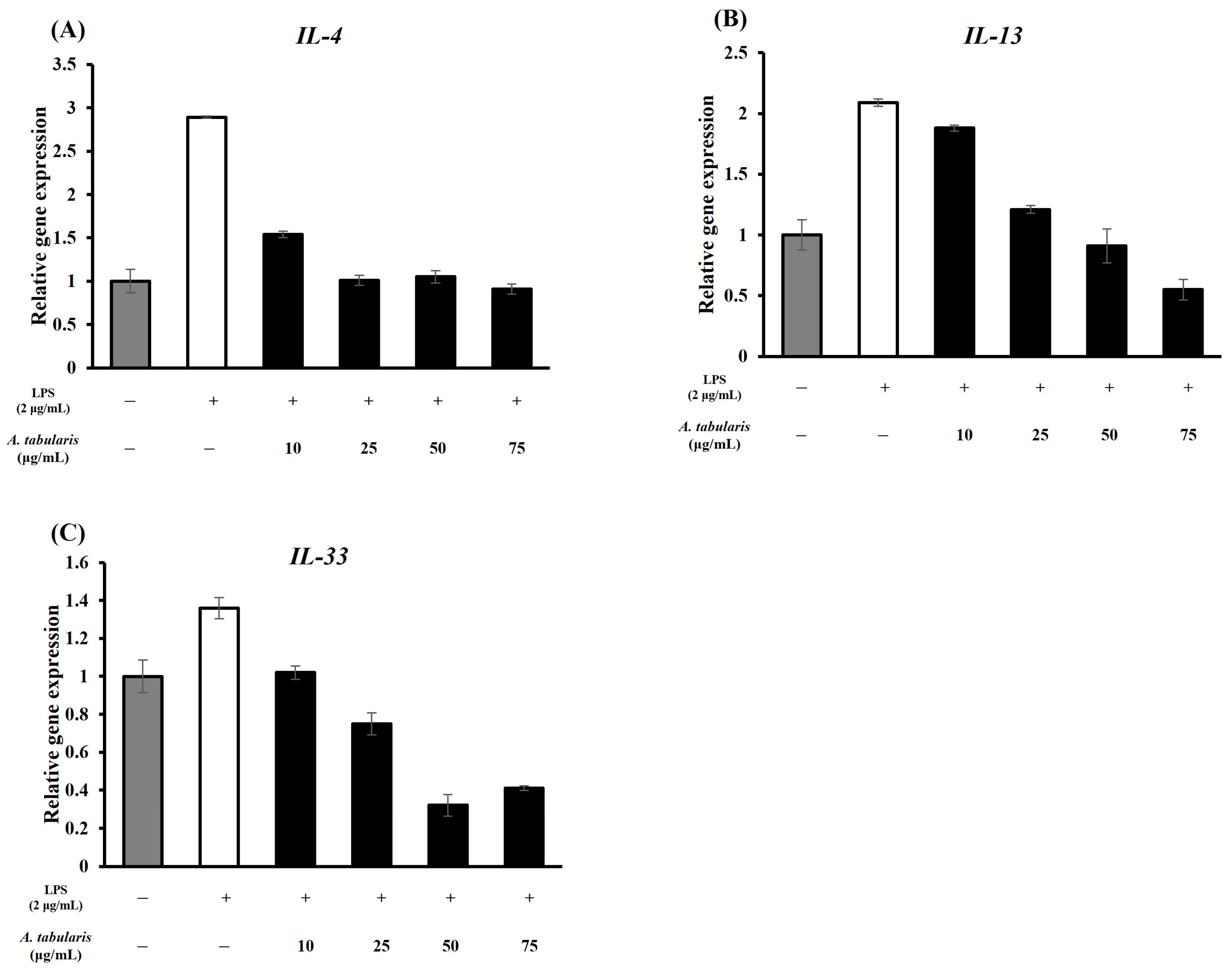
2.4.2. T-Helper 2 (TH2) Inflammation Suppression
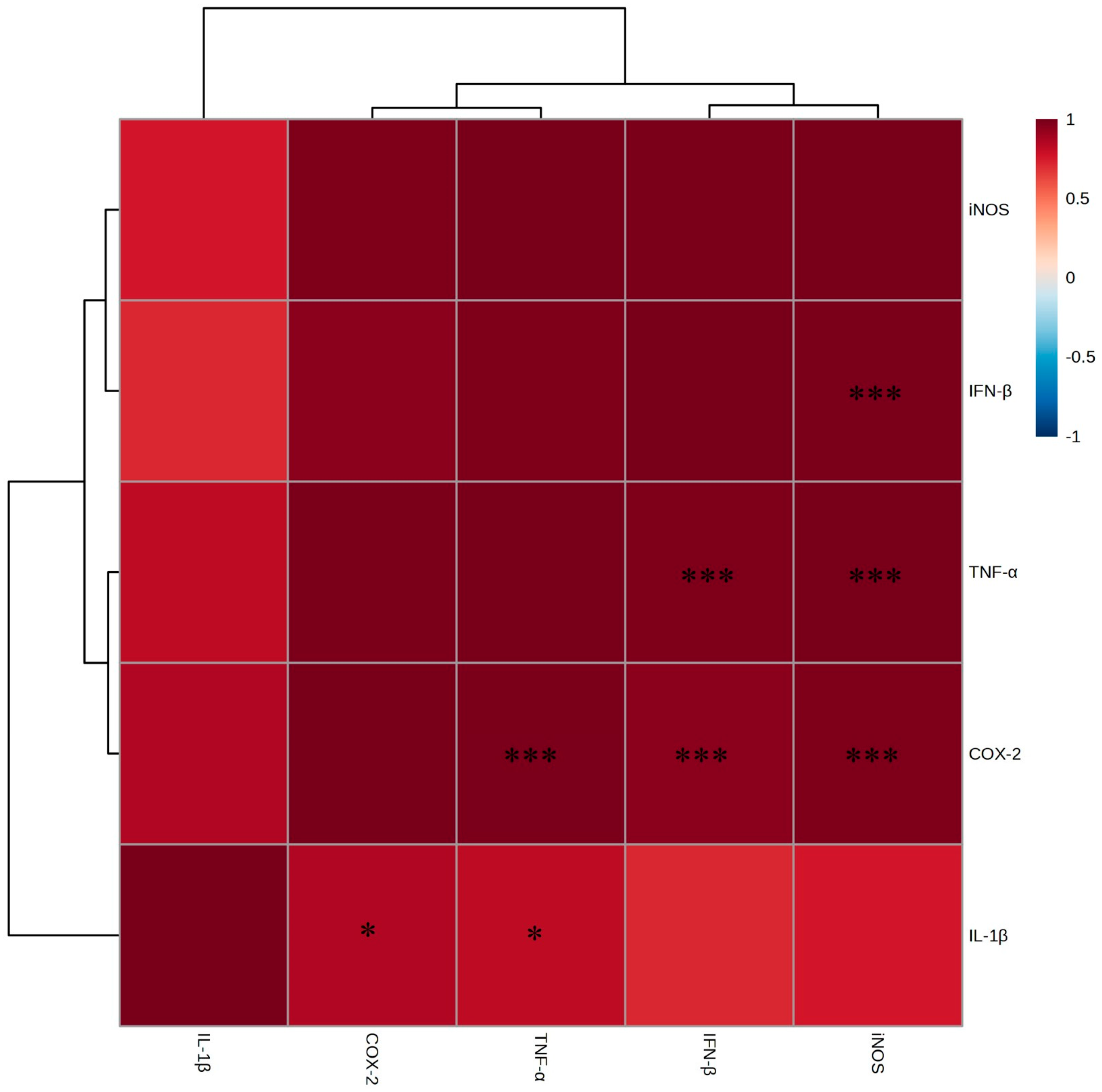
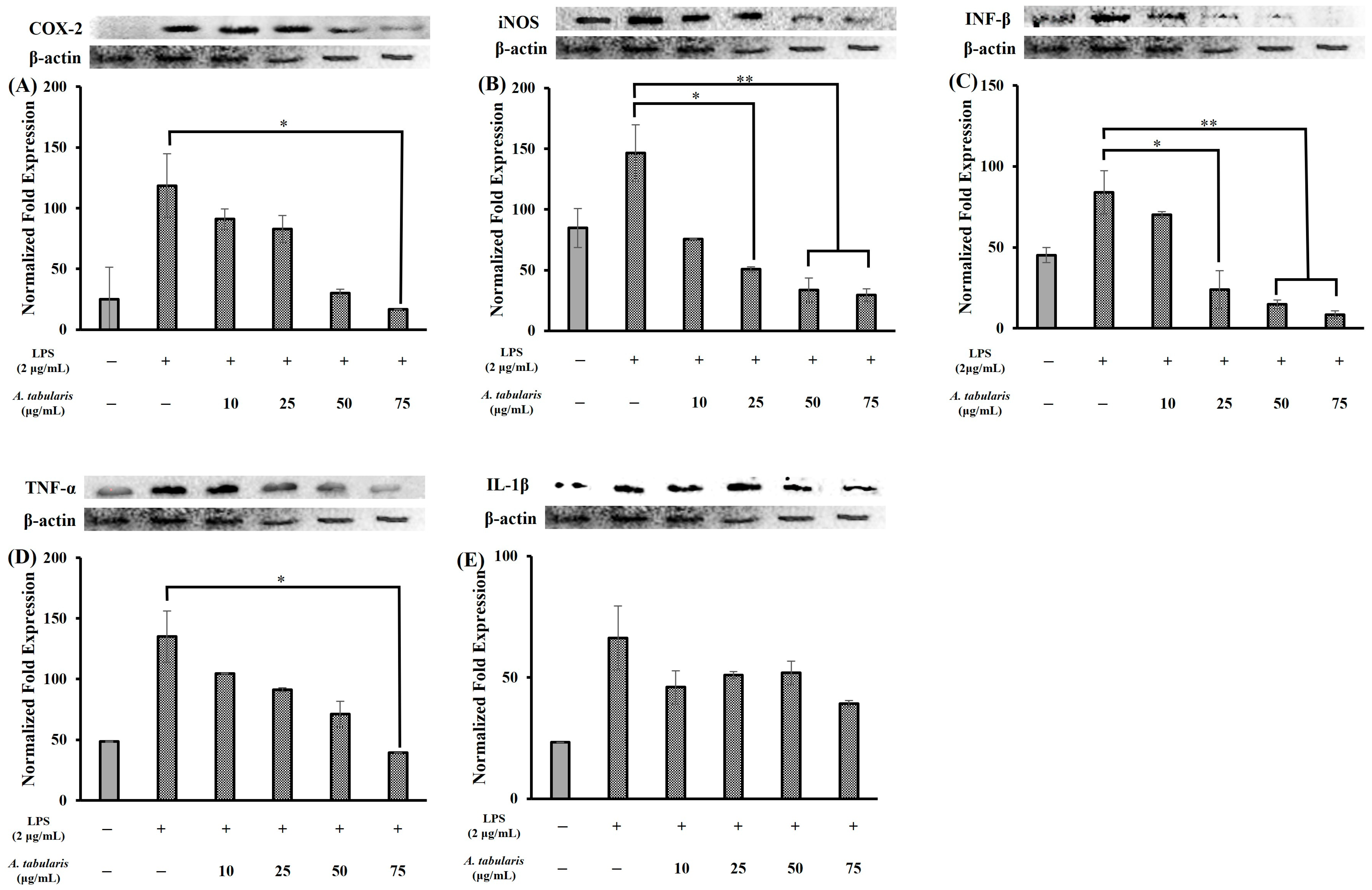
2.5. Inhibition of Inflammatory Protein Expression
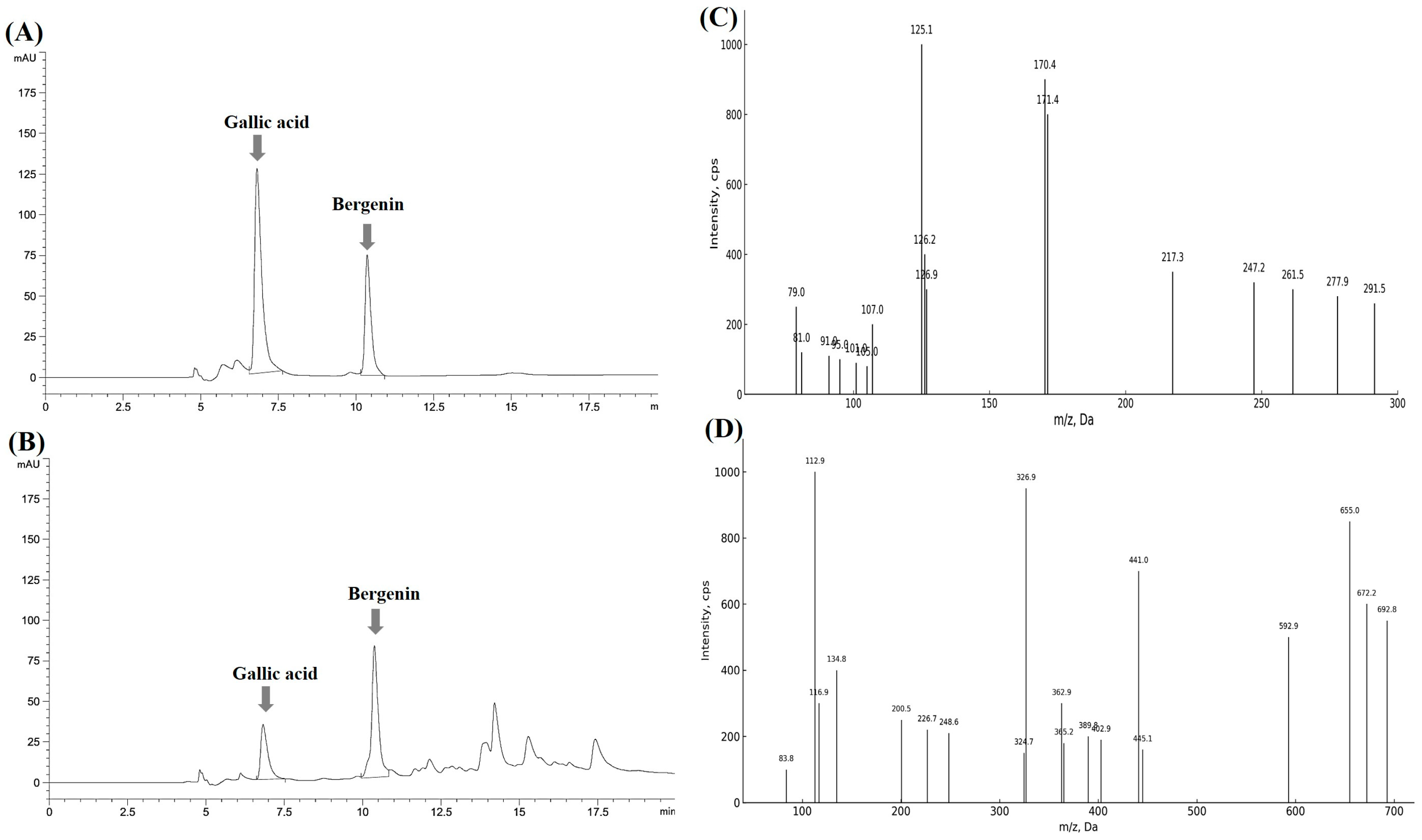
2.6. Analysis of Antioxidant and Anti-Inflammatory Compounds from the Extract
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Astilboides tabularis (Hemsl.) Engl. Root Extracts
4.3. Determination of Total Phenolic Content
4.4. Liquid Chromatography Analysis
4.4.1. Primary Compound Screening via LC-QTOF-MS
4.4.2. Secondary Compound Confirmation via LC-MS/MS
4.4.3. Qualitative and Quantitative Analysis Using LC-UV
4.5. Antioxidant Activity
4.5.1. DPPH Radical Scavenging Activity Assays
4.5.2. ATBS Radical Scavenging Activity Assays
4.6. Reducing Power Assay
4.7. MTT Assays
4.8. Protective Effect Against H₂O₂-Induced Oxidative Stress
4.9. Determination of NO Production in RAW 264.7 Macrophages
4.10. Real-Time PCR (RT-PCR)
4.11. Western Blot Analysis
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.I.; Hwang, H.Y.; Kim, J.S.; Park, S.W. Analysis of the Functional Components and Various Biological Activities of Korean Medicinal Herbs. J. Korean Soc. Food Sci. Nutr. 2019, 48, 1345. [Google Scholar] [CrossRef]
- Cho, Y.J.; Ju, I.S.; Kwon, O.J.; Chun, S.S.; An, B.J.; Kim, J.H. Biological and Antimicrobial Activity of Portulaca oleracea. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 49–54. [Google Scholar]
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; McCord, J.M.; Harman, D. Oxygen Radicals and Human Disease. Ann. Intern. Med. 1987, 107, 526–545. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, S.J.; Hwang, J.W.; Kim, E.H.; Park, P.J.; Jeong, J.H. Anti-Inflammatory Effects of Extracts from Ligustrum ovalifolium H. Leaves on RAW 264.7 Macrophages. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1205–1210. [Google Scholar] [CrossRef]
- Gramlich, T.; Petras, R.E. Pathology of Inflammatory Bowel Disease. Semin. Pediatr. Surg. 2007, 16, 154–163. [Google Scholar] [CrossRef]
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P. Anti-Inflammatory Effects of Schisandrin Isolated from the Fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008, 591, 293–299. [Google Scholar] [CrossRef]
- Song, H.J.; Lee, S.O. Anti-Inflammatory Effect of Beluga Lentil Extract in RAW 264.7 Macrophages. Food Sci. Preserv. 2024, 31, 462–473. [Google Scholar] [CrossRef]
- Fridovich, I. The Biology of Oxygen Radicals: The Superoxide Radical Is an Agent of Oxygen Toxicity; Superoxide Dismutases Provide an Important Defense. Science 1978, 201, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Kim, K.Y.; Yoo, K.O. Environmental Characteristics of Astilboides tabularis (Hemsl.) Engl. Habitats. Korean J. Plant Res. 2015, 28, 64–78. [Google Scholar] [CrossRef]
- Ku, Y.B.; Oh, H.K.; Shim, K.Y.; Kim, M.S.; Lee, S.M. Growth Characteristics, Genetic Diversity and Conservation of Endangered Plants (I): The Case of Astilboides tabularis, Euchresta japonica, Echinosophora koreensis and Lilium cernuum; National Institute of Environmental Research: Incheon, Republic of Korea, 2006; pp. 1–60. [Google Scholar]
- Lee, C.H. Studies on Chemical Compositions and Bioactivities of Astilboides tabularis (Hemsl.) Engl. Ph.D. Thesis, Department of Applied Plant Sciences, Graduate School, Kangwon National University, Chuncheon, Republic of Korea, 2016. [Google Scholar]
- Yang, J.; Lee, C.; Kim, H.; Kwon, Y.S.; Kim, M.J. Biological Activities and Phenolic Compound Content of Astilboides tabularis (Hemsl.) Engler Extracts. Curr. Pharm. Biotechnol. 2020, 21, 1070–1078. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P.K.; Wanasundara, U.N. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by Reactive Oxygen and Nitrogen Species: Role in Inflammatory Disease and Progression to Cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-Like Receptor Signaling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-Activated Protein Kinases in Innate Immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. Thymoquinone: An IRAK1 Inhibitor with In Vivo and In Vitro Anti-Inflammatory Activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef]
- Trinchieri, G.; Sher, A. Cooperation of Toll-Like Receptor Signals in Innate Immune Defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef]
- Murphy, K.M.; Ouyang, W.; Farrar, J.D. Signaling and Transcription in T Helper Development. Annu. Rev. Immunol. 2000, 18, 439–482. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Nat. Rev. Immunol. 2010, 10, 850–860. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Nat. Rev. Immunol. 2010, 10, 593–607. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, A.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-Like Cytokine That Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Nature 2005, 435, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Won, J.H.; Im, H.T.; Kim, Y.H.; Yun, K.J.; Park, H.J.; Choi, J.W.; Lee, K.T. Anti-Inflammatory Effect of Buddlejasaponin IV through the Inhibition of iNOS and COX-2 Expression in RAW 264.7 Macrophages via the NF-κB Inactivation. Br. J. Pharmacol. 2006, 148, 216. [Google Scholar] [CrossRef]
- Baldwin, A.S. The NF-κB and IκB Proteins: New Discoveries and Insights. Annu. Rev. Immunol. 1996, 14, 649–681. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Taniguchi, T.; Yanai, H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2018, 10, a028423. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gong, Q.; Lu, B.; Huang, K.; Tong, Y.; Mutsvene, T.E.; Lin, M.; Xu, Z.; Lu, F.; Li, X.; et al. Anti-Inflammatory and Antioxidative Effects of Gallic Acid on Experimental Dry Eye: In Vitro and In Vivo Studies. Eye Vis. 2023, 10, 17. [Google Scholar] [CrossRef]
- Koul, B.; Kumar, A.; Yadav, D.; Jin, J.O. Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology. Molecules 2020, 25, 5555. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Araujo, A.K.; Pacheco, G.; Oliveira, A.P.; Carvalho, J.L.; Chaves, L.S.; Sousa, G.C.; Lopes, A.L.; Silva, P.C.; Leódido, A.C.M.; et al. Anti-Inflammatory Properties of Bergenin in Mice. J. Appl. Pharm. Sci. 2019, 9, 69–77. [Google Scholar]
- Luo, J.-F.; Yue, L.; Wu, T.-T.; Zhao, C.-L.; Ye, J.-H.; He, K.; Zou, J. Triterpenoid and Coumarin Isolated from Astilbe grandis with Anti-Inflammatory Effects through Inhibiting the NF-κB Pathway in LPS-Induced RAW264.7 Cells. Molecules 2023, 28, 5731. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Wang, Y.; Zhai, J.; Chen, G.; Hu, K. Gambogic Acid Induces HO-1 Through Nrf2 Signaling Pathway and Inhibits NF-κB and MAPK Activation to Reduce Inflammation in LPS-Activated RAW264.7 Cells. Biomed. Pharmacother. 2019, 109, 555–562. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, X.; Liang, P.; Zhang, L.; Xu, Y.; Gong, M.; Qiu, X.; Zhang, J.; Xu, W. Integrating Approach to Discover Novel Bergenin Derivatives and Phenolics with Antioxidant and Anti-Inflammatory Activities from Bio-Active Fraction of Syzygium brachythyrsum. Arab. J. Chem. 2022, 15, 103507. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reactions: Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in Inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Agrawal, S.K. Pre- and Post-Treatment with Curcumin and Resveratrol Protects Astrocytes after Oxidative Stress. Brain Res. 2018, 1692, 45–55. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. Pharmacological Manipulation of Cell Death: Clinical Applications in Sight? J. Clin. Investig. 2005, 115, 2610–2617. [Google Scholar] [CrossRef]


| Sample | DPPH Radical Scavenging Activity IC50 (1) (μg/mL) | ATBS Radical Scavenging Activity IC50 (μg/mL) |
|---|---|---|
| EtOAc | 11.38 ± 0.48 | 7.46 ± 0.58 |
| Ascorbic acid | 2.83 ± 0.01 | 1.72 ± 0.01 |
| BHA | 8.89 ± 0.32 | 4.12 ± 0.13 |
| α-Tocopherol | 12.57 ± 0.24 | 23.12 ± 0.81 |
| BHT | 131.18 ± 0.62 | 74.28 ± 0.85 |
| Sample | Total Phenolic Content (1) |
|---|---|
| EtOAc | 407.02 ± 13.56 |
| Compounds | Calibration Curve | Correlation Coefficient (R2) | Retention Time (min) | Content (mg/g·Ext) |
|---|---|---|---|---|
| Gallic acid | Y = 21.34153X − 96.99437 | 0.99933 | 6.825 | 29.75 ± 0.10 |
| Bergenin | Y = 10.29642X − 44.28700 | 0.99940 | 10.375 | 123.12 ± 0.52 |
| Primer Name | Sequences | |
|---|---|---|
| iNOS | Forward | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ |
| Reverse | 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ | |
| IL-1β | Forward | 5′-TGGACGGACCCCAAAAGATG-3′ |
| Reverse | 5′-AGAAGGTGCTCATGTCCTCA-3′ | |
| COX-2 | Forward | 5′-CACTACATCCTGACCCACTT-3′ |
| Reverse | 5′-ATGCTCCTGCTTGAGTATGT-3′ | |
| TNF-α | Forward | 5′-TTGACCTCAGCGCTGAGTTG-3′ |
| Reverse | 5′-CCTGTAGCCCACGTCGTAGC-3′ | |
| IFN-β | Forward | 5′-GGGCTGCTTCCAAACCTTTG-3′ |
| Reverse | 5′-AAGACACAGGTAGTCGCCAC-3′ | |
| IL-4 | Forward | 5′-GTCATCCTGCTCTTCTTTCTCG-3′ |
| Reverse | 5′-GTCATCCTGCTCTTCTTTCTCG-3′ | |
| IL-13 | Forward | 5′-CTGAGCAACATCACACAAGACC-3′ |
| Reverse | 5′-AATCCAGGGCTACACAGAACC-3′ | |
| IL-33 | Forward | 5′-TCCAACTCCAAGATTTCCCCG-3′ |
| Reverse | 5′-CATGCAGTAGACATGGCAGAA-3′ | |
| GPDPH | Forward | 5′-TGCTGAGTATGTCGTGGAGT-3′ |
| Reverse | 5′-GTTCACACCCATCACAAACA-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, N.H.; Baek, Y.S.; Kim, H.K.; Lee, C.O.; Kim, M.J. Antioxidant and Anti-Inflammatory Activities of Astilboides tabularis (Hemsl.) Engl. Root Extract. Molecules 2025, 30, 1892. https://doi.org/10.3390/molecules30091892
Yoo NH, Baek YS, Kim HK, Lee CO, Kim MJ. Antioxidant and Anti-Inflammatory Activities of Astilboides tabularis (Hemsl.) Engl. Root Extract. Molecules. 2025; 30(9):1892. https://doi.org/10.3390/molecules30091892
Chicago/Turabian StyleYoo, Nam Ho, Young Sun Baek, Hee Kyu Kim, Chan Ok Lee, and Myong Jo Kim. 2025. "Antioxidant and Anti-Inflammatory Activities of Astilboides tabularis (Hemsl.) Engl. Root Extract" Molecules 30, no. 9: 1892. https://doi.org/10.3390/molecules30091892
APA StyleYoo, N. H., Baek, Y. S., Kim, H. K., Lee, C. O., & Kim, M. J. (2025). Antioxidant and Anti-Inflammatory Activities of Astilboides tabularis (Hemsl.) Engl. Root Extract. Molecules, 30(9), 1892. https://doi.org/10.3390/molecules30091892