Influence of Metal Composition and Support Material on Carbon Yield and Quality in the Direct Decomposition of Methane
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Screening Based on Carbon Yields
2.2. Catalyst Physicochemical Properties
2.3. Catalytic Activity in DDM
2.4. Characterization of CNT
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalytic Reaction
3.4. Acid Treatment for the Purification of CNT
3.5. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashik, U.P.M.; Wan Daud, W.M.A.; Abbas, H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane—A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef]
- Kim, D.H.; Youn, J.R.; Seo, J.C.; Kim, S.B.; Kim, M.J.; Lee, K. One-pot synthesis of NiCo/MgAl2O4 catalyst for high coke-resistance in steam methane reforming: Optimization of Ni/Co ratio. Catal. Today 2023, 411, 113910. [Google Scholar] [CrossRef]
- Kim, H.; Eissa, A.A.S.; Kim, S.B.; Lee, H.; Kim, W.; Seo, D.j.; Lee, K.; Yoon, W. One-pot synthesis of a highly mesoporous Ni/MgAl2O4 spinel catalyst for efficient steam methane reforming: Influence of inert annealing. Catal. Sci. Technol. 2021, 11, 4447–4458. [Google Scholar] [CrossRef]
- Zhang, T.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over silica-supported nickel catalysts. Appl. Catal. A Gen. 1998, 167, 161–172. [Google Scholar] [CrossRef]
- Oku, T. Hydrogen storage in boron nitride and carbon nanomaterials. Energies 2015, 8, 319–337. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, X.; Lu, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-nanomaterial-based flexible batteries for wearable electronics. Adv. Mater. 2019, 31, 1800716. [Google Scholar] [CrossRef]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Ali, M.; Ngo, S.I.; Lim, Y.I.; An, S.; Lee, Y.J.; Lee, U.D. Experiment and simulation of non-catalytic thermal decomposition of CH4 for CO2-free hydrogen production in a vertical tube. Int. J. Hydrogen Energy 2024, 63, 580–595. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Methane cracking for hydrogen production: A review of catalytic and molten media pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Makris, T.D.; Giorgi, L.; Giorgi, R.; Lisi, N.; Salernitano, E. CNT growth on alumina supported nickel catalyst by thermal CVD. Diam. Relat. Mater. 2005, 14, 815–819. [Google Scholar] [CrossRef]
- Ammendola, P.; Chirone, R.; Lisi, L.; Ruoppolo, G.; Russo, G. Copper catalysts for H2 production via CH4 decomposition. J. Mol. Catal. A Chem. 2007, 266, 31–39. [Google Scholar] [CrossRef]
- Chen, C.M.; Dai, Y.M.; Huang, J.G.; Jehng, J.M. Intermetallic catalyst for carbon nanotubes (CNTs) growth by thermal chemical vapor deposition method. Carbon 2006, 44, 1808–1820. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Ismagilov, Z.R.; Chuvilin, A.L.; Ushakov, V.A. Carbon capacious Ni-Cu-Al2O3 catalysts for high-temperature methane decomposition. Appl. Catal. A Gen. 2003, 247, 51–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Smith, K.J. CH4 decomposition on Co catalysts: Effect of temperature, dispersion, and the presence of H2 or CO in the feed. Catal. Today 2002, 77, 257–268. [Google Scholar] [CrossRef]
- Solymosi, F.; Cserényi, J.; Szöke, A.; Bánsági, T.; Oszkó, A. Aromatization of methane over supported and unsupported Mo-based catalysts. J. Catal. 1997, 165, 150–161. [Google Scholar] [CrossRef]
- Wu, S.L.; Chen, C.M.; Kuo, J.H.; Wey, M.Y. Synthesis of carbon nanotubes with controllable diameter by chemical vapor deposition of methane using Fe@Al2O3 core–shell nanocomposites. Chem. Eng. Sci. 2020, 217, 115541. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Y.; Chu, W. Catalytic chemical vapor deposition of methane to carbon nanotubes: Copper promoted effect of Ni/MgO catalysts. J. Nanotechnol. 2014, 2014, 547030. [Google Scholar] [CrossRef]
- Pérez-Mendoza, M.; Vallés, C.; Maser, W.K.; Martínez, M.T.; Benito, A.M. Influence of molybdenum on the chemical vapour deposition production of carbon nanotubes. Nanotechnology 2005, 16, 550–556. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Khassin, A.A.; Kustova, G.N.; Ushakov, V.A.; Moroz, E.M.; Shmakov, N.; Kriventsov, V.V.; Kochubey, D.I.; Pavlyukhin, Y.T.; et al. Coprecipitated iron-containing catalysts (Fe-Al2O3, Fe-Co-Al2O3, Fe-Ni-Al2O3) for methane decomposition at moderate temperatures I. Genesis of calcined and reduced catalysts. Appl. Catal. A Gen. 2004, 268, 127–138. [Google Scholar] [CrossRef]
- Choi, J.B.; Im, J.S.; Kang, S.C.; Lee, Y.S.; Lee, C.W. Effect of metal–support interaction in Ni/SiO2 catalysts on the growth of carbon nanotubes by methane decomposition. Carbon Lett. 2023, 33, 477–488. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lua, A.C. Methane decomposition using Ni-Cu alloy nanoparticle catalysts and catalyst deactivation studies. Chem. Eng. J. 2015, 262, 1077–1089. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Chai, S.P.; Zein, S.H.S.; Mohamed, A.R. The effect of reduction temperature on Co-Mo/Al2O3 catalysts for carbon nanotubes formation. Appl. Catal. A Gen. 2007, 326, 173–179. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, K.D.; Park, Y.S.; Go, K.S.; Kim, W.; Lim, M.; Nho, N.S.; Lee, D.H. Effect of reduction conditions of Mo-Fe/MgO on the formation of carbon nanotube in catalytic methane decomposition. J. Ind. Eng. Chem. 2022, 109, 384–396. [Google Scholar] [CrossRef]
- Magrez, A.; Smajda, R.; Seo, J.W.; Horváth, E.; Ribic, P.R.; Andresen, J.C.; Acquaviva, D.; Olariu, A.; Laurenczy, G.; Forró, L. Striking influence of the catalyst support and its acid–base properties: New insight into the growth mechanism of carbon nanotubes. ACS Nano 2011, 5, 3428–3437. [Google Scholar] [CrossRef]
- Hoque, A.; Nawarathne, C.P.; Alvarez, N.T. Vertically aligned carbon nanotubes from premade binary metal oxide nanoparticles on bare SiO2. Carbon 2025, 235, 120086. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.J.; Kim, D.H.; Mnoyan, A.; Lee, K. Enhanced methane dry reforming with Ni/SiO2 catalysts featuring hierarchical external nanostructures. Catalysts 2024, 14, 265. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.; Kim, Y.J.; Kim, J.; Youn, J.R.; Kim, D.H.; Shapiro, D.; Guo, J.; Lee, K. Surface control of Ni–Al2O3 dry reforming of methane catalyst by composition segregation. J. CO2 Util. 2024, 81, 102721. [Google Scholar] [CrossRef]
- Somanathan, T.; Krishna, V.M.; Saravanan, V.; Kumar, R.; Kumar, R. MgO nanoparticles for effective uptake and release of doxorubicin drug: pH sensitive controlled drug release. J. Nanosci. Nanotechnol. 2016, 16, 9421–9431. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, J.; Guo, B.; Liu, H.; Jin, Y.; Ran, J. Effect of Cu doping on Ni surface on CO formation pathways during the methane dry reforming reaction. Mol. Catal. 2024, 560, 114125. [Google Scholar] [CrossRef]
- Dai, Y.M.; Lu, C.Y.; Chang, C.J. Catalytic activity of mesoporous Ni/CNT, Ni/SBA-15 and (Cu, Ca, Mg, Mn, Co)-Ni/SBA-15 catalysts for CO2 reforming of CH4. RSC Adv. 2016, 6, 73887–73896. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; No, M.L.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Dry reforming of methane over sub-stoichiometric NiAl2O4-mediated Ni/Al2O3 catalysts. Fuel 2024, 358, 130166. [Google Scholar] [CrossRef]
- Tan, M.; Wang, X.; Hu, Y.; Shang, X.; Zhang, L.; Zou, X.; Dinga, W.; Lu, X. Influence of nickel content on structural and surface properties, reducibility and catalytic behavior of mesoporous γ-alumina-supported Ni–Mg oxides for pre-reforming of liquefied petroleum gas. Catal. Sci. Technol. 2016, 6, 3049–3063. [Google Scholar] [CrossRef]
- Melgunov, M.S. Application of the simple Bayesian classifier for the N2 (77 K) adsorption/desorption hysteresis loop recognition. Adsorption 2023, 29, 199–208. [Google Scholar] [CrossRef]
- Schreiter, N.; Kirchner, J.; Kureti, S. A DRIFTS and TPD study on the methanation of CO2 on Ni/Al2O3 catalyst. Catal. Commun. 2020, 140, 105988. [Google Scholar] [CrossRef]
- Ahmad, M. Tailored Ni-MgO catalysts: Unveiling temperature-driven synergy in CH4-CO2 reforming. Catalysts 2023, 14, 33. [Google Scholar] [CrossRef]
- Vandevyvere, T.; Sabbe, M.K.; Thybaut, J.W.; Lauwaert, J. Enhancing stability of γ-Al2O3-supported NiCu catalysts by impregnating basic oxides in the hydrodeoxygenation of anisole. Catalysts 2024, 14, 166. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, P.; Yang, J.; Zhu, Y.A.; Chen, D. C–H bond activation in light alkanes: A theoretical perspective. Chem. Soc. Rev. 2021, 50, 4299–4358. [Google Scholar] [CrossRef]
- Caravaggio, G.; Nossova, L.; Turnbull, M.J. Nickel–magnesium mixed oxide catalyst for low temperature methane oxidation. Chem. Eng. J. 2021, 405, 126862. [Google Scholar] [CrossRef]
- Dijon, J.; Szkutnik, P.D.; Fournier, A.; Monsabert, T.G.; Okuno, H.; Quesnel, E.; Muffato, V.; Vito, E.; Bendiab, N.; Bogner, A.; et al. How to switch from a tip to base growth mechanism in carbon nanotube growth by catalytic chemical vapour deposition. Carbon 2010, 48, 3953–3963. [Google Scholar] [CrossRef]
- Shen, J.; Olfert, J.; Abbasi-Atibeh, E.; Semagina, N. The effect of catalyst particle size and temperature on CNT growth on supported Fe catalysts during methane pyrolysis. Catal. Today 2025, 453, 115275. [Google Scholar] [CrossRef]
- Jodin, L.; Dupuis, A.C.; Rouvière, E.; Reiss, P. Influence of the catalyst type on the growth of carbon nanotubes via methane chemical vapor deposition. J. Phys. Chem. B 2006, 110, 7328–7333. [Google Scholar] [CrossRef] [PubMed]
- Moodley, P.; Loos, J.; Niemantsverdriet, J.W.; Thüne, P.C. Is there a correlation between catalyst particle size and CNT diameter? Carbon 2009, 47, 2002–2013. [Google Scholar] [CrossRef]
- Allaedini, G.; Aminayi, P.; Tasirin, S.M. Methane decomposition for carbon nanotube production: Optimization of the reaction parameters using response surface methodology. Chem. Eng. Res. Des. 2016, 112, 163–174. [Google Scholar] [CrossRef]
- Santhosh, C.; Saranya, M.; Felix, S.; Ramachandran, R.; Pradeep, N.; Uma, V.; Grace, A.N. Growth of carbon nanotubes using MgO supported Mo–Co catalysts by thermal chemical vapor deposition technique. J. Nano Res. 2013, 24, 46–57. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Xie, X.; Liu, J.; Xu, Y.; Shen, W. Novel Ni catalysts for methane decomposition to hydrogen and carbon nanofibers. J. Catal. 2006, 238, 412–424. [Google Scholar] [CrossRef]
- Al-Hilfi, S.H.; Kinloch, I.A.; Derby, B. Chemical vapor deposition of graphene on Cu–Ni alloys: The impact of carbon solubility. Coatings 2021, 11, 892. [Google Scholar] [CrossRef]
- Maheswaran, R.; Shanmugavel, B.P. A critical review of the role of carbon nanotubes in the progress of next-generation electronic applications. J. Electron. Mater. 2022, 51, 2786–2800. [Google Scholar] [CrossRef]
- Flygare, M.; Svensson, K. Influence of crystallinity on the electrical conductivity of individual carbon nanotubes. Carb. Trends 2021, 5, 100125. [Google Scholar] [CrossRef]
- Deng, J.; Liu, C.; Song, D.; Madou, M. Fabrication of crystalline submicro-to-nano carbon wire for achieving high current density and ultrastable current. Microsyst. Nanoeng. 2022, 8, 15. [Google Scholar] [CrossRef] [PubMed]

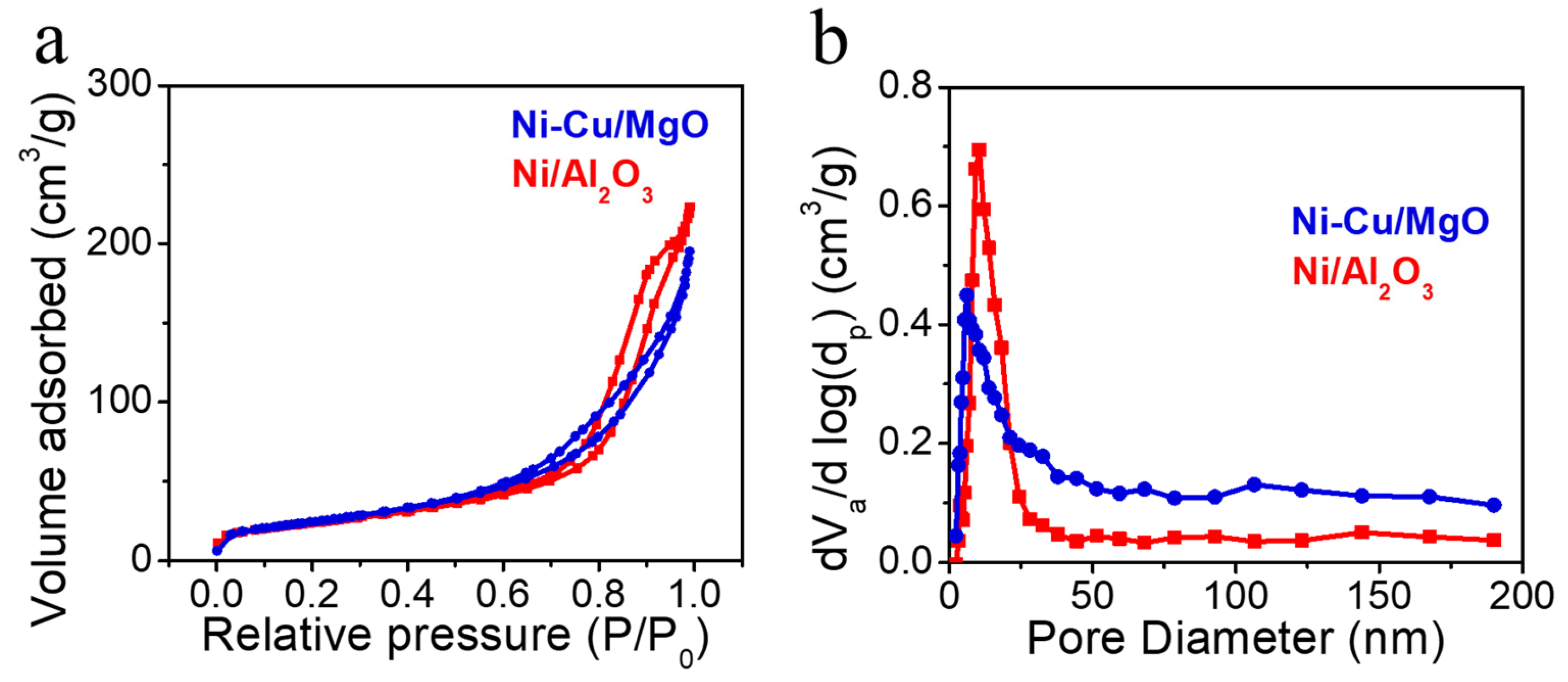
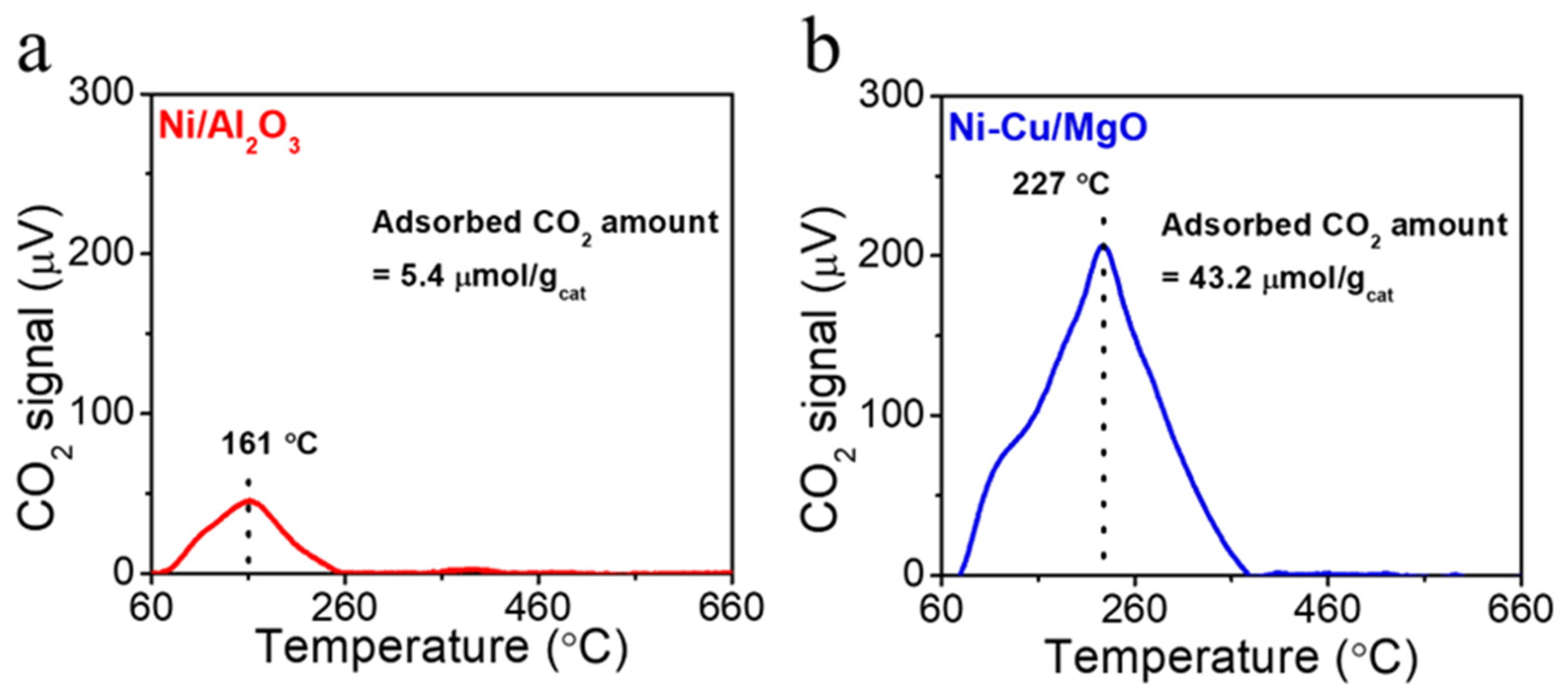
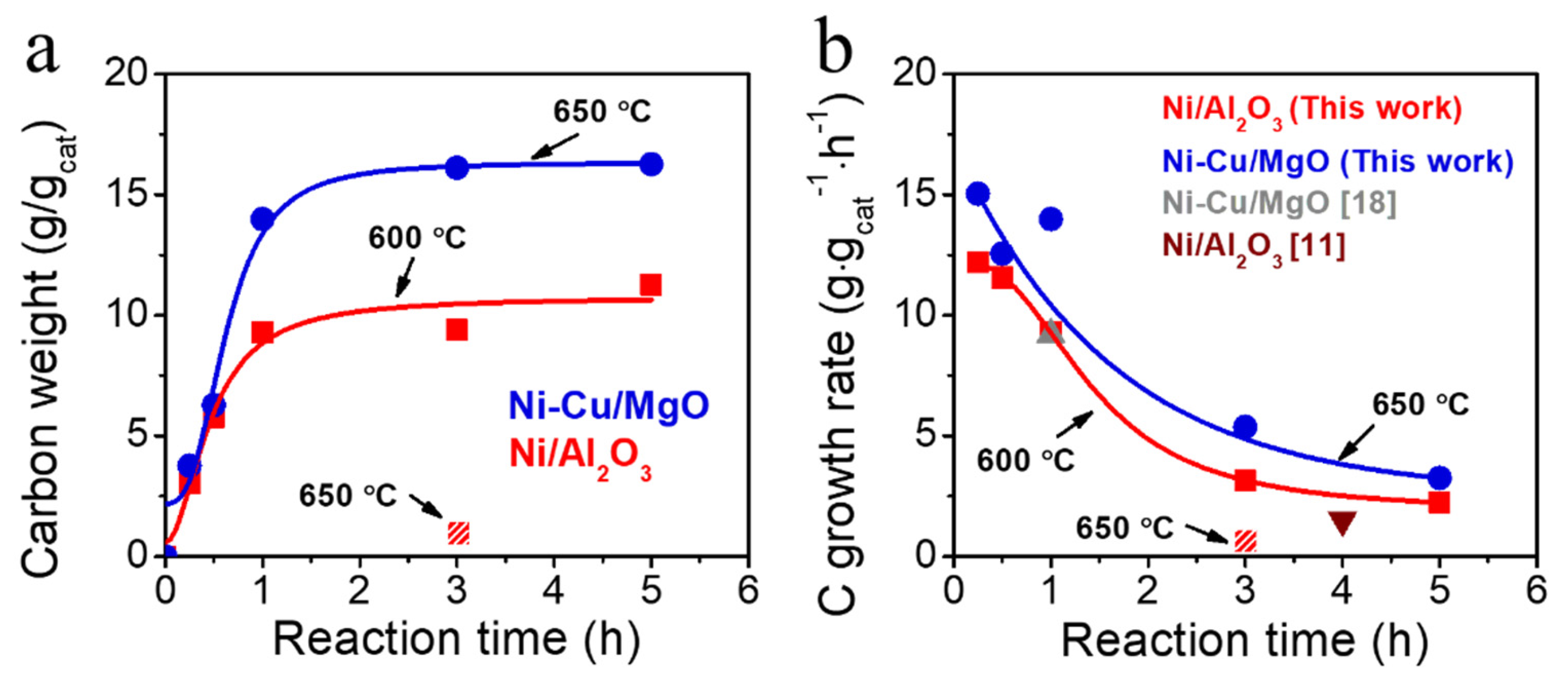




| Catalysts | Contents (wt%) | Reaction Time (h) | Carbon Yield (g·gcat−1·h−1) |
|---|---|---|---|
| Ni/Al2O3 | Ni (40), Al2O3 (60) | 3 | 3.13 |
| Ni-Cu/MgO | Ni (50), Cu (5), MgO (45) | 3 | 5.37 |
| Co-Mo/MgO | Co (3), Mo (17), MgO (80) | 3 | 0.31 |
| Ni/SiO2 | Ni (10), SiO2 (90) | 3 | 0 |
| Fe/Al2O3 | Fe (29), Al2O3 (71) | 3 | 0.26 |
| Ni/MgO | Ni (71), MgO (29) | 3 | 0.12 |
| Catalysts | Crystallite Size (nm) |
|---|---|
| Ni/Al2O3 | 15.64 |
| Ni-Cu/MgO | 9.04 |
| Catalysts | S.S.A. (m2 gcat−1) | T.P.V. (cm3 gcat−1) | M.P.D. (nm) |
|---|---|---|---|
| Ni/Al2O3 | 85.53 | 0.34 | 16.01 |
| Ni-Cu/MgO | 87.03 | 0.30 | 13.86 |
| Catalysts | CNT Yield at 1 h Reaction (g·gcat−1) a | CNT Diameter (nm) b | CNT Crystallinity (ID/IG) c | CNT Resistivity at 1 g/cc Density (Ω·cm) d |
|---|---|---|---|---|
| Ni/Al2O3 | 9.29 | 32.36 | 1.25 | 0.05 |
| Ni-Cu/MgO | 13.99 | 38.61 | 1.63 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, U.; Ku, B.-J.; Gwon, Y.; Kim, D.-H.; Kim, M.; Jeon, I.-J.; Lee, H.; Shim, J.-O.; Lee, K. Influence of Metal Composition and Support Material on Carbon Yield and Quality in the Direct Decomposition of Methane. Molecules 2025, 30, 1903. https://doi.org/10.3390/molecules30091903
Jun U, Ku B-J, Gwon Y, Kim D-H, Kim M, Jeon I-J, Lee H, Shim J-O, Lee K. Influence of Metal Composition and Support Material on Carbon Yield and Quality in the Direct Decomposition of Methane. Molecules. 2025; 30(9):1903. https://doi.org/10.3390/molecules30091903
Chicago/Turabian StyleJun, Uidam, Bon-Jun Ku, Yeji Gwon, Dong-Hyun Kim, Mansu Kim, I-Jeong Jeon, Hongjin Lee, Jae-Oh Shim, and Kyubock Lee. 2025. "Influence of Metal Composition and Support Material on Carbon Yield and Quality in the Direct Decomposition of Methane" Molecules 30, no. 9: 1903. https://doi.org/10.3390/molecules30091903
APA StyleJun, U., Ku, B.-J., Gwon, Y., Kim, D.-H., Kim, M., Jeon, I.-J., Lee, H., Shim, J.-O., & Lee, K. (2025). Influence of Metal Composition and Support Material on Carbon Yield and Quality in the Direct Decomposition of Methane. Molecules, 30(9), 1903. https://doi.org/10.3390/molecules30091903







