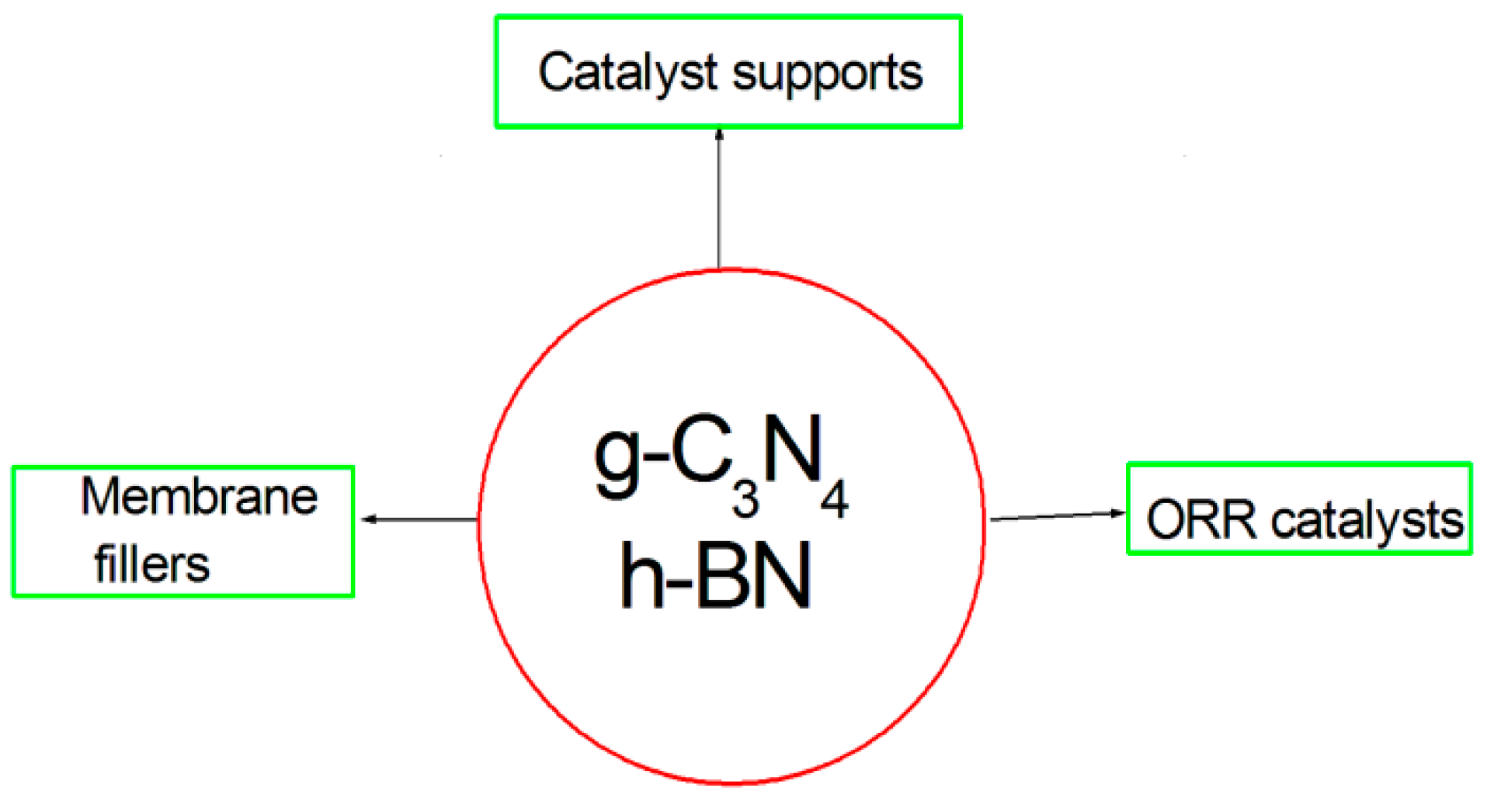
The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers
Abstract
1. Introduction
2. Catalyst Supports
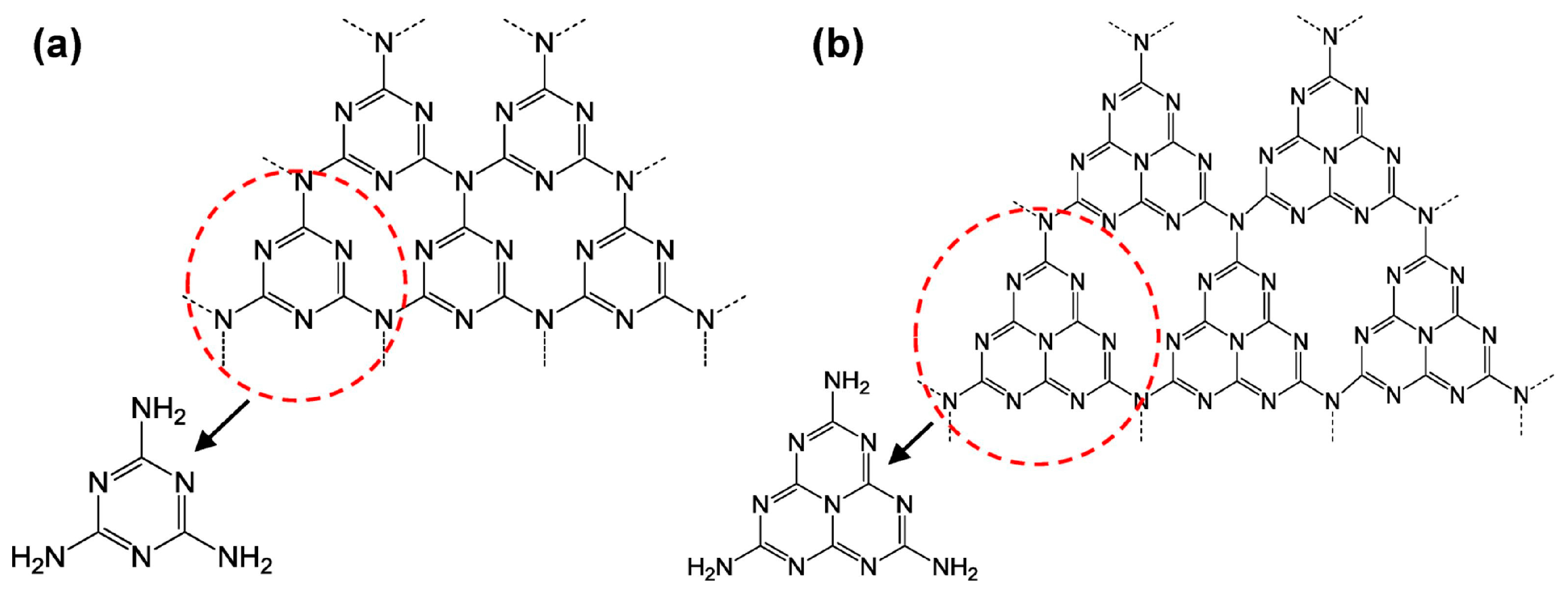
2.1. Carbon Nitrides
2.1.1. Pristine g-C3N4
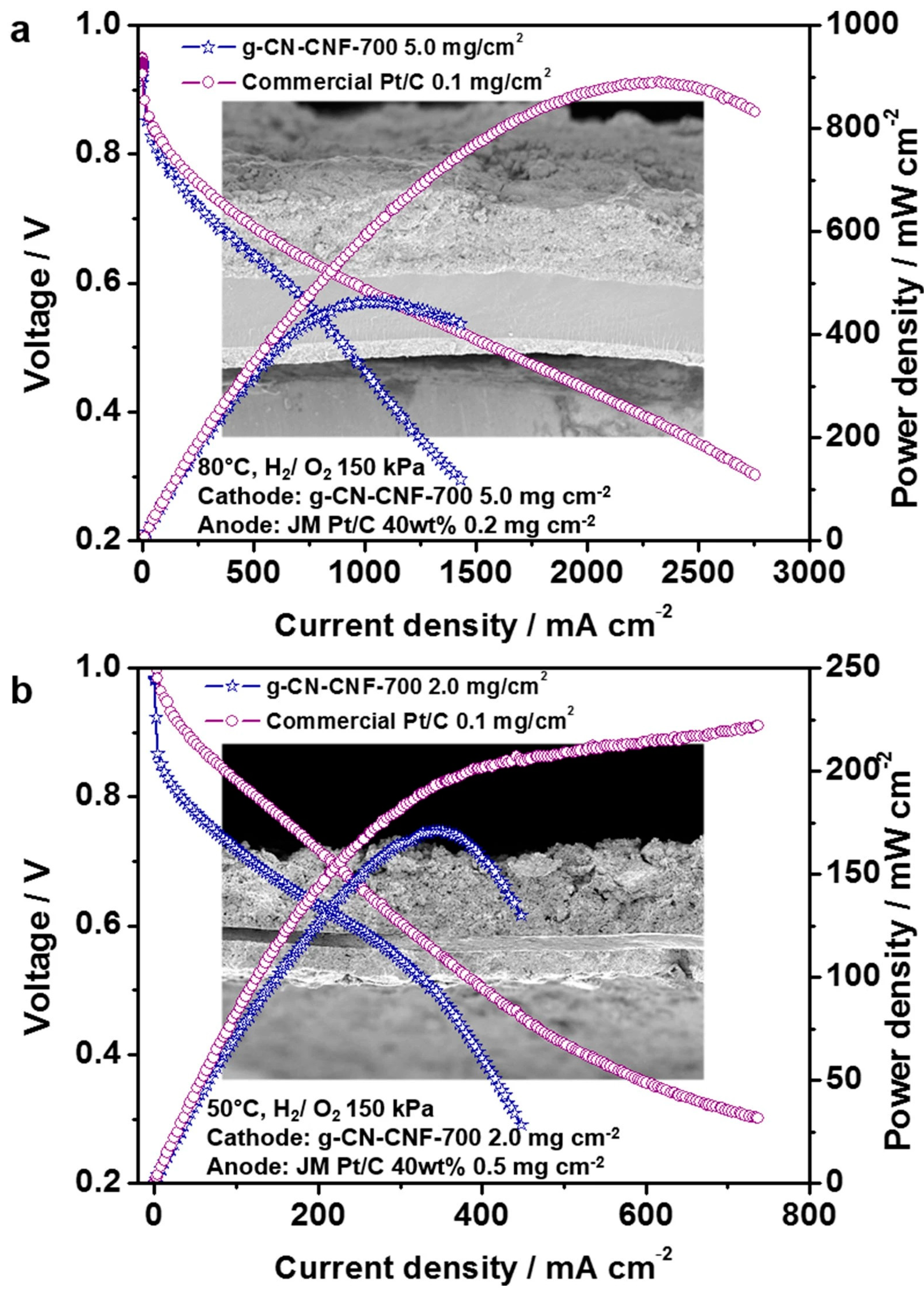
2.1.2. g-C3N4-Carbon Black Composites
2.1.3. g-C3N4–Graphene (Reduced Graphene Oxide) Composites
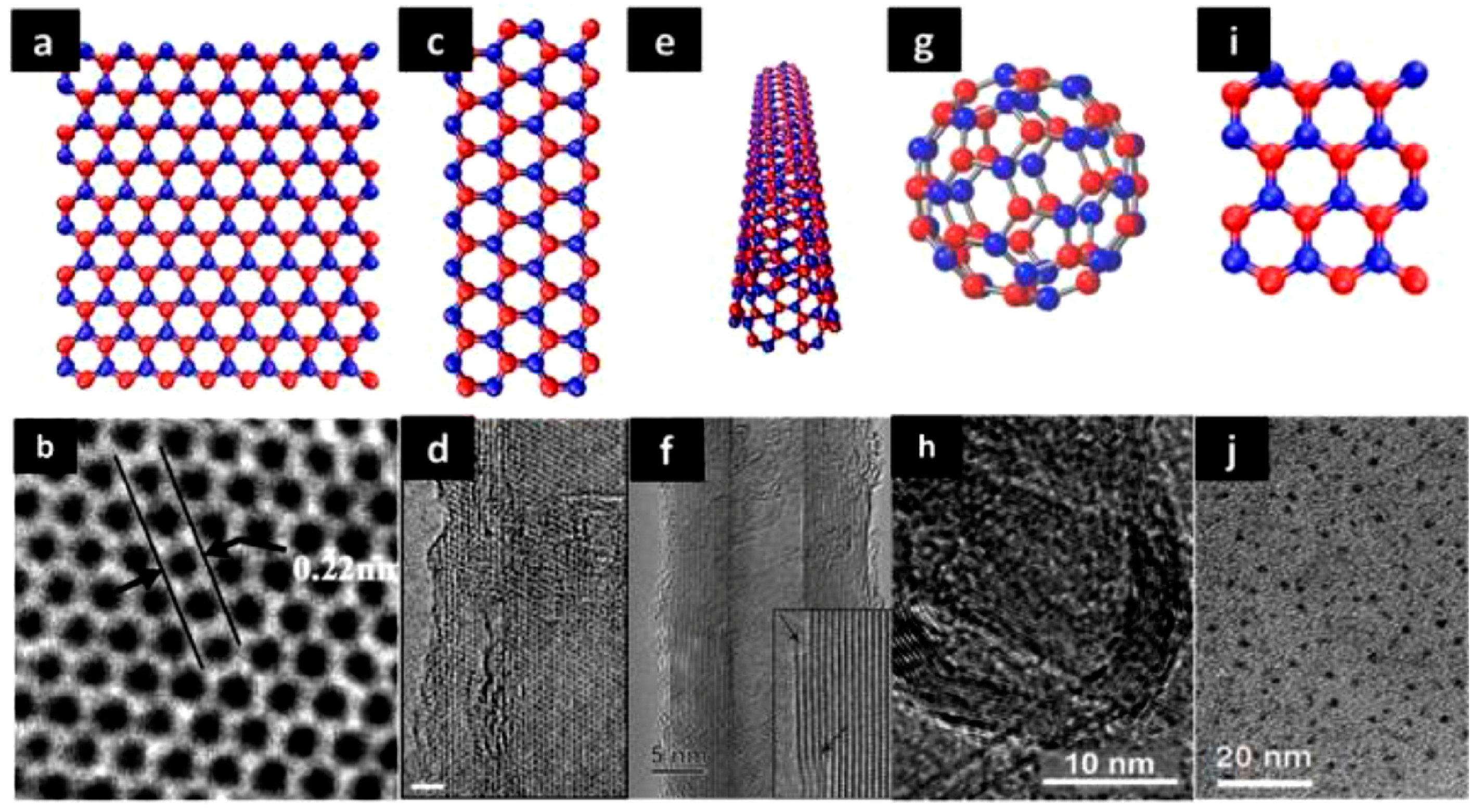
2.2. h-BN and h-BN Nanosheets (h-BNNSs)
3. ORR Catalysts
3.1. g-C3N4
3.1.1. g-C3N4–Carbon Composites
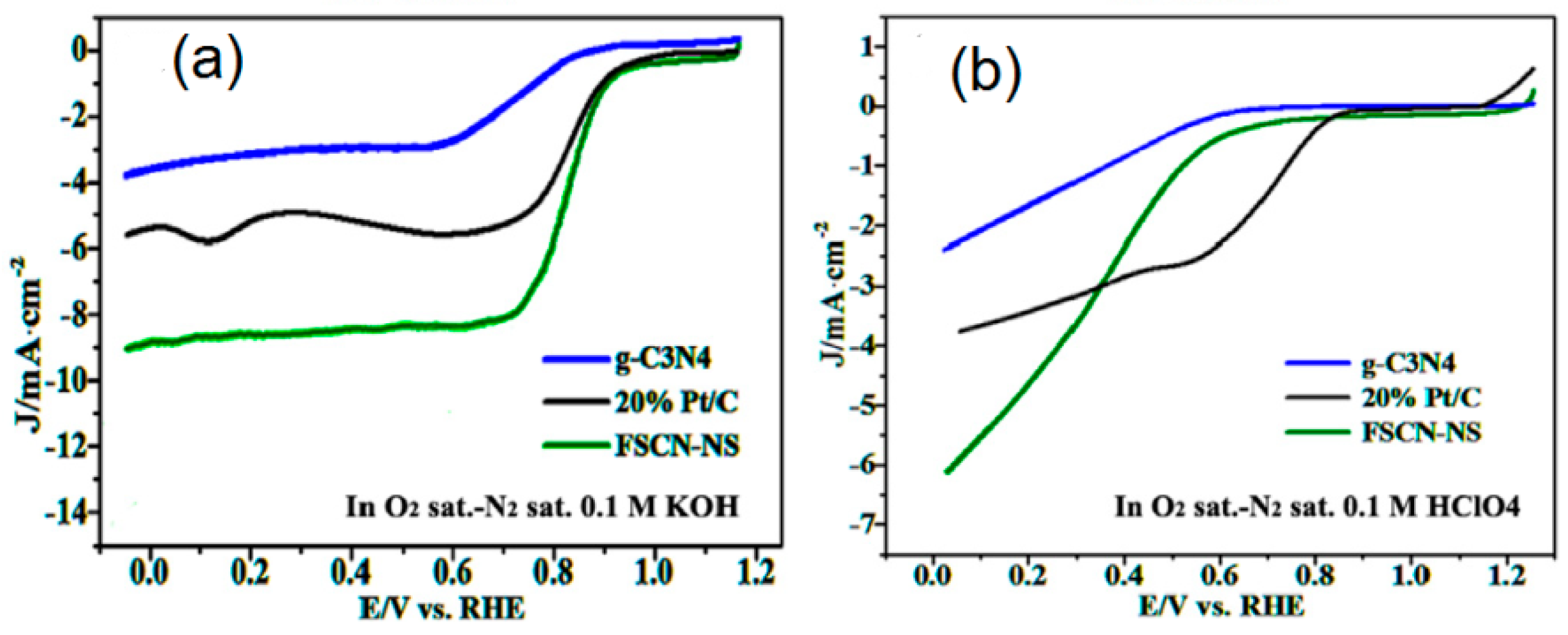
3.1.2. Element-Doped g-C3N4 and g-C3N4–Carbon Composites
3.2. h-BN
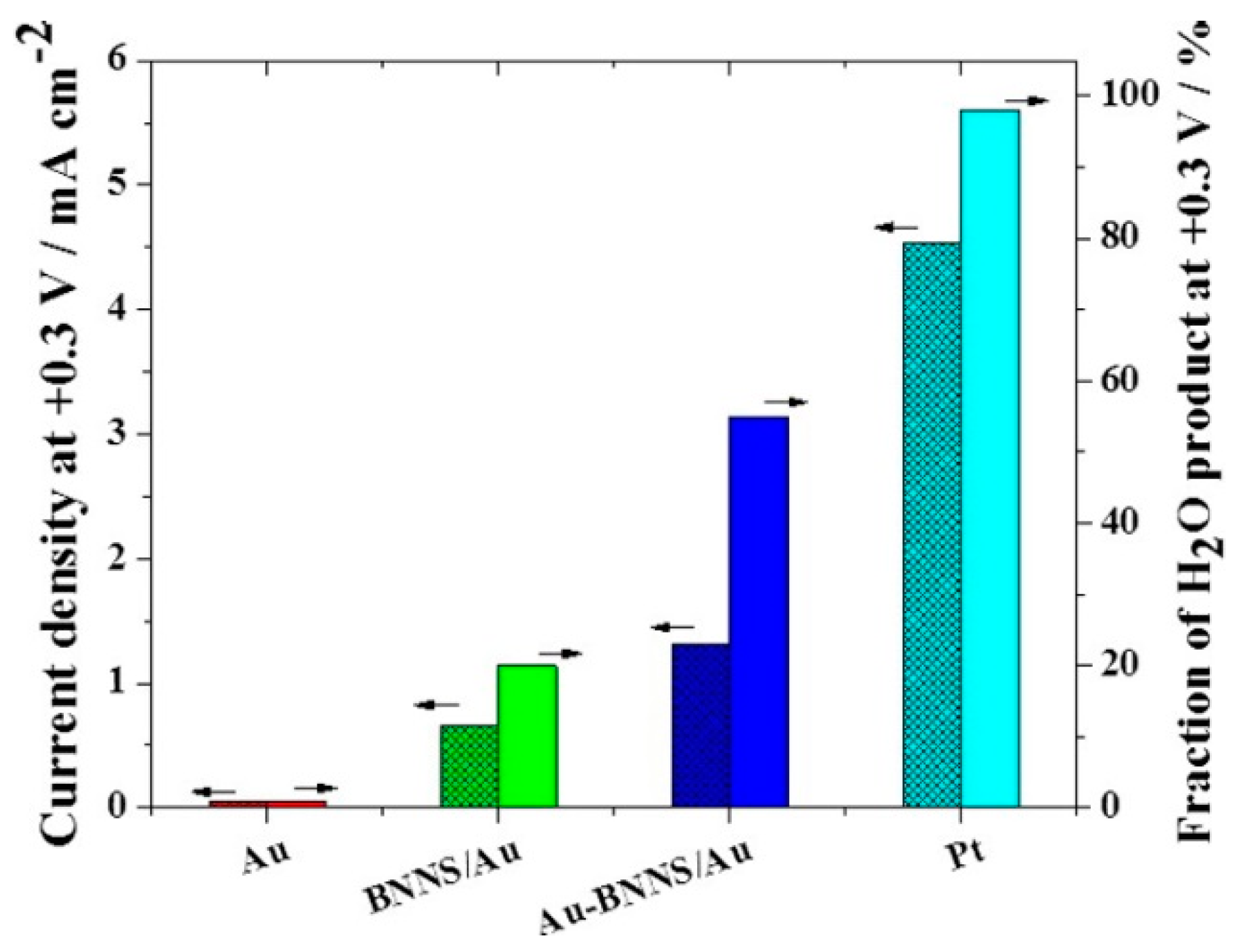
3.2.1. Metal-Supported h-BN
3.2.2. Metal-Doped h-BN
3.2.3. Carbon-Doped h-BN
3.2.4. Graphene-h-BN Composites
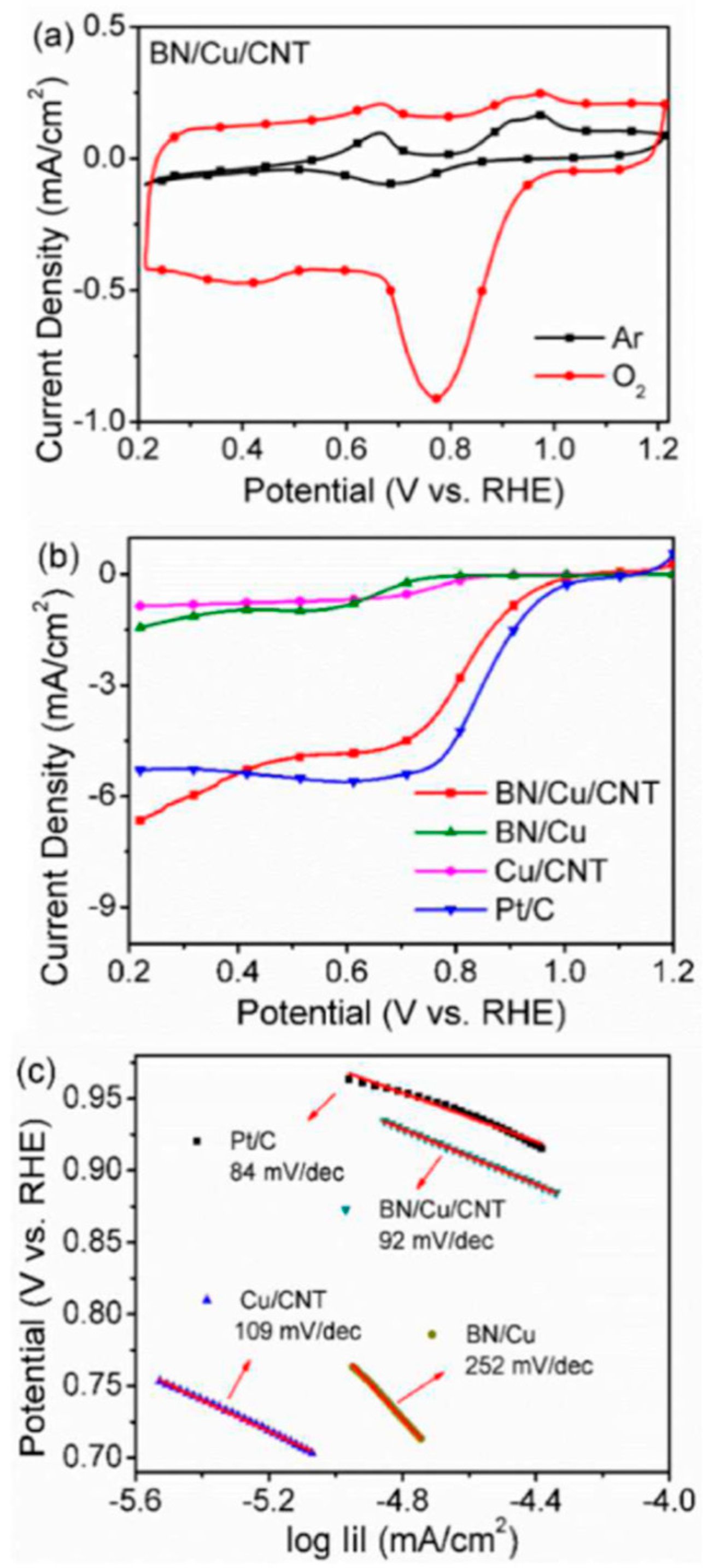
3.2.5. Tri-Functional h-BN-Based Electrocatalysts
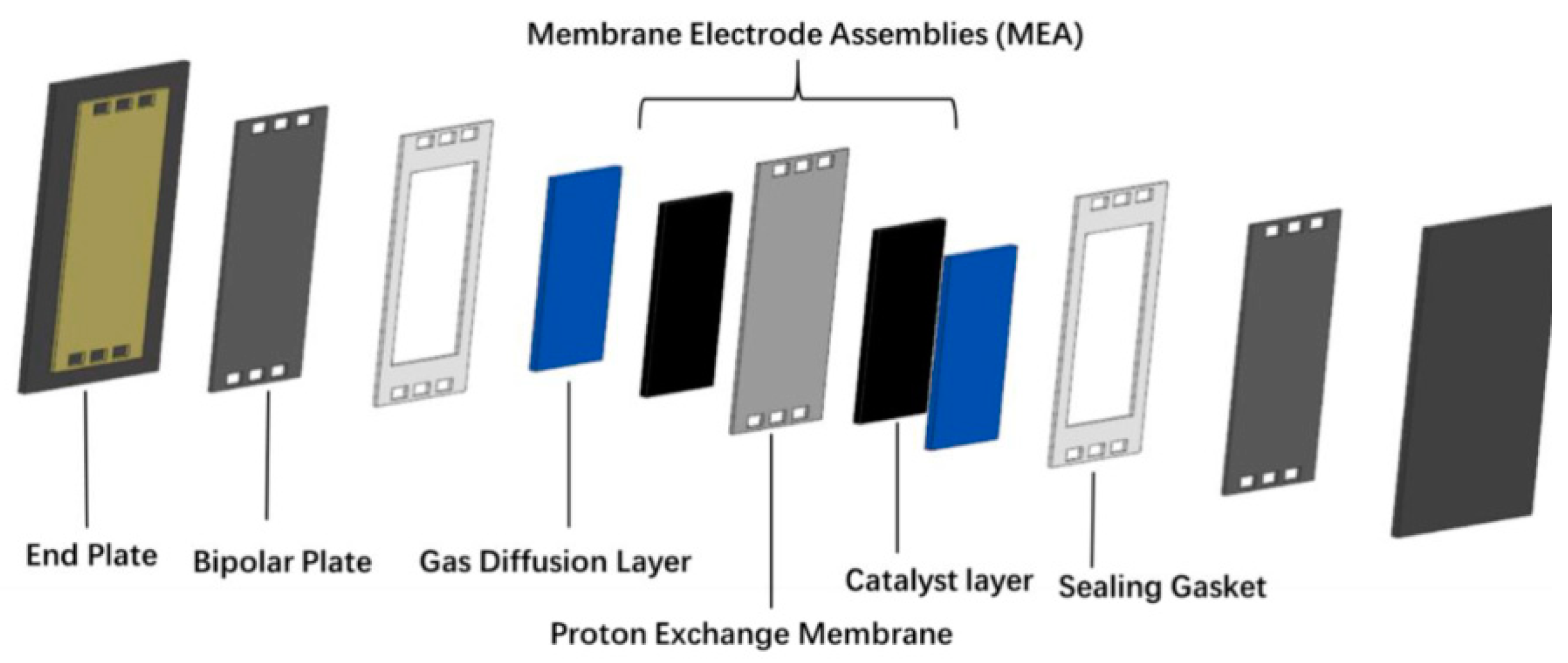
4. Composite Membrane
4.1. h-BN and h-BN-Containing Composite Membranes
4.1.1. h-BN Membranes
4.1.2. h-BN-Nafion Composite Membranes
4.1.3. h-BN-Non-Perfluorinated Polymer Composite Membranes
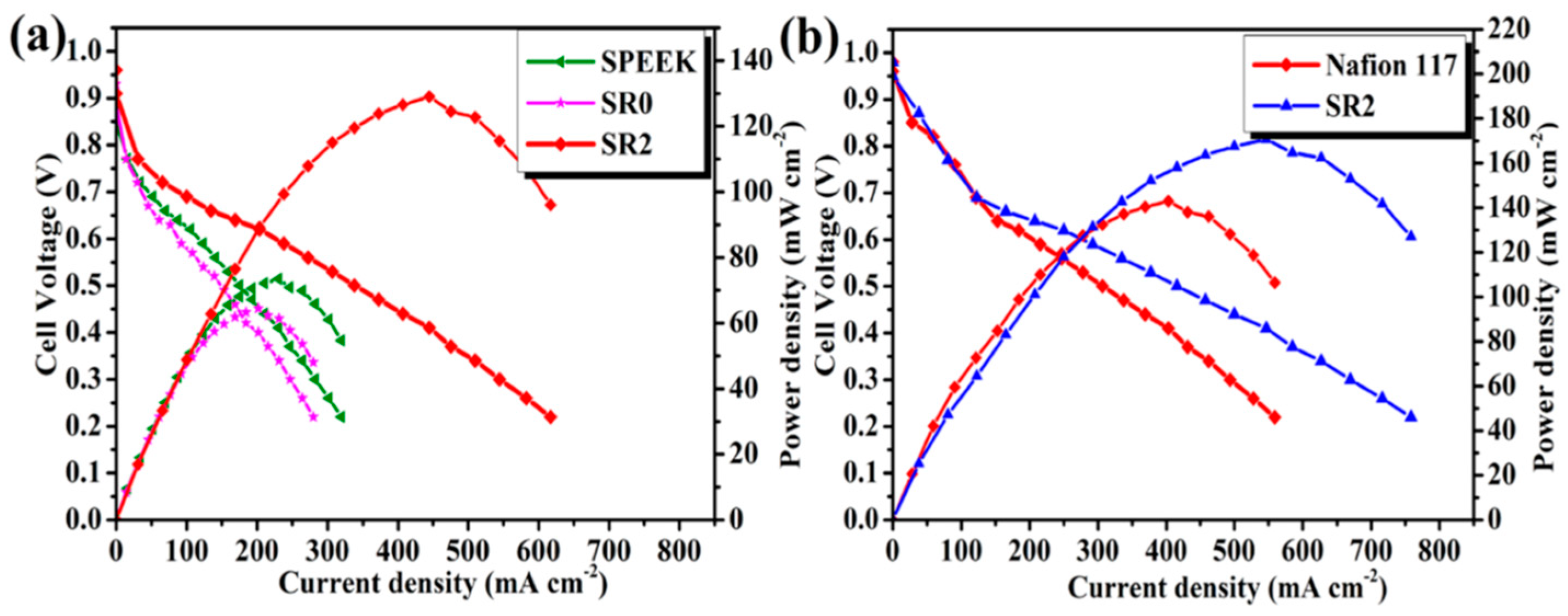
4.2. g-C3N4-Containing Composite Membranes
5. Summary and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Joy, J.; George, E.; Vijayan, P.P.; Anas, S.; Thomas, S. An overview of synthesis, morphology, and versatile applications of nanostructured graphitic carbon nitride (g-C3N4). J. Ind. Eng. Chem. 2024, 133, 74–89. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Hamdy, M.S.; Taha, T.A.; AlSalem, H.S.; Alenad, A.M.; Amin, M.A.; Shah, R.; Palamanit, A.; Khan, J.; et al. Fabrication, characteristics, and applications of boron nitride and their composite nanomaterials. Surf. Interfaces 2022, 29, 101725. [Google Scholar] [CrossRef]
- Xing, L.; Shi, W.; Su, H.; Xu, Q.; Das, P.K.; Mao, B.; Scott, K. Membrane electrode assemblies for PEM fuel cells: A review of functional graded design and optimization. Energy 2019, 177, 445–464. [Google Scholar] [CrossRef]
- Shi, D.; Cai, L.; Zhang, C.; Chen, D.; Pan, Z.; Kang, Z.; Liu, Y.; Zhang, J. Fabrication methods, structure design and durability analysis of advanced sealing materials in proton exchange membrane fuel cells. Chem. Eng. J. 2023, 454, 139995. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Abdelghani, E.A.M.; Olabi, A.G. Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 2021, 46, 23529–23547. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Taniguchi, A.; Akita, T.; Yasuda, K.; Miyazaki, Y. Analysis of electrocatalyst degradation in PEMFC caused by cell reversal during fuel starvation. J. Power Sources 2004, 130, 42–49. [Google Scholar] [CrossRef]
- Roen, L.M.; Paik, C.H.; Jarvi, T.D. Electrocatalytic corrosion of carbon support in PEMFC cathodes. Electrochem. Sol. State Lett. 2004, 7, A19–A23. [Google Scholar] [CrossRef]
- Antolini, E. Graphene as a new carbon support for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2012, 123–124, 52–68. [Google Scholar] [CrossRef]
- Antolini, E. Ceramic materials as supports for low-temperature fuel cell catalysts. Solid State Ionics 2009, 180, 746–763. [Google Scholar] [CrossRef]
- Sinniah, J.D.; Wong, W.Y.; Loh, K.S.; Yunus, R.M.; Timmiati, S.N. Perspectives on carbon-alternative materials as Pt catalyst supports for a durable oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2022, 534, 231422. [Google Scholar] [CrossRef]
- Mansor, N.; Miller, T.S.; Dedigama, I.; Jorge, A.B.; Jia, J.; Brázdová, V.; Mattevi, C.; Gibbs, C.; Hodgson, D.; Shearing, P.R.; et al. Graphitic Carbon Nitride as a Catalyst Support in Fuel Cells and Electrolyzers. Elecrochim. Acta 2016, 222, 44–57. [Google Scholar] [CrossRef]
- Prabakaran, K.; Jandas, P.J.; Luo, J.; Fu, C. Graphitic carbon nitride for fuel cells. In Nanoscale Graphitic Carbon Nitride; Pandikumar, A.C., Murugan, S.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 341–365. [Google Scholar]
- Antolini, E. Composite materials: An emerging class of fuel cell catalyst supports. Appl. Catal. B: Environ. 2010, 100, 413–426. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-based catalyst supports for oxygen reduction in proton-exchange membrane fuel cells. Trends Chem. 2022, 4, 886–906. [Google Scholar] [CrossRef]
- Antolini, E. Nitrogen-doped carbons by sustainable N- and C-containing natural resources as nonprecious catalysts and catalyst supports for low temperature fuel cells. Renew. Sust. Energy Rev. 2016, 58, 34–51. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Wei, X.; Lin, R. Effect of nitrogen-doped carbon support on the performance of catalysts for the oxygen reduction reaction. ACS Appl. Energy Mater. 2024, 7, 8705–8714. [Google Scholar] [CrossRef]
- Brandiele, R.; Durante, C.; Zerbetto, M.; Vicentini, N.; Kosmala, T.; Badocco, D.; Pastore, P.; Rizzi, G.A.; Isse, A.A.; Gennaro, A. Probing the correlation between Pt-support interaction and oxygen reduction reaction activity in mesoporous carbon materials modified with Pt-N active sites. Electrochim. Acta 2018, 277, 287–300. [Google Scholar] [CrossRef]
- Hornberger, E.; Merzdorf, T.; Schmies, H.; Hübner, J.; Klingenhof, M.; Gernert, U.; Kroschel, M.; Anke, B.; Lerch, M.; Schmidt, J.; et al. Impact of Carbon N-Doping and Pyridinic-N Content on the Fuel Cell Performance and Durability of Carbon-Supported Pt Nanoparticle Catalysts. ACS Appl. Mater. Interfaces 2022, 14, 18420. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wang, Y.-G.; Chen, X.; Liu, M.; Zheng, Z.; Peng, X. Carbon corrosion mechanism on nitrogen-doped carbon support —A density functional theory study. Int. J. Hydrogen Energy 2021, 46, 13273–13282. [Google Scholar] [CrossRef]
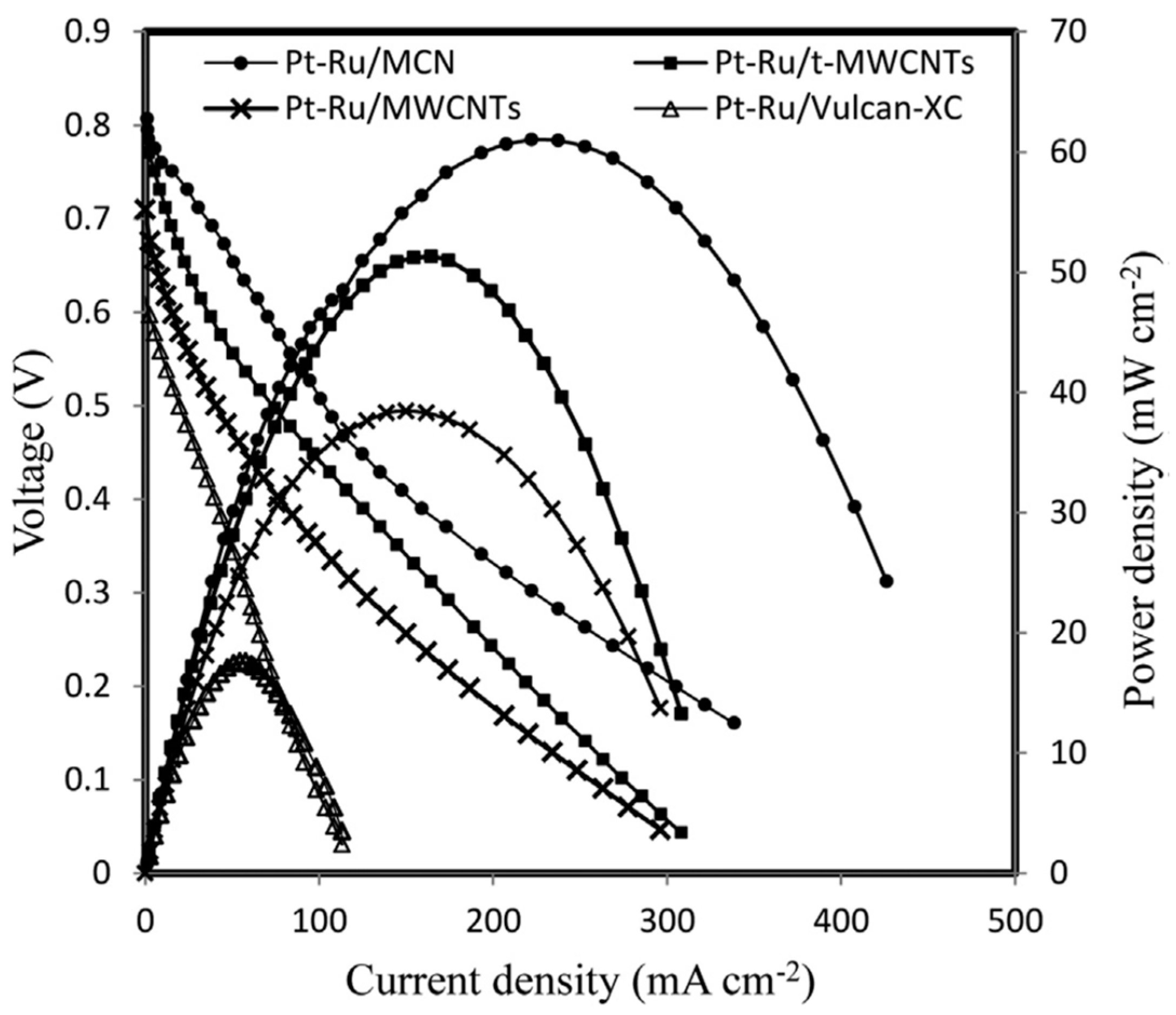
- Kim, M.; Hwang, S.; Yu, J.-S. Novel ordered nanoporous graphitic C3N4 as a support for Pt–Ru anode catalyst in direct methanolfuel cell. J. Mater. Chem. 2007, 17, 1656–1659. [Google Scholar] [CrossRef]
- Yue, B.; Ma, Y.; Tao, H.; Yu, L.; Jian, G.; Wang, X.; Wang, X.; Lu, Y.; Hu, Z. CNxnanotubes as catalyst support to immobilize platinum nanoparticles for methanol oxidation. J. Mater. Chem. 2008, 18, 1747–1750. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, S.; Jian, G.; Tao, H.; Yu, L.; Wang, X.; Wang, X.; Zhu, J.; Hu, Z.; Chen, Y. CNx nanofibers converted from polypyrrole nanowires as platinum support for methanol oxidation. Energy Environ. Sci. 2009, 2, 224–229. [Google Scholar] [CrossRef]
- Sadhukhan, M.; Kundu, M.K.; Bhowmik, T.; Barman, S. Highly dispersed platinum nanoparticles on graphitic carbon nitride: A highly active and durable electrocatalyst for oxidation of methanol, formic acid and formaldehyde. Int. J. Hydrogen Energy 2017, 42, 9371–9383. [Google Scholar] [CrossRef]
- Mansor, N.; Jorge, A.B.; Corà, F.; Gibbs, C.; Jervis, R.; McMillan, P.F.; Wang, X.; Brett, D.J.L. Graphitic carbon nitride supported catalysts for polymer electrolyte fuel cells. J. Phys. Chem. C 2014, 118, 6831–6838. [Google Scholar] [CrossRef]
- Goel, J.; Basu, S. Effect of support materials on the performance of direct ethanol fuel cell anode catalys. Int. J. Hydrogen Energy 2014, 39, 15956–15966. [Google Scholar] [CrossRef]
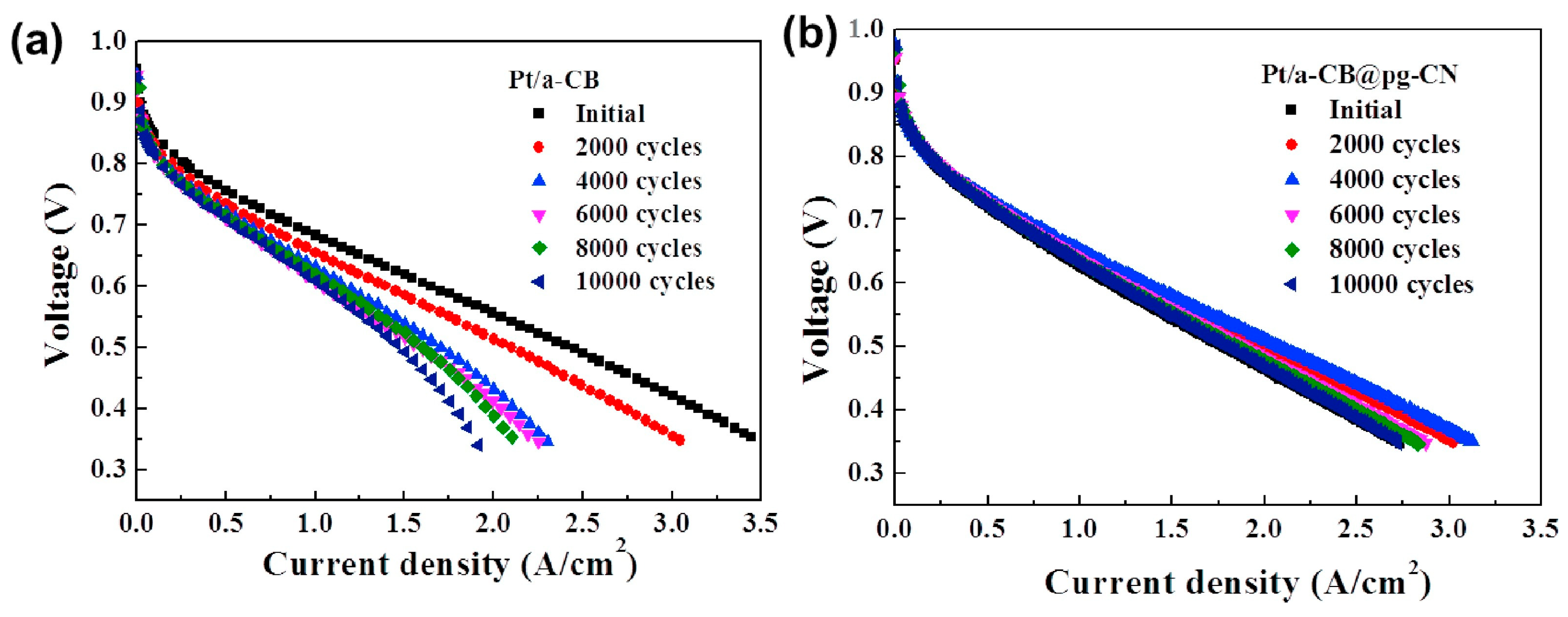
- Zhang, W.; Cao, Z.; Zhang, J.; Peng, K.; Ma, Q.; Xu, Q.; Su, H. Enhanced durability of Pt-based electrocatalysts in high-temperature polymer electrolyte membrane fuel cells using a graphitic carbon nitride nanosheet support. ACS Sustain. Chem. Eng. 2020, 8, 9195–9205. [Google Scholar] [CrossRef]
- Qian, H.; Huang, H.; Wang, X. Design and synthesis of palladium/graphitic carbon nitride/carbon black hybrids as high-performance catalysts for formic acid and methanol electrooxidation. J. Power Sources 2015, 275, 734–741. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, J.; Chae, K.H.; Cho, M.K.; Jung, J.; Cho, J.; Lee, H.J.; Ham, H.C.; Kim, J.Y. Polymeric graphitic carbon nitride nanosheet-coated amorphous carbon supports for enhanced fuel cell electrode performance and stability. Appl. Catal. B Environ. 2018, 237, 318. [Google Scholar] [CrossRef]
- Fang, B.; Daniel, L.; Bonakdarpour, A.; Govindarajan, R.; Sharman, J.; Wilkinson, D.P. Pt nanowire electrocatalyst for improved fuel cell performance using a graphitic carbon nitride-decorated hierarchical nanocarbon support. Nano-Micro Small 2021, 17, 2102288. [Google Scholar] [CrossRef]
- Yu, D.; Lu, C.; Yan, Z.; Yang, X.; Yang, W.; Lv, Z.; Tian, Z.; Tian, Q. Palladium nanoparticles supported on phosphorus-doped graphitic carbon nitride/carbon black hybrids for formic acid electro-oxidation. Int. J. Electrochem. Sci. 2021, 16, 211040. [Google Scholar] [CrossRef]
- Alawadhi, H.; Abdelkareem, M.A.; Hussain, N.; Wilberforce, T.; Sayed, E.T. A composite of graphitic carbon nitride and Vulcan carbon as an effective catalyst support for Ni in direct urea fuel cells. J. Taiwan Inst. Chem. Eng. 2020, 116, 160. [Google Scholar] [CrossRef]
- Liang, X.; Dong, F.; Tang, Z.; Wang, Q. The significant promotion of g-C3N4 on Pt/CNS catalyst for the electrocatalytic oxidation of methanol. Int. J. Hydrogen Energy 2021, 46, 39645–39657. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Vajtai, R.; Wang, X.; Ajayan, P.M. Pt-decorated 3D architectures built from graphene and graphitic carbon nitride nanosheets as efficient methanol oxidation catalysts. Adv. Mater. 2014, 26, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Z.; Wang, Z.-B.; Sui, X.-L.; Zhang, L.-M.; Gu, D.-M. Ultrathin graphitic carbon nitride nanosheets and graphene composite material as high-performance PtRu catalyst support for methanol electro-oxidation. Carbon 2016, 93, 105115. [Google Scholar] [CrossRef]
- Song, C.; Kim, S. Preparation and electrochemical characterization of Pt-supported flake-like graphitic carbon nitride on reduced graphene oxide as fuel cell catalysts. J. Electrochem. Soc. 2015, 162, F1181–F1186. [Google Scholar] [CrossRef]
- Song, C.; Park, S.-J.; Kim, S. Effect of modification by polydopamine and polymeric carbon nitride on methanol oxidation ability of Pt catalysts-supported on reduced graphene oxide. J. Electrochem. Soc. 2016, 163, F668–F674. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, H.; Li, F.; Deng, K.; Wang, X. Palladium nanoparticles supported on graphitic carbon nitride-modified reduced graphene oxide as highly efficient catalysts for formic acid and methanol electrooxidation. J. Mater. Chem. A 2014, 2, 19084–19094. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Q.; Wu, X.; Fu, Y.; Deng, K.; Wang, X. Intimately coupled hybrid of graphitic carbon nitride nanoflakelets with reduced graphene oxide for supporting Pd nanoparticles: A stable nanocatalyst with high catalytic activity towards formic acid and methanol electrooxidation. Electrochim. Acta 2016, 200, 131–141. [Google Scholar] [CrossRef]
- Fang, D.; Yang, L.; Yang, G.; Yi, G.; Feng, Y.; Shao, P.; Shi, H.; Yu, K.; You, D.; Luo, X. Electrodeposited graphene hybridized graphitic carbon nitride anchoring ultrafine palladium nanoparticles for remarkable methanol electrooxidation. Int. J. Hydrogrn Enrtgy 2020, 45, 21483–21492. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Liu, D.; Cui, G.; Dou, B.; Wang, J. Efficient anchoring of nanoscale Pd on three-dimensional carbon hybrid as highly active and stable catalyst for electro-oxidation of formic acid. Appl. Catal. B Environ. 2020, 263, 118304. [Google Scholar] [CrossRef]
- Shen, B.; Wei, Y.; Sun, P.; He, H.; Ying, G.; Huang, H. Immobilizing ultrasmall Pt nanocrystals on 3D interweaving BCN nanosheet-graphene networks enables efficient methanol oxidation reaction. Dalton Trans. 2023, 52, 13644–13652. [Google Scholar] [CrossRef] [PubMed]
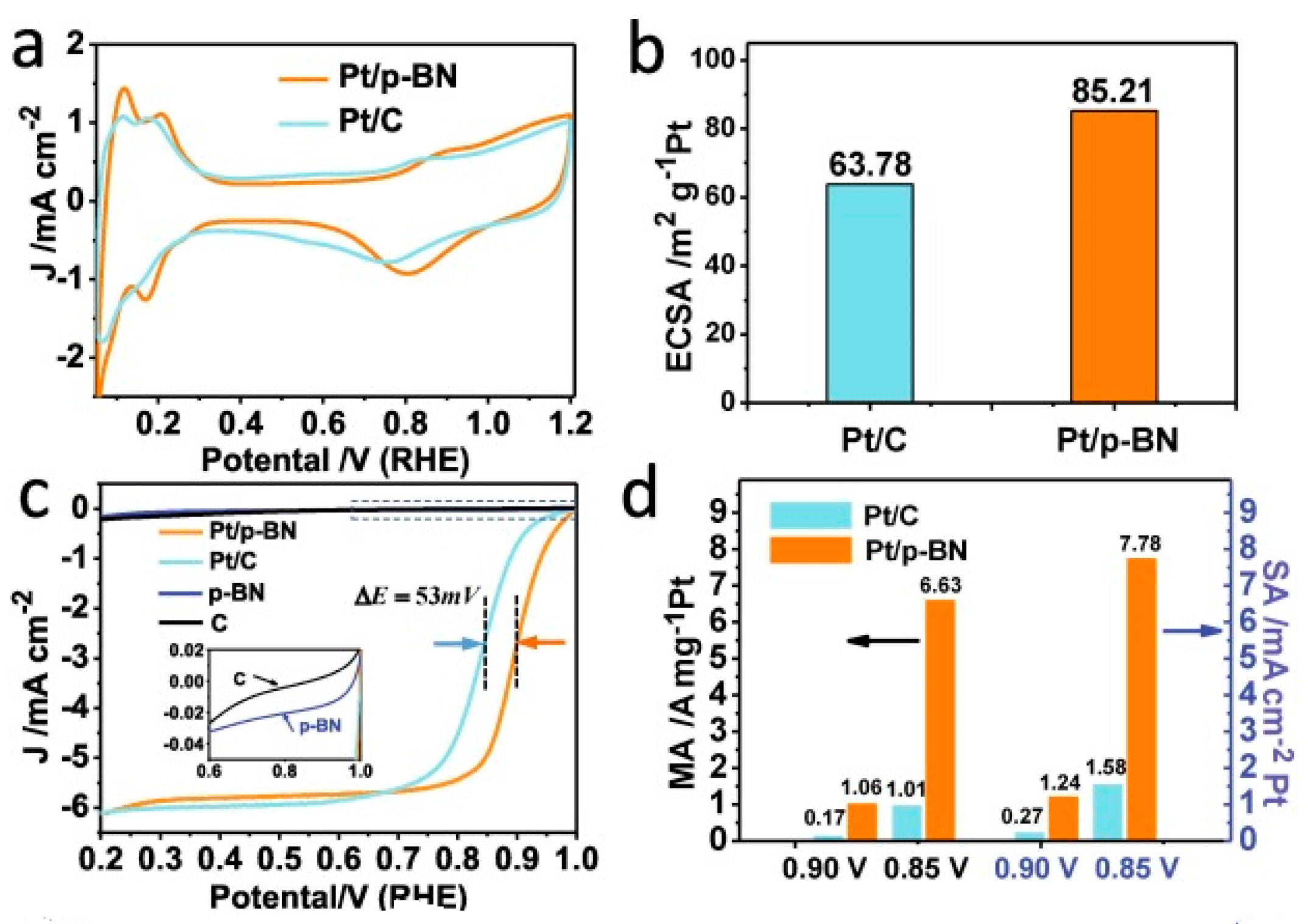
- Li, Q.; Li, L.; Yu, X.; Wu, X.; Xie, Z.; Wang, X.; Lu, Z.; Zhang, X.; Huang, Y.; Yang, X. Ultrafine platinum particles anchored on porous boron nitride enabling excellent stability and activity for oxygen reduction reaction. Chem. Eng. J. 2020, 399, 125827. [Google Scholar] [CrossRef]
- Lyalin, A.; Nakayama, A.; Uosaki, K.; Taketsugu, T. Theoretical predictions for hexagonal BN based nanomaterials as electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2013, 15, 2809–2830. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.-H.; Li, Y.-X.; Li, X.; Dong, C.-K.; Wu, D.-Y.; Tang, C.-C.; Chou, S.-L.; Fang, F.; Du, X.-W. Conductive boron nitride as promising catalyst support for the oxygen evolution reaction. Adv. Energy Mater. 2020, 10, 1902521. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Z.; Foo, G.S.; Gao, X.; Zhou, M.; Liu, B.; Veith, G.M.; Wu, P.; Browning, K.L.; Lee, H.N.; et al. Taming interfacial electronic properties of platinum nanoparticles on vacancy-abundant boron nitride nanosheets for enhanced catalysis. Nat. Commun. 2017, 8, 15291. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; Li, P.; Zhao, G.; Wang, G.; Jiang, Y.; Chen, J.; Dou, S.X.; Pan, H.; Sun, W. Hexagonal boron nitride as a multifunctional support for engineering efficient electrocatalysts toward the oxygen reduction reaction. Nano Lett. 2020, 20, 6807–6814. [Google Scholar] [CrossRef]
- Zuo, L.-X.; Jiang, L.-P. Electrocatalysis of the oxygen reduction reaction and the formic acid oxidation reaction on BN/Pd composites prepared sonochemically. J. Electrochem. Soc. 2017, 164, H805–H811. [Google Scholar] [CrossRef]
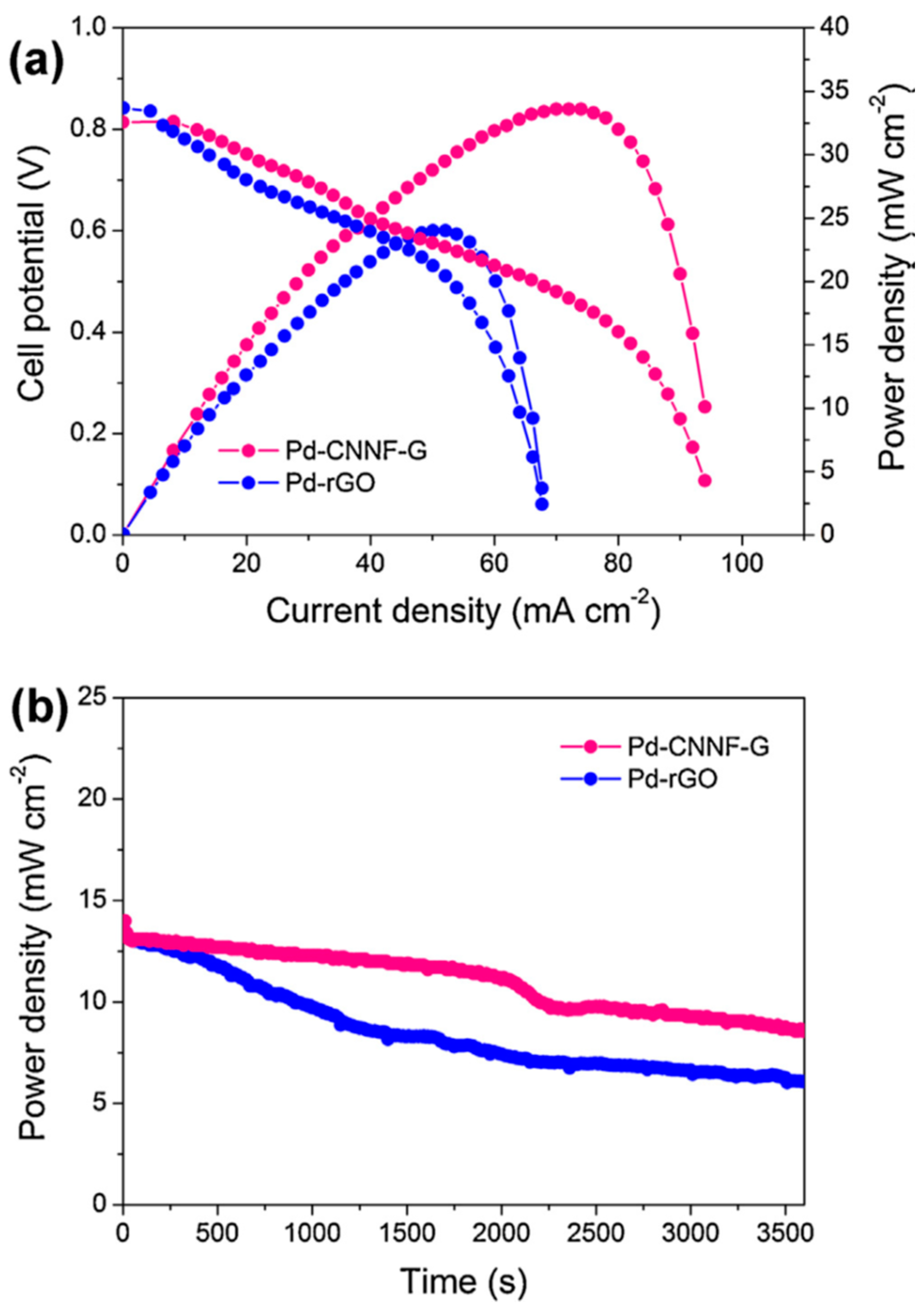
- Zhang, H.; Xu, L.; Tian, Y.; Jiao, A.; Li, S.; Liu, M.; Chen, X.; Chen, F. Convenient synthesis of 3D fluffy PtPd nanocorals loaded on 2D h-BN supports as highly efficient and stable electrocatalysts for alcohol oxidation reaction. ACS Omega 2019, 4, 11163–11172. [Google Scholar] [CrossRef]
- Zhang, H.; Li, D.; Li, Q.; Guo, K.; Yu, C.; Lin, J.; Tang, C.; Huang, Y. Pd nanoparticles anchored on porous boron nitride nanofibers as highly active and stable electrocatalysts for formic acid oxidation. Coll. Surf. A Physicochem. Eng. Aspects 2022, 646, 128947. [Google Scholar] [CrossRef]
- Li, R.; Lin, J.; Zhang, H.; Gong, Y.; Zhang, X.; Yu, C.; Tang, C.; Huang, Y. Improving electrocatalytic formic acid oxidation performance via catalyst layer design utilizing porous boron nitride-supported Pd catalysts. J. Power Sources 2025, 629, 236039. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of metal catalysts for oxygen reduction reaction: From nanoscale engineering to atomic design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Li, S.; Shi, L.; Guo, Y.; Wang, J.; Liu, D.; Zhao, S. Selective oxygen reduction reaction: Mechanism understanding, catalyst design and practical application. Chem. Sci. 2024, 15, 11188–11228. [Google Scholar] [CrossRef] [PubMed]
- Tarasevich, M.R.; Korchagin, O.V. Electrocatalysis and pH (a review). Russ. J. Electrochem. 2013, 49, 600–618. [Google Scholar] [CrossRef]
- Ishihara, A.; Ohgi, Y.; Matsuzawa, K.; Mitsushima, S.; Ota, K. Progress in non-precious metal oxide-based cathode for polymer electrolyte fuel cells. Electrochim. Acta 2010, 55, 8005–8012. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, S.; Luo, R.; Tang, H.; Wang, R.; Zhang, R.; Tian, T.; Tang, H. Transition metal nitrides for electrocatalytic application: Progress and rational design. Nanomaterials 2022, 12, 2660. [Google Scholar] [CrossRef]
- Bidault, F.; Brett, D.J.L.; Middleton, P.H.; Brandon, N.P. Review of gas diffusion cathodes for alkaline fuel cells. J. Power Sources 2009, 187, 39–48. [Google Scholar] [CrossRef]
- Kalyani, A.K.M.; Rajeev, R.; Benny, L.; Cherian, A.R.; Varghese, A. Surface tuning of nanostructured graphitic carbon nitrides for enhanced electrocatalytic applications: A review. Mater. Chem. 2023, 30, 101523. [Google Scholar]
- Govindaraju, V.R.; Sureshkumar, K.; Ramakrishnappa, T.; Muralikrishna, S.; Samrat, D.; Pai, R.K.; Kumar, V.; Vikrant, K.; Kim, K.-H. Graphitic carbon nitride composites as electro catalysts: Applications in energy conversion/storage and sensing system. J. Clean. Prod. 2021, 320, 128693. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, D.; Wang, F.; Chen, M. Element-doped graphitic carbon nitride: Confirmation of doped elements and applications. Nanoscale Adv. 2021, 3, 4370–4387. [Google Scholar] [CrossRef]
- Kong, L.; Wang, J.; Ma, F.; Sun, M.; Quan, J. Graphitic carbon nitride nanostructures: Catalysis. Appl. Mater. 2019, 16, 388–424. [Google Scholar] [CrossRef]
- Kwon, K.; Sa, Y.J.; Cheon, J.Y.; Joo, S.H. Ordered mesoporous carbon nitrides with graphitic frameworks as metal-free, highly durable, methanol-tolerant oxygen reduction catalysts in an acidic medium. Langmuir 2012, 28, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Mahmood, N.; Zhu, J.; Mahmood, A.; Butt, F.K.; Rizwan, S.; Aslam, I.; Tanveer, M.; Idrees, F.; Shakir, I.; et al. One dimensional graphitic carbon nitrides as effective metal-free oxygen reduction catalysts. Sci. Rep. 2015, 5, 12389. [Google Scholar] [CrossRef] [PubMed]
- Lyth, S.M.; Nabae, Y.; Moriya, S.; Kuroki, S.; Kakimoto, M.-A.; Ozaki, J.-I.; Miyata, S. Carbon nitride as a nonprecious catalyst for electrochemical oxygen reduction. J. Phys. Chem. C 2009, 113, 20148–20151. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous graphitic-C3N4@carbon metal-free electrocatalysts for highly efficient oxygen reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, Y.; Chen, J.; Liu, J.; Hulicova-Jurcakova, D.; Jaroniec, M.; Qiao, S.Z. Facile oxygen reduction on a three-dimensionally ordered macroporous graphitic C3N4/carbon composite electrocatalyst. Angew Chem. 2012, 51, 3892–3896. [Google Scholar] [CrossRef]
- Qin, Y.; Li, J.; Yuan, J.; Kong, Y.; Tao, Y.; Lin, F.; Li, S. Hollow mesoporous carbon nitride nanosphere/three-dimensional graphene composite as high efficient electrocatalyst for oxygen reduction reaction. J. Power Sources 2014, 272, 696–702. [Google Scholar] [CrossRef]
- Fu, X.; Hu, X.; Yan, Z.; Lei, K.; Li, F.; Cheng, F.; Chen, J. Template-free synthesis of porous graphitic carbon nitride/carbon composite spheres for electrocatalytic oxygen reduction reaction. Chem. Commun. 2016, 52, 1725–1728. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Wang, X.; Müllen, K. Graphene-based carbon nitride nanosheets as efficient metal-free electrocatalysts for oxygen reduction reactions. Angew. Chem. 2011, 50, 5339–5343. [Google Scholar] [CrossRef]
- Selvarajan, P.; Fawaz, M.; Sathish, C.I.; Li, M.; Chu, D.; Yu, X.; Breesec, M.B.H.; Yi, J.; Vinu, A. Activated graphene nanoplatelets decorated with carbon nitrides for efficient electrocatalytic oxygen reduction reaction. Adv. Energy. Sust. Res. 2021, 2, 2100104. [Google Scholar] [CrossRef]
- Garcia, J.L.; Miyao, T.; Inukai, J.; Tongol, B.J.V. Graphitic carbon nitride on reduced graphene oxide prepared via semi-closed pyrolysis as electrocatalyst for oxygen reduction reaction. Mater. Chem. Phys. 2022, 288, 126415. [Google Scholar] [CrossRef]
- Mane, R.S.; Periyasamy, G.; Jha, N. Sunlight assisted Lewis base enriched 2D/2D g-C3N4-graphene oxide composite as an efficient ORR electrocatalyst. Electrochim. Acta 2024, 481, 143916. [Google Scholar] [CrossRef]
- Kim, O.-H.; Cho, Y.-H.; Chung, D.Y.; Kim, M.J.; Yoo, J.M.; Park, J.E.; Choe, H.; Sung, Y.-E. Facile and gram-scale synthesis of metal-free catalysts: Toward realistic applications for fuel cells. Sci. Rep. 2015, 5, 8376. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Ma, L.; An, L.; Wang, F.; Xi, J.; Sun, H.; Luo, Z.; Wu, Q. Layered spongy-like O-doped g-C3N4: An efficient non-metal oxygen reduction catalyst for alkaline fuel cells. J. Electrochem. Soc. 2017, 164, F354–F359. [Google Scholar] [CrossRef]
- Xu, J.; Li, B.; Li, S.; Liu, J. From melamine sponge towards 3D sulfur-doping carbon nitride as metal-free electrocatalysts for oxygen reduction reaction. Mater. Res. Express 2017, 4, 076305. [Google Scholar] [CrossRef]
- He, Q.; Zhou, F.; Zhan, S.; Huang, N.; Tian, Y. Photoassisted oxygen reduction reaction on mpg-C3N4: The effects of elements doping on the performance of ORR. Appl. Surf. Sci. 2018, 430, 325–334. [Google Scholar] [CrossRef]
- Qin, X.; Huang, Y.; Wang, K.; Xu, T.; Wang, Y.; Dong, W. Dual core-shell structured g-C3N4@Fe/Sr@g-C3N4 porous nanosphere as high efficient oxygen reduction reaction electrocatalyst in both acidic and alkaline media for fuel cells. Electrochim. Acta 2019, 322, 134745. [Google Scholar] [CrossRef]
- Xu, C.; Han, Q.; Zhao, Y.; Wang, L.; Li, Y.; Qu, L. Sulfur-doped graphitic carbon nitride decorated with graphene quantum dots for an efficient metal-free electrocatalyst. J. Mater. Chem. A 2015, 3, 1841–1846. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Jia, F.; Xie, J.; Li, W. Three-dimensional phosphorus-doped graphitic-C3N4 self-assembly with NH2-functionalized carbon composite materials for enhanced oxygen reduction reaction. Langmuir 2016, 32, 12569–12578. [Google Scholar] [CrossRef]
- Sarkar, S.; Sumukh, S.S.; Roy, K.; Kamboj, N.; Purkait, T.; Das, M.; Dey, R.S. Facile one step synthesis of Cu-g-C3N4 electrocatalyst realized oxygen reduction reaction with excellent methanol crossover impact and durability. J. Coll. Interface Sci. 2020, 558, 182–189. [Google Scholar] [CrossRef]
- Sarkar, S.; Kamboj, N.; Das, M.; Purkait, T.; Biswas, A.; Dey, R.S. Universal approach for electronically tuned transition-metal-doped graphitic carbon nitride as a conductive electrode material for highly efficient oxygen reduction reaction. Inorg. Chem. 2020, 59, 1332–1339. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, R.K.; Ubaidullah, M.; Al-Enizi, A.M.; Pandit, B.; Nangan, S.; Angadi, V.J.; Yasin, G. Engineering of hollow mesoporous Fe-graphitic carbon Nitride@CNTs for superior electrocatalytic oxygen reduction reaction. Fuel 2024, 357A, 129809. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, J.; Wu, C.; Guan, L. Strong-coupled Co-g-C3N4/SWCNTs composites as high-performance electrocatalysts for oxygen reduction reaction. RSC Adv. 2015, 5, 65303–65307. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, M. Metal–nitrogen (Co-g-C3N4) doping of surface-modified single-walled carbon nanohorns for use as an oxygen reduction electrocatalyst. RSC Adv. 2016, 6, 25670–25677. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, T.; Bo, X.; Zhou, M. Cobalt-doped carbon nitride supported on ordered mesoporous carbon as noble metal-free oxygen reduction electrocatalysts. J. Phis. Chem. Solids 2019, 131, 111–118. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J. Graphene supported Co-g-C3N4 as a novel metal–macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Chen, S.-N.; Wang, Y.-Z.; Ho, K.-S.; Chuang, J.-K.; Ho, L.-C. Cobalt-doped carbon nitride frameworks obtained from calcined aromatic polyimines as cathode catalyst of anion exchange membrane fuel cells. Membranes 2022, 12, 74. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Lee, D.-E.; Tonda, S. Cobalt- and iron-coordinated graphitic carbon nitride on reduced graphene oxide: A nonprecious bimetallic M–Nx–C analogue electrocatalyst for efficient oxygen reduction reaction in acidic media. Appl. Surf. Sci. 2020, 531, 147367. [Google Scholar] [CrossRef]
- Wasey, A.H.M.; Chakrabarty, S.; Das, G.P.; Majumder, C. h-BN monolayer on the Ni(111) surface: A potential catalyst for oxidation. ACS Appl. Mater. Interfaces 2013, 5, 10404–10408. [Google Scholar] [CrossRef]
- Lyalin, A.; Nakayama, A.; Uosaki, K.; Taketsugu, T. Functionalization of monolayer h-BN by a metal support for the oxygen reduction reaction. J. Phys. Chem. C 2013, 117, 21359–21370. [Google Scholar] [CrossRef]
- Koitz, R.; Nørskov, J.K.; Studt, F. A systematic study of metal-supported boron nitride materials for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 12722–12727. [Google Scholar] [CrossRef]
- Gao, X.; Wang, S.; Lin, S. Defective hexagonal boron nitride nanosheet on Ni(111) and Cu(111): Stability, electronic structures, and potential applications. ACS Appl. Mater. Interfaces 2016, 8, 24238–24247. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Siahrostami, S. Noble metal supported hexagonal boron nitride for the oxygen reduction reaction: A DFT study. Nanoscale Adv. 2019, 1, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, G.; Noguchi, H.; Uosaki, K. Electrocatalytic activity of various types of h-BN for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 13755–13761. [Google Scholar] [CrossRef] [PubMed]
- Uosaki, K.; Elumalai, G.; Noguchi, H.; Masuda, T.; Lyalin, A.; Nakayama, A.; Taketsugu, T. Boron nitride nanosheet on gold as an electrocatalyst for oxygen reduction reaction: Theoretical suggestion and experimental Proof. J. Am. Chem. Soc. 2014, 136, 6542–6545. [Google Scholar] [CrossRef]
- Elumalai, G.; Noguchi, H.; Lyalin, A.; Taketsugu, T.; Uosaki, K. Gold nanoparticle decoration of insulating boron nitride nanosheet on inert gold electrode toward an efficient electrocatalyst for the reduction of oxygen to water. Electrochem. Commun. 2016, 66, 53–57. [Google Scholar] [CrossRef]
- Elumalai, G.; Noguchi, H.; Dinh, H.C.; Uosaki, K. An efficient electrocatalyst for oxygen reduction to water—Boron nitride nanosheets decorated with small gold nanoparticles (~5 nm) of narrow size distribution on gold substrate. J. Electroanal. Chem. 2018, 819, 107–113. [Google Scholar] [CrossRef]
- Dinh, H.C.; Elumalai, G.; Noguchi, H.; Lyalin, A.; Taketsugu, T.; Uosaki, K. Size dependent electrocatalytic activities of h-BN for oxygen reduction reaction to water. J. Chem. Phys. 2023, 158, 134713. [Google Scholar] [CrossRef]
- Lin, S.; Ye, X.; Johnson, R.S.; Guo, H. First-principles investigations of metal (Cu, Ag, Au, Pt, Rh, Pd, Fe, Co, and Ir) doped hexagonal boron nitride nanosheets: Stability and catalysis of CO oxidation. J. Phys. Chem. C 2013, 117, 17319–17326. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Zhao, J. Iron-embedded boron nitride nanosheet as a promising electrocatalyst for the oxygen reduction reaction (ORR): A density functional theory (DFT) study. J. Power Sources 2015, 287, 431–438. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Nematollahi, P. Potential of Si-doped boron nitride nanotubes as a highly active and metal-free electrocatalyst for oxygen reduction reaction: A DFT study. Synt. Met. 2017, 226, 129–138. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Saraswat, S.K.; Lagum, A.A.; Al-Ma’abreh, A.M.; Molani, F.; Al-Musawi, T.J.; Mohamed, A.M.A.; Kadhim, M.M. Study the single-atom Mn-doped catalysts on boron nitride sheet surface as cathode for oxygen reduction reaction in proton-exchange membrane fuel cells. Sust. Chem. Pharm. 2023, 33, 101115. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Jia, B.; Hao, J.; Zhang, C.; Wu, G.; Yuan, Y.; Ma, Y.; Li, Y.; Lu, P. Transition metal embedded in two-dimensional bi-BN as high activity single atom electrocatalyst for oxygen reduction reactions. Quantum Chem. 2023, 123, e27218. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Z.; Chu, J.; Peng, X.; Kong, A. Mesoporous carbon-doped boron nitrides for cathodic and anodic hydrogen peroxide electrosynthesis. Carbon 2024, 228, 119383. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z. Carbon-doped boron nitride nanosheet: An efficient metal-free electrocatalyst for the oxygen reduction reaction. J. Phys. Chem. C 2015, 119, 26348–26354. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Tran, L.N. Assessment of electrocatalytic performance of metal-free C-doped BN nanoflakes for oxygen reduction and hydrogen evolution reactions: A comparative study. J. Phys. Chem. C 2018, 122, 21124–21131. [Google Scholar] [CrossRef]
- Gao, M.; Adachi, M.; Lyalin, A.; Taketsugu, T. Long range functionalization of h-BN monolayer by carbon doping. J. Phys. Chem. C 2016, 120, 15993–16001. [Google Scholar] [CrossRef]
- Marbaniang, P.; Patil, I.; Lokanathan, M.; Parse, H.; Sesu, D.C.; Ingavale, S.; Kakade, B. Nanorice-like structure of carbon-doped hexagonal boron nitride as an efficient metal-free catalyst for oxygen electroreduction. ACS Sustain. Chem. Eng. 2018, 6, 11115–11122. [Google Scholar] [CrossRef]
- Zhan, W.; Gao, J.; Li, X.; Wang, H.; Gao, W.; Yin, H. High-efficient OER/ORR bifunctional electrocatalysts based on hexagonal boron nitride enabled by co-doping of transition metal and carbon. Appl. Phys. Lett. 2023, 123, 073901. [Google Scholar] [CrossRef]
- Patil, I.M.; Lokanathan, M.; Kakade, B. Three dimensional nanocomposite of reduced graphene oxide and hexagonal boron nitride as an efficient metal-free catalyst for oxygen electroreduction. J. Mater. Chem. A 2016, 4, 4506–4515. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, C.; Du, A.; Dou, S.; Li, Z. In-plane graphene/boron-nitride heterostructures as an efficient metal-free electrocatalyst for the oxygen reduction reaction. Nanoscale 2016, 8, 14084–14091. [Google Scholar] [CrossRef]
- Patil, I.M.; Lokanathan, M.; Ganesan, B.; Swam, A.; Kakade, B. Carbon nanotube/boron nitride nanocomposite as a significant bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Chem. Eur. J. 2017, 23, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.K.; Sahoo, K.R.; Thakur, P.; Sharma, R.; Bawan, S.; Podila, R.; Narayanan, T.N. Graphene–hBN non-van der Waals vertical heterostructures for four- electron oxygen reduction reaction. Phys. Chem. Chem. Phys. 2019, 21, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, S.; Zhang, H. Screening of single-atom catalysts sandwiched by boron nitride sheet and graphene for oxygen reduction and oxygen evolution. Renew. Energy 2022, 189, 502–509. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Z.; Huang, T.; Tang, C. BN/Cu/CNT nanoparticles as an efficient tri-functional electrocatalyst for ORR and OER. Int. J. Hydrogen Energy 2023, 48, 20368–20377. [Google Scholar] [CrossRef]
- Maiti, T.K.; Singh, J.; Dixit, P.; Majhi, J.; Bhushan, S.; Bandyopadhyay, A.; Chattopadhyay, S. Advances in perfluorosulfonic acid-based proton exchange membranes for fuel cell applications: A review. Chem. Eng. J. Adv. 2022, 12, 100372. [Google Scholar] [CrossRef]
- Sun, D.; Sun, Z.; Yang, D.; Jiang, X.; Tang, J.; Wang, X. Advances in boron nitride-based materials for electrochemical energy storage and conversion. Eco Energy 2023, 1, 375–404. [Google Scholar] [CrossRef]
- Yoon, S.I.; Seo, D.-J.; Kim, G.; Kim, M.; Jung, C.-Y.; Yoon, Y.-G.; Joo, S.H.; Kim, T.-Y.; Shin, H.S. AA′-stacked trilayer hexagonal boron nitride membrane for proton exchange membrane fuel cells. ACS Nano 2018, 12, 10764–10771. [Google Scholar] [CrossRef]
- Jia, W.; Wu, P. Stable boron nitride nanocomposites based membranes for high-efficiency proton conduction. Electrochim. Acta 2018, 273, 162–169. [Google Scholar] [CrossRef]
- Jia, W.; Tang, B.; Wu, P. Novel composite proton exchange membrane with connected long-range ionic nanochannels constructed via exfoliated nafion–boron nitride nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 14791–14800. [Google Scholar] [CrossRef]
- Akel, M.; Celik, S.U.; Bozkurt, A.; Ata, A. Nano hexagonal boron nitride–Nafion composite membranes for proton exchange membrane fuel cells. Polym Compos. 2016, 37, 422–428. [Google Scholar] [CrossRef]
- Parthiban, V.; Sahu, A. Performance enhancement of direct methanol fuel cells using a methanol barrier boron nitride–Nafion hybrid membrane. New J. Chem. 2020, 44, 7338–7349. [Google Scholar] [CrossRef]
- Lee, S.; Jang, W.; Kim, M.; Shin, J.E.; Park, H.B.; Jung, N.; Whang, D. Rational design of ultrathin gas barrier layer via reconstruction of hexagonal boron nitride nanoflakes to enhance the chemical stability of proton exchange membrane fuel cells. Nano-Micro Small 2019, 15, 1903705. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.A.M.; Shaari, N.; Zaiman, N.F.H.N. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly(ether ether ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- Oh, K.-H.; Lee, D.; Choo, M.J.; Park, K.H.; Jeon, S.; Hong, S.H.; Park, J.-K.; Choi, J.W. Enhanced durability of polymer electrolyte membrane fuel cells by functionalized 2D boron nitride nanoflakes. ACS Appl. Mater. Interfaces 2014, 6, 7751–7758. [Google Scholar] [CrossRef]
- Huang, Z.; Lv, B.; Zhou, L.; Wei, T.; Qin, X.; Shao, Z. Ultra-thin h-BN doped high sulfonation sulfonated poly(ether-ether-ketone) of PTFE-reinforced proton exchange membrane. J. Membrane Sci. 2022, 644, 120099. [Google Scholar] [CrossRef]
- Yadav, V.; Rajput, A.; Rathod, N.H.; Kulshrestha, V. Enhancement in proton conductivity and methanol cross-over resistance by sulfonated boron nitride composite sulfonated poly (ether ether ketone) proton exchange membrane. Int. J. Hydrogen Energy 2020, 45, 17017–17028. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Jaafar, J.; Ismail, A.F.; Goh, P.S.; Gangasalam, A.; Hanifah, N.F.R.; Wong, K.C.; Subramaniam, M.N.; Peter, J. Functionalized boron nitride embedded sulfonated poly (ether ether ketone) proton exchange membrane for direct methanol fuel cell applications. J. Environ. Chem. Eng. 2021, 9, 105876. [Google Scholar] [CrossRef]
- Gahlot, S.; Kulshrestha, V. Functionalized White graphene based composite proton exchange membrane: Improved durability and proton conductivity. Int. J. Hydrogen Energy 2018, 43, 21683–21689. [Google Scholar] [CrossRef]
- Kumar, G.R.; Pugalenthi, M.R.; Cao, G.; Manimuthu, R.P. Reinforced hydroxylated boron nitride on porous sulfonated poly(ether sulfone) with excellent electrolyte properties for H2/O2 fuel cells. Energy Fuels 2022, 36, 6445–6458. [Google Scholar] [CrossRef]
- Hussin, D.E.; Budak, Y.; Devrim, Y. Development and performance analysis of polybenzimidazole/boron nitride composite membranes for high-temperature PEM fuel cell. Int. J. Energy Res. 2021, 45, 4174. [Google Scholar]
- Pugalenthi, M.R.; Gayathri, R.; Cao, G.; Prabhu, M.R. Study of amine customized exfoliated BN sheets in SPEEK-PES based blend membrane for acid-base cation exchange membrane fuel cells. J. Environ. Chem. Eng. 2022, 10, 107025. [Google Scholar]
- Harameen, H.M.A.; Akay, R.G. Investigation into the influence of boron nitride addition on the properties of SPEEK/PBI based electrolyte membrane. Int. J. Hydrogen Energy 2024, 54, 189–199. [Google Scholar] [CrossRef]
- Ingabire, P.B.; Pan, X.; Haragirimana, A.; Li, N.; Hu, Z.; Chen, S. Enhanced conduction capability of nanocomposite membrane of quaternized poly (arylene ether sulfone)s covalently bonded with graphitic carbon nitride nanosheets for fuel cells. React. Funct. Polym. 2019, 144, 104260. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Q.; Cong, C.; Meng, X.; Zhou, Q. Influence of alkaline 2D carbon nitride nanosheets as fillers for anchoring HPW and improving conductivity of SPEEK nanocomposite membranes. Int. J. Hydrogen Energy 2017, 42, 10317–10328. [Google Scholar] [CrossRef]
- Shabani, M.; Entezari, M.H. Designing continuous proton-conductive channels for direct methanol fuel cell through the sulfonated poly(ether ether ketone)/carbon quantum dot/graphitic carbon nitride nanosheet. Eur. Polym. J. 2024, 202, 112641. [Google Scholar] [CrossRef]
- Gang, M.; He, G.; Li, Z.; Cao, K.; Li, Z.; Yin, Y.; Wu, H.; Jiang, Z. Graphitic carbon nitride nanosheets/sulfonated poly(ether ether ketone) nanocomposite membrane for direct methanol fuel cell application. J. Membrane Sci. 2016, 507, 1–11. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Jaafar, J.; Ismail, A.F.; Junoh, H.; Samavati, A.; Goh, P.S.; Gangasalam, A.; Peter, J. Synthesis and characterization of conductive polymer coated graphitic carbon nitride embedded sulfonated poly (ether ether ketone) membranes for direct methanol fuel cell applications. Int. J. Energy Res. 2021, 45, 16649–16666. [Google Scholar] [CrossRef]
- Zhai, S.; Song, H.; Jia, X.; Yang, K.; Feng, M.; He, S.; Lin, J. Fabrication of water-insoluble phosphotungstic acid-carbon nitride nanohybrids for promoting proton transport of nanocomposite proton exchange membranes. J. Power Sources 2021, 506, 230195. [Google Scholar] [CrossRef]
- Li, M.; Qu, S.; Zhang, C.; Duan, J.; Wang, W. Low-dimensional protonated carbon nitride incorporated sulfonated poly (ether ether ketone) for PEMFC applications. Ionics 2020, 26, 5629–5636. [Google Scholar] [CrossRef]
- Liu, L.; Shi, L.; Lv, J.; Sun, Q.; Zhang, Y.; Huang, Z.; Hu, Z.; Chen, S. Advanced composite membranes based on multifunctional fillers constructed by covalently linking phosphotungstic acid with mesoporous carbon nitride for high-performance and durable fuel cell under low humidity. J. Membrane Sci. 2024, 689, 122154. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Liu, Y.; Li, N.; Hu, Z.; Chen, S. Double-filler composite sulfonated poly(aryl ether ketone) membranes with graphite carbon nitride and graphene oxide as polyelectrolyte for fuel cells. Polymer 2022, 238, 124426. [Google Scholar] [CrossRef]
- Ingabire, P.B.; Haragirimana, A.; Liu, Y.; Li, N.; Hu, Z.; Chen, S. Titanium oxide/graphitic carbon nitride nanocomposites as fillers for enhancing the performance of SPAES membranes for fuel cells. J. Ind. Eng. Chem. 2020, 91, 213–222. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Haragirimana, A.; Ingabire, P.B.; Li, N.; Hu, Z.; Chen, S. Immobilized phosphotungstic acid for the construction of proton exchange nanocomposite membranes with excellent stability and fuel cell performance. Int. J. Hydrogen Energy 2020, 45, 17782–17794. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, X.; Li, N.; Hu, Z.; Chen, S. Improved performance of quaternized poly(arylene ether ketone)s/graphitic carbon nitride nanosheets composite anion exchange membrane for fuel cell applications. Appl. Surf. Sci. 2020, 503, 144071. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Simultaneous improvement of anion conductivity and cell durability through the formation of dense ion clusters of F-doped graphitic carbon nitride/quaternized poly(phenylene oxide) composite membrane. J. Membrane Sci. 2022, 650, 120384. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Li, N.; Hu, Z.; Chen, S. Sulfonated graphitic carbon nitride nanosheets as proton conductor for constructing long-range ionic channels proton exchange membrane. J. Membrane Sci. 2020, 601, 117908. [Google Scholar] [CrossRef]
- Lv, B.; Geng, K.; Yin, H.; Yang, C.; Hao, J.; Luan, Z.; Huang, Z.; Qin, X.; Song, W.; Li, N.; et al. Polybenzimidazole/cerium dioxide/graphitic carbon nitride nanosheets for high performance and durable high temperature proton exchange membranes. J. Membrane Sci. 2021, 639, 119760. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Son, B.; Shanmugam, S. Porous gC3N4–Gd2Zr2O7 enables the high-temperature operation of Nafion membranes in polymer electrolyte fuel cells over 500 hours. J. Mater. Chem. A 2022, 10, 8975–8988. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Gikunoo, E.K.; Shanmugam, S. Toward extended durability and power output of high temperature proton exchange membrane fuel cells with Gd2Zr2O7-C3N4 composite membrane. Ionics 2025, in press. [Google Scholar] [CrossRef]
- Songfeng, E.; Liu, J.; Zhao, R.; Ning, D.; Lu, Z. Formation mechanisms of hexagonal boron nitride nanosheets in solvothermal exfoliation. Langmuir 2023, 39, 1619–1628. [Google Scholar]
- Hosseini, S.S.; Mehrpooya, M.; Jahangir, M.H. Combination of g-C3N4 and triplex metal oxides for enhanced performance of oxygen reduction reaction. Anal. Bioanal. Electrochem. 2023, 15, 1098–1115. [Google Scholar]
- Shi, X.; Wang, W.; Miao, X.; Tian, F.; Xu, Z.; Li, N.; Jing, M. Constructing conductive channels between platinum nanoparticles and graphitic carbon nitride by gamma irradiation for an enhanced oxygen reduction reaction. ACS Appl. Mater. Interfaces 2020, 12, 46095–46106. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Luo, Y.; Zeng, L.; Huang, P.; Xu, S.; Liu, Y.; Wang, Y.; Li, X.; Xie, Y. Ag/g-C3N4 nanosheets as a progressive support of Pt catalyst for improved electrocatalytic oxidation of methanol. J. Mater. Sci. 2024, 59, 3573–3584. [Google Scholar] [CrossRef]


| Cell Component | Compound | Advantages | Disadvantages | |
|---|---|---|---|---|
| g-C3N4 | Catalyst support | Pristine g-C3N4 | High catalytic activity of supported catalysts, high stability, | Poor conductivity |
| g-C3N4–CB and g-C3N4–rGO composites | High catalytic activity of supported catalysts, high conductivity | Moderate stability | ||
| ORR catalyst | OMCN and 1D g-C3N4 | Good ORR activity in alkaline media, high stability, high methanol tolerance | Poor ORR activity in acid media, moderate conductivity, 2e− transfer | |
| g-C3N4–carbon composites | Good ORR activity in alkaline and acid media, High conductivity, 4e− transfer | Moderate stability | ||
| O-, S-, and P-doped g-C3N4 and g-C3N4–carbon composites | High ORR activity, close to Pt, high conductivity, 4e− transfer | Moderate stability of carbon composites | ||
| Cu-, Fe-, and Co-doped g-C3N4 and g-C3N4–carbon composites | High ORR activity, higher than Pt, high stability, 4e− transfer, high methanol tolerance | Moderate stability of carbon composites | ||
| Composite membrane | SPEEK, SPAES, and QPAEK/ mesoporous g-C3N4, F-, O-, P-, and H-doped g-C3N4, and sulfonic acid functionalized g-C3N4Double filler GO, CeO2, TiO2, Gd2Zr2O7, and HPW/g-C3N4 | High proton conductivity, high stability, high fuel cell performance, used with PEM and AEM | ||
| h-BN | Catalyst support | p-h-BN and h-BNNSs | Good activity of supported catalysts, co-catalytic activity, high durability | |
| ORR catalyst | Ni(111)- and Cu(111)-supported h-BN and defective h-BNNS | High ORR activity, close to Pt, high stability | Poor conductivity | |
| TM-doped h-BN and h-BNNs | High ORR activity, close to Pt., 4e− transfer, high stability | Only theoretical calculations | ||
| Carbon- and graphene-doped h-BN | High ORR activity, close to Pt, 4e− transfer, high methanol tolerance | |||
| Tri-functional h-BN/Cu/G | High ORR activity, close to Pt, 4e− transfer | |||
| Composite membrane | Nafion, SPEEK-, SPES-, and PBI-functionalized h-BN and h-BNNSs | High proton conductivity, high stability, high fuel cell performance | Only used with PEM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antolini, E. The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers. Molecules 2025, 30, 1852. https://doi.org/10.3390/molecules30081852
Antolini E. The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers. Molecules. 2025; 30(8):1852. https://doi.org/10.3390/molecules30081852
Chicago/Turabian StyleAntolini, Ermete. 2025. "The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers" Molecules 30, no. 8: 1852. https://doi.org/10.3390/molecules30081852
APA StyleAntolini, E. (2025). The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers. Molecules, 30(8), 1852. https://doi.org/10.3390/molecules30081852






