New Cu(II), Cu(I) and Ag(I) Complexes of Phenoxy-Ketimine Schiff Base Ligands: Synthesis, Structures and Antibacterial Activity
Abstract
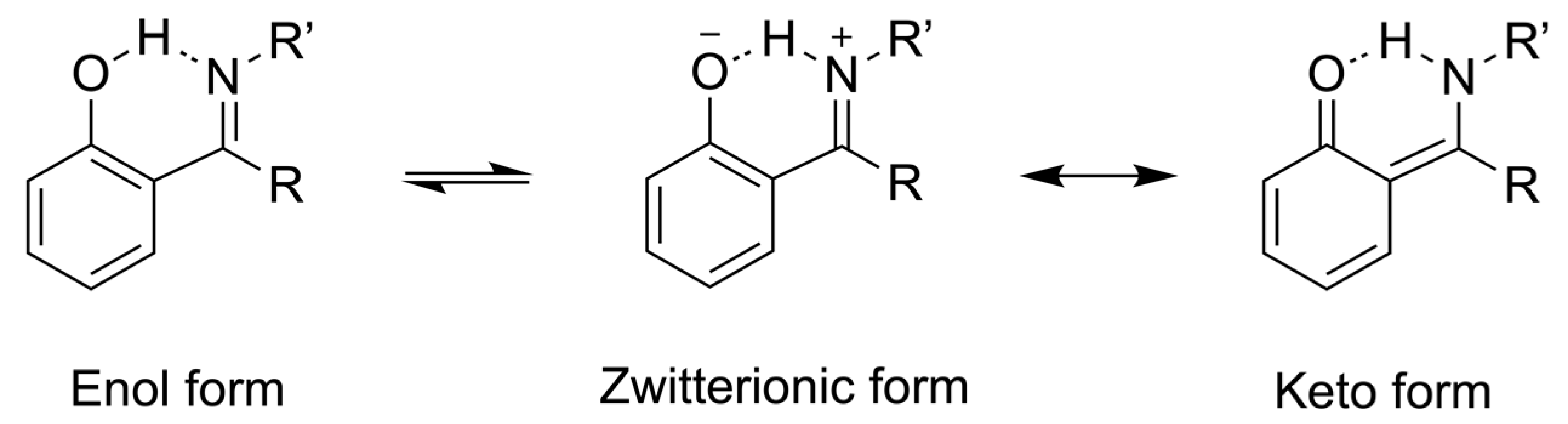
1. Introduction
2. Results and Discussion
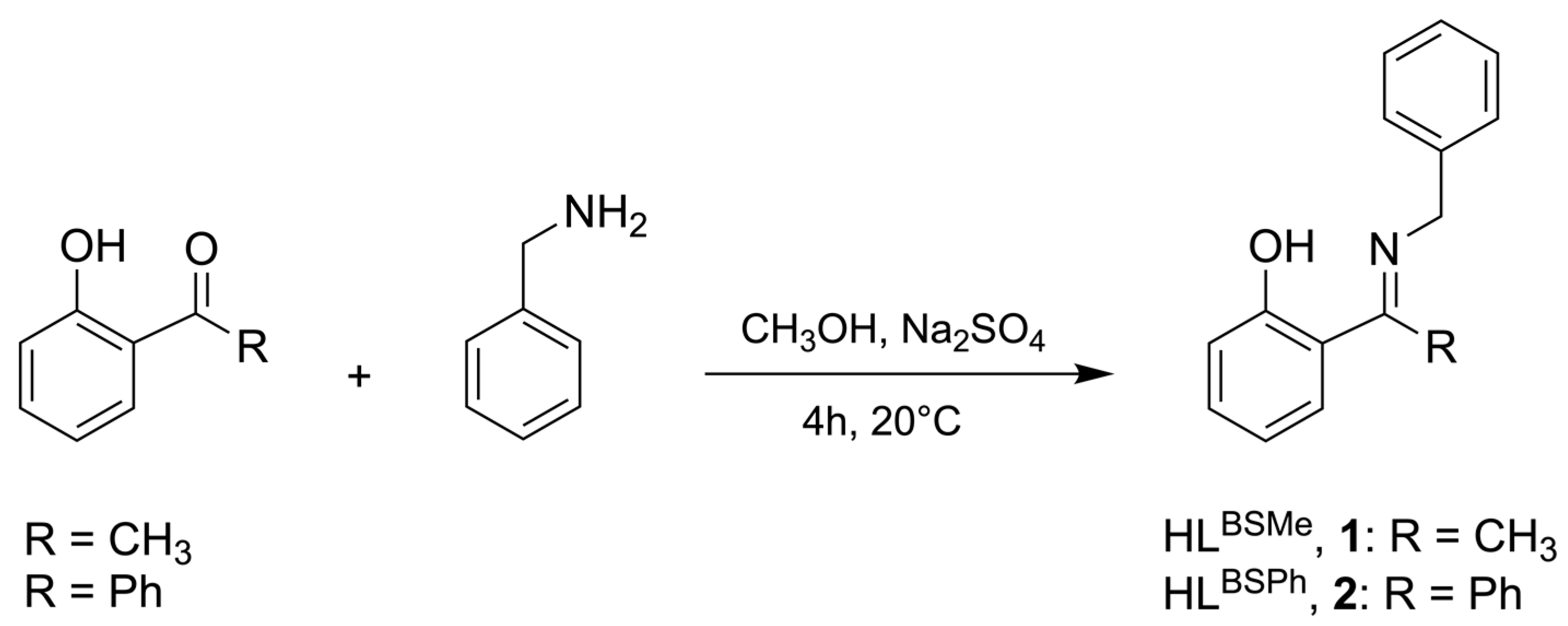
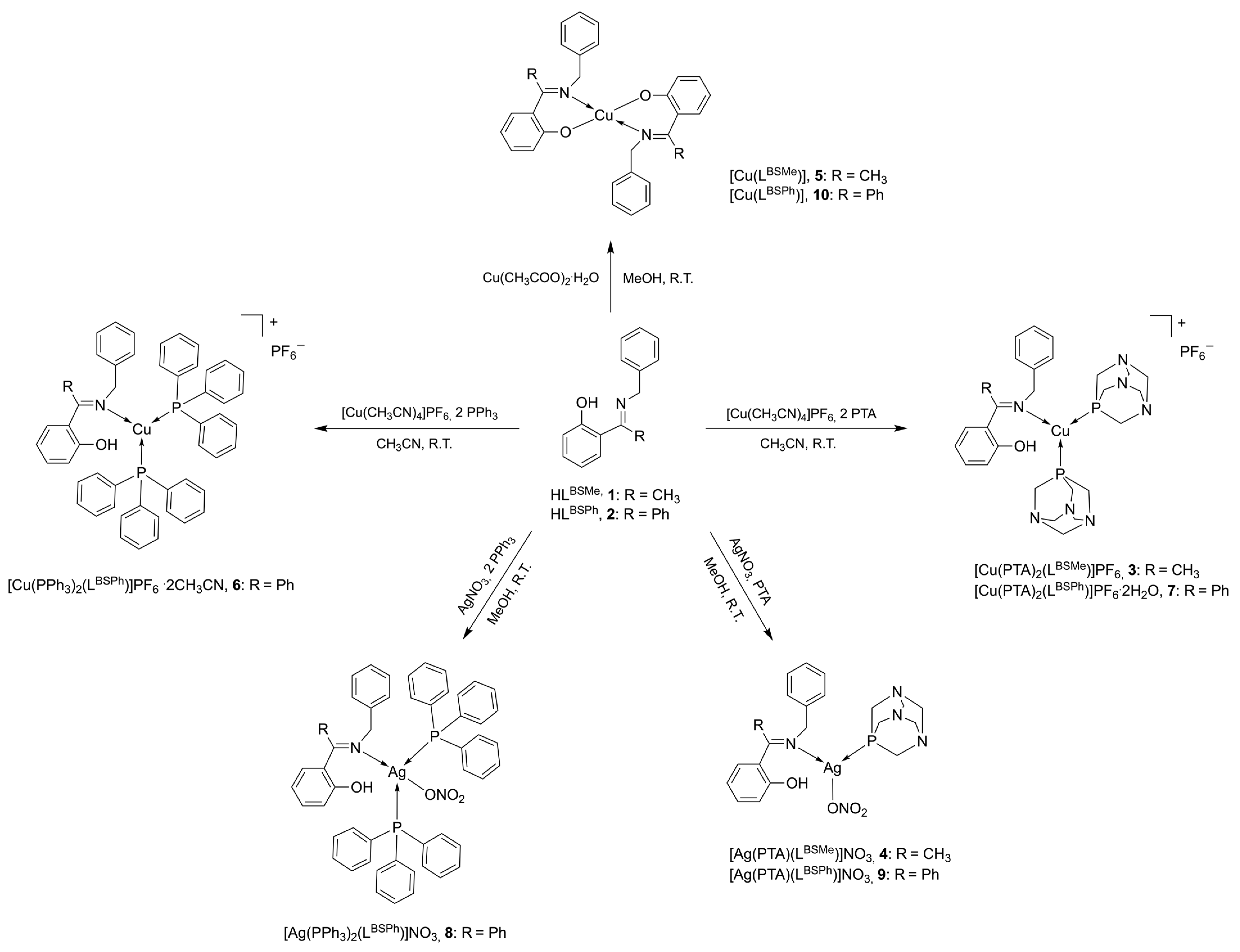
2.1. Synthesis and Characterization
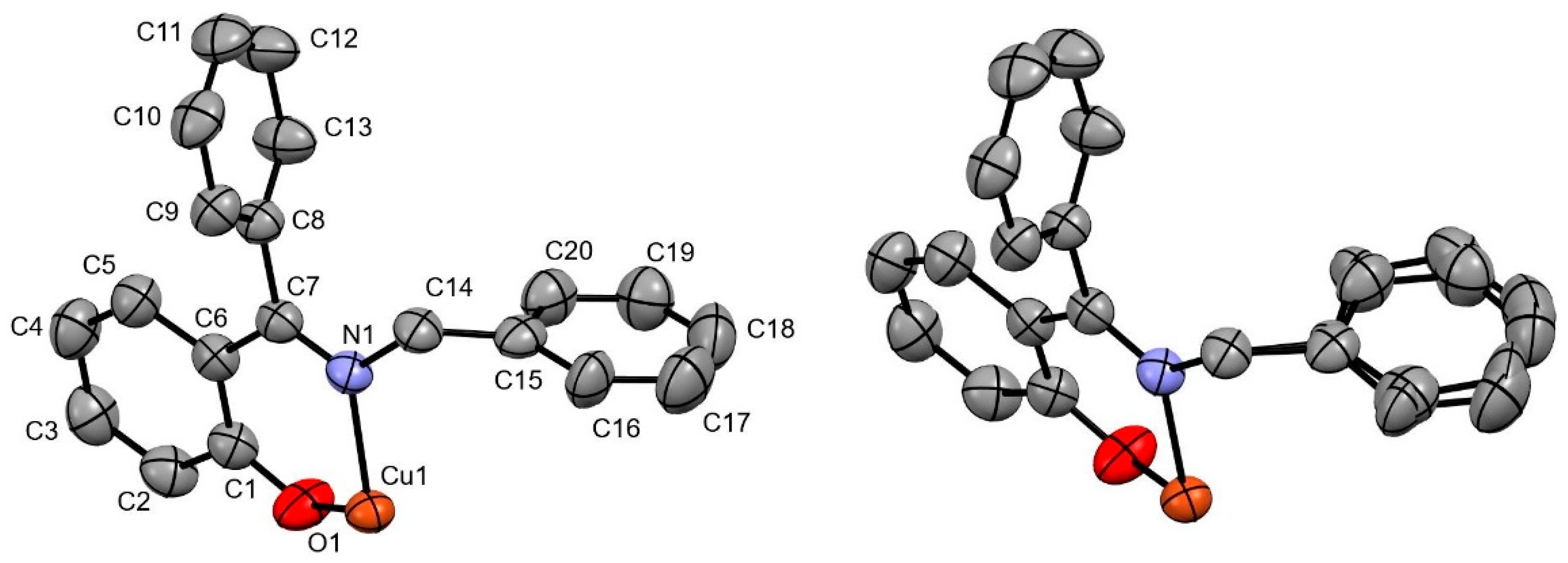
2.2. X-Ray Crystallography
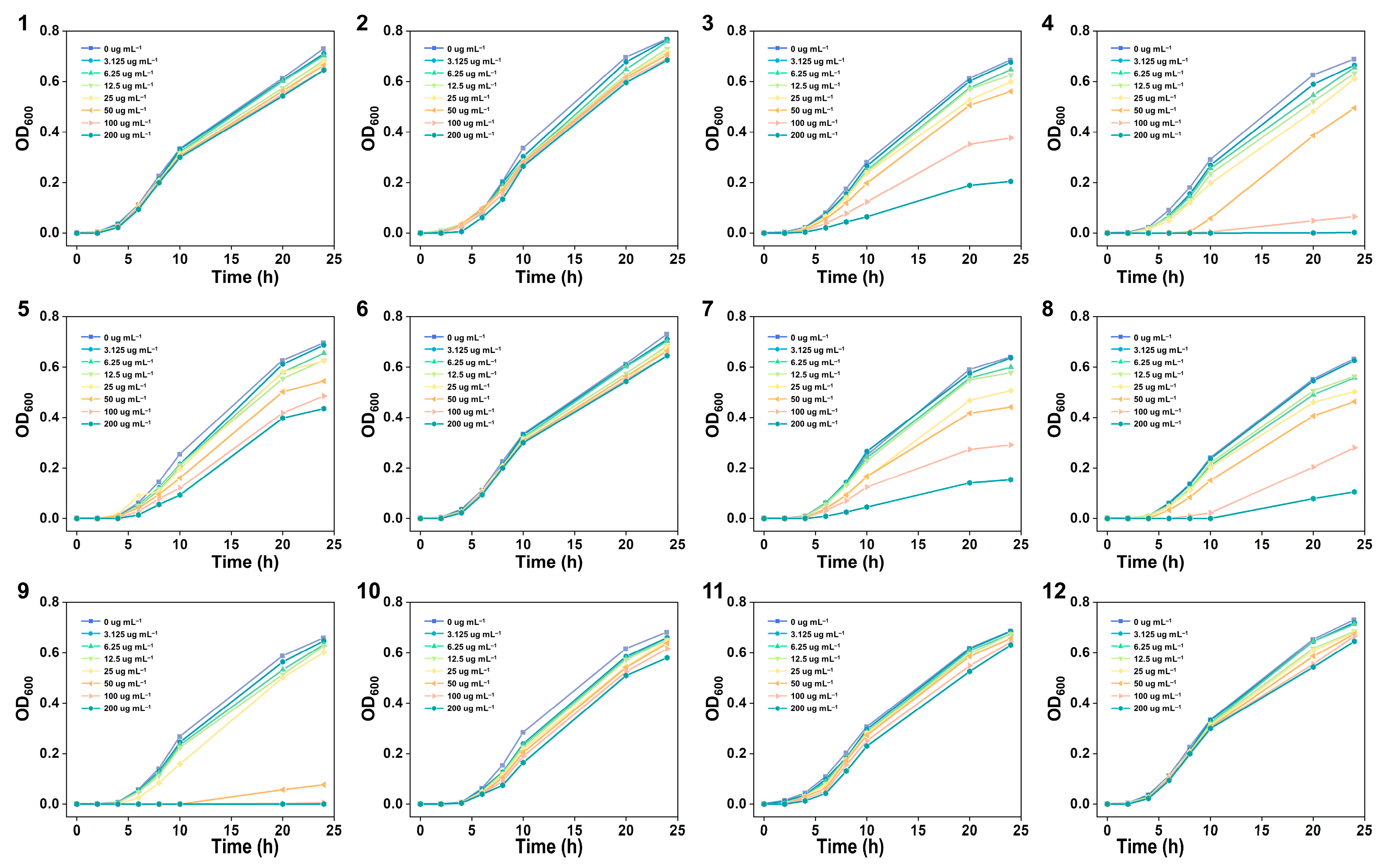
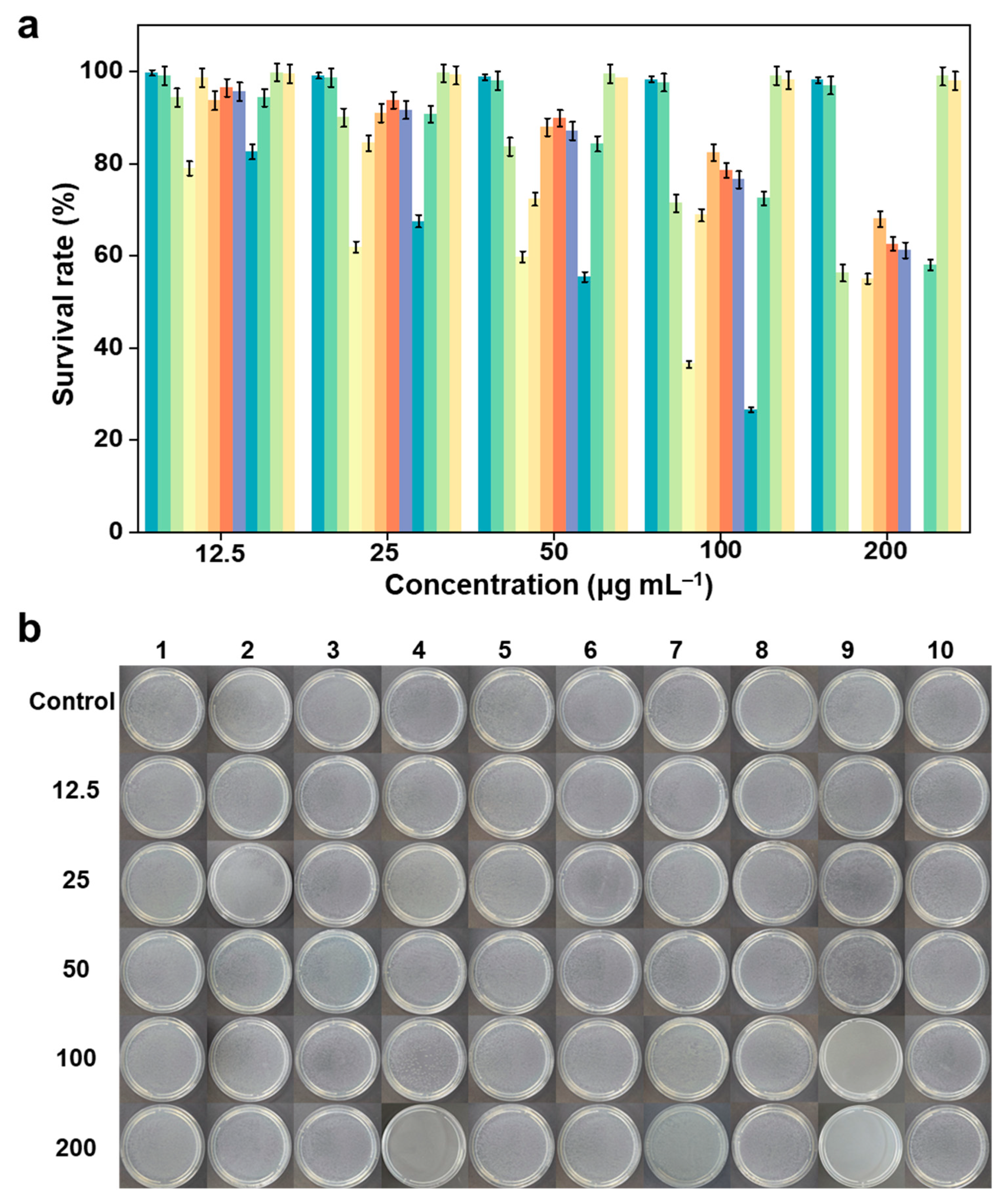
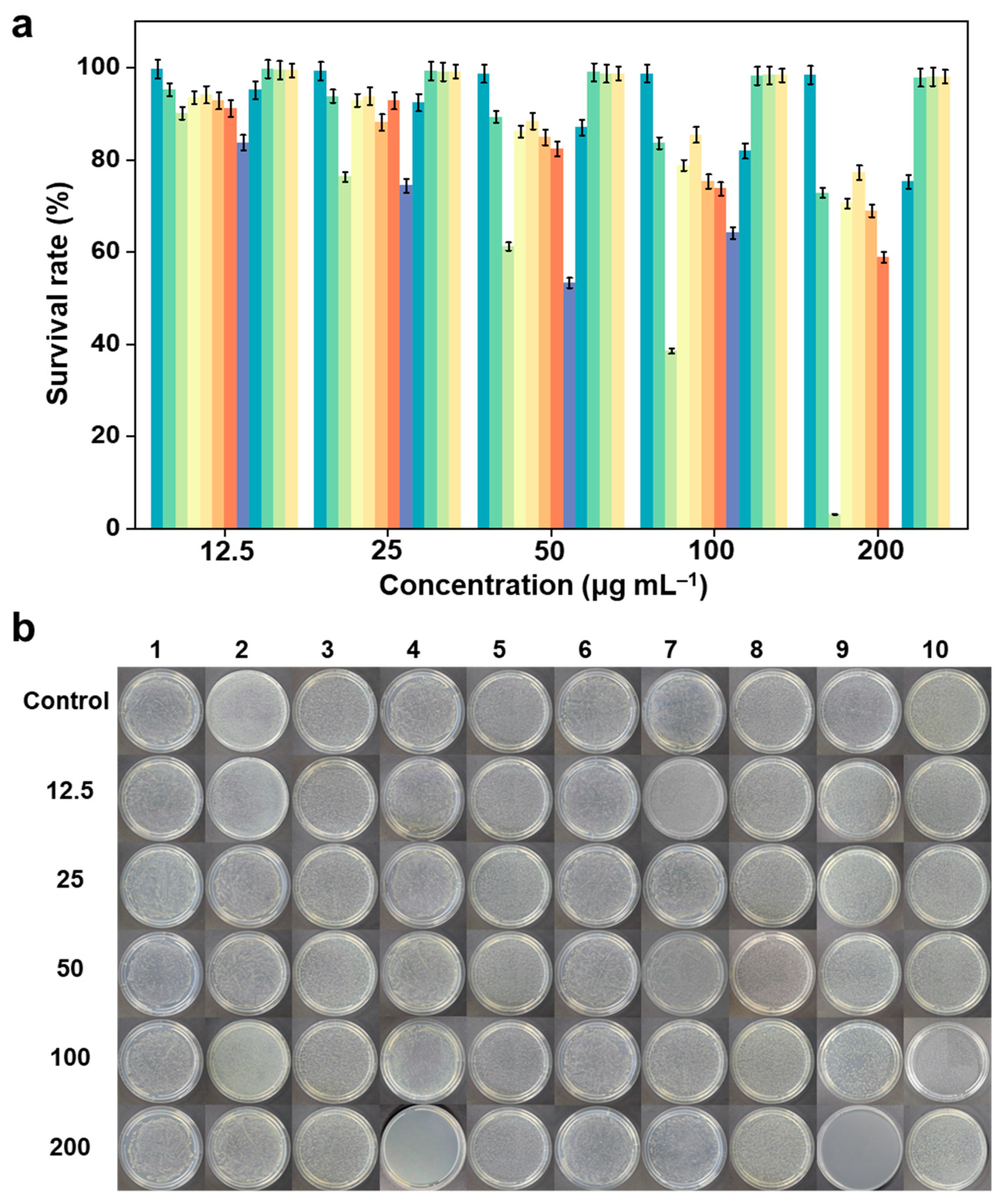
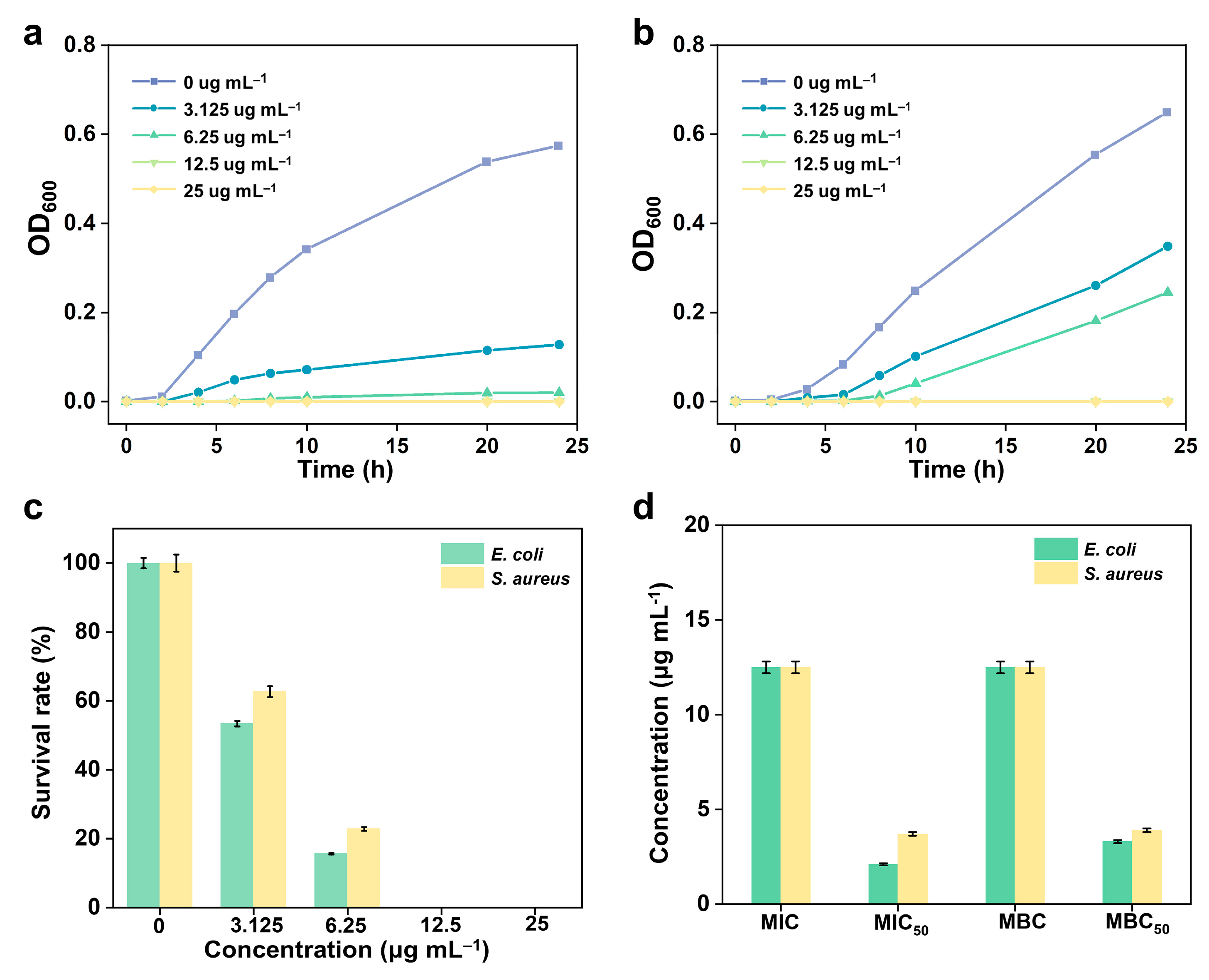
2.3. In-Vitro Antibacterial Activity
3. Experimental Section
3.1. Materials and Instruments
3.2. Synthesis
3.2.1. Synthesis of the Ligand HLBSMe (1)
3.2.2. Synthesis of the Ligand HLBSPh (2)
3.2.3. Synthesis of [Cu(HLBSMe)(PTA)2]PF6 (3)
3.2.4. Synthesis of [Ag(HLBSMe)(PTA)]NO3 (4)
3.2.5. Synthesis of [Cu(LBSMe)2] (5)
3.2.6. Synthesis of [Cu(HLBSPh)(PPh3)2]PF6·2CH3CN (6)
3.2.7. Synthesis of [Cu(HLBSPh)(PTA)2]PF6·2H2O (7)
3.2.8. Synthesis of [Ag(HLBSPh)(PPh3)2]NO3 (8)
3.2.9. Synthesis of [Ag(HLBSPh)(PTA)]NO3 (9)
3.2.10. Synthesis of [Cu(LBSPh)2] (10)
3.3. Crystallographic Data Collection and Refinement
3.4. Antibacterial Screening
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 1 November 2024).
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 November 2024).
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, F.; Compagne, N.; Antraygues, K.; Eveque, M.; Flipo, M.; Willand, N. Antibiotics with novel mode of action as new weapons to fight antimicrobial resistance. Eur. J. Med. Chem. 2023, 256, 115413. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Lin, Y.; Betts, H.; Keller, S.; Cariou, K.; Gasser, G. Recent developments of metal-based compounds against fungal pathogens. Chem. Soc. Rev. 2021, 50, 10346–10402. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planetary Health 2018, 2, E398–E405. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Cvijan, B.B.; Jacic, J.K.; Bajcetic, M. The Impact of Copper Ions on the Activity of Antibiotic Drugs. Molecules 2023, 28, 5133. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Ghanghas, P.; Choudhary, A.; Kumar, D.; Poonia, K. Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorg. Chem. Commun. 2021, 130, 108710. [Google Scholar] [CrossRef]
- Alkis, M.E.; Kelestemür, Ü.; Alan, Y.; Turan, N.; Buldurun, K. Cobalt and ruthenium complexes with pyrimidine based Schiff base: Synthesis, characterization, anticancer activities and electrochemotherapy efficiency. J. Mol. Struct. 2021, 1226, 129402. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef] [PubMed]
- Magyari, J.; Holló, B.B.; Vojinovic-Jesic, L.S.; Radanovic, M.M.; Armakovic, S.; Armakovic, S.J.; Molnár, J.; Kincses, A.; Gajdács, M.; Spengler, G.; et al. Interactions of Schiff base compounds and their coordination complexes with the drug cisplatin. New J. Chem. 2018, 42, 5834–5843. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al-Hamdani, A.A.S.; Kaseem, M. Synthesis and antioxidant activities of Schiff bases and their complexes: A review. Appl. Organomet. Chem. 2016, 30, 810–817. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Organometallic complexes of Schiff bases: Recent progress in oxidation catalysis. J. Organomet. Chem. 2016, 822, 173–188. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.B. Molecular Assembly of Schiff Base Interactions: Construction and Application. Chem. Rev. 2015, 115, 1597–1621. [Google Scholar] [CrossRef]
- Qin, W.L.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Nakayama, Y.; Saito, J.; Bando, H.; Fujita, T. Propylene Polymerization Behavior of Fluorinated Bis(phenoxy-imine) Ti Complexes with an MgCl2-Based Compound (MgCl2-Supported Ti-Based Catalysts). Macromol. Chem. Phys. 2005, 206, 1847–1852. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Akitsu, T. Novelties in Schiff Bases; IntechOpen: London, UK, 2024; p. 160. [Google Scholar]
- Younus, H.A.; Saleem, F.; Hameed, A.; Al-Rashida, M.; Al-Qawasmeh, R.A.; El-Naggar, M.; Rana, S.; Saeed, M.; Khan, K.M. Part-II: An update of Schiff bases synthesis and applications in medicinal chemistry-a patent review (2016–2023). Expert Opin. Ther. Patents 2023, 33, 841–864. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.D.; Islam, M.M.; Karim, M.R.; Rahman, S.; Shaikh, M.A.A.; Georghiou, P.E.; Menelaou, M. Recent Progress in Metal-Incorporated Acyclic Schiff-Base Derivatives: Biological Aspects. ChemistrySelect 2022, 7, e202104290. [Google Scholar] [CrossRef]
- Soroceanu, A.; Bargan, A. Advanced and Biomedical Applications of Schiff-Base Ligands and Their Metal Complexes: A Review. Crystals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. A review on biological activities of Schiff base, hydrazone, and oxime derivatives of curcumin. RSC Adv. 2020, 10, 30186–30202. [Google Scholar] [CrossRef]
- Parveen, S. Recent advances in anticancer ruthenium Schiff base complexes. Appl. Organomet. Chem. 2020, 34, e5687. [Google Scholar] [CrossRef]
- Khan, A.M.; Abid, O.U.R.; Mir, S. Assessment of biological activities of chitosan Schiff base tagged with medicinal plants. Biopolymers 2020, 111, e23338. [Google Scholar] [CrossRef]
- Hameed, A.; al-Rashida, M.; Uroos, M.; Ali, S.A.; Khan, K.M. Schiff bases in medicinal chemistry: A patent review (2010–2015). Expert Opin. Ther. Patents 2017, 27, 63–79. [Google Scholar] [CrossRef]
- Nayab, P.S.; Akrema; Ansari, I.A.; Shahid, M.; Rahisuddin. New phthalimide-appended Schiff bases: Studies of DNA binding, molecular docking and antioxidant activities. Luminescence 2017, 32, 829–838. [Google Scholar] [CrossRef]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological Properties of Schiff Bases and Azo Derivatives of Phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Lv, L.; Zheng, T.P.; Tang, L.; Wang, Z.R.; Liu, W.K. Recent advances of Schiff base metal complexes as potential anticancer agents. Coord. Chem. Rev. 2025, 525, 216327. [Google Scholar] [CrossRef]
- Kadhum, A.M.; Mallah, S.H.; Waheeb, A.S.; Salman, A.W.; Zafar, A.; Ahmad, N.S.; Siraj, S.; Iqbal, M.A. Advancement in Schiff base complexes for treatment of colon cancer. Rev. Inorg. Chem. 2024, 1–21. [Google Scholar] [CrossRef]
- Thakur, S.; Jaryal, A.; Bhalla, A. Recent advances in biological and medicinal profile of Schiff bases and their metal complexes: An updated version (2018–2023). Results Chem. 2024, 7, 101350. [Google Scholar] [CrossRef]
- Jorge, J.; Santos, K.F.D.; Timoteo, F.; Vasconcelos, R.R.P.; Caceres, O.I.A.; Granja, I.J.A.; De Souza, D.M., Jr.; Frizon, T.E.A.; Botteselle, G.D.; Braga, A.L.; et al. Recent Advances on the Antimicrobial Activities of Schiff Bases and their Metal Complexes: An Updated Overview. Curr. Med. Chem. 2024, 31, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Sovari, S.N.; Zobi, F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry 2020, 2, 418–452. [Google Scholar] [CrossRef]
- Malik, M.A.; Dar, O.A.; Gull, P.; Wani, M.Y.; Hashmi, A.A. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. MedChemComm 2018, 9, 409–436. [Google Scholar] [CrossRef]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef]
- Low, M.L.; Maigre, L.; Dorlet, P.; Guillot, R.; Pagès, J.M.; Crouse, K.A.; Policar, C.; Delsuc, N. Conjugation of a New Series of Dithiocarbazate Schiff Base Copper(II) Complexes with Vectors Selected to Enhance Antibacterial Activity. Bioconjug. Chem. 2014, 25, 2269–2284. [Google Scholar] [CrossRef]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Bagihalli, G.B.; Avaji, P.G.; Patil, S.A.; Badami, P.S. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur. J. Med. Chem. 2008, 43, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, T.; Ali, B.; Qayyum, H.; Haroone, M.S.; Shabbir, G. Pharmacological aspects of Schiff base metal complexes: A critical review. Inorg. Chem. Commun. 2023, 150, 110449. [Google Scholar] [CrossRef]
- Routaray, A.; Nath, N.; Maharana, T.; Sahoo, P.K.; Das, J.P.; Sutar, A.K. Salicylaldimine Copper(II) complex catalyst: Pioneer for ring opening Polymerization of Lactide. J. Chem. Sci. 2016, 128, 883–891. [Google Scholar] [CrossRef]
- John, A.; Katiyar, V.; Pang, K.; Shaikh, M.M.; Nanavati, H.; Ghosh, P. Ni(II) and Cu(II) complexes of phenoxy-ketimine ligands: Synthesis, structures and their utility in bulk ring-opening polymerization (ROP) of L-lactide. Polyhedron 2007, 26, 4033–4044. [Google Scholar] [CrossRef]
- Felemban, M.F.; Tayeb, F.J.; Alqarni, A.; Ashour, A.A.; Shafie, A. Recent advances in Schiff base coinage metal complexes as anticancer agents: A comprehensive review (2021–2025). Dyes Pigment. 2025, 237, 112710. [Google Scholar] [CrossRef]
- Babic, S.; Marjanovic, J.S.; Divac, V.M.; Klisuric, O.R.; Milivojevic, D.; Bogojeski, J.V.; Rakovic, I.; Zaric, M.; Jovanovic, M.; Zaric, R.Z.; et al. Molecular docking study and in vitro evaluation of apoptotic effect of biogenic-amine-based N, O-Cu(II) complexes as potent antitumor agents. J. Coord. Chem. 2025, 78, 1007–1026. [Google Scholar] [CrossRef]
- Richa; Kumar, V.; Kataria, R. Phenanthroline and Schiff Base associated Cu(II)-coordinated compounds containing N, O as donor atoms for potent anticancer activity. J. Inorg. Biochem. 2024, 251, 112440. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Panova, E.V.; Voronina, J.K.; Safin, D.A. Copper(II) Chelates of Schiff Bases Enriched with Aliphatic Fragments: Synthesis, Crystal Structure, In Silico Studies of ADMET Properties and a Potency against a Series of SARS-CoV-2 Proteins. Pharmaceuticals 2023, 16, 286. [Google Scholar] [CrossRef]
- Mohan, N.; Vidhya, C.V.; Suni, V.; Ameer, J.M.; Kasoju, N.; Mohanan, P.V.; Sreejith, S.S.; Kurup, M.R.P. Copper(II) salen-based complexes as potential anticancer agents. New J. Chem. 2022, 46, 12540–12550. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Oladipo, S.D.; Zamisa, S.; Kumalo, H.M.; Lawal, I.A.; Lawal, M.M.; Mabuba, N. Design of New Schiff-Base Copper(II) Complexes: Synthesis, Crystal Structures, DFT Study, and Binding Potency toward Cytochrome P450 3A4. ACS Omega 2021, 6, 13704–13718. [Google Scholar] [CrossRef] [PubMed]
- Kargar, H.; Behjatmanesh-Ardakani, R.; Torabi, V.; Sarvian, A.; Kazemi, Z.; Chavoshpour-Natanzi, Z.; Mirkhani, V.; Sahraei, A.; Tahir, M.N.; Ashfaq, M. Novel copper(II) and zinc(II) complexes of halogenated bidentate N,O-donor Schiff base ligands: Synthesis, characterization, crystal structures, DNA binding, molecular docking, DFT and TD-DFT computational studies. Inorg. Chim. Acta 2021, 514, 120004. [Google Scholar] [CrossRef]
- Guo, Y.N.; Hu, X.B.; Zhang, X.L.; Pu, X.H.; Wang, Y. The synthesis of a Cu(II) Schiff base complex using a bidentate N2O2 donor ligand: Crystal structure, photophysical properties, and antibacterial activities. RSC Adv. 2019, 9, 41737–41744. [Google Scholar] [CrossRef]
- Lian, W.J.; Wang, X.T.; Xie, C.Z.; Tian, H.; Song, X.Q.; Pan, H.T.; Qiao, X.; Xu, J.Y. Mixed-ligand copper(II) Schiff base complexes: The role of the co-ligand in DNA binding, DNA cleavage, protein binding and cytotoxicity. Dalton Trans. 2016, 45, 9073–9087. [Google Scholar] [CrossRef] [PubMed]
- Ei-Sherif, A.A.; Eldebss, T.M.A. Synthesis, spectral characterization, solution equilibria, in vitro antibacterial and cytotoxic activities of Cu(II), Ni(II), Mn(II), Co(II) and Zn(II) complexes with Schiff base derived from 5-bromosalicylaldehyde and 2-aminomethylthiophene. Spectrochim. Acta Part A 2011, 79, 1803–1814. [Google Scholar] [CrossRef]
- Li, Y.P.; Wu, Y.B.; Zhao, J.; Yang, P. DNA-binding and cleavage studies of novel binuclear copper(II) complex with 1,1′-dimethyl-2,2′-biimidazole ligand. J. Inorg. Biochem. 2007, 101, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, T.T.; Cai, S.L.; Wang, X.; Liu, L.; Wang, Y.M. Synthesis, structure and biological activity of cobalt(II) and copper(II) complexes of valine-derived Schiff bases. J. Inorg. Biochem. 2006, 100, 1888–1896. [Google Scholar] [CrossRef]
- Costamagna, J.; Ferraudi, G.; Matsuhiro, B.; Campos-Vallette, M.; Canales, J.; Villagrán, M.; Vargas, J.; Aguirre, M.J. Complexes of macrocycles with pendant arms as models for biological molecules. Coord. Chem. Rev. 2000, 196, 125–164. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, Q.W.; Hu, Z.H.; Wang, S. Synthesis, structural studies and antimicrobial activity of copper(II) complexes derived from 2,4-difluoro-6-(((2-(pyrrolidin-1-yl)ethyl)imino) methyl)phenol. Polyhedron 2024, 259, 117071. [Google Scholar] [CrossRef]
- Sonawane, H.R.; Vibhute, B.T.; Aghav, B.D.; Deore, J.V.; Patil, S.K. Versatile applications of transition metal incorporating quinoline Schiff base metal complexes: An overview. Eur. J. Med. Chem. 2023, 258, 115549. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, A.A.; Kumar, U.; Jain, P.; Sharma, A.K.; Kant, C.; Faizi, M.S.H. Recent advances in synthesis of heterocyclic Schiff base transition metal complexes and their antimicrobial activities especially antibacterial and antifungal. J. Mol. Struct. 2023, 1294, 136346. [Google Scholar] [CrossRef]
- Devi, J.; Sharma, S.; Kumar, S.; Kumar, B.; Kumar, D.; Jindal, D.K.; Das, S. Synthesis, characterization, in vitro antimicrobial and cytotoxic studies of Co(II), Ni(II), Cu(II), and Zn(II) complexes obtained from Schiff base ligands of 1, 2, 3, 4-tetrahydro-naphthalen-1-ylamine. Appl. Organomet. Chem. 2022, 36, e6760. [Google Scholar] [CrossRef]
- Peewasan, K.; Merkel, M.P.; Zarschler, K.; Stephan, H.; Anson, C.E.; Powell, A.K. Tetranuclear Cu(II)-chiral complexes: Synthesis, characterization and biological activity. RSC Adv. 2019, 9, 24087–24091. [Google Scholar] [CrossRef] [PubMed]
- Dar, O.A.; Lone, S.A.; Malik, M.A.; Wani, M.Y.; Ahmad, A.; Hashmi, A.A. New transition metal complexes with a pendent indole ring: Insights into the antifungal activity and mode of action. RSC Adv. 2019, 9, 15151–15157. [Google Scholar] [CrossRef]
- Kargar, H.; Aghaei-Meybodi, F.; Behjatmanesh-Ardakani, R.; Elahifard, M.R.; Torabi, V.; Fallah-Mehrjardi, M.; Tahir, M.N.; Ashfaq, M.; Munawar, K.S. Synthesis, crystal structure, theoretical calculation, spectroscopic and antibacterial activity studies of copper(II) complexes bearing bidentate Schiff base ligands derived from 4-aminoantipyrine: Influence of substitutions on antibacterial activity. J. Mol. Struct. 2021, 1230, 129908. [Google Scholar] [CrossRef]
- Kargar, H.; Aghaei-Meybodi, F.; Elahifard, M.R.; Tahir, M.N.; Ashfaq, M.; Munawar, K.S. Some new Cu(II) complexes containing O,N-donor Schiff base ligands derived from 4-aminoantipyrine: Synthesis, characterization, crystal structure and substitution effect on antimicrobial activity. J. Coord. Chem. 2021, 74, 1534–1549. [Google Scholar] [CrossRef]
- Alturiqi, A.S.; Alaghaz, A.; Zayed, M.E.; Ammar, R.A. Synthesis, characterization, biological activity, and corrosion inhibition in acid medium of unsymmetrical tetradentate N2O2 Schiff base complexes. J. Chin. Chem. Soc. 2018, 65, 1060–1074. [Google Scholar] [CrossRef]
- Regojevic, M.S.; Zoric, M.Z.; Radnovic, N.D.; Bogdanovic, M.G.; Holló, B.B.; Rodic, M.V.; Raievic, V.; Borisev, I.D.; Vojinovic-Jesic, L.S.; Hozjan, M.; et al. Anion-directed synthesis of copper(I/II) complexes with a Schiff base derived from 2-(diphenylphosphino)benzaldehyde and aminoguanidine. J. Mol. Struct. 2025, 1336, 14. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Weil, M.; Hadadzadeh, H.; Daryanavard, M. Two different 1D-chains in the structures of the copper(I) coordination polymers based on bidentate Schiff-base building units and thiocyanate anions as bridging ligands. Inorg. Chim. Acta 2009, 362, 4837–4842. [Google Scholar] [CrossRef]
- Morshedi, M.; Amirnasr, M.; Triki, S.; Khalaji, A.D. New (NS)2 Schiff base with a flexible spacer: Synthesis and structural characterization of its first coordination polymer [Cu2(l-I)2(l-(thio)2dapte)]n (1). Inorg. Chim. Acta 2009, 362, 1637–1640. [Google Scholar] [CrossRef]
- Ferraro, V.; Fuhr, O.; Bizzarri, C.; Braese, S. Substituted Pyrrole-based Schiff Bases: Effect on the Luminescence of Neutral Heteroleptic Cu(I) Complexes. Eur. J. Inorg. Chem. 2024, 27, e202400080. [Google Scholar] [CrossRef]
- Crestani, M.G.; Manbeck, G.F.; Brennessel, W.W.; McCormick, T.M.; Eisenberg, R. Synthesis and Characterization of Neutral Luminescent Diphosphine Pyrrole- and Indole-Aldimine Copper(I) Complexes. Inorg. Chem. 2011, 50, 7172–7188. [Google Scholar] [CrossRef]
- Lv, J.; Wu, X.Y.; Wang, R.; Wu, Y.Q.; Xu, S.X.; Zhao, F.; Wang, Y.B. Schiff base-type Cu(I) complexes containing naphthylpyridyl-methanimine ligands featuring higher light-absorption capability: Synthesis, structures, and photophysical properties. Polyhedron 2022, 224, 116002. [Google Scholar] [CrossRef]
- Lv, J.; Lu, Y.F.; Wang, J.L.; Zhao, F.; Wang, Y.B.; He, H.F.; Wu, Y.Q. Schiff base-type copper(I) complexes exhibiting high molar extinction coefficients: Synthesis, characterization and DFT studies. J. Mol. Struct. 2022, 1249, 131638. [Google Scholar] [CrossRef]
- Lv, J.; Li, Q.Q.; Wang, J.L.; Xu, S.X.; Zhao, F.; He, H.F.; Wang, Y.B. Orange-red emissive Cu(I) complexes bearing Schiff base ligands: Synthesis, structures, and photophysical properties. J. Mol. Struct. 2022, 1252, 132180. [Google Scholar] [CrossRef]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Huang, Y.Q.; Duan, Y.Y.; Liu, Q.; Xu, Q.L.; Jia, J.; Wang, J.M.; Tong, Q.; Luo, P.P.; Wen, Y.J.; et al. Schiff-base silver nanocomplexes formation on natural biopolymer coated mesoporous silica contributed to the improved curative effect on infectious microbes. Nano Res. 2021, 14, 2735–2748. [Google Scholar] [CrossRef]
- Azócar, M.I.; Gómez, G.; Levín, P.; Paez, M.; Muñoz, H.; Dinamarca, N. Review: Antibacterial behavior of carboxylate silver(I) complexes. J. Coord. Chem. 2014, 67, 3840–3853. [Google Scholar] [CrossRef]
- Nomiya, K.; Tsuda, K.; Sudoh, T.; Oda, M. Ag(I)-N bond-containing compound showing wide spectra in effective antimicrobial activities: Polymeric silver(I) imidazolate. J. Inorg. Biochem. 1997, 68, 39–44. [Google Scholar] [CrossRef]
- Khan, E.; Hanif, M.; Akhtar, M.S. Schiff bases and their metal complexes with biologically compatible metal ions; biological importance, recent trends and future hopes. Rev. Inorg. Chem. 2022, 42, 307–325. [Google Scholar] [CrossRef]
- Njogu, E.M.; Omondi, B.; Nyamori, V.O. Silver(I)-pyridinyl Schiff base complexes: Synthesis, characterisation and antimicrobial studies. J. Mol. Struct. 2017, 1135, 118–128. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Oladipo, S.D.; Zamisa, S.J.; Sanusi, I.A.; Omondi, B. DNA/BSA binding studies and in vitro anticancer and antibacterial studies of isoelectronic Cu(I)- and Ag(I)-pyridinyl Schiff base complexes incorporating triphenylphosphine as co-ligands. Inorg. Chim. Acta 2023, 558, 121760. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases-Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xu, Z.L.; Bao, S.S.; Wang, T.T.; Zheng, Z.H.; Ferreira, R.A.S.; Zheng, L.M.; Carlos, L.D. Lanthanide salen-type complexes exhibiting single ion magnet and photoluminescent properties. Dalton Trans. 2016, 45, 2974–2982. [Google Scholar] [CrossRef]
- Kitamura, F.; Sawaguchi, K.; Mori, A.; Takagi, S.; Suzuki, T.; Kobayashi, A.; Kato, M.; Nakajima, K. Coordination Structure Conversion of Hydrazone-Palladium(II) Complexes in the Solid State and in Solution. Inorg. Chem. 2015, 54, 8436–8448. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.T.; Lo, K.M.; Sinniah, S.K.; Sim, K.S.; Tan, K.W. Synthesis, characterization and biological evaluation of cationic hydrazone copper complexes with diverse diimine co-ligands. RSC Adv. 2014, 4, 61232–61247. [Google Scholar] [CrossRef]
- Su, W.; Qian, Q.Q.; Li, P.Y.; Lei, X.L.; Xiao, Q.; Huang, S.; Huang, C.S.; Cui, J.G. Synthesis, Characterization, and Anticancer Activity of a Series of Ketone-N4-Substituted Thiosemicarbazones and Their Ruthenium(II) Arene Complexes. Inorg. Chem. 2013, 52, 12440–12449. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassa, G.; Kleij, A.W. Recent advances with π-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Sathyadevi, P.; Butorac, R.R.; Cowley, A.H.; Bhuvanesh, N.S.P.; Dharmaraj, N. Copper(I) and nickel(II) complexes with 1:1 vs. 1:2 coordination of ferrocenyl hydrazone ligands: Do the geometry and composition of complexes affect DNA binding/cleavage, protein binding, antioxidant and cytotoxic activities? Dalton Trans. 2012, 41, 4423–4436. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Zare, M. Synthesis and characterization of NaY zeolite-encapsulated Mn-hydrazone Schiff base: An efficient and reusable catalyst for oxidation of olefins. J. Coord. Chem. 2012, 65, 4054–4066. [Google Scholar] [CrossRef]
- Chellan, P.; Land, K.M.; Shokar, A.; Au, A.; An, S.H.; Clavel, C.M.; Dyson, P.J.; de Kock, C.; Smith, P.J.; Chibale, K.; et al. Exploring the Versatility of Cycloplatinated Thiosemicarbazones as Antitumor and Antiparasitic Agents. Organometallics 2012, 31, 5791–5799. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Kalaivani, P.; Poornima, P.; Dallemer, F.; Paramaguru, G.; Padma, V.V.; Renganathan, R.; Huang, R.; Natarajan, K. One pot synthesis of structurally different mono and dimeric Ni(II) thiosemicarbazone complexes and N-arylation on a coordinated ligand: A comparative biological study. Dalton Trans. 2012, 41, 9323–9336. [Google Scholar] [CrossRef]
- Minkin, V.I.; Tsukanov, A.V.; Dubonosov, A.D.; Bren, V.A. Tautomeric Schiff bases: Iono-, solvato-, thermo- and photochromism. J. Mol. Struct. 2011, 998, 179–191. [Google Scholar] [CrossRef]
- Hansen, P.E.; Filarowski, A. Characterisation of the PT-form of o-hydroxy acylarornatic Schiff bases by NMR spectroscopy and DFT calculations. J. Mol. Struct. 2004, 707, 69–75. [Google Scholar] [CrossRef]
- Dominiak, P.M.; Grech, E.; Barr, G.; Teat, S.; Mallinson, P.; Wozniak, K. Neutral and ionic hydrogen bonding in Schiff bases. Chem. Eur. J. 2003, 9, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Król-Starzomska, I.; Filarowski, A.; Rospenk, M.; Koll, A.; Melikova, S. Proton transfer equilibria in Schiff bases with steric repulsion. J. Phys. Chem. A 2004, 108, 2131–2138. [Google Scholar] [CrossRef]
- Filarowski, A.; Glowiaka, T.; Koll, A. Strengthening of the intramolecular O⋯H⋯N hydrogen bonds in Schiff bases as a result of steric repulsion. J. Mol. Struct. 1999, 484, 75–89. [Google Scholar] [CrossRef]
- Jinno, S.; Senoo, T.; Mori, K. Access to ortho-Hydroxyphenyl Ketimines via Imine Anion-Mediated Smiles Rearrangement. Org. Lett. 2022, 24, 4140–4144. [Google Scholar] [CrossRef]
- Guerrero-Corella, A.; Esteban, F.; Iniesta, M.; Martín-Somer, A.; Parra, M.; Díaz-Tendero, S.; Fraile, A.; Alemán, J. 2-Hydroxybenzophenone as a Chemical Auxiliary for the Activation of Ketiminoesters for Highly Enantioselective Addition to Nitroalkenes under Bifunctional Catalysis. Angew. Chem. Int. Ed. 2018, 57, 5350–5354. [Google Scholar] [CrossRef]
- Al-Qaisi, F.; Genjang, N.; Nieger, M.; Repo, T. Synthesis, structure and catalytic activity of bis(phenoxyiminato)iron(III) complexes in coupling reaction of CO2 and epoxides. Inorg. Chim. Acta 2016, 442, 81–85. [Google Scholar] [CrossRef]
- Sibaouih, A.; Ryan, P.; Axenov, K.V.; Sundberg, M.R.; Leskelä, M.; Repo, T. Efficient coupling of CO2 and epoxides with bis(phenoxyiminato) cobalt(III)/Lewis base catalysts. J. Mol. Catal. A Chem. 2009, 312, 87–91. [Google Scholar] [CrossRef]
- Marcazzan, P.; Patrick, B.O.; James, B.R. Catalyst poisoning in catalyzed imine hydrogenation: A novel zwitterionic Rh(I)/o-hydroxy-substituted imine complex. J. Mol. Catal. A Chem. 2006, 257, 26–30. [Google Scholar] [CrossRef]
- Cimarelli, C.; Palmieri, G.; Volpini, E. Synthesis of enantiopure 2-aminoalkylphenols by stereoselective addition of Grignard reagents to chiral 2-imidoylphenols. Tetrahedron Asymmetry 2002, 13, 2011–2018. [Google Scholar] [CrossRef]
- Mondal, B.; Chakraborty, S.; Munshi, P.; Walawalkar, M.G.; Lahiri, G.K. Ruthenium-(II)/-(III) terpyridine complexes incorporating imine functionalities. Synthesis, structure, spectroscopic and electrochemical properties. J. Chem. Soc. Dalton Trans. 2000, 2327–2335. [Google Scholar] [CrossRef]
- Keerthi, K.D.; Santra, B.K.; Lahiri, G.K. Ruthenium(II) bipyridine complexes with modified phenolic Schiff base ligands. Synthesis, spectroscopic characterization and redox properties. Polyhedron 1998, 17, 1387–1396. [Google Scholar] [CrossRef]
- Choudhary, N.; Hughes, D.L.; Kleinkes, U.; Larkworthy, L.F.; Leigh, G.J.; Maiwald, M.; Marmion, C.J.; Sanders, J.R.; Smith, G.W.; Sudbrake, C. New tetradentate Schiff bases, their oxovanadium(IV) complexes, and some complexes of bidentate Schiff bases with vanadium(III). Polyhedron 1997, 16, 1517–1528. [Google Scholar] [CrossRef]
- Pellei, M.; Santini, C.; Bagnarelli, L.; Caviglia, M.; Sgarbossa, P.; De Franco, M.; Zancato, M.; Marzano, C.; Gandin, V. Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management. Int. J. Mol. Sci. 2023, 24, 4091. [Google Scholar] [CrossRef]
- Del Bello, F.; Pellei, M.; Bagnarelli, L.; Santini, C.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Battocchio, C.; Iucci, G.; Schiesaro, I.; et al. Cu(I) and Cu(II) Complexes Based on Lonidamine-Conjugated Ligands Designed to Promote Synergistic Antitumor Effects. Inorg. Chem. 2022, 61, 4919–4937. [Google Scholar] [CrossRef]
- Pellei, M.; Bagnarelli, L.; Luciani, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; De Franco, M.; Gandin, V.; Marzano, C.; et al. Synthesis and Cytotoxic Activity Evaluation of New Cu(I) Complexes of Bis(pyrazol-1-yl) Acetate Ligands Functionalized with an NMDA Receptor Antagonist. Int. J. Mol. Sci. 2020, 21, 2616. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Santoni, G.; Pellei, M.; Santini, C.; Cimarelli, C.; Marcantoni, E.; Petrini, M.; Del Bello, F.; Giorgioni, G.; et al. Novel antitumor copper(II) complexes designed to act through synergistic mechanisms of action, due to the presence of an NMDA receptor ligand and copper in the same chemical entity. New J. Chem. 2018, 42, 11878–11887. [Google Scholar] [CrossRef]
- Gandin, V.; Ceresa, C.; Esposito, G.; Indraccolo, S.; Porchia, M.; Tisato, F.; Santini, C.; Pellei, M.; Marzano, C. Therapeutic potential of the phosphino Cu(I) complex (HydroCuP) in the treatment of solid tumors. Sci. Rep. 2017, 7, 13936. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Peruzzo, V.; Tegoni, M.; Giorgetti, M.; Damjanovic, M.; Trapananti, A.; Bagno, A.; Santini, C.; Pellei, M.; et al. Insights into the cytotoxic activity of the phosphane copper(I) complex Cu(thp)(4) PF6. J. Inorg. Biochem. 2016, 165, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Papini, G.; Bandoli, G.; Dolmella, A.; Gioia Lobbia, G.; Pellei, M.; Santini, C. New homoleptic carbene transfer ligands and related coinage metal complexes. Inorg. Chem. Commun. 2008, 11, 1103–1106. [Google Scholar] [CrossRef]
- Räisänen, M.T.; Elo, P.; Kettunen, M.; Klinga, M.; Leskelä, M.; Repo, T. Practical method for 2-hydroxyphenylketimine synthesis. Synth. Commun. 2007, 37, 1765–1777. [Google Scholar] [CrossRef]
- Cimarelli, C.; Palmieri, G.; Volpini, E. An improved solvent-free preparation of 2-imidoylphenols. Org. Prep. Proced. Int. 2001, 33, 369–371. [Google Scholar] [CrossRef]
- Cimarelli, C.; Palmieri, G. Alkylation of dianions derived from 2-(1-iminoalkyl) phenols: Synthesis of functionalized 2-acyl phenols. Tetrahedron 1998, 54, 15711–15720. [Google Scholar] [CrossRef]
- Filarowski, A.; Koll, A.; Głowiak, T. Steric Modification of the Intramolecular Hydrogen Bond in 2-(Methylimino-phenyl-methyl)-phenols. Monatshefte Chem. 1999, 130, 1097–1108. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; St Petkov, P.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Nakamoto, K. Applications in Coordination Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–273. [Google Scholar]
- Effendy; Healy, P.C.; Marchetti, F.; Pettinari, C.; Pettinari, R.; Tombesi, A.; Skelton, B.W.; White, A.H. Synthesis and structural characterization of some 1:1 and 1:2 adducts of silver(I) salts with hindered Pmes3, PPhmes2 and PPh2mes bases (Ph = phenyl, mes=2,4,6-trimethylpheny1)). Inorg. Chim. Acta 2022, 535, 120857. [Google Scholar] [CrossRef]
- Meijboom, R.; Bowen, R.J.; Berners-Price, S.J. Coordination complexes of silver(I) with tertiary phosphine and related ligands. Coord. Chem. Rev. 2009, 253, 325–342. [Google Scholar] [CrossRef]
- Barron, P.F.; Dyason, J.C.; Healy, P.C.; Engelhardt, L.M.; Skelton, B.W.; White, A.H. Lewis Base Adducts of Group 11 Metal Compounds. Part 24. Co-ordination of Triphenylphosphine with Silver Nitrate. A Solid-state Cross-polarization Magic Angle Spinning 31P Nuclear Magnetic Resonance, Crystal Structure, and Infrared Spectroscopic Study of Ag(PPh3)nNO3 (n = 1–4). J. Chem. Soc.-Dalton Trans. 1986, 9, 1965–1970. [Google Scholar]
- Pellei, M.; Del Gobbo, J.; Caviglia, M.; Karade, D.V.; Gandin, V.; Marzano, C.; Poyil, A.N.; Dias, H.V.R.; Santini, C. Synthesis and cytotoxicity studies of Cu(I) and Ag(I) complexes based on sterically hindered β-diketonates with different degrees of fluorination. Dalton Trans. 2023, 52, 12098–12111. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.V.R.; Flores, J.A.; Pellei, M.; Morresi, B.; Gioia Lobbia, G.; Singh, S.; Kobayashi, Y.; Yousufuddin, M.; Santini, C. Silver(I) and copper(I) complexes supported by fully fluorinated 1,3,5-triazapentadienyl ligands. Dalton Trans. 2011, 40, 8569–8580. [Google Scholar] [CrossRef]
- Pellei, M.; Alidori, S.; Papini, G.; Gioia Lobbia, G.; Gorden, J.D.; Dias, H.V.R.; Santini, C. Silver(I)-organophosphane complexes of electron withdrawing CF3- or NO2-substituted scorpionate ligands. Dalton Trans. 2007, 4845–4853. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Alidori, S.; Gioia Lobbia, G.; Papini, G.; Pellei, M.; Santini, C. Small Scorpionate Ligands: Silver(I)-Organophosphane Complexes of 5-CF3-Substituted Scorpionate Ligand Combining a B−H···Ag Coordination Motif. Inorg. Chem. 2007, 46, 9708–9714. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands:: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Cryst. 2002, B58, 380–388. [Google Scholar] [CrossRef]
- Wang, D.J.; Fan, L.; Wang, G.H. Crystal structure of N-(diphenylmethylene)diphenylmethanamine, C26H21N. Z. Krist.-New Cryst. Struct. 2009, 224, 186–188. [Google Scholar]
- Liu, X.L.; Gao, A.; Ding, L.; Xu, J.; Zhao, B.G. Aminative Umpolung Synthesis of Aryl Vicinal Diamines from Aromatic Aldehydes. Org. Lett. 2014, 16, 2118–2121. [Google Scholar] [CrossRef] [PubMed]
- Vorontsova, N.V.; Bystrova, G.S.; Antonov, D.Y.; Vologzhanina, A.V.; Godovikov, I.A.; Il’in, M.M. Novel ligands based on bromosubstituted hydroxycarbonyl 2.2 paracyclophane derivatives: Synthesis and application in asymmetric catalysis. Tetrahedron Asymmetry 2010, 21, 731–738. [Google Scholar] [CrossRef]
- Blackwell, J.M.; Piers, W.E.; Parvez, M.; McDonald, R. Solution and solid-state characteristics of imine adducts with tris(pentafluorophenyl)borane. Organometallics 2002, 21, 1400–1407. [Google Scholar] [CrossRef]
- Rozenberg, V.; Danilova, T.Y.; Sergeeva, E.; Vorontsov, E.; Starikova, Z.; Lysenko, K.; Belokon’, Y. Regioselective Fries Rearrangement and Friedel−Crafts Acylation as Efficient Routes to Novel Enantiomerically Enriched ortho-Acylhydroxy [2.2]paracyclophanes. Eur. J. Org. Chem. 2000, 2000, 3295–3303. [Google Scholar] [CrossRef]
- Ito, M.; Kasuga, N.C.; Matsuse, R.; Hirotsu, M. Crystal structures and circular dichroism of {2,20-[(1S,2S)-1,2-diphenylethane-1,2-diylbis(nitrilo-phenylmethanylylidene)]diphenolato} nickel(II) and its ethanol solvate. Acta Cryst. 2024, E80, 1259–1265. [Google Scholar]
- Hirotsu, M.; Kuwamura, N.; Kinoshita, I.; Kojima, M.; Yoshikawa, Y.; Ueno, K. Steric, geometrical and solvent effects on redox potentials in salen-type copper(II) complexes. Dalton Trans. 2009, 7678–7683. [Google Scholar] [CrossRef]
- Hirotsu, M.; Kojima, M.; Nakajima, K.; Kashino, S.; Yoshikawa, Y. Stereochemistry and electrochemistry of cobalt(II) and cobalt(III) complexes containing optically active tetradentate Schiff base ligands. Bull. Chem. Soc. Jpn. 1996, 69, 2549–2557. [Google Scholar] [CrossRef]
- Hirotsu, M.; Nakajima, K.; Kojima, M.; Yoshikawa, Y. Manganese(III) Complexes Containing Optically Active Tetradentate Schiff Base Ligands. Effect of Phenyl Substituents. Inorg. Chem. 1995, 34, 6173–6178. [Google Scholar] [CrossRef]
- Hirotsu, M.; Kojima, M.; Nakajima, K.; Kashino, S.; Yoshikawa, Y. Steric Control of Redox Potentials of Cobalt(II) Schiff Base Complexes with Phenyl Substituents. Chem. Lett. 1994, 23, 2183–2186. [Google Scholar] [CrossRef]
- Cifuentes-Vaca, O.L.; Andrades-Lagos, J.; Campanini-Salinas, J.; Laguna, A.; Vásquez-Velásquez, D.; Gimeno, M.C. Silver(I) and copper(I) complexes with a Schiff base derived from 2-aminofluorene with promising antibacterial activity. Inorg. Chim. Acta 2019, 489, 275–279. [Google Scholar] [CrossRef]
- Berthon, G. Critical evaluation of the stability constants of metal complexes of amino acids* with polar side chains (TechnicaI report). Pure Appl. Chem. 1995, 67, 1117–1240. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Singh, S.; Singh, A.K.; Rao, P.K.; Yadav, R.K.; Singh, A.P.; Tripathi, U.N. Exploration of iron(III) complexes with bidentate N, O-donor Schiff base ligands through synthesis, characterization, DFT, and antibacterial studies. J. Mol. Struct. 2025, 1319, 139496. [Google Scholar] [CrossRef]
- Salah, N.; Adly, O.M.I.; Ibrahim, M.A.; Abdelaziz, M.; Abdelrhman, E.M. New Metal Complexes Incorporating Schiff Base Ligand Based on Pyridine Moiety: Synthesis, Spectral Characterization, DFT, Biological Evaluation, and Molecular Docking. Appl. Organomet. Chem. 2025, 39, e7751. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Mocktar, C.; Omondi, B. In vitro biological studies of heteroleptic Ag(I) and Cu(I) unsymmetrical N,N’-diarylformamidine dithiocarbamate phosphine complexes; the effect of the metal center. Arab. J. Chem. 2020, 13, 6379–6394. [Google Scholar] [CrossRef]
- Reddy, T.S.; Privér, S.H.; Ojha, R.; Mirzadeh, N.; Velma, G.R.; Jakku, R.; Hosseinnejad, T.; Luwor, R.; Ramakrishna, S.; Wlodkowic, D.; et al. Gold(I) complexes of the type [AuL{κC-2-C6H4P(S)Ph2}] [L = PTA, PPh3, PPh2(C6H4-3-SO3Na) and PPh2(2-py)]: Synthesis, characterisation, crystal structures, and In Vitro and In Vivo anticancer properties. Eur. J. Med. Chem. 2025, 281, 117007. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, V.K.; Mishra, R.; Sharma, A.; Pandey, A.; Srivastava, S.K.; Chaurasia, H. Design, Synthesis, DFT, docking Studies, and antimicrobial evaluation of novel benzimidazole containing sulphonamide derivatives. Bioorg. Chem. 2024, 149, 107473. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, X.X.; Fatima, A.; Zhang, L.; Yuan, G.J.; Lian, F.X.; Wang, Y. Antibacterial Activity and Mechanisms of Plant Flavonoids against Gram-Negative Bacteria Based on the Antibacterial Statistical Model. Pharmaceuticals 2024, 17, 292. [Google Scholar] [CrossRef]
- Hasan, A.; Varna, D.; Chakraborty, I.; Angaridis, P.A.; Raptis, R.G. Synthesis, structure and antibacterial properties of a mononuclear Ag(I) complex, [Ag(OBz)(PTA)2] (OBz =benzoate, PTA =1,3,5-triaza-7-phospadamantane). Results Chem. 2022, 4, 100580. [Google Scholar] [CrossRef]
- Pervaiz, M.; Sadiq, A.; Sadiq, S.; Saeed, Z.; Imran, M.; Younas, U.; Bukhari, S.M.; Khan, R.R.M.; Rashid, A.; Adnan, A. Design and synthesis of Schiff base Homobimetallic-Complexes as promising antimicrobial agents. Inorg. Chem. Commun. 2022, 137, 109206. [Google Scholar] [CrossRef]
- Ejidike, I.P. Cu(II) Complexes of 4-[(1E)-N-{2-[(Z)-Benzylidene-amino]ethyl}ethanimidoyl]benzene-1,3-diol Schiff Base: Synthesis, Spectroscopic, In-Vitro Antioxidant, Antifungal and Antibacterial Studies. Molecules 2018, 23, 1581. [Google Scholar] [CrossRef]
- Calu, L.; Badea, M.; Korosin, N.C.; Chifiriuc, M.C.; Bleotu, C.; Stanica, N.; Silvestro, L.; Maurer, M.; Olar, R. Spectral, thermal and biological characterization of complexes with a Schiff base bearing triazole moiety as potential antimicrobial species. J. Therm. Anal. Calorim. 2018, 134, 1839–1850. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, Q.; Jing, H.R.; Cai, Y.J.; Wang, Q.; Li, Y.G. Synthesis, characterization, and antimicrobial activity of two Schiff base silver(I) complexes derived from 4-carboxybenzaldehyde. J. Coord. Chem. 2017, 70, 1066–1076. [Google Scholar] [CrossRef]
- CrysAlisPro Versions 1.171.43.141a; Rigaku Oxford Diffraction: Oxford, UK, 2024.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Liu, J.M.; Guo, C.P.; Liu, Z.Z.; Cheng, F.; Zhang, S.; Zhang, Z.H. Simultaneous sterilization and biosensing of pathogenic bacteria via copper phthalocyanine-based COF embedded with Cu-N4 single atomic sites and silver nanoparticles. Chem. Eng. J. 2024, 494, 153139. [Google Scholar] [CrossRef]
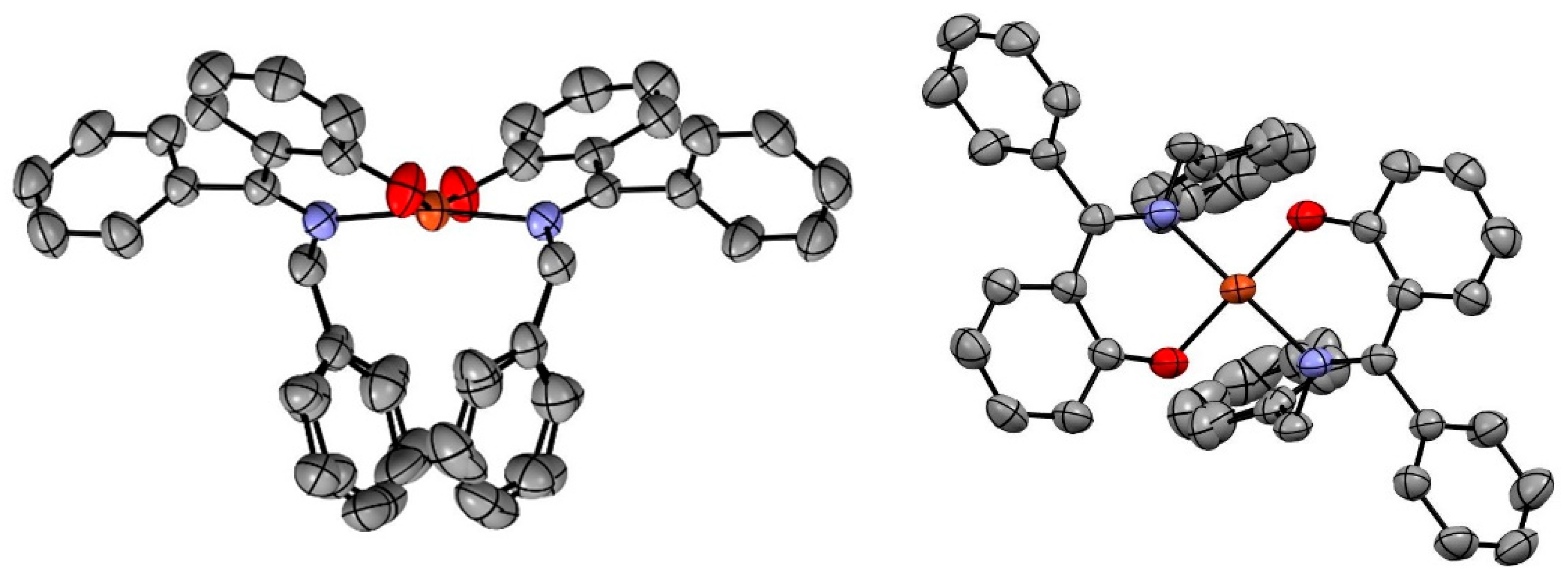
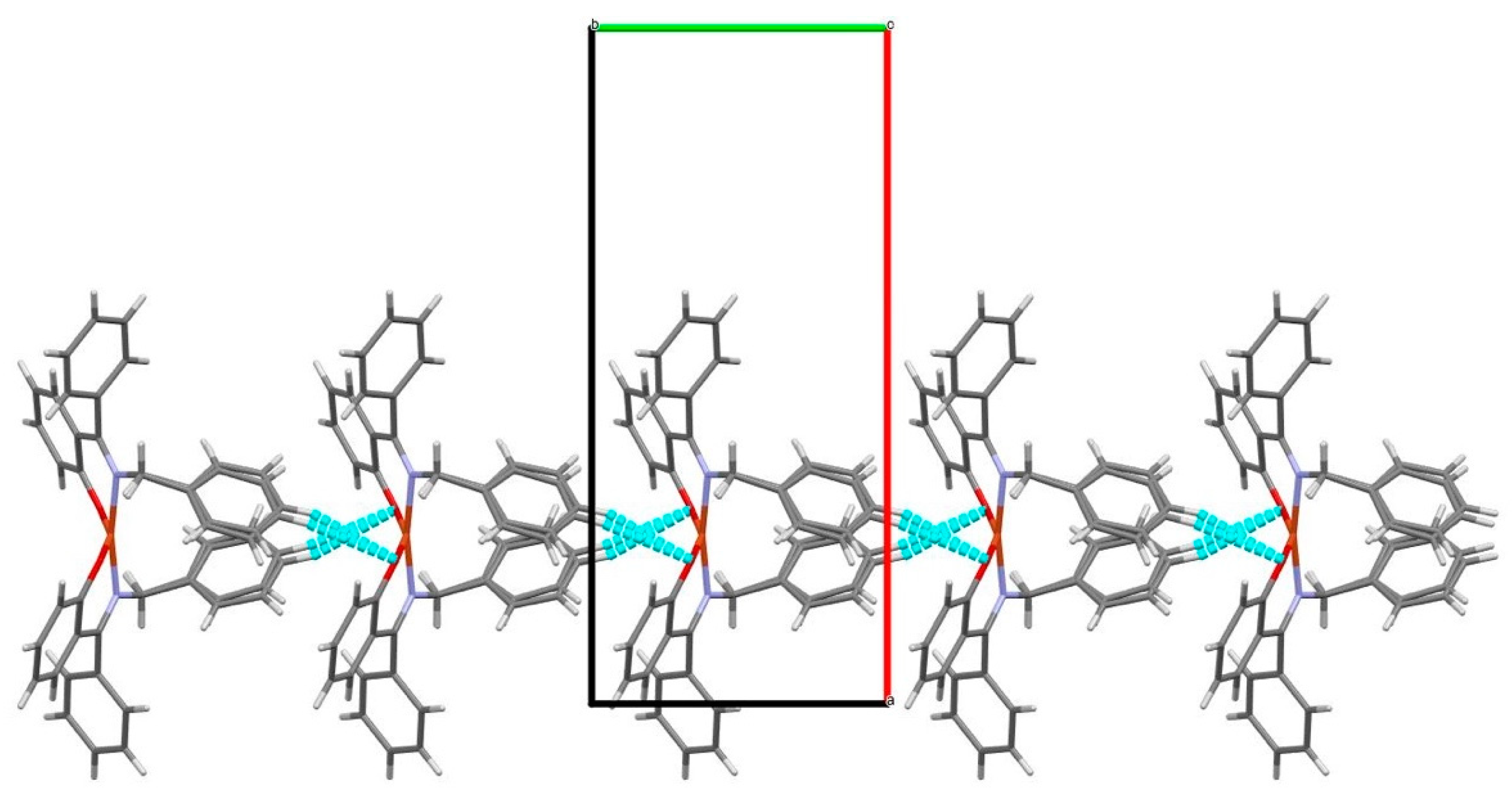

| A Atom | D Atom | P Atom a | A····D (Å) | A····D–P (°) | Symmetry Op. b |
|---|---|---|---|---|---|
| O1 | H18 | C18 | 2.81 | 171.8 | 3/2 − x, y, 1 − z |
| O1 | H18A | C18 | 2.85 | 159.9 | 3/2 − x, y, 1 − z |
| C15/C20 | H11 | C11 c | 2.78 d | 168.8 d | 1 − x, −1/2 + y, 1/2 − z |
| C15A/C20A | H11 | C11 c | 2.78 d | 165.0 d | 1 − x, −1/2 + y, 1/2 − z |
| C1 | H12 | C12 c | 2.91 | 147.7 | 1 − x, 1 − y, 1 − z |
| C2 | H12 | C12 c | 2.91 | 145.7 | 1 − x, 1 − y, 1 − z |
| C15/C20 | C15/C20 e | 4.20 f | 26.6 g | 1/2 − x, y, 1 − z | |
| C15A/C20A | C15A/C20A e | 4.03 f | 23.4 g | 1/2 − x, y, 1 − z |
| E. coli | S. aureus | ||||
|---|---|---|---|---|---|
| N. | Tested Compounds | MIC | MIC50 | MIC | MIC50 |
| 1 | HLBSMe | / | / | / | / |
| 2 | HLBSPh | / | / | / | / |
| 3 | [Cu(HLBSMe)(PTA)2]PF6 | / | 0.057 (0.076) | / | 0.116 (0.149) |
| 4 | [Ag(HLBSMe)(PTA)]NO3 | 0.050 (0.090) | 0.012 (0.021) | 0.100 (0.181) | 0.061 (0.110) |
| 5 | [Cu(LBSMe)2] | / | 0.110 (0.210) | / | 0.381 (0.738) |
| 6 | [Cu(HLBSPh)(PPh3)2]PF6·2CH3CN | / | / | / | / |
| 7 | [Cu(HLBSPh)(PTA)2]PF6·2H2O | / | 0.114 (0.130) | / | 0.084 (0.100) |
| 8 | [Ag(HLBSPh)(PPh3)2]NO3 | / | 0.098 (0.100) | / | 0.080 (0.079) |
| 9 | [Ag(HLBSPh)(PTA)]NO3 | 0.025 (0.040) | 0.010 (0.016) | 0.050 (0.081) | 0.036 (0.058) |
| 10 | [Cu(LBSPh)2] | / | / | / | / |
| 11 | PPh3 | / | / | / | / |
| 12 | PTA | / | / | / | / |
| E. coli | S. aureus | ||||
|---|---|---|---|---|---|
| N. | Tested Compounds | MBC | MBC50 | MBC | MBC50 |
| 1 | HLBSMe | / | / | / | / |
| 2 | HLBSPh | / | / | / | / |
| 3 | [Cu(HLBSMe)(PTA)2]PF6 | / | 0.262 (0.350) | / | 0.642 (0.858) |
| 4 | [Ag(HLBSMe)(PTA)]NO3 | 0.200 (0.362) | 0.048 (0.086) | 0.200 (0.362) | 0.062 (0.112) |
| 5 | [Cu(LBSMe)2] | / | 0.243 (0.474) | / | 0.578 (1.128) |
| 6 | [Cu(HLBSPh)(PPh3)2]PF6·2CH3CN | / | 0.566 (0.513) | / | 1.125 (1.020) |
| 7 | [Cu(HLBSPh)(PTA)2]PF6·2H2O | / | 0.319 (0.377) | / | 0.551 (0.651) |
| 8 | [Ag(HLBSPh)(PPh3)2]NO3 | / | 0.322 (0.327) | / | 0.294 (0.299) |
| 9 | [Ag(HLBSPh)(PTA)]NO3 | 0.100 (0.163) | 0.046 (0.075) | 0.200 (0.326) | 0.068 (0.110) |
| 10 | [Cu(LBSPh)2] | / | 0.282 (0.443) | / | 0.875 (1.375) |
| 11 | PPh3 | / | / | / | / |
| 12 | PTA | / | / | / | / |
| Empirical Formula | C40H32CuN2O2 |
|---|---|
| Formula weight | 636.21 |
| Temperature/K | 295.1(3) |
| Radiation | Cu Kα (λ = 1.54184) |
| Crystal system | monoclinic |
| Space group | I2/a |
| a/Å | 20.4267(4) |
| b/Å | 8.7751(2) |
| c/Å | 18.3240(3) |
| α/° | 90 |
| β/° | 101.135(2) |
| γ/° | 90 |
| Volume/Å3 | 3222.68(11) |
| Z | 4 |
| ρcalc g/cm3 | 1.311 |
| μ/mm−1 | 1.246 |
| F (000) | 1324.0 |
| Crystal size/mm3 | 0.42 × 0.4 × 0.2 |
| 2θ range for data collection/° | 8.824 to 155.422 |
| Index ranges | −20 ≤ h ≤ 25, −11 ≤ k ≤ 11, −23 ≤ l ≤ 23 |
| Reflections collected | 36,327 |
| Independent reflections | 3416 [Rint = 0.0377] |
| Data/restraints/parameters | 3416/0/247 |
| Goodness-of-fit on F2 | 1.034 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0331, wR2 = 0.0927 |
| Largest diff. peak/hole/e Å−3 | 0.17/−0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caviglia, M.; Li, Z.; Santini, C.; Del Gobbo, J.; Cimarelli, C.; Du, M.; Dolmella, A.; Pellei, M. New Cu(II), Cu(I) and Ag(I) Complexes of Phenoxy-Ketimine Schiff Base Ligands: Synthesis, Structures and Antibacterial Activity. Molecules 2025, 30, 1893. https://doi.org/10.3390/molecules30091893
Caviglia M, Li Z, Santini C, Del Gobbo J, Cimarelli C, Du M, Dolmella A, Pellei M. New Cu(II), Cu(I) and Ag(I) Complexes of Phenoxy-Ketimine Schiff Base Ligands: Synthesis, Structures and Antibacterial Activity. Molecules. 2025; 30(9):1893. https://doi.org/10.3390/molecules30091893
Chicago/Turabian StyleCaviglia, Miriam, Zhenzhen Li, Carlo Santini, Jo’ Del Gobbo, Cristina Cimarelli, Miao Du, Alessandro Dolmella, and Maura Pellei. 2025. "New Cu(II), Cu(I) and Ag(I) Complexes of Phenoxy-Ketimine Schiff Base Ligands: Synthesis, Structures and Antibacterial Activity" Molecules 30, no. 9: 1893. https://doi.org/10.3390/molecules30091893
APA StyleCaviglia, M., Li, Z., Santini, C., Del Gobbo, J., Cimarelli, C., Du, M., Dolmella, A., & Pellei, M. (2025). New Cu(II), Cu(I) and Ag(I) Complexes of Phenoxy-Ketimine Schiff Base Ligands: Synthesis, Structures and Antibacterial Activity. Molecules, 30(9), 1893. https://doi.org/10.3390/molecules30091893









